Abstract
Salmonella typhimurium expresses two antigenically distinct flagellins, each containing a different H antigen (i and 1,2), the combination of which is highly specific for this serotype. In this study, overlapping recombinant flagellin fragments were constructed from the fliC (H:i) and fljB (H:1,2) flagellin genes, and the expression products were tested for binding to H antigen-specific monoclonal and polyclonal antibodies. A minimal area, 86 amino acids for H:i and 102 amino acids for H:1,2, located in the central variable domain of each flagellin was required for the binding of serotype-specific antibodies, providing further evidence for the presence of a discontinuous H epitope. Two peptides comprising these areas were shown to be highly suitable for application as antigens in an enzyme-linked immunosorbent assay detecting S. typhimurium-specific antibody.
Worldwide, Salmonella typhimurium is a major cause of human food poisoning. The consumption of food products originating from infected animals is a primary source of human infection. To efficiently detect and ultimately eliminate the presence of Salmonella from animal reservoirs such as pigs and poultry, there is a need for a rapid and sensitive assay. Flagella are immunodominant antigenic surface structures of Salmonella that, upon infection, elicit an early short-lived humoral response in the host, including very young animals (19), making them ideal for the detection of infections in livestock of all ages. Flagellin is the major structural protein of flagella and carries the serotype-specific H-antigenic determinants (5). These H antigens are located in the central variable domain comprising flagellin regions IV, V, and VI (11, 20) and probably induce the production of serotype-specific antibodies in the infected host. On the other hand, antibodies against the conserved N- and C-terminal flagellin domains or regions I, II, and VIII give rise to cross-reactions between Salmonella serotypes and other Enterobacteriaceae in serological tests based on whole purified flagellum antigen (3). Furthermore, flagellar proteins are easily produced through heterologous expression systems and therefore can be obtained in large quantities that are relatively pure (7, 16). This is an advantage over the lipopolysaccharide (O antigens), the other commonly used antigen in Salmonella-specific serological assays. In a previous study we determined the location of the serotype-specific H:gm antigen of Salmonella enteritidis through the construction of overlapping recombinant peptides from its flagellin (16). Testing of these peptides with gm-specific antibodies resulted in the selection of a peptide that contained the H:gm epitope specific for S. enteritidis and allowed the development of an S. enteritidis-specific enzyme-linked immunosorbent assay (ELISA).
S. typhimurium (H:i:1,2) carries two distinct flagellin genes (fliC and fljB), and through phase variation it can express two antigenically different flagella on its surface (9). In the present study we have isolated both flagellin genes of S. typhimurium and cloned overlapping fragments of these genes in an expression vector. We produced monoclonal antibodies (MAbs) to both S. typhimurium flagellins and used these to select two recombinant flagellin fragments of 86 and 102 amino acids, specific to the H:i and H:1,2 antigens, respectively. These peptides appeared to be highly suitable antigens in an ELISA detecting S. typhimurium-specific antibodies.
Production and validation of H:i- and H:1,2-specific MAbs.
Purified S. typhimurium flagellins enriched in H:i or H:1,2 were prepared as described by van Zijderveld et al. (19). MAb-producing hybridoma cell lines were obtained from mice immunized with the purified flagellin. Three H:i-specific MAbs (IH10, IC11, and VB5) and three H:1,2-specific MAb-producing cell lines (XA1, XA9, and VIID6) were selected (19) and used for further analyses. The H:gm-specific MAb (gm3) from our previous studies (16, 19) was used as a negative control.
In order to confirm the specificities of the anti-i and anti-1,2 MAbs for their respective H antigens, serotyping of isolates of various biphasic Salmonella serotypes (Table 1) was performed on Western blots, both with MAbs and with commercially obtained H:i- H:1,2-, or H:gm-specific serotyping sera (absorbed agglutinating rabbit sera; Murex Diagnostics, Dartford, United Kingdom). Salmonella strains were cultured overnight at 37°C in Luria-Bertani broth, and whole-protein cell extracts were separated on a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel and then electrophoretically transferred (1) onto a Hybond C nitrocellulose filter (Amersham, Buckinghamshire, United Kingdom). Binding of commercial typing antiserum (1:400) or MAbs (IH10, 1:4,000; IC11 and VB5, 1:1,000; XA1, XA9, and VIID6, 1:4) to different Salmonella serotypes was analyzed by a Western blot assay performed essentially as described by Ausubel et al. (1).
