This cohort study assesses the safety and medium-term outcomes of percutaneous intramyocardial septal radiofrequency ablation in a large patient cohort with drug-refractory hypertrophic obstructive cardiomyopathy.
Key Points
Question
Can percutaneous intramyocardial septal radiofrequency ablation (PIMSRA) be an effective nonsurgical treatment of hypertrophic obstructive cardiomyopathy?
Findings
In this single-arm cohort study of 200 patients with symptomatic hypertrophic obstructive cardiomyopathy, despite medical therapy, PIMSRA was associated with a durable reduction in peak left ventricular outflow tract gradients and with a significant improvement in symptoms at medium-term follow-up with low morbidity and mortality.
Meaning
PIMSRA may be an alternative to surgical intervention in patients who remain symptomatic despite medical therapy.
Abstract
Importance
Patients with hypertrophic obstructive cardiomyopathy (HOCM) and drug-refractory symptoms and outflow gradients have limited nonsurgical treatment options. The feasibility of percutaneous intramyocardial septal radiofrequency ablation (PIMSRA) has been reported previously; however, procedural and medium-term outcomes are unknown.
Objective
To describe the safety and medium-term outcomes of PIMSRA in a large patient cohort with drug-refractory HOCM.
Design, Setting, and Participants
This was a single-arm, open-label study of PIMSRA in patients with drug-refractory HOCM. Patients presenting to the Xijing Hospital in Xi’an, China, between October 2016 to June 2020 with hypertrophic cardiomyopathy. Of 1314 patients presenting with HOCM, 244 fulfilled inclusion criteria of severe resting/provoked outflow gradients of 50 mm Hg or higher, and symptoms of New York Heart Association functional class of II or higher refractory to maximum tolerated medications. After discussion among the heart team, 40 patients underwent surgical or alcohol septal reduction therapy and 4 required treatment of significant coronary artery disease.
Interventions
PIMSRA performed in patients.
Main Outcomes and Measures
The primary outcome was 30-day major adverse clinical events: death, emergency surgery, severe effusion requiring intervention, procedure-related stroke, bleeding, and stroke. Secondary outcomes included 30-day technical success and 90-day improvement in outflow obstruction.
Results
The mean (SD) age of 200 patients was 46.9 (14.0) years, and 125 (62.5%) were men. Resting or provoked left ventricular outflow tract gradients were 50 mm Hg or higher. The median (IQR) follow-up for all patients was 19 (6-50) months. Thirty-day major adverse clinical events rate was 10.5% (n = 21): there were 2 in-hospital/30-day deaths (1.0%), 7 patients (3.5%) with pericardial effusion requiring mini-thoracotomy, 12 patients (6%) with pericardial effusion requiring pericardiocentesis, and no bleeding or strokes. Other periprocedural complications included permanent right bundle branch block in 5 patients (2.5%), resuscitated ventricular fibrillation in 2 (1.0%), and septal branch aneurysm in 2 (1.0%). There were no permanent pacemaker implantations. At follow-up, maximum septal thickness was reduced from a mean (SD) of 24.0 (5.1) mm to 17.3 (4.4) mm (P < .001), and left ventricular outflow tract gradient was decreased from a mean (SD) of 79.0 (53.0) mm Hg to 14.0 (24.0) mm Hg (P < .001). Overall, 190 patients (96%) with HOCM were in New York Heart Association functional class I or II at last follow-up.
Conclusions and Relevance
This study found that PIMSRA in patients with drug-refractory HOCM may be an effective procedure for relief of left ventricular outflow tract obstruction and symptoms with acceptable complication rates. These results are encouraging and support the design of a randomized clinical trial against well-established septal reduction therapies.
Introduction
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant inherited cardiomyopathy, with an adult incidence rate of 1:500 to 1:200.1,2 Up to 75% of patients with HCM have left ventricular outflow tract (LVOT) obstruction under resting conditions (defined as gradients ≥30 mm Hg) or with provocation (<30 mm Hg at rest and ≥30 mm Hg with physiologic provocation).1 Dynamic LVOT hypertrophic obstructive cardiomyopathy (HOCM) is the key mechanism leading to clinical symptoms in patients and significantly increased mortality.3 Asymmetric myocardial hypertrophy with systolic anterior motion of the mitral valve is the pathophysiologic cause of LVOT obstruction.4 Although medical treatment with β-blockers, nondihydropyridine calcium channel blockers, and disopyramide have a class I indication in current guidelines,1 they are effective in reducing LVOT obstruction in less than 50% of patients resulting in a large patient population with drug-refractory sypmtoms.5 Therefore, effective ventricular septal reduction with either surgical myectomy or alcohol septal ablation (ASA) is the main invasive treatment for LVOT obstruction. Unfortunately, the success of ASA is limited by septal coronary anatomy and alternative nonsurgical techniques have had limited success.6,7
We previously reported our initial experience with percutaneous intramyocardial septal radiofrequency ablation (PIMSRA) treating HOCM in 15 patients (NCT02888132) with follow-up time of 6 months8 and now have accumulated medium-term follow-up data, focusing on the efficacy, safety, and complications.
Methods
Patient Population
A total of 1314 consecutive patients with HCM were evaluated at Xijing Hospital in Xi’an, China, between October 2016 to June 2020, of whom 857 had resting or provoked LVOT gradients less than 50 mm Hg. Among the remaining 457 patients with resting or provoked LVOT gradients of 50 mm Hg or higher, 213 patients with HOCM were not receiving adequate medical therapy or had not undergone a comprehensive evaluation for PIMSRA procedure (Video; eFigure in the Supplement). The final patient cohort of 244 individuals had drug-refractory severe HOCM defined as a resting or provoked LVOT gradient of 50 mm Hg or higher, treatment with maximum tolerated β-blocker and/or calcium channel blockers, and persistent symptoms of New York Heart Association (NYHA) functional class of II or higher. All patients with HOCM participated in shared decision-making with a multidisciplinary heart team, and 40 patients elected to undergo one of the guideline-recommended septal reduction therapies (surgical myectomy [n = 32] or ASA [n = 8]).9 An additional 4 patients with significant coronary lesions felt to be the cause of symptoms underwent percutaneous coronary interventions and were excluded from the trial. The remaining patients with drug-refractory HOCM elected to enter the PIMSRA trial (NCT04355260 and NCT02888132). All patients signed informed consent after a discussion with the heart team and consideration of guideline recommended alternative therapies. The Institutional Ethics Committee (KY20162042-1) of Xijing Hospital approved the PIMSRA (Liwen procedure) in accordance with the ethical standards of the Declaration of Helsinki.10
Video. Percutaneous Intramyocardial Septal Radiofrequency Ablation (PIMSRA) .
