Abstract
Objective
Previous studies have demonstrated that weight loss has been shown to improve pain in weight‐bearing joints, and more recent studies suggest that weight loss may be accompanied by improvements in pain in non–weight‐bearing regions. In previous work, we demonstrated that these symptoms improve substantially in patients with obesity undergoing 12 weeks of a very low‐energy diet (VLED) restricted to 800 kcal as part of a weight‐loss program. Preclinical models also have shown analgesic effects of calorie restriction. The purpose of the current observational study was to determine the time course and trajectory of improvement in pain and other symptoms, especially during the early phase of a VLED intervention, prior to major weight loss.
Methods
Participants were 195 individuals with obesity who had elevated levels of pain and associated symptoms at baseline (score of ≥4 on Fibromyalgia Survey Criteria) and completed a minimum of 3 weeks of a VLED intervention. The primary outcome was improvement in Fibromyalgia Survey Criteria at week 3. In secondary analyses, we created groups of those showing little/no improvement, moderate improvement, and high improvement (little/no improvement mean, 2.21; SD, 1.02; moderate improvement mean, 2.25; SD, 0.81; high improvement mean, 2.42; SD, 0.95; F2,189 = 1.01, P = 0.37), then compared baseline characteristics.
Results
A large proportion of study participants (72%) experienced symptom reductions of 30% or greater by week 3, but there were no differences in the amount of weight lost at this time point. Those who showed little or no improvement (less than 30%) had a higher body mass index at baseline and were more likely to report a diagnosis of depression (both P < 0.05).
Conclusion
This degree of improvement after 3 weeks of a VLED is encouraging. These findings help establish the temporal pattern of symptom improvement associated with caloric restriction and suggest that the palliative effects of this diet are at least partly due to the diet itself, rather than the weight loss that ensues.
INTRODUCTION
Individuals with obesity are approximately twice as likely to experience persistent pain when compared with those with a normal body mass index (BMI) (1). The National Health Interview Survey found that 19% of Americans report persistent pain and that obesity increases this risk by 60% (2). Furthermore, with comorbid pain conditions, obesity tends to significantly exacerbate painful symptoms (3). Given the nature of this complex relationship, there has been increased interest in understanding the benefits of weight loss for pain and other relevant outcomes.
Previous studies investigating the associations of caloric restriction with fibromyalgia symptoms have shown very interesting findings. In one study, patients enrolled in a hypocaloric diet of no more than 1500 kcal/day over a 24‐week intervention showed improvement in total Fibromyalgia Impact Questionnaire (FIQ) scores; however, these improvements were stabilized at the 12‐ to 24‐week period. When dissecting this further, patients on the hypocaloric diet intervention achieved only slight improvements in pain severity. There were no significant differences in other fibromyalgia cognitive symptoms, such as depression and anxiety (4). Similar studies have shown improvement in FIQ scores with caloric restriction when performed over longer periods (5, 6). However, no robust trials have been performed to investigate early effects (less than 12 weeks) of a very low‐calorie diet on fibromyalgia symptoms.
In a recent study, we examined the impact of an approximately 12‐week very low‐energy diet (VLED) (approximately 800 kcal/day) on pain and comorbid symptoms in 123 individuals reporting pain prior to the initiation of treatment. Symptoms such as pain, fatigue, and unrefreshing sleep and cognitive symptoms such as depression were improved by the intervention in those who lost at least 10% of their body weight (7). Improvement in pain was not confined to weight‐bearing joints. This finding has subsequently been observed in other studies examining the effects of weight loss (8). These broad improvements in pain and related symptoms make clear that the benefits seemingly conferred by weight loss cannot be attributed to a reduction in mechanical stress on weight‐bearing joints alone; rather, it seems likely that at least a subset of patients experience a global reduction in pain sensitivity.
Such a reduction in pain sensitivity has been described in animal models employing calorie restriction. For instance, intermittent fasting reduced experimental pain measures, although these animals were of normal weight and did not involve induction of chronic pain (9). These studies raise the possibility that calorie restriction, per se, may have analgesic effects in humans, as was recently shown by a substantial reduction in migraine days during calorie restriction in a cross‐over study of migraine patients with obesity (10). The purpose of the current observational study was to provide a preliminary assessment of the effects of VLED on symptoms of fibromyalgia such as widespread pain and fatigue prior to major weight loss. These estimates will ultimately be useful for informing a definitive controlled clinical trial comparing a VLED and a standard diet in individuals with Fibromyalgia (FM) or related chronic pain conditions.
