Abstract
Objective
Axial disease is common and burdensome in patients with psoriatic arthritis (PsA). Human leukocyte antigen‐B27 (HLA‐B27) is a risk factor for axial PsA; treatment response by HLA‐B27 status is inadequately characterized. This study evaluated responses to biologic disease‐modifying antirheumatic drugs (bDMARDs) or targeted synthetic DMARDs (tsDMARDs) overall and by HLA‐B27 status in patients with PsA axial disease.
Methods
This observational study included participants in the CorEvitas (formerly Corrona) PsA/Spondyloarthritis Registry who initiated bDMARD or tsDMARD treatment at baseline, had a 6‐month follow‐up visit, fulfilled Classification Criteria for Psoriatic Arthritis, had a baseline Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score of ≥4, and had known HLA‐B27 status. Disease characteristics at baseline and 6 months were evaluated overall and by HLA‐B27 status. Association between HLA‐B27 status and treatment response was evaluated using an analysis of covariance model.
Results
The analysis included 173 bDMARD or tsDMARD treatment initiations (54 [31.2%] among patients with HLA‐B27+ status and 119 [68.8%] among patients with HLA‐B27− status). BASDAI total and component scores decreased by ≤0.84 across groups after 6 months of bDMARD or tsDMARD therapy; these changes are not considered clinically meaningful. HLA‐B27 status was not statistically significantly associated with changes in axial‐related outcomes.
Conclusion
In patients with PsA axial disease, 6 months of bDMARD or tsDMARD therapy provided only mild improvements in axial‐related outcomes, irrespective of HLA‐B27 status. This continued high disease activity reflects a critical unmet need for focus on the axial domain of PsA and for additional safe and effective therapies for psoriatic axial disease.
INTRODUCTION
Psoriatic arthritis (PsA) is a chronic inflammatory musculoskeletal disease in patients with active or latent psoriasis. PsA presentation is heterogeneous, with clinical manifestations that can include peripheral arthritis, axial disease, skin and nail disease, dactylitis, and enthesitis, as well as inflammatory bowel disease and uveitis (1, 2). PsA disease progression is highly variable, with some patients experiencing only mild, nondestructive joint symptoms for many years, whereas other patients develop severe, irreversible, debilitating, and erosive joint damage within a few years of diagnosis (3, 4). As a result of the broad spectrum of PsA disease activity and severity, treatment recommendations range from the use of physiotherapy, nonsteroidal anti‐inflammatory drugs, and local glucocorticoid injections to conventional synthetic disease‐modifying antirheumatic drugs (DMARDs), targeted synthetic DMARDs (tsDMARDs), or biologic DMARDs (bDMARDs). The choice of treatment is also dependent on which domain(s) are predominantly involved (1, 2).
PsA axial disease is characterized by symptoms of inflammatory back and/or neck pain that improve with physical activity and worsen with prolonged periods of rest, as well as morning pain and/or stiffness that lasts for more than 30 minutes each day (3, 5). Axial PsA occurs as a result of spinal and/or sacroiliac changes that can lead to permanent joint damage, if untreated. As untreated axial disease worsens over time, patients can develop significant sacroiliac joint and/or buttock pain, decreased cervical spinal mobility, and decreased lateral flexion (3). The reported prevalence of axial disease in patients with PsA has been highly variable. Depending on the criteria used to define spinal disease in PsA, it has been estimated that less than 25% to more than 70% of patients have axial involvement (5). Nevertheless, the presence of axial disease is associated with significant reductions in quality of life related to back pain, fatigue, and morning stiffness. These symptoms have been shown to negatively affect patients’ overall physical functioning (including their ability to walk and perform self‐care and other usual activities), to increase feelings of anxiety and depression, and to reduce work productivity (6, 7).
Human leukocyte antigen‐B27 (HLA‐B27) is a major histocompatibility complex (MHC) allele that has been identified as a strong genetic susceptibility marker for ankylosing spondylitis (AS) (8). AS is the most common type of spondyloarthritis (SpA) and predominantly affects the axial skeleton and sacroiliac joints (9). More than 80% of patients with AS are HLA‐B27–positive compared with 6% of the general US population (10, 11). In contrast, HLA‐B27 positivity has been reported in only 14% to 40% of patients with axial PsA and in less than 10% of patients with predominantly peripheral PsA (11, 12, 13). These observations suggest that the association between HLA‐B27 positivity and axial PsA is not as strong as the association with AS (11, 12, 13).
Several different biologic mechanisms have been identified through which HLA‐B27 positivity may contribute to the etiopathogenesis of AS and possibly other spondyloarthritides. It is not known whether these mechanisms could affect treatment response. In HLA‐B27–positive individuals, HLA‐B27 misfolding has been hypothesized to trigger increased production of pro‐inflammatory cytokines that are therapeutic targets in the treatment of PsA and AS. Specifically, the accumulation of misfolded HLA‐B27 heavy chains can cause endoplasmic reticulum stress, which activates an unfolded protein response that stimulates innate immune signaling and may alter cellular responses to key cytokines (14). Other proposed mechanisms include a “cell surface HLA‐B27 homodimers” hypothesis (in which heavy chains form homodimers that bind to immunoreceptors), an “arthritogenic” peptide hypothesis (in which HLA‐B27–specific autoimmune response is initiated for structurally unique peptide–MHC complexes), a “molecular mimicry” theory (which suggests that cross‐reactive peptides produced during bacterial infection can stimulate T cells to respond to HLA‐B27–associated self‐peptides), and aberrant peptide processing (which may result in generation of extended peptides that are highly immunogenic when bound to HLA‐B27) (14).
Real‐world data on axial PsA treatment response are limited, particularly on whether treatment response differs by HLA‐B27 status. In this analysis, we evaluated responses to 6 months of treatment with a bDMARD or a tsDMARD in patients with PsA axial disease enrolled in the CorEvitas (formerly known as Corrona) PsA/SpA Registry and determined whether treatment responses differed by HLA‐B27 status.
PATIENTS AND METHODS
Data source
The registry is a large, independent, prospective, multicenter, observational cohort of patients with PsA or SpA who are treated at private or academic practice sites across the United States. As of April 1, 2020, the registry included information on 3597 patients with PsA. Within the registry, data are collected from both patients and their treating rheumatologists on numerous variables related to disease duration, prognosis, disease severity and activity, medical comorbidities, use of medications, and safety. Follow‐up assessments are requested at least once every 6 months during routine clinical visits. The data used for the current analysis were collected from April 1, 2013, to March 26, 2020.