TABLE 1.
Validation of H antigen-specific MAbs compared to commercial serotyping antisera (Murex) with whole-protein cell extracts of various Salmonella serotypes in Western blots
| Common H antigen | Salmonella strain (serotype) | H:i-specific antibody bindingd
|
H:1,2-specific antibody bindingd
|
||||
|---|---|---|---|---|---|---|---|
| Serotyping antisera
|
MAbs | Serotyping antisera
|
MAbs | ||||
| 1b | 2b | 1c | 2c | ||||
| H:i:1,2 | S. typhimurium (i:1,2) human isolatesa | + | + | + | + | + | + |
| S. typhimurium (i) human isolatesa | + | + | + | + | + | + | |
| S. typhimurium (1,2) human isolatesa | ± | + | ± | + | + | + | |
| S. typhimurium (1,2) human isolatesa | − | − | − | + | + | + | |
| S. typhimurium (i) ATCC 14028a | + | + | + | ± | − | − | |
| S. aberdeena (i) | + | + | + | ± | ± | − | |
| H:i | S. bonariensis (i:enx) | + | + | + | − | − | − |
| S. kentucky (i:z6) | + | + | + | ± | ± | − | |
| S. bergen, S. jukestown (i:enz15) | + | + | + | − | ± | − | |
| S. takoradi (i:1,5) | + | + | + | ± | − | − | |
| S. bandia, S. kedougou (i:lw) | + | + | + | ± | − | − | |
| S. schalkwijk (i:en) | + | + | + | ± | ± | − | |
| H:1,2 | S. heidelberg, S. virchow (r:1,2) | ± | ± | − | + | + | + |
| S. muenchen (d:1,2) | ± | ± | − | + | ± | ± | |
| S. newport (eh:1,2) | ± | ± | − | + | + | + | |
| H:gm | S. enteritidis (gm:−) | − | − | − | − | − | − |
The dominant H antigen, determined by agglutination, is shown in parentheses.
Serum batch 1, lot no. K002510; serum batch 2, lot no. K973010.
Serum batch 1, lot no. 846810; serum batch 2, lot no. K506610.
Antibody binding by Western blotting: +, strong; ±, weak; −, negative.
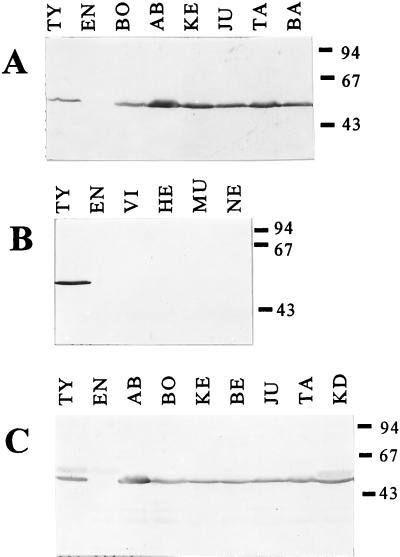
As expected, the commercial serotyping antisera showed only weak nonspecific binding to heterologous flagellins or other unrelated protein bands (Fig. 1C and Table 1). When we tested different batches of commercial typing H antigen-specific serum, the H:1,2 antigen-specific sera in particular showed some differences in their binding patterns to the Salmonella cell extracts (Table 1). All selected MAbs showed clear positive and negative recognition of their corresponding H antigens in whole-cell extracts of diverse Salmonella serotypes, with no detectable nonspecific binding (Table 1 and Fig. 1A and B). No H:1,2 flagellin was recognized in S. typhimurium ATCC 14028 or Salmonella aberdeen preparations (both serotype H:i:1,2) by H:1,2-specific MAbs, while H:i-specific MAbs strongly bound to both serotypes (Table 1). Subsequent agglutination of both strains with commercial typing sera confirmed the absence of H:1,2 flagellin. MAbs were highly suitable for the detection of H:i or H:1,2 Salmonella serotypes and, compared to serotyping sera MAbs, more specific in recognizing their H antigens, since they did not bind at all to heterologous flagellins or other proteins (Table 1 and Fig. 1).
FIG. 1.