This animation and additional live case show the steps to perform the novel PIMSRA procedure. With the patient under general anesthesia, real-time echocardiographic guidance is used to position a radiofrequency electrode needle using a puncture guide over the apex of the heart. The needle is inserted into the interventricular septum via a percutaneous transapical intramyocardial approach. High-frequency alternating current is applied, resulting in irreversible coagulation necrosis of the tissue and regional blood vessels. Basal and mid portions of anterior and posterior septum can be targeted to ensure efficacy. Contrast echocardiography is performed to assess the ablation region, and a comprehensive Doppler assessment is performed to assess hemodynamic improvement.
Echocardiography
Transthoracic echocardiography (TTE) was performed with the EPIQ 7C Ultrasound System (Philips Medical Systems) with a S5-1 and X5-1 transducer (1.0 to 5.0 MHz). Measurements of the septal thickness, LVOT diameter, systolic anterior motion of the mitral valve, mitral regurgitation, LVOT peak gradient, left atrial volume index, left ventricular end diastolic volume, left ventricular end systolic volume, and left ventricular ejection fraction were obtained according to the recommendations of the American Society of Echocardiography.11 LVOT diameter was measured at the narrowest point of apical 5- or 3-chamber view in systole on the echo. LVOT gradient was measured with color-guided continuous-wave Doppler.12,13 Exercise stress echocardiography was performed with a supine bicycle exercise (semi-recumbent and tilting bicycle Ergometer; Lode BV) according to a standard protocol14 for assessment of a provocable LVOT gradient and exercise time. The provoked peak gradient of all patients before and after the procedure was assessed with the stress echo.
Myocardial Contrast Echocardiography for Left Ventricular Chamber Opacification
The contrast agent used was 2 mL of sulfur hexafluoride microbubbles (SonoVue; Bracco), which was injected through a large peripheral vein. We gave a 0.5-mL bolus injection, then pushed the remaining 1.5 mL at the rate of 1 mL/min and added 5 mL normal saline to flush the tubing. Meanwhile, EPIQ 7C Ultrasound System (Philips Medical Systems) with a S5-1 and X5-1 transducer (1.0 to 5.0 MHz) was used to record echocardiographic images of different myocardial segments.
Preprocedural Computed Tomography Angiography
Retrospectively electrocardiogram-triggered spiral acquisition of cardiac computed tomography angiography imaging was performed with 128-slice dual-source computed tomography (Somatom Definition Flash; Siemens Healthcare). An intravenous bolus of 1 mL/kg of iopromide 370 (Bayer Schering Pharma) was administrated at a flow rate of 5 mL/s followed by 40 mL of a saline solution. All images were reconstructed with a slice thickness of 0.75 mm and increment of 0.5 mm and then transferred to an external workstation (Syngo MMWP VE 36A; Siemens Healthcare) for analysis.
Electrocardiogram and Rhythm Monitoring
Standard 12-lead electrocardiogram (ECG-1250P; Nihon Kohden) and 24-hour Holter (DMS300-4A; DM Software) were performed in all patients.
Genetic Testing
Genetic testing for known HCM variants was performed, and pathogenicity analysis was reported according to the American College of Medical Genetics and Genomics guidelines.15
PIMSRA
The patients placed in the left lateral decubitus position after general anesthesia were monitored with continuous electrocardiographic, blood pressure, and oxygen saturation. Under the real-time guidance of TTE (Figure 1), a radiofrequency electrode needle (17G, Cool-tip RF Ablation System; Medtronic Minimally Invasive Therapies or 18G, Liwen RF Radiofrequency Ablation System) was inserted into the hypertrophied septum percutaneously via the transapical intramyocardial approach: only the needle tip (not the insulating body) emits radiofrequency energy. Color Doppler TTE guidance was used intraprocedurally to avoid vascular injury at the point of needle insertion.
Figure 1. Percutaneous Intramyocardial Septal Radiofrequency Ablation (PIMSRA) Procedure Illustration and Echo Imaging.