To achieve these objectives, we assessed pain and comorbid symptoms multiple times throughout VLED in patients with obesity. We hypothesized that some patients would show early symptom improvement prior to significant weight loss. We also conducted secondary analyses to assess patient characteristics at baseline that predicted early response to a VLED.
PATIENTS AND METHODS
Sample. Individuals participated in the Weight Management Program (WMP) at the University of Michigan Health System. The WMP is a 2‐year, multicomponent, multidisciplinary behavioral lifestyle program that is intended to help individuals with obesity achieve and maintain long‐term weight loss. Primary eligibility is determined by a BMI in excess of 32 kg/m2 (27 kg/m2 for Asian Americans). In the first phase of the program, patients are asked to incorporate a VLED in the form of total liquid meal replacement (800 kcal/day or less). Total liquid meal replacement limits meal choices, reduces unreported meals, reduces participant unhealthy food consumption, allows for faster absorption of nutrients, and augments short‐term and long‐term weight loss. The primary weight‐loss goal during the first phase is equal to or exceeding 15% of baseline body weight. Participants with difficulty adhering to the intervention and do not lose weight receive counseling at visits. An additional 4 weeks may be added to the intervention phase to improve the likelihood of weight loss. The program has been described in detail elsewhere (7, 11).
For the current analyses, participants needed only to have completed the first 3 weeks of the VLED, because we were primarily interested in effects on symptoms that occur prior to significant weight loss. Additionally, to prevent floor effects, we restricted the sample to those who reported a score of 4 or greater on the Fibromyalgia Survey Criteria (see below) at baseline, representing at least moderate issues with pain and pain‐related comorbid symptoms. Out of 233 participants who completed the 3‐week visit, 195, or 84%, met this criterion and were included in the primary analyses. Of these 195 individuals, 119, or 61%, completed the 12‐week course of VLED and were examined in secondary analyses of 12‐week trends in symptoms.
Measures
Age, sex, and level of education were collected by participant self‐report. A standardized form was administered to capture self‐reported physician diagnoses of 16 common conditions, including hypertension, dyslipidemia, osteoarthritis, and depression.
Four additional metabolic risk factors were calculated from the baseline physical examination. These were the following: high triglyceride level (150 mg/dL or higher), low high‐density lipoprotein cholesterol level (less than 40 mg/dL for men; less than 50 mg/dL for women), high blood pressure (blood pressure of 130/85 mm Hg or greater), and high fasting glucose level (100 mg/dL or higher).
Pain and comorbid symptoms
The spatial distribution (or “widespreadedness”) of pain—as well as the severity of symptoms like fatigue and nonrestorative sleep that often accompany chronic pain—were assessed by the 2016 revision to the 2010/2011 Fibromyalgia Survey Criteria (12). This measure assesses the presence of pain at 19 sites across the body, which can be summed to form a Widespread Pain Index (WPI). Additionally, current levels of fatigue, unrefreshing sleep, cognitive difficulties, abdominal pain, headache, and depression are assessed and can be summed to form a Symptom Severity (SS) scale. The total score is the sum of these two subscales and can range between 0 and 31 points; this measure can either be used to diagnose the presence of fibromyalgia (by using a cut‐point) or form a continuously scaled metric of FM symptom severity. These criteria were administered at baseline prior to the initiation of VLED and at 3, 5, 8, and 12 weeks. For the purposes of this study, we focused on the baseline and 3‐week timepoints.
Statistical analyses
The primary outcome of interest in this study was the magnitude of improvement in FM survey scores at week 3. We conducted paired‐sample t tests on baseline and 3‐week fibromyalgia survey scores and calculated the percentage of participants experiencing 30% improvement.
In secondary analyses, we calculated the percentage change in fibromyalgia criteria scores at week 3 and divided the sample into the following three groups: those who either did not improve or improved less than 30%, those who showed improvement of 30%‐50%, and those who improved 50% or more. These cutoffs were selected because they have been used to define clinically meaningful change for pain outcomes in clinical trials (13). We subsequently compared baseline characteristics between these groups using one‐way analysis of variance with post hoc comparisons corrected with the Sidak adjustment for continuous variables and Pearson χ2 tests for categorical variables. We compared baseline Fibromyalgia Survey Criteria total scores, WPI scores, and SS scores between groups, as well as BMI and the total number of conditions associated with obesity (eg, diabetes, hypertension). We also compared the proportion of female patients, those with a physician diagnosis of depression, those with obstructive sleep apnea, those with osteoarthritis, and metabolic risk factors between groups.
