Highlights
-
•
Clinical guidelines recommend > 20 adjuvant or neoadjuvant breast cancer options.
-
•
Randomised data on 1000–10,000 women are available for most options.
-
•
Breast cancer mortality or recurrence was reduced by 10–25% for most options.
-
•
Anthracycline chemotherapy and radiotherapy increased non-breast-cancer mortality.
-
•
Radiotherapy-related risks increased with increasing organ dose.
Keywords: Breast cancer, Adjuvant treatments, Neoadjuvant treatments, Treatment benefits, Treatment harms
Abstract
Background
Adjuvant and neoadjuvant breast cancer treatments can reduce breast cancer mortality but may increase mortality from other causes. Information regarding treatment benefits and risks is scattered widely through the literature. To inform clinical practice we collated and reviewed the highest quality evidence.
Methods
Guidelines were searched to identify adjuvant or neoadjuvant treatment options recommended in early invasive breast cancer. For each option, systematic literature searches identified the highest-ranking evidence. For radiotherapy risks, searches for dose–response relationships and modern organ doses were also undertaken.
Results
Treatment options recommended in the USA and elsewhere included chemotherapy (anthracycline, taxane, platinum, capecitabine), anti-human epidermal growth factor 2 therapy (trastuzumab, pertuzumab, trastuzumab emtansine, neratinib), endocrine therapy (tamoxifen, aromatase inhibitor, ovarian ablation/suppression) and bisphosphonates. Radiotherapy options were after breast conserving surgery (whole breast, partial breast, tumour bed boost, regional nodes) and after mastectomy (chest wall, regional nodes).
Treatment options were supported by randomised evidence, including > 10,000 women for eight treatment comparisons, 1,000–10,000 for fifteen and < 1,000 for one. Most treatment comparisons reduced breast cancer mortality or recurrence by 10–25%, with no increase in non-breast-cancer death.
Anthracycline chemotherapy and radiotherapy increased overall non-breast-cancer mortality. Anthracycline risk was from heart disease and leukaemia. Radiation-risks were mainly from heart disease, lung cancer and oesophageal cancer, and increased with increasing heart, lung and oesophagus radiation doses respectively. Taxanes increased leukaemia risk.
Conclusions
These benefits and risks inform treatment decisions for individuals and recommendations for groups of women.
Introduction
In early invasive breast cancer, neoadjuvant treatments may be recommended before surgery and adjuvant treatments recommended after surgery. These treatments can reduce breast cancer recurrence and mortality, but may increase the risk of death from some other diseases. The evidence on benefits and risks of these treatments is not static but accumulates continuously and is scattered throughout the literature. Therefore, an up-to-date summary is needed for clinical training and to inform treatment decisions in the clinic today.
The highest quality evidence regarding the causal effect of treatment usually arises from a meta-analysis of randomised trials or, occasionally, from a single randomised trial [1], [2]. The measure of the causal effect of a treatment that has proved most useful is the rate ratio (RR). The RR is the rate at which a particular endpoint occurs in women allocated to one specific treatment option divided by the corresponding rate in women allocated to a different treatment option, but for whom all other aspects of care are identical. It is important to be aware that RRs compare treatments on a proportional scale, so if the RR for treatment A versus treatment B is 2, then the rate at which events are occurring among patients receiving treatment A is double the rate among patients receiving treatment B. It has been observed that in most scenarios RRs are remarkably stable across different groups of patients. This is in contrast to the absolute differences in rates, i.e. the rate for treatment A minus the rate for treatment B, which often vary substantially across different trials, across patients diagnosed in different calendar years, and across groups of patients with different characteristics within a single trial [3], [4].
RRs can be calculated for overall mortality, for mortality from specific causes, and for non-fatal endpoints eg cancer recurrence. For breast cancer mortality, RRs can be used to compare the proportional benefits of different treatments and can inform commissioning of treatments. RRs are also a fundamental component in decision-making at the individual patient level, and several breast cancer decision aids use them in conjunction with mortality rates from regional or national mortality data to provide quantitative estimates of the absolute breast cancer mortality benefits of systemic therapies for individual women. These are widely used in clinics and multidisciplinary team meetings throughout the world [5], [6]
At the present time, decision aids do not provide direct quantitative estimates of the risks of systemic therapies or of the benefits and risks of radiotherapy, although they are referred to in guidelines [7], [8], [9], [10], [11], [12]. The opportunity to fill this gap may arise in the near future, as several new breast cancer decision aids are under development, and updates of existing aids are planned [5], [13], [14].
The benefits and risks of most cancer treatments vary according to dose. For systemic therapy, a few standard regimens and doses are usually used in which each patient receives a similar dose per unit surface area (mg/m2) for chemotherapy, or a similar total dose for anti-human epidermal growth factor 2 (HER2) therapies, endocrine therapies and bisphosphonates. RRs from meta-analyses of randomised data that assess benefits and risks of these standard systemic therapy regimens are likely to apply to women receiving the same regimens today. For radiotherapy, there is usually little variation in biologically effective doses delivered to the target regions (breast, chest wall and lymph nodes) [15]. However, breast cancer mortality RRs for radiotherapy are unusual in that they vary according to the surgery that a woman has received, her tumour characteristics and the regions irradiated, so different RRs apply in different scenarios. For example, radiotherapy following mastectomy reduces breast cancer mortality substantially in women with positive lymph nodes but not in women with negative nodes [16].
When considering the risks of radiotherapy, the distribution of radiation dose within the patient needs to be considered. Randomised trials have identified heart disease, lung cancer and oesophageal cancer as the main diseases where breast cancer radiotherapy can increase mortality risks. They have also shown that the increased risks are likely to last for many decades after exposure [17]. However, the RRs obtained in the trials are unlikely to be directly relevant for patients being treated today. This is because the radiation-related risk of these diseases depends on the incidental radiation doses received by the heart, lung and oesophagus respectively [17], [18], [19]. Doses to these organs from typical modern radiotherapy are usually lower than for women irradiated in the past, as radiotherapy can now be delivered more precisely. In addition, organ doses from modern radiotherapy vary substantially according to the regimen used [15], [20], [21]. A further complication is that information on incidental heart, lung and oesophagus doses is unavailable in most of the randomised trials of radiotherapy carried out in the past. Therefore the main source of useful information on the magnitude of the radiation risks has proved to be carefully designed observational studies of individuals for whom the relevant organ has been exposed to radiation at a range of doses. These individuals were then followed over several decades to estimate the rate at which they developed or died from the disease in question. These studies have enabled dose–response relationships to be derived in the form of estimated increases in the RR per gray organ dose (Gy) for heart disease, lung cancer and oesophagus cancer. The increase in RR per Gy can then be combined with typical modern organ doses in Gy to provide estimates of RRs from typical modern radiotherapy regimens.
We present a systematic review of the information needed to estimate proportional benefits and risks of modern adjuvant and neoadjuvant treatment options recommended in current clinical guidelines for early breast cancer. For each treatment option, the literature was searched for randomised evidence and the highest-ranking study was identified. RRs for breast cancer and non-breast-cancer mortality were then collated and summarised.
