Abstract
Brain size and IQ are positively correlated. However, multiple meta-analyses have led to considerable differences in summary effect estimations, thus failing to provide a plausible effect estimate. Here we aim at resolving this issue by providing the largest meta-analysis and systematic review so far of the brain volume and IQ association (86 studies; 454 effect sizes from k = 194 independent samples; N = 26 000+) in three cognitive ability domains (full-scale, verbal, performance IQ). By means of competing meta-analytical approaches as well as combinatorial and specification curve analyses, we show that most reasonable estimates for the brain size and IQ link yield r-values in the mid-0.20s, with the most extreme specifications yielding rs of 0.10 and 0.37. Summary effects appeared to be somewhat inflated due to selective reporting, and cross-temporally decreasing effect sizes indicated a confounding decline effect, with three quarters of the summary effect estimations according to any reasonable specification not exceeding r = 0.26, thus contrasting effect sizes were observed in some prior related, but individual, meta-analytical specifications. Brain size and IQ associations yielded r = 0.24, with the strongest effects observed for more g-loaded tests and in healthy samples that generalize across participant sex and age bands.
Keywords: in vivo brain volume, intelligence, meta-analysis, specification curve analysis, multiverse analysis, systematic review
1. Introduction
Associations between brain volume and intelligence have attracted the interest of the scientific community at least since the early 1800s [1]. This potential association is important for our understanding of (human) intelligence because brain volume is considered to be among the best-replicated correlates of psychometric g (i.e. general cognitive ability; [2]). In this vein, larger brains have been assumed to accommodate more neurons and glial cells which in turn may allow for more complex and quicker information processing. Other researchers have argued that larger brains provide surplus brain tissue that serves as a type of brain reserve, which may protect against a deterioration of cognitive abilities due to ageing- or fitness-related factors (for an overview, see [3]). However, to determine the potential relevance of these mechanisms, it is important to obtain a plausible estimate of the brain volume and IQ association.
Early empirical studies had to rely on proxies for intelligence (e.g. educational achievement) and brain size measures (such as head height, width, circumference or composites of it; e.g. [4]), which introduced considerable statistical noise in the data, thus leading to imprecise estimates of associations. Even with the advent of the first psychometric tests in the early 1900s, the issue of reliable brain volume measurement pertained, thus failing to alleviate validity problems of the observed associations.
It was only the development of neuroimaging methods, such as magnetic resonance imaging (MRI), that allowed reliable assessments of in vivo brain volumes and consequently their link with intelligence. While the direction of the seemingly positive brain size and intelligence link was frequently reproduced, the observed strength varied considerably. The first available report of MRI-assessed brain volume and IQ correlations in healthy men suggested that this association explained an impressive 25% of variance (r = 0.51; [5]), thus representing a large effect according to the well-established effect size classification of Cohen [6]. However, subsequent replications appeared to yield predominantly effects in the small-to-moderate range.
Several narrative reviews have been published that aimed at clarifying the brain-volume and IQ link [7–12]. Although all of them concluded that there is overwhelming evidence for a positive relationship, the strength and consequently meaning of the effect remained unclear. However, this is unsurprising because narrative reviews (i) lack formalized procedures that would allow systematic syntheses of effect sizes or influences of potential moderators and (ii) are vulnerable to various forms of bias (e.g. [13], pp. 301–302).
Systematic numerical effect syntheses by means of meta-analytical approaches are often considered to represent a means to provide definite conclusions about a given research question (i.e. assuming that they have been adequately performed) because they can address both moderator effects and estimate potentially confounding dissemination biases. However, meta-analytic researchers that investigate an identical research question may arrive at surprisingly different conclusions.
It has been demonstrated that design and analytical choices as well as researcher degrees of freedom (decisions that are being made that affect e.g. the specification of inclusion criteria or the selected statistical approach) may lead to substantially varying results from meta-analyses although they are based on similar databases and examine identical research questions (see the multiverse and specification-curve approach to meta-analysis introduced by [14], adopting analogous approaches for primary data analysis, i.e. multiverse analysis, by [15], and specification-curve-analysis, by [16,17]). Typically, there are a variety of reasonable choices that need to be made in regard to any type of study (for an overview, see [18]) which different researchers may (dis)agree about. For meta-analyses in this context, it matters most which studies are included and how they are analysed [14].
Researchers typically have conceptual or methodological reasons to prefer certain specifications over others. However, other researchers may apply different but equally reasonable selection criteria and analysis approaches which necessarily will lead to different summary effect estimations. One prominent example of the effects that such differing methodological choices can have is the widely received but heavily criticized paper that seemingly showed larger death-tolls of hurricanes with female as opposed to those with male names [19] in the prestigious journal PNAS. Although the methodological choices of this study had been well-justified, they represented an extreme specification which represented a single possibility out of a total of 1728 reasonable specifications (i.e. based on a number of published opinions about how these data should have been analyzed) to analyze these data (i.e. under the assumption that all possible meaningful kinds of data to analyze and how to do it had been identified in this study), only 37 of which (i.e. 2.1%) would have led to significantly deadlier hurricanes with female names [16,17].
Such observations have led researchers to argue that data analyses in general and research syntheses in particular should not only rely on a single reasonable specification which may represent only one of many justifiable approaches to treat and analyze data. Providing a distribution of these reasonable approaches allows a multi-faceted evaluation of potential moderating variables in terms of how they affect an effect size in terms of accuracy and strength.
Naturally, researchers may be unaware of all potentially reasonable ways to analyze their data (i.e. both in terms of which data to analyze and how to do so) because they may be unaware about influential factors such as moderating variables. By means of a brute-force approach, all possible (but not necessarily reasonable) ways to calculate meta-analytical summary effects can inform a researcher about potentially meaningful specifications that she may have missed. This means that clustering of summary effects of certain data subsets within the framework of a combinatorial meta-analysis may allow an identification of previously unobserved moderators ([20]; for discussion and an application, [14]).