Serotyping of Salmonella strains with MAb VB5 and commercial serotyping serum (batch 2) on Western blots. (A) H:i flagellin-containing Salmonella stained with VB5. (B) H:1,2 flagellin-containing Salmonella stained with VB5. (C) H:i flagellin-containing Salmonella stained with commercial H:i-specific serotyping serum. The Salmonella strains used (with the H serotype in parentheses) are as follows: AB, S. aberdeen (i:1,2); BA, S. bandia (i:lw); BE, S. bergen (i:enz15); BO, S. bonariensis (i:enx); EN, S. enteritidis (gm:-); HE, S. heidelberg (r:1,2); JU, S. jukestown (i:enz15); KE, S. kentucky (i:z6); KD, S. kedougou (i:lw); NE, S. newport (eh:1,2); MU, S. muenchen (d:1,2); TA, S. takoradi (i:1,5); TY, S. typhimurium (i:1,2); VI, S. virchow (r:1,2). Molecular size markers in kilodaltons are shown at the right.
Construction of flagellin gene clones.
The fliC gene (Fig. 2) was amplified from chromosomal DNA of S. typhimurium SL3261 (i,1,2 [14]) with the conserved flagellin domain primers E1 and E2 (Table 2 and Fig. 2). PCR was performed in a 50-μl reaction volume with 1 U of DNA polymerase (PrimeZyme kit; Biometra, Göttingen, Germany) and 25 pmol of each primer. For each reaction, 35 cycles, each consisting of three steps (94°C for 1 min, 50°C for 2 min, and 72°C for 3 min), were carried out, followed by a single step of 10 min at 72°C to complete elongation of the products. The amplified fliC gene was cloned into pEX11 (6) and electroporated into Escherichia coli POP2136 (8).
FIG. 2.
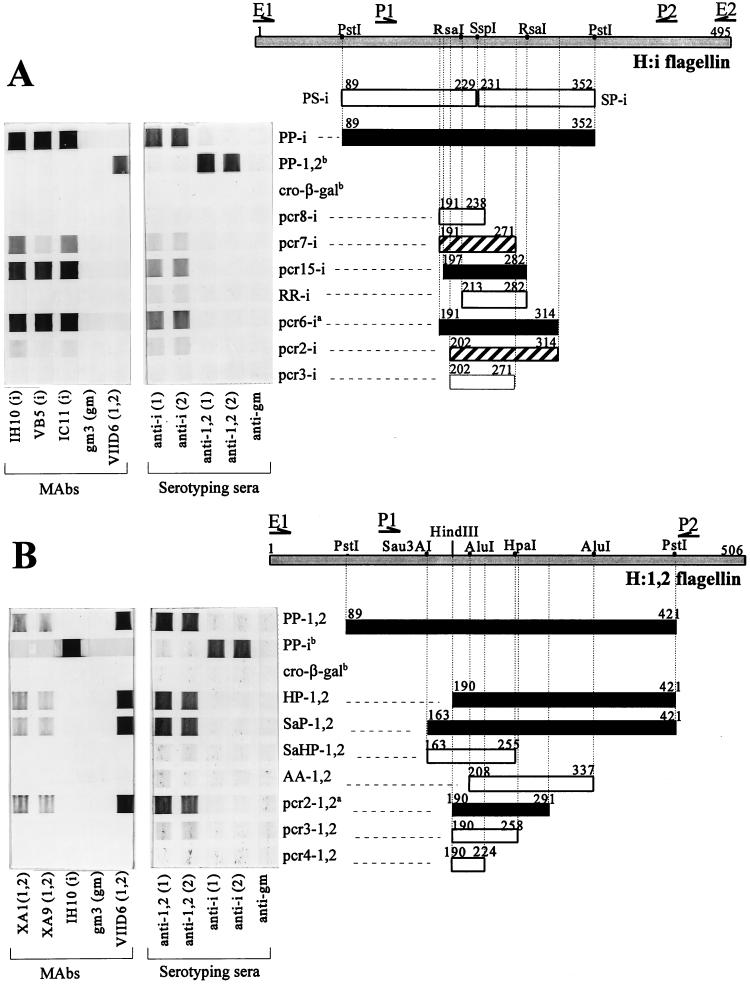
Immunostained cross blots showing the binding of H-specific antibodies to recombinant flagellin expression products. Binding of the H:i (A) and H:1,2 (B) flagellins with H-specific MAbs and commercial serotyping sera. Binding of the MAbs is indicated by the bar (filled, strong; hatched, weak; open, negative). Restriction sites used for the creation of fragments are indicated on the top bar of each panel, which represents the complete flagellin protein. The positions of the primers used for the cloning of the flagellin genes (E1, E2, P1, and P2) are depicted by arrows. Fragments indicated by superscript a were also cloned in pGEX and applied in an ELISA. Both a large fragment of the heterologous flagellin and cro-β-galactosidase (cro-β-gal) expressed from the empty vector pEX13 (indicated by superscript b) were always included as negative control fragments.