All participating investigators were required to obtain full board approval for conducting research involving human subjects. Sponsor approval and continuing review were obtained through a central Institutional Review Board (IRB; New England Independent Review Board No. 120160939). For academic investigative sites that did not receive a waiver to use the central IRB, approval was obtained from the respective governing IRBs, and documentation of approval was submitted to the sponsor prior to initiating any study procedures. All patients were required to provide written informed consent prior to participating in the registry.
Study population
For this analysis, patients were considered to have axial PsA if they had a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score of ≥4 at baseline. Eligible patients were also required to meet the Classification Criteria for Psoriatic Arthritis (CASPAR) and to have a known HLA‐B27 genotype. HLA‐B27 genotyping was undertaken as part of routine medical care at the discretion of the investigator in the registry. Therefore, HLA‐B27 status data were not available for all patients with PsA, including those with axial PsA.
This analysis included patients with PsA who initiated bDMARD or tsDMARD treatment at a registry visit (baseline) and had a subsequent 6‐month follow‐up visit (occurring between 3 and 9 months post initiation). Treatments initiated by patients in this analysis included the bDMARDs adalimumab, etanercept, certolizumab pegol, infliximab, golimumab, abatacept, ustekinumab, ixekizumab, and secukinumab and the tsDMARDs tofacitinib and apremilast.
At the time of this analysis, there were 3597 patients in the registry with PsA, and there were 2694 initiations of bDMARD or tsDMARD treatments during the study period. Of bDMARD or tsDMARD initiators, 1660 had a BASDAI score of ≥4 at initiation, and 1620 of these patients met CASPAR criteria at initiation. A total of 538 initiators meeting BASDAI and CASPAR eligibility criteria had known HLA‐B27 status, and 173 of these treatment initiators had a 6‐month follow‐up visit (Figure 1). Among the 173 treatment initiations included in this analysis, 54 (31.2%) were by patients with positive HLA‐B27 status and 119 (68.8%) were by patients with negative HLA‐B27 status. Of the 173 treatment initiations, 141 (81.5%) were with bDMARDs and 32 (18.5%) were with tsDMARDs. The 173 treatment initiations occurred in 135 patients (39 [28.9%] who were HLA‐B27 positive and 96 [71.1%] who were HLA‐B27 negative).
Figure 1.
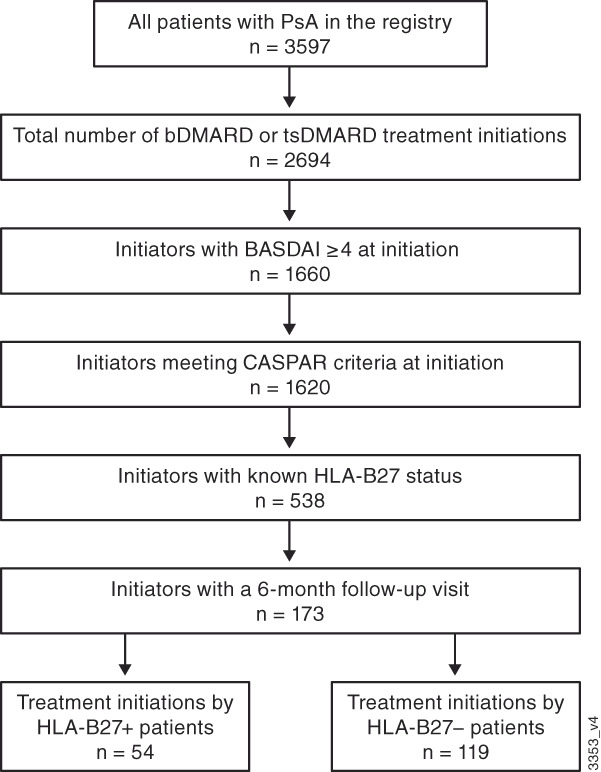
Flow diagram of treatment initiations included in this analysis. Patients could initiate >1 treatment. BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; bDMARD, biologic disease‐modifying antirheumatic drug; CASPAR, Classification Criteria for Psoriatic Arthritis; HLA, human leukocyte antigen; PsA, psoriatic arthritis; tsDMARD, targeted synthetic disease‐modifying antirheumatic drug.
Criteria for evaluation
For this analysis, the baseline visit was defined as the visit at which a patient initiated treatment with a bDMARD or tsDMARD. If bDMARD or tsDMARD treatment was initiated between visits, baseline data were used from the last visit with non‐missing data occurring within 4 months prior to initiation to 3 months post initiation. The 6‐month follow‐up visit was defined as a routine clinical visit within 3 to 9 months after bDMARD or tsDMARD initiation. If more than one visit occurred during this time frame, the visit closest to the nominal day of 6 months from baseline was selected as the 6‐month visit. Patients could be included more than once in this analysis if they initiated multiple bDMARDs or tsDMARDs during the study period (ie, data from the same patient were included for each bDMARD and tsDMARD initiation).
Assessments
Demographic characteristics, history of comorbidities, PsA disease measures, treatments initiated, and concomitant therapies were collected at baseline. Disease‐specific outcomes assessed at baseline and at 6 months post‐treatment initiation included the following: the percentage of body surface area affected by psoriasis; Clinical Disease Activity Index (CDAI; 0‐76); swollen joint count (0‐66); tender joint count (0‐68); Physician Global Assessment of PsA (0‐100 mm visual analog scale [VAS]); patient‐reported pain, spine pain, and nocturnal spine pain (0‐100 mm VAS); BASDAI (whereby scores ≥4 indicate suboptimal control of disease (15) and changes from baseline are considered clinically meaningful if scores decrease by ≥50% or by ≥2 points) (16); modified BASDAI (Question 3 removed; ie, How would you describe the overall level of pain/swelling in joints other than the neck, back or hips you have had? [from 0 = none to 10 = very severe]); BASDAI Question 2 (ie, how would you describe the overall level of AS neck, back or hip pain you have had? [from 0 = none to 10 = very severe]) (17, 18); Health Assessment Questionnaire for the Spondyloarthropathies (0‐3 scale) (19); Health Assessment Questionnaire‐Disability Index (0‐3 scale) (20); and Ankylosing Spondylitis Disease Activity Score with C‐reactive protein (ASDAS‐CRP; calculated using a formula based on weighted measures of total back pain, duration of morning stiffness, patient global assessment, peripheral pain and swelling, and CRP) (21, 22). ASDAS‐CRP scores of <1.3 are defined as inactive disease, scores of ≥1.3 to <2.1 are defined as moderate disease activity, scores ≥2.1 to ≤3.5 are defined as high disease activity, and scores >3.5 are defined as very high disease activity. Absolute changes in ASDAS‐CRP scores of ≥1.1 are considered clinically important, and changes of ≥2.0 are considered major improvement (21).
