Abstract
Threats elicit physiological responses, the frequency and intensity of which have implications for survival. Ethical and practical limitations on human laboratory manipulations present barriers to studying immersive threat. Furthermore, few investigations have examined group effects and concordance with subjective emotional experiences to threat. The current preregistered study measured electrodermal activity in 156 adults while they participated in small groups in a 30-min haunted-house experience involving various immersive threats. Results revealed positive associations between (a) friends and tonic arousal, (b) unexpected attacks and phasic activity (frequency and amplitude), (c) subjective fear and phasic frequency, and (d) dissociable sensitization effects linked to baseline orienting response. Findings demonstrate the relevance of (a) social dynamics (friends vs. strangers) for tonic arousal and (b) subjective fear and threat predictability for phasic arousal.
Keywords: fear, physiology, skin conductance, social, threat, open data, open materials, preregistered
Physiological threat responses help coordinate defensive reactions to promote safety (Lang et al., 2000). Arousal of the sympathetic nervous system supports “fight or flight” reactions, and intensity of sympathetic arousal is commonly indexed through electrodermal activity (EDA; Dawson et al., 2017). Threat features and the surrounding environment may alter sympathetic responding, as can subjective emotional experiences and sensitivity to threat. Studying threat is challenging given ethical constraints on human laboratory experiments. Laboratory threat stimuli are frequently mild (noise, shock), homogeneous, and discretely administered in a way that is less dynamic than in the real world.
In this study, groups of participants went through a 30-min immersive haunted-house threat experience. The haunted house included 17 rooms, each comprising various threats that were temporally and thematically linked to form a coherent narrative and uninterrupted experience. Real-time physiological-monitoring wristbands continuously measured EDA, which incorporates slow shifting tonic components (skin conductance level, or SCL) and more rapid transient events (skin conductance responses, or SCRs). EDA was examined in relation to four factors: external factors of group composition and threat imminence as well as intrapersonal factors of subjective fear and baseline orienting response (a measure of sensitivity to threat).
Under threat, the presence of other people can act as a safety signal, danger signal, or both. Social contagion and fear buffering have been extensively observed in rodents (Hernandez-Lallement et al., 2022) and humans (Oliveira & Faustino, 2017). Both phenomena have roots in ecological models. Larger group sizes can reduce fear and danger through risk dilution whereby the presence of other individuals increases threat detection, deters predators, and provides secondary predation targets (Beauchamp, 2015; Mobbs et al., 2015). The mere presence of a friend can reduce physiological responses to aversive events, a phenomenon referred to as social buffering (Heinrichs et al., 2003). Alternatively, friends may increase physiological arousal through social contagion. During social contagion, the threat responding of other people triggers a response despite the absence of direct threat detection (Pereira et al., 2012). Relationships matter for social contagion; closer relationships are more effective at transferring aversive experiences (Martin et al., 2015). Despite studies of social-threat effects, how facing threat in a group relates to human physiological responding is largely unknown because investigation of human social contagion and buffering does not typically involve collective threat experiences (e.g., Gomes & Semin, 2020). Uncovering more about social-group effects on human threat physiology is important because humans frequently encounter threats in groups, humans experience both social affiliation and threat from conspecifics (Schindler & Radford, 2018), and skin conductance is higher in response to threatening than to nonthreatening social information (Adolph et al., 2010).
Features of the threat itself also influence physiological responding (Davies & Craske, 2015). Threat features and being in social groups may differentially contribute to physiology because they prepare the body for different actions. Social others influence the general need for vigilance, whereas threat features convey explicit information about combatting present danger. Among threat features, a key consideration is imminence, or the spatial and temporal proximity of threat (Fanselow & Lester, 1988). Attack probability varies along the imminence continuum ranging from safe states, during which attack probability is almost zero, to circa strike (CS), during which a predator is about to attack or is attacking. Defensive responses intensify as imminence increases (Mobbs et al., 2007). Difficulty in estimating attack probability because of unpredictability can also increase responsivity. Unpredictability itself is generally aversive and can lead to sustained threat responding (Grillon et al., 2004; Kirschner et al., 2016).
Although physiological reactivity to threat has been largely treated as a proxy for the subjective experience of fear (Kreibig, 2010), subjective and objective measures do not always align (Taschereau-Dumouchel et al., 2020). Individuals report fear without corresponding changes in physiology and experience physiological arousal without conscious fear (Rosebrock et al., 2016; Tooley et al., 2017). Lack of emotional coherence is linked to vulnerability to psychopathology and problematic emotion regulation (Dan-Glauser & Gross, 2013; Rosebrock et al., 2016), whereas stronger coherence is thought to reflect better emotion recognition (Kret & De Gelder, 2012). Prescriptive social norms regarding how certain emotions should be expressed may also play a role (Rattel et al., 2020). The current study provided a unique opportunity to measure concordance during recreational threat exposure, which included dynamics of enjoyment together with fear (Andersen et al., 2020).
Statement of Relevance.
When we encounter threats, our bodies respond with increased heart rate, faster pulse, and sweating. These physiological responses help organisms survive—they prepare us to flee or fight. Controlled research on how these responses unfold in different real-world and interpersonal contexts is rare because of ethical and practical barriers to exposing humans to immersive and intense threats in the laboratory. To overcome these barriers, we examined the intensity and frequency of bodily responses during a multifaced haunted-house threat experience. We found that unexpected scares produced more frequent and higher intensity responses than predictable scares. We also found that the presence of friends in the experience increased overall physiological arousal. And people who had more frequent physiological responses also reported feeling more afraid. This unique study provides important information about how the human body dynamically responds to different features of immersive threat experiences.
Across species, animals respond to new stimuli with an orienting response frequently characterized by increased arousal (Bradley, 2009). Over time, habituation (response decrement) or sensitization (response amplification) may occur (Çevik, 2014). Orienting serves to ensure that new stimuli are attended to, whereas habituation ensures that the brain and body do not spend resources unnecessarily attending to repeated events (Bradley, 2009; Rankin et al., 2009). Although the utility of sensitization is less well understood, it is commonly observed in pain disorders (Ursin, 2014) and can be an indicator of anxiety in humans (Campbell et al., 2014). Typically, studies of physiological sensitization and habituation use repeated presentation of identical stimuli. In the current study, stimuli (threats in each room of the haunted house) were not identically repeated, allowing us to test whether baseline orienting response is associated with sensitization to repeated novel threats. Additionally, different indices of EDA were tested to determine how SCR amplitude and frequency relate to baseline orienting response during immersive threat.