TABLE 2.
Nucleotide sequences of primers used for amplification of flagellin genes, sequencing analysis of gene fljB, and construction of overlapping fragments
| Primer functionc and name | Sequencea | Primer positionb | Gene |
|---|---|---|---|
| FS E1 | 5′AAGGAATTCATCATGGCACAAG-3′ | >−12 | fliC or fljB |
| F E2 | 5′-GAAGAATTCAACGCAGTAAAGAGAG-3′ | <1471 | fliC |
| F P1 | 5′-AAAGTCCTGGCGCAGGACAACA-3′ | >406 | fliC or fljB |
| F P2 | 5′-ATAGCGGAGTTGAAACGGTTCT-3′ | <1289 | fliC |
| <1322 | fliB | ||
| S S1,2 | 5′-TGGATGGAGTCGAGGTCAGACT-3′ | <317 | fljB |
| O ST i-191D2 | 5′-TGTGAATTCATATGCCGATACTACGATTG-3′ | >561 | fliC |
| O ST i-197D | 5′-TACGAATTCTGCTTTAGACAATAGTACTT-3′ | >582 | fliC |
| O ST i-202D | 5′-GACGAATTCACTTTTAAAGCCTCGGCT-3′ | >595 | fliC |
| O ST i-238R | 5′-CCAGTCGACCCCGTAACGGTAACTTT-3′ | <697 | fliC |
| O ST i-274R | 5′-TTTGTCGACTCCTCAGTTGCTGTCGCAGGTAGT-3′ | <810 | fliC |
| O ST i-282R | 5′-AACGTCGACATTTTTCACATCCTCAGT-3′ | <826 | fliC |
| O ST i-314R | 5′-ACCGTCGACATCAGTATAAGACATCTTAAC-3′ | <922 | fliC |
| O ST1,2-190D | 5′-ACAGAATTCGCTTATGCCAATAATGGT-3′ | >559 | fljB |
| O ST1,2-224R | 5′-TTTGTCGACACCACCGGTTACAGAAGC-3′ | <655 | fljB |
| O ST1,2-258R | 5′-ACCGTCGACAGCAACGTTAACTTCATA-3′ | <757 | fljB |
| O ST1,2-291R | 5′-ACAGTCGACGGTGTATCTTTTAACTC-3′ | <856 | fljB |
The EcoRI (GAATTC) and SalI (GTCGAC) sites are indicated in bold. Start or stop codons are underlined.
Numbering refers to position of the 5′ end of the last perfect matching nucleotide in forward primers (indicated by >) or the last matching nucleotide in the 3′ end in reverse primers (indicated by <) and is according to an alignment performed with Clustal-W (15) with sequence D13689 from the NCBI database.
Primers were used for amplification of flagellin genes (F), sequencing analysis of gene fljB (S), and construction of overlapping fragments (O). All primers were ordered from Pharmacia.
The HindIII site typically present in the fliB gene (13) could not be detected in the PCR product obtained with primers E1 and E2. Therefore, a central part of both the fliC and fliB flagellin genes was amplified from S. typhimurium SL3261 chromosomal DNA with primers P1 and P2 (13) (Table 2 and Fig. 2). HindIII digestion produced a 750-bp fragment containing the variable domain and part of the downstream conserved domain of the fljB gene. This probe was 32P labeled (1) and used in Southern blotting, where it identified two larger fljB fragments; a 980-bp HindIII-EcoRI fragment and a 999-bp PstI-PstI fragment (PP-1,2 [Fig. 2B]). These fragments were cloned into pUC18 and used for further subcloning of fljB flagellar fragments. The amino acid sequences deduced from the fliC and fljB genes had an overall identity of 75% (data not shown). As expected, the highest variability in amino acids was found in the central domain of the flagellin protein.
Subcloning and expression of flagellin fragments.
The fliC and fljB gene fragments were digested in several single and double digestions as outlined in Fig. 2, separated on an agarose gel, and isolated from the gel (QIAEX kit; Qiagen, London, United Kingdom). Smaller fragments were created by PCR amplification of either a PstI-PstI fliC fragment (PP-i [Fig. 2A]) or the 980-bp HindIII-EcoRI fljB fragment. All fragments were cloned into expression vector pEX11, pEX12, or pEX13 (6), and clones in E. coli POP2136 were selected by culturing at 30°C on agar supplemented with 100 μg of ampicillin per ml. Subclones containing the correct inserts were identified by restriction enzyme analysis of the plasmids. The expression and purification of fusion proteins were carried out as described previously (8). The expression products were separated by SDS-polyacrylamide gel electrophoresis and tested for recognition by specific MAbs and typing sera in Western blots. For each fragment, one representative subclone was selected for further analysis.
Epitope mapping of the H:i and H:1,2 flagellins.
The purified recombinant flagellin fragments were screened for binding to H:i- and H:1,2-specific typing sera and MAbs in cross blots. For this procedure, a Hybond C nitrocellulose filter (Amersham) was soaked in 0.5 M Tris-HCl (pH 6.8) and then placed in a Miniblotter 16 (Immunetics, Cambridge, Mass.) according to the manufacturer’s instructions. Purified fliC and fliB flagellin fragments (Fig. 2) were resuspended in 1 M Tris-HCl (pH 6.8)–6% SDS–30% glycerol–0.75% dithiothreitol and boiled for 5 min. Each of a maximum 10 channels of the Miniblotter was filled with 110 μl of a suspension containing 15 μg of protein and incubated for 1 h at room temperature. SDS was removed by electroelution for 10 min at 50 V in 25 mM Tris-HCl–192 mM glycine–20% methanol. Immunostaining was performed essentially as with Western blotting, except that incubation with typing antisera and MAbs was carried out with the filter placed crosswise in the immunoblotter.
The smallest H:i and H:1,2 flagellin fragments that were still strongly recognized by H antigen-specific MAbs were 86 (pcr15-i) and 102 (pcr2-1,2) amino acids, respectively (Fig. 2), and contained 67 to 70% of the variable residues identified by the above-mentioned sequence comparison of the H:i and H:1,2 flagellins. When 5 N-terminal (pcr2-i) or 11 C-terminal (pcr7-i) amino acids were deleted from pcr15-i, a significant decrease in antibody binding was observed (Fig. 2A). The simultaneous removal of both these ends (pcr3-i) or the deletion of 17 amino acids from the N terminus (RR-i) resulted in a complete loss of all detectable binding. When a larger fragment, PP-i (89-352), was cut into two parts at amino acid position 230, no binding by specific MAbs to fragment PS-i or SP-i could be detected (Fig. 2A). However, SP-i (data not shown) and RR-i (Fig. 2) were still weakly recognized by specific typing sera. Removing 18 N-terminal (AA-1,2) or 33 C-terminal (pcr3-1,2) amino acids from the minimal fragment pcr2-1,2 eliminated binding of all specific MAbs (Fig. 2B). However, pcr3-1,2 was still recognized weakly by commercial typing sera, but this binding disappeared after the removal of 34 additional C-terminal amino acids (pcr4-1,2).
Obviously, the most critical parts of the i and 1,2 antigenic determinants of S. typhimurium are contained within the pcr15-i and pcr2-1,2 fragments and are located between positions 190 and 291 on the H:i and H:1,2 flagellins (Fig. 2), a site which is in the hypervariable flagellin region IV (20). The localization of the H:i- and H:1,2-specific antigenic determinants in this part of the flagellin is in accordance with the results of a study of Yoshioka et al. (22). These authors showed that a spontaneous deletion mutant, lacking a part of the flagellin that corresponded to our fragment pcr15-i, was motile in the presence of H:i-specific antisera, in contrast to wild-type S. typhimurium. A similar location for the H:d antigen was inferred for Salmonella muenchen by Newton et al. (11), who presented evidence that region IV codes for the major determinants.
