Abstract
Background
Airway inflammation produced by neutrophils is a critical factor in the development of chronic obstructive pulmonary disease (COPD). Poor or excessive neutrophil polarization and chemotaxis may lead to pathogen accumulation and tissue damage. However, it is unclear how cigarette smoke extract (CSE) attracts neutrophils and to what extent COPD is affected by the improper polarization of these abnormal neutrophils. This study sought to assess the polarization and migration dynamics of neutrophils isolated from patients with different severities of COPD compared to healthy smoking and non-smoking control subjects, and to detect how CSE triggers the polarization of neutrophils.
Methods
The neutrophils were freshly isolated using standard isolation protocol. The polarization of the neutrophils was observed using a Zigmond chamber when stimulated by a linear concentration gradient of CSE or N-formyl-methionine-leucine-phenylalanine (fMLP). Confocal laser-scanning microscopy was used to observe the intracellular calcium of the neutrophils. The experimental data are presented as the mean ± standard deviation. SPSS 20.0 software was used for the statistical analysis. A P value <0.05 was considered statistically significant.
Results
The neutrophils from the COPD patients showed a higher frequency of spontaneous polarization and a lower prevalence of directionality polarization than those from the healthy control (HC) and smoker subjects. The abnormal polarization of the neutrophils from the COPD patients was altered by the influence of store-operated calcium entry (SOCE) component matrix interaction molecules 1 and 2 and calcium release-activated calcium channel protein 1 [stromal interaction molecule 1 (STIM1), Stromal interaction molecule 2 (STIM2), and calcium release-activated calcium modulator 1 (ORAI1)].
Conclusions
The COPD neutrophils exhibited unique polarization and migration patterns compared to those of the cells examined from other populations. The attraction of CSEs to neutrophils was mediated by the SOCE/Akt/Src pathway.
Keywords: Chronic obstructive pulmonary disease (COPD), neutrophil polarization, cigarette smoke extract (CSE), store-operated calcium entry (SOCE)
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most common long-term diseases and causes of death worldwide, and accounts for approximately 1/4 of all diseases. COPD affects approximately 210 million people worldwide and is responsible for about 5% of all deaths (1). The abnormal polarity (and polarization) of neutrophils leads to the migration and accumulation of more neutrophils in the lungs of COPD patients. COPD patients are afflicted by chronic neutrophil inflammation of the airways and lung parenchyma, which leads to phagocytic growth and tissue remodeling (2).
Neutrophils play a critical role in the immunological response to infection and chronic inflammation, and it is well accepted that neutrophils contribute significantly to COPD (3). Neutrophils display plasticity, with the ability to adapt their function in different inflammatory contexts. In the tumor microenvironment, neutrophils have varied functions and have been classified using different terms, including N1/N2 neutrophils, tumor-associated neutrophils, and polymorphonuclear neutrophil myeloid-derived suppressor cells (PMN-MDSCs). These populations of neutrophils are primarily defined by their functional phenotype, because few specific cells surface markers have been identified (4). The inflammatory response in COPD involves both innate and adaptive immunity with neutrophilic inflammation the commonest inflammatory phenotype in COPD. Neutrophils are recruited as the predominant cells with consequent release of proteases and airway damage as well as activation of ILC3s. While the main inflammatory pathway in COPD is neutrophilic in nature, studies targeting neutrophilic inflammation have been disappointing to date (5). Previous studies in vivo have shown that two serine proteinases, elastase and proteinase-3, which are released by neutrophils, can induce in animals’ pathologic changes that resemble human emphysema (6,7). Furthermore, neutrophil sequestration in the pulmonary circulation also will lead to the development of emphysema in dogs (8). These studies, therefore, confirm that the neutrophil and its proteinases have the potential to produce human emphysema. The neutrophil also has been implicated in other manifestations of COPD since experimental application of neutrophil elastase (NE) can reproduce many of the features of patients with this syndrome (9).
Thus, the number of neutrophils in the lungs of COPD patients is higher than that of asymptomatic smokers (10,11). Increased neutrophil counts are associated with airway constriction (12), reduced gas transfer (13), an early decline in forced expiratory volume in the first second (FEV1) (14), and increases in the severity of emphysema (13,15). More neutrophils in the lungs may be a marker of disease progression (due to enhanced inflammatory signaling) or a factor in its (COPD patients) pathogenesis (due to defective apoptosis or increased or abnormal migration). In studying neutrophil migration in COPD, there is evidence of altered chemotactic behavior. Current apoptosis studies have not demonstrated enhanced neutrophil lifespan in the lungs (16,17).
Neutrophil migration is crucial for a proper immune response. This migration requires the coordination of many signaling pathways at the front and rear of the cell that led to neutrophil polarization and motility. Laurel E and colleagues have discussed how the neutrophil uropod has emerged as a crucial structure for proper neutrophil motility that can also be the driving center for neutrophil polarization and migration, which involves the Rho GTPase signaling and front-rear polarity, cell intrinsic polarity and neutrophil reverse migration, myosin II, adhesion, ERM proteins, microtubules and so on (18).
After stimulation with chemoattractants, the longitudinal axis of neutrophils alters, and wide lamellipodium forms at the leading edge and a constricted tail forms at the posterior edge. The neutrophils then begin to migrate toward the chemoattractants (19). The polarization of the cytoskeleton and the asymmetric distribution of signaling molecules are responsible for the migration of neutrophils (20). Neutrophil polarization and chemotaxis affect the polymerization of F-actin, which affects the development of pseudopods. Several chemotactic agents have been linked to both phosphoinositide 3-kinase (PI3K)-dependent and -independent signaling pathways in vitro (21-23). In COPD, smoking is the most common cause of airway inflammation. Chronic bronchitis is significantly associated with smoking, and it is well established that cigarette smoke extract (CSE) activates macrophages in the airway.
Nuzzi et al. demonstrated that calpain is an intracellular calcium-dependent protease responsible for regulating cell migration and neutrophil chemotaxis. In resting neutrophils, a decrease in calpain activity has been shown to enhance the stochastic movement of neutrophils, which is also known as chemotaxis (24). Further, the catalytic activity of calpain may have a role in pseudopod formation toward the chemotactic agent, and this activity may be crucial to the occurrence of effective chemotaxis. Additionally, calpain’s catalytic activity plays a role in biasing pseudopod formation in the direction of the chemoattractant, and this activity is required for efficient chemotaxis. However, it is unknown how neutrophils localize CSE and whether the calcium-dependent protease calpain is essential for its polarization.
Sapey et al. found that neutrophils migrated significantly faster when compared with neutrophils isolated from Healthy control; however, the precision of the movement was more decreased, and the distance traveled was more increased in COPD patients than in healthy control (HC) and healthy smoker (HS) subjects, which implies that the migration of COPD neutrophils may be excessive and imprecise in moving through the body (1). Yoshikawa et al. found that patients with moderate to severe COPD have lower chemotaxis in response to N-formyl-methionine-leucine-phenylalanine (fMLP) and interleukin 8 (IL-8) than healthy non-smoker subjects and mild COPD patients (25). Most eukaryotic cells, including neutrophils, move by protruding actin-rich pseudopods at the leading edge of the cell body, while retracting back into the rest of the cell, which is a frequent form of movement in most eukaryotic cells (26). It has not yet been determined whether neutrophil polarization in COPD patients’ peripheral blood is increased, nor has the exact process by which neutrophil polarization leads to abnormal migration been established.
Increased cytoplasmic Ca2+ concentration in neutrophils may be responsible for the pro-polarizing and pro-motile effects of inflammation (19,27). Phospholipases C and 1,2-diacylglycerol (DAG) are broken down in neutrophils by fMLP, which activates the fMLP receptor and causes the breakdown of phosphatidylinositol 4,5-bisphosphate into inositol triphosphate (IP3) and DAG (28,29). When IP3 binds to its receptor, Ca2+ is released from the endoplasmic reticulum (ER) reserve in a short period. The store-operated calcium entry (SOCE) mechanism leads to extracellular Ca2+ influx across the plasma membrane when the extracellular Ca2+ concentration in the ER is low. SOCE was initially proposed in the 1980s by Putney et al. (30). As part of this study, we sought to examine whether the polarization activity of neutrophils in COPD is correlated with the severity of COPD and whether defects in the composition or function of the SOCE affect intracellular calcium concentrations, leading to the paradoxical polarization of these cells.
This study had the following 3 main objectives: (I) to examine whether neutrophils from COPD patients differed to those from HC and non-smoker subjects in terms of their migratory dynamics by quantitatively comparing the neutrophils from the COPD patients to those from the HC and non-smoker subjects; (II) to determine whether abnormal polarization is driven by intracellular calcium concentrations triggered by SOCE and its components, stromal interaction molecule 1 (STIM1), STIM2, and ORAI1, or if a more central cell signaling component is involved, as while it has been established that cigarette smoking is the primary risk factor for COPD, it is still unknown why only 15% of smokers develop the disease (31-33); (III) to learn more about how CSE attracts neutrophils and what potential pathways are involved in this process.