Moreover, the time-point a certain meta-analysis is performed at can affect its outcomes because of the so-called decline effect (i.e. effect sizes of early studies investigating a given research question are more often than not inflated, thus leading to systematic decreases of effect strengths over time; [21,22]).
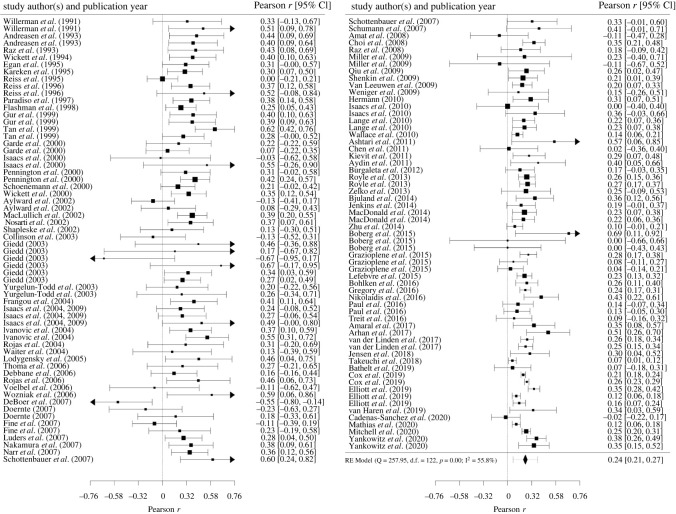
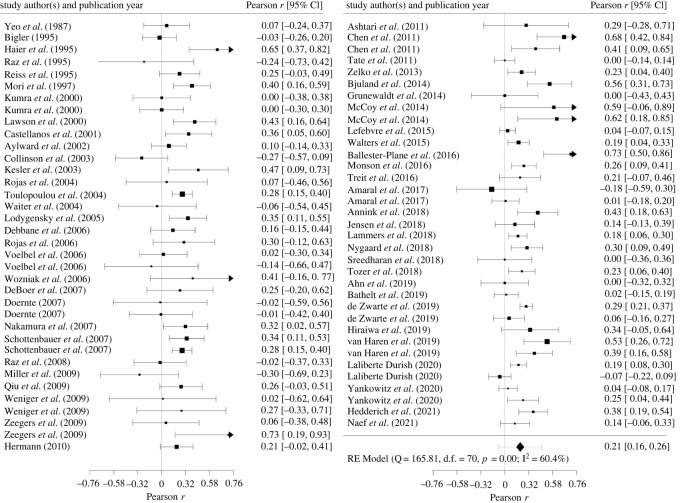
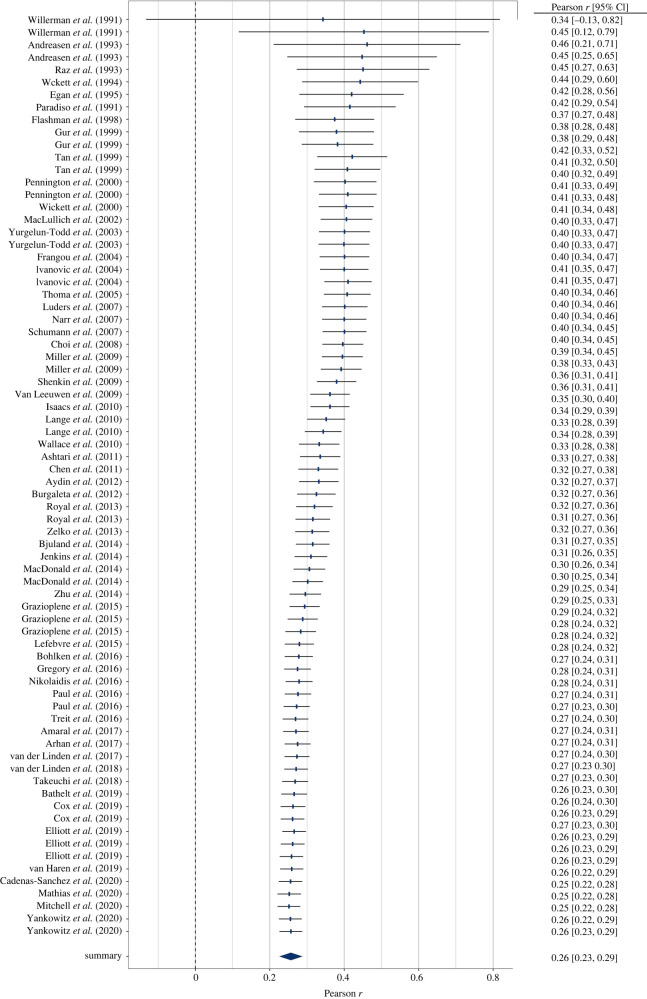
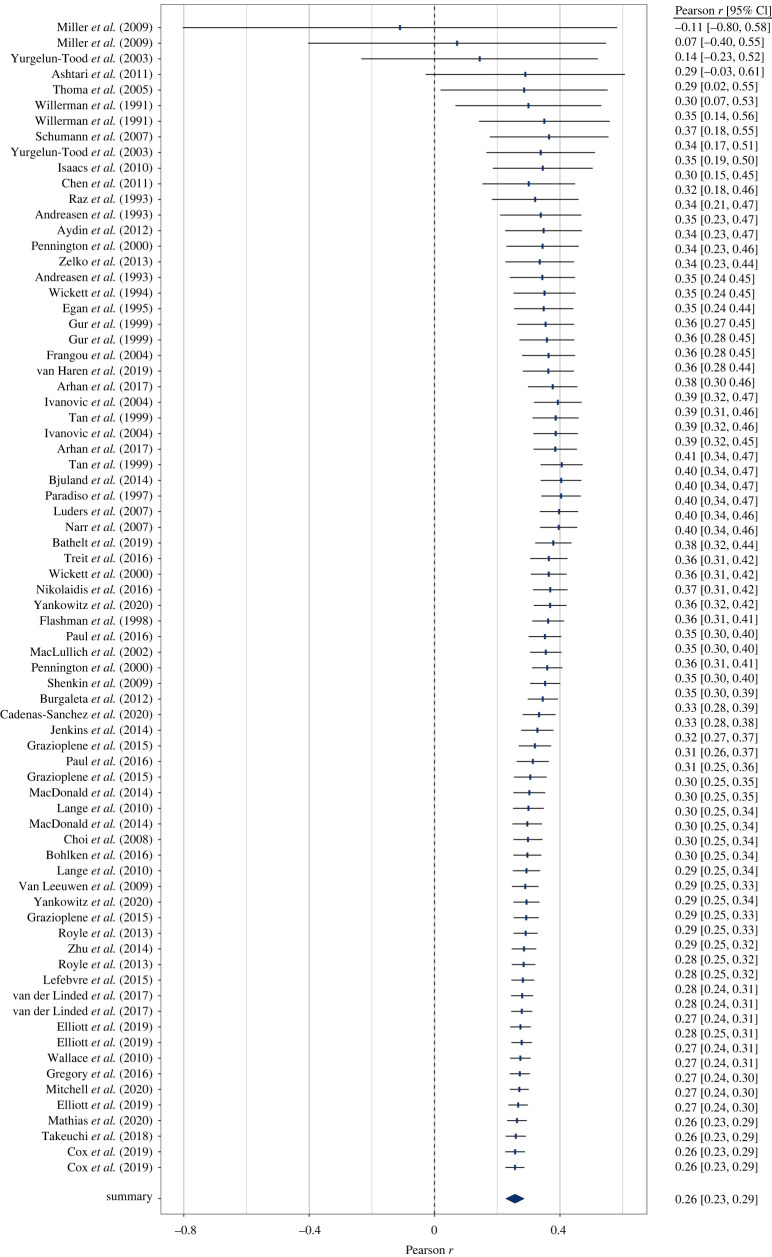
So far three meta-analyses have been published about the association between brain size and IQ. They are an illustrative example of the influences on meta-analytical results in terms of which studies have been included as well as when and how a meta-analysis was performed. The first formal meta-analysis about in vivo brain size and IQ associations was published in 2005 [23], yielding evidence for a moderate effect of r = 0.33 (i.e. explaining about 11% of variance; k = 33 independent samples, N = 1530).
About a decade later, this account was updated in the wake of another meta-analysis [24] which included an extension of the search strategy to further literature databases and the revision of the inclusion criteria (i.e. extending the inclusion from associations of only healthy to patient-based samples as well). This investigation yielded a considerably lower summary effect, indicating a small-to-moderate association between brain volume and IQ of r = 0.24 (i.e. explaining about 6% of variance; k = 120 independent samples, N = 6778). Moreover, an application of several dissemination bias assessment methods indicated that this summary effect was likely an inflated estimate due to confounding dissemination bias.
Finally, in another meta-analysis [25], a subset of study effects that had been reported in Pietschnig et al. [24] was synthesized anew. This reanalysis of a selection of studies, focusing merely on a part of the extant research evidence (i.e. about a fifth of the available independent effect sizes, representing a mere fifth of the evidence that was available in 2015), yielded a moderate effect of r = 0.31 (explaining about 10% of variance; k = 32 independent samples, N = 1758), although the authors concluded that the true association with highly g-loaded tests (i.e. g-loadings indicate how close a given test is related with the general factor of intelligence; henceforth referred to as: g-ness) should amount to about r = 0.40 (i.e. corresponding to about 16% of explained variance). Importantly, the analysis of Gignac & Bates [25] represents a comparatively small data subset based on one particular (out of many reasonable) specifications from the data of Pietschnig et al. [24].