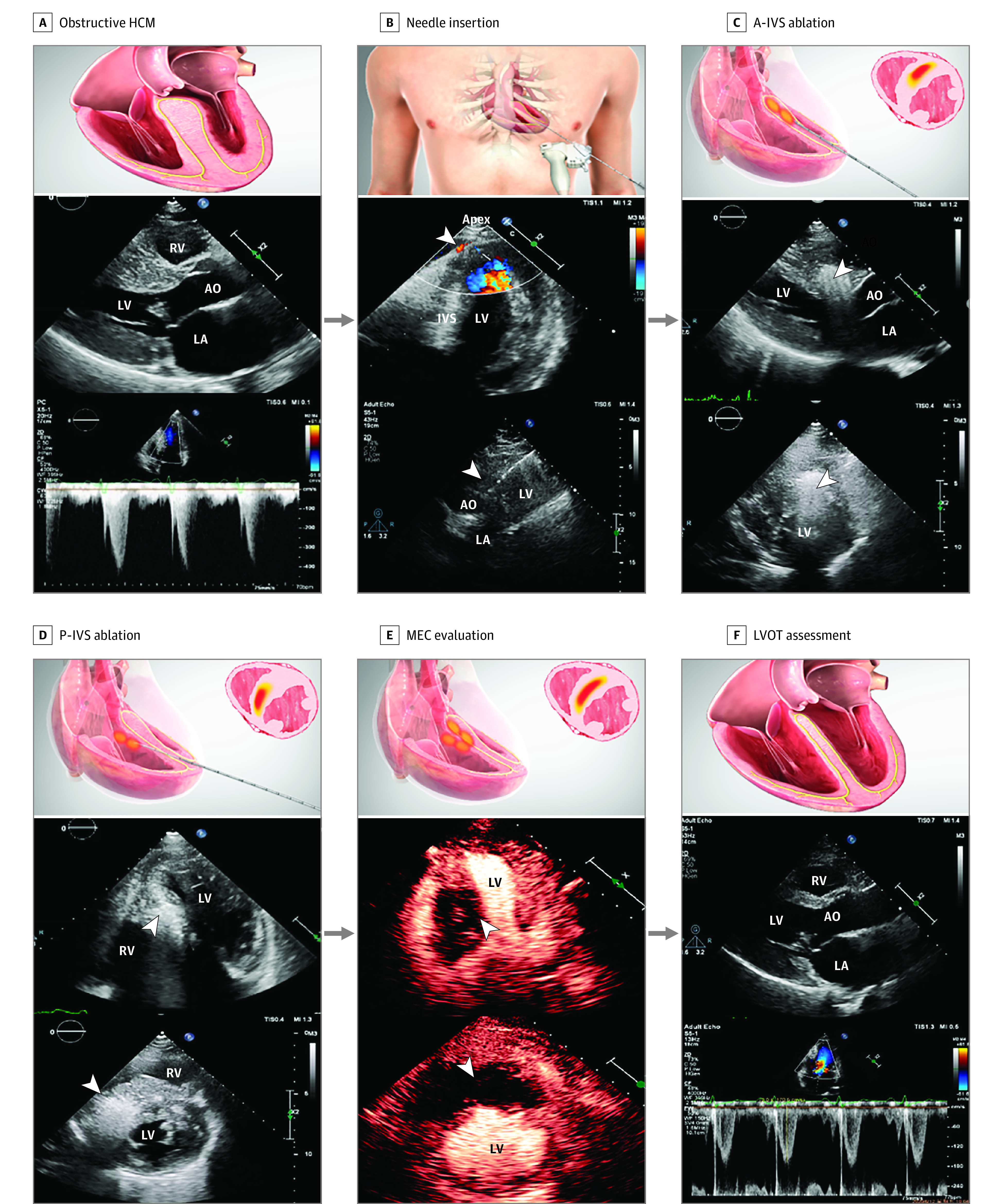
A, Before PIMSRA, the ventricular septum was hypertrophic and left ventricular outflow tract (LVOT) was obstructive. B, The needle was percutaneously inserted through the left ventricular (LV) apex, while using color Doppler guidance to avoid vascular injury. The arrowhead showed vessels at the apex and the needle in the intraventricular septum (IVS) through the guide frame. C, Basal and midanterior ventricular septum was ablated. The bright echo signal as the arrowhead showed was the ablation area. D, Then, the needle was withdrawn and repositioned in the hypertrophic posterior septum for ablation. E, After the basal and mid portion of anterior IVS (A-IVS) and posterior IVS (P-IVS) ablation, contrast echo was used to assess whether the ablation range was adequate. F, Nine months after PIMSRA, the ventricular septum became thinner and LVOT peak gradient decreased. AO indicates aorta; HCM, hypertrophic cardiomyopathy; LA, left atrium; MEC, myocardial echo contrast; RV, right ventricle.
Using the high-frequency alternating current from the front end of the radiofrequency needle, the ions in the myocardial cells were energized to generate heat. The local tissue temperatures recorded by the radiofrequency system could reach above 80 °C. The tissue around the radiofrequency electrode needle was dehydrated, resulting in irreversible coagulation necrosis of the tissue as well as coagulation of regional blood vessels resulting in reduced regional blood supply.16 The close circuit of the water cooling system was used to cool the radiofrequency needle. Once the tissue impedance rose to 150% of the baseline value, the radiofrequency machine output would automatically shut off and enter the hibernating state for 15 seconds. If a prolonged episode of heart block or tachyarrhythmia occurred, the ablation procedure was paused until normal rhythm resumed, either spontaneously or following lidocaine treatment.
For hypertrophy of both the anterior and posterior septum associated with LVOT obstruction, basal and mid portion of both regions were ablated to ensure efficacy. To protect the cardiac conduction system, the ablation area was 3 to 5 mm from the endocardium of both left and right ventricular cavities. After ablation, the radiofrequency needle was gently removed and local pressure was applied for 5 to 10 minutes to achieve hemostasis. The patient’s vital signs and symptoms were closely monitored, with echocardiographic imaging to assess for the development of a pericardial effusion. After at least 15 minutes of observation, a final TTE hemodynamic assessment was performed.
End Points
The primary end point was 30-day major adverse clinical events following the procedure including death, emergency surgery, significant pericardial effusion requiring pericardiocentesis or surgery, significant bleeding, and surgery-related stroke.
The secondary end points included (1) radiofrequency ablation system instruments reaching the desired treatment site and successfully completing ablation and removal of the system; (2) improvement of LVOT gradient by more than 50% within 90 days after operation; and (3) no major adverse events associated with instruments or surgery within 90 days of instrument use.
We established follow-up at immediately postprocedure, 1 week, and 1, 3, 6, and 12 months after PIMSRA and every 6 months thereafter with documentation of the physical examination, 12-lead electrocardiogram, dynamic electrocardiogram monitor, and TTE.
Statistical Analysis
SPSS statistical software, version 17.0 (SPSS Inc) was used for all statistical analysis. We presented baseline and in-hospital patient characteristics and follow-up data of those undergoing PIMSRA. Continuous normal distribution variables were presented as mean (SD) and comparison between pre- and postprocedure was analyzed by paired sample t test. Continuous non-normal distribution variables were presented as median (IQR) and comparison between pre- and postprocedure was analyzed by Wilcoxon signed rank test. Categorical variables were expressed as frequencies (percentages) and pre- and postprocedure comparison of binary scale variables was analyzed by the paired McNemar test. Ranked ordinal variables was analyzed by the Wilcoxon signed rank test. Statistical significance was presented with a 2-sided P value less than .05.
Results
Baseline Characteristics
The baseline characteristics of 200 patients with HOCM who underwent the PIMSRA are listed in Table 1. All patients were Chinese. The mean (SD) age was 46.9 (14.0) years and 125 (62.5%) were men. All patients had drug-refractory symptoms: 58 (29%) were categorized as NYHA II; 126 (63%), NYHA III; and 16 (8%), NYHA IV.
