Abstract
Objectives
The aim of this systematic review was to examine the effectiveness of pre-anaesthesia assessment clinics (PACs) in improving the quality and safety of perioperative patient care.
Design
Systematic review.
Data sources
The electronic databases CINAHL Plus with Full Text (EBSCOhost), Medline and Embase (OvidSP) were systematically searched on 11 September 2018 and updated on 3 February 2020 and 4 February 2021.
Eligibility criteria
The inclusion criteria for this study were studies published in English or Scandinavian language and scientific original research that included randomised or non-randomised prospective controlled studies. Additionally, studies that reported the outcomes from a PAC consultation with the patient present were included.
Data extraction and synthesis
Titles, abstracts and full texts were screened by a team of three authors. Risk of bias was assessed using the Joanna Briggs Institute critical appraisal checklist for quasi-experimental studies. Data extraction was performed by one author and checked by four other authors. Results were synthesised narratively owing to the heterogeneity of the included studies.
Results
Seven prospective controlled studies on the effectiveness of PACs were included. Three studies reported a significant reduction in the length of hospital stay and two studies reported a significant reduction in cancellation of surgery for medical reasons when patients were seen in the PAC. In addition, the included studies presented mixed results regarding anxiety in patients. Most studies had a high risk of bias.
Conclusion
This systematic review demonstrated a reduction in the length of hospital stay and cancellation of surgery when the patients had been assessed in the PAC. There is a need for high-quality prospective studies to gain a deeper understanding of the effectiveness of PACs.
PROSPERO registration number
CRD42019137724.
Keywords: Adult anaesthesia, Health & safety, Quality in health care, Adult surgery
Strengths and limitations of this study.
An extensive database search was conducted with no limitations on outcomes and the type of pre-anaesthetic assessment clinic.
Only randomised or non-randomised prospective controlled studies were included.
The Joanna Briggs Institute critical appraisal checklist for quasi-experimental studies was used.
The included studies were heterogeneous and had a high risk of bias, which is a major limitation of this review.
Introduction
Anaesthesia is crucial in surgery. However, it may activate physiological changes that increase morbidity and mortality,1 depending on the patients’ preoperative health status and age.2 Hospitals treat patients with complex, comorbid healthcare problems, undergoing progressively extensive surgeries and interventions.3 4 To ensure the quality and safety of anaesthesia and surgery, precise knowledge of the clinical characteristics of patients is critical to the perioperative management.2 Over the past 50 years, perioperative mortality, including anaesthesia-related mortality, has declined, which is significant in developed countries,1 5 mainly because of new anaesthetics, improved monitoring equipment and training, availability of recovery rooms and improved airway management.4 However, a previous review found higher rates of morbidity and mortality in non-operating room anaesthesia, which was attributed to limited preoperative evaluation.6 A retrospective study found significant associations between perioperative mortality and age <1 year or >65 years, American Society of Anesthesiologists (ASA) Physical Status Classification System, emergency case status and operative start time after 18:00.7 This might indicate that risk factors are both patient and surgery related and may be linked to organisational structures.8
Currently, an increasing number of pre-anaesthesia assessment clinics (PACs) support hospitals internationally in handling the rising number of patients and complexity of surgical procedures.9 The design of PACs differs critically based on location, organisational structure, timing and patient groups. They primarily function as a service unit for surgeons, patients and the anaesthetic team.10 The PAC consultation, by the anaesthesiologist, anaesthesia nurse or both, is a globally recognised evaluation method and optimises the patients’ medical condition prior to surgery and anaesthesia.2 Thus, it is essential for secure anaesthetic practice since it detects anaesthesia-related risk factors and high-risk patients, improves patient outcomes, prepares the patient physically and psychologically for anaesthesia, and ensures the patient’s most favourable condition for surgery and anaesthesia.11–13 This is primarily performed by interviewing and examining the patient; reviewing previous medical, surgical and anaesthesia issues; evaluating current medication; and obtaining and reviewing preoperative tests.10 PACs also allow increased communication between healthcare providers and coordination with postoperative care.14 15 Because of well-prepared patients and staff, several researchers have indicated that with PAC, the number of surgical cancellations, length of hospital stay, laboratory tests and mortality rate have reduced.7 16 17 Others assert that patients feel less anxious regarding the subsequent anaesthetic and surgical processes and are highly satisfied with the service with PAC consultations.16 18 19
As Turunen et al stated, research on PACs regarding costs, financial savings, the impact on patient safety and quality of care, accuracy of the number of operative patients and effect on preoperative nursing levels is scarce.20 Survey results indicate that anaesthesiologists perceive day of surgery delays due to missing information as common, even with PAC consultations.21 This systematic review aimed to examine the effectiveness of PACs in improving the quality and safety of perioperative patient care. Further, we aimed to determine the gaps in existing knowledge for future research.
Methods
Our systematic review followed the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions22 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.23 The protocol was registered in PROSPERO: CRD42019137724.24
The two review questions were:
Is PAC effective in improving patient satisfaction and safety, while reducing anxiety?
Is PAC effective in reducing cancellation rate and cost of surgery, and improving the efficiency of perioperative patient care?