Inflammation in the lungs is associated with COPD; however, it is impossible to determine whether any differences between HC subjects and COPD patients are due to COPD, lung inflammation per se, smoking, or the medications used during the treatment of COPD, as all of these can alter neutrophil behavior. Thus, we carefully selected controls, including HC subjects who had never smoked, smokers with normal lung function and no respiratory symptoms (the so-called “HS” subjects). Additionally, as various aspects of neutrophil function, including migration (34), are affected by age, all the subjects were age- and gender-matched before enrollment in the study.
To date, the study of abnormal neutrophils’ chemotaxis and migration are mainly focus on neutrophil chemotaxis phenomena and aberrant cell surface adhesion molecules, such as PI3K pathway. However, the initiating point of this abnormal migration, that is, the abnormal polarization of neutrophils, has not been reported. Our research has conducted an in-depth study on the relationship between abnormal polarization of neutrophils and SOCE in COPD patients, which provides a new vision and target for clinical diagnosis, treatment and prevention for COPD patients. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1480/rc).
Methods
Reagents and methods
N-formyl-met-leu-phe (fMLP), SKF96365, 2-Aminoethoxydiphenyl Borate (2-APB), thapsigargin (TG), and CaCl2 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fluo-4 AM was purchased from Invitrogen (Thermo Fisher Scientific Corporation, Waltham, MA, USA). Goat polyclonal anti-protein kinase B (Akt) antibody, rabbit polyclonal anti-phosphor-Akt, and anti-STIM1 antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit monoclonal anti-Src antibody and rabbit polyclonal anti-phosphor-Src antibody were purchased from Cell Signaling Technology (Boston, MA, USA). CSE was extracted from lit cigarettes, leached, dissolved in 2.5 mL of phosphate buffered solution, and then sterilized by filtration.
Study subjects
The recruited volunteers were divided into the following 6 groups according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (35) and the results of exhaustive medical history and lung function tests: HC subjects (n=10), HS subjects (n=10), GOLD stage I patients (n=11), GOLD stage II patients (n=13), GOLD stage III patients (n=14), and GOLD stage IV patients (n=14). The COPD patients were all ex-smokers, aged 40–80 years. All patients had been clinically stable for at least 8 weeks without any changes in medication before the commencement of the study. All alternative and concomitant diseases were excluded clinically, physiologically, and radiologically. The study included age- and gender-matched HC and HS subjects. The HC subjects showed no evidence of substance use, were non-smokers, and had no other health problems. The HS subjects had a significant smoking age, normal lung function, no respiratory problems, and were not taking any medications at the time of the examination. The questionnaire elicited information about the status of the smoker. All the recruited volunteers are from the Huizhou Municipal Central Hospital. The study was approved by the Huizhou Municipal Central Hospital Ethics Committee (No. LLBA201913A). All participants signed informed consent forms. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Isolation of neutrophils from blood
The neutrophils taken from the subjects were treated with ethylenediaminetetraacetic acid sodium salt to prevent coagulation. The neutrophils were separated from the whole blood samples using a density-gradient method (36). Previously purified neutrophils were resuspended in D-Hanks without CaCl2 at a 1.0×106 cells/mL concentration. When the cells were isolated, Wright-Giemsa staining showed that >95% of the cells were neutrophils. The use of trypan blue exclusion revealed a survival rate of >98%. The cells were kept on ice and used for research within 4 hours of isolation.
Evaluation of cell shape
Cell morphology was determined using an OLYMPUS IX51 microscope and a 40X objective, and digital images of the cells were taken and saved using an OLYMPUS DP71 Color Microscope Camera and its software (Olympus Corporation, Tokyo, Japan). After treating the cells with gradient concentrations of fMLP or CSE in a Zigmond chamber for 15 minutes (the time point at which the cell polarization rate reached peak levels), at least 250 cells were observed by inverted microscopy to determine the proportion of polarized cells and to classify the cells into the following 3 categories: spherical, nonpolar, and polar (37). In this study, 3 independent experiments were set up for each treatment, and shape polarization was defined by a ratio of cell length to cell width >1.5 (38). Apoptotic cells with blebs were not included in the above observations.
Analysis of cell polarity
A total of 1 million dHL-60 cells from Hank’s (2 mL) were placed on coverslips (48 mm × 60 mm) in 20 µL of D-type suspension and allowed to attach for 5 minutes at room temperature. Immediately after placing the coverslips into the Zigmond chambers, a fMLP gradient of 0–100 nM was applied to the surface of the coverslips (Neuroprobe, Gaithersburg, MD, USA). Every 30 seconds for 15 minutes, digital snapshots of the cells were taken with an Olympus IX71 inverted microscope using a digital camera (Olympus, Japan). Neutrophil polarization was measured using 5 randomly selected fields (see details below).
Spontaneous polarization
The number of cells that had been polarized before activation by the fMLP concentration gradient was counted.
Random polarization
After 15 minutes of fMLP stimulation, the percentage of polarized cells in random directions was calculated.
Directional polarization
The proportion of polarized cells within ±45° along the gradient was calculated (39). Immunosuppressive neutrophils were stimulated with fMLP, and cell polarization was scored on slides as spherical, polarized (with distinct head-to-tail polarization), or irregular (non-spherical cells without distinct head-to-tail polarization) (40,41). Photographs were evaluated with the help of the Image-pro Plus 6.0 program (Media Cybernetics Inc., USA). The identity of each subject was masked by a single analyst who undertook all the work in 1 place.
The Ca2+ concentration of the cells
The Fluo-4/AM, a Ca2+-sensitive fluorescent indicator, was used to assess intracellular Ca2+ levels under an inverted laser-scanning confocal microscope (Olympus, FV1000-IX71, Japan). To prepare the neutrophils for the loading of 2 µM Fluo-4/AM, they were preincubated with SKF96365 and 2-APB in Hanks’ buffered salt solution (pH 7.4) for 30 minutes at 37 °C. CaCl2 was then removed from the buffered solution, and 0.3 mM EGTA [glycol-bis-(2-aminoethylether)-N,N,N',N'-tetra] was added to create a Ca2+-free solution. In various experiments, the neutrophils were kept in a Ca2+-free buffer for 30 minutes before imaging with Ca2+. The fluo-4 exhibited an argon laser-induced fluorescence at 488 nm, and the resulting fluorescence was measured using a 525-nm channel. Background subtraction was used for Fluo-4 imaging to show the change in Ca2+ as ΔF/F0 ratios after subtracting the fluorescence signal intensity estimated from the average results of the first 10 frames of the stimulus application, where ΔF represented the change in fluorescence signal intensity after the removal of F0.
Western blot analysis
The protease inhibitors (Roche, Switzerland) were added to 0.2 mL of ristocetin-induced platelet aggregation lysis buffer (Beyotime Biotech, Nantong, China), and centrifuged at 13,200 rpm for 15 minutes. The protein concentration of the supernatant was determined. Sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE; 10%) was used on the protein samples and in the polyvinylidene fluoride membrane transfer, and heated at 100 °C for 10 minutes before loading onto the gels. Afterward, the membranes were blocked with tris buffered saline with tween buffer for 1 hour at room temperature, and gently shaken before exposure to the dye (20 mM Tris, 500 mM NaCl, and 0.1% Tween-20). The membranes were incubated with a series of primary antibodies overnight at 4 °C, after which the membranes were washed. The primary antibodies used in the experiments were as follows: 1:1,000 mouse polyclonal anti-TGF-b1 (ab64715; Abcam, Cambridge, UK), 1:1,000 rabbit polyclonal anti MMP-2 (SAB4501891; Sigma-Aldrich, St. Louis, MO, USA), 1:1,000 rabbit polyclonal anti-collagen I (ab34710; Abcam, Cambridge, UK), and 1:1,000 rabbit polyclonal anti-a-SMA (sc-130619; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membranes were incubated with a 1:5,000 dilution of peroxidase-conjugated secondary antibody solution purchased from Zhongshan Biotechnology (Beijing, China) before the application of enhanced chemiluminescence (WBKLS0100, Millipore, Inc., USA). To estimate the density of each band, a gel-pro analyzer and a densitometer scan (Media Cybernetics) were used.
Statistical analysis
For the statistical analysis, the IBM SPSS Statistics 23 application was used. The standard error of the mean was minus the mean. Results were expressed as mean ± SEM. The analysis of the data required the use of the t-test or the 1-way analysis of variance (ANOVA). When comparing 2 groups side by side, the Student-Newman-Keuls test was used to assess whether there was a statistically significant difference in the results. Differences were considered statistically significant if the P value was <0.05.
Results
Demographic data
The recruited volunteers were classified as healthy non-smoker subjects (n=10) and HS subjects (n=10), patients with GOLD stage I (n=11), II (n=13), III (n=14), and IV (n=14). Complete medical histories and pulmonary function tests were used to allocate the subjects to groups (35,42). Patients’ post-bronchodilator FEV1 and FEV1/forced vital capacity (FVC) are shown as a percentage of their expected values for age, gender, and height (for FEV1 and FEV1/FVC) (43). There was no significant difference in age between the HC or HS or GOLD stage I subjects, or between the GOLD stage II, III, or IV subjects. This suggests that the age of the donor affects neutrophil properties, including migration (34).
All the data are expressed as the median (interquartile range) unless otherwise stated. HS subjects smoked significantly more packs per year than the rest of the population, but we ensured that there were no differences in the number of packs smoked per year between healthy smoker and COPD patients, to eliminate the effect of smoking exposure. The lung functions (predicted FEV1%, and FEV1/FVC) and medication parameters differed significantly. Significant differences of lung functions (predicted FEV1%, and FEV1/FVC) were found between the COPD groups (P<0.001). AS shown in Table 1.