These inconsistent results of the meta-analytical effect estimates illustrate how different procedural choices in conducting a meta-analysis are. In fact, the inconsistencies between results of these very three meta-analyses have recently been highlighted as a textbook example of specification-dependent outcomes [14]. The considerable differences of the observed summary effects have obviously meaningful implications for the meaning of the brain volume and IQ link. For instance, when assuming 16% of explained variance of the brain volume and IQ association (i.e. corresponding to the interpretation of [25]), this would mean that 2.4 IQ points of this difference are merely due to their difference in brain size, when observing two individuals with a 10 IQ point difference. But when assuming 6% of explained variance (i.e. corresponding to the estimate of [24]), only 0.9 IQ points are explained by this correlation.
Obviously, such inconsistencies in effect estimations are undesirable. Although brain volume and IQ have consistently been shown to be positively linked in prior research syntheses, the strength and therefore meaningfulness of this link remains unresolved.
In the present case, these differing estimates may be predominantly attributed to two components. On the one hand, a larger estimate of the first published meta-analysis [23] compared to subsequent updates could be attributed to the decline effect, because published primary study effects and consequently meta-analytical summary effects tend to decrease over time [22]. However, a decline effect can be ruled out as the sole cause for these differences because the most recent meta-analysis about this topic [25] yielded a larger effect than the previous estimate. On the other hand, these inconsistencies may be attributable to the different (reasonable) choices that researchers make when conceptualizing their study (or, in certain cases, analysing their data).
In these past three meta-analyses, several differences in terms of such choices can be identified. For instance, the inclusion criteria differed considerably in terms of sample characteristics (healthy versus patients; children/adolescents versus adults). Similarly, the analyses differed in terms of their methodological approaches. While two meta-analyses [23,25] used the so-called psychometric approach as developed by Hunter & Schmidt (e.g. [26]), the third one used the approach as introduced by Hedges & Olkin [27]. These two approaches most notably differ in terms of their underlying philosophy. While the former focuses on potential summary effect underestimations due to suboptimal sampling and measurement inaccuracies in the primary studies, the latter focuses on effect inflation and confounding bias. Consequently, in the former approach larger summary effects are typically obtained because individual study effects are corrected for artefacts such as range restriction or unreliability prior to the effect synthesis. The latter approach yields smaller summary effects because uncorrected estimates are synthesized.