Table 1. Baseline Clinical Characteristics.
| Characteristic | No. (%) |
|---|---|
| Clinical characteristic | |
| Male | 125 (62.5) |
| Female | 75 (37.5) |
| Age, mean (SD), y | 46.9 (14.0) |
| BSA, mean (SD), m2 | 1.8 (0.2) |
| Clinical symptoms | |
| Shortness of breath | 151 (75.5) |
| Chest pain or tightness | 195 (97.5) |
| Syncope or presyncope | 78 (39.0) |
| NYHA functional classification | |
| II | 58 (29.0) |
| III | 126 (63.0) |
| IV | 16 (8.0) |
| Family history of HCM | 67 (33.5) |
| Medications | |
| β-Blocker | 188 (94.0) |
| Calcium channel blocker | 23 (11.5) |
| History of other cardiac procedure | |
| Alcohol septal ablation | 5 (2.5) |
| ICD implantation | 2 (1.0) |
| Genetic characteristics | |
| No. (%) | 193 (96.5) |
| Pathogenic/likely pathogenic | 89 (46.1) |
| MYH7 | 57 (64.0) |
| MYBPC3 | 29 (32.6) |
| TNNI3 | 2 (2.2) |
| PTPN11 | 1 (1.1) |
Abbreviations: BSA, body surface area; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter-defibrillator; MYBPC3, cardiac myosin-binding protein C variant; MYH7, beta-myosin heavy chain gene variant; NYHA, New York Heart Association; PTPN11, part of the RAS-MAPK signaling genes variant; TNNI3, cardiac troponin I variant.
Procedural Outcomes
Procedural, in-hospital, and 30-day parameters and clinical events are shown in Table 2. Radiofrequency ablation was applied a mean (SD) of 7.0 (3.0) times per each session. The mean (SD) total energy of ablation was 166.5 (138.6) kilojoules; mean (SD) total ablation time, 71.0 (34.0) minutes; mean (SD) maximum power, 60.0 (40.0) Watts; and mean (SD) power, 41.4 (31.4) Watts. The estimated ablation volume was 4.1 cm × 3.6 cm × 1.7 cm.
Table 2. Procedural, In-Hospital, and 30-Day Parameters and Clinical Events.
| Characteristic | No. (%) |
|---|---|
| Procedural ablation parameters, median (IQR) | |
| Total energy, kJ | 166.5 (138.6) |
| Mean ablation times, No. | 7.0 (3.0) |
| Total ablation time, min | 71.0 (34.0) |
| Maximum power, Watts | 60.0 (40.0) |
| Mean power, Watts | 41.4 (31.4) |
| Ablation range, mean (SD), cm | |
| Length | 4.1 (0.9) |
| Width | 3.6 (1.2) |
| Thickness | 1.7 (0.7) |
| Major adverse clinical events | 21 (10.5) |
| In-hospital and 30-d mortality | 2 (1.0) |
| Pericardial effusion | |
| Requiring surgery | 7 (3.5) |
| Requiring pericardiocentesis | 12 (6.0) |
| Procedure-related stroke | 0 |
| Bleeding | 0 |
| Stroke | 0 |
| Other procedural complications | |
| Right bundle branch block | 11 (5.5) |
| Left bundle branch block | 2 (1.0) |
| Permanent pacemaker after the procedure | 0 |
| Accelerated idioventricular rhythm | 45 (22.5) |
| Ventricular fibrillation requiring brief resuscitation | 2 (1.0) |
| Aneurysm of septal branch | 2 (1.0) |
| Other in-hospital and 30-d clinical events | |
| Infection | 0 |
| Ventricular tachycardia | 1 (0.5) |
| Permanent pacemaker implantation | 0 |
| Ventricular septal defect | 0 |
Thirty-day major adverse clinical event rate was 10.5% (n = 21): there were 2 in-hospital/30-day deaths (1.0%), 7 patients (3.5%) had pericardial effusion requiring mini thoracotomy in the setting of tamponade physiology, 12 patients (6%) had pericardial effusion requiring pericardiocentesis, and no other bleeding events or strokes. There were no clinically significant pericardial effusions during the procedure after institution of preprocedural computed tomography angiography planning and intraprocedural color Doppler guidance. One cardiac death occurred 1 week postprocedure owing to cardiogenic shock in the setting of severe, persistent systolic anterior motion of the mitral valve, which retrospectively may have been associated with excessive ablation and resulting myocardial edema. Another patient was found unresponsive in bed on day 6 after discontinuation of telemetry on postoperative day 5. The rapid response team defibrillation monitor showed asystole. This was then presumed to be sudden cardiac death due to either ventricular or bradycardia arrhythmia. Since this event, telemetry monitoring is performed throughout the hospital stay.
Right bundle branch block occurred intraprocedurally in 11 patients (5.5%), transient in 6 (3%), and permanent in 5 (2.5%). Other conduction disturbances included transient left bundle branch block in 2 (1.0%); transient asymptomatic accelerated idioventricular rhythm in 45 (22.5%); and ventricular fibrillation in 2 (1.0%) responding to defibrillation. Subsequent use of lower initial radiofrequency ablation energy (5-10 Watts) appeared to eliminate the occurrence of ventricular fibrillation. There were 2 patients (1%) who developed an aneurysm of the left anterior descending septal branch; 1 was successfully treated with coil occlusion, and the other recovered without treatment or other cardiac issues. The procedure was aborted prior to full septal ablation in 9 patients (4.5%) owing to intraoperative hypotension or ventricular arrhythmia.
Association of Treatment With Outcomes
Results of a median (IQR) follow-up of 19.0 (6-50) months are shown in Table 3. At the last follow-up, most patients had significant improvement in symptoms (percent with NYHA III/IV symptoms decreased from 71% to 4%). A reduction in symptoms of 1 or more classes occurred in 185 of 198 patients (93.4%). In the subgroup of patients with NYHA II symptoms, there was an improvement to NYHA I in 52 of 58 patients (89.7%). In patients who remained in NYHA class III, 2 of 8 (25%) had a reduction in symptoms of 1 class or more.
Table 3. Clinical Outcomes of PIMSRA at Last Follow-up.
| Variable | No. (%) | P value | |
|---|---|---|---|
| Baseline (n = 200) | At last follow-up (n = 198)a | ||
| Clinical results | |||
| NYHA functional classification | |||
| I | 0 | 167 (84.3) | <.001 |
| II | 58 (29.0) | 23 (11.6) | |
| III | 126 (63.0) | 8 (4.0) | |
| IV | 16 (8.0) | 0 | |
| 6-min Walk distance, mean (SD) | 422.3 (89.0) | 451.1 (81.9) | <.001 |
| Echocardiographic results | |||
| Maximum septal thickness, mean (SD), mm | 24.0 (5.1) | 17.3 (4.4) | <.001 |
| LVOT diameter, median (IQR), mm | 6.0 (3.0) | 11.0 (5.9) | <.001 |
| Mitral valve SAM grade | |||
| 0 | 18 (9.0) | 115 (58.1) | <.001 |
| 1 | 11 (5.5) | 34 (17.2) | |
| 2 | 65 (32.5) | 43 (21.7) | |
| 3 | 106 (53.0) | 6 (3.0) | |
| LVOT peak gradient, median (IQR), mm Hg | |||
| Resting | 79.0 (53.0) | 14.0 (24.0) | <.001 |
| Provoked | 127.0 (65.5) | 47.0 (76.5) | <.001 |
| Left atrial volume index, mean (SD), mL/m2 | 49.5 (15.0) | 35.9 (11.7) | <.001 |
| LVEDV, mean (SD), mL | 76.4 (17.1) | 78.0 (16.3) | .20 |
| LVESV, mean (SD), mL | 30.9 (8.5) | 31.9 (8.6) | .11 |
| LVEF, mean (SD), % | 60.2 (4.7) | 59.2 (3.8) | .008 |
| Exercise time, median (IQR), s | 360 (120) | 540 (225) | <.001 |
| Electrocardiographic results | |||
| Left bundle branch block | 1 (0.5) | 1 (0.5) | >.99 |
| Right bundle branch block | 10 (5.0) | 15 (7.6) | .06 |
| Atrioventricular block (all types) | 5 (2.5) | 5 (2.5) | >.99 |
| Nonsustained VT | 12 (6) | 16 (8.1) | .45 |
| Ventricular premature beat, median (IQR) | 11.0 (43.0) | 8.5 (43.5) | .82 |
Abbreviations: LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; LVOT, left ventricular outflow tract; NYHA, New York Heart Association; PIMSRA, percutaneous intramyocardial septal radiofrequency ablation; SAM, systolic anterior motion; VT, ventricular tachycardia.
Median (IQR) follow-up of 19.0 (6-50) months.
There was a significant increase in mean (SD) 6-minute walking distance from 422.3 (89.0) m to 451.1 (81.9) m (difference, −28.7 [95% CI, −40.9 to −16.6]; P < .001). LVOT peak gradient at rest was decreased from 79.0 (53.0) mm Hg to 14.0 (24.0) mm Hg (Figure 2A) and provoked peak gradient was decreased from 127.0 (65.5) mm Hg to 47.0 (76.5) mm Hg (P < .001) (Figure 2B). Mean (SD) maximum septal thickness was reduced from 24.0 (5.1) mm to 17.3 (4.4) mm (P < .001) (Figure 2C).
Figure 2. Patient-Level Outcomes for the Percutaneous Intramyocardial Septal Radiofrequency Ablation (PIMSRA) Procedure.
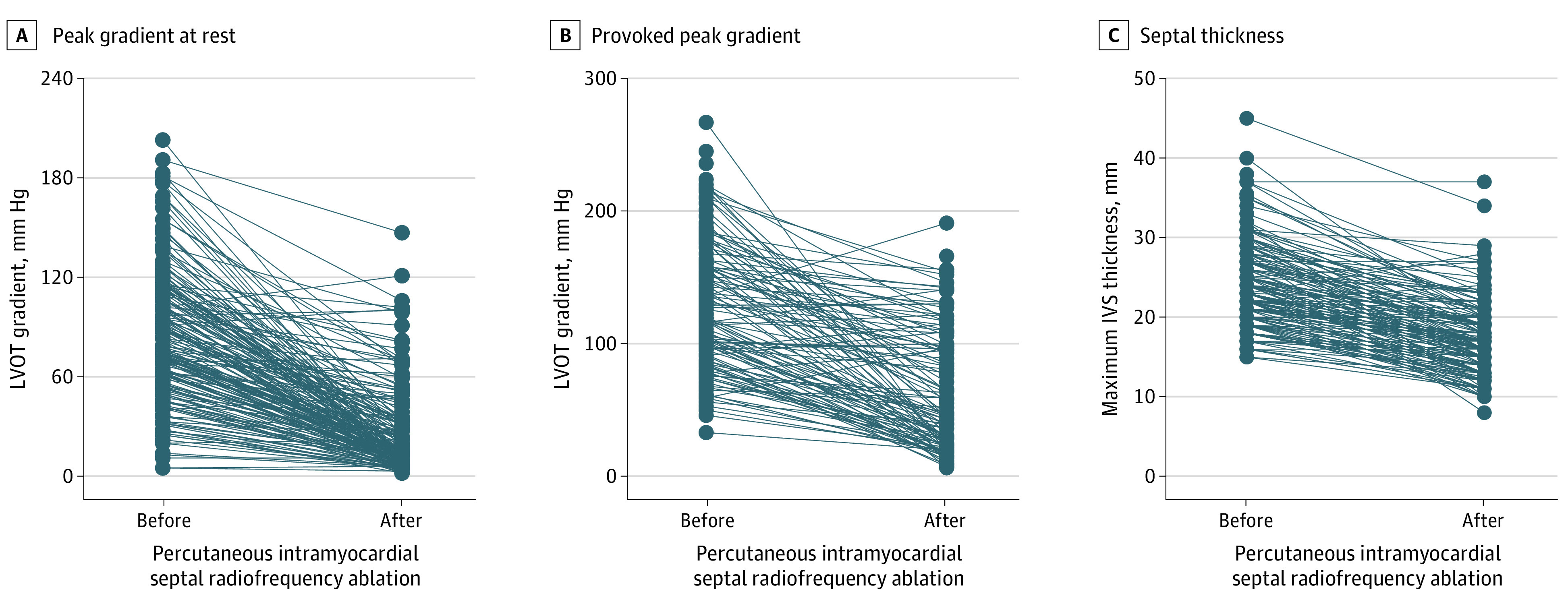
A, Left ventricular outflow tract (LVOT) resting peak gradient in the individual patients before and after PIMSRA. B, LVOT-provoked peak gradient in the individual patients before and after PIMSRA. C, Maximum intraventricular septal thickness in the individual patients before and after PIMSRA.