Search strategies
We performed a scoping search in different databases to identify the key terms.25 26 The final search was planned and conducted in close collaboration with the university librarian. On 11 September 2018, we searched CINAHL Plus with Full Text (EBSCOhost), Medline and Embase (OvidSP) databases, which were updated on 3 February 2020 and 4 February 2021. Considering the lack of subject headings (e.g.Medical Subject Headings) for PAC, we combined text words, such as ‘pre-anaesthesia’, ‘nurse’, ‘surgery’, ‘anaesthesia’, ‘preoperative’, ‘assessment’, ‘measurement’, ‘evaluate’, ‘preadmission’, ‘centre’, ‘clinic’, ‘ward’, ‘unit’ and ‘outpatient’. The searches are detailed in online supplemental appendix 1. The search mode in CINAHL was Boolean/Phase, which supports Boolean searching or exact phrase searching. For comprehensiveness, we used both the truncation and proximity operators. We limited the search to 1996, the year one of the first known articles in this area was published.27 Complementary methods to identify studies included following up on citations via Scopus, scanning the reference lists of relevant papers and included articles, and checking for relevant studies in clinical trials.25
bmjopen-2021-054206supp001.pdf (103.6KB, pdf)
Eligibility criteria
The main inclusion criterion was that the study, using empirical quantitative methods, addressed the effectiveness of PACs. Specific eligibility criteria were: (a) published in English or Scandinavian language, (b) scientific publication of original research, (c) reported the outcomes of PAC, (d) PAC consultation with the patient present, (e) randomised or non-randomised prospective controlled studies, and (f) newly established PAC. The following were excluded: (a) editorials, discussions and conference abstracts, (b) reviews, (c) instrument testing, (d) studies on children and (e) retrospective studies.
Study selection
All references identified in the search were transferred to EndNote (version X9), where the duplicates were removed. Subsequently, all unique references were transferred to the Covidence screening tool.28 Study eligibility was ascertained independently, first at the title and abstract level, and subsequently at the full-text level. Three of the authors screened all the articles (EWK, AO and MF). Inclusion was determined by consensus, and disagreements were resolved by consulting two other authors (RCB and TT).
Quality assessment
Risk of bias in studies was assessed using design-specific checklists. Given the methodological similarity of the studies, only the Joanna Briggs Institute critical appraisal checklist for quasi-experimental studies was used.29 Author EWK performed the risk of bias assessment, and RCB confirmed its accuracy. Disagreements were resolved through discussion with MF and AO. Each of the nine checklist questions was answered no, yes, unclear or not applicable.
Data extraction and analysis
Author EWK extracted data from each study onto a predesigned Excel spreadsheet. All the authors confirmed the accuracy, consistency and completeness of the extracted data that included publication details; study design; setting; and characteristics of the patients, interventions, comparisons and outcome (PICO). We requested information on the missing data however received no response from the authors. If the PICO elements had been sufficiently similar and statistical data were available, we had intended to conduct a meta-analysis. However, the extracted data revealed substantial heterogeneity. Therefore, we performed a narrative synthesis. We described the findings in text, stratified by outcome, with descriptions of the effects of interventions for each study, classification of the effect direction, and we looked across contributing studies to develop a summary of findings for each outcome.22
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research. However, the project was initiated by health professionals.
Results
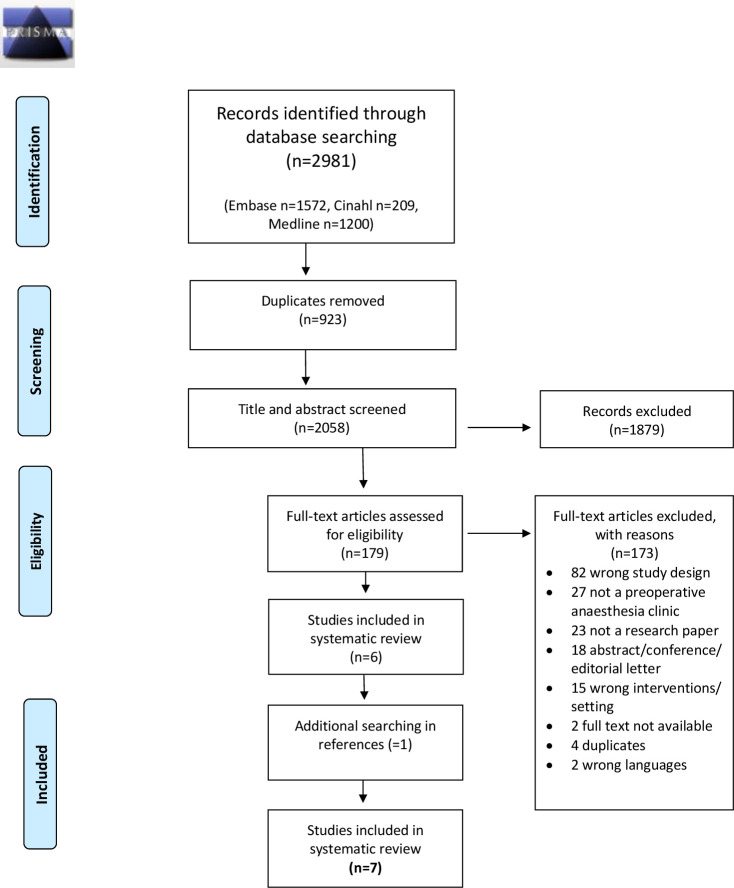
Figure 1 provides the details of the study selection process. A total of 2981 records were identified in the final search (2021). After removing duplicates, we screened 2058 records based on the title and abstract; 179 records passed the full-text screening. After applying the inclusion criteria, seven studies were selected for the final analysis.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).23
Overall characteristics of the studies
The seven studies are listed in table 1. All seven studies were in English and were published between 2000 and 2017, with data collected between 1997 and 2015 (one study did not report data collection information).30 Based on the inclusion criteria, all were prospective controlled studies; however, no randomised controlled trials were found. There was one controlled before–after study.31 The remaining six studies had control groups; assessments followed PAC implementation, without baseline assessments. There were three two-group non-parallel after-only studies32–34 and three two-group parallel after-only studies30; one had a matched control group35 and one had three follow-up assessments of one arm.36 One study had only cancellation rate as prospective data.32 The studies included 77 411 patients.