Current Study
This preregistered study used a unique, immersive experience to study human subjective and physiological response to threat. The haunted house was set in a fictitious penitentiary that involved a variety of threatening encounters. Although participants knew they were not in actual danger, this type of immersive threat manipulation is not replicable in the lab. Each of the 17 contiguous rooms involved distinct threats, including the inability to escape an oncoming car, mimicked suffocation, actual electric shocks, and being shot with pellets by a firing squad while blindfolded. Threat imminence was independently scored for each room to assess how EDA varied as a function of attack predictability. Participants attended the experience in groups of varying size and composition, with some degree of random assignment. This created a unique opportunity to examine whether group composition was associated with EDA. This study also tested effects of fear ratings and baseline orienting response. Phasic and tonic EDA were measured as indicators of sympathetic arousal to threat. The design and analysis plans for the study were preregistered at https://osf.io/wxek6/. 1
Method
Participants
Participants who first paid an entrance fee and signed a legal waiver to participate in the haunted house were then invited to participate in this study. Data were collected over 8 days from 157 adults in 59 groups. Only one group had only participants; every other group contained a mix of participants and nonparticipants. Data from one participant were excluded because of a trigger failure during data collection (age = 24 years, male). The resulting 156 participants were included in the analyses (age: M = 25.79 years, SD = 5.90, range = 18–59; 85 females). One participant did not report age. The sample size was based on collecting the maximum available data given experimental constraints, including a limited run season for the experience and the number of wearable devices. All participants provided written consent in accordance with the policies of the institutional review board, and study procedures were conducted in accordance with the American Psychological Association guidelines for human research.
Haunted-house threat manipulation
The 17th Door haunted-house attraction is an established haunted-house experience run by a professional production company. The haunted house consisted of 17 rooms, each of which included distinct threats (e.g., electric taser, suffocation, firing squad). Many threats were more threatening and/or pain inducing than is ethically allowed in campus laboratory experiences in the United States. Rooms were linked to a theme about a dangerous prisoner in a fictitious prison. As part of the story arc, participants were guided from room to room in a continuous sequence by one of the haunted-house personnel (not involved in the scares) without a temporal or spatial break and without experimenter interruption. Although the experience is discussed as a collection of rooms, the experience did not involve breaks or exits from the experience between rooms. As in other guided attractions and tours, participants were moved through the experience on a fixed schedule by a haunted-house employee who was not involved in the scares. One group of participants entered the experience at a time, and there was a delay before the next group began, eliminating the potential for emotional contagion between groups. The entire experience lasted approximately 30 min. Room and threat descriptions are provided in Table S1 in the Supplemental Material available online. Personnel in the haunted house were blind to the existence of study participants and study hypotheses.
Electrodermal reactivity
EDA was measured continuously throughout the experience using a wrist-worn wireless sensor that records skin conductance exosomatically (E4 system, Empatica, Boston, MA; sampling frequency: 4 Hz, resolution: 1 digit ~900 picoSiemens). Data were downsampled to 1 Hz for processing. Using LedaLab (Version 3.4.9; Benedek & Kaernbach, 2010), we removed artifacts using a first-order Butterworth filter with a cutoff frequency of 0.05 Hz. Next, the skin conductance signal was decomposed into tonic and phasic components using continuous decomposition analysis. Phasic SCRs are short-term responses to specific stimuli (e.g., SCR will ramp up if you hear a loud noise). Tonic SCL is less reactive to external stimuli and represents slow drifts in general physiological responding. Continuous decomposition analysis is particularly useful for data with high phasic activity, as is the case in a continuous threat experience. A threshold value of .05 μs was applied to SCRs (Boucsein et al., 2012). Metrics were z transformed to facilitate between-events and between-subjects comparison by reducing variance due to peripheral factors unrelated to the experiment (e.g., skin properties). For each event, we assessed average SCL, frequency of SCRs, and summed amplitude of SCRs. All metrics are expressed in microseconds (μs). Amplitude refers to the amplitude across all trials for which there were valid responses (i.e., SCRs in excess of .05 μs).
A manual trigger was initiated by an experimenter as participants entered the experience. Timing of the experience was tightly controlled by the haunted-house personnel, who provided a detailed timeline of events to the experimenters, including start and stop timing for each room and timing of scares within rooms. Part of the experiment team also participated in a pilot run (no participant data collected) of the study to verify timing. The first 30 s after trigger start were cropped from the experience time series to account for instructions and walking from the entrance to Room 1. Timing was subsequently locked to the haunted-house schedule. The time series ended at 31 min from the trigger start (i.e., the duration of the experience). An additional 10 s were cropped between each room to account for small possible deviations in timing. Continuous decomposition analyses used data from the entire room with the exception of these 10-s buffers. Data for each room were approximately 1.5 min in duration. All analyses except for threat-imminence models were agnostic to differences in threats between rooms and treated the experience as a single immersive experiment comprising multiple repeated threat events.
Group composition
Participants self-reported the number of friends and strangers in their group during the experience. The entire group composition was beyond experimenter control (although an effort was made to recruit both smaller and larger friend groups). A ratio of friends to strangers was calculated by subtracting the proportion of strangers from the proportion of friends. Positive values indicate more friends than strangers, and negative values indicate more strangers than friends.
Threat imminence
Imminence is typically defined to include four phases of threat: safety, before encounter, after encounter, and CS (Fanselow & Lester, 1988). CS threat exists when a predator is prepared to attack or has attacked. Imminence was independently coded by two experimenters familiar with the threat-imminence continuum, one of whom went through the haunted-house experience. Coding was based on room descriptions provided by the haunted house. Coders agreed on 71% of rooms, and average scores from the raters were used in the analyses. Results remained the same when scores from each rater independently were used. The haunted house was designed to include scares; thus, the coders took into account the presence and predictability of a CS instead of including all phases of the threat-imminence continuum. Imminence was scored on a scale as follows: no CS = 0, expected CS = 1, unexpected CS = 2, expected and unexpected CS = 3. Higher scores were given to compound fear experiences consisting of both expected and surprise scares.