Statistical analyses
Statistical analyses were performed using SAS 9.4 software. Baseline characteristics were summarized descriptively as mean (standard deviation [SD]) and counts (%) for the overall population and by HLA‐B27 status. Mean (SD) changes from baseline to 6 months were calculated for joint‐, skin‐, and axial‐related outcomes.
A separate analysis of covariance model was used to test for an association between HLA‐B27 status and changes in each joint‐, skin‐, and axial‐related outcome after 6 months of treatment. Differences between the baseline characteristic values of the HLA‐B27–positive group and the HLA‐B27–negative group were calculated. The model was adjusted for the variables with absolute standardized differences of >0.2 between the HLA‐B27–positive and HLA‐B27–negative groups; these included age at initiation, insurance (private or other), alcohol use (never or ever consumed), history of cardiovascular disease (yes or no), prior conventional synthetic DMARD use (0, 1, or ≥2 therapies used), prior tsDMARD use (0, 1, or 2 therapies used), and prior bDMARD use (0, 1, or ≥2 therapies used). The standardized difference between groups for the type of therapy initiated was 0.0956 (ie, did not meet the threshold of >0.2). Within‐patient correlations were accounted for using sandwich standard error estimates. Differences in ASDAS‐CRP by HLA‐B27 status were summarized descriptively because laboratory data (CRP levels) were only available in a small subset of patients for which this information was requested by a provider as part of routine medical care.
A descriptive analysis was performed to evaluate BASDAI‐related outcomes for a subgroup of patients with imaging (x‐ray or magnetic resonance imaging) confirmation of axial involvement in the sacroiliac joints.
RESULTS
Baseline demographics, history of comorbidities, and PsA axial disease characteristics overall and by HLA‐B27 status are shown in Table 1. Axial disease‐related outcome measures, including BASDAI score, BASDAI Question 2 score, modified BASDAI score, and ASDAS‐CRP score, consistently reflected active disease or high disease activity at baseline (ie, BASDAI scores ≥4 and ASDAS‐CRP scores ≥2.1).
Table 1.
Baseline characteristics of PsA treatment initiators with axial disease and known HLA‐B27 genotype
| Characteristic | HLA‐B27+ (n = 54) | HLA‐B27− (n = 119) | Overall (N = 173) | Standardized difference |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age at initiation, years a | 54.2 (11.5) | 49.4 (12.4) | 50.9 (12.3) | 0.3990 |
| Male, n (%) | 19 (35.8) | 51 (42.9) | 70 (40.7) | 0.1438 |
| Race, n (%) | −0.1311 | |||
| White | 50 (96.2) | 110 (93.2) | 160 (94.1) | |
| Other | 2 (3.8) | 8 (6.8) | 10 (5.9) | |
| BMI, kg/m2 | 33.0 (8.4) | 32.5 (7.4) | 32.7 (7.7) | 0.0583 |
| Time since PsA symptom onset, years | 8.8 (8.9) | 9.1 (8.2) | 9.0 (8.4) | −0.0352 |
| Time since PsA diagnosis, years | 4.4 (4.5) | 5.2 (5.6) | 5.0 (5.3) | −0.1491 |
| Current psoriasis, n (%) | 43 (79.6) | 102 (85.7) | 145 (83.8) | −0.1613 |
| Insurance, n (%) a | 0.2570 | |||
| Private | 36 (66.7) | 93 (78.2) | 129 (74.6) | |
| Other | 18 (33.3) | 26 (21.9) | 44 (25.4) | |
| Smoking status, n (%) | 0.0683 | |||
| Never | 23 (44.2) | 56 (47.1) | 79 (46.2) | |
| Current | 11 (21.2) | 25 (21.0) | 36 (21.1) | |
| Previous | 18 (34.6) | 38 (31.9) | 56 (32.7) | |
| Alcohol use, n (%) a | −0.4126 | |||
| Never | 32 (60.4) | 47 (40.2) | 79 (46.5) | |
| Ever | 21 (39.6) | 70 (59.8) | 91 (53.5) | |
| History of comorbidities, n (%) | ||||
| Ulcerative colitis | 0 | 0 | 0 | b |
| Crohn's disease | 0 | 1 (0.8) | 1 (0.6) | −0.1302 |
| Inflammatory bowel disease | 0 | 1 (0.8) | 1 (0.6) | −0.1302 |
| Uveitis | 1 (1.9) | 1 (0.8) | 2 (1.2) | 0.0879 |
| Osteoporosis | 1 (1.9) | 4 (3.4) | 5 (2.9) | −0.0948 |
| Fibromyalgia | 3 (5.6) | 16 (13.4) | 19 (11.0) | −0.2715 |
| Cardiovascular disease a | 13 (24.1) | 10 (8.4) | 23 (13.3) | 0.4348 |
| Treatment history | ||||
| Prior csDMARD, n (%) a | 0.3902 | |||
| 0 | 9 (16.7) | 22 (18.5) | 31 (17.9) | |
| 1 | 19 (35.2) | 60 (50.4) | 79 (45.7) | |
| ≥2 | 26 (48.1) | 37 (31.1) | 63 (36.4) | |
| Prior bDMARD, n (%) a | 0.2001 | |||
| 0 | 3 (5.6) | 8 (6.7) | 11 (6.4) | |
| 1 | 22 (40.7) | 35 (29.4) | 57 (32.9) | |
| ≥2 | 29 (53.7) | 76 (63.9) | 105 (60.7) | |
| Prior tsDMARD, n (%) a | 0.2744 | |||
| 0 | 37 (68.5) | 70 (58.8) | 107 (61.8) | |
| 1 | 17 (31.5) | 47 (39.5) | 64 (37.0) | |
| 2 | 0 | 2 (1.7) | 2 (1.2) | |
| PsA axial disease measures | ||||
| BASDAI score (0‐10) c | 6.3 (1.4) | 6.5 (1.5) | 6.4 (1.4) | −0.1166 |
| BASDAI Question 2, spine pain (0‐10) | 6.2 (2.4) | 6.2 (2.6) | 6.2 (2.6) | 0.0229 |
| Modified BASDAI score (0‐10) d | 6.2 (1.5) | 6.4 (1.5) | 6.4 (1.5) | −0.1748 |
| ASDAS‐CRP score (0‐5) e | 3.4 (1.0) | 3.1 (0.9) | 3.2 (0.9) | b |
| Therapy initiated, n (%) f | 0.0956 | |||
| bDMARD | 46 (85.2) | 95 (79.8) | 141 (81.5) | |
| TNFi | 26 (48.1) | 50 (42.0) | 76 (43.9) | |
| Non‐TNFi | 20 (37.0) | 45 (37.8) | 65 (37.6) | |
| tsDMARD | 8 (14.8) | 24 (20.2) | 32 (18.5) | |
| Concomitant therapy, n (%) | −0.0693 | |||
| Monotherapy | 35 (68.6) | 84 (71.8) | 119 (70.8) | |
| Combination with methotrexate | 16 (31.4) | 33 (28.2) | 49 (29.2) |
Abbreviations: ASDAS‐CRP, Ankylosing Spondylitis Disease Activity Score with C‐reactive protein; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; bDMARD, biologic disease‐modifying antirheumatic drug; BMI, body mass index; csDMARD, conventional synthetic disease‐modifying antirheumatic drug; HLA, human leukocyte antigen; PsA, psoriatic arthritis; SD, standard deviation; TNFi, tumor necrosis factor‐α inhibitor; tsDMARD, targeted synthetic disease‐modifying antirheumatic drug.