It is evident that these differences in how to synthesize which primary study data necessarily lead to differing results. However, evidently any individual choice of a certain way to synthesize the available data may be criticized in its own right, even if this choice has been reasonably justified, thus leaving the meaning of contradictory findings of isolated meta-analytical summary estimates unresolved.
Novel methods provide a means to explore the multiverse of different design choices by allowing the assessment of a large number of (reasonable) specifications for the effect size synthesis in any given research question, thus providing a range of plausible estimates instead of an isolated point estimate (see [14]).
In the present preregistered meta-analysis of the associations between in vivo brain volume and cognitive abilities (intelligence, IQ), we aimed at resolving the ambiguity of available effect estimates by (i) updating the available meta-analytical data base, (ii) assessing subgroup analysis- and meta-regression-based influences of moderators, (iii) investigating evidence of dissemination bias and (iv) providing a range of effect estimates based on a large number of (reasonable) effect syntheses based on evidence from combinatorial, multiverse analysis, and specification-curve approaches to meta-analysis (see [28], for a similarly designed research synthesis).
2. Methods
The present study was preregistered at the Open Science Framework (OSF; https://osf.io/r6gnk). Study materials, R-codes, and all data are available at https://osf.io/y6msp/.
2.1. Inclusion and exclusion criteria
In order to be included in the present meta-analysis the primary studies were required to fulfil four criteria. First, they had to assess the association between in vivo brain volume and IQ. Second, in vivo brain volume has had to be measured by either MRI or CT. Studies had to provide measurements of the whole brain volume (TBV or ICV). If both were reported, TBV was preferred over ICV. Associations with partial brain area volume were excluded. Third, intelligence had to be measured with psychometric intelligence tests. Fourth, in cases of data dependencies, studies with the most comprehensive account of parameters were preferred. If no such hierarchy could be established, the earliest published account was coded.
Effect sizes were coded separately according to intelligence domain (full-scale, verbal and performance IQ). We used two different analysis methods when multiple subtest correlations within a certain domain were reported. First, in line with standard approaches, we selected those estimates that were judged to be conceptually more closely related to the respective domain (e.g. preferring verbal comprehension over working memory correlations for verbal IQ analyses). Second, we used robust variance estimations (RVE; [29]) to account for data dependencies while retaining the information of all coded information.
In cases where potentially eligible studies did not provide sufficient information to calculate effect sizes, corresponding study authors were contacted. When no response was received, the respective study was excluded (when effect sizes were reported to be non-significant in published papers, but no correlation coefficient was given and no response was received, coefficients were set to zero according to standard meta-analytical procedures; [30], pp. 408–409; in our study 30 out of 454 effect sizes).
2.2. Literature search
To update the so far most comprehensive available data set of IQ and brain size correlations [24], we first searched the online literature databases PubMed, ISI Web of Science, Scopus and Google Scholar using the string: (brain size AND intelligen*) OR (brain volume AND intelligen*) OR (brain size AND IQ) OR (brain volume AND IQ).
Second, we used a forward citation search of all three published meta-analyses on the subject [23–25]. Third, we conducted an extensive search for unpublished accounts in grey literature databases, search engines and repositories, sources dedicated to theses and dissertations, conference materials and registries for active studies. Finally, reference lists of all identified studies were handsearched for additional potentially eligible studies. Individual search strings and covered databases are available from https://osf.io/7z2u3/. Sample sex ratio, sample type (healthy versus patient samples), test instrument, publication year, mean sample age, participant numbers, effect size estimates and volumetric as well as IQ test standard deviations, number of corrections to the correlation coefficient (e.g. controlling for body height or mass), publication status (i.e. published versus grey literature versus personal communication), sample age (children/adolescents versus adults), brain volume measurement type (TBV or ICV), IQ domain and the assumed g-loadedness of the respectively used intelligence test (g-ness: fair versus good versus excellent; these categories correspond to the three rating criteria of [25], indicating a rank score based on included test number, dimensions and correlation with g; testing time was presently not used as an evaluation criterion; for details refer to [25]) were recorded independently by two researchers (D.G., M.Z.). A coding book containing data sheets and a coding manual explaining all variables and their categories is available at https://osf.io/fr8g7/. Inconsistencies were resolved by discussion with a third independent coder (J.P.). All searches were updated on April 14, 2021.
2.3. Synthesis methods
Meta-analytical calculations were performed in R by means of the metafor package [31].
2.3.1. Hedges and Olkin meta-analysis
A random-effects meta-analysis in the tradition of Hedges & Olkin [27] was conducted based on independent effect sizes. Effect syntheses were calculated based on Fisher's z-transformed values to account for the skewed distributions of Pearson rs. For ease of interpretation, results were back-transformed into the r-metric prior to reporting. Some researchers have criticized this procedure because it may introduce a substantial upward bias (see, [26]). Therefore, we conducted a sensitivity analysis with correlations corrected for this negative bias [32].
Effect sizes were weighted according to study precision. Precision of the effect size estimates is illustrated by 95% confidence intervals (CI) based on the Knapp-Hartung adjustment [33,34]. In sensitivity analyses, we conducted leave-one-out analyses to evaluate influences of individual studies on the overall effect size and assessed potential influences of outliers following the approach of Viechtbauer & Cheung [35].
2.3.2. Psychometric meta-analysis
Within the framework of psychometric meta-analyses, researchers aim at accounting for potential measurement inaccuracy and sampling errors. Following the specifications of two previous meta-analyses [23,25], we accounted for direct range restriction only (i.e. using the Case II formula of [36], to correct correlations and the approach of [37], to estimate their standard errors; see, [38]), because (i) reliabilities of MRI and IQ measures are typically high and (ii) reliabilities were infrequently reported [23,25]. Here, we used the n-weighted Hunter and Schmidt estimator (HS) to calculate effect syntheses.