Table 1. Characteristics of study participants in the trial who contributed neutrophil blood.
| Group characteristics | Healthy control | Healthy smokers | COPD (GOLD stage) | |||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| Total number of participants | 10 | 10 | 11 | 13 | 14 | 14 |
| Age, years; mean [range] | 53 [51–54] | 54 [50–57] | 59 [50–69] | 73 [63–74] | 67 [59–78] | 72 [62–77] |
| Number of males | 8 | 10 | 9 | 10 | 12 | 12 |
| Smoking status | Non-smokers | Current smokers | Ex-smokers | Ex-smokers | Ex-smokers | Ex-smokers |
| Pack-years cigarettes; mean [range] | 0 [0–0] | 28 [20–36] | 33 [20–45] | 31 [8–55] | 40 [24–56] | 40 [27–55] |
| Predicted FEV1, %; mean [range] | 98 [92–104] | 101 [91–110] | 87 [82–91] | 60 [56–64] | 39 [36–42] | 22 [19–24] |
| FEV1/FVC; mean [range] | 88 [83–92] | 82 [78–86] | 68 [66–70] | 64 [59–69] | 60 [54–65] | 52 [45–59] |
| Patients receiving inhaled steroids, % | 0 | 0 | 0 | 30 | 100 | 100 |
| Patients receiving theophylline, % | 0 | 0 | 0 | 30 | 33 | 33 |
| Patients receiving long-acting b2-agonists, % | 0 | 0 | 0 | 66 | 83 | 100 |
| Patients receiving long-acting anticholinergic drugs, % | 0 | 0 | 0 | 66 | 83 | 100 |
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity.
Abnormal polarization of neutrophils in COPD
After 15 minutes, the cells were placed in a Zigmond chamber and subjected to a fMLP gradient to measure their polarization and chemotactic activity. The neutrophils of the COPD patients were more active than those of the normal subjects.
Spontaneous polarization
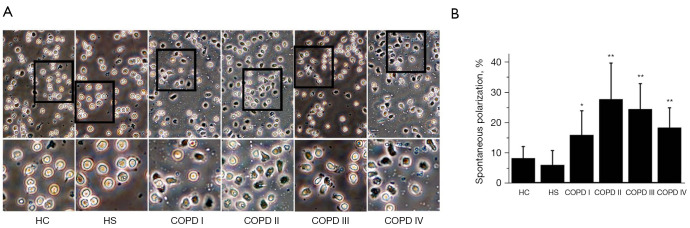
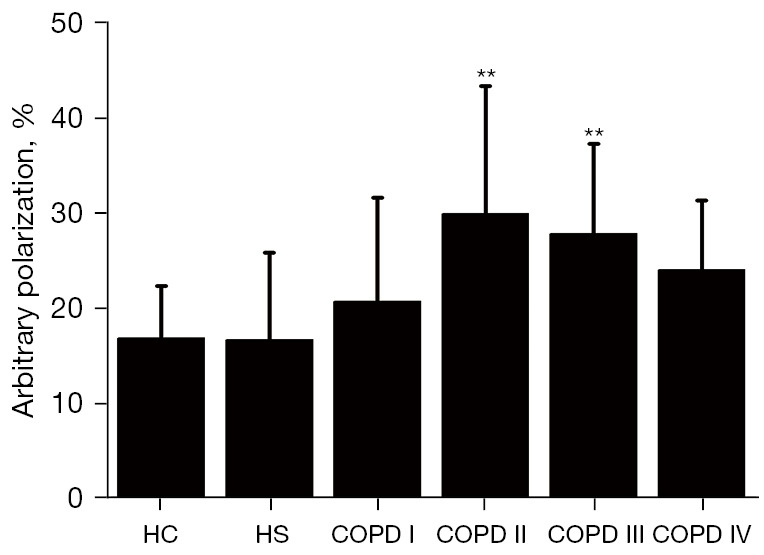
Compared to the HC (7.99%±3.91%) and HS (5.76%±4.81%) subjects, neutrophils from COPD patients demonstrate a more spontaneous polarization, especially the GOLD stage II COPD (27.50%±11.99%; P<0.01), GOLD stage III (24.21%±8.45%; P<0.01), and GOLD stage IV (18.13%±6.56%; P<0.01) patients. There was no significant difference of spontaneous polarization between the HC subjects and GOLD stage I patients; however, there was a significant difference of neutrophils’ spontaneous polarization between the stage I patients (15.70%±8.01%) and the HS subjects (5.76%±4.81%). However, there was no difference of neutrophils’ spontaneous polarization between the HC and HS subjects. The severity of COPD ranged from GOLD stage I to IV; however, the neutrophils of GOLD stage II and III patients showed higher spontaneous polarization than those of GOLD stages I and IV patients (Figure 1A,1B).
Figure 1.
The average percentage of cells polarized in the spontaneous direction without gradients of fMLP. (A) The polarization and migratory dynamics of the peripheral neutrophils from the COPD patients along the fMLP gradient according to disease severity. Time-lapse microscopy was used to record cell morphology on the bridge at a 30 s interval and the direction of the fMLP gradient (0–100 nmol/L) was indicated by the wedge, and cell images were acquired at 20× object by optical microscope. A higher magnification image of the boxed section is shown below each panel of images for a clearer view of cell polarization. (B) A comparison of polarization in HC (never-smoker) subjects, HS subjects, and COPD patients with different degrees of disease severity. The SEM is given as the standard error bar. *, P<0.05; **, P<0.01. The neutrophils of the COPD patients moved with higher spontaneous polarization than those of age-matched the HC and HS subjects. Specifically, the neutrophils of the COPD stage II, III, and IV patients moved with a significantly more percentage than those of the age-matched HC and HS patients (P<0.05), and the neutrophils of the COPD stage I patients differed significantly to those of the HS patients. fMLP, N-formyl-methionine-leucine-phenylalanine; COPD, chronic obstructive pulmonary disease; HC, healthy control; HS, healthy smoker. SEM, Standard error of mean.
Random polarization
GOLD stage II (22.93%±13.39%) and GOLD stage III (27.25%±9.41%) patients had significantly higher neutrophil mobility than HC (16.85%±5.52%; P<0.01) and HS (16.72%±9.16%; P<0.01) subjects. There was no statistically significant difference between GOLD stage I (20.71%±10.93%), and IV patients (24.03%±7.34%), or HC and HS subjects; however, there was a significant difference between GOLD stage I and II patients (20.71%±10.93%). Further, the peripheral venous blood of the COPD patients exhibited more random polarization neutrophils when stimulated by fMLP in a random direction (see Figure 2).
Figure 2.
The average percentage of cells polarized in random directions in gradients of fMLP. The polarization dynamics of the peripheral neutrophils from the COPD patients in the fMLP concentration gradient according to disease severity. The SEM is given as the standard error bar. Significant differences from the COPD stage I. **, P<0.01. The neutrophils of the COPD stage II (29.93%±13.39%) and III (27.85%±9.41%) patients moved with a significantly more percentage than those of the HC (16.85%±5.52%; P<0.01) and HS (16.72%±9.16%; P<0.01) subjects. fMLP, N-formyl-methionine-leucine-phenylalanine; COPD, chronic obstructive pulmonary disease; HC, healthy control; HS, healthy smoker. SEM, Standard error of mean.
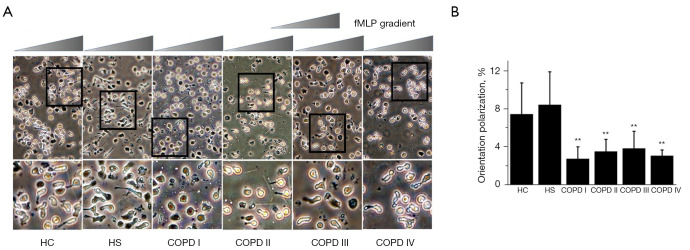
Directional polarization
As shown by the percentage of neutrophils polarized in the fMLP direction, the neutrophils from the COPD patients migrated at a significantly lower rate than the neutrophils from the general population. Additionally, the neutrophils from the stage I (2.75%±1.27%), and IV (3.06%±0.62%) subjects were significantly less directionally polarized than those from the HS (8.44%±3.47%; P<0.01) and HC (7.44%±3.31%; P<0.05) subjects. Second, the directional polarization of the neutrophils from the stage II (3.52%±1.29%) and III (3.83%±1.82%) patients was significantly lower than that of the HS subjects (P<0.05), but there was no statistically significant difference between stages II and III COPD patients and HC patients (see Figure 3A,3B). Further, no age- and gender-matched differences in the number of polarized (spontaneous, random, or directional) neutrophils were found between the HC and HS subjects, indicating that tobacco use had no effect on neutrophil polarization or migration in HC and HS subjects.
Figure 3.