Discussion
The current study builds on the initial early feasibility report of the PIMSRA procedure by reporting the medium-term outcomes for 200 treated patients and is the largest study of radiofrequency ablation for treatment of HOCM reported to date and to our knowledge. The study showed (1) PIMSRA may be an effective nonsurgical therapy for reduction in LVOT gradients in medication-refractory HOCM, which is not dependent on coronary anatomy; (2) PIMSRA is associated with a low incidence of permanent conduction abnormalities with no pacemaker requirement; and (3) pericardial effusion is the most common procedural complication that has declined with improvements in procedural planning and technique.
Medical treatments of HOCM have limited success, reducing LVOT obstruction in less than 50% of patients resulting in a large patient population with drug-refractory symptoms.5 Although newer cardiac myosin inhibitor drugs hold promise,17,18 a substantial number of patients (approximately 25%) continue to have severe LVOT gradients of 50 mm Hg or higher and would likely require septal reduction therapy. Left ventricular septal myectomy continues to be the most efficacious and safest septal reduction therapy, although mortality remains similar between surgery and ASA.19 Nonetheless, there is limited access to centers with adequate surgical expertise and patient reluctance to undergo open heart procedures, making a less invasive procedure attractive. Nonsurgical septal reduction using alcohol injection into the first major septal artery was developed in 1995 as an alternative to surgery in drug-refractory patients,20 and ASA has become an acceptable alternative for patients who are not ideal candidates for surgical myectomy.1,9 Its main limitation is the reliance on the size and distribution of the septal perforator branches.21 Injecting ethanol into a proximal branch of the left anterior descending artery does not guarantee the distribution of alcohol will be confined to the basal septum and is likely the basis for the 10% to 15% rate of complete heart block and permanent pacemaker implantation.22,23,24 The largest multinational ASA registry (the Euro-ASA registry) included a total of 1275 highly symptomatic patients treated with ASA and reported a 30-day post-ASA mortality of 1%,25 comparable with PIMSRA. The most frequent complication of ASA was transient periprocedural complete heart block in 468 patients (37%) at 30 days after ASA, with 151 (12%) subsequently requiring permanent pacemaker implantation. More recent studies suggest the pacemaker implantation rates are as low as 6.1% for patients undergoing ASA and 5.0% in patients undergoing surgical myectomy.19 Other arrhythmic complications of ASA include supraventricular tachycardia, ventricular tachycardia, and bradyarrhthmias.26 Patients undergoing ASA have intraprocedural or follow-up rates of ventricular arrhythmias between 1.8% and 7%.24,26,27,28 Finally, a predictor of poor outcomes is the need for a repeat ASA, which may be required in 7% to 13% of patients.19,24
The PIMSRA procedure can precisely target the region causing the obstruction while avoiding the right and left endocardial borders to protect the conduction system and prevent excessive thinning/scarring of the myocardium. Although there was permanent right bundle branch block in 5.5% and left bundle branch block in 1.0% of patients, no patients required permanent pacemaker placement, an improvement over both ASA and surgical myectomy rates. We encountered 2 cases of intraprocedural ventricular fibrillation, successfully cardioverted to sinus rhythm, which we believe was caused by high initial radiofrequency ablation power output and excessive ventricular stimulation. Since reducing the starting radiofrequency energy output from 40 Watts to 5 to 10 Watts, ventricular fibrillation has not been seen. One of the 2 deaths within 30 days occurred while the patient was asleep, raising the suspicion of malignant tachyarrhythmia or bradyarrhythmia, both of which have been documented in patients undergoing ASA days to months following the procedure.26 Although we have not seen any malignant arrhythmias in any monitored patients after PIMSRA in our database, the PIMSRA procedure does cause intramyocardial scar formation during the healing process16; thus long-term rhythm monitoring may be warranted. Finally, the procedure was aborted prior to full septal ablation in 4.5% of patients owing to intraoperative hypotension or ventricular arrhythmia. These patients may require repeat PIMSRA procedures or surgical myectomy in the future if the LVOT obstruction and symptoms are not adequately relieved.
Although other transapical procedures have been associated with poor outcomes,29 the PIMSRA procedure uses a 17- to 18-gauge needle (approximately 1.2-1.4 mm outer diameter) and the needle remains intramyocardial. Thus, the injury to the myocardium from the needle remains limited. Nonetheless, this apical approach resulted in bleeding and pericardial effusion primarily early in our experience, thus attributable in part to learning curve, but also preprocedural planning. We now use preprocedural computed tomography angiography and intraoperative color Doppler flow imaging during needle insertion to determine the appropriate site of needle entry allowing us to minimize vessel injury. In the last 120 cases, no patient required mini thoracotomy to drain the pericardial effusion.
Despite a significant overall reduction in LVOT gradient, about 4% of patients still had symptoms of exertional chest tightness and shortness of breath (NYHA class III), which was mainly associated with incomplete relief of LVOT obstruction but not to excessive septal thickness. These patients theoretically could be retreated or alternatively have surgical myectomy. One of the 2 patients who died within 30 days after the procedure had significant persistent systolic anterior motion, despite the initiation of fluid therapy and vasoconstrictors. Systolic anterior motion is a well-recognized cause for unexplained or sudden hypotension in the cardiac perioperative setting.30,31,32 The 2020 American Heart Association/American College of Cardiology Guideline for HCM emphasized maximizing preload and afterload to avoid hypotension,1 which may also apply to patients following the PIMSRA procedure.