2.3.3. Robust variance estimation
By means of this approach, we were able to include more than one effect size per sample within a particular study and intelligence domain, following current recommendations [39]. Data dependencies within domain-specific analyses (i.e. full-scale versus verbal versus performance IQ) were modelled using robust variance estimation within meta-regressions, thus allowing for including a maximum amount of available information of primary studies (i.e. inclusion of multiple effect sizes from identical participants; this is reasonable because full-scale, verbal and performance IQ results are typically highly intercorrelated), while avoiding inappropriate effect size weighting due to dependent data [40].
We used a correlated-effects model because data dependence was primarily caused by correlations between participants' domain intelligence scores from different (sub)domains. We used τ2 (random-effects maximum likelihood) and ω2 by means of simplistic methods of moments estimators to estimate weights [41]. Fisher's z-transformed correlation coefficients were used for analyses.
2.4. Moderator analyses
2.4.1. Subgroup analyses
Influences of categorical variables were examined in a series of mixed-effects subgroup analyses. We assessed group differences according to sample type (healthy versus clinical), age (children/adolescents versus adults), sex (men versus women) and g-ness. Furthermore, another two supplemental subgroup analyses were performed to (i) further corroborate results of bias analyses (i.e. published in a peer-reviewed journal versus obtained from grey literature or through personal communication) and (ii) assess possible influences of different brain volume measurement types (i.e. total brain volume versus intracranial volume).
2.4.2. Meta-regressions
2.4.2.1. Single regressions
Initially, a series of single regressions of effect sizes on study publication years was calculated for each IQ domain, to assess evidence for a potential decline effect. Differences of associations between intelligence domains were evaluated by means of a RVE-based meta-regression. For each RVE-regression, we ran one model with and one model without intercept to examine differences of coefficients compared to full-scale IQ and regression-based effect estimates for each domain, respectively.
2.4.2.2. Multiple regressions
Subsequently, theory-guided hierarchical multiple precision-weighted mixed-effects meta-regressions were calculated for each domain. In three blocks we included (i) g-ness (only for full-scale IQ analyses; fair/good versus excellent), publication year, publication status; (ii) male ratio, mean sample age and (iii) study goal (i.e. assessment of IQ and brain volume association was the primary study goal versus not), number of included covariates in study. Model fit between blocks was compared by means of likelihood ratio tests. In supplemental analyses, we repeated these calculations only in studies that reported total brain volume (results omitted for brevity).
2.5. Dissemination bias
We used several dissemination bias detection methods to assess potential influences of summary effect-biasing artefacts. The use of multiple detection methods is sensible because different methods have been shown to not be equally sensitive to different bias scenarios and sources [42,43].
Results of bias analyses were based on published data only (excepting direct comparisons) and we focused on healthy samples (i.e. following the specifications of [25], and [23]), because bias may be expected to be stronger in neurotypical samples (i.e. because in non-neurotypical samples, expectations of certain effect strength observations may be lower and reporting of effect sizes therefore less dependent on conforming to preceding observations). Fisher's r-to-z transformed correlation coefficients were used (excepting p-value-based analyses) and analyses were carried out separately for full-scale, verbal and performance IQ.
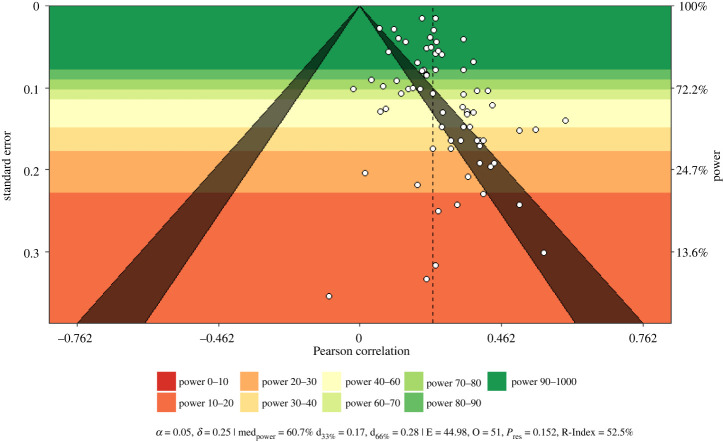
First, we provide power-enhanced funnel plots (i.e. sunset plots, see [44]; using the R package metaviz, [45]) comprising estimates for the median power of all studies, necessary true effect estimates for a median power reduction to 33% or 66%, results of the test of excess significance (TES; observed random-effects summary effects were used for study power calculations; [46]), as well as the R-Index for expected replicability [47].
Second, we used Sterne & Egger's regression approach [48] by regressing effect sizes on the standard normal deviate effect size of the inverse standard error (p-values < 0.10 were considered indicative of bias). Third, the trim-and-fill method [49] was applied to detect potential funnel plot asymmetry. Resulting bias-adjusted estimates were interpreted in terms of a sensitivity analysis rather than a corrected summary effect estimate.
Fourth, we used three novel methods that are based on the distribution of published p-values and provide a means to estimate summary effect sizes and detect evidence for p-hacking and dissemination bias. The methods p-curve [50], p-uniform [51] and p-uniform* [52] are based on the idea that p-values follow a uniform distribution when the true investigated effect of any given research question is zero. In p-curve and p-uniform, only significant p-values are included in the analyses. This makes sense, because significant effects should have an identical publication probability. Observed right-skewness of these distributions are interpreted to be indicative of a true non-zero effect while observed left-skewness indicates confounding effects of p-hacking. Examinations of p-curves allow consequently a formal test of p-hacking by examining the shape of the observed p-value distribution (p-curve) or comparing observed with expected conditional p-values (p-uniform). By minimizing a loss function resulting from inspection of the observed p-value distribution (p-curve) or assessing the value for which the conditional p-value distribution is uniform (p-uniform), summary effects can be estimated.