The average percentage of cells polarized in a directional direction in gradients of fMLP. (A) A comparison of directional polarization in the HC subjects, HS subjects, and COPD patients with different degrees of disease severity. Time-lapse microscopy was used to record cell morphology on the bridge at a 30 s interval and the direction of the fMLP gradient (0–100 nmol/L) was indicated by the wedge, and cell images were acquired at 20× object by optical microscope. The icon above the images indicates the direction of fMLP gradient. A higher magnification image of the boxed section is shown below each panel of images for a clearer view of cell polarization. (B) The SEM is given as the standard error bar. Significant differences from the COPD stage I. **, P<0.01. The neutrophils of the COPD stage I and IV patients demonstrated significantly less directional polarization than those of the HS (P<0.01), and HC (P<0.05) subjects. The neutrophils of the COPD stage II and III patients also moved with significantly less directional polarization than those of the HS subjects (P<0.05), but no statistically significant difference was found between the COPD stage II and III patients and the HC subjects. fMLP, N-formyl-methionine-leucine-phenylalanine; COPD, chronic obstructive pulmonary disease; HC, healthy control; HS, healthy smoker. SEM, Standard error of mean.
Impaired calcium influx in COPD
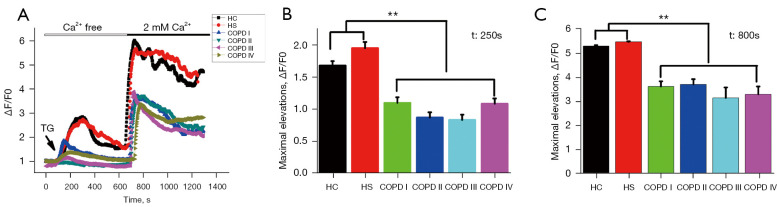
Fluo-4-mediated Ca2+ imaging techniques were applied in experiments in which the Sarcoendoplasmic Reticulum Calcium ATPase (SERCA) pump inhibitor TG was introduced (an effective tool for studying SOCE) to clarify whether the abnormal neutrophil polarization and signaling in COPD patients were due to changes in cell membrane Ca2+ influx (44,45). As Figure 4 shows, the supplementation of 2 mM of Ca2+ in the medium may indicate SOCE. The basal levels of cytoplasmic Ca2+ were restored by supplementation with 2 mM of Ca2+ before the addition of TG, normalized to ∆F/F0~0, and ∆F/F0 was used to measure increased cytoplasmic Ca2+ influx. As the right panel of Figure 4 shows, TG caused an initial, relatively small, increase in cytosolic Ca2+, and the small increase in cytosolic Ca2+ in the neutrophils from the COPD patients was approximately 30% lower than that in the neutrophils from HC and HS subjects. Reintroducing external Ca2+ resulted in a rapid Ca2+influx, albeit this increase in cytoplasmic Ca2+ was reduced by approximately 60% in COPD patients compared to HC and HS subjects.
Figure 4.
Impaired calcium influx in COPD. (A) The initial [Ca2+] i rise caused the calcium store (endoplasmic reticulum) to empty, which was triggered by TG, and the subsequent [Ca2+] i rise resulting from re-addition of extracellular Ca2+ was lower in the neutrophils of COPD patients than those of the age-matched HC and HS subjects. Cytoplasmic Ca2+ was assessed in Fluo-4/AM-loaded human neutrophils. Ca2+ (2 mM) was added to the extracellular medium as described, and the influx of divalent cations was monitored by laser-scanning confocal microscopy. Each point shows the mean of at least 8 observations. The corresponding bar charts (B,C) show the maximal elevation of the curve in Fluo-4/AM imaging after the addition of Ca2+. These results are representative of 3 independent experiments. The bars show the mean ± SEM; **, P<0.01. TG, thapsigargin; COPD, chronic obstructive pulmonary disease; HC, healthy control; HS, healthy smoker. SEM, standard error of mean.
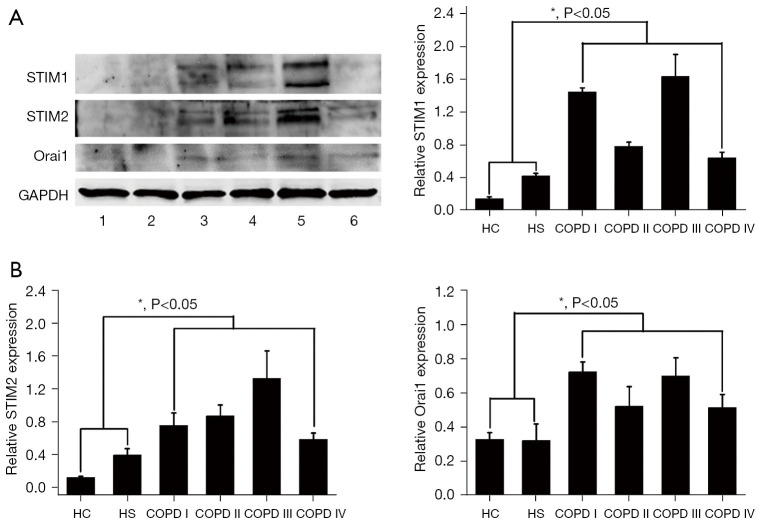
Expression of important components of SOCE (STIM1, STIM2, and ORAI2) in the neutrophils of COPD patients
COPD patients had higher levels of STIM1, STIM2, and ORAI2 than age- and gender-matched HS and HC subjects (see Figure 5). These results were replicated in 3 independent experiments.
Figure 5.
Expression of the vital component of SOCE in the neutrophils of COPD patients. The levels of STIM1, STIM2, and ORAI1 were more enhanced in the COPD patients than those of the age-matched HS and HC subjects. Western blot was used to evaluate the expression and distribution of STIM1, STIM2, and ORAI1. (A) Western blot showed that the expression of STIM1, STIM2, and ORAI1 in the neutrophils of the COPD patients was more upregulated that of the age-matched HS and HC subjects. (B) Densitometric quantification of the corresponding protein levels. The data are presented as the mean ± SEM (n=3). SOCE, store-operated calcium entry; STIM1, stromal interaction molecule 1; STIM2, stromal interaction molecule 2; ORAI1, calcium release-activated calcium modulator 1; COPD, chronic obstructive pulmonary disease; HC, healthy control; HS, healthy smoker; SEM, standard error of mean.
CSE triggered the polarization of neutrophils
The morphology of neutrophils changed due to CSE stimulation, such that the leading edge (pseudopod) became more polarized and the tail became more prominent (uropod). The neutrophils were stimulated with 5 different concentrations of CSE (i.e., 0.5%, 1%, 2%, 5%, and 10%) in Zigmond chambers to find an optimal concentration that would induce the maximum random polarization, which would better represent the response of neutrophils to stimulation with a gradient of CSE concentration. The results showed that CSE produced the largest percentage of shape polarization at 0.5% (see Figure 6).
Figure 6.
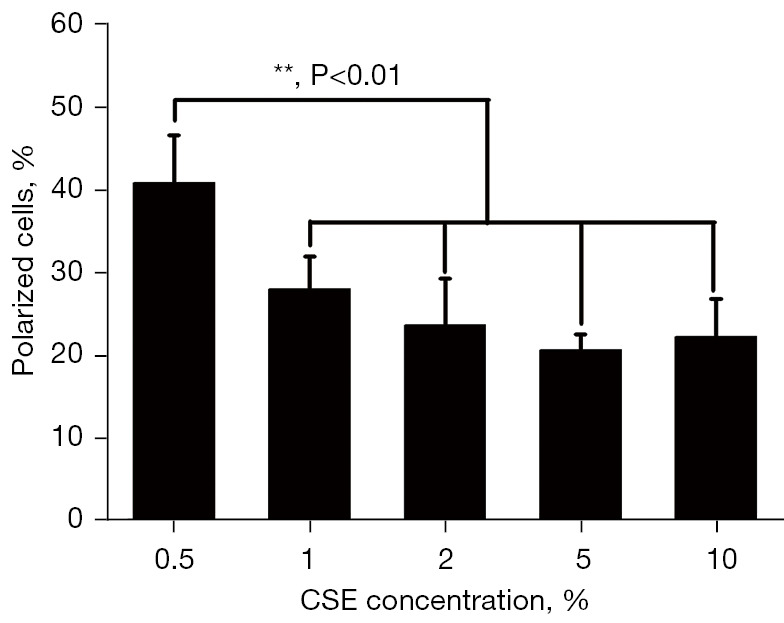
Neutrophil polarization induced by CSE. The median percentage values of the polarization cells triggered by CSE for each group. The cells were harvested at different intervals for the Zigmond assays. The data are presented as the mean ± SD (n=3). When stimulated by 5% CSE, the neutrophils demonstrated a higher random polarization than any other concentrations of CSE. This concentration was used in the subsequent experiments. CSE, cigarette smoke extract.