Methods
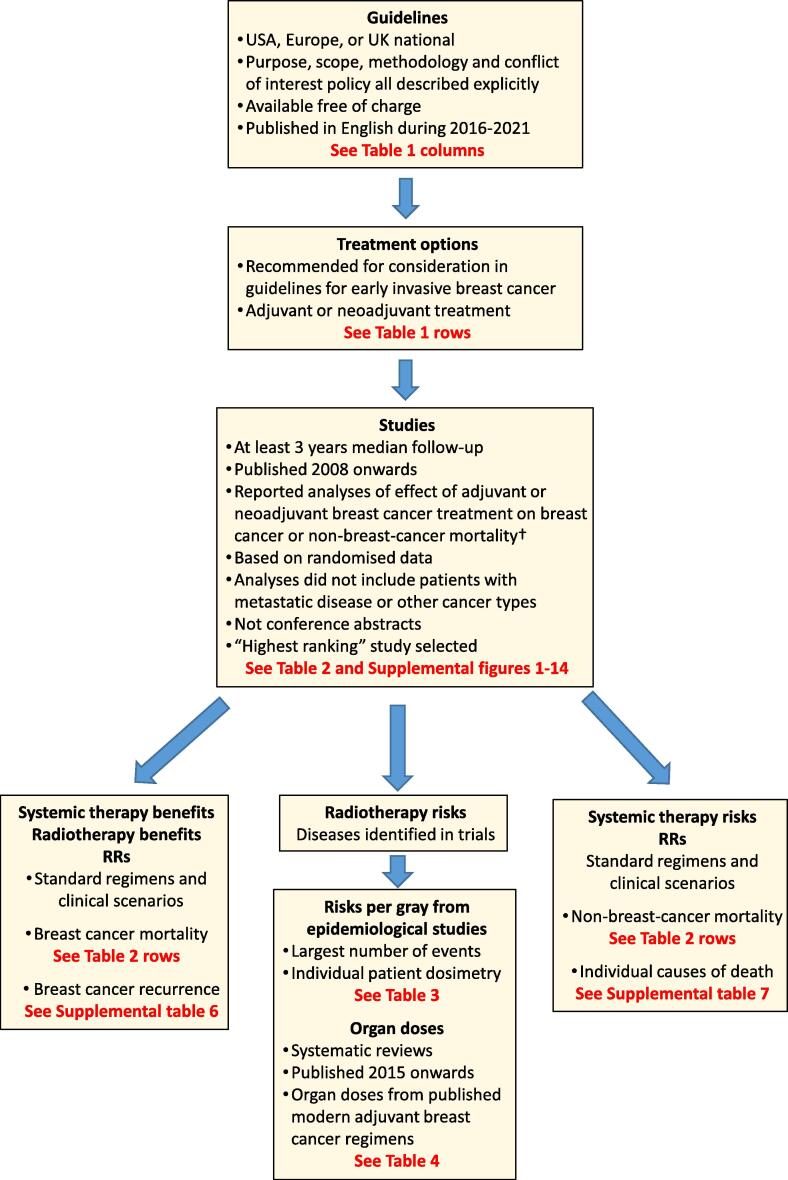
The methods used in the present study are explained below and summarised in Fig. 1.
Fig. 1.
Flowchart for study with criteria applied at each stage. † If no eligible trial was found, the trial referenced in the guidelines was used. Abbreviations: RR = rate ratio.
Guidelines
US, European and UK national breast cancer guidelines were identified. Guidelines were included if their purpose, scope, methodology and conflict of interest policy were all clearly stated, and they were freely available and published in English during 2016 to 2021. Emergency guidelines recommending temporary changes to practice during the COVID-19 pandemic were not included, nor were guidelines specifically for hereditary or non-invasive breast cancer.
Treatment options
Adjuvant and neoadjuvant treatment options recommended in guidelines for consideration in early invasive breast cancer were listed. Early invasive breast cancer was defined as cancer that has not spread beyond the breast or axillary lymph nodes. This included stages I, IIA, IIB and IIIA breast cancers, and excluded carcinoma in situ [22]. Adjuvant and neoadjuvant treatments were those intended to reduce risks of cancer recurrence and/or death. Adjuvant treatment was delivered after curative-intent surgery, and neoadjuvant treatment before curative-intent surgery.
Searches
For each treatment option, systematic literature searches were conducted to identify the highest-ranking evidence of its effects on mortality. New breast cancer treatments are sometimes recommended in the USA before they are endorsed by European or UK guidelines. Searches were not performed for treatments not yet recommended outside the USA.
Database searches were conducted for meta-analyses of trials of each treatment option compared with a less intensive treatment option (Supplemental Table 2a; Supplemental Figs. 1-14) [23]. Eligible studies compared treatment effects on breast cancer or non-breast-cancer mortality, included at least 3 years median follow-up and were published 2008 and onwards, as earlier studies would inevitably assess the effects of older treatment regimens. Non-randomised studies comparing different treatments were excluded because they may provide misleading estimates of treatment effects [24]. We also excluded studies of patients with metastatic disease, or other cancer types. Conference abstracts were excluded since they are not usually peer-reviewed; and most have insufficient detail on methods for study ranking.
If a search identified more than one eligible meta-analysis, they were ranked to identify the one providing the strongest evidence for each treatment option using the following criteria:
-
1.
Individual patient data meta-analysis including all relevant randomised trials, both published and unpublished.
-
2.
Individual patient data meta-analysis omitting some relevant randomised trials.
-
3.
Published data meta-analysis including all relevant randomised trials.
-
4.
Published data meta-analysis omitting some relevant randomised trials.
For treatment options with no eligible meta-analysis, additional database searches were conducted for individual randomised trials. If more than one randomised trial was found, the trial with the largest number of women randomised was used. If no eligible trial was found, the largest meta analysis or trial referenced in the guidelines was used.
Rate ratios
For each selected meta-analysis or trial, RRs for breast cancer mortality, non-breast-cancer mortality and individual causes of death were extracted. For chemotherapy, anti-HER2 therapy, endocrine therapy and bisphosphonates, RRs were extracted for standard dose regimens recommended in breast cancer guidelines (Supplemental Table 1). For radiotherapy, RRs were extracted for clinical scenarios quoted in guidelines. Where RRs were unavailable, hazard ratios or odds ratios were used or risk ratios were calculated (Supplemental Table 3). If guidelines recommended treatment options that were not yet reported to reduce breast cancer mortality, RRs for breast cancer recurrence were also extracted.
RRs were extracted by two oncologists (CT and DD) and checked by two other oncologists and a breast surgeon (FH, FD and GM). Discrepancies were resolved by consensus. For each endpoint the time period studied was extracted, defined as the time period covered by the RRs, as was the number of women on which the RR was based. RRs which differed from 1.00 with a two-sided p-value of ≤ 0.05 were regarded as significantly increased or decreased. 95% confidence intervals (CIs) are shown.
Risks of radiotherapy
For each individual cause of death with a significantly raised RR from radiotherapy, additional searches were performed for relevant dose–response relationships (Supplemental Table 2b). The dose–response relationship with the largest number of events based on individual patient radiation dosimetry was selected, and details extracted. For each dose–response relationship, a search was performed for systematic reviews of radiation doses to the relevant organs from typical modern radiotherapy regimens (Supplemental Table 2b). Eligible reviews were those that included organ doses from all available published modern early breast cancer regimens and were published 2015 onwards.
Results
Guidelines
Guidelines from six organisations were used to extract treatment options (Table 1). USA guidelines included the National Comprehensive Cancer Network (NCCN) [25], American Society of Clinical Oncology (ASCO) [26], [27], [28], [29], [30] and American Society for Radiation Oncology (ASTRO) [8], [9], [10]. European guidelines included the European Society for Medical Oncology (ESMO) [11] and St Gallen [31], [32]. UK guidelines were produced by the National Institute for Health and Care Excellence (NICE) [7], [33], [34], [35]. Two organisations, European Society for Radiotherapy and Oncology (ESTRO) and the Royal College of Radiologists (RCR), were not used to identify options because their guidance informs technical aspects of the radiotherapy options included in ESMO and NICE.