Flagellin fragments were recognized exclusively by homologous MAbs and not by heterologous MAbs (Fig. 2). The binding by H-specific typing antisera showed similar results. Apart from the overall pattern of strongly positive and clearly negative antibody binding to flagellin fragments, two H:i fragments (pcr2-i and pcr7-i) bound only weakly to both MAbs and serotyping sera. Since these two fragments were slightly smaller or shifted in position with respect to the smallest strongly positive fragment pcr15-i, we assume that they contain only part of the antigenic determinants. Furthermore, H:i and H:1,2 typing sera bound to some flagellin fragments (SP-i, RR-i, and pcr3-1,2), none of which was recognized by the corresponding MAbs (Fig. 2). Although we do not have conclusive evidence, we believe that due to incomplete absorption of the commercial typing sera, residual antibodies that recognize flagellin epitopes unrelated to the H antigen are still present and are probably the cause of this weak binding. All other flagellin fragments, however, showed similar binding of both MAbs and serotyping sera. It is evident that both MAbs and serotyping sera have one antibody binding site on the S. typhimurium flagellin which probably consists of a single epitope.
Evidently, H:i (pcr15-i) and H:1,2 (pcr2-1,2) flagellin fragments, with minimum lengths of 86 and 102 amino acids, respectively, are required for the strong binding of H-specific MAbs. The lengths of these fragments correspond well to the length of our previously found flagellin fragment, 91 amino acids, the minimal fragment for the H:gm flagellar determinant of S. enteritidis (16). Since the binding site of an antibody cannot contain more than 15 amino acid residues (18), it is obvious from our data that the residues involved in the formation of the H epitope are dispersed over a flagellin area of approximately 100 amino acids and therefore must represent a discontinuous epitope. Support for this position comes from two other studies in which sequence comparisons of Salmonella flagellins could not identify a single stretch of amino acid residues as a possible epitope site for the H antigen (10, 17). Furthermore, Joys and Schödel (4) showed that synthetic octameric peptides recognized by specific antibodies originated from sites widely dispersed on the flagellin of S. muenchen. The presence of such a discontinuous epitope suggests that some conformation of the recombinant flagellin fragments is required for antibody binding. Apparently, this conformation is maintained during or recovered after exposure to the denaturing conditions of our assays. Indeed, in our cross blots, a significant reduction of antibody binding to the recombinant flagellin fragments was observed when the electroelution of the denaturing agent SDS was omitted (data not shown), indicating that regaining the conformation of the epitope is necessary for optimal binding. An important consequence of the discontinuity of the H-specific epitope is that we could determine only the flagellin region in which a significant portion of the H-antigenic determinants was dispersed. No conclusion could be drawn about the individual amino acid residues that interact with the antibody. Also, the outer termini of the smallest binding fragments do not necessarily represent amino acid residues that are part of the antibody binding site, since their sole function could well be to maintain the flagellin fragment in the conformation required for the formation of the three-dimensional antibody binding site. In an attempt to identify the amino acids that are involved in the binding of specific antibodies, alignments between flagellar amino acid sequences of various Salmonella serotypes present in the National Center for Biotechnology Information (NCBI) database were determined (data not shown). This analysis resulted in the identification of a considerable number of hypervariable residues, which may be important for the differences in antigenic properties of flagellins of different serotypes. Aside from the fact that the majority of these residues were located on the H:i- and H:1,2-containing flagellin fragments, no solid conclusions could be drawn from this alignment, and further studies are needed to establish which amino acid residues contribute to the formation of the H antigen-determining epitope.
Use of H-specific flagellin fragments in ELISA for the detection of antibodies in chickens.