Table 1.
Description of the included studies
| Author, year, country |
Study design | Sampling time | Population | Intervention | Comparison | Outcomes |
| Farasatkish et al, 2009, Iran33 |
Two-group after study | May 2007 through August 2007 |
N=1716, open-heart surgery, ASA class III–IV | Pre-anaesthesia consultation clinic (3–10 days before surgery) | Usual care (within 24 hours of surgery) | Cancellations |
| Kamal et al, 2011, England34 |
Two-group after study | April 2005 through April 2009 |
N=1445, complex elective orthopaedic surgery, ASA class III–IV | Preoperative anaesthetic assessment clinic (timing not stated) | Usual care (day of surgery) | Admissions, length of stay, mortality, cost |
| Kamau et al, 2017, Kenya31 |
CBA | August 2000, April 2001, November 2001 | N=51, elective non-cardiac surgery, ASA class not stated | Pre-anaesthesia clinic consultation (≥48 hours before surgery) | Usual care (day before surgery) | Anxiety (STAI score) |
| Klopfenstein et al, 2000, Switzerland30 |
Two-group after study (parallel) | No data | N=40, elective endoscopic urological surgery, ASA class I–III | Pre-anaesthetic consultation (1–2 weeks before surgery) | Usual care (the evening before surgery) | Anxiety (MAACL, VAS) |
| Lee et al, 2012, China35 |
Two-group after study (parallel) | March 2007 through November 2009 | N=352, elective surgery, ASA class I–IV | Anaesthesia consultation clinic (≤3 months before surgery) | Usual care (the evening before surgery) | Quality of recovery score, cost, cancellations, length of stay, satisfaction, anxiety (VAS), willingness to pay |
| Mendes et al, 2005, Brazil36 |
Two-group after study (parallel) | April 2007 through June 2007 |
N=52 254, surgery, ASA class not stated | Preoperative outpatient evaluation clinic (timing not stated) | Usual care (timing not stated) | Cancellations, length of stay, PAC evaluations, outpatient procedures |
| van Klei et al, 2002, The Netherlands32 |
Two-group after study | November 2012 | N=21 553, elective surgery, ASA class not stated | Preoperative outpatient evaluation clinic (average 3 weeks before surgery) | Usual care (day before surgery) | Cancellations |
ASA, American Society of Anesthesiologists; CBA, controlled before–after; MAACL, Multiple Affect Adjective Check List; PAC, Preanesthetic assessment clinic; STAI, State-Trait Anxiety Inventory; VAS, Visual Analogue Scale.
Of the 77 411 patients in the studies, 9626 and 15 531 patients were in the intervention and control groups, respectively. One study did not specify the number of patients in the intervention and control groups, but only the total number of surgeries performed.36 Five studies reported data for sex, showing that 51% of the patients were women and 49% were men (12 129 vs 11 583).30–33 35 There were more women than men in both the intervention (4345 vs 4134) and control groups (7784 vs 7449). Five studies reported data for age showing that all patients were over 20 years of age30–33 35 and four studies had grouped within the ASA category.30 33–35
The patients were scheduled to undergo a variety of surgeries, including orthopaedic,31 32 34 35 urology,30–32 35 general,31 32 35 heart,33 gynaecology/obstetrics,31 32 35 vascular surgery,32 ophthalmology,32 maxillofacial/dental surgery31 32 and neurological surgery,32 while one did not specify the type.36 In five studies, the type of anaesthesia was not specified,31–34 36 and two studies reported patients for general and/or regional supplement.30 35
The patients included had previous anaesthetic experience in one study,30 previous and no previous anaesthetic experience in another,31 and five studies did not report these data.32–36 Limited background characteristics of the patients were reported in two studies.34 36 One stated that the patients included had ASA 3 or 4 and a body mass index >40; however, no ASA number, sex or age was reported.34 Mendes et al did not report any background characteristics of the included patients.36
Considering the intervention, the PACs in all studies comprised an outpatient service whereby patients were examined for medical conditions important for anaesthesia and informed regarding expectations on the day of surgery. Nevertheless, the terminology used for PACs varied, as they served different surgical specialties and conducted pre-anaesthesia consultation from ≥48 hours to ≤3 months before the surgery. The settings included a university hospital (n=3),31 35 36 teaching hospital (n=1),32 medical centre (n=1)33 and general hospital (n=1)34; one study did not specify the context.30 The staff conducting the pre-anaesthetic consultation also varied: in five studies, it was the anaesthesiologists30 32 34–36; in the other studies, it was (also) the orthopaedic senior house officer,34 consultant or resident,31 or physician.33 In three studies, nurses were part of the team.32 34 35 The comparison in all studies was usual care, which generally involved a preoperative anaesthetic evaluation of the admitted patients the day before the surgery.