Table 1.
Guidelines and treatment options in adjuvant and neoadjuvant breast cancer during 2016–2021.
 |
Abbreviations NCCN National Comprehensive Cancer Network; ASCO American Society of Clinical Oncology; ASTRO American Society for Radiation Oncology; Bisphos Bisphosphonates; ESMO European Society of Medical Oncology; St Gallen St Gallen International Consensus Guidelines; NICE National Institute for Health and Care Excellence; P page; HER2 human epidermal growth factor receptor 2; ER oestrogen receptor; PR progesterone receptor; yr years; OS/OA ovarian suppression or ablation; AI aromatase inhibitors; RT radiotherapy
*Patient groups are listed only if recommendations are similar in all relevant guidelines
†Section numbers refer to NICE Guideline for all rows apart from “Appraisal” which refer to NICE Technology Appraisal Guidance
‡NCCN and ASCO list chemotherapy regimens. ESMO, St Gallen and NICE list general categories only (see Supplemental Table 1 for further details).
§NCCN, ESMO and St Gallen: HER2-/ER- disease only. ASCO: HER2- disease with any ER-status
¶Pembrolizumab was recommended in NCCN 2022 guidelines. It was stated as “not recommended” in St Gallen 2021 and ASCO 2021. It is currently under consideration by NICE (2022). It was not mentioned in ESMO. It was only recommended in USA guidelines therefore it is not included in subsequent tables.
**High risk was defined as: ≥4 positive nodes, or 1–3 positive nodes with one or more of the following: Grade 3, tumour size ≥ 5 cm, Ki-67 score ≥ 20%.
††Abemaciclib was recommended by ASCO 2022 and mentioned as an option in NCCN 2022. St Gallen 2021 stated “the panel was divided on whether to endorse abemaciclib adjuvant therapy” and “longer term follow-up from trials is awaited to settle this question”. Abemaciclib was not mentioned in ESMO and NICE. It was only recommended in USA guidelines therefore it is not included in subsequent tables.
‡‡NCCN and ASCO: Inoperable cancer, or operable cancer if high risk HER2+ or triple negative or to reduce the extent of surgery or patients in whom surgery may be delayed. NICE: High risk HER2+ or ER- or to reduce tumour size. ESMO and St Gallen: Inoperable cancer, or operable cancer if high risk HER2+ or triple negative or to reduce the extent of surgery.
Treatment options
Treatment types recommended for consideration in adjuvant or neoadjuvant early breast cancer included chemotherapy, anti-HER2 therapy, other targeted therapy, endocrine therapy, bisphosphonates and radiotherapy (Table 1). Within each type, there were several treatment options. Most options were recommended in all relevant guidelines, although some entered different guidelines at different times (Supplemental Table 4). Two options, pembrolizumab and abemaciclib were recommended only in the USA. European and UK guidelines either did not mention them, or stated that insufficient evidence was available to recommend them.
For chemotherapy, USA guidelines listed categories and individual regimens whereas European guidelines usually listed just categories: anthracycline-based, anthracycline + taxane, taxane-based and platinum-based (Supplemental Table 1). Most chemotherapy options were recommended for use in either the adjuvant or neoadjuvant setting. Neoadjuvant delivery was recommended for women at higher risk of breast cancer recurrence, who had HER2 positive or ER negative cancers, and adjuvant delivery was recommended for other women. For two chemotherapy types, the recommended timing of delivery was specified as adjuvant for capecitabine and neoadjuvant for platinum-based chemotherapy.
Recommended anti-HER2 therapies in HER2 positive cancer were trastuzumab, pertuzumab, trastuzumab emtansine and neratinib, in neoadjuvant or adjuvant settings. For other targeted therapies, the recommended timing of delivery was specified as neoadjuvant for pembrolizumab and adjuvant for abemaciclib.
Endocrine therapies in oestrogen receptor (ER) positive disease were tamoxifen and aromatase inhibitors (AIs) given with varying durations and sequences and, in pre-menopausal women, ovarian suppression or ablation. Endocrine therapy in ER positive disease, was usually recommended after surgery, but neoadjuvant delivery could be given for women at low risk of recurrence (Table 1). Bisphosphonates were recommended in postmenopausal women in the adjuvant setting.
Adjuvant radiotherapy after breast conserving surgery was recommended in most women. This involved either whole breast radiotherapy, sometimes with an additional radiation boost to the tumour bed, or partial breast radiotherapy. Radiotherapy recommended after mastectomy included the chest wall. Radiotherapy to the regional nodes may be recommended after either breast conserving surgery or mastectomy.
Treatment comparisons: Systemic therapies
Treatment comparisons in randomised trials provided direct evidence for most treatment options (Table 2, Supplemental table 5). Searches were not performed for pembrolizumab and abemaciclib since they were only recommended in the USA.
Table 2.
Studies and rate ratios for breast cancer and non-breast-cancer mortality from randomised trials comparing different adjuvant or neoadjuvant breast cancer treatments (see also Supplemental Tables 5–7).
 |
Abbreviations: RR rate ratio; CI confidence interval; vs versus; HER2 human epidermal growth factor 2; ER oestrogen receptor; PR progesterone receptor; path CR pathological complete response; AI aromatase inhibitor; RT radiotherapy
*The time period following diagnosis that RRs relate to. In most studies, this starts soon after time of diagnosis, as randomisation took place soon after diagnosis. For studies where randomisation did not take place until several years after diagnosis, both time from diagnosis to randomisation and time studied following randomisation are given in footnotes.
†The number of women was the same for assessment of breast cancer mortality and non-breast-cancer mortality unless indicated.
‡ Anthracycline breast cancer mortality rate ratio is for four or more cycles of any anthracycline regimen e.g. 4AC (doxorubicin and cyclophosphamide) versus no chemotherapy. The non-breast-cancer mortality rate ratio is for any anthracycline chemotherapy versus no chemotherapy.
§Taxane + anthracycline vs anthracycline breast cancer mortality rate ratio is for the addition of four taxane cycles to anthracycline-based chemotherapy (usually 4AC). An estimate of the RR for taxane + anthracycline vs no chemotherapy can be derived by multiplying the RRs for anthracycline vs nil and taxane + anthracycline vs nil, i.e. 0.79 × 0.86 = 0.68 (95% CI 0.59–0.77). The non-breast-cancer mortality rate ratio is for taxane + anthracycline vs the same or more anthracycline-based non-taxane chemotherapy.
¶For capecitabine versus not, a reduction in overall mortality was reported (RR 0.59, 95% CI 0.39–0.90, P = 0.01) so it is likely that capecitabine did, reduce breast cancer mortality, although this was not reported specifically.
**Rate ratio not published. Values shown are risk ratios calculated from published data, see Supplemental Table 3 for details.
††Meta-analysis of 4 published trials. All women received 5 years of tamoxifen before randomisation. Median follow-up after randomisation varied from 4.2 to 7.6 years. Reported measure of reduction in breast cancer mortality is odds ratio.
‡‡In the two largest trials, the RRs for non-breast-cancer mortality were 0.99 (95% CI 0.89–1.10) (ATLAS) [55] and 0.94 (0.82–1.07) (aTTom) [56].
§§Treatments diverged 2–3 years after diagnosis. Time-period studied is 8 years starting at 2 years after diagnosis.