Absolute standardized difference between the HLA‐B27–positive and HLA‐B27–negative groups was >0.2; these variables were included in the adjusted analysis of covariance model.
Not evaluated because of small sample size or not applicable.
Scores ≥4 indicate suboptimal control of disease.
Excludes Question 3.
Scores <1.3 indicate inactive disease, ≥1.3 to <2.1 indicate moderate disease activity, ≥2.1 to ≤3.5 indicate high disease activity, and >3.5 indicate very high disease activity; only measured if requested by a provider as part of routine medical care.
Patients may have initiated treatment with >1 drug over time and may have been included in >1 group.
Note: Data are mean (SD) unless otherwise stated. Some entries have missing patient values; observations are based on unique therapy initiation, and thus patients may have contributed more than one observation.
At 6 months after treatment initiation, mean scores decreased in both HLA‐B27 groups for many outcomes (Table 2). However, BASDAI and ASDAS‐CRP axial disease‐related measures reflected only mild improvement at 6 months after treatment initiation and were still indicative of active disease or high disease activity, irrespective of HLA‐B27 status (Figures 2 and 3). Across groups, changes from baseline to 6 months in the BASDAI‐related endpoints ranged from −0.63 to −0.84, which are not considered clinically meaningful (16). For example, the mean (SD) change from baseline to 6 months in BASDAI score was −0.80 (1.75) in the HLA‐B27–positive group and − 0.84 (2.24) in the HLA‐B27–negative group. Among patients with ASDAS‐CRP data at both baseline and 6 months, mean (SD) change from baseline to 6 months after treatment initiation was −0.36 (1.11) in the HLA‐B27–positive group (n = 12) and − 0.73 (1.16) in the HLA‐B27–negative group (n = 13).
Table 2.
Mean (SD) PsA disease characteristics of treatment initiators with axial disease and known HLA‐B27 genotype at baseline and 6 months post treatment initiation
| Characteristic | HLA‐B27+ (n = 54) | HLA‐B27− (n = 119) | Overall (N = 173) |
|---|---|---|---|
| TJC, 0‐68 | |||
| Baseline | 9.3 (9.5) | 10.6 (13.0) | 10.2 (12.0) |
| 6 months | 6.5 (9.1) | 8.4 (12.6) | 7.8 (11.6) |
| Change from baseline | −2.4 (8.5) | −2.2 (10.0) | −2.3 (9.5) |
| SJC, 0‐66 | |||
| Baseline | 4.0 (5.2) | 4.7 (6.8) | 4.5 (6.3) |
| 6 months | 2.5 (4.1) | 3.0 (5.1) | 2.8 (4.8) |
| Change from baseline | −1.5 (5.2) | −1.7 (6.1) | −1.6 (5.8) |
| Physician Global Assessment of PsA, 0‐100 mm VAS | |||
| Baseline | 43.5 (20.0) | 40.3 (24.3) | 41.3 (23.1) |
| 6 months | 30.7 (27.1) | 26.9 (22.1) | 28.0 (23.6) |
| Change from baseline | −12.9 (28.3) | −14.0 (26.7) | −13.7 (27.1) |
| Patient‐reported pain, 0‐100 mm VAS | |||
| Baseline | 67.4 (20.9) | 66.7 (18.6) | 66.9 (19.3) |
| 6 months | 57.9 (26.1) | 57.7 (25.1) | 57.8 (25.3) |
| Change from baseline | −9.2 (27.8) | −8.8 (28.6) | −8.9 (28.3) |
| Patient‐reported spine pain, 0‐100 mm VAS | |||
| Baseline | 49.8 (31.7) | 50.7 (29.5) | 50.4 (30.1) |
| 6 months | 39.7 (29.9) | 42.5 (29.6) | 41.6 (29.6) |
| Change from baseline | −10.2 (28.3) | −8.6 (32.0) | −9.1 (30.8) |
| Patient‐reported nocturnal spine pain, 0‐100 mm VAS | |||
| Baseline | 39.5 (30.3) | 46.6 (32.0) | 44.3 (31.5) |
| 6 months | 38.2 (31.0) | 40.5 (29.5) | 39.8 (29.9) |
| Change from baseline | −1.2 (24.9) | −7.0 (31.1) | −5.2 (29.3) |
| HAQ‐DI, 0‐3 | |||
| Baseline | 1.2 (0.6) | 1.1 (0.7) | 1.1 (0.6) |
| 6 months | 1.1 (0.7) | 1.0 (0.7) | 1.1 (0.7) |
| Change from baseline | −0.05 (0.5) | −0.07 (0.5) | −0.07 (0.5) |
| HAQ‐S, 0‐3 | |||
| Baseline | 1.2 (0.6) | 1.1 (0.7) | 1.1 (0.6) |
| 6 months | 1.1 (0.7) | 1.1 (0.7) | 1.1 (0.7) |
| Change from baseline | −0.03 (0.5) | −0.07 (0.5) | −0.06 (0.5) |
| BSA, % a | |||
| Baseline | 5.1 (6.8) | 5.1 (8.9) | 5.1 (8.3) |
| 6 months | 2.9 (4.5) | 3.7 (7.8) | 3.4 (6.9) |
| Change from baseline | −2.2 (6.4) | −1.5 (10.5) | −1.7 (9.4) |
| CDAI, 0‐76 | |||
| Baseline | 18.6 (10.2) | 19.7 (13.6) | 19.4 (12.7) |
| 6 months | 14.5 (12.2) | 15.0 (11.9) | 14.9 (12.0) |
| Change from baseline | −4.0 (13.5) | −4.6 (11.0) | −4.4 (11.7) |
Abbreviations: BSA, body surface area affected by psoriasis; CDAI, Clinical Disease Activity Index; HAQ‐DI, Health Assessment Questionnaire‐Disability Index; HAQ‐S, Health Assessment Questionnaire for the Spondyloarthropathies; HLA, human leukocyte antigen; PsA, psoriatic arthritis; SD, standard deviation; SJC, swollen joint count; TJC, tender joint count; VAS, visual analog scale.
<3%, mild; 3%‐10%, moderate; >10%, severe psoriasis.
Note: Some entries have missing patient values; observations are based on unique therapy initiation, and thus patients may have contributed more than one observation.
Figure 2.
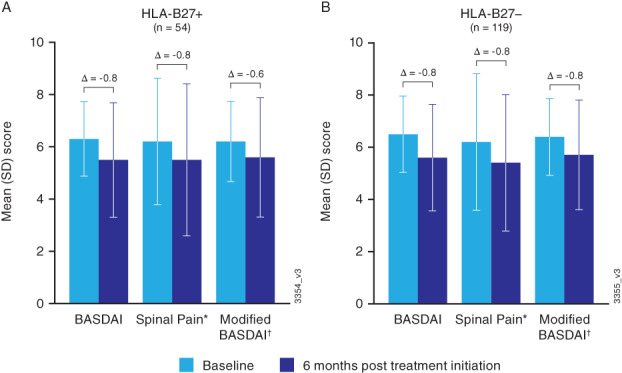
Mean (SD) and change from baseline to 6‐month post‐treatment‐initiation scores for BASDAI‐related endpoints in HLA‐B27–positive (A) and HLA‐B27–negative (B) patients with axial PsA. Scores ≥4 indicate suboptimal control of disease. *BASDAI Question 2 (How would you describe the overall level of AS neck, back or hip pain you have had?). †Excludes BASDAI Question 3 (How would you describe the overall level of pain/swelling in joints other than neck, back, hips you have had?). BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; HLA, human leukocyte antigen; PsA, psoriatic arthritis; SD, standard deviation.
Figure 3.
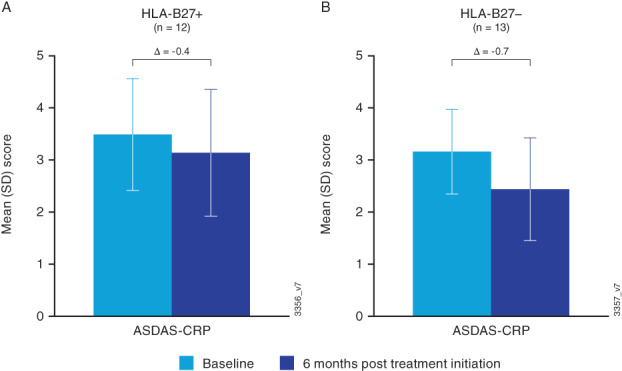
Mean (SD) and change from baseline to 6‐month post‐treatment‐initiation score for ASDAS‐CRP in HLA‐B27–positive (A) and HLA‐B27–negative (B) patients with axial PsA and available CRP data. Scores <1.3 indicate inactive disease; ≥1.3 to <2.1 indicate moderate disease activity; ≥2.1 to ≤3.5 indicate high disease activity; and >3.5 indicate very high disease activity; only measured if requested by a provider as part of routine medical care. ASDAS‐CRP, Ankylosing Spondylitis Disease Activity Score with C‐reactive protein; HLA, human leukocyte antigen; SD, standard deviation.
Covariate‐adjusted differences between patients with negative HLA‐B27 status and those with positive HLA‐B27 status from baseline to 6 months after treatment initiation are shown in Table 3 for PsA disease‐related outcomes. HLA‐B27 status was not statistically significantly associated with changes from baseline for any of these disease measures.
Table 3.
Adjusted difference between HLA‐B27+ and HLA‐B27− treatment initiators in PsA disease outcomes (mean change from baseline to 6 months)
| Disease characteristic | β Estimate for HLA‐B27+ status (95% CI) | P value |
|---|---|---|
| BASDAI (0‐10) | 0.10 (−0.56 to 0.75) | 0.78 |
| BASDAI Question 2: spine pain | 1.52 (−6.30 to 9.34) | 0.70 |
| Modified BASDAI score (0‐10) | 0.11 (−0.56 to 0.78) | 0.75 |
| Tender joint count (0‐68 joints) | −0.19 (−2.80 to 2.42) | 0.88 |
| Swollen joint count (0‐66 joints) | −0.15 (−1.48 to 1.19) | 0.83 |
| Physician Global Assessment of PsA (0‐100 mm VAS) | 1.73 (−7.27 to 10.73) | 0.71 |
| Patient‐reported pain (0‐100 mm VAS) | 2.95 (−5.70 to 11.60) | 0.50 |
| Patient‐reported spine pain (0‐100 mm VAS) | −0.07 (−8.47 to 8.33) | 0.99 |
| Patient‐reported nocturnal spine pain (0‐100 mm VAS) | 2.98 (−6.56 to 12.53) | 0.54 |
| HAQ‐DI (0‐3) | −0.01 (−0.17 to 0.15) | 0.90 |
| HAQ‐S (0‐3) | 0.00 (−0.16 to 0.16) | >0.99 |
| BSA (%) | −1.30 (−3.18 to 0.57) | 0.17 |
| CDAI (0‐76) | 0.18 (−3.69 to 4.05) | 0.93 |
Abbreviations: BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; bDMARD, biologic disease‐modifying antirheumatic drug; BSA, body surface area of psoriasis; CDAI, Clinical Disease Activity Index; CI, confidence interval; HAQ‐DI, Health Assessment Questionnaire‐Disability Index; HAQ‐S, Health Assessment Questionnaire for the Spondyloarthropathies; HLA, human leukocyte antigen; PsA, psoriatic arthritis; tsDMARD, targeted synthetic disease‐modifying antirheumatic drug; VAS, visual analog scale.