Subjective fear
Before the experience, participants reported expected fear on a scale from 1 (low) to 10 (high). After the experience, participants reported experienced fear on the same scale. To avoid artificial skew due to floor or ceiling effects, we conducted analyses for experienced fear controlling for expected fear.
Baseline orienting response
The baseline orienting response was operationalized by calculating SCR frequency and amplitude during Room 1 of the haunted-house experience using continuous decomposition analysis for the entire first room and taking the average. Only one scare event occurred in Room 1 (see Table S1). Change from this baseline orienting response across subsequent rooms of the experience (Rooms 2–17) was operationalized as sensitization. Sensitization was quantified by extracting individual slope coefficients from two linear growth-curve models with room as a predictor of SCR frequency and amplitude, respectively. Positive slopes are referred to as sensitization, and negative slopes are referred to as habituation. Habituation was observed in only 11% of participants for SCR frequency and 2% for SCR amplitude. Thus, results focus on sensitization (high positive slopes) and blunted sensitization (positive slopes close to zero).
Analytic approach
Data analyses were conducted in the R programming environment (Version 3.6.1; R Core Team, 2019) using the lme4 (Version 1.1-21; Bates et al., 2015) and reghelper (Version 1.0.1; Hughes, 2020) packages. Mixed-effects models were tested using the lmer function in lmerTest (Version 3.1-3; Kuznetsova et al., 2017), which assessed t tests using Satterthwaite’s method. Effect sizes reported as R2 are conditional effects of variance explained by the entire model (Nakagawa et al., 2017). Model comparisons were conducted using the anova function in lme4. Linear models were tested using the lm function in the stats package for R (Version 4.0.3; R Core Team, 2020).
EDA ij reactivity for the jth participant at the ith room was modeled as a function of time (room order) and factors of interest (group composition, threat imminence, subjective fear). Using linear models, we modeled sensitization as a function of baseline orienting response. Because baseline orienting response was an independent question of interest, all models excluded baseline orienting response data from Room 1 and used data only for Rooms 2 to 17. Model diagnostics are provided in Figure S1 in the Supplemental Material. Covariates were included for day of testing and group to account for potential variability between experiences, but neither effect was significant, and no results were affected by covariate inclusion.
Results
Correlations among EDA metrics are provided in Table S2 in the Supplemental Material. Initial fit statistics for mixed-effects models assessing EDA are depicted in Table 1. First, an unconditional model was run specifying separate random intercepts for individuals to confirm that there were significant individual differences in EDA. Intraclass correlation coefficients indicated that it was appropriate to include random intercepts in subsequent models (Koo & Li, 2016). Next, fixed effects of time (room order) were added, and model fit significantly improved for all models. Thus, effects of time were included in subsequent models.
Table 1.
Fit Statistics and Comparisons for Mixed-Effects Models Assessing Electrodermal Activity
| Model and DV | Observations (N) | Individuals (N) | AIC | BIC | –2LL | ICC | χ2 | p |
|---|---|---|---|---|---|---|---|---|
| Random intercepts | ||||||||
| Tonic SCL | 2,496 | 156 | 16,018.0 | 16,035.4 | −8,006.0 | .81 | ||
| SCR frequency | 2,496 | 156 | 15,397.5 | 15,415.0 | −7,695.8 | .55 | ||
| SCR amplitude | 2,496 | 156 | 13,864.9 | 13,882.4 | −6,929.5 | .59 | ||
| Random intercepts + time | ||||||||
| Tonic SCL | 2,496 | 156 | 14,474.4 | 14,497.7 | −7,233.2 | 1,545.6 | < .001 | |
| SCR frequency | 2,496 | 156 | 15,011.8 | 15,035.1 | −7,501.9 | 387.7 | < .001 | |
| SCR amplitude | 2,496 | 156 | 13,457.4 | 13,480.7 | −6,724.7 | 409.6 | < .001 |
Note: DV = dependent variable; AIC = Akaike information criterion; BIC = Bayesian information criterion; LL = log likelihood; ICC = intraclass correlation coefficient; SCL = skin conductance level; SCR = skin conductance response.
Group composition
The number of friends per group ranged from one to eight, excluding the responding participant (M = 3.47, SD = 1.60). The number of strangers per group ranged from zero to seven (M = 3.23, SD = 1.85). The numbers of friends and strangers were highly correlated because the haunted-house management preferred similarly sized groups of eight to 10 individuals, so that more friends resulted in fewer strangers, r(156) = −.70, p < .001. This high correlation motivated the use of the difference ratio between friends and strangers, which ranged from −.75 to 1 (M = .07, SD = .49).
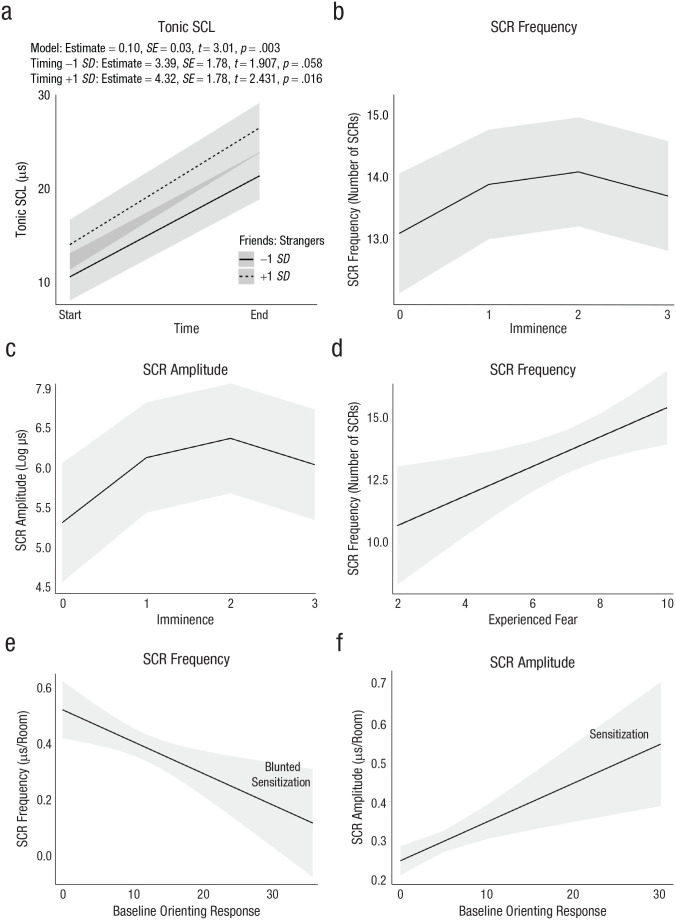
An increased ratio of friends to strangers was significantly associated with higher tonic SCL in the model controlling for effects of time (Table 2, Model A). Time and friends:strangers ratio interacted such that tonic SCL deviated as a function of group composition at the end of the experience but did not significantly differ at the beginning (Fig. 1a).