¶¶Women randomised after around 5 years of AI preceded by 5 years of tamoxifen. Time-period studied is 10 years starting at randomisation.
***Women received about 5 years of AI or of tamoxifen → AI before randomisation. Time-period studied is 7 years starting at randomisation.
†††Includes women of all ages
‡‡‡76% of patients had invasive breast cancer and 24% had ductal carcinoma in situ.
§§§ Includes all trials of RT versus no RT and also trials of RT versus more extensive surgery.
Searches yielded eligible meta-analyses for: anthracycline, taxane + anthracycline, trastuzumab, timing of systemic therapy, 5 years of tamoxifen and extended tamoxifen (ER positive cancer); AI versus tamoxifen (in premenopausal women with ovarian suppression or ablation and in postmenopausal women, both with ER positive cancer) and tamoxifen/AI versus tamoxifen alone (postmenopausal women with ER positive cancer); and bisphosphonates (postmenopausal women) (Supplemental Figs. 1,2,5a,8–11,13) [4], [38], [42], [43], [44], [46], [47], [51].
For treatment comparisons with no eligible meta-analysis, searches yielded eligible randomised trials for: pertuzumab and trastuzumab emtansine in HER2 positive cancer (Supplemental Figs. 5c,6b) [39], [40]. In endocrine therapy for pre-menopausal women with ER positive cancer there was an eligible trial for ovarian suppression, (Supplemental Fig. 12b) [45]. In endocrine therapy for post-menopausal women with ER positive cancer there was an eligible trial for AI versus not after 5 years of tamoxifen [48]. For extended AI, two randomised trials with differing designs were included because guidelines differed in their recommendations as to the optimal AI duration (Supplemental Fig. 11) [49], [50].
For neoadjuvant platinum in triple negative cancer, for capecitabine, in HER2 negative residual cancer after neoadjuvant chemotherapy, and for neratinib in HER2 positive cancer, no trials reported breast cancer mortality. Therefore the effects of platinum on pathological complete response and of capecitabine and neratinib on breast cancer recurrence were extracted from the studies cited in the guidelines (Supplemental Figs. 3a, 4b, 7b, Supplemental Table 6) [36], [37], [41].
The number of women randomised in treatment comparisons providing direct evidence regarding the efficacy of systemic treatment was>10,000 for six comparisons, between 1,000 and 10,000 for eleven comparisons and<1000 for one comparison (Table 2).
For taxanes, only indirect evidence was available. There were no trials of taxane-based treatment without inclusion of an anthracycline, versus no chemotherapy. Also, few women were randomised to taxane + anthracycline versus no chemotherapy. Instead the overall effect of taxane + anthracycline can be assessed by multiplying the RRs for taxane + anthracycline versus anthracycline and anthracycline versus no chemotherapy.
Treatment comparisons: Radiotherapy
The guidelines recommended a total of five radiotherapy options (Table 1). Treatment comparisons in randomised trials provided direct evidence for all of these options (Table 2, Supplemental table 5).
Searches yielded eligible meta-analyses for radiotherapy after breast conserving surgery and radiotherapy after mastectomy (Supplemental Fig. 14a) [3], [16]. For treatment comparisons with no eligible meta-analyses, searches identified one eligible randomised trial for each of regional node, partial breast and tumour bed boost radiotherapy (Supplemental Fig. 14b-d) [52], [53], [54]. The number of women randomised in treatment comparisons of the efficacy of radiotherapy was >10,000 for one comparison, and between 1,000 and 10,000 for four comparisons (Table 2).
Evidence identifying causes of non-breast-cancer mortality affected by radiotherapy came from a meta-analysis including over 40,000 women in randomised trials of radiotherapy versus not following any surgery, and with any targets, and trials of radiotherapy versus more extensive surgery [17].
Rate ratios
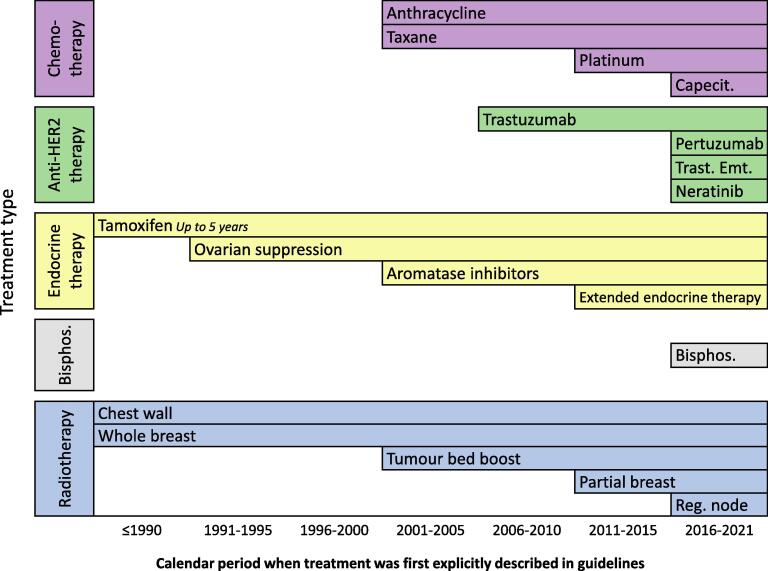
For treatments first recommended in 1990 or earlier (Fig. 2) (up to 5 years of tamoxifen, whole breast RT, chest wall RT), trials had lengthy follow-up and RRs compared treatments over a period of at least 15 years following diagnosis (Table 2). For newer treatments first recommended after 2015 (capecitabine, pertuzumab, trastuzumab emtansine, neratinib, bisphosphonates, regional node radiotherapy), follow-up was shorter and most RRs compared treatments over <10 years following diagnosis. For other treatments, first recommended during 1991 to 2015, the length of follow-up was variable and RRs compared treatments over < 1 to 20 years after diagnosis.
Fig. 2.
Calendar period when early breast cancer treatments were first explicitly described in clinical guidelines. For further details, see Supplemental table 4. Abbreviations: Reg. node = regional node, Bisphos. = bisphosphonates, Trast. Emt. = trastuzumab emtansine, Capecit = capecitabine.
Benefits in breast cancer mortality
For eight systemic therapy comparisons (anthracycline versus no chemotherapy; taxane + anthracycline versus anthracycline; trastuzumab versus not; 5 years of tamoxifen versus not; extended tamoxifen ≥ 10 versus 5 years; AI versus tamoxifen in postmenopausal women; tamoxifen/AI versus tamoxifen; and bisphosphonate versus not) there were statistically significant reductions in breast cancer mortality, with RRs varying from 0.67 (95% CI 0.61–0.73) to 0.88 (95% CI 0.80–0.97) (Table 2). Eight of the ten remaining systemic therapy options significantly reduced breast cancer recurrence (Supplemental Table 6). Neoadjuvant platinum chemotherapy increased pathological complete response rate, but trials are not mature enough to assess its effect on breast cancer mortality. A meta analysis assessed the effect of timing of chemotherapy relative to surgery. It included all trials randomising women to the same chemotherapy given neoadjuvantly versus adjuvantly. Delivery of chemotherapy neoadjuvantly had no significant effect on breast cancer mortality. It did however increase local breast cancer recurrence (RR 1.37, 95% CI 1.17–1.61) (Supplemental table 6) [42].
For five treatment comparisons (pertuzumab versus not; trastuzumab emtansine versus trastuzumab; ovarian suppression versus not (both with tamoxifen); AI versus not (both after 5 years of tamoxifen); and extended AI), RRs for breast cancer mortality were less than one, but the 95% CIs included one. For capecitabine versus not, a reduction in overall mortality was reported (RR 0.59, 95% CI 0.39–0.90, P = 0.01) so it is likely that it did, in fact, reduce breast cancer mortality, although this was not reported specifically. For platinum chemotherapy versus other chemotherapy and for neratinib versus not, breast cancer mortality by treatment allocation has not yet been reported.
In radiotherapy, there were significant reductions in breast cancer mortality for radiotherapy after breast conserving surgery (RR = 0.82, 95% CI 0.75–0.90), radiotherapy after mastectomy and axillary dissection in node positive disease (RR = 0.84, 95% CI 0.76–0.94) and regional node radiotherapy (RR = 0.81, 95% CI 0.74–0.94).
The other two radiotherapy options — partial breast versus whole breast radiotherapy and tumour bed boost after whole breast radiotherapy — did not increase or reduce breast cancer mortality significantly (Table 2). Partial breast radiotherapy was recommended in guidelines due to the theoretical benefits of irradiating less tissue than whole breast radiotherapy, and for its potential to reduce overall treatment time [7], [9], [11]. It did not significantly affect recurrence at any site or ipsilateral breast tumour recurrence [52]. Tumour bed boost was recommended in addition to whole breast radiotherapy because it has been shown to reduce ipsilateral breast tumour recurrence [10], [11] (Supplemental Table 6).
Risks of non-breast-cancer mortality and individual causes of death
For systemic therapies, anthracycline chemotherapy significantly increased non-breast-cancer mortality (RR = 1.20, 95% CI 1.00–1.43) (Table 2), with significant increases in mortality from heart disease and acute myeloid leukaemia (heart disease RR = 1.61, 95% CI 1.00–2.22 and acute myeloid leukaemia 8/4754 versus 0/4733 deaths, p = 0.004) (Supplemental Tables 3, 7). The cumulative dose of anthracycline in these trials ranged from 240 to 360 mg/m2 doxorubicin or 400–800 mg/m2 epirubicin [4]. For taxane chemotherapy, the RR for leukaemia was 11.00, 95% CI 1.42–85.17, based on 10/22,128 leukaemias in women randomised to taxane + anthracycline and 1/22,123 in women randomised to anthracycline. Leukaemia is rare, and there were too few events for this excess to increase non-breast cancer mortality (RR = 0.99, 95% CI 0.83–1.15). AI versus tamoxifen in premenopausal women increased mortality from cancers other than breast based on 22/3528 cancer deaths in women randomised to AI versus 10/3502 in women randomised to tamoxifen, but with no significant increase in overall non-breast-cancer mortality (RR 1.30, 95% CI 0.75–2.25) [46]. There were no reports of increased mortality from non-breast-cancer causes for other systemic therapies.
For radiotherapy, a meta-analysis of all available randomised trials comparing radiotherapy versus no radiotherapy yielded an overall non-breast-cancer mortality RR of 1.15 (95% CI 1.09–1.22) [17] (Table 2). This increase was mainly due to heart disease (RR 1.30, 95% CI 1.15–1.46), lung cancer (RR 1.64, 95% CI 1.22–2.21), oesophageal cancer (RR = 2.51, 95% CI 1.08–5.72) and thromboembolism (RR = 2.10, 95% CI 1.11–3.90) (Supplemental Table 7).
Radiotherapy risks per unit dose
For heart disease the dose–response relationship based on the largest number of cardiac events with individual patient dosimetry, included 963 cases and the RR for major coronary events increased by 7.4% per Gy (95% CI 2.9–14.5) [18] (Table 3, Supplemental Table 8). For lung cancer there were five dose–response relationships based on individual patient or trial-level dosimetry. These were combined in a published data meta-analysis [17] and the RR per Gy per mean whole lung dose was 11% (95% CI 6–19). For oesophageal cancer, the risk increased by 7.1% per Gy (95% CI 1.8–20.6) median oesophagus dose [19]. For thromboembolism, no dose–response relationships were available and the mechanism of radiation-related disease remains unknown.
Table 3.
Dose-response relationships for individual causes of non-breast-cancer mortality that are significantly increased by radiotherapy.
| Endpoint | Organ | Dose measure |
Number of events |
Percentage increase in RR per Gy* |
Reference† | Table/figure in reference |
|---|---|---|---|---|---|---|
| Major coronary events | Whole heart | Mean | 963 | 7.4% (95% CI 2.9–14.5) | Darby 201318 | Fig. 1 |
| Lung cancer | Lung‡ | Mean | 475 all studies combined | 11% (95% CI 6–19) | EBCTCG 201717 | Fig. S8 |
| Oesophageal cancer | Whole oesophagus | Median | 156 | 7.1% (95% CI 1.8–20.6)§ | Journy 202019 | Table 2 |
Abbreviations: RR rate ratio; Gy gray; CI confidence interval
i.e. excess RR per Gy (lung cancer and major coronary events) or excess odds ratio per Gy (oesophageal cancer). Models are of the form Bs(1 + KX/100) where S denotes a group, or stratum, of individuals for whom the rate at which the endpoint occurs in the absence of radiation exposure is likely to be similar. Bs is the rate at which the endpoint occurs in that stratum in the absence of radiotherapy, X is the dose measure in Gy and K is the percentage increase in the rate ratio or the odds ratio per Gy.
See also Supplemental Table 8.
Based on published data meta-analysis of five studies where doses were allocated to individuals based on trial-level or individual patient doses. Organ doses were for both lungs combined in one study, ipsilateral lung in two studies and location of second cancer in two studies.
The dose–response relationship based on whole oesophagus dose is listed because it is based on median oesophagus dose, which is assessable for patients being considered for breast cancer radiotherapy.
Radiotherapy risks and organ doses
Four systematic reviews were identified reporting typical regimen-specific organ doses, ie the average of the mean organ doses measured in CT plans for that regimen. Some regimen-specific doses were based on radiotherapy plans from multiple patients, others were based on a single radiotherapy plan.
Two systematic reviews of heart radiation doses from modern breast cancer radiotherapy were identified (Table 4). The first included all regimens published during 2003–2013 which reported mean heart dose from breast cancer radiotherapy [15]. There were 45 right and 357 left radiotherapy regimens in 167 studies from 28 countries. A second systematic review included heart doses from 32 right and 196 left regimens published in 99 studies during 2014–2017 [57]. For lung doses, one systematic review included mean whole lung doses (right and left lungs combined) from 218 regimens in 88 studies published during 2010–2015 [20]. For whole oesophagus doses, one systematic review included mean oesophagus doses from 89 regimens in 33 studies published during 2010–2020 [21]. In these reviews, the main determinants of heart, lung and oesophagus dose were regions irradiated and techniques used. For all these organs the range of doses was substantial and for many regimens, organ doses of over 10 Gy had been reported.
Table 4.