Although 29% of patients were in NYHA class II before PIMSRA, they had limiting angina without epicardial coronary artery disease on coronary computed tomography angiography and likely related to drug-refractory severe LVOT obstruction. Symptoms improved in 89.7% of patients with NYHA II symptoms supporting the use of PIMSRA despite absence of NYHA class III and IV symptoms. Similar to our results, the Euro-ASA registry published their outcomes in 161 patients with NYHA class II symptoms and severe LVOT gradients treated with ASA.33 In that study, the median (IQR) follow-up was 4.8 (1.7-8.5) years with a sustained reduction in LVOT gradient (mean [SD], 63 [32] to 15 [19] mm Hg; P < .01) and reduction in NYHA class from a mean (SD) of 2.0 (0) to 1.3 (0.1) (P < .01).
Another transcatheter approach to septal reduction therapy is endocardial radiofrequency ablation of septal hypertrophy.6,7 Lawrenz et al6 reported their experience in 19 patients, 9 undergoing left ventricular septal ablation (using a retrograde transaortic approach) and 10 with right ventricular septal ablation (using an inferior caval approach). Although successfully reducing the LVOT gradient and improving symptoms, there was a 21% rate of complete atrioventricular block requiring permanent pacemaker implantation. Other investigators have reported similar results using an antegrade, transseptal approach to the left ventricular endocardium34 with a 17% rate of permanent pacemaker for complete atrioventricular block. However, using similar methods, a series of 20 patients35 and 25 patients7 have recently reported successful reduction in LVOT gradient despite no significant reduction in septal wall thickness, with no serious complications or complete atrioventricular block. Given these discordant outcomes, endocardial radiofrequency ablation of septal hypertrophy requires further study.
Finally, our genetic analysis of the study cohort showed a high percentage (67%) with the MYH7 variant. A recent meta-analysis suggested the mean frequency of the MYH7 variant was 14%.36 Many other authors have suggested that the MYH7 variant is associated with more severe disease with 1 study suggesting presence of systolic anterior motion was significantly more frequent in patients carrying the MYH7 variant (33% with MYH7 vs 10% with MYBPC3; P = .025).37 The high percentage of MYH7 variant in our patient population may be due both ethnic/racial differences in the prevalence of this variant but also selection bias because our patient population presented to the hospital for diagnosis and treatment and thus may represent a more severe form of the disease.
Limitations
Although the procedure has been standardized and is reproducible, the current study represents the results of a single site that developed this innovative procedure and thus may not be generalizable to other sites. Because this was a single-arm observational study from a single center without a comparison group, further studies are clearly warranted to substantiate our findings. Although we have accumulated significant experience in PIMSRA to treat patients with HOCM with satisfactory outcomes within our research protocol, it has been done in a limited number of patients with midterm follow-up. It will be important to follow patients for more than 5 years to observe the risks of cardiac arrhythmia based on the European Society of Cardiology and American Heart Association guidelines. Sequential magnetic resonance imaging studies are necessary in these patients to assess the degree of myocardial scar induced by this procedure.
Conclusions
In this single-site study, PIMSRA in patients with HOCM may be a safe and effective procedure for relief of LVOT obstruction and symptoms at midterm follow-up, supporting the initiation of a randomized clinical trial. Until further data are available comparing these results to well-established alternatives such as surgical myectomy or ASA procedures, this procedure remains investigational.
eFigure. Study flow chart
References
- 1.Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142(25):e533-e557. [DOI] [PubMed] [Google Scholar]
- 2.Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi: 10.1016/j.jacc.2015.01.019 [DOI] [PubMed] [Google Scholar]
- 3.Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295-303. doi: 10.1056/NEJMoa021332 [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic obstructive cardiomyopathy: surgical myectomy and septal ablation. Circ Res. 2017;121(7):771-783. doi: 10.1161/CIRCRESAHA.116.309348 [DOI] [PubMed] [Google Scholar]
- 5.Maron MS, Ommen SR. Exploring new and old therapies for obstructive hypertrophic cardiomyopathy: mavacamten in perspective. Circulation. 2021;143(12):1181-1183. doi: 10.1161/CIRCULATIONAHA.120.051330 [DOI] [PubMed] [Google Scholar]
- 6.Lawrenz T, Borchert B, Leuner C, et al. Endocardial radiofrequency ablation for hypertrophic obstructive cardiomyopathy: acute results and 6 months’ follow-up in 19 patients. J Am Coll Cardiol. 2011;57(5):572-576. doi: 10.1016/j.jacc.2010.07.055 [DOI] [PubMed] [Google Scholar]
- 7.Kong L, Zhao Y, Pan H, Ma J, Qian J, Ge J. A modified endocardial radiofrequency ablation approach for hypertrophic obstructive cardiomyopathy guided by transthoracic echocardiography: a case series. Ann Transl Med. 2021;9(12):1006. doi: 10.21037/atm-21-2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Li J, Zuo L, et al. Percutaneous intramyocardial septal radiofrequency ablation for hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2018;72(16):1898-1909. doi: 10.1016/j.jacc.2018.07.080 [DOI] [PubMed] [Google Scholar]
- 9.Elliott PM, Anastasakis A, Borger MA, et al. ; Authors/Task Force members . 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733-2779. doi: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 10.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 12.Panza JA, Petrone RK, Fananapazir L, Maron BJ. Utility of continuous wave Doppler echocardiography in the noninvasive assessment of left ventricular outflow tract pressure gradient in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1992;19(1):91-99. doi: 10.1016/0735-1097(92)90057-T [DOI] [PubMed] [Google Scholar]