We examined the association between early change in pain and comorbid symptoms and change at 12 weeks by calculating the change in Fibromyalgia Survey Criteria scores at 3 weeks (baseline to week 3) and at 12 weeks (baseline to week 12). The association of these values was tested with Pearson correlations.
RESULTS
Sample
At baseline, the majority of the 195 participants analyzed were female (74%), had an average age of 45 years, and had severe obesity with an average BMI of 41 kg/m2. Participants lost approximately 2 kg/m2 at the week‐3 visit (mean, 2.32; SD, 0.94), or 5.6% of their body weight.
Early change in pain and comorbid symptoms
Total Fibromyalgia Survey Criteria scores declined from an average of 8.39 (SD, 3.49) at baseline to 4.78 (SD, 3.20) at week 3 (t = 16.72; P < 0.001). A similar pattern was observed for the WPI subscale (baseline mean, 2.82 [SD, 2.43]; week‐3 mean, 1.31 [SD, 1.86]; t = 9.82; P < 0.001) and the SS subscale (baseline mean, 5.57 [SD, 2.14]; week‐3 mean, 3.47 [SD, 2.04]; t = 13.85; P < 0.001). Eighty‐nine percent of participants showed at least one point of improvement at week 3. A substantial proportion of patients achieved at least a 30% reduction in symptoms (n, 140; 72%) (see Figure 1).
Figure 1.
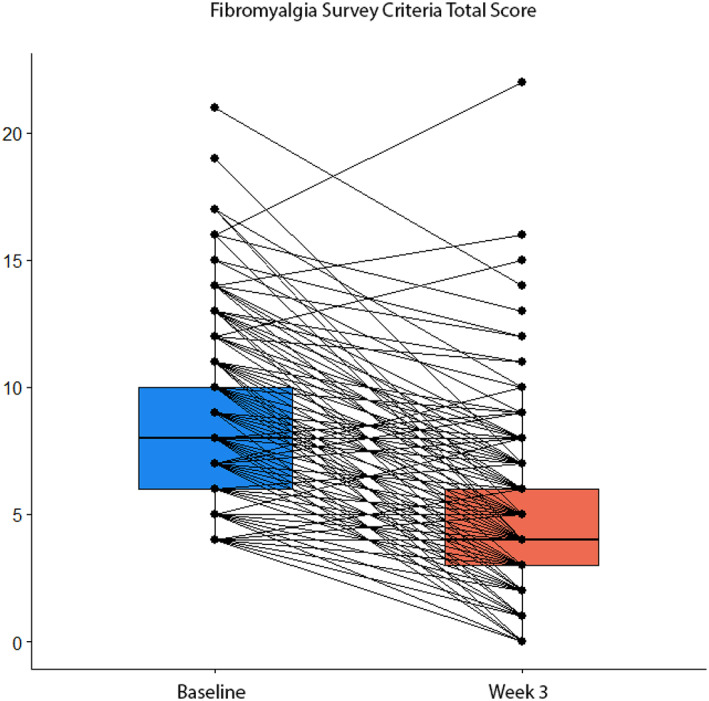
Box plots showing paired Fibromyalgia Survey Criteria total scores prior to and after three weeks after very low‐energy diet calorie restriction.
Secondary analyses
When comparing baseline characteristics among those with little or no improvement, moderate improvement, and high improvement, the following three statistically significant differences were observed: BMI was higher in patients who achieved little or no improvement, a higher proportion of female patients achieved moderate improvement, and physician‐diagnosed depression was more common in patients who showed little or no improvement (all P < 0.05). There were no differences in the number of obesity‐associated conditions, the proportion of patients with sleep apnea or osteoarthritis, or the number of metabolic syndrome risk factors between groups (all P > 0.05) (see Table 1). When comparing change in BMI units between the three groups, there were no significant differences (little/no improvement mean, 2.21; SD, 1.02; moderate improvement mean, 2.25; SD, 0.81; high improvement mean, 2.42; SD, 0.95; F2,189 = 1.01; P = 0.37). Additionally, there was no significant difference in percentage weight loss between the three groups (see Table 2).
Table 1.