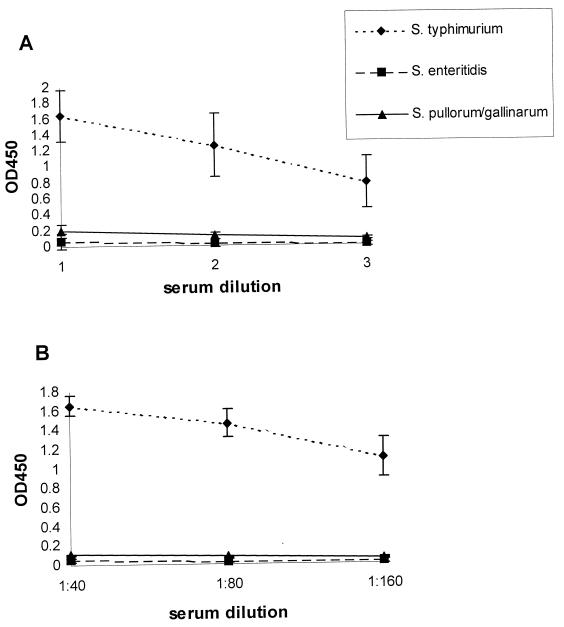
To obtain a soluble protein antigen suitable for use in ELISA, two small flagellin fragments of fliC (pcr6-i) and fljB (pcr2-1,2) (Fig. 2) were cloned into pGEX4T-1 (12) (Pharmacia, Uppsala, Sweden). Expression in E. coli PC2495 (21) and purification of the fusion proteins were carried out as described before (12) (Pharmacia). The wells of a microtiter plate (Greiner, Alphen a/d Rijn, The Netherlands) were each coated with 100 μl of a solution containing 5 μg of either the pcr6-i or the pcr2-1,2 flagellin peptide per ml. ELISAs were performed as described previously (2). Sixteen-week-old SPF White Leghorn chickens were infected orally with S. typhimurium, S. enteritidis, or Salmonella enterica Pullorum and Gallinarum, and serum samples were obtained on day 14, 21, or 28 after infection. Only sera from S. typhimurium-infected chickens bound to both recombinant flagellin fragments by ELISA (Fig. 3).
FIG. 3.
Results of an indirect ELISA with experimental sera from chickens orally infected with S. typhimurium (n = 8), S. enteritidis (n = 5), or S. enterica Pullorum and Gallinarum (n = 7) on H:i-specific fragment pcr6-i (A) or H:1,2-specific fragment pcr2-1,2 (B). OD, optical density.
In conclusion, this study provides evidence that all H-specific MAbs and commercial typing sera recognize the same epitope on their corresponding flagellin. This antibody binding site most probably consists of a single epitope, since both MAbs and serotyping sera showed the same binding to the various flagellin fragments. The finding that relatively large flagellin peptides are minimally required for binding of H:i and H:1,2 serotype-specific antibodies provides further evidence for the existence of a discontinuous H-specific epitope. Furthermore, ELISA results indicate that H-specific flagellin fragments bind specifically to antibodies from S. typhimurium-infected chickens. This indicates that the use of these fragments in an ELISA is a convenient method for the detection of S. typhimurium antibodies in animal reservoirs. A clear advantage is that the use of separate H:i and H:1,2 antigens in a test allows discrimination between infections with S. typhimurium and other Salmonella serotypes that have only one of these two H antigens in common with S. typhimurium. The application of these flagellar antigens as a diagnostic tool for the detection of S. typhimurium antibodies in commercial flocks of chicken and swine is currently being evaluated.
Nucleotide sequence accession number.
The amino acid sequences deduced from the fliC gene and the fliB gene were deposited in GenBank under accession no. AF045151.
Acknowledgments
We thank the Veterinary Health Inspection (VHI) and Food Inspection (HIGB) of the Dutch Ministry of WVS for financial support for this study.