- 13.Sasson Z, Yock PG, Hatle LK, Alderman EL, Popp RL. Doppler echocardiographic determination of the pressure gradient in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1988;11(4):752-756. doi: 10.1016/0735-1097(88)90207-0 [DOI] [PubMed] [Google Scholar]
- 14.Lancellotti P, Pellikka PA, Budts W, et al. The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30(2):101-138. doi: 10.1016/j.echo.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 15.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu F, Fu J, Hsi D, et al. Percutaneous intramyocardial septal radiofrequency ablation for interventricular septal reduction: an ovine model with 1-year outcomes. Cardiology. 2020;145(1):53-62. doi: 10.1159/000502973 [DOI] [PubMed] [Google Scholar]
- 17.Saberi S, Cardim N, Yamani M, et al. Mavacamten favorably impacts cardiac structure in obstructive hypertrophic cardiomyopathy: EXPLORER-HCM cardiac magnetic resonance substudy analysis. Circulation. 2021;143(6):606-608. doi: 10.1161/CIRCULATIONAHA.120.052359 [DOI] [PubMed] [Google Scholar]
- 18.Chuang C, Collibee S, Ashcraft L, et al. Discovery of aficamten (CK-274), a next-generation cardiac myosin inhibitor for the treatment of hypertrophic cardiomyopathy. J Med Chem. 2021;64(19):14142-14152. doi: 10.1021/acs.jmedchem.1c01290 [DOI] [PubMed] [Google Scholar]
- 19.Kimmelstiel C, Zisa DC, Kuttab JS, et al. Guideline-based referral for septal reduction therapy in obstructive hypertrophic cardiomyopathy is associated with excellent clinical outcomes. 2019;12(7):e007673. doi: 10.1161/CIRCINTERVENTIONS.118.007673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet. 1995;346(8969):211-214. doi: 10.1016/S0140-6736(95)91267-3 [DOI] [PubMed] [Google Scholar]
- 21.Spirito P, Rossi J, Maron BJ. Alcohol septal ablation: in which patients and why? Ann Cardiothorac Surg. 2017;6(4):369-375. doi: 10.21037/acs.2017.05.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald P, Kusumoto F. The effects of septal myectomy and alcohol septal ablation for hypertrophic cardiomyopathy on the cardiac conduction system. J Interv Card Electrophysiol. 2018;52(3):403-408. doi: 10.1007/s10840-018-0433-0 [DOI] [PubMed] [Google Scholar]
- 23.Liebregts M, Vriesendorp PA, Steggerda RC, et al. Effect of alcohol dosage on long-term outcomes after alcohol septal ablation in patients with hypertrophic cardiomyopathy. Catheter Cardiovasc Interv. 2016;88(6):945-952. doi: 10.1002/ccd.26448 [DOI] [PubMed] [Google Scholar]
- 24.Nagueh SF, Groves BM, Schwartz L, et al. Alcohol septal ablation for the treatment of hypertrophic obstructive cardiomyopathy. a multicenter North American registry. J Am Coll Cardiol. 2011;58(22):2322-2328. doi: 10.1016/j.jacc.2011.06.073 [DOI] [PubMed] [Google Scholar]
- 25.Veselka J, Jensen MK, Liebregts M, et al. Long-term clinical outcome after alcohol septal ablation for obstructive hypertrophic cardiomyopathy: results from the Euro-ASA registry. Eur Heart J. 2016;37(19):1517-1523. doi: 10.1093/eurheartj/ehv693 [DOI] [PubMed] [Google Scholar]
- 26.Bleszynski PA, Goldenberg I, Fernandez G, et al. Risk of arrhythmic events after alcohol septal ablation for hypertrophic cardiomyopathy using continuous implantable cardiac monitoring. Heart Rhythm. 2021;18(1):50-56. doi: 10.1016/j.hrthm.2020.08.013 [DOI] [PubMed] [Google Scholar]
- 27.Balt JC, Wijffels MC, Boersma LV, Wever EF, ten Berg JM. Continuous rhythm monitoring for ventricular arrhythmias after alcohol septal ablation for hypertrophic cardiomyopathy. Heart. 2014;100(23):1865-1870. doi: 10.1136/heartjnl-2014-305593 [DOI] [PubMed] [Google Scholar]
- 28.Sorajja P, Valeti U, Nishimura RA, et al. Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2008;118(2):131-139. doi: 10.1161/CIRCULATIONAHA.107.738740 [DOI] [PubMed] [Google Scholar]
- 29.Banks A, Gaca J, Kiefer T. Review of alternative access in transcatheter aortic valve replacement. Cardiovasc Diagn Ther. 2020;10(1):72-82. doi: 10.21037/cdt.2019.10.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381(9862):242-255. doi: 10.1016/S0140-6736(12)60397-3 [DOI] [PubMed] [Google Scholar]
- 31.Makhija N, Magoon R, Balakrishnan I, Das S, Malik V, Gharde P. Left ventricular outflow tract obstruction following aortic valve replacement: a review of risk factors, mechanism, and management. Ann Card Anaesth. 2019;22(1):1-5. doi: 10.4103/aca.ACA_226_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujita Y, Kagiyama N, Sakuta Y, Tsuge M. Sudden hypoxemia after uneventful laparoscopic cholecystectomy: another form of SAM presentation. BMC Anesthesiol. 2015;15:51. doi: 10.1186/s12871-015-0031-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veselka J, Faber L, Liebregts M, et al. Outcome of alcohol septal ablation in mildly symptomatic patients with hypertrophic obstructive cardiomyopathy: a long-term follow-up study based on the Euro-Alcohol Septal Ablation Registry. J Am Heart Assoc. 2017;6(5):e005735. doi: 10.1161/JAHA.117.005735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crossen K, Jones M, Erikson C. Radiofrequency septal reduction in symptomatic hypertrophic obstructive cardiomyopathy. Heart Rhythm. 2016;13(9):1885-1890. doi: 10.1016/j.hrthm.2016.04.018 [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, Qiu H, Jiang R, et al. Selective interventricular septal radiofrequency ablation in patients with hypertrophic obstructive cardiomyopathy: who can benefit? Front Cardiovasc Med. 2021;8:743044. doi: 10.3389/fcvm.2021.743044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sedaghat-Hamedani F, Kayvanpour E, Tugrul OF, et al. Clinical outcomes associated with sarcomere mutations in hypertrophic cardiomyopathy: a meta-analysis on 7675 individuals. Clin Res Cardiol. 2018;107(1):30-41. doi: 10.1007/s00392-017-1155-5 [DOI] [PubMed] [Google Scholar]
- 37.Velicki L, Jakovljevic DG, Preveden A, et al. Genetic determinants of clinical phenotype in hypertrophic cardiomyopathy. BMC Cardiovasc Disord. 2020;20(1):516. doi: 10.1186/s12872-020-01807-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Study flow chart