Baseline characteristics compared between participants achieving little/no, moderate, and high improvement in fibromyalgia symptoms at week 3
| All patients (n = 195) | Little/no improvement (n = 55) | Moderate improvement (n = 47) | High improvement (n = 93) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F2,192 | P | |
| Age | 45.08 | 10.97 | 43.75 | 11.98 | 45.23 | 9.06 | 45.80 | 11.27 | 0.607 | 0.546 |
| Weight ‐ lbs | 260.72 | 43.03 | 267.68 | 44.17 | 246.09 | 30.98 | 258.67 | 44.88 | 7.015 | <0.001 |
| BMI ‐ kg/m2 | 41.49 | 5.22 | 43.44 | 5.41 | 40.74 | 3.87 | 40.72 | 5.44 | 5.585 | 0.004 |
| WPI | 2.82 | 2.43 | 2.91 | 2.43 | 2.77 | 2.29 | 2.80 | 2.52 | 0.053 | 0.949 |
| SSI | 5.57 | 2.14 | 5.13 | 2.10 | 6.02 | 2.31 | 5.60 | 2.05 | 2.253 | 0.108 |
| FM total | 8.39 | 3.49 | 8.04 | 3.65 | 8.79 | 3.48 | 8.40 | 3.42 | 0.584 | 0.559 |
| # Comorbid conditions | 2.90 | 1.76 | 3.18 | 1.79 | 2.87 | 1.74 | 2.74 | 1.75 | 1.089 | 0.339 |
| Count | % | Count | % | Count | % | Count | % | Χ 2 | P | |
| Female | 145 | 74.4 | 39 | 70.9 | 42 | 89.4 | 64 | 68.8 | 7.390 | 0.025 |
| Race | 9.083 | 0.696 | ||||||||
| White | 166 | 85.1 | 49 | 89.0 | 39 | 83.0 | 78 | 83.9 | ||
| Black/African American | 19 | 9.7 | 3 | 5.5 | 5 | 10.6 | 11 | 11.8 | ||
| Asian American | 2 | 1.0 | 1 | 1.8 | 0 | 0 | 1 | 1.1 | ||
| Indian/Alaska Native | 1 | 0.5 | 0 | 0 | 1 | 0.5 | 0 | 0 | ||
| Not reported | 7 | 3.6 | 2 | 4.3 | 2 | 1.0 | 3 | 3.3 | ||
| Ethnicity | 4.147 | 0.844 | ||||||||
| Hispanic or Latino | 7 | 3.6 | 1 | 1.8 | 3 | 3.2 | 3 | 3.2 | ||
| Non‐Hispanic | 181 | 92.8 | 53 | 96.4 | 43 | 91.5 | 85 | 91.4 | ||
| Not reported | 6 | 3.1 | 1 | 1.8 | 1 | 1.8 | 4 | 4.3 | ||
| Education | ||||||||||
| High school/GED | 1 | 0.5 | 0 | 0 | 0 | 0 | 1 | 1.1 | 7.802 | 0.453 |
| Vocational school/some college | 36 | 18.5 | 5 | 9.1 | 12 | 25.5 | 19 | 20.4 | ||
| College degree | 74 | 37.9 | 22 | 40.0 | 18 | 38.3 | 34 | 36.6 | ||
| Professional/graduate degree | 83 | 42.6 | 28 | 50.9 | 17 | 36.2 | 38 | 40.9 | ||
| Not reported | 1 | 0.5 | 0 | 0 | 0 | 0 | 1 | 1.1 | ||
| Depression | 94 | 48.1 | 35 | 63.6 | 23 | 48.9 | 36 | 38.7 | 8.614 | 0.013 |
| OSA | 78 | 40 | 25 | 45.5 | 16 | 34.0 | 37 | 39.8 | 1.379 | 0.502 |
| OA | 34 | 17.4 | 10 | 18.2 | 5 | 10.6 | 19 | 20.4 | 2.109 | 0.348 |
| # Metabolic syndromes | 12.823 | 0.118 | ||||||||
| 0 | 27 | 13.8 | 5 | 9.1 | 5 | 10.6 | 17 | 18.3 | ||
| 1 | 53 | 27.1 | 15 | 27.3 | 11 | 23.4 | 27 | 29.0 | ||
| 2 | 62 | 31.7 | 19 | 34.5 | 16 | 34.0 | 27 | 29.0 | ||
| 3 | 43 | 22 | 11 | 20.0 | 10 | 21.3 | 22 | 23.7 | ||
| 4 | 10 | 5.1 | 5 | 9.1 | 5 | 10.6 | 0 | 0 | ||
Abbreviations: BMI, body mass index; FM, Fibromyalgia; GED, general educational development; OA, Osteoarthritis; OSA, Obstructive Sleep Apnea; SD, standard deviation; SSI, Symptom Severity Index; WPI, Widespread Pain Index.
Table 2.
Percentage weight loss by visit and group
| % Loss (mean/SD) | All patients | Little/no improvement | Moderate improvement | High improvement |
|---|---|---|---|---|
| Week 3 (n = 195) | 5.63/2.23 | 5.22/2.41 | 5.45/1.78 | 5.96/2.29 |
| Week 5 (n = 180) | 10.74/3.79 | 10.63/3.95 | 9.81/3.66 | 11.24/3.71 |
| Week 8 (n = 161) | 13.53/4.57 | 13.20/4.00 | 13.18/5.07 | 13.86/4.64 |
| Week 12 (n = 138) | 15.02/6.48 | 14.83/5.77 | 15.73/7.45 | 14.79/6.42 |
Abbreviation: SD, standard deviation.
There was a statistically significant correlation between the magnitude of improvement experienced at week 3 and the magnitude of improvement experienced at week 12 (r = 0.68; P < 0.001; n = 119). Figure 2 shows smoothed trajectories for improvement in Fibromyalgia Survey Criteria total scores between baseline and week 12 alongside the same changes in BMI.
Figure 2.
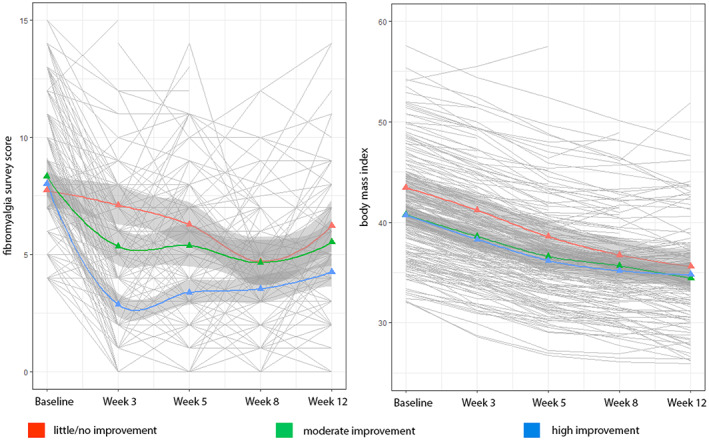
Left panel: Smoothed trajectories for patients showing high improvement, moderate improvement, and little or no improvement for fibromyalgia survey scores. Right panel: Smoothed trajectories for the same groups on body mass index.
DISCUSSION
Our results show, for the first time, that individuals following aggressive calorie restriction, ie, a VLED, had rapid and significant improvements in pain distribution and common pain‐related comorbid symptoms and, importantly, prior to the achievement of significant weight loss. A large proportion of study participants (72%) experienced symptom reductions of 30% or greater in the first 3 weeks of a VLED, a response rate that is highly encouraging given the challenges of treating chronic pain. Furthermore, improvement at week 3 was strongly associated with improvement over the entire 12‐week course of VLED, suggesting that patients who respond are likely to show these effects early in the process. These findings provide preliminary support for the hypothesis that calorie restriction, per se, can reduce pain and comorbid symptoms in individuals with obesity.
Participants who experienced the greatest benefit were less likely to have a depression diagnosis at study entry. This suggests that symptom improvement related to a VLED is not likely to be driven by changes in affect and echoes what we found in the initial study, in which the change in widespread pain was not associated with the magnitude of improvement in depressive symptoms (7). Those who experienced the greatest improvement in symptoms had a lower baseline BMI (although all participants had severe obesity) compared with those who showed little or no benefit, but there was no clear indication that other metabolic risk factors confer risk or benefit with respect to noting a symptomatic improvement associated with calorie restriction. Examining the trends in symptom reduction compared with changes in BMI make it clear that symptom improvement occurs rapidly and in a nonlinear fashion, whereas BMI decreases steadily over the entire period of observation.