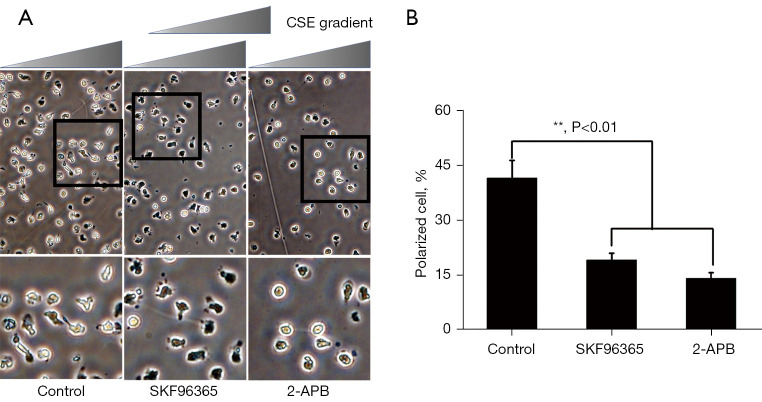
SKF96365 and 2-APB prohibited prevented the shape polarization of neutrophils stimulated with uniform concentrations of CSE
There is evidence that the inhibition of SOCE negatively regulates the response of various cells to fMLP, including the shape polarization of neutrophils (38). In the present study, 2 modulators (i.e., SKF96365 and 2-APB) that inhibit SOCE were used to investigate whether this effect also occurred when CSE-stimulated neutrophils. The cells were exposed to SKF96365 (40 µM) and 2-APB (100 µM) before stimulation with a uniform concentration (0.5%) of CSE. As expected, the inhibition of SOCE by SKF96365 (40 µM) and 2-APB (100 µM) impaired the ability of CSE stimulation to induce neutrophil polarization (see Figure 7). These results suggest that Ca2+ influx is necessary for neutrophil polarization. Based on these observations, it can be concluded that SOCE is necessary for fMLP-generated neutrophil polarization.
Figure 7.
Effects of SKF96365 and 2-APB on the direct polarization of neutrophils and CSE gradient in the Zigmond chamber. (A) Neutrophils were given 30-minutes pretreatment with phosphate buffered solution, 40 µM of SKF96365, or 100 µM of 2-APB, and then assembled in Zigmond assays. Time-lapse microscopy was used to record cell morphology on the bridge at a 30 s interval and the direction of the fMLP gradient (0–100 nmol/L) was indicated by the wedge, and cell images were acquired at 20× object by optical microscope. The icon above the images indicates the direction of fMLP gradient. A higher magnification image of the boxed section is shown below each panel of images for a clearer view of cell polarization. The percentage of cells polarized in each panel was quantified as described under the “Methods” section. Images were acquired at 20× objective. (B) The data are expressed as the mean ± SD from 3 independent experiments. CSE, cigarette smoke extract; fMLP, N-formyl-methionine-leucine-phenylalanine.
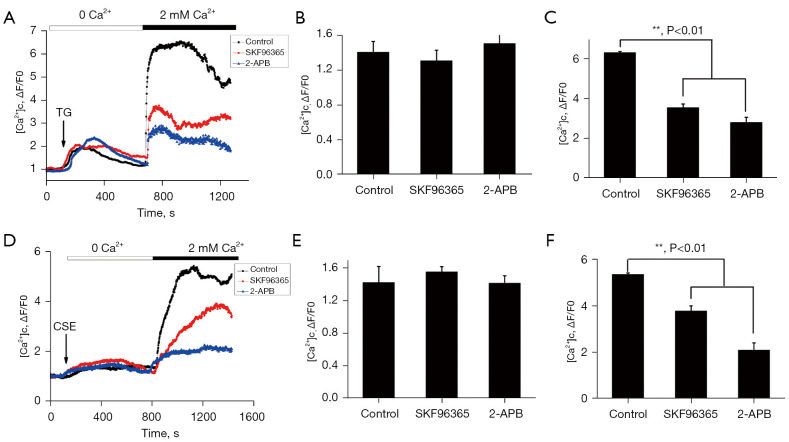
SKF96365 and 2-APB reduced the Ca2+ influx of neutrophils
The SERCA pump inhibitor TG was added to determine whether the inhibitory effects of SKF96365 and 2-APB on cell polarization and signal transduction were due to changes in cytosolic Ca2+ influx (44,45). As Figure 8A shows, basal Ca2+ stores were depleted after TG use, and after supplementation with 2 mM Ca2+, cytoplasmic Ca2+ levels were restored to the medium, revealing SOCE. Basal levels of cytoplasmic Ca2+ before the addition of CSE or TG were normalized to ΔF/F0~0 to measure increased cytoplasmic Ca2+ influx. As the right panels of Figure 8B,8C show, the pretreatment of cells with SKF96365 (40 µM) 2-APB (100 µM) significantly inhibited apparent Ca2+ influx by 60% and 70%, respectively, while the depletion of Ca2+ stores was not affected. As Figure 8D shows, CSE triggered an initial slight rise in cytosolic Ca2+ in the presence or absence of SKF96365 and 2-APB, but this rise was quickly reversed. As Figure 8E,8F show, reintroducing external Ca2+ resulted in a rapid increase in Ca2+ by 30% and 60% in cells pretreated with SKF96365 (40 µM) or 2-APB (100 µM), respectively, affecting the release of the Ca2+ reserves.
Figure 8.
Effects of SKF96365 and 2-APB on [Ca2+] i in neutrophils. The neutrophils were pretreated under Hank’s balanced salt solution with 40 µΜ SKF96365, or with 100 µΜ 2-APB for 30 minutes. (A) The pretreated cells were suspended in Ca2+-free medium and stimulated with TG (2 µΜ) followed by the addition of 2 mM of extracellular Ca2+. The cells pretreated with SKF96365 or 2-APB showed a significant reduction in TG-induced Ca2+ influx. **, P<0.01, by a 1-way ANOVA. (D) The pretreated cells were suspended in Ca2+-free medium and stimulated with CSE followed by the addition of 2 mM of extracellular Ca2+. The cells pretreated with SKF96365 or 2-APB showed a significant inhibition of CSE-induced Ca2+ influx without a change in Ca2+ store depletion. (B,C,E,F) shows the quantification of Ca2+ at 500 s or 800 s during Ca2+ influx when stimulated by TG and CSE respectively. **, P<0.01, by a 1-way ANOVA. The figures on the right show the means ± SD from 3 independent experiments. TG, thapsigargin; CSE, cigarette smoke extract.
Neutrophil polarization and chemotactic activity associated with Akt activity
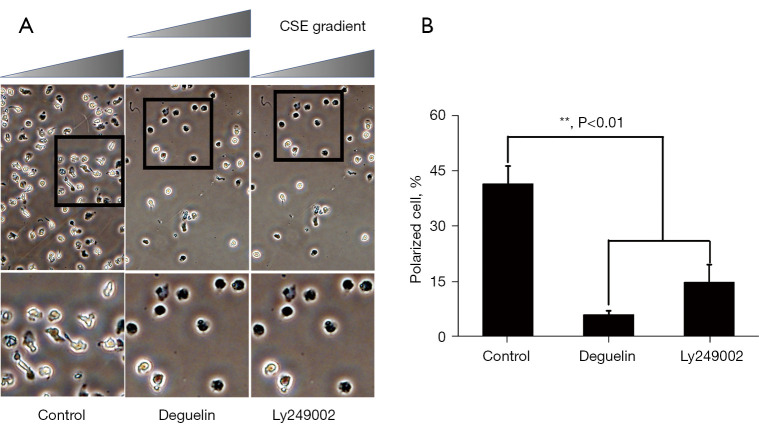
The phosphorylation of Ser473 and Thr308 in Akt, which is commonly used as a marker of PI3K activation, is a hallmark of PI3K activation (46). We then investigated whether CSE-induced cell polarization requires Akt activation. Specifically, we examined the effects of PI3K and Akt inhibitors on neutrophil polarization and chemotaxis. We used the Akt inhibitor deguelin, which produced substantial Akt activity inhibition but little PI3K activity. As Figure 9 shows, all the PI3K/Akt inhibitors reduced the proportion of cells exposed to CSE concentration gradients by 0–50%. The Akt inhibitor reduced the phosphorylation of Src, but the PI3K inhibitor did not show a significant reduction in Src and Akt phosphorylation (see Figure 10).
Figure 9.
Effects of Akt/PI3K inhibitors on the direct polarization of neutrophils and CSE gradient in the Zigmond chamber. (A) The neutrophils were pretreated with PI3K/Akt inhibitors or PBS for 30 minutes at 37 °C, and then induced to polarized and quantified in 3 independent experiments. Time-lapse microscopy was used to record cell morphology on the bridge at a 30 s interval and the direction of the fMLP gradient (0–100 nmol/L) was indicated by the wedge, and cell images were acquired at 20× object by optical microscope. The icon above the images indicates the direction of fMLP gradient. A higher magnification image of the boxed section is shown below each panel of images for a clearer view of cell polarization. (B) The data are presented as the mean ± SD (n=3). **, P<0.01 indicated a statistically significant difference. CSE, cigarette smoke extract.
Figure 10.