Effect-size estimations that are based on p-uniform* rely on both significant and non-significant p-values (here, it is assumed that publication probabilities are identical within, but not across, groups). P-uniform has been shown to be more precise than both p-curve and p-uniform in the presence of non-trivial between-studies heterogeneity and allows assessment of the extent of unobserved heterogeneity [52]. P-uniform and p-uniform* were calculated by means of the R package puniform [53] and the R code available from www.p-curve.com/Supplement.
Fifth, we used two selection-model approaches that were either based on the four identical weight functions for p-values, as used in Pietschnig et al. [24]), or on the standard error of the effect sizes [54]. By means of these approaches, the robustness of the summary effect can be assessed when primary study effects are weighted according to them being more or less significant (p-values were weighted according to the approach of [55]) and more or less accurate [54]. We used metasens [56] to conduct the Copas and Shi analysis.
Sixth, we inspected the overlap between two DerSimonian-Laird estimator-based confidence intervals by means of the approach of Henmi & Copas [57]. This method can be seen as a type of sensitivity analysis in which conventionally calculated confidence intervals are compared with those of a hybrid estimation resulting from the use of fixed-effect weights but random-effects heterogeneity estimates. Large discrepancies between effect and confidence interval estimates are considered to be indicative of bias, although there is no consensus about a suitable hard-and-fast decision criterion.
Seventh, we directly assessed potential bias by regressing effect sizes on publication type (published versus unpublished effects). Moreover, we assessed cross-temporal changes in effect size in another meta-regression model, in order to assess the evidence for a potentially confounding decline effect in the data, as evidenced in a predecessor meta-analysis on this topic [24].
Finally, we conducted two cumulative meta-analyses according to (i) sample size and (ii) publication year to further illustrate potential influences of small-study effects and cross-temporal changes.
2.6. Exploring the multiverse
To untangle specific analytical design choices (i.e. reflecting researcher degrees of freedom) from the general pattern of brain size and IQ associations, we explored the multiverse of possible (reasonable) meta-analytic design specifications [14] using the R Code available from https://osf.io/kqgey/.
2.6.1 . Combinatorial meta-analysis
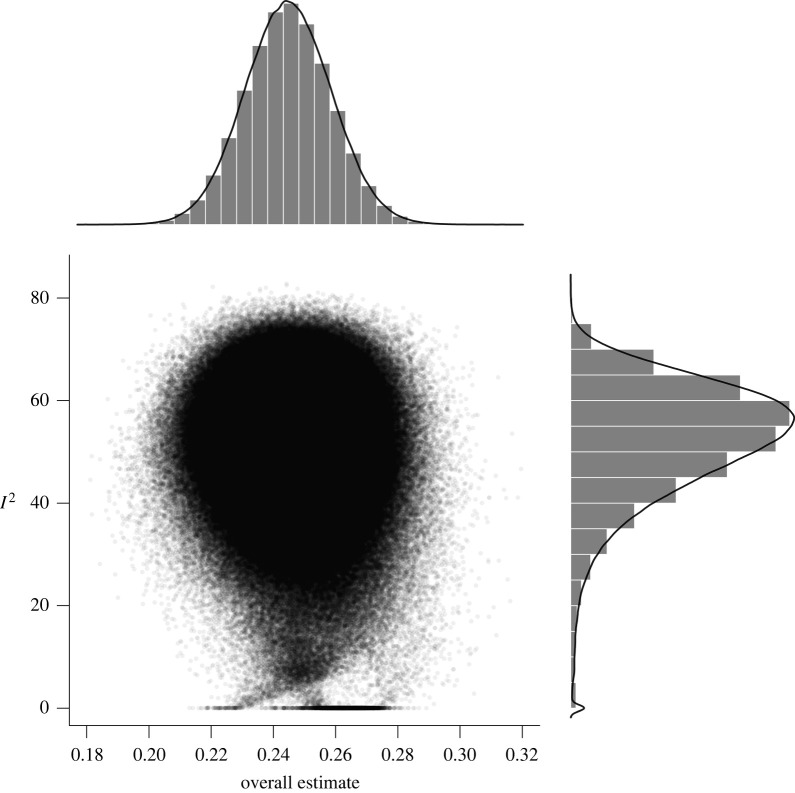
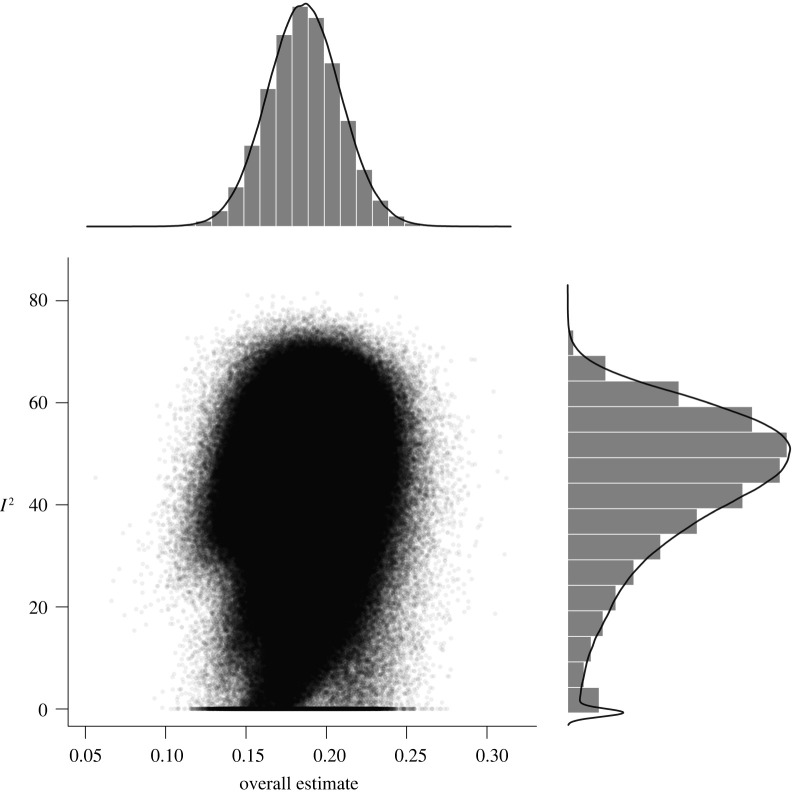
To this end, we first used a combinatorial meta-analysis to examine estimates for a large random selection of any possible subset of the 2k − 1 possible (not necessarily reasonable) combinations of the available data [20]. This approach can be interpreted as a sensitivity analysis by allowing the identification of outlier studies that overproportionally influence effect estimations.