Table 2.
Significant Results From Linear and Mixed-Effects Models Predicting Electrodermal Activity
| Model | Estimate | SE | t | p | 95% CI | R 2 | σ2 | τ00 |
|---|---|---|---|---|---|---|---|---|
| A. Friend ratio predicting tonic SCL | 3.86 | 1.77 | 2.18 | .029 | [0.39, 7.33] | .90 | 14.28 | 115.23 |
| B. Imminence predicting SCR frequency a | −13.96 | 4.46 | −3.13 | .002 | [−22.69, −5.23] | .62 | 19.51 | 28.45 |
| C. Imminence predicting SCR amplitude a | −13.65 | 3.24 | −4.22 | < .001 | [−20.00, −7.31] | .66 | 10.30 | 17.96 |
| D. Experienced fear predicting SCR frequency | 0.58 | 0.21 | 2.71 | .007 | [0.16, 0.99] | .62 | 19.60 | 27.21 |
| E. Baseline orienting response predicting SCR frequency sensitization | −0.01 | 0.004 | −2.86 | .005 | [−0.02, −0.003] | .05 | ||
| F. Baseline orienting response predicting SCR amplitude sensitization | 0.01 | 0.003 | 3.20 | .002 | [0.004, 0.02] | .06 |
Note: Models A to D are mixed-effects models including random intercepts and controlling for the effects of time. Models E and F are linear regression models. For each model, the estimate is unstandardized, and the t is standardized. CI = confidence interval; SCL = skin conductance level; SCR = skin conductance response.
This model predicted quadratic effects.
Fig. 1.
Model estimates of effects of context and endogenous variables on electrodermal-activity measures. The top row shows (a) mean tonic skin conductance level (SCL) as a function of measurement time and friends:strangers ratio and (b) mean skin conductance response (SCR) frequency as a function of threat imminence. The middle row shows (c) mean SCR amplitude as a function of threat imminence and (d) mean SCR frequency as a function of experienced fear. The bottom row shows (e) mean SCR frequency as a function of baseline orienting response and (f) mean SCR amplitude as a function of baseline orienting response. Values in (e) and (f) index sensitization outcomes. Lines depict predicted values (marginal effects) for the regression model; error bands indicate 95% confidence intervals.
Threat imminence
Of the 16 rooms (Rooms 2–17), 12.50% were coded as no CS, 18.75% as expected CS, 31.25% as unexpected CS, and 37.50% as a combination of expected and unexpected CS. Threat imminence was not significantly associated with time, estimate: b = −0.031, SE = 0.06, t = −0.52, p = .61, 95% confidence interval (CI) = [−0.16, 0.10]; thus, imminence was not confounded with sensitization effects.
Threat imminence was linearly associated with SCR amplitude, estimate: b = 0.15, SE = 0.06, t = 2.42, p = .016, 95% CI = [0.03, 0.28], R2 = .66, σ2 = 10.38, τ00 = 17.96. Linear effects of imminence were not associated with SCR frequency. Imminence was quadratically associated with both SCR frequency and amplitude (Table 2, Models B and C, Figs. 1b and 1c), and quadratic models were a better fit—SCR frequency: χ2 = 9.81, p = .002; SCR amplitude: χ2 = 17.74, p < .001. Post hoc tests comparing unexpected CS with other imminence categories revealed that unexpected CS alone evoked higher reactivity than other imminence categories (Table 3). See Table S3 in the Supplemental Material for comparison with exponential and logarithmic models, against which the quadratic model also produced better fit.
Table 3.
Results From Post Hoc Mixed-Effects Models Comparing Unexpected Circa Strike (CS) With Other Threat-Imminence Levels
| Outcome and model | Estimate | SE | t | p | 95% CI | R 2 | σ2 | τ00 |
|---|---|---|---|---|---|---|---|---|
| SCR frequency | ||||||||
| A. Unexpected CS vs. no CS | 0.48 | 0.16 | 3.10 | .002 | [0.18, 0.79] | .61 | 20.15 | 28.35 |
| B. Unexpected CS vs. expected CS | 0.55 | 0.29 | 1.91 | .057 | [−0.02, 1.12] | .60 | 21.05 | 28.13 |
| C. Unexpected CS vs. combined CS | −0.59 | 0.21 | −2.74 | .006 | [−1.01, −0.17] | .62 | 19.07 | 26.60 |
| SCR amplitude | ||||||||
| D. Unexpected CS vs. no CS | 0.50 | 0.12 | 4.36 | < .001 | [0.28, 0.73] | .66 | 11.06 | 20.27 |
| E. Unexpected CS vs. expected CS | 0.56 | 0.02 | 2.80 | .005 | [0.17, 0.95] | .67 | 10.14 | 18.48 |
| F. Unexpected CS vs. combined CS | −0.49 | 0.16 | −3.08 | .002 | [−0.80, −0.18] | .66 | 10.55 | 17.91 |
Note: Models A to C are linear mixed-effects models including random intercepts for skin conductance response (SCR) frequency (number of SCRs) and restricting threat-imminence levels to unexpected CS and each of the other imminence levels. Models D to F are linear mixed-effects models including random intercepts for SCR amplitude (log μs) and restricting threat-imminence levels to unexpected CS and each of the other imminence levels. All models control for effects of time. For each model, the estimate is unstandardized, and the t is standardized. Bonferroni correction should be interpreted as p < .008. CI = confidence interval.