Typical modern radiotherapy organ doses for heart, lung and oesophagus in systematic reviews of breast cancer radiotherapy published during 2015–2020.
| Organ | Years of radiotherapy | Number regimens/laterality of cancer | Regions irradiated |
Organ doses* Average/range |
Reference | Table/Figure in reference | |
|---|---|---|---|---|---|---|---|
| Whole heart | 2003–2013 | 45 right 357 left |
Right, all regimens Left without IMN Left + IMN |
3.3 4.2 8.4 |
0.4–21.6 <0.1–23.0 0.7–28.6 |
Taylor 201515 | Table 1 Fig. 3 |
| Whole heart | 2014–2017 | 32 right 196 left |
Right whole breast Left whole breast |
1.9 3.6 |
0.2–8.8 0.1–18.7 |
Drost 201857 | Table 1 |
| Both lungs combined | 2010–2015 | 218 right + left combined |
Partial breast Breast/chest wall Breast/chest wall + axilla/SCF Breast/chest wall + axilla/SCF + IMN |
1.6 5.6 7.3 8.6 |
0.3–5.1 0.2–13.1 2.2–12.1 3.0–12.1 |
Aznar 201820 | Fig. E4 |
| Whole oesophagus | 2010–2020 | 89 right + left combined |
Partial breast Breast/chest wall Breast/chest wall + SCF/axilla/IMN |
0.2 1.8 11.4 |
0.1–0.4 0.1–10.4 1.1–29.3 |
Duane 202121 | Fig. 3 |
Abbreviations: SCF supraclavicular fossa; IMN internal mammary node
Average and range of reported regimen-specific doses for radiotherapy to different target regions. Each regimen-specific dose is the average of the mean organ doses in individual patient CT plans for the regimen.
Discussion
This review of adjuvant and neoadjuvant treatments for early breast cancer provides a resource for clinical practice and training. It summarises the treatment options recommended in USA, European and UK national guidelines, and the benefits and risks of those options compared, in most cases, with an already established standard, less intensive, treatment. The proportional benefits of most treatment options compared with standard treatment were a 10–25% reduction in breast cancer mortality or recurrence, with no overall increase in non-breast-cancer death.
Two treatments, anthracycline chemotherapy and radiotherapy, did increase overall non-breast-cancer death. For anthracycline, the main risks were from heart disease and acute myeloid leukaemia. For individual causes of death, taxanes increased leukaemia risk and aromatase inhibitors in premenopausal women were associated with increased mortality from second cancers, but this was based on few events. The risks from current systemic therapies are likely to be similar to those described here since similar doses of these drugs are used today. Radiation-risks were mainly from heart disease, lung cancer, oesophageal cancer and thromboembolism. The radiation risks from current treatment are likely to be lower than those in the evidence presented here, as organ-specific doses are lower. This review summarises the information needed to estimate proportional radiation-risks from modern radiotherapy.
Strengths and weaknesses
This is the first published review to summarise systematically the quantitative evidence on the proportional mortality benefits and risks from adjuvant and neoadjuvant breast cancer treatments. It has several strengths.
First, it collates multiple estimates that were previously scattered widely throughout the literature. Most of these estimates were in tables, graphs, footnotes, or in the text of publications and were time-consuming to locate.
Second, wherever possible the same endpoints — breast cancer and non-breast-cancer mortality — were presented for all studies, so the effects of different treatments could be compared. Wherever possible we have presented the results in terms of RRs. However, in one study, only odds ratios were available and so we provided them. For some other studies only numbers of cause-specific deaths were available and for these we calculated and provided the relevant risk ratios. For these trials in which rates are small and the treatments arms being compared have usually been followed for similar lengths of time, both of these approximations are likely to perform well.
Third, our searches were extensive, with inclusive terms. We searched > 13,000 publications in total, with > 100 publications for each treatment option. Therefore we are likely to have identified and assessed all relevant studies.
Fourth, ranking of search results enabled identification of the highest quality estimates, with the least risk of bias and the greatest number of women randomised. Measures of strength of evidence were abstracted, including the number of women randomised, confidence intervals, and the time period studied. For 12 of the 24 treatment comparisons (Table 2) an individual patient data meta-analysis was identified including all relevant available randomised trials. Most adjuvant and neoadjuvant breast cancer treatments have modest effects on mortality that can be difficult to quantify in individual trials due to lack of statistical power. However, meta-analyses of all available randomised data can enable reliable assessment of treatment effects up to 10 years. Even using the highest quality randomised evidence, there is uncertainty about the effects of treatment beyond this since most trials do not usually follow patients beyond 10 years.
Fifth, the review brings together all evidence types needed to assess quantitatively the proportional risks from modern breast cancer radiotherapy, including meta-analyses of randomised data, dose–response relationships and systematic dosimetry reviews. These may be used to estimate risks from modern radiotherapy regimens using typical regimen-specific organ doses. Radiation-risks can also be estimated for individual women, using organ doses from their radiotherapy planning CT-scan. It is not known how radiotherapy causes thromboembolism, so that at present it is difficult to quantify with any confidence the thromboembolic risk from current radiotherapy.
A limitation of our study is that we considered only the mortality benefits and risks of breast cancer treatments, not their effects on breast cancer recurrence or on side-effects that are not usually fatal, such as endometrial cancer after tamoxifen. The exception is the inclusion of more recent trials mentioned in guidelines and relevant to current clinical practice, but which had not reported an improvement in breast cancer mortality at the time of writing. In addition, even using all available randomised evidence, there were few events for reliable assessment of rare side-effects. For example, the risk of acute myeloid leukaemia after taxane chemotherapy was significantly increased in a meta-analysis including 44,251 women, but this was based on just 12 events. A recent abstract of a meta-analysis of trials comparing taxane + anthracycline versus taxane alone reported no increase in leukaemias, although longer follow-up is needed [58].
A further limitation is that the review can inevitably only assess current guidelines and evidence. As existing trials mature, and new trials are reported, guidelines will change, and updated reviews will be needed.
Clinical implications
The RRs in our review may be used by health care professionals to estimate benefits and risks of adjuvant and neoadjuvant breast cancer treatments for patients today. For breast cancer mortality, RRs can be used to compare the proportional benefits of different treatments and can inform prioritisation of treatments at a national level.
At an individual patient level, the trade off between absolute benefits and risks of treatments is complex. Absolute benefits and risks differ according to patient and tumour characteristics. Estimation of these effects requires data on women in the general population, and it is outside the scope of this review. It was recently addressed in a systematic review of breast cancer decision aids [12]. The RRs in our review may however be used in breast cancer decision aids. First, the list of early breast cancer treatments recommended in current clinical guidelines informs which new treatments may be included when decision aids are updated. For example capecitabine, pertuzumab, trastuzumab emtansine and neratinib, are recommended in clinical guidelines but are not included in current decision aids, so clinicians cannot easily estimate their absolute benefits and risks for individual patients. Second, our summary of the highest-ranking RRs may be used to update current decision aids. Third, our review provides quantitative estimates of the risks from systemic therapy and of the benefits and risks from radiotherapy. These are not currently available in decision aids.
Our review of clinical guidelines illustrates the successive improvements in adjuvant and neoadjuvant breast cancer treatments that have occurred during the past 30 years (Fig. 2). During 2001–2005, taxane and anthracycline chemotherapy were first recommended, as were AIs and tumour bed radiotherapy boost. These were followed by trastuzumab in 2006–2010, platinum chemotherapy, extended tamoxifen and partial breast RT during 2011–2015. Then during 2016–2021 several new anti-HER2 agents were approved for use, as were bisphosphonates and capecitabine, and the role of regional node RT was established. The resulting changes in clinical practice are likely to have reduced breast cancer mortality. Since the 1990s, breast cancer mortality in high income countries has approximately halved, and a considerable part of this is likely to be due to improved treatments [59].