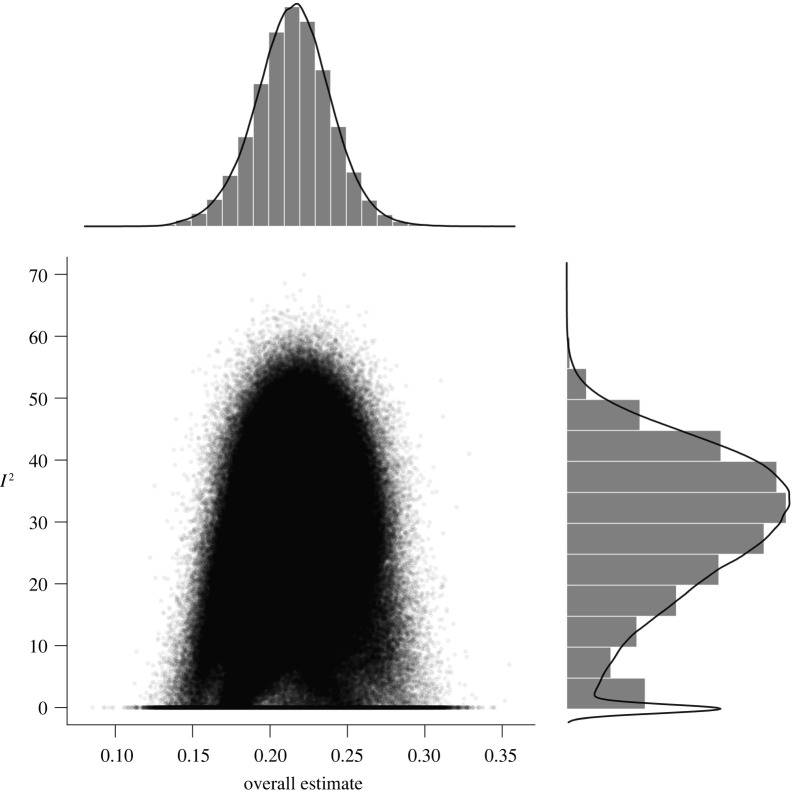
Consequently, 2123 and 271 (about 10 undecillions and 2 undecillions) combinations were possible for an exhaustive selection of full-scale IQ subsets for healthy and patient samples, respectively (270 and 245 as well as 249 and 233 combinations were possible for verbal and performance IQ). For our analyses, we randomly drew 100 000 subsets out of these possible combinations to illustrate outlier influences in GOSH plots (Graphic Display of Heterogeneity). Specifically, GOSH plots allow a visual inspection of summary effect distributions and their associated between-studies heterogeneity when any kind of (un)reasonable specification has been used. Moreover, numerical inspections of dispersion values (e.g. interquartile ranges) and the effect distribution permit an evaluation of the influence of moderating variables (i.e. narrow intervals and symmetrical distributions indicated well-interpretable summary effects).
2.6.2 . Specification-curve meta-analysis
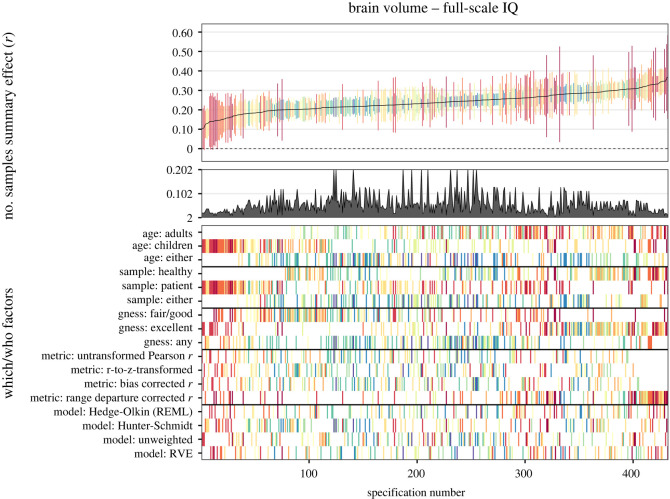
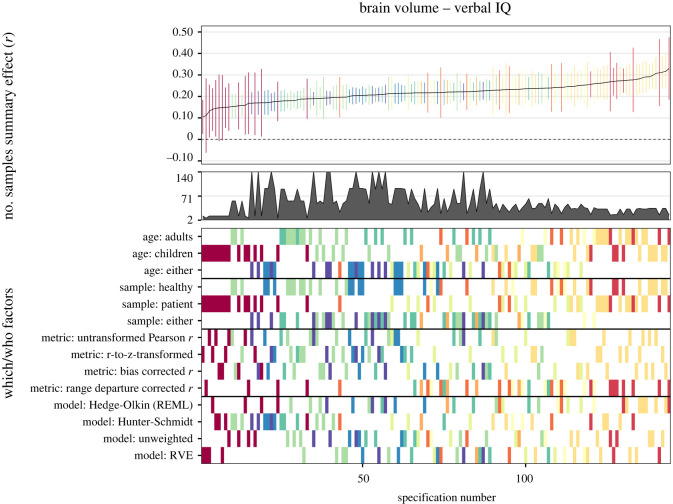
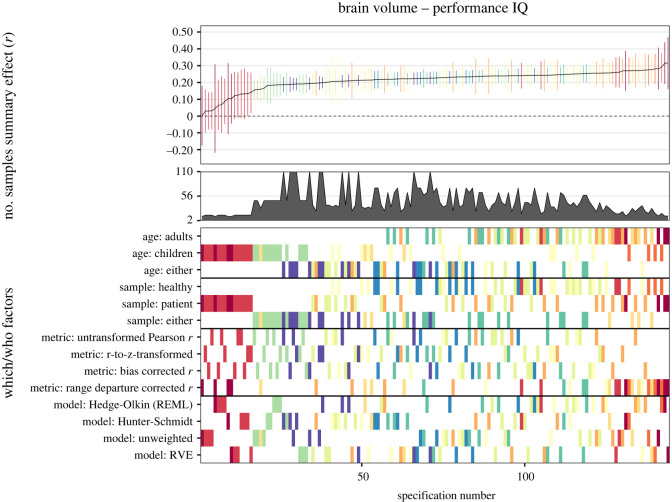
By means of another method, we examined several possible reasonable specifications. In our meta-analytic specification-curve analyses (see [14]), we introduced three which factors: (1) sample age: adults versus children/adolescents versus either; (2) sample type: healthy versus patient versus either; (3) g-ness (for full-scale IQ only): fair or good versus excellent versus either; and two how factors: (1) effect size type: non-transformed Pearson rs versus r-to-z-transformed effect size versus small sample bias corrected rs versus range departure corrected rs; (2) analysis approach: Hedges-Olkin random-effects estimation versus Hunter-Schmidt effect estimation versus unweighted estimation versus RVE).
Consequently, these potentially influential analytic choices yielded 3 × 3 × 3 = 27 ways for which data to analyze and 4 × 4 = 16 ways for how to do it, thus totaling 27 × 16 = 432 reasonable specifications. In our further analyses in the subsets of verbal and performance data only, this number was reduced to 144 reasonable specifications because g-ness was not relevant in these cases. Specifications that comprised less than two effect sizes were dropped from analyses.
2.7. Final sample
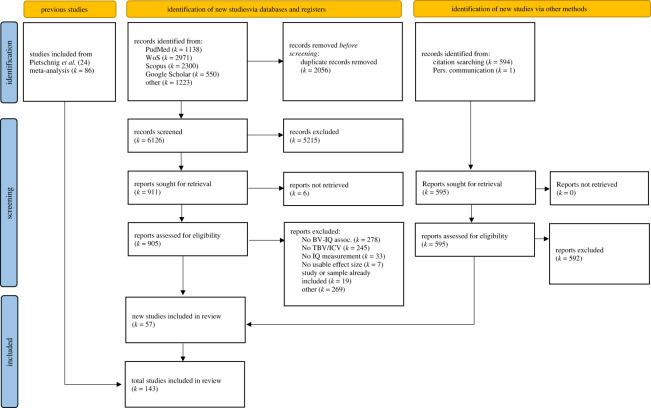