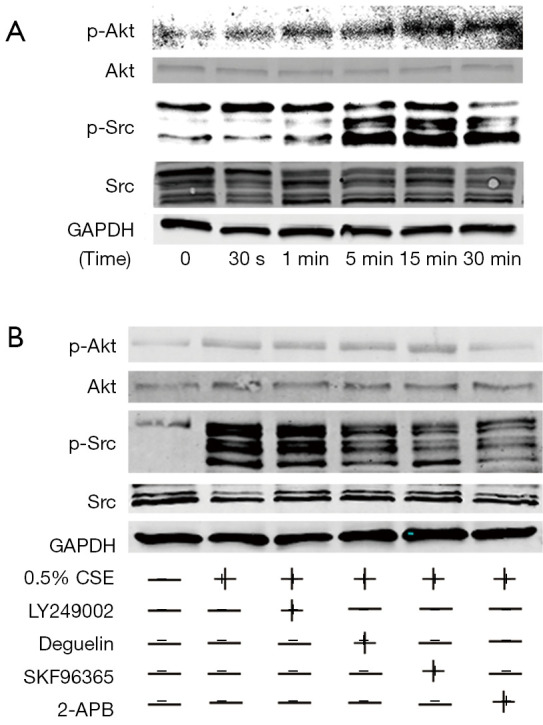
SKF96365 and 2-APB downregulated the CSE-stimulated activation of Akt and Src. The effects of SKF96365 and 2-APB on the CSE-induced activation of Akt and Src. The neutrophils were stimulated with 0.5% CSE at 37 °C. The cell lysates were analyzed by SDS-PAGE followed by Western blot using antibodies against pAkt, Akt, pSrc, and Src. The phosphorylation of the Akt and Src of the neutrophils after being stimulated by CSE for 30 s, 1 min, 5 min, 15 min, and 30 min was observed (A). The effects of SKF96365 and 2-APB on the levels of the phosphorylation of Akt and Src after being exposed to CSE were also observed. (B) The relative quantities of the activation of Akt and Src. The data are expressed as the mean ± SD from 3 experiments, P<0.05, by a 1-way ANOVA, compared to the group with CSE stimulation in the absence of modulators. CSE, cigarette smoke extract; pAkt, phosphorylated Akt; pSrc, phosphorylated Src.
SKF96365 and 2-APB downregulate the CSE-stimulated activation of Akt and Src
The PI3K inhibitor LY294002 has been shown to reduce neutrophil response to fMLP (47,48). Additionally, 4-amino-5-(-4-chlorophenyl)-7-(t-butyl) pyrazolo [3,4-d] pyrimidine (PP2) has been shown to inhibit fMLP-induced neutrophil migration (49). The PI3K inhibitor LY294002 has been shown to reduce the neutrophil response to fMLP (47,48). Additionally, a selective Src kinase inhibitor, 4-amino-5-(-4-chlorophenyl)-7-(t-butyl) pyrazolo[3,4-d] pyrimidine (PP2), has been shown to inhibit fMLP-induced neutrophil migration (49). These results suggest that both PI3K-dependent and Src-dependent pathways may play an important role in neutrophil polarization. Akt is a serine/threonine-protein kinase involved in cell migration and the AKT-mediated regulation of polarization in human neutrophil-like HL-60 cells (50). Thus, in the present study, the phosphorylation of Akt and Src was measured after CSE stimulation to determine whether CSE activates these 2 signaling pathways in neutrophils.
A Western blot analysis was performed using antibodies against phosphor-Akt, phosphor-Src, total Akt, or total Src. As demonstrated in a time-dependent manner, the reactivity of both proteins peaked at 15 minutes (see Figure 9A). These findings suggest CSE activates both PI3K-dependent and Src-dependent pathways in neutrophils and lends credence to previous reports that these 2 pathways regulate cell polarization and chemotaxis in parallel (23).
Based on the above-mentioned results, we hypothesized that PI3K (LY249002 at 10 µM) and Akt (deguelin at 100 nM) inhibitors might reduce the CSE-stimulated activation of Akt and Src. As hypothesized in the present study, SKF96365 and 2-APB may inhibit neutrophil shape polarization by modulating both signaling pathways (see Figure 7). SKF96365 and 2-APB were again used to assess their effects on the CSE-induced activation of Akt and Src in neutrophils at the concentrations described above to inhibit CSE-induced shape polarization. As Figure 10 shows, deguelin, SKF96365, and 2-APB showed significant inhibition at the level of phospho-Src, but only 2-APB showed significant inhibition at the level of phospho-Akt.
Discussion
The coordinated remodeling of the cytoskeleton and adhesion complexes is essential for neutrophil migration to tissues during the immune response (51). Cell motility is governed by the production and breakdown of actin and adhesion structures, while polarity and gradient sensing contribute to the directionality of cell motility (52,53). When compared with the HC and HS subjects, the COPD patients had 3 different neutrophil polarization patterns.
First, during the experiment, the proportion of polarized neutrophils that were not triggered by the fMLP concentration gradient differed between the Zigmond chambers and the control chamber. As a result, compared to the age-matched HC and HS subjects, the COPD patients had a higher proportion of moving neutrophils, especially in stages II and III, which suggests that COPD neutrophils may not be migrating correctly in vivo, but may initiate their attack on tissues by releasing no stimulant.
Second, neutrophils from the COPD patients exhibited a greater proportion of polarization in the random direction than those from the age-matched HC and HS subjects. However, there was a significantly smaller proportion of polarization along the fMLP direction, which suggests that in-vivo COPD neutrophil migration is excessive and inaccurate when stimulated by inflammatory stimuli, such as fMLP, and infection and colonization, as fMLP is a bacterial protein. These observations are consistent with previous findings that COPD is associated with increased and continued tissue damage and a poor response to infection (1,54,55). Research has shown that the lungs of COPD patients produce excessive amounts of chemotactic chemicals, which direct neutrophils to migrate to the lungs (56). The lungs of COPD patients suffer more damage during neutrophil migration, abundant but inaccurate polarization and chemotaxis of neutrophils of COPD patients may partially explain this. As this study showed, the increased polarization of COPD neutrophils when infection occurs increases the likelihood of collateral damage, which increases the secretion of other pro-inflammatory mediators, such as LTB4 (leukotriene B4) and IL-8 (57), which amplifies the migration of inflammatory cells to surrounding tissues and their subsequent pro-inflammatory interactions (58). The reduced polarization and migration accuracy of this population makes it more susceptible to bacterial colonization, which leads to increased morbidity and mortality from infection (55).
It should also be noted that activated neutrophils may also have many deleterious effects on the respiratory system (59). Anti-inflammatory glucocorticoids become ineffective due to pro-inflammatory reactive oxygen production. Additionally, neutrophils secrete serine proteinases whose activities in the lung may contribute to emphysema. These include NE and proteinase-3, which degrade elastin fibers and stimulate mucus secretion, and matrix metalloproteinase-8 (MMP-8) and MMP-9, which degrade elastin and collagen. Neutrophils produce substances that attract neutrophils, which continues the inflammation associated with COPD.
Aberrant migration is present at all stages of COPD. However, the disorder does not appear to be progressive phenomenon or represent a response to disease severity. As neutrophil inflammation, the length of the disease, and the severity of the disease are all factors that affect the loss of lung function, we can only speculate that extensive but inaccurate migration causes excessive tissue damage.
Abnormal polarization may be a function of SOCE-driven Ca2+ influx. Elevated Ca2+ is critical for some responses of neutrophils to various stimuli (60,61). Several recent studies have shown that Ca2+ influx occurs through SOCE and Receptor-operated Ca 2+ entry (ROCE) (62,63), and there are numerous reports that cellular functions, such as the cell polarization, survival, migration, and transfer of non-excitable cells, are affected by Ca2+ influx through SOCE, which is an important component of the Ca2+ influx pathway (38,56,64,65). We found that the first peak of the TG-triggered a Ca2+ rise, and the subsequent peak of calcium influx was more reduced in the neutrophils from the COPD patients than those from the HS and HC subjects. A previous study has shown that SOCE may play an important role in fMLP-induced cell polarization and the activation of the Akt/Src/Rho GTPase pathway (66). Thus, we can infer that dysfunctional Ca2+ influx driven by SOCE contributes to the abnormal polarization of neutrophils in COPD patients, as all samples were collected from stable period of COPD patients.
Our clinical investigations also demonstrated that STIM1, STIM2, and Orai1 were overexpressed in the neutrophils from the COPD patients compared to those from the age-matched HS and HC subjects, but no significance difference was found between the HS and HS subjects in the Western blot analysis. In 2005, STIM1 was identified as a mammalian ER Ca2+sensor (67,68). Shortly thereafter, in 2006, Orai1 was discovered to be a component of the mammalian calcium release-activated channels (68-70). As stated above, the neutrophils from the COPD patients exhibited a decrease in intracellular calcium influx induced by TG, also known as SOCE, but an increase in the expression of a critical component of SOCE (STIM1, STIM2, and ORAI2). Thus, the long-term dysfunction of SOCE leads to the compensatory overexpression of its components, which further increases the levels of STIM1, STIM2, and ORAI2 in stable COPD patients.