Our findings are consistent with some extant findings in clinical populations and animal models. We noted previously that the anti‐inflammatory cytokine interleukin‐10 increased substantially following 12 weeks of a VLED, although we did not find that the magnitude of this increase was associated with the degree of symptom improvement (7). A recent study examining the impact of 4 weeks of calorie restriction in women with obesity found that markers of inflammation, including high sensitivity C‐reactive protein and lipopolysaccharide‐binding protein, were substantially reduced by the intervention. Conversely, a 2‐week period of VLED did not result in significant reductions of inflammatory markers in another recent study of individuals with overweight. Pain and other somatic symptoms were not addressed in this study (14). Nonobese animals showed dampening immune responses to various experimental insults, such as plantar incision after calorie restriction (15). Similarly, in nonobese animals, caloric restriction has also been shown to promote hypothalamic mRNA expression of anti‐inflammatory inhibitory factor κB‐α and IL‐10, as well as to inhibit lipopolysaccharide‐induced microglial activation, suggesting that neuroinflammation may be another potential mechanism of action (16). The role of inflammation in calorie‐restriction–induced analgesia requires more investigation.
Another potential group of mechanisms through which aggressive calorie restriction could promote analgesic effects is through central alteration of neurotransmission. Short‐term calorie restriction increases expression of neuropeptide Y (NPY) in the arcuate nucleus of the hypothalamus, and centrally expressed NPY shows substantial analgesic properties (17). Baseline central μ‐opioid receptor (MOR) availability is decreased in men with obesity compared with lean controls, a pattern we have previously observed in patients with FM, and MOR availability increased in several areas of the brain after calorie‐restriction–induced weight loss (18, 19). It is possible that calorie restriction partially rectifies deficiencies in the endogenous opioid system, although this hypothesis will require further research.
The implications of this study suggest an early association between caloric restriction, through a VLED, and fibromyalgia symptoms. Although a larger study with a control group would be the next step in investigating this association, this provides important information for clinicians who counsel patients on alternatives to pharmacologic treatments for pain and other somatic symptoms.
The limitation of this study is the lack of control. Without a control group, which would allow for a comparison of the specific effects of a VLED on these symptoms with the effects of a standard dietary intervention, the discussion of potential mechanisms should be considered preliminary. Clearly, randomized controlled clinical trials will be necessary to confirm these results. We also acknowledge that no minimal detectable change or minimal clinically important differences have yet been derived for the FM total score or the constituent subscales. This is a limitation in the interpretation of the current data.
The current study provides preliminary support that calorie restriction is associated with symptom improvement in patients with obesity, although these results should be viewed as provisional because of the lack of a control condition. In ongoing studies, we are exploring inflammatory mechanisms as a potential explanation, but the existing literature strongly suggests that several biological systems might contribute to these effects. Two parallel avenues of research are needed to harness the potential of calorie restriction as a treatment for pain, one demonstrating the mechanism of effects and the other identifying which patients are most likely to clinically benefit.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Aaron Stubbs had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Stubbs, Rothberg, Schrepf, Clauw, Williams, Miller, Nay, and Brown
Acquisition of data
Miller, Nay, and Brown
Analysis and interpretation of data
Harte and McAfee
Supporting information
Disclosure Form
ACKNOWLEDGMENTS
This project was supported in part by Grant Number P30DK020572 (MDRC) from the National Institute of Diabetes and Digestive and Kidney Diseases, Grant Number K12 DE023574 from the National Institute of Dental and Craniofacial Research, Dr. Stubbs has received support in part by Grant Number T32AR007080 from the National Institute of Health. Dr. Harte has received research funding from Aptinyx, Cerephex, Forest Laboratories, Eli Lily and Merck; and served as a consultant for Pfizer, Analgesic Solutions, Aptinyx, and deCode Genetics. Dr. Williams is currently the President of the American Pain Society; he has served as a consultant with Pfizer Inc. and with Community Health Focus Inc. Dr. Clauw has received research funding from Cerephex, Forest, Merck, and Pfizer, and serves as a consultant for Tonix, Theravance, Cerephex, Pfizer, Abbott, Merck, Eli Lilly, UCB, Johnson & Johnson, Forest Laboratories, and Purdue Pharma.
Drs. Rothberg and Schrepf contributed equally to this work.