We included effect sizes of 86 studies from Pietschnig et al. [24] ([58] and [59] were presently excluded because (i) of duplicate effect size publication and (ii) brain-volume measurements had not been performed in vivo) as well as 57 newly identified studies. Because more recent studies were based on larger samples, this update more than tripled the included participant numbers compared to Pietschnig et al. [24], thus representing a crucial expansion of the empirical knowledge base. In all, we extracted 454 effect sizes including 194 independent samples for full-scale, 115 for verbal, and 82 for performance IQ associations (Ns = 26 764; 7667; and 5984; respectively). The majority of samples comprised healthy participants and about a third comprised patients (ks = 123 versus 71; 70 versus 45; 49 versus 33; Ns = 23 403 versus 5361; 5440 versus 2237; 4162 versus 1858 for healthy and patient samples for full-scale, verbal and performance IQ, respectively). A PRISMA flowchart for the study inclusion process is provided in figure 1 (for study characteristics, table 1).
Figure 1.
PRISMA flowchart.
Table 1.
Characteristics of included studies. Note. NA = info not available; Review: 1 = included in McDaniel [23], 2 = included in Pietschnig et al. [24], 3 = included in Gignac & Bates [25], 4 = included in present update; Reporting: reported = published in a journal article, grey = published as thesis/dissertation, PC = result obtained via personal communication; FSIQ = full-scale IQ; Type of test: IQ assessment used in study; subtest abbreviations: arith = arithmetic, bd = block design, com = comprehension, ds = digit symbol, inf = information, lm = logical memory, lns = letter-number sequencing, mr = matrix reasoning, obj = object assembly, pc = picture completion, pic = picture arrangement, sim = similarities, span = digit span (b stand for backwards), ss = symbol search, ss p + f = spatial span forwards and backwards, vpa = verbal pair associates; domain indices of the Wechsler scales are abbreviated as follows: POI = perceptual organization index, PRI = perceptual reasoning index, PSI = processing speed index, VCI = verbal comprehension index, WMI = working memory index; full information explaining all abbreviations are available in the codebook and data files in supplemental materials. Published study outcomes with r = exactly 0 represent correlations set to zero, because no eligible numerical value was available.
| study | year | review | sample type | mean age | male ratio (%) | reporting | IQ domain | test | n | r |
|---|---|---|---|---|---|---|---|---|---|---|
| Yeo et al. [60] | 1987 | 2, 4 | patients | 38.40 | 34.00 | reported | FSIQ | WAIS | 41 | 0.007 |
| Yeo et al. [60] | 1987 | 2, 4 | patients | 38.40 | 34.00 | reported | verbal | WAIS verbal | 41 | