Abstract
Silver resistance of sensitive Escherichia coli J53 and resistance plasmid-containing J53(pMG101) was affected by halides in the growth medium. The effects of halides on Ag+ resistance were measured with AgNO3 and silver sulfadiazine, both on agar and in liquid. Low concentrations of chloride made the differences in MICs between sensitive and resistant strains larger. High concentrations of halides increased the sensitivities of both strains to Ag+.
Silver salts and compounds are used widely as environmental biocides and as clinical antimicrobial agents (2, 7, 10, 13, 17). Ag+-resistant bacteria have been isolated (2, 8, 18), and resistance has sometimes been plasmid linked (5, 12, 19). However, most reports have been preliminary (e.g., references 5, 8, and 16) and the existence of silver-resistant bacteria in clinics has been questioned (10, 17), largely because of the compounding effects of environmental factors on toxicity and resistance (2, 3). Silver-binding components such as microbial biomass, proteins, lignin, and chloride are such factors. Among silver salts, AgNO3 is water soluble, Ag2SO4 and Ag-acetate are sparingly soluble, and AgCl, AgBr, AgI, and Ag2S are basically insoluble (4).
The purpose of this report is to set out easy-to-use conditions for measuring silver sensitivity and resistance in familiar and widely used media, Luria-Bertani (LB) agar and broth (14), so as to facilitate wider identification of silver resistance in nature.
Metal resistances including silver resistance are found in environments exposed to compounds that might provide for selection (3, 7, 17). Environmental toxicity of heavy metals to microorganisms is affected by both biotic and abiotic factors (3, 13, 16). These factors, for example, alter the bioavailability of metal ions (1, 3, 15). For Hg2+, and as we will show for Ag+ below, addition of Cl− increases toxicity to bacteria (6), presumedly by increasing membrane permeability (11). The amount of bioavailable Hg2+ decreased when Cl− levels exceeded 1 mM (1). This reduction was suggested to be due to an increase in the proportion of negatively charged mercury or Hg(II) complexes (HgCl3−/HgCl42− [1]). Similarly, in early and preliminary studies, the difference between Ag+-sensitive and -resistant cells was most clear in the presence of Cl−, while in the absence of Cl−, cells with or without the resistance plasmid were both sensitive to Ag+ (16).
The genes for Ag+ resistance have been identified recently (9). The cluster of seven genes (and two open reading frames of unknown function) is organized into three divergently transcribed units. The gene products are tentatively identified by homologies with other available sequences, but frequently these are also for proteins that have not been directly isolated. SilE is a periplasmic metal-binding protein that has been purified and measured for Ag+-binding properties (9). SilS and SilR are a presumed two-component membrane sensor and transcriptional responder, and SilCBA and SilP are a presumed Ag+ efflux chemiosmotic cation-proton antiporter and a P-type ATPase, respectively.
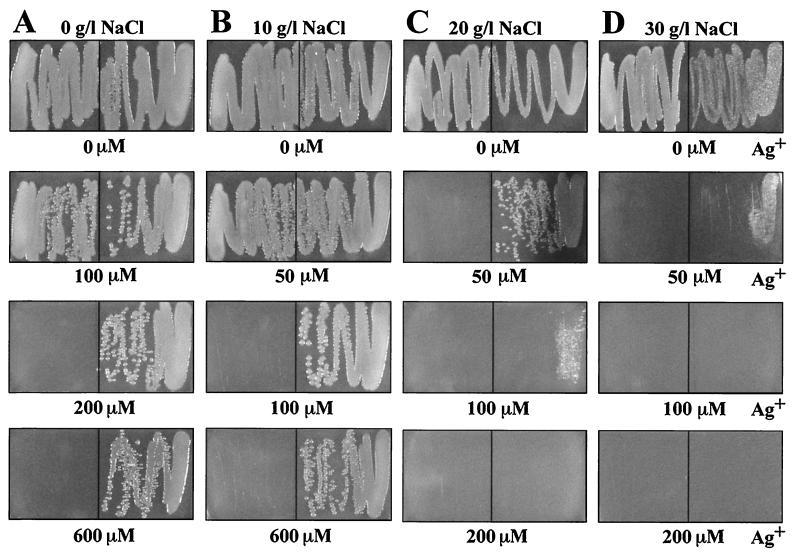