Description of risk of bias in the studies
Figure 2 shows the results of the risk of bias assessment. In all seven included studies, the cause and effect were clear. Most of the studies measured outcomes similarly and used appropriate statistical analyses. Several studies had limitations of follow-up and similarity in care and participants. None of the patients had multiple pre- and post-measurements.
Figure 2.
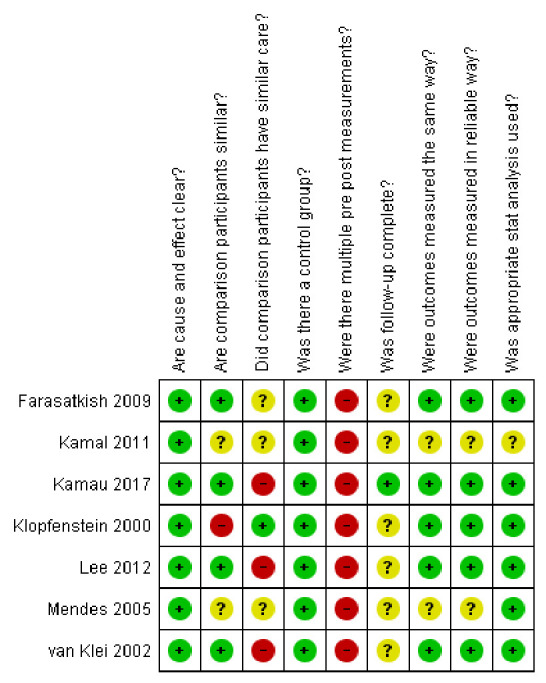
The Joanna Briggs Institute critical appraisal checklist for quasi-experimental studies was used for the risk of bias assessment.29
Outcomes of the included studies
The outcomes of the included studies are each described separately below.
Satisfaction
One study reported satisfaction as an outcome.35 The summarised patient satisfaction with the anaesthetic consultation score out of 100 showed that the patients in the PAC group were more satisfied (mean difference: 2.10%; 95% CI: 0.51% to 3.70%; p=0.01).35 There was no statistically significant difference between the two groups in mean patient satisfaction with perioperative anaesthesia care score after surgery (mean difference: 0.01%, p=0.94).35 The quality of recovery (QoR) measure referred to the patients’ QoR score.37 The mean QoR score (range: 0–18) following anaesthesia on the first day after surgery was similar between the intervention (13.17±2.73) and control (13.31±2.65) groups (p=0.67).35
Anxiety
Three studies reported anxiety.30 31 35 Two studies reported the Visual Analogue Scale (VAS), one rated from 0 (no anxiety) to 10 (very high anxiety),30 another used a 100 mm horizontal line with ‘not anxious at all’ to ‘extremely anxious’.35 In one study, the median VAS anxiety score was 3 (0–5) in the intervention group and 5 (2–8) in the control group (p=0.0038).30 In another study, there were no significant differences between the control and intervention groups for levels of anxiety (VAS), surgery (26 vs 25, respectively, p=0.12) and anaesthesia (20 vs 19, respectively, p=0.60).35 The median Multiple Affect Adjective Check List score, with possible range of scores from 0 to 21 (higher scores indicating greater levels of anxiety), was 3 (0–9) in the intervention group and 6.5 (2–12) in the control group (p=0.0053).30 The difference in the State-Trait Anxiety Index score, which comprised 40 questions rated on a 4-point Likert scale, was 1.51 (95% CI: 1.02 to 2.02, p=0.0051).31 The results on anxiety in these two studies were significant. However, Kamau et al found no differences on examining anxiety and the influences of sex, duration of hospital stay and prior anaesthesia experience.31
Mortality
One study reported the mortality rates.34 Patients attending the high dependency unit (HDU), intensive care unit (ICU) and post-anaesthesia care unit (PACU) following complex orthopaedic surgery had a significant reduction in mortality rate after being assessed at the PAC, from 18 (6.1%) of 298 patients to 14 (1.2%) of 1147 patients (p=0.001).34
Cancellation rate
Four studies reported reduced cancellation rates following the establishment of a PAC.32 33 35 36 One of the included studies had 316 (2.0%) cancellations for medical reasons before the introduction of PAC, and 79 (0.9%) after, with a difference of 1.02% (95% CI: 0.31% to 1.31%). After adjustment, the OR was 0.7 (95% CI: 0.5 to 0.9).32 The overall cancellation of surgery reduced from 1027 (6.3%) to 393 (4.6%) following PAC introduction, with a difference of 0.9% (95% CI: 0.3% to 1.0%).32 Mendes et al36 found a decrease in overall cancellations from year 1 (39.3%) to year 4 (15.9%), p≤0.05. In the first year of their study, there were 469 cancellations per 10 639 surgeries performed. The following year, a considerable increase above the baseline in the intervention group was observed, followed by a progressive decrease in the last year with 391 cancellations per 10 397 surgeries performed.36 Farasatkish et al reported that of the 1716 patients studied, a mean of 15.1% cancelled in the two groups. The cancellation rates in the control and intervention groups were 16.8% (146 (number of cancellations)/866 (number of surgeries)) and 13.29% (113 of 850) (p=0.046), respectively. The most common reason for cancellation was incomplete medical work-up; 51 of 146 (35%) in the control group and 32 of 113 (28%) in the intervention group (p=0.03).33 Lee et al found similar rates for surgery being cancelled on the scheduled date for the intervention group compared with the control group (2.3% vs 3.4%, p=0.75).35
Costs and willingness to pay
Two studies reported the costs.34 35 One study reported a total saving of £486.62 per patient after establishing a PAC.34 Another reported a significantly lower preoperative cost per patient in the intervention group compared with that of the control group (mean difference: $463; 95% CI: −$648 to −$278 per patient, p<0.01).35 However, the mean difference in the total perioperative treatment cost was not significant, even after adjusting for cancellation on the day of surgery costs.35 Compared with the control group, the willingness to pay (WTP) among the intervention group patients was significantly more than the median WTP (US$13) for a clinic consultation at the PAC.35
Length of stay
The length of stay was reported in three studies.34–36 Mendes et al36 found a significant decrease in mean hospital stay of patients from 6.2 to 5.0 days (p≤0.001) during the 4 years of this study. Kamal et al34 found a significant reduction in the length of stay in the HDU from 2.1 days to 1.6 days (p=0.01), and in the ICU from 2.3 days to 1.9 days (p=0.01). In the last study, no significant changes were found in the median duration of postoperative stay between the intervention and control groups.35
Organisation planning and efficiency
Organisation planning and efficiency have been reported in two studies.34 36 One study found statistically significant changes in the reduction of unplanned admissions to the PACU (65 of 298 (22%), 111 of 1147 (10%), p=0.001), ICU (4 of 298 (1.3%), 4 of 1147 (0.4%), p=0.01) and HDU (4 of 298 (1.34%), 20 of 1147 (1.7%), p=0.01) after implementing a PAC.34 The planned admissions in the ICU (4 of 298 (1.3%), 18 of 1147 (1.6%), p=0.01) and HDU (14 of 298 (4.7%), 85 of 1147 (7.4%), p=0.1) increased after implementing a PAC.34 The number of PAC evaluations increased from 14 704 (year 1) to 413 990 (year 4) (p≤0.001).36 The number of outpatient procedures increased from baseline 1510 to 2170 (year 1) to 1943 (year 4) (p≤0.001), and the inpatient procedures decreased from 9556 (year 1) to 8449 (year 4) (p≤0.001).36
Discussion
This systematic review summarises the effectiveness of PACs in improving quality and safety of pre-anaesthetic patient care in general hospitals and determines the gaps in existing knowledge for future research. Herein, we present the main results of the review and infer the implications for research and practice.
Seven studies met the inclusion criteria, and the main findings were reduction in the length of stay and surgery cancellation rate in hospitals. However, the studies were of low quality, making it difficult to draw any conclusion. The evidence from our systematic review is insufficient to conclude whether patients have reduced anxiety because of PAC. This is because the included studies used different instruments for measuring anxiety levels, and the results could not be pooled.38
A major purpose of establishing a PAC in a hospital is to better prepare the patients for the anticipated surgery. Healthcare professionals and policymakers are exploring strategies to reduce unnecessary investigations without compromising quality of care and patient safety.39 Transition of evidence-based interventions to the hospital systems can provide substantial benefits to patient care.40 According to the ASA and the National Institute of Health and Care Excellence, routine preoperative laboratory tests are not recommended for relatively healthy patients. Instead, they encourage patient and surgery-specific investigations.15 40 This recommendation is not always implemented in hospital protocols or practice. An observational study showed that routine preoperative testing to predict abnormalities found at least one abnormal test result in most of the relatively healthy patients. Only 0.67% of the abnormalities had a significant impact on changing the perioperative management.41 Blitz et al argued that PACs should focus on early patient engagement strategies, interdisciplinary team communication, detailed perioperative care plans and patient documentation using electronic health record, which should be open for review by the perioperative team.14 Furthermore, a previous study mentioned that the risk factors are not only patient related but also organisation related,7 and that some hospitals have perioperative care teams that are better at identifying and relieving perioperative complications.42 43 Thus, the value of PACs lies in their ability to improve the quality of the perioperative process by designing a more robust system for preoperative assessment and preparation.14 A narrative review found higher rates of morbidity and mortality in non-operating room anaesthesia, and one of the main reasons was limited preoperative evaluation.6 In this systematic review, the assessment of PAC was significantly associated with reduced mortality following complex orthopaedic surgery in only one study.34 Retrospective studies have reported similar results, but with different surgeries.14 44
Cancellation on the day of surgery has undesirable effects on both the patients and the hospital system.13 Late patient-related cancellations can totally or partially be prevented,45 if addressed during preoperative evaluations.16 This has been confirmed by only three studies in this systematic review that found a reduction in surgery cancellation after implementing a PAC.32 33 36 However, Lee et al found no significant changes between the intervention and control groups.35 Mendes et al found that the number of cancellations for medical reasons after PAC implementation decreased in the first year of implementation. In the second and third years, they were high before the number dropped to below baseline.36 These conflicting findings indicate that hospitals operate in specific contexts, with unique populations, processes and microsystems, encountering unique obstacles and making implementation difficult. Patient-focused interventions should consider barriers, facilitators, and inter-relationships between systems, staff, and interventions to increase the likelihood of sustainable success.46 Additionally, Kamal et al indicated that PACs lead to more planned admissions to the ICU, HDU and PACU, which is more predictable for patients, staff and administration.34
Another finding of this review was a significant reduction in the length of hospital stay following patients’ examination in a PAC; however, a small number of studies with low quality were considered. Nevertheless, similar results were found in another systematic review claiming that perioperative systems support the hospitals by addressing the expected growth in the number and complexity of surgical procedures.16 When patients are examined in the PAC and well prepared with information, consultations and tests, they need not be hospitalised until the day of surgery. A survey on operated patients showed that given a choice, 75% do not wish admission to the hospital until the day of operation; a major reason being shorter hospital stay.47 An updated systematic review on the effectiveness of nurse-led preoperative assessment services for elective surgery found that the included articles demonstrated a reduced length of stay; these studies had low methodological quality, and therefore, the authors could not conclude that this service leads to reduced length of hospital stay.17
Strengths and limitations of the study
The review was performed in duplicate or independently by two researchers, and consensus was reached through discussion. However, grey literature, such as government and institutional documents, was excluded and might be a limitation of this study. Since organisation of healthcare systems varies among countries, the type of staff who performed the preoperative assessment was not considered as an inclusion criterion. The European Society of Anaesthesiology guidelines recommend that the anaesthesiologists must complete the preoperative assessment while trained nurses or anaesthesia trainees should perform the screening.8 A preoperative evaluation performed by an internist was associated with increased length of stay and increased postoperative mortality.48 The results of this systematic review may have been affected by the heterogeneity in the types of staff performing the preoperative assessment.