We thank H. Maassen of the National Institute of Public Health and Environment (RIVM), Bilthoven, The Netherlands, for supplying strains of various serotypes of Salmonella and D. Mekkes of the Animal Health Service, Deventer, The Netherlands, for supplying chicken sera for ELISA. N. Hendriks is acknowledged for her technical assistance.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. 1 to 3. Brooklyn, N.Y: John Wiley & Sons, Inc.; 1997. [Google Scholar]
- 2.Baay M F D, Huis in’t Veld J H J. Alternative antigens reduce cross-reactions in an ELISA for the detection of Salmonella enteritidis in poultry. J Appl Bacteriol. 1993;74:243–247. doi: 10.1111/j.1365-2672.1993.tb03021.x. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim G F, Fleet G H, Lyons M J, Walker R A. Immunological relationships between Salmonella flagellins and between these and flagellins from other species of Enterobacteriaceae. Med Microbiol Immunol. 1985;174:101–113. doi: 10.1007/BF02123231. [DOI] [PubMed] [Google Scholar]
- 4.Joys T M, Schödel F. Epitope mapping of the d flagellar antigen of Salmonella muenchen. Infect Immun. 1991;59:3330–3332. doi: 10.1128/iai.59.9.3330-3332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kauffmann F. Das Kauffmann-White schema. In: van Oye E, editor. The world problem of salmonellosis. The Hague, The Netherlands: Dr. W. Junk Publishers; 1964. pp. 21–66. [Google Scholar]
- 6.Kusters J G, Jager E J, van der Zeijst B A M. Improvement of the cloning linker of the bacterial expression vector pEX. Nucleic Acids Res. 1989;17:8007. doi: 10.1093/nar/17.19.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwang J, Littledike E T. Production and identification of recombinant proteins of Salmonella typhimurium and their use in detection of antibodies in experimentally challenged animals. FEMS Microbiol Lett. 1995;130:25–30. doi: 10.1016/0378-1097(95)00179-9. [DOI] [PubMed] [Google Scholar]
- 8.Lenstra J A, Kusters J G, Koch G, van der Zeijst B A M. Antigenicity of the peplomer protein of infectious bronchitis virus. Mol Immunol. 1989;1:7–15. doi: 10.1016/0161-5890(89)90014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macnab R M. Flagella. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C: American Society for Microbiology; 1987. pp. 70–83. [Google Scholar]
- 10.Masten B J, Joys T M. Molecular analyses of the Salmonella g… flagellar antigen complex. J Bacteriol. 1993;175:5359–5365. doi: 10.1128/jb.175.17.5359-5365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newton S M C, Wasley R D, Wilson A, Rosenberg L T, Miller J F, Stocker B A D. Segment IV of a Salmonella flagellin gene specifies flagellar antigen epitopes. Mol Microbiol. 1991;5:419–425. doi: 10.1111/j.1365-2958.1991.tb02124.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 13.Smith N H, Selander R K. Sequence invariance of the antigen-coding central region of the phase 1 flagellar filament gene (fliC) among strains of Salmonella typhimurium. J Bacteriol. 1990;172:603–609. doi: 10.1128/jb.172.2.603-609.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stocker B A. Aromatic-dependent Salmonella as live vaccine presenters of foreign epitopes as inserts in flagellin. Res Microbiol. 1990;141:787–796. doi: 10.1016/0923-2508(90)90112-4. [DOI] [PubMed] [Google Scholar]
- 15.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Asten A J A M, Zwaagstra K A, Baay M F, Kusters J G, Huis in’t Veld J H J, van der Zeijst B A M. Identification of the domain which determines the g,m serotype of the flagellin of Salmonella enteritidis. J Bacteriol. 1995;177:1610–1613. doi: 10.1128/jb.177.6.1610-1613.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanegas R A, Joys T M. Molecular analyses of the phase-2 antigen complex 1,2, · · of Salmonella spp. J Bacteriol. 1995;177:3863–3864. doi: 10.1128/jb.177.13.3863-3864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Regenmortel M H V. Structural and functional approaches to the study of protein antigenicity. Immunol Today. 1989;10:266–272. doi: 10.1016/0167-5699(89)90140-0. [DOI] [PubMed] [Google Scholar]
- 19.van Zijderveld F G, van Zijderveld-van Bemmel A M, Anakotta J. Comparison of four different enzyme-linked immunosorbent assays for serological diagnosis of Salmonella enteritidis infections in experimentally infected chickens. J Clin Microbiol. 1992;30:2560–2566. doi: 10.1128/jcm.30.10.2560-2566.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei L N, Joys T M. Covalent structure of three phase-1 flagellar filament proteins of Salmonella. J Mol Biol. 1985;186:791–803. doi: 10.1016/0022-2836(85)90397-3. [DOI] [PubMed] [Google Scholar]
- 21.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 22.Yoshioka K, Aizawa S, Yamaguchi S. Flagellar filament structure and cell motility of Salmonella typhimurium mutants lacking part of the outer domain of flagellin. J Bacteriol. 1995;177:1090–1093. doi: 10.1128/jb.177.4.1090-1093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]