No potential conflicts of interest relevant to this article were reported.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr2.11418&file=acr211418‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Higgins DM, Kerns RD, Brandt CA, Haskell SG, Bathulapalli H, Gilliam W, et al. Persistent pain and comorbidity among Operation Enduring Freedom/Operation Iraqi Freedom/operation New Dawn veterans. Pain Med 2014;15:782‐90. [DOI] [PubMed] [Google Scholar]
- 2. Kennedy J, Roll JM, Schraudner T, Murphy S, McPherson S. Prevalence of persistent pain in the U.S. adult population: new data from the 2010 National Health Interview Survey. J Pain 2014;15:979‐84. [DOI] [PubMed] [Google Scholar]
- 3. Neumann L, Lerner E, Glazer Y, Bolotin A, Shefer A, Buskila D. A cross‐sectional study of the relationship between body mass index and clinical characteristics, tenderness measures, quality of life, and physical functioning in fibromyalgia patients. Clin Rheumatol 2008;27:1543‐7. [DOI] [PubMed] [Google Scholar]
- 4. Slim M, Calandre EP, Garcia‐Leiva JM, Rico‐Villademoros F, Molina‐Barea R, Rodriguez‐Lopez CM, et al. The effects of a gluten‐free diet versus a hypocaloric diet among patients with fibromyalgia experiencing gluten sensitivity–like symptoms. J Clin Gastroenterol 2017:51:500‐7. [DOI] [PubMed] [Google Scholar]
- 5. Senna MK, Sallam RA, Ashour HS, Elarman M. Effect of weight reduction on the quality of life in obese patients with fibromyalgia syndrome: a randomized controlled trial. Clin Rheumatol 2012. Nov;31:1591‐7. [DOI] [PubMed] [Google Scholar]
- 6. Shapiro JR, Anderson DA, Danoff‐Burg S. A pilot study of the effects of behavioral weight loss treatment on fibromyalgia symptoms. J Psychosom Res 2005;59:275‐82. [DOI] [PubMed] [Google Scholar]
- 7. Schrepf A, Harte SE, Miller N, Fowler C, Nay C, Williams DA, et al. Improvement in the spatial distribution of pain, somatic symptoms, and depression after a weight loss intervention. J Pain 2017;18:1542‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stefanik JJ, Felson DT, Apovian CM, Niu J, Clancy MM, LaValley MP, et al. Changes in pain sensitization after bariatric surgery. Arthritis Care Res (Hoboken) 2018;70:1525‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de los Santos‐Arteaga M, Sierra‐Domínguez SA, Fontanella GH, Delgado‐García JM, Carrión ÁM. Analgesia induced by dietary restriction is mediated by the κ‐opioid system. J Neurosci 2003;23:111206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Lorenzo C, Pinto A, Ienca R, Coppola G, Sirianni G, Di Lorenzo G, et al. A randomized double‐blind, cross‐over trial of very low‐calorie diet in overweight migraine patients: a possible role for ketones? Clinical trial Nutrients 2019;11:1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rothberg AE, McEwen LN, Fraser T, Burant CF, Herman WH. The impact of a managed care obesity intervention on clinical outcomes and costs: a prospective observational study. Obesity (Silver Spring) 2013;21:2157‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, et al. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46:319‐29. [DOI] [PubMed] [Google Scholar]
- 13. Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9‐19. [DOI] [PubMed] [Google Scholar]
- 14. Strasser B, Berger K, Fuchs D. Effects of a caloric restriction weight loss diet on tryptophan metabolism and inflammatory biomarkers in overweight adults. Eur J Nutr 2015;54:101‐7. [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Ni Y, Zhang W, Sun YE, Ma Z, Gu X. Antinociceptive effects of caloric restriction on post‐incisional pain in nonobese rats. Sci Rep 2017;7:1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radler ME, Hale MW, Kent S. Calorie restriction attenuates lipopolysaccharide (LPS)‐induced microglial activation in discrete regions of the hypothalamus and the subfornical organ. Brain Behav Immun 2014;38:13‐24. [DOI] [PubMed] [Google Scholar]
- 17. Diaz‐delCastillo M, Woldbye DPD, Heegaard AM. Neuropeptide Y and its involvement in chronic pain. Neuroscience 2018;387:162‐9. [DOI] [PubMed] [Google Scholar]
- 18. Schrepf A, Harper D, Harte SE, Wang H, Ichesco E, Hampson JP, et al. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain 2016;157:2217‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burghardt PR, Rothberg AE, Dykhuis KE, Burant CF, Zubieta JK. Endogenous opioid mechanisms are implicated in obesity and weight loss in humans. J Clin Endocrinol Metab 2015;100:3193‐3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form