We exclusively included studies with high internal validity. Therefore, several retrospective studies were excluded. Nonetheless, as the remaining studies’ risk of bias was fairly high, and they were heterogeneous, a meta-analysis was not statistically appropriate.26 The included studies’ designs could not rule out selection bias and confounders; thus, the strength of the evidence should be assessed cautiously. Many studies did not adjust for several confounders, which could be responsible for the observed effects. Several studies lacked descriptions of the methods used and the patients included, lowering transparency. The results are relevant to healthcare services, focusing on the well-being and safety of the patients.
Implications for future research and practice
This systematic review identified the ambiguity in the PAC interventions offered to the intervention group. In many studies, it was evident that the methods used lacked clarity, and high-quality research is needed in this field. The included studies did not demonstrate earlier surgical room entry time49 50 or reduction in the number of preoperative tests for patients attending the PAC, similar to the results of the retrospective studies.27
Other implications may include the organisation structure of different PACs and their functioning. Additionally, the tests that should be part of the assessment at the PACs should be investigated. The use of technology, such as streaming services, facilitates different patient groups and might become crucial for reducing human contact and spread of infection in the context of COVID-19.
Conclusion
PAC use has reduced the length of stay and surgery cancellation rate at hospitals. However, the effectiveness of PAC, the major review question, remains unclear and requires further research. There is a demand for high-quality studies capturing robust data describing the quality of care and clinical outcomes for patients requiring anaesthesia. This requires increased focus and funding for this specific area of health services research and could, therefore, lead to implementation of PACs in healthcare services and improve patient safety and perioperative care.
Supplementary Material
Acknowledgments
We thank the librarian Ellen Sejerstedt, University of Agder, Kristiansand, for helping with the search strategy and elimination of duplicate articles.
Footnotes
Contributors: EWK—guarantor for this study. EWK, MF, AO and TT—study design. EWK, MF and AO—search and screening of the articles. EWK—data extraction. MF, AO, RCB and TT—control of data extraction. EWK, MF, AO and RCB—quality assessment of the included articles. EWK—drafting of the manuscript. MF, AO, RCB and TT—contribution to and review of the final version of the manuscript. MF, AO, RCB and TT—supervision of the study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information. All data relevant to the study are included within the article or have been uploaded within supplemental files.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Not applicable.