Despite the above findings about neutrophils, the methods by which neutrophils discover CSE and the mechanisms by which SOCE mediates cell polarization and other activities remain unknown. Cigarette smoke causes direct injury of airway epithelial cells, which leads to the release of endogenous intracellular molecules or danger-associated molecular patterns. These signals are identified by pattern-recognition receptors, such as Toll-like receptors 4 and 2 on epithelial cells, and a non-specific inflammatory response is triggered. Upon the release of early cytokines (tumor necrosis factor α and interleukins 1 and 8), macrophages, neutrophils, and dendritic cells are recruited to the site of inflammation to orchestrate the innate immune response (71). SKF96365 and 2-APB inhibited calcium influx following neutrophil activation with TG, which is a SERCA pump inhibitor that is thought to activate SOCE. Similarly, in experiments in which CSE-induced cell polarization and calcium influx, SKF96365 and 2-APB inhibited both processes. Thus, SOCE appears to be involved in TG- and CSE-induced Ca2+ influx. Both SKF96365 and 2-APB were observed to abrogate the CSE-stimulated phosphorylation of Akt and Src at the same concentrations as those that inhibited cell morphological polarization. These results suggest that the activation of CSE-stimulated Akt and Src may depend on SOCE. The results of the Western blot analysis showed that the Akt inhibitor deguelin abolished the CSE-induced phosphorylation of Akt and Src. However, LY249002, an inhibitor of PI3K, rarely exhibits Akt and Src phosphorylation inhibition. Inconsistent with this, neutrophils incubated with LY249002 and deguelin showed a decrease in the proportion of polarization when stimulated with appropriate concentrations of CSE. Additionally, we observed a similar alteration in calpain1 but not calpain2 following stimulation with CSE.
The inhibition of extracellular Ca2+ entry by 2 SOCE inhibitors suggests that Ca2+ influx via SOCE is a critical step in controlling neutrophil polarization. PI3K-dependent and Src-dependent pathways have been identified as upstream signaling pathways for Rho GTPases that regulate cell polarization (23). In the present study, CSE-activated cell polarization and the signaling pathways were significantly inhibited by SOCE inhibitors and Akt inhibitors. Thus, we propose a model in which CSE receptors initially induce Ca2+ entry via SOCE, resulting in the activation of the PI3K-dependent and non-dependent pathways (i.e., the phosphorylation of Akt and Src), which leads to the activation of calpain 1, which in turn leads to cell polarization.
In conclusion, the current study identified 3 differences in the polarization behavior of peripheral neutrophils between COPD patients and HC subjects and high-risk individuals. The cells from the COPD patients migrated with greater spontaneous polarization and arbitrary polarization but less directional polarization toward fMLP. Despite significant differences in the expression of important components of SOCE (STIM1, STIM2, and ORAI1), the cells produced less intracellular calcium influx triggered by TG (i.e., SOCE). Further, CSE-induced neutrophil polarization, which were donated from HC, and SOCE, PI3K, and Akt inhibitors decreased these polarizations and intracellular calcium influx, which provides insights into the role of the signaling pathway. Our findings support theories of neutrophil-induced tissue damage in COPD and provide novel insights into the potential mechanisms underlying abnormal cell behavior in this important disease. Based on our findings, Akt inhibitors should be further studied clinically to determine their potential use in the treatment of inflammatory diseases.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by funding from the National Natural Science Foundation of China (Nos. 81700034, 81670026, 81470228, 81770033, and 81500023), the Natural Science Foundation of Guangdong Province (Nos. 2017A030310106, S2013040013505, 2014A030310325, 2015A030313236, 2014A030310325, and 2017A030313849), and the Priority Support Foundation of the Huizhou Municipal Central Hospital.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Huizhou Municipal Central Hospital Ethics Committee (No. LLBA201913A) and all participants signed the informed consent form.
Footnotes
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1480/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1480/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1480/coif). The authors have no conflicts of interest to declare.
References
- 1.Sapey E, Stockley JA, Greenwood H, et al. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011;183:1176-86. 10.1164/rccm.201008-1285OC [DOI] [PubMed] [Google Scholar]
- 2.Saetta M. Airway inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:S17-20. 10.1164/ajrccm.160.supplement_1.6 [DOI] [PubMed] [Google Scholar]
- 3.Lange P, Ahmed E, Lahmar ZM, et al. Natural history and mechanisms of COPD. Respirology 2021;26:298-321. 10.1111/resp.14007 [DOI] [PubMed] [Google Scholar]
- 4.Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood 2019;133:2159-67. 10.1182/blood-2018-11-844548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brightling C, Greening N. Airway inflammation in COPD: progress to precision medicine. Eur Respir J 2019;54:1900651. 10.1183/13993003.00651-2019 [DOI] [PubMed] [Google Scholar]
- 6.Snider GL, Stone PJ, Lucey EC, et al. Eglin-c, a polypeptide derived from the medicinal leech, prevents human neutrophil elastase-induced emphysema and bronchial secretory cell metaplasia in the hamster. Am Rev Respir Dis 1985;132:1155-61. [DOI] [PubMed] [Google Scholar]
- 7.Kao RC, Wehner NG, Skubitz KM, et al. Proteinase 3, A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Invest 1988;82:1963-73. 10.1172/JCI113816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guenter CA, Coalson JJ, Jacques J. Emphysema associated with intravascular leukocyte sequestration. Comparison with papain-induced emphysema. Am Rev Respir Dis 1981;123:79-84. [DOI] [PubMed] [Google Scholar]
- 9.Stockley RA. Neutrophils and the pathogenesis of COPD. Chest 2002;121:151S-5S. 10.1378/chest.121.5_suppl.151S [DOI] [PubMed] [Google Scholar]
- 10.Rutgers SR, Timens W, Kaufmann HF, et al. Comparison of induced sputum with bronchial wash, bronchoalveolar lavage and bronchial biopsies in COPD. Eur Respir J 2000;15:109-15. 10.1183/09031936.00.15110900 [DOI] [PubMed] [Google Scholar]
- 11.Stănescu D, Sanna A, Veriter C, et al. Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax 1996;51:267-71. 10.1136/thx.51.3.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilette C, Colinet B, Kiss R, et al. Increased galectin-3 expression and intra-epithelial neutrophils in small airways in severe COPD. Eur Respir J 2007;29:914-22. 10.1183/09031936.00073005 [DOI] [PubMed] [Google Scholar]
- 13.Parr DG, White AJ, Bayley DL, et al. Inflammation in sputum relates to progression of disease in subjects with COPD: a prospective descriptive study. Respir Res 2006;7:136. 10.1186/1465-9921-7-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest 2005;128:1995-2004. 10.1378/chest.128.4.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshioka A, Betsuyaku T, Nishimura M, et al. Excessive neutrophil elastase in bronchoalveolar lavage fluid in subclinical emphysema. Am J Respir Crit Care Med 1995;152:2127-32. 10.1164/ajrccm.152.6.8520785 [DOI] [PubMed] [Google Scholar]
- 16.Rytilä P, Plataki M, Bucchieri F, et al. Airway neutrophilia in COPD is not associated with increased neutrophil survival. Eur Respir J 2006;28:1163-9. 10.1183/09031936.00149005 [DOI] [PubMed] [Google Scholar]
- 17.Voynow JA, Shinbashi M. Neutrophil Elastase and Chronic Lung Disease. Biomolecules 2021;11:1065. 10.3390/biom11081065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hind LE, Vincent WJ, Huttenlocher A. Leading from the Back: The Role of the Uropod in Neutrophil Polarization and Migration. Dev Cell 2016;38:161-9. 10.1016/j.devcel.2016.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niggli V. Signaling to migration in neutrophils: importance of localized pathways. Int J Biochem Cell Biol 2003;35:1619-38. 10.1016/S1357-2725(03)00144-4 [DOI] [PubMed] [Google Scholar]
- 20.Onishi K, Higuchi M, Asakura T, et al. The PI3K-Akt pathway promotes microtubule stabilization in migrating fibroblasts. Genes Cells 2007;12:535-46. 10.1111/j.1365-2443.2007.01071.x [DOI] [PubMed] [Google Scholar]
- 21.Inoue T, Meyer T. Synthetic activation of endogenous PI3K and Rac identifies an AND-gate switch for cell polarization and migration. PLoS One 2008;3:e3068. 10.1371/journal.pone.0003068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singhal A, Dhankani P, Gupta KL, et al. Rho signaling inhibition mitigates lung injury via targeting neutrophil recruitment and selectin-AKT signaling. Biochim Biophys Acta Mol Cell Res 2021;1868:119122. 10.1016/j.bbamcr.2021.119122 [DOI] [PubMed] [Google Scholar]
- 23.Sai J, Raman D, Liu Y, et al. Parallel phosphatidylinositol 3-kinase (PI3K)-dependent and Src-dependent pathways lead to CXCL8-mediated Rac2 activation and chemotaxis. J Biol Chem 2008;283:26538-47. 10.1074/jbc.M805611200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuzzi PA, Senetar MA, Huttenlocher A. Asymmetric localization of calpain 2 during neutrophil chemotaxis. Mol Biol Cell 2007;18:795-805. 10.1091/mbc.e06-09-0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshikawa T, Dent G, Ward J, et al. Impaired neutrophil chemotaxis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:473-9. 10.1164/rccm.200507-1152OC [DOI] [PubMed] [Google Scholar]