Future research
Breast cancer practice is likely to continue to improve as the results of more trials become available. Several ongoing trials are investigating treatment de-escalation. The timing of treatments relative to surgery is changing and an increasing number of women now receive neoadjuvant chemotherapy. More information is needed on whether RRs from adjuvant regimens also apply to the same regimens if they are used before surgery. Chemotherapy regimens may also change. A few recent trials have assessed the impact of avoiding anthracycline in chemotherapy regimens, but at present the randomised evidence on this is limited [58].
In the future, new chemotherapy and anti-HER2 therapies may be recommended. For currently recommended treatments, increased follow-up of trials may provide additional information on long-term outcomes. This is particularly relevant to toxicity, which can occur several decades after treatment. As evidence increases, so will the need for accurate up-to-date summaries of it.
CRediT authorship contribution statement
Amanda J. Kerr: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – review & editing. David Dodwell: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. Paul McGale: Conceptualization, Methodology, Writing – review & editing. Francesca Holt: Data curation, Writing – review & editing. Fran Duane: Data curation, Writing – review & editing. Gurdeep Mannu: Data curation, Writing – review & editing. Sarah C. Darby: Analysis, Conceptualization, Data curation, Investigation, Methodology, Supervision, Visualization, Writing – review & editing. Carolyn W. Taylor: Conceptualization, Data curation, Investigation, Methodology, Supervision, Visualization, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors would like to thank patient contributors Hilary Stobart and Mairead MacKenzie on behalf of Independent Cancer Patients' Voice for helpful discussions. They would also like to thank Paul Pharoah, University of Cambridge, and Alexandra Freeman and Gabriel Recchia both at the Winton Centre for Risk and Evidence Communication for their ideas and input.
This work was supported by Cancer Research UK [grant C8225/A21133], the National Institute for Health Research [grant 300676] and the National Institute for Health Research Oxford Biomedical Research Centre. The study sponsors had no role in study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the paper for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctrv.2022.102375.
Contributor Information
Amanda J. Kerr, Email: amanda.kerr@ndph.ox.ac.uk.
David Dodwell, Email: david.dodwell@ndph.ox.ac.uk.
Paul McGale, Email: paul.mcgale@ndph.ox.ac.uk.
Francesca Holt, Email: francesca.holt@ndph.ox.ac.uk.
Fran Duane, Email: fduane@tcd.ie.
Gurdeep Mannu, Email: gurdeep.mannu@ndph.ox.ac.uk.
Sarah C. Darby, Email: sarah.darby@ndph.ox.ac.uk.
Carolyn W. Taylor, Email: carolyn.taylor@ndph.ox.ac.uk.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Puhan M.A., Schunemann H.J., Murad M.H., Li T., Brignardello-Petersen R., Singh J.A., et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 2.Centre for Evidence-based Medicine. Centre for Evidence-based medicine – Levels of evidence. https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ 2009 [accessed 22 Aug 2019].
- 3.Early Breast Cancer Trialists' Collaborative Group. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011b;378:1707-16. 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed]
- 4.Early Breast Cancer Trialists' Collaborative Group. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432-44. 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed]
- 5.PREDICT, 2017. https://breast.predict.nhs.uk/ [accessed 22 Aug 2019].
- 6.Cancermath.net. http://www.lifemath.net/cancer/2019 [accessed 22 Aug 2019].
- 7.National Institute for Health and Care Excellence (NICE). Early and locally advanced breast cancer: diagnosis and management 2018 (NICE Clinical Guideline ng101). https://www.nice.org.uk/guidance/ng101/chapter/Recommendations#adjuvant-chemotherapy-for-invasive-breast-cancer 2019 [accessed 22 Aug 2019].
- 8.Recht A., Comen E.A., Fine R.E., Fleming G.F., Hardenbergh P.H., Ho A.Y., et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. Pract Radiat Oncol. 2016;6:e219–e234. doi: 10.1016/j.prro.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Correa C., Harris E.E., Leonardi M.C., Smith B.D., Taghian A.G., Thompson A.M., et al. Accelerated Partial Breast Irradiation: Executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Practical Radiation Oncol. 2017;7:73–79. doi: 10.1016/j.prro.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Smith B.D., Bellon J.R., Blitzblau R., Freedman G., Haffty B., Hahn C., et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018;8:145–152. doi: 10.1016/j.prro.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 12.Zhao A., Larbi M., Miller K., O’Neill S., Jayasekera J. A scoping review of interactive and personalized web-based clinical tools to support treatment decision making in breast cancer. Breast. 2022;61:43–57. doi: 10.1016/j.breast.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zdenkowski N., Butow P., Tesson S., Boyle F. A systematic review of decision aids for patients making a decision about treatment for early breast cancer. Breast. 2016;26:31–45. doi: 10.1016/j.breast.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Mühlbauer V., Berger-Höger B., Albrecht M., Mühlhauser I., Steckelberg I. Communicating prognosis to women with early breast cancer – overview of prediction tools and the development and pilot testing of a decision aid. BMC Health Serv Res. 2019;19:171. doi: 10.1186/s12913-019-3988-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor C.W., Wang Z., Macaulay E., Jagsi R., Duane F., Darby S.C. Exposure of the Heart in Breast Cancer Radiation Therapy: A Systematic Review of Heart Doses Published During 2003 to 2013. Int J Radiat Oncol Biol Phys. 2015;93:845–853. doi: 10.1016/j.ijrobp.2015.07.2292. [DOI] [PubMed] [Google Scholar]
- 16.Early Breast Cancer Trialists' Collaborative Group. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed]
- 17.Taylor C., Correa C., Duane F.K., Aznar M.C., Anderson S.J., Bergh J., et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence From Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J Clin Oncol. 2017;35:1641–1649. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Brønnum D., et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 19.Journy N., Schonfeld S.J., Hauptmann M., Roberti S., Howell R.M., Smith S.A., et al. Dose-volume effects of breast cancer radiation therapy on the risk of second oesophageal cancer. Radioth Oncol. 2020;151:33–39. doi: 10.1016/j.radonc.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Aznar M.C., Duane F.K., Darby S.C., Wang Z., Taylor C.W. Exposure of the lungs in breast cancer radiotherapy: A systematic review of lung doses published 2010–2015. Int J Radiat Oncol Biol Phys. 2018;126:148–154. doi: 10.1016/j.radonc.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duane F.K., Kerr A., Wang Z., Darby S.C., Ntentas G., Aznar M.C., et al. Exposure of the oesophagus in breast cancer radiotherapy: A systematic review of oesophagus doses published 2010–2020. Radioth Oncol. 2021;164:261–267. doi: 10.1016/j.radonc.2021.09.032. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute. NCI Dictionary of Cancer Terms https://www.cancer.gov/publications/dictionaries/cancer-terms [accessed 22 Oct 2019].
- 23.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGale P., Cutter D., Darby S.C., Henson K.E., Jagsi R., Taylor C.W. Can Observational Data Replace Randomized Trials? J Clin Oncol. 2016;34:3355–3357. doi: 10.1200/JCO.2016.68.8879. [DOI] [PubMed] [Google Scholar]
- 25.National Comprehensive Cancer Network Guidelines Version 2.2022 Breast Cancer. https://www.nccn.org/ [accessed 4th Feb 2022].