Subjective fear
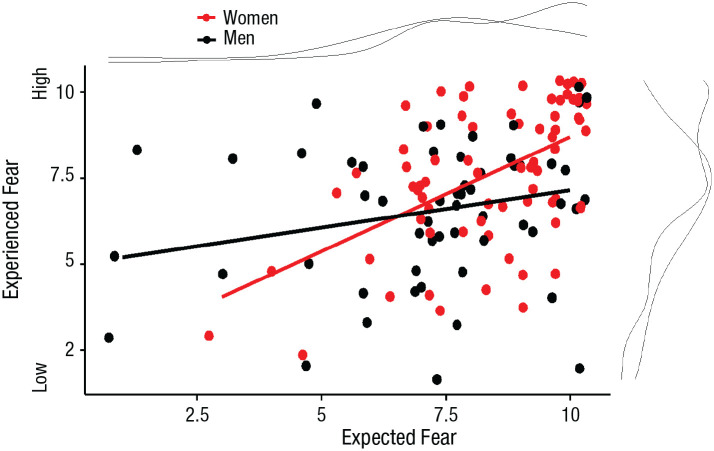
Expected fear, measured in the preexperience survey, ranged from 1 to 10 (M = 7.87, SD = 2.03). One participant did not report their expected fear. Experienced fear, measured in the postexperience survey, ranged from 2 to 10 (M = 7.16, SD = 2.17). Findings were consistent with prior work (McLean & Anderson, 2009): Compared with men, women reported higher subjective expected fear (women: M = 8.45, men: M = 7.19), t(153) = 4.01, p < .001, and higher experienced fear (women: M = 7.57, men: M = 6.68), t(154) = 2.59, p = .010 (Fig. 2). Expected and experienced fear were significantly different, t(154) = 3.76, p < .001; expected fear was higher than experienced fear.
Fig. 2.
Scatterplot (with best-fitting regression lines) showing the relation between subjective expected and experienced fear, separately for men and women. The curved lines at the top and right of the plot indicate the density of the data.
Participants also rated fear of a series of real-world threats on a scale from 1 to 10 prior to entering the haunted house. Anticipated haunted-house fear was significantly higher than fear of all of the real-world threat scenarios. Experienced fear was significantly higher than fear of the real-world scenarios with the exception of a near-miss car accident and a severe animal threat (Table 4).
Table 4.
Results From Paired-Samples t Tests Comparing Haunted-House Expected and Experienced Fear With Estimated Fear of Real-World Threats
| Real-world threat | Fear of real-world threat (M) | Comparison with expected fear | Comparison with experienced fear | ||
|---|---|---|---|---|---|
| t(147) | p | t(148) | p | ||
| Near-miss car accident | 7.32 | 2.55 | .012 | 0.81 | .419 |
| Severe animal threat (e.g., dog, bear, shark) | 6.96 | 3.78 | < .001 | 0.62 | .536 |
| Riding a large roller coaster | 3.72 | 16.06 | < .001 | 12.94 | < .001 |
| Speaking before a big crowd (e.g., wedding toast) | 4.71 | 11.29 | < .001 | 8.71 | < .001 |
| Severe airplane turbulence | 5.09 | 10.27 | < .001 | 7.54 | < .001 |
| Possible house break-in (e.g., a window breaking) | 6.50 | 5.17 | < .001 | 2.25 | .026 |
Note: Mean expected fear was 7.87, and mean experienced fear was 7.16. Bonferroni-corrected p = .004 for 12 comparisons at α = .05.
Greater experienced fear was associated with greater frequency of SCR reactivity in the model controlling for expected fear (Table 2, Model D, Fig. 1d). Gender did not significantly moderate effects of experienced fear on SCR frequency, estimate: b = 0.14, SE = 0.41, t = 0.35, p = .73, 95% CI = [−0.65, 0.94].
Baseline orienting response and sensitization
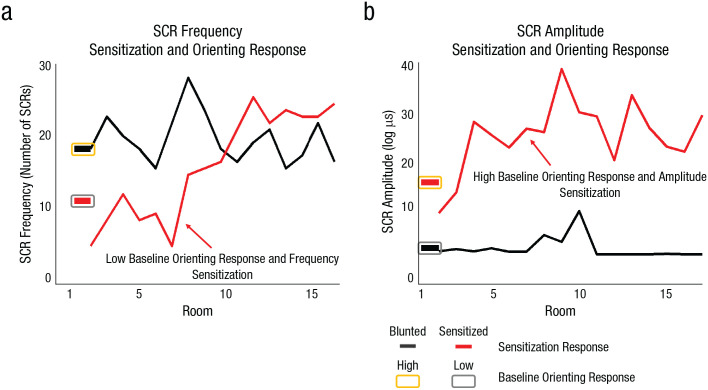
Random slopes were extracted for SCR frequency and amplitude as a measure of sensitization. SCR frequency in Room 1 was significantly associated with blunted SCR frequency sensitization (more sensitization in earlier rooms; Table 2, Model E). SCR amplitude in Room 1 was significantly associated with increased SCR amplitude sensitization (more sensitization in later rooms; Table 2, Model F). Although higher baseline orienting response is generally associated with habituation, the amplitude of SCRs in Room 1 was associated with greater subsequent amplitude, whereas frequency of SCRs in Room 1, indicative of reactive sensitivity, was associated with blunted sensitization (Figs. 1e and 1f). Illustrative time-course plots are provided in Figure 3.
Fig. 3.
Skin conductance response (SCR) frequency (a) and amplitude (b) as a function of room number, sensitization, and baseline orienting response. Results are shown separately for two example participants, one showing a blunted sensitization response (black) and the other showing a sensitized response (red).
Discussion
For humans, threat responses encompass physiological arousal, subjective fear experiences, and defensive behavior. Ethical restrictions provide limited allowance for studying how sympathetic arousal functions in immersive threat settings. This study leveraged advances in wearable technology to measure EDA during a haunted-house experience, which was carefully designed to create an immersive threat experience that was not actually dangerous (much as a horror writer or filmmaker does). The haunted house excluded performance demands common to the frequently used Trier Social Stress Test, involved small social groups, included threats of greater variety than classical conditioning tasks, and was rated as more fear inducing than real-world threat scenarios. These features increased the ecological validity of the haunted-house experience to aid understanding of how humans process threats in dynamic contexts.