- 26.Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol 2007;9:193-200. 10.1038/ncb1536 [DOI] [PubMed] [Google Scholar]
- 27.Condliffe AM, Kitchen E, Chilvers ER. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin Sci (Lond) 1998;94:461-71. 10.1042/cs0940461 [DOI] [PubMed] [Google Scholar]
- 28.Froghi S, Grant CR, Tandon R, et al. New Insights on the Role of TRP Channels in Calcium Signalling and Immunomodulation: Review of Pathways and Implications for Clinical Practice. Clin Rev Allergy Immunol 2021;60:271-92. 10.1007/s12016-020-08824-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvatici R, Falzarano S, Mollica A, et al. Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur J Pharmacol 2006;534:1-11. 10.1016/j.ejphar.2006.01.034 [DOI] [PubMed] [Google Scholar]
- 30.Putney JW, Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986;7:1-12. 10.1016/0143-4160(86)90026-6 [DOI] [PubMed] [Google Scholar]
- 31.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977;1:1645-8. 10.1136/bmj.1.6077.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keatings VM, Collins PD, Scott DM, et al. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 1996;153:530-4. 10.1164/ajrccm.153.2.8564092 [DOI] [PubMed] [Google Scholar]
- 33.O'Donnell RA, Richter A, Ward J, et al. Expression of ErbB receptors and mucins in the airways of long term current smokers. Thorax 2004;59:1032-40. 10.1136/thx.2004.028043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulop T, Larbi A, Douziech N, et al. Signal transduction and functional changes in neutrophils with aging. Aging Cell 2004;3:217-26. 10.1111/j.1474-9728.2004.00110.x [DOI] [PubMed] [Google Scholar]
- 35.Montes de Oca M, Pérez-Padilla R. Global Initiative for Chronic Obstructive Lung Disease (GOLD)-2017: The alat perspective. Arch Bronconeumol 2017;53:87-8. 10.1016/j.arbr.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 36.Nauseef WM. Isolation of human neutrophils from venous blood. Methods Mol Biol 2014;1124:13-8. 10.1007/978-1-62703-845-4_2 [DOI] [PubMed] [Google Scholar]
- 37.Keller HU, Zimmermann A. Shape, movement and function of neutrophil granulocytes. Biomed Pharmacother 1987;41:285-9. [PubMed] [Google Scholar]
- 38.Schaff UY, Dixit N, Procyk E, et al. Orai1 regulates intracellular calcium, arrest, and shape polarization during neutrophil recruitment in shear flow. Blood 2010;115:657-66. 10.1182/blood-2009-05-224659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heit B, Liu L, Colarusso P, et al. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J Cell Sci 2008;121:205-14. 10.1242/jcs.020412 [DOI] [PubMed] [Google Scholar]
- 40.McKay DA, Kusel JR, Wilkinson PC. Studies of chemotactic factor-induced polarity in human neutrophils. Lipid mobility, receptor distribution and the time-sequence of polarization. J Cell Sci 1991;100 (Pt 3):473-9. 10.1242/jcs.100.3.473 [DOI] [PubMed] [Google Scholar]
- 41.Zhao D, Meng X, Cai C, et al. Temperature pretreatment alters the polarization response of human neutrophils to the chemoattractant N-formyl-Met-Leu-Phe. Inflammation 2009;32:47-56. 10.1007/s10753-008-9101-3 [DOI] [PubMed] [Google Scholar]
- 42.Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256-76. 10.1164/ajrccm.163.5.2101039 [DOI] [PubMed] [Google Scholar]
- 43.Bush A, Cramer D. Guidelines for the measurement of respiratory function. Respir Med 1994;88:798. 10.1016/S0954-6111(05)80210-0 [DOI] [PubMed] [Google Scholar]
- 44.Shibukawa Y, Suzuki T. Ca2+ signaling mediated by IP3-dependent Ca2+ releasing and store-operated Ca2+ channels in rat odontoblasts. J Bone Miner Res 2003;18:30-8. 10.1359/jbmr.2003.18.1.30 [DOI] [PubMed] [Google Scholar]
- 45.Liu X, O'Connell A, Ambudkar IS. Ca2+-dependent inactivation of a store-operated Ca2+ current in human submandibular gland cells. Role of a staurosporine-sensitive protein kinase and the intracellular Ca2+ pump. J Biol Chem 1998;273:33295-304. 10.1074/jbc.273.50.33295 [DOI] [PubMed] [Google Scholar]
- 46.Burelout C, Naccache PH, Bourgoin SG. Dissociation between the translocation and the activation of Akt in fMLP-stimulated human neutrophils--effect of prostaglandin E2. J Leukoc Biol 2007;81:1523-34. 10.1189/jlb.0406256 [DOI] [PubMed] [Google Scholar]
- 47.Servant G, Weiner OD, Herzmark P, et al. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 2000;287:1037-40. 10.1126/science.287.5455.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knall C, Worthen GS, Johnson GL. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci U S A 1997;94:3052-7. 10.1073/pnas.94.7.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fumagalli L, Zhang H, Baruzzi A, et al. The Src family kinases Hck and Fgr regulate neutrophil responses to N-formyl-methionyl-leucyl-phenylalanine. J Immunol 2007;178:3874-85. 10.4049/jimmunol.178.6.3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou W, Chu X, Cai C, et al. AKT-mediated regulation of polarization in differentiated human neutrophil-like HL-60 cells. Inflamm Res 2012;61:853-62. 10.1007/s00011-012-0478-y [DOI] [PubMed] [Google Scholar]
- 51.Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science 2003;302:1704-9. 10.1126/science.1092053 [DOI] [PubMed] [Google Scholar]
- 52.Szczur K, Xu H, Atkinson S, et al. Rho GTPase CDC42 regulates directionality and random movement via distinct MAPK pathways in neutrophils. Blood 2006;108:4205-13. 10.1182/blood-2006-03-013789 [DOI] [PubMed] [Google Scholar]
- 53.Adams W, Espicha T, Estipona J. Getting Your Neutrophil: Neutrophil Transepithelial Migration in the Lung. Infect Immun 2021. [Epub ahead of print]. 10.1128/IAI.00659-20 [DOI] [PubMed] [Google Scholar]
- 54.Li QY, Huang SG, Wan HY, et al. Effect of smoking cessation on airway inflammation of rats with chronic bronchitis. Chin Med J (Engl) 2007;120:1511-6. 10.1097/00029330-200709010-00009 [DOI] [PubMed] [Google Scholar]
- 55.Restrepo MI, Mortensen EM, Pugh JA, et al. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J 2006;28:346-51. 10.1183/09031936.06.00131905 [DOI] [PubMed] [Google Scholar]
- 56.Sun S, Li W, Zhang H, et al. Requirement for store-operated calcium entry in sodium butyrate-induced apoptosis in human colon cancer cells. Biosci Rep 2012;32:83-90. 10.1042/BSR20110062 [DOI] [PubMed] [Google Scholar]
- 57.Hubbard RC, Fells G, Gadek J, et al. Neutrophil accumulation in the lung in alpha 1-antitrypsin deficiency. Spontaneous release of leukotriene B4 by alveolar macrophages. J Clin Invest 1991;88:891-7. 10.1172/JCI115391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferry G, Lonchampt M, Pennel L, et al. Activation of MMP-9 by neutrophil elastase in an in vivo model of acute lung injury. FEBS Lett 1997;402:111-5. 10.1016/S0014-5793(96)01508-6 [DOI] [PubMed] [Google Scholar]
- 59.Barnes PJ. Medicine. Neutrophils find smoke attractive. Science 2010;330:40-1. 10.1126/science.1196017 [DOI] [PubMed] [Google Scholar]
- 60.Chen LW, Jan CR. Mechanisms and modulation of formyl-methionyl-leucyl-phenylalanine (fMLP)-induced Ca2+ mobilization in human neutrophils. Int Immunopharmacol 2001;1:1341-9. 10.1016/S1567-5769(01)00066-2 [DOI] [PubMed] [Google Scholar]
- 61.Krause KH, Campbell KP, Welsh MJ, et al. The calcium signal and neutrophil activation. Clin Biochem 1990;23:159-66. 10.1016/0009-9120(90)80030-M [DOI] [PubMed] [Google Scholar]
- 62.Salmon MD, Ahluwalia J. Pharmacology of receptor operated calcium entry in human neutrophils. Int Immunopharmacol 2011;11:145-8. 10.1016/j.intimp.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 63.Salmon MD, Ahluwalia J. Actions of calcium influx blockers in human neutrophils support a role for receptor-operated calcium entry. Cell Immunol 2010;262:6-10. 10.1016/j.cellimm.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 64.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 2009;15:124-34. 10.1016/j.ccr.2008.12.019 [DOI] [PubMed] [Google Scholar]
- 65.Lee C, Xu DZ, Feketeova E, et al. Store-operated calcium channel inhibition attenuates neutrophil function and postshock acute lung injury. J Trauma 2005;59:56-63; discussion 63. 10.1097/01.TA.0000171456.54921.FE [DOI] [PubMed] [Google Scholar]
- 66.Zou W, Meng X, Cai C, et al. Store-operated Ca2+ entry (SOCE) plays a role in the polarization of neutrophil-like HL-60 cells by regulating the activation of Akt, Src, and Rho family GTPases. Cell Physiol Biochem 2012;30:221-37. 10.1159/000339059 [DOI] [PubMed] [Google Scholar]
- 67.Liou J, Kim ML, Heo WD, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 2005;15:1235-41. 10.1016/j.cub.2005.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roos J, DiGregorio PJ, Yeromin AV, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 2005;169:435-45. 10.1083/jcb.200502019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feske S, Gwack Y, Prakriya M, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006;441:179-85. 10.1038/nature04702 [DOI] [PubMed] [Google Scholar]
- 70.Vig M, Peinelt C, Beck A, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006;312:1220-3. 10.1126/science.1127883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet 2012;379:1341-51. 10.1016/S0140-6736(11)60968-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as