References
- 1.Braz LG, Braz DG, Cruz DSda, et al. Mortality in anesthesia: a systematic review. Clinics 2009;64:999–1006. 10.1590/S1807-59322009001000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos ML, Novaes CdeO, Iglesias AC. [Epidemiological profile of patients seen in the pre-anesthetic assessment clinic of a university hospital]. Rev Bras Anestesiol 2017;67:457–67. 10.1016/j.bjan.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 3.Amaya F, Shimamoto S, Matsuda M, et al. Preoperative anesthesia clinic in Japan: a nationwide survey of the current practice of preoperative anesthesia assessment. J Anesth 2015;29:175–9. 10.1007/s00540-014-1918-3 [DOI] [PubMed] [Google Scholar]
- 4.Whitaker DK, Brattebø G, Smith AF, et al. The Helsinki Declaration on patient safety in Anaesthesiology: putting words into practice. Best Pract Res Clin Anaesthesiol 2011;25:277–90. 10.1016/j.bpa.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 5.Bainbridge D, Martin J, Arango M, et al. Perioperative and anaesthetic-related mortality in developed and developing countries: a systematic review and meta-analysis. Lancet 2012;380:1075–81. 10.1016/S0140-6736(12)60990-8 [DOI] [PubMed] [Google Scholar]
- 6.Herman AD, Jaruzel CB, Lawton S, et al. Morbidity, mortality, and systems safety in non-operating room anaesthesia: a narrative review. Br J Anaesth 2021;127:729–44. 10.1016/j.bja.2021.07.007 [DOI] [PubMed] [Google Scholar]
- 7.Whitlock EL, Feiner JR, Chen L-L. Perioperative mortality, 2010 to 2014: a retrospective cohort study using the National anesthesia clinical outcomes registry. Anesthesiology 2015;123:1312–21. 10.1097/ALN.0000000000000882 [DOI] [PubMed] [Google Scholar]
- 8.De Hert S, Staender S, Fritsch G, et al. Pre-Operative evaluation of adults undergoing elective noncardiac surgery: updated guideline from the European Society of Anaesthesiology. Eur J Anaesthesiol 2018;35:407–65. 10.1097/EJA.0000000000000817 [DOI] [PubMed] [Google Scholar]
- 9.Chan FWK, Wong FYY, Cheung YS, et al. Utility of a preoperative assessment clinic in a tertiary care hospital. Hong Kong Med J 2011;17:441–5doi:<br> [PubMed] [Google Scholar]
- 10.Gupta A, Gupta N. Setting up and functioning of a preanaesthetic clinic. Indian J Anaesth 2010;54:504–7. 10.4103/0019-5049.72638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hariharan S, Chen D, Merritt-Charles L. Evaluation of the utilization of the preanaesthetic clinics in a university teaching hospital. BMC Health Serv Res 2006;6:59. 10.1186/1472-6963-6-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg E, Saffary R, Schmiesing C. New role for the anesthesia preoperative clinic: helping to ensure that surgery is the right choice for patients with serious illness. Anesth Analg 2019;129:311–5. 10.1213/ANE.0000000000004178 [DOI] [PubMed] [Google Scholar]
- 13.Emanuel A, Macpherseon R. The anaesthetic pre-admission clinic is effective in minimising surgical cancellation rates. Anaesth Intensive Care 2013;41:90–4. 10.1177/0310057X1304100115 [DOI] [PubMed] [Google Scholar]
- 14.Blitz JD, Kendale SM, Jain SK, et al. Preoperative evaluation clinic visit is associated with decreased risk of in-hospital postoperative mortality. Anesthesiology 2016;125:280–94. 10.1097/ALN.0000000000001193 [DOI] [PubMed] [Google Scholar]
- 15.Committee on Standards and Practice Parameters, Apfelbaum JL, Connis RT, et al. Practice Advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task force on Preanesthesia evaluation. Anesthesiology 2012;116:522–38. 10.1097/ALN.0b013e31823c1067 [DOI] [PubMed] [Google Scholar]
- 16.Lee A, Kerridge RK, Chui PT, et al. Perioperative systems as a quality model of perioperative medicine and surgical care. Health Policy 2011;102:214–22. 10.1016/j.healthpol.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 17.Hines S, Munday J, Kynoch K. Effectiveness of nurse-led preoperative assessment services for elective surgery: a systematic review update. JBI Database System Rev Implement Rep 2015;13:279–317. 10.11124/01938924-201513060-00016 [DOI] [PubMed] [Google Scholar]
- 18.Delaney D, Bayley EW, Olszewsky P, et al. Parental satisfaction with pediatric preoperative assessment and education in a presurgical care center. J Perianesth Nurs 2015;30:290–300. 10.1016/j.jopan.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 19.Davidson S, McKendrick D, French T. Preassessment clinic interview and patient anxiety. Saudi J Anaesth 2016;10:402–8. 10.4103/1658-354X.177339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turunen E, Miettinen M, Setälä L, et al. The impact of a structured preoperative protocol on day of surgery cancellations. J Clin Nurs 2018;27:288–305. 10.1111/jocn.13896 [DOI] [PubMed] [Google Scholar]
- 21.Holt NF, Silverman DG, Prasad R, et al. Preanesthesia clinics, information management, and operating room delays: results of a survey of practicing anesthesiologists. Anesth Analg 2007;104:615–8. 10.1213/01.ane.0000255253.62668.3a [DOI] [PubMed] [Google Scholar]
- 22.Cochrane Handbook for systematic reviews of interventions, 2019. Available: http://www.training.cochrane.org/handbook [Accessed 8 Dec 2018].