Note: Linear regression model estimates were adjusted for baseline value and other potential risk factors (age at initiation, insurance, alcohol use, history of cardiovascular disease, prior conventional synthetic DMARD use, prior bDMARD use, and prior tsDMARD use). The HLA‐B27− group is the reference group for this comparison.
In a descriptive analysis of bDMARD or tsDMARD treatment initiators with imaging confirmation of axial involvement (n = 21), changes from baseline in BASDAI‐related endpoints were consistent with those in the overall population, suggesting only mild improvement for most outcomes. BASDAI scores in both the HLA‐B27–positive and HLA‐B27–negative groups remained >4 after 6 months of treatment, indicating suboptimal control of disease and improvements that were not clinically meaningful (data not shown).
DISCUSSION
Results of the current analysis of PsA/SpA registry data suggest that, despite the use of bDMARD or tsDMARD therapies, continued unmet need exists in providing clinically meaningful improvements in axial disease‐related outcomes in patients with PsA axial disease. After 6 months of treatment with bDMARD or tsDMARD therapies, axial‐related disease activity remained high. Across all groups, scores on all BASDAI‐related measures ranged from 5.8 to 6.9, which are well above the threshold for active, moderate to severe disease (defined as scores ≥4) (15, 23). Mean changes from baseline for BASDAI‐related endpoints ranged from −0.63 to −0.84; these changes do not fall within the definition of clinically meaningful response to treatment (≥50% or ≥2‐point improvement in BASDAI score) (16, 23). Results were similar in patients with positive and negative HLA‐B27 status. This observed lack of clinically meaningful response and continued unmet need may be at least partly attributable to the established nature of disease in this population (mean time since onset of PsA symptoms was 9.0 years, and mean time since PsA diagnosis was 5.0 years [Table 1]). These patients may be more challenging to treat and may need more aggressive therapy.
Although bDMARDs are currently recommended for the treatment of axial PsA (1, 2), data on the clinical efficacy of bDMARDs in axial PsA are limited. Current evidence‐based axial PsA treatment guidelines are largely based on efficacy data in patients with AS (1), with reference to only one recent study in patients with PsA (24) in 2019 guidelines (2). Within the small number of studies we identified in the literature reporting on the efficacy of bDMARDs in axial PsA (8, 24, 25, 26, 27, 28, 29, 30), only two publications (8, 30) evaluated efficacy by HLA‐B27 status. In the 24‐week PSUMMIT‐1 and PSUMMIT‐2 studies of ustekinumab in tumor necrosis factor inhibitor–naive patients with active PsA and physician‐identified spondylitis (8), numerically greater improvements in neck, back, and hip pain and fatigue were observed in patients with positive HLA‐B27 status compared with patients with negative HLA‐B27 status. However, consistent with findings from the current study, improvements in entheseal pain and morning stiffness severity and duration, as well as mean changes in total modified BASDAI scores, were generally similar in patients with positive and negative HLA‐B27 status (8). In the DISCOVER‐1 and DISCOVER‐2 studies of guselkumab (approved for the treatment of active PsA in July 2020 and therefore not included as a bDMARD in the current study), clinically meaningful and statistically significant improvements in axial symptoms—measured as changes from baseline to week 24 and week 52 in BASDAI scores, BASDAI Question 2 (spinal pain), modified BASDAI scores, and ASDAS scores—were observed in guselkumab‐treated patients irrespective of HLA‐B27 status (30).
There are significant unmet needs in the characterization and treatment of axial PsA. Establishing more robust criteria for the diagnosis of axial PsA, clearly defining the axial PsA phenotype, evaluating the efficacy of treatments in axial PsA, and understanding axial involvement have been identified as important topics for future PsA research (2). An evaluation of the published literature and findings from the current study showing minimal differences in treatment outcomes between patients with positive HLA‐B27 status and those with negative HLA‐B27 status further highlight the unmet need to better understand whether the role of HLA‐B27 differs in PsA compared with AS and other spondyloarthritides. Although HLA‐B27 positivity is more common in patients with axial PsA than in the general population, it is considerably less common in axial PsA than in AS, and data indicate that axial PsA and AS may be distinct clinical entities (10, 11, 12).
The current study has a number of strengths. First, the registry strives to provide comprehensive patient‐level data. Patients are characterized fully using information available on important clinical and patient‐reported outcomes. Second, the real‐world data collected in the registry are likely more representative of daily clinical practice than findings from clinical trials. Finally, the registry collects data from a geographically and clinically diverse patient population from across the United States. As such, findings from the current study are likely generalizable to broad populations of patients with PsA.
A limitation of this study is that, of the 3597 patients with PsA in the registry, only a small fraction (n = 135 patients with 173 treatment initiations) met the inclusion criteria for this analysis, in large part because HLA‐B27 status was only available for 538 eligible treatment initiations, and only 173 of 538 initiators had a 6‐month follow‐up visit. The lack of follow‐up for a large proportion of treatment initiators may have introduced selection bias if patients who were responding better to treatment were less likely to have a follow‐up visit. Of note, patients excluded from this study because they did not have known HLA‐B27 status had largely similar baseline characteristics (data not shown) to those included in the analysis, with few small differences: excluded patients had longer mean time since PsA diagnosis (7.1 vs. 5.0 years); lower prevalence of psoriasis (73% vs. 84%); and lower mean tender joint count (7.8 vs. 10.2), CDAI score (16.8 vs. 19.4), and patient‐reported spine pain (43.6 vs. 50.4). The sample size was especially small for patients with imaging‐confirmed axial PsA and known HLA‐B27 status (n = 21). Furthermore, in the registry, laboratory data for CRP levels are only available if requested by a provider as part of routine medical care; thus, the sample sizes of patients with ASDAS‐CRP results at both baseline and 6 months were also small (HLA‐B27+, n = 12; HLA‐B27−, n = 13). Another limitation of this analysis is that, in the absence of a clear and universally accepted definition of axial involvement in PsA (2), axial disease was identified based on a BASDAI score of ≥4 at baseline and the decision of the treating rheumatologist to obtain HLA‐B27 status, as HLA‐B27 positivity is more common in patients with axial involvement (11). Eleven percent of patients in this analysis had a history of fibromyalgia; thus, it was not possible to rule out that this and other comorbid conditions such as mechanical back pain contributed to a BASDAI score of ≥4, especially in this population of older adults who predominantly had obesity (mean age of approximately 50 years; mean body mass index >30 kg/m2).