- 26.Denduluri N., Chavez-MacGregor M., Telli M.L., Eisen A., Graff S.L., Hassett M.J., et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2018;36:2433–2443. doi: 10.1200/JCO.2018.78.8604. [DOI] [PubMed] [Google Scholar]
- 27.Korde L.A., Somerfield M.R., Carey L.A., Crews J.R., Denduluri N., Hwang E.S., et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. JCO. 2021;39:1485–1505. doi: 10.1200/JCO.20.03399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giordano S.H., Freedman R.A., Somerfield M.R. Optimal Adjuvant Chemotherapy and Targeted Therapy Guideline Expert Panel. Abemaciclib With Endocrine Therapy in the Treatment of High-Risk Early Breast Cancer: ASCO Optimal Adjuvant Chemotherapy and Targeted Therapy Guideline Rapid Recommendation Update. J Clin Oncol. 2022;40:307–309. doi: 10.1200/JCO.21.02677. [DOI] [PubMed] [Google Scholar]
- 29.Burstein H.J., Lacchetti C., Anderson H., Buchholz T.A., Davidson N.E., Gelmon K.A., et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol. 2019;37:423–438. doi: 10.1200/JCO.18.01160. [DOI] [PubMed] [Google Scholar]
- 30.Dhesy-Thind S., Fletcher G.G., Blanchette P.S., Clemons M.J., Dillmon M.S., Frank E.S., et al. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:2062–2081. doi: 10.1200/JCO.2016.70.7257. [DOI] [PubMed] [Google Scholar]
- 31.Burstein H.J., Curigliano G., Thurlimann B., Weber W.P., Poortmans P., Regan M.M., et al. Customising local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32:1216–1235. doi: 10.1016/j.annonc.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burstein H.J., Curigliano G., Loibl S., Dubsky P., Gnant M., Poortmans P., et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30:1541–1557. doi: 10.1093/annonc/mdz235. [DOI] [PubMed] [Google Scholar]
- 33.NICE Technology Appraisal Guidance TA569, 2019. Pertuzumab for adjuvant treatment of HER2-positive early stage breast cancer. https://www.nice.org.uk/guidance/ta569 [accessed 7th Nov 2020].
- 34.NICE Technology Appraisal Guidance TA612, 2019. Neratinib for extended adjuvant treatment of hormone receptor-positive, HER2-positive early stage breast cancer after adjuvant trastuzumab. https://www.nice.org.uk/guidance/ta612 [accessed 7th Nov 2020].
- 35.NICE Technology Appraisal Guidance TA632. Trastuzumab emtansine for adjuvant treatment of HER2-positive early breast cancer. 2020. https://www.nice.org.uk/guidance/ta632 [accessed 7th Nov 2020].
- 36.Poggio F., Bruzzone M., Ceppi M., Pondé N.F., La Valle G., Del Mastro L., et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29:1497–1508. doi: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 37.Masuda N., Lee S.-J., Ohtani S., Im Y.-H., Lee E.-S., Yokota I., et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Eng J Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 38.Early Breast Cancer Trialists’ Collaborative Group. Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13,864 women in seven randomised trials. Lancet Oncol 2021;22:1139-50. 10.1016/S1470-2045(21)00288-6. [DOI] [PMC free article] [PubMed]
- 39.Piccart M., Procter M., Fumagalli D., de Azambuja E., Clark E., Ewer M.S., et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. JCO. 2021;39:1448–1457. doi: 10.1200/JCO.20.01204. [DOI] [PubMed] [Google Scholar]
- 40.von Minckwitz G., Huang C.-S., Mano M.S., Loibl S., Mamounas E.P., Untch M., et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Eng J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 41.Martin M., Holmes F.A., Ejlertsen B., Delaloge S., Moy B., Iwata H., et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1688–1700. doi: 10.1016/S1470-2045(17)30717-9. [DOI] [PubMed] [Google Scholar]
- 42.Early Breast Cancer Trialists’ Collaborative Group. Neo-adjuvant versus adjuvant chemotherapy in early breast cancer: long-term outcomes among 4756 women in 10 randomised trials. Lancet Oncol 2018;19: 27-39. 10.1016/S1470-2045(17)30777-5. [DOI] [PMC free article] [PubMed]
- 43.Early Breast Cancer Trialists' Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011a;378:771-84. 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed]
- 44.Ibrahim E.M., Al-Hajeili M.R., Bayer A.M., Abulkhair O.A., Refae A.A. Extended adjuvant endocrine therapy in early breast cancer: a meta-analysis of published randomized trials. Med Onc. 2017;34:131. doi: 10.1007/s12032-017-0986-2. [DOI] [PubMed] [Google Scholar]
- 45.Francis P.A., Pagani O., Fleming G.F., Walley B.A., Colleoni M., Láng I., et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Eng J Med. 2018;379:122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in premenopausal women with estrogen receptor positive early stage breast cancer treated with ovarian suppression: patient-level meta-analysis of 7,030 women in four randomised trials. Lancet Oncol 2022 https://org/10.1016/S1470-2045(21)00758-0. [DOI] [PMC free article] [PubMed]
- 47.Early Breast Cancer Trialists' Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015a;386:1341-52. 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed]
- 48.Ingle J.N., Tu D., Pater J.L., Muss H.B., Martino S., Robert N.J., et al. Intent-to-treat analysis of the placebo-controlled trial of letrozole for extended adjuvant therapy in early breast cancer: NCIC CTG MA.17. Ann Oncol. 2008;19:877–882. doi: 10.1093/annonc/mdm566. [DOI] [PubMed] [Google Scholar]
- 49.Goss P.E., Ingle J.N., Pritchard K.I., Robert N.J., Muss H., Gralow J., et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med. 2016;375:209–219. doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mamounas E.P., Bandos H., Lembersky B.C., Jeong J.-H., Geyer C.E., Rastogi P., et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:88–99. doi: 10.1016/S1470-2045(18)30621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Early Breast Cancer Trialists' Collaborative Group. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 2015b;386:1353-61. 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed]
- 52.Vicini F.A., Cecchini R.S., White J.R., Arthur D.W., Julian T.B., Rabinovitch R.A., et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet. 2019;394:2155–2164. doi: 10.1016/S0140-6736(19)32514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartelink H., Maingon P., Poortmans P., Weltens C., Fourquet A., Jager J., et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16:47–56. doi: 10.1016/S1470-2045(14)71156-8. [DOI] [PubMed] [Google Scholar]
- 54.Poortmans P.M., Weltens C., Fortpied C., Kirkove C., Peignaux-Casasnovas K., Budach V., et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I-III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial. Lancet Oncol. 2020;21:1602–1610. doi: 10.1016/S1470-2045(20)30472-1. [DOI] [PubMed] [Google Scholar]
- 55.Davies C., Pan H., Godwin J., Gray R., Arriagada R., Raina V., et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gray R.G., Rea D., Handley K., Bowden S.J., Perry P., Earl H.M., et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol. 2013;31:5. doi: 10.1200/jco.2013.31.18_suppl.5. [DOI] [Google Scholar]
- 57.Drost L., Yee C., Lam H., Zhang L., Wronski M., McCann C., et al. A Systematic Review of Heart Dose in Breast Radiotherapy. Clin Breast Cancer. 2018;18:e819–e824. doi: 10.1016/j.clbc.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Early Breast Cancer Trialists’ Collaborative Group. Abstract GS2-06: Taxane with anthracycline versus taxane without anthracycline: An individual patient-level meta-analysis of 16,500 women with early-stage breast cancer in 13 randomised trials. Cancer Res 2022:82; G2-06. 10.1158/1538-7445.SABCS21-GS2-06. [DOI]
- 59.Collins R, “Big Data, Better Treatment”: The work of the Early Breast Cancer Trialists' Collaborative Group. Available from: https://slideplayer.com/slide/8910693/ [accessed: 12 May 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.