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristoffersen EW, Opsal A, Tveit TO. The effect of preanaesthetic assessment clinic: a systematic review of randomised and non-randomised prospective controlled studies. Prospero CRD42019137724 2019. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019137724doi:CRD42019137724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Booth A, Sutton A, Papaioannou D. Systematic approaches to a successful literature review. Los Angeles, California: Sage, 2016. [Google Scholar]
- 26.Møller AM, Myles PS. What makes a good systematic review and meta-analysis? Br J Anaesth 2016;117:428–30. 10.1093/bja/aew264 [DOI] [PubMed] [Google Scholar]
- 27.Fischer SP. Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital. Anesthesiology 1996;85:196–206. 10.1097/00000542-199607000-00025 [DOI] [PubMed] [Google Scholar]
- 28.Covidence systematic review software,Veritas Health Innovation , 2018,Melbourne, Australia. Available: https://www.covidence.org [Google Scholar]
- 29.Tufanaru C, Munn Z, Aromataris E. Systematic review of effectiveness. JBI Manual for evidence synthesis. In: Aromataris E, Munn Z, eds. JBI manual for evidence synthesis, 2020. [Google Scholar]
- 30.Klopfenstein CE, Forster A, Van Gessel E. Anesthetic assessment in an outpatient consultation clinic reduces preoperative anxiety. Can J Anaesth 2000;47:511–5. 10.1007/BF03018941 [DOI] [PubMed] [Google Scholar]
- 31.Kamau A, Mung'ayi V, Yonga G. The effect of a preanaesthesia clinic consultation on adult patient anxiety at a tertiary hospital in Kenya: a cohort study. Afr Health Sci 2017;17:138–46. 10.4314/ahs.v17i1.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Klei WA, Moons KGM, Rutten CLG, et al. The effect of outpatient preoperative evaluation of hospital inpatients on cancellation of surgery and length of hospital stay. Anesth Analg 2002;94:644–9. 10.1097/00000539-200203000-00030 [DOI] [PubMed] [Google Scholar]
- 33.Farasatkish R, Aghdaii N, Azarfarin R. Can preoperative anesthesia consultation clinic help to reduce operating room cancellation rate of cardiac surgery on the day of surgery? Middle East J Anaesthesiol 2009;20:93–6 https://pubmed.ncbi.nlm.nih.gov/19266833/doi:20 [PubMed] [Google Scholar]
- 34.Kamal T, Conway RM, Littlejohn I, et al. The role of a multidisciplinary pre-assessment clinic in reducing mortality after complex orthopaedic surgery. Ann R Coll Surg Engl 2011;93:149–51. 10.1308/003588411X561026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee A, Chui PT, Chiu CH, et al. The cost-effectiveness of an outpatient anesthesia consultation clinic before surgery: a matched Hong Kong cohort study. Perioper Med 2012;1:3. 10.1186/2047-0525-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendes FF, Mathias LAdaST, Duval Neto GF, et al. [Impact of preoperative outpatient evaluation clinic on performance indicators.]. Rev Bras Anestesiol 2005;55:175–87. 10.1590/s0034-70942005000200004 [DOI] [PubMed] [Google Scholar]
- 37.Myles PS, Hunt JO, Nightingale CE, et al. Development and psychometric testing of a quality of recovery score after general anesthesia and surgery in adults. Anesth Analg 1999;88:83–90. 10.1213/00000539-199901000-00016 [DOI] [PubMed] [Google Scholar]
- 38.Mitchell M. Anaesthesia type, gender and anxiety. J Perioper Pract 2013;23:41–7. 10.1177/175045891302300301 [DOI] [PubMed] [Google Scholar]
- 39.Karim HMR, Singha SK, Neema PK, et al. Information technology-based joint preoperative assessment, risk stratification and its impact on patient management, perioperative outcome, and cost. Discoveries 2021;9:e130. 10.15190/d.2021.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National guideline C . National Institute for health and care excellence: clinical guidelines. preoperative tests (update): routine preoperative tests for elective surgery. London: National Institute for Health and Care Excellence, UK, 2016. [PubMed] [Google Scholar]
- 41.Reazaul Karim HM, Prakash A, Sahoo SK, et al. Abnormal routine pre-operative test results and their impact on anaesthetic management: an observational study. Indian J Anaesth 2018;62:23–8. 10.4103/ija.IJA_223_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savoldelli GL. Perioperative care is a hazardous business: Let’s make it safer! Trends in Anaesthesia and Critical Care 2016;7-8:3–4. 10.1016/j.tacc.2016.05.003 [DOI] [Google Scholar]
- 43.Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet 2012;380:1059–65. 10.1016/S0140-6736(12)61148-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantlay KL, Baker S, Parry A, et al. The impact of a consultant anaesthetist led pre-operative assessment clinic on patients undergoing major vascular surgery. Anaesthesia 2006;61:234–9. 10.1111/j.1365-2044.2005.04514.x [DOI] [PubMed] [Google Scholar]
- 45.Hänninen-Khoda L, Koljonen V, Ylä-Kotola T. Patient-Related reasons for late surgery cancellations in a plastic and reconstructive surgery department. JPRAS Open 2018;18:38–48. 10.1016/j.jpra.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geerligs L, Rankin NM, Shepherd HL, et al. Hospital-Based interventions: a systematic review of staff-reported barriers and facilitators to implementation processes. Implementation Sci 2018;13:36. 10.1186/s13012-018-0726-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harries RL, Bradshaw CA, Jones EA, et al. To admit or not to admit on the morning of surgery patients' perspectives on day of surgery admission. J Perioper Pract 2013;23:56–8. 10.1177/175045891302300304 [DOI] [PubMed] [Google Scholar]
- 48.Wijeysundera DN, Austin PC, Beattie WS, et al. Outcomes and processes of care related to preoperative medical consultation. Arch Intern Med 2010;170:1365–74. 10.1001/archinternmed.2010.204 [DOI] [PubMed] [Google Scholar]
- 49.Ferschl MB, Tung A, Sweitzer B, et al. Preoperative clinic visits reduce operating room cancellations and delays. Anesthesiology 2005;103:855–9. 10.1097/00000542-200510000-00025 [DOI] [PubMed] [Google Scholar]
- 50.Correll DJ, Bader AM, Hull MW, et al. Value of preoperative clinic visits in identifying issues with potential impact on operating room efficiency. Anesthesiology 2006;105:1254–9. 10.1097/00000542-200612000-00026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-054206supp001.pdf (103.6KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information. All data relevant to the study are included within the article or have been uploaded within supplemental files.