Recently, it has been suggested that it is not appropriate to use Assessment of Spondyloarthritis international Society (ASAS) classification criteria for axial SpA in patients with axial PsA (31) because ASAS clinical criteria include HLA‐B27 status (32). Given the notably lower prevalence of HLA‐B27 positivity in patients with PsA compared with AS, new classification criteria for axial PsA are needed to clearly differentiate from classical AS (31, 33).
In conclusion, in this registry study of patients with axial PsA, only mild improvements were observed in axial disease‐related outcomes after 6 months of treatment with bDMARD or tsDMARD therapy. Results were similar in patients with positive HLA‐B27 status and negative HLA‐B27 status, suggesting that HLA‐B27 positivity is not predictive of bDMARD or tsDMARD treatment response in patients with axial PsA. The continued high disease activity of these patients reflects critical unmet needs for a focus on the axial domain of PsA and for additional safe and effective therapies for psoriatic axial disease. Filling these gaps will require advancing the ongoing initiatives within the rheumatology community to develop a validated, consensus definition of axial PsA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Mease had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Mease, Chakravarty, McLean, Blachley, Lin, Kavanaugh, Ogdie.
Acquisition of data
Mease, McLean, Blachley, Kavanaugh, Ogdie.
Analysis and interpretation of data
Mease, Chakravarty, McLean, Blachley, Kawashima, Lin, Ogdie.
ROLE OF STUDY SPONSORS
Authors employed by CorEvitas, LLC or Janssen Scientific Affairs, LLC were involved in the study design and in the collection, analysis, and interpretation of the data, the writing of the manuscript, and the decision to submit the manuscript for publication. Access to study data was limited to CorEvitas, and CorEvitas statisticians completed all the analyses. All authors contributed to the interpretation of the results, drafted or revised the manuscript, and approved the manuscript for publication. Publication of this article was not contingent upon approval by CorEvitas or by Janssen Scientific Affairs.
Supporting information
Disclosure Form
ACKNOWLEDGMENTS
Editorial and writing support was provided by Cherie Koch, PhD (Janssen Scientific Affairs, LLC). The authors would like to thank Sean Quinn (Janssen Scientific Affairs, LLC) and Celeste A. Lemay (CorEvitas, LLC) for administrative support of this manuscript and Jonathan Uy, MD (Janssen Scientific Affairs, LLC) for his review of this manuscript.
This study was sponsored by CorEvitas, LLC, and the analysis was funded by Janssen Scientific Affairs, LLC. CorEvitas has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai, Eli Lilly and Company, Genentech, Gilead, GlaxoSmithKline, Janssen, LEO, Novartis, Ortho Dermatologics, Pfizer Inc., Regeneron, Sanofi, Sun, and UCB.
Dr. Mease has received research support, consulting fees, and/or speaker bureau support from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, SUN, and UCB. Drs. Chakravarty and Lin are employees of Janssen Scientific Affairs, LLC, and own stock in Johnson & Johnson, of which Janssen Scientific Affairs is a wholly owned subsidiary. Dr. McLean and Mr. Blachley are employees of CorEvitas, LLC. Ms. Kawashima was an employee of CorEvitas, LLC when this study was conducted. Dr. Kavanaugh has received consulting fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, and UCB. Dr. Ogdie has received grants to the University of Pennsylvania from AbbVie, Novartis, and Pfizer and to Forward from Amgen, and has received consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, CorEvitas, Eli Lilly, Gilead, Happily Health, Janssen, Novartis, Pfizer, and UCB. Her husband has received royalties from Novartis.
CorEvitas’ PsA/SpA Registry dataset is based on a large North American multicenter study adhering to a number of institutional review boards, with complex logistics. Patients did not provide consent to raw data sharing during the data collection for this purpose, and the CorEvitas data sharing policies do not permit raw data sharing for this purpose. Data are available from CorEvitas, LLC through a commercial subscription agreement and are not publicly available. No additional data are available from the authors.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr2.11416&file=acr211416‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Coates LC, Soriano ER, Corp N, Bertheussen H, Callis‐Duffin K, Barbosa Campanholo C, et al. OP0229 The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) treatment recommendations 2021. Ann Rheum Dis 2021;80:139‐40. [Google Scholar]
- 2. Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gottlieb AB, Merola JF. Axial psoriatic arthritis: an update for dermatologists. J Am Acad Dermatol 2021;84:92‐101. [DOI] [PubMed] [Google Scholar]
- 4. Gottlieb A, Korman NJ, Gordon KB, Feldman SR, Lebwohl M, Koo JYM, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol 2008;58:851‐64. [DOI] [PubMed] [Google Scholar]
- 5. Gladman DD. Axial disease in psoriatic arthritis. Curr Rheumatol Rep 2007;9:455‐60. [DOI] [PubMed] [Google Scholar]
- 6. Mease PJ, Palmer JB, Liu M, Kavanaugh A, Pandurengan R, Ritchlin CT, et al. Influence of axial involvement on clinical characteristics of psoriatic arthritis: analysis from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. J Rheumatol 2018;45:1389‐96. [DOI] [PubMed] [Google Scholar]
- 7. Wervers K, Luime JJ, Tchetverikov I, Gerards AH, Kok MR, Appels CWY, et al. Influence of disease manifestations on health‐related quality of life in early psoriatic arthritis. J Rheumatol 2018;45:1526‐31. [DOI] [PubMed] [Google Scholar]
- 8. Helliwell PS, Gladman DD, Chakravarty SD, Kafka S, Karyekar CS, You Y, et al. Effects of ustekinumab on spondylitis‐associated endpoints in TNFi‐naïve active psoriatic arthritis patients with physician‐reported spondylitis: pooled results from two phase 3, randomised, controlled trials. RMD Open 2020;6:e001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia‐Montoya L, Gul H, Emery P. Recent advances in ankylosing spondylitis: understanding the disease and management. F1000Res 2018;7:F1000 Faculty Rev‐1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reveille JD, Hirsch R, Dillon CF, Carroll MD, Weisman MH. The prevalence of HLA‐B27 in the US: data from the US National Health and Nutrition Examination Survey, 2009. Arthritis Rheum 2012;64:1407‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jadon DR, Sengupta R, Nightingale A, Lindsay M, Korendowych E, Robinson G, et al. Axial Disease in Psoriatic Arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis 2017;76:701‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feld J, Ye JY, Chandran V, Inman RD, Haroon N, Cook R, et al. Is axial psoriatic arthritis distinct from ankylosing spondylitis with and without concomitant psoriasis? [Clinical Science Article] Rheumatology (Oxford) 2020;59:1340‐6. [DOI] [PubMed] [Google Scholar]
- 13. Chandran V, Tolusso DC, Cook RJ, Gladman DD. Risk factors for axial inflammatory arthritis in patients with psoriatic arthritis. J Rheumatol 2010;37:809‐15. [DOI] [PubMed] [Google Scholar]
- 14. Chen B, Li J, He C, Li D, Tong W, Zou Y, et al. Role of HLA‐B27 in the pathogenesis of ankylosing spondylitis (Review). Mol Med Rep 2017;15:1943‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen JD, Cunin P, Farrenq V, Oniankitan O, Carton L, Chevalier X, et al. Estimation of the Bath Ankylosing Spondylitis Disease Activity Index cutoff for perceived symptom relief in patients with spondyloarthropathies. J Rheumatol 2006;33:79‐81. [PubMed] [Google Scholar]
- 16. Zochling J, van der Heijde D, Burgos‐Vargas R, Collantes E, Davis JC Jr., Dijkmans B, et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2006;65:442‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calin A, Nakache JP, Gueguen A, Zeidler H, Mielants H, Dougados M. Defining disease activity in ankylosing spondylitis: is a combination of variables (Bath Ankylosing Spondylitis Disease Activity Index) an appropriate instrument? [Original Paper] Rheumatology (Oxford) 1999;38:878‐82. [DOI] [PubMed] [Google Scholar]
- 18. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286‐91. [PubMed] [Google Scholar]
- 19. Daltroy LH, Larson MG, Roberts NW, Liang MH. A modification of the Health Assessment Questionnaire for the spondyloarthropathies. J Rheumatol 1990;17:946‐50. [PubMed] [Google Scholar]
- 20. Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137‐45. [DOI] [PubMed] [Google Scholar]
- 21. Machado P, Landewé R, Lie E, Kvien TK, Braun J, Baker D, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut‐off values for disease activity states and improvement scores. Ann Rheum Dis 2011;70:47‐53. [DOI] [PubMed] [Google Scholar]
- 22. Lukas C, Landewé R, Sieper J, Dougados M, Davis J, Braun J, et al. Development of an ASAS‐endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18‐24. [DOI] [PubMed] [Google Scholar]
- 23. Sidiropoulos P, Kritikos HD, Siakka P, Mamoulaki M, Kouroumali H, Voudouris K, et al. Low dose of infliximab is inadequate in most patients with spondylarthropathies. Clin Exp Rheumatol 2005;23:513‐6. [PubMed] [Google Scholar]
- 24. Baraliakos X, Gossec L, Pournara E, Jeka S, Mera‐Varela A, D'Angelo S, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double‐blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis 2021;80:582‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gratacós J, Casado E, Real J, Torre‐Alonso JC. Prediction of major clinical response (ACR50) to infliximab in psoriatic arthritis refractory to methotrexate. Ann Rheum Dis 2007;66:493‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Favalli EG, Selmi C, Becciolini A, Biggioggero M, Ariani A, Santilli D, et al. Eight‐year retention rate of first‐line tumor necrosis factor inhibitors in spondyloarthritis: a multicenter retrospective analysis. Arthritis Care Res (Hoboken) 2017;69:867‐74. [DOI] [PubMed] [Google Scholar]
- 27. Kavanaugh A, Puig L, Gottlieb AB, Ritchlin C, You Y, Li S, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician‐reported spondylitis: post‐hoc analyses from two phase III, multicentre, double‐blind, placebo‐controlled studies (PSUMMIT‐1/PSUMMIT‐2). Ann Rheum Dis 2016;75:1984‐8. [DOI] [PubMed] [Google Scholar]
- 28. Lubrano E, Spadaro A, Marchesoni A, Olivieri I, Scarpa R, D'Angelo S, et al. The effectiveness of a biologic agent on axial manifestations of psoriatic arthritis. A twelve‐month observational study in a group of patients treated with etanercept. Clin Exp Rheumatol 2011;29:80‐4. [PubMed] [Google Scholar]
- 29. Behrens F, Koehm M, Arndt U, Wittig BM, Greger G, Thaҫi D, et al. Does concomitant methotrexate with adalimumab influence treatment outcomes in patients with psoriatic arthritis? Data from a large observational study. J Rheumatol 2016;43:632‐9. [DOI] [PubMed] [Google Scholar]
- 30. Mease PJ, Helliwell PS, Gladman DD, Poddubnyy D, Baraliakos X, Chakravarty SD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post‐hoc analysis of the phase 3 DISCOVER‐1 and DISCOVER‐2 studies. Lancet Rheumatol 2021;3:E715–23. [DOI] [PubMed] [Google Scholar]
- 31. Coates LC, Baraliakos X, Blanco FJ, Blanco‐Morales EA, Braun J, Chandran V, et al. The phenotype of axial spondyloarthritis: is it dependent on HLA‐B27 status? [Original Article] Arthritis Care Res (Hoboken) 2021;73:856‐60. [DOI] [PubMed] [Google Scholar]
- 32. Rudwaleit M, Landewé R, van der Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770‐6. [DOI] [PubMed] [Google Scholar]
- 33. Stober C, Jadon DR, Armstrong AW, Chandran V, de Wit M, Helliwell PS, et al. Proceedings of the 2020 GRAPPA Collaborative Research Network (CRN) meeting. J Rheumatol Suppl 2021;97:4‐9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form