Increased tonic responding was associated with being among more friends and fewer strangers, a social-contagion effect. The relationship-type effect is consistent with prior work demonstrating greater contagion in both positive and negative contexts for individuals with closer relationships (Palumbo et al., 2017). It is possible that arousal projected by friends was more relevant than that of strangers (Ma et al., 2011). Additionally, friends may have upregulated the excitement of the experience. Notably, this effect was for tonic SCL, which represents a general state of preparatory hyperactivity to confront stress. Thus, the presence of friends increased arousal in a nonspecific manner.
Individuals who reported greater subjective fear also demonstrated increased phasic frequency but did not show greater amplitude. Heightened SCR frequency can aid learning about threats by orienting people toward relevant stimuli (Yiend, 2010). Experienced fear may therefore correspond with conscious attention toward threat as opposed to overall arousal (Lau & Rosenthal, 2011). The positive association between SCR frequency and amplitude indicates that individuals who respond less frequently tend to exhibit smaller amplitudes when they do respond. Subjective fear may reflect projection bias, whereby participants undergoing increased physiological arousal recalled their experiences as more fear inducing. Projection bias is associated with maladaptive emotion regulation and has deleterious effects on well-being (Chang et al., 2018). Experienced fear was reported at the conclusion of the experience. Thus, reported fear may reflect a peak-end bias, a cognitive bias that heavily weights events at the end of an experience to influence how other events are remembered (Kemp et al., 2008). Despite the protective nature of identifying threat and mounting a physiological response, biases that amplify subjective perceptions of fear may contribute to psychological profiles observed in psychopathology (Rozenman et al., 2017). Future work including video coding would expand understanding of how subjective fear relates to behavioral responses such as vocalizations and locomotion.
Unexpected attacks elicited greater SCR frequency and amplitude compared with expected attacks and combined expected and unexpected attacks. Heightened physiological responsivity to unexpected threats may be due to underlying processes, including hypervigilance, inflated threat estimates, and deficient safety-threat discrimination (Grupe & Nitschke, 2013). Quadratic effects revealed weaker phasic responding to combined expected and unexpected attacks. This blunted responding may reflect physiological restriction when fear (response to perceptible threat) and anxiety (response to future threat) are simultaneously experienced (Davies & Craske, 2015). Quadratic effects should not be interpreted to suggest that increasing threat imminence would result in eventual nonresponding but, rather, that our scoring scale indicates that unexpected attacks produced the greatest response.
The baseline orienting response had dissociable effects on later responding with increased amplitude sensitization and blunted frequency sensitization. Increased sensitization to repeated threat exposure is linked to anxiety and may be an identifiable vulnerability factor (Campbell et al., 2014). SCR amplitude is thought to be more sensitive to peripheral factors such as sweat-gland density, which may account for the sensitization observed in this study. Few studies include both measures of phasic SCR, requiring caution in interpretation of these conflicting patterns. A prior large twin-cohort study supports the assertion that these measures represent distinct phenotypes that are differentially linked to psychopathology risk (Isen et al., 2012). In that study, frequency, but not amplitude, was inversely associated with externalizing psychopathology risk. SCR should not be treated as a homogeneous measure, but rather, both frequency and amplitude may be important for fully understanding links between physiology and psychopathology (Dawson et al., 2017).
There are limits to the inferences that can be drawn from this study. Although the novel field-experimental context is a major strength of this study, as in any field setting it is possible that arousal observed was due to various emotional and cognitive experiences, including excitement, nervousness, fear, anticipation, attention, and sensory inputs. Findings are interpreted in relation to fear, given subjective reports of high anticipated and experienced fear, although debate exists as to how best to define and measure fear (Mobbs et al., 2019). Despite the inherent reduction in experimental control, the use of an immersive experience makes a substantial contribution to the understanding of how social context relates to physiological arousal under immersive threat. Prior exposure to similar threats (e.g., a speeding oncoming vehicle) or threat-related phobia may bias responding and should be assessed in future work as potential confounds, both in and out of the laboratory. Participants in this study also self-selected to attend the haunted-house experience. They are likely to be an unusual sample because they sought out horror-related entertainment. Timing constraints also limited the number of individual-difference measures collected. Examinations of state and trait anxiety would illuminate how differences in phasic and tonic responding confer risk for psychopathology. However, this study provides an important proof of concept and guide for field experiments to probe contributors to threat physiology (see also Andersen et al., 2020).
The current study substantially furthers understanding of human physiological responses to threats in a social context. Foundational concepts in behavioral ecology motivated preregistered hypotheses regarding contextual and intrapersonal factors posited to influence arousal. Friends increased overall arousal, whereas subjective fear and unexpected attacks increased phasic responding. Frequency and amplitude of SCR differentially related to sensitization after baseline orienting response. These findings highlight the dynamic nature of sympathetic nervous system responses and identify important factors influencing threat responsivity. Insights from this work suggest the need for additional investigations to further detail social and intrapersonal contributions to threat physiology.
Supplemental Material
Supplemental material, sj-docx-1-pss-10.1177_09567976211032231 for Physiological Responses to a Haunted-House Threat Experience: Distinct Tonic and Phasic Effects by Sarah M. Tashjian, Virginia Fedrigo, Tanaz Molapour, Dean Mobbs and Colin F. Camerer in Psychological Science
Acknowledgments
We thank numerous research assistants from the Mobbs and Camerer teams and especially Heather and Robbie Luther for their extraordinary partnership.
ORCID iDs: Sarah M. Tashjian  https://orcid.org/0000-0002-0946-6662
https://orcid.org/0000-0002-0946-6662
Colin F. Camerer  https://orcid.org/0000-0003-4049-1871
https://orcid.org/0000-0003-4049-1871
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/09567976211032231
There was one deviation from the preregistered analysis plan, which is that we examined rooms with combined threat-imminence types. Our preregistered plan was to analyze rooms of only one imminence type and exclude rooms of combined types. No analyses were conducted in which rooms were excluded.
Transparency
Action Editor: Daniela Schiller
Editor: Patricia J. Bauer
Author Contributions
C. F. Camerer and D. Mobbs developed the study concept. V. Fedrigo, C. F. Camerer, T. Molapour, and D. Mobbs designed the study. V. Fedrigo conducted data collection and preprocessing. S. M. Tashjian analyzed and interpreted the data. S. M. Tashjian drafted the manuscript, and C. F. Camerer provided critical revisions. All the authors provided revisions to the manuscript and approved the final version for submission.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: C. F. Camerer, D. Mobbs, and S. M. Tashjian were supported by National Institute of Mental Health Grant No. 2P50MH094258. C. F. Camerer was also supported by a Behavioral and Economics Discovery Fund.