The effects of halide ions on Ag+ resistance of Escherichia coli K-12 strains J53 (Ag+ sensitive) and J53(pMG101) (Ag+ resistant) (9, 12) were measured on LB agar (14) streaked with log-phase cultures at a density of 200 Klett turbidity units (which corresponds to 2 g [wet weight] of cells per liter) (see Fig. 1 and 2). Since Ag+ is bactericidal rather than bacteriostatic, as are some other toxic heavy metals, the numbers of cells streaked and their growth phase have great influence on growth appearing on the agar plate (reference 16 and data not shown). NaCl alone, without Ag+, was toxic at high concentrations: at 40 g of NaCl per liter, the resistant J53(pMG101) cells showed reduced growth whereas the sensitive strain J53 reproducibly showed unimpaired growth (data not shown). A similar difference in NaCl sensitivity was found in liquid media (see below). We do not know whether this enhanced sensitivity to NaCl is associated with the silver resistance determinant or another region on the 180-kb (9) plasmid. In LB agar with added Ag+ and no added NaCl, E. coli J53 grew with up to 100 μM Ag+ and J53(pMG101) grew at 600 μM Ag+ (Fig. 1A). E. coli J53 grew with up to 50 μM Ag+ with 10 g of NaCl per liter (171 mM [Fig. 1B]), but there was no growth at 50 μM Ag+ with higher NaCl levels (Fig. 1C and D). Growth of E. coli J53(pMG101) was barely reduced at 600 μM Ag+ and 10 g of NaCl per liter (Fig. 1B), but in 20 g of NaCl per liter there was no growth at 200 μM Ag+ and a marked reduction in growth at 100 μM Ag+ (Fig. 1C). In 30 g of NaCl per liter, slight growth of E. coli J53(pMG101) was observed only at 50 μM Ag+ (Fig. 1D). These results on agar media (Fig. 1) were quantitatively reproducible from experiment to experiment and were consistent with those obtained in liquid media (see below). It seemed likely that the effects of NaCl on silver toxicity result from the low solubility of AgCl (solubility product, 1.56 × 10−10 mol/liter [20]) and the formation of water-soluble complex anions, AgCl2− and AgCl32− (4), at higher chloride levels. Similar chemistry occurs with the other halides, bromide and iodide (solubility products of AgBr and AgI, 7.7 × 10−13 and 1.5 × 10−16 mol/liter, respectively [20]), and formation of anionic complexes (AgX2− and AgX32−, where X is Cl, Br, or I) with relative stabilities of I− > Br− > Cl− (4). In addition to the anionic silver complexes, cationic species (Ag2X+ and Ag3X2+) are formed in aqueous solutions (4). The water-soluble ionic Ag-halide complexes might have increased access to the cell membrane, resulting in increased bioavailability of Ag+. At low halide levels, silver is excluded by precipitating as AgX, but at higher concentrations there is an increase in the proportion of accessible anionic complexes, resulting in decreased Ag+ resistance.
FIG. 1.
Growth of E. coli J53 (sensitive, left) and J53(pMG101) (resistant, right) on LB agar with NaCl and AgNO3 after 20 h at 37°C. LB was supplemented with AgNO3 and no added NaCl (A), AgNO3 and 10 g of NaCl per liter (B), AgNO3 and 20 g of NaCl per liter (C), and AgNO3 and 30 g of NaCl per liter (D). The numbers below the panels represent the AgNO3 concentration added to each petri dish.
FIG. 2.