Open Practices: Deidentified data, analysis code, and all procedural details have been made publicly available via OSF and can be accessed at https://osf.io/bw69r/. The design and analysis plans for the study were preregistered at https://osf.io/wxek6/. Analyses and aims were preregistered after data were recorded and prior to any inspection of the data. This article has received the badges for Open Data, Open Materials, and Preregistration. More information about the Open Practices badges can be found at http://www.psychologicalscience.org/publications/badges.



References
- Adolph D., Schlösser S., Hawighorst M., Pause B. M. (2010). Chemosensory signals of competition increase the skin conductance response in humans. Physiology & Behavior, 101(5), 666–671. 10.1016/j.physbeh.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Andersen M. M., Schjoedt U., Price H., Rosas F. E., Scrivner C., Clasen M. (2020). Playing with fear: A field study in recreational horror. Psychological Science, 31(12), 1497–1510. 10.1177/0956797620972116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. (2015). Lme4: Linear mixed-effects models using ‘Eigen’ and S4 (Version 1.1-21) [Computer software]. https://cran.r-project.org/web/packages/lme4/index.html
- Beauchamp G. (2015). Animal vigilance: Monitoring predators and competitors. Academic Press. [Google Scholar]
- Benedek M., Kaernbach C. (2010). A continuous measure of phasic electrodermal activity. Journal of Neuroscience Methods, 190(1), 80–91. 10.1016/j.jneumeth.2010.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucsein W., Fowles D. C., Grimnes S., Ben-Shakhar G., Roth W. T., Dawson M. E., Filion D. L., & Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures. (2012). Publication recommendations for electrodermal measurements. Psychophysiology, 49(8), 1017–1034. 10.1111/j.1469-8986.2012.01384.x [DOI] [PubMed] [Google Scholar]
- Bradley M. M. (2009). Natural selective attention: Orienting and emotion. Psychophysiology, 46(1), 1–11. 10.1111/j.1469-8986.2008.00702.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. L., Gorka S. M., McGowan S. K., Nelson B. D., Sarapas C., Katz A. C., Robison-Andrew E. J., Shankman S. A. (2014). Does anxiety sensitivity correlate with startle habituation? An examination in two independent samples. Cognition & Emotion, 28(1), 46–58. 10.1080/02699931.2013.799062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çevik M. Ö. (2014). Habituation, sensitization, and Pavlovian conditioning. Frontiers in Integrative Neuroscience, 8, Article 13. 10.3389/fnint.2014.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang V. T., Overall N. C., Madden H., Low R. S. T. (2018). Expressive suppression tendencies, projection bias in memory of negative emotions, and well-being. Emotion, 18(7), 925–941. 10.1037/emo0000405 [DOI] [PubMed] [Google Scholar]
- Dan-Glauser E. S., Gross J. J. (2013). Emotion regulation and emotion coherence: Evidence for strategy-specific effects. Emotion, 13(5), 832–842. 10.1037/a0032672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. D., Craske M. G. (2015). Psychophysiological responses to unpredictable threat: Effects of cue and temporal unpredictability. Emotion, 15(2), 195–200. 10.1037/emo0000038 [DOI] [PubMed] [Google Scholar]
- Dawson M. E., Schell A. M., Filion D. L. (2017). The electrodermal system. In Cacioppo J. T., Tassinary L. G., Berntson G. (Eds.), Handbook of psychophysiology (4th ed., pp. 217–243). Cambridge University Press. [Google Scholar]
- Fanselow M. S., Lester L. S. (1988). A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In Bolles R. C., Beecher M. D. (Eds.), Evolution and learning (pp. 185–212). Erlbaum. [Google Scholar]
- Gomes N., Semin G. R. (2020). Mapping human vigilance: The influence of conspecifics. Evolution and Human Behavior, 41(1), 69–75. 10.1016/j.evolhumbehav.2019.10.002 [DOI] [Google Scholar]
- Grillon C., Baas J. P., Lissek S., Smith K., Milstein J. (2004). Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience, 118(5), 916–924. 10.1037/0735-7044.118.5.916 [DOI] [PubMed] [Google Scholar]
- Grupe D. W., Nitschke J. B. (2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14(7), 488–501. 10.1038/nrn3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry, 54(12), 1389–1398. 10.1016/S0006-3223(03)00465-7 [DOI] [PubMed] [Google Scholar]
- Hernandez-Lallement J., Gómez-Sotres P., Carrillo M. (2022). Towards a unified theory of emotional contagion in rodents—a meta-analysis. Neuroscience & Biobehavioral Reviews, 132, 1229–1248. 10.1016/j.neubiorev.2020.09.010 [DOI] [PubMed] [Google Scholar]
- Hughes J. (2020). reghelper: Helper functions for regression analysis (Version 1.0.1) [Computer software]. https://CRAN.R-project.org/package=reghelper
- Isen J. D., Iacono W. G., Malone S. M., McGue M. (2012). Examining electrodermal hyporeactivity as a marker of externalizing psychopathology: A twin study. Psychophysiology, 49(8), 1039–1048. 10.1111/j.1469-8986.2012.01394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp S., Burt C. D. B., Furneaux L. (2008). A test of the peak-end rule with extended autobiographical events. Memory & Cognition, 36(1), 132–138. 10.3758/MC.36.1.132 [DOI] [PubMed] [Google Scholar]
- Kirschner H., Hilbert K., Hoyer J., Lueken U., Beesdo-Baum K. (2016). Psychophsyiological [sic] reactivity during uncertainty and ambiguity processing in high and low worriers. Journal of Behavior Therapy and Experimental Psychiatry, 50, 97–105. 10.1016/j.jbtep.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Koo T. K., Li M. Y. (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15(2), 155–163. 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig S. D. (2010). Autonomic nervous system activity in emotion: A review. Biological Psychology, 84(3), 394–421. 10.1016/j.biopsycho.2010.03.010 [DOI] [PubMed] [Google Scholar]
- Kret M. E., De Gelder B. (2012). A review on sex differences in processing emotional signals. Neuropsychologia, 50(7), 1211–1221. 10.1016/j.neuropsychologia.2011.12.022 [DOI] [PubMed] [Google Scholar]
- Kuznetsova A., Brockhoff P. B., Christensen R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13). 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Lang P. J., Davis M., Öhman A. (2000). Fear and anxiety: Animal models and human cognitive psychophysiology. Journal of Affective Disorders, 61(3), 137–159. 10.1016/S0165-0327(00)00343-8 [DOI] [PubMed] [Google Scholar]
- Lau H., Rosenthal D. (2011). Empirical support for higher-order theories of conscious awareness. Trends in Cognitive Sciences, 15(8), 365–373. 10.1016/j.tics.2011.05.009 [DOI] [PubMed] [Google Scholar]
- Ma Q., Shen Q., Xu Q., Li D., Shu L., Weber B. (2011). Empathic responses to others’ gains and losses: An electrophysiological investigation. NeuroImage, 54(3), 2472–2480. 10.1016/j.neuroimage.2010.10.045 [DOI] [PubMed] [Google Scholar]
- Martin L. J., Hathaway G., Isbester K., Mirali S., Acland E. L., Niederstrasser N., Slepian P. M., Trost Z., Bartz J. A., Sapolsky R. M., Sternberg W. F., Levitin D. J., Mogil J. S. (2015). Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Current Biology, 25(3), 326–332. 10.1016/j.cub.2014.11.028 [DOI] [PubMed] [Google Scholar]
- McLean C. P., Anderson E. R. (2009). Brave men and timid women? A review of the gender differences in fear and anxiety. Clinical Psychology Review, 29(6), 496–505. 10.1016/j.cpr.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Mobbs D., Adolphs R., Fanselow M. S., Barrett L. F., LeDoux J. E., Ressler K., Tye K. M. (2019). Viewpoints: Approaches to defining and investigating fear. Nature Neuroscience, 22(8), 1205–1216. 10.1038/s41593-019-0456-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Hagan C. C., Dalgleish T., Silston B., Prévost C. (2015). The ecology of human fear: Survival optimization and the nervous system. Frontiers in Neuroscience, 9, Article 55. 10.3389/fnins.2015.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Petrovic P., Marchant J. L., Hassabis D., Weiskopf N., Seymour B., Dolan R. J., Frith C. D. (2007). When fear is near: Threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science, 317(5841), 1079–1083. 10.1126/science.1144298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Johnson P. C. D., Schielzeth H. (2017). The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. Journal of the Royal Society Interface, 14(134), Article 20170213. 10.1098/rsif.2017.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira R. F., Faustino A. I. (2017). Social information use in threat perception: Social buffering, contagion and facilitation of alarm responses. Communicative & Integrative Biology, 10(3), Article e1325049. 10.1080/19420889.2017.1325049 [DOI] [Google Scholar]
- Palumbo R. V., Marraccini M. E., Weyandt L. L., Wilder-Smith O., McGee H. A., Liu S., Goodwin M. S. (2017). Interpersonal autonomic physiology: A systematic review of the literature. Personality and Social Psychology Review, 21(2), 99–141. 10.1177/1088868316628405 [DOI] [PubMed] [Google Scholar]
- Pereira A. G., Cruz A., Lima S. Q., Moita M. A. (2012). Silence resulting from the cessation of movement signals danger. Current Biology, 22(16), R627–R628. 10.1016/j.cub.2012.06.015 [DOI] [PubMed] [Google Scholar]
- Rankin C. H., Abrams T., Barry R. J., Bhatnagar S., Clayton D., Colombo J., Coppola G., Geyer M. A., Glanzman D. L., Marsland S., McSweeney F., Wilson D. A., Wu C.-F., Thompson R. F. (2009). Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory, 92(2), 135–138. 10.1016/j.nlm.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattel J. A., Mauss I. B., Liedlgruber M., Wilhelm F. H. (2020). Sex differences in emotional concordance. Biological Psychology, 151, Article 107845. 10.1016/j.biopsycho.2020.107845 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2019). R: A language and environment for statistical computing (Version 3.6.1) [Computer software]. http://www.R-project.org
- R Core Team. (2020). stats (Version 4.0.3) [Computer software]. https://www.r-project.org/
- Rosebrock L. E., Hoxha D., Norris C., Cacioppo J. T., Gollan J. K. (2016). Skin conductance and subjective arousal in anxiety, depression, and comorbidity. Journal of Psychophysiology, 31(4), 145–157. 10.1027/0269-8803/a000176 [DOI] [Google Scholar]
- Rozenman M., Vreeland A., Piacentini J. (2017). Thinking anxious, feeling anxious, or both? Cognitive bias moderates the relationship between anxiety disorder status and sympathetic arousal in youth. Journal of Anxiety Disorders, 45, 34–42. 10.1016/j.janxdis.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler S., Radford A. N. (2018). Factors influencing within-group conflict over defence against conspecific outsiders seeking breeding positions. Proceedings of the Royal Society B: Biological Sciences, 285(1893), Article 20181669. 10.1098/rspb.2018.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschereau-Dumouchel V., Kawato M., Lau H. (2020). Multivoxel pattern analysis reveals dissociations between subjective fear and its physiological correlates. Molecular Psychiatry, 25(10), 2342–2354. 10.1038/s41380-019-0520-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley M. D., Carmel D., Chapman A., Grimshaw G. M. (2017). Dissociating the physiological components of unconscious emotional responses. Neuroscience of Consciousness, 2017(1), Article nix021. 10.1093/nc/nix021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin H. (2014). Brain sensitization to external and internal stimuli. Psychoneuroendocrinology, 42, 134–145. 10.1016/j.psyneuen.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Yiend J. (2010). The effects of emotion on attention: A review of attentional processing of emotional information. Cognition & Emotion, 24(1), 3–47. 10.1080/02699930903205698 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-pss-10.1177_09567976211032231 for Physiological Responses to a Haunted-House Threat Experience: Distinct Tonic and Phasic Effects by Sarah M. Tashjian, Virginia Fedrigo, Tanaz Molapour, Dean Mobbs and Colin F. Camerer in Psychological Science