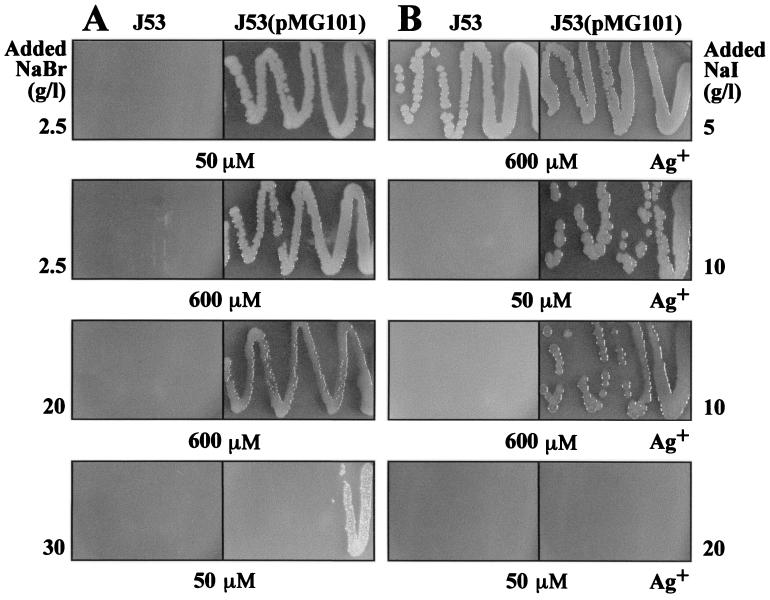
Growth of E. coli J53 and J53(pMG101) on LB agar plates supplemented with NaBr and AgNO3 (A) or NaI and AgNO3 (B). The numbers on the sides of the panels represent the NaBr or NaI concentration, and the numbers below the panels represent the AgNO3 concentration added to each petri dish.
Growth experiments were conducted with NaBr- and NaI-supplemented LB agar and AgNO3 (Fig. 2). The Ag+ sensitivity of E. coli J53 increased with the addition of low levels (2.5 g/liter) of NaBr (Fig. 2A), although NaBr alone was not toxic up to 30 g/liter (data not shown). A high level (30 g/liter) of NaBr reduced the Ag+ resistance of J53(pMG101) substantially (Fig. 2A), as was found with 30 g of NaCl per liter (Fig. 1D). The addition of 5 g of NaI per liter (35 mM) reduced the Ag+ sensitivity of E. coli J53 (Fig. 2B), allowing growth in 0.6 mM Ag+, presumably by removing the Ag+ as precipitated AgI. Higher I− levels (10 or 20 g/liter [Fig. 2B]) increased the sensitivity of the resistant strain, presumably by solubilizing Ag+ in a more “bioavailable” anionic complex.
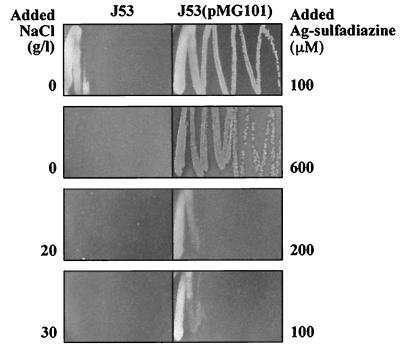
Since Ag-sulfadiazine is the primary silver compound in clinical use (7, 13), it is appropriate to measure differences between AgNO3 and Ag-sulfadiazine. Sulfadiazine in this compound is thought to function not as an antibiotic, but rather to complex silver in a water-insoluble form that is “slow released” for bioactivity (2, 13). The blackening on the skin from Ag+ reduction with AgNO3 is not seen with Ag-sulfadiazine. In the absence of added NaCl, Ag-sulfadiazine was slightly more toxic than AgNO3 (compare Fig. 3 with Fig. 1A). Growth of sensitive E. coli J53 was reduced at 100 μM Ag-sulfadiazine, and resistant J53(pMG101) grew at up to 600 μM Ag-sulfadiazine. The toxicity of Ag-sulfadiazine to sensitive and resistant cells increased with the addition of NaCl (Fig. 3), as had been the case with AgNO3 (Fig. 1). Comparing the toxicity of AgNO3 with that of Ag-sulfadiazine, the latter was less (roughly twofold) toxic after the addition of NaCl (compare Fig. 3 with Fig. 1). For example, growth of resistant J53(pMG101) was reduced at 200 μM Ag-sulfadiazine, compared to 100 μM AgNO3 (with 20 g of NaCl per liter), and growth of resistant J53(pMG101) occurred at 100 μM Ag-sulfadiazine, compared to 50 μM AgNO3 (with 30 g of NaCl per liter [Fig. 3]).
FIG. 3.
Growth of E. coli J53 and J53(pMG101) on LB agar supplemented with NaCl and Ag-sulfadiazine. The numbers on the sides of the panels represent the NaCl or Ag-sulfadiazine concentration added to each petri dish.
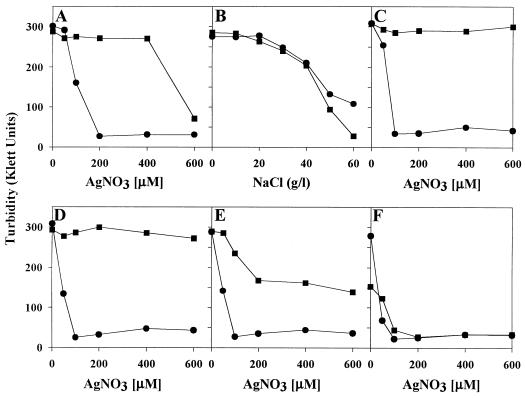
Although growth on agar plates is the more ready means of distinguishing Ag+-sensitive from Ag+-resistant E. coli (Fig. 1 to 3), liquid growth experiments showed basically similar results (Fig. 4). The effects of chloride ions on Ag+ resistance of E. coli J53 and J53(pMG101) were measured in LB broth (14) containing NaCl and/or AgNO3, inoculated with 2 Klett turbidity units (20 μg [wet weight] of cells per ml) of log-phase cells. Low NaCl levels enhanced Ag+ resistance of E. coli J53(pMG101) from 400 μM Ag+ to more than 600 μM Ag+ (Fig. 4A, C, and D). Growth of sensitive E. coli was unaffected at 50 μM Ag+, but turbidity was reduced at 100 μM Ag+. With added higher NaCl concentrations, there was reduced growth of sensitive cells at 50 μM Ag+ (Fig. 4D to F). Addition of 5 or 10 g of NaCl per liter (85 or 171 mM) to LB allowed growth of E. coli J53(pMG101) in 600 μM Ag+ (Fig. 4C and D), but growth was reduced by Ag+ at higher levels (20 or 30 g/liter) of NaCl (Fig. 4E and F). In LB liquid cultures, NaCl (50 or 60 g/liter) by itself, without added silver, was more toxic for strain J53(pMG101) than for the plasmidless strain J53 (Fig. 4B).
FIG. 4.
Growth of E. coli J53 (•) and J53(pMG101) (■) in LB liquid broth. LB media were supplemented with AgNO3 (no added NaCl) (A), NaCl (no added AgNO3) (B), AgNO3 and 5 g of NaCl per liter (C), AgNO3 and 10 g of NaCl per liter (D), AgNO3 and 20 g of NaCl per liter (E), and AgNO3 and 30 g of NaCl per liter (F). Turbidity was measured after 20 h of growth at 37°C by a Klett-Summerson colorimeter with a Kodak 56 green filter.
These results provide a basis for measurement of Ag+ sensitivity and resistance by bacterial isolates, which is useful since silver compounds are increasingly being used as microbicidal agents (2, 10, 13, 17). Environmental silver-binding components are quite diverse and radically affect the proportion of silver that is bioavailable. In clinical settings, Cl− and proteins bind Ag+; in soil and most aqueous environments, Cl− and organic S and N ligands predominate for Ag(I) binding.
Acknowledgments
This work was supported by a grant from the Department of Energy.
We thank A. O. Summers (University of Georgia, Athens) for E. coli J53(pMG101) and Portia Harris (Xavier University, New Orleans, La.) for assistance during this work.
REFERENCES
- 1.Barkay T, Gillman M, Turner R R. Effects of dissolved organic carbon and salinity on bioavailability of mercury. Appl Environ Microbiol. 1997;63:4267–4271. doi: 10.1128/aem.63.11.4267-4271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clement J L, Jarrett P S. Antibacterial silver. Metal-Based Drugs. 1994;1:467–482. doi: 10.1155/MBD.1994.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins Y E, Stotzky G. Factors affecting the toxicity of heavy metals to microbes. In: Beveridge T J, Doyle R J, editors. Metal ions and bacteria. New York, N.Y: John Wiley and Sons, Inc.; 1989. pp. 31–90. [Google Scholar]
- 4.Cotton F A, Wilkinson G. Advanced inorganic chemistry. 5th ed. New York, N.Y: John Wiley and Sons, Inc.; 1988. Silver and gold: group IB(11) pp. 937–949. [Google Scholar]
- 5.Deshpande L M, Chopade B A. Plasmid mediated silver resistance in Acinetobacter baumannii. Biometals. 1994;7:49–56. doi: 10.1007/BF00205194. [DOI] [PubMed] [Google Scholar]
- 6.Farrell R E, Germida J J, Huang P M. Biotoxicity of mercury as influenced by mercury(II) speciation. Appl Environ Microbiol. 1990;56:3006–3016. doi: 10.1128/aem.56.10.3006-3016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George N, Faoagali J, Muller M. Silvazine™ (silver sulfadiazine and chlorhexidine) activity against 200 clinical isolates. Burns. 1997;23:493–495. doi: 10.1016/s0305-4179(97)00047-8. [DOI] [PubMed] [Google Scholar]
- 8.Goddard P A, Bull A T. The isolation and characterization of bacteria capable of accumulating silver. Appl Microbiol Biotechnol. 1989;31:308–313. [Google Scholar]
- 9.Gupta, A., K. Matsui, J.-F. Lo, and S. Silver. Molecular basis for resistance to silver cations in Salmonella. Nature Med., in press. [DOI] [PubMed]
- 10.Gupta A, Silver S. Silver as a biocide: will resistance become a problem? Nat Biotechnol. 1998;16:888. doi: 10.1038/nbt1098-888. [DOI] [PubMed] [Google Scholar]
- 11.Gutknecht J. Inorganic mercury (Hg2+) transport through lipid bi-layer membranes. J Membr Biol. 1981;61:61–66. [Google Scholar]
- 12.McHugh S L, Moellering R C, Hopkins C C, Swartz M N. Salmonella typhimurium resistant to silver nitrate, chloramphenicol, and ampicillin. Lancet. 1975;i:235–240. doi: 10.1016/s0140-6736(75)91138-1. [DOI] [PubMed] [Google Scholar]
- 13.Russell A D, Hugo W B. Antimicrobial activity and action of silver. Prog Med Chem. 1994;31:351–370. doi: 10.1016/s0079-6468(08)70024-9. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 15.Selifonova O, Burlage R, Barkay T. Bioluminescent sensors for detection of bioavailable Hg(II) in the environment. Appl Environ Microbiol. 1993;59:3083–3090. doi: 10.1128/aem.59.9.3083-3090.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silver S. Mechanisms of plasmid-determined heavy metal resistances. In: Levy S B, Clowes R C, Koenig E L, editors. Molecular biology, pathogenicity, and ecology of bacterial plasmids. New York, N.Y: Plenum Publishing Corp.; 1981. pp. 179–189. [Google Scholar]
- 17.Silver, S., A. Gupta, K. Matsui, and J.-F. Lo. Resistance to Ag(I) cations in bacteria: environments, genes and proteins. Metal-Based Drugs, in press. [DOI] [PMC free article] [PubMed]
- 18.Slawson R M, Van Dyke M I, Lee H, Trevors J T. Germanium and silver resistance, accumulation, and toxicity in microorganisms. Plasmid. 1992;27:72–79. doi: 10.1016/0147-619x(92)90008-x. [DOI] [PubMed] [Google Scholar]
- 19.Starodub M E, Trevors J T. Mobilization of Escherichia coli R1 silver-resistance plasmid pJT1 by Tn5-Mob into Escherichia coli C600. Biol Metals. 1990;3:24–27. doi: 10.1007/BF01141173. [DOI] [PubMed] [Google Scholar]
- 20.Weast R C, editor. CRC handbook of chemistry and physics. 49th ed. Cleveland, Ohio: The Chemical Rubber Company; 1968. pp. B–307. [Google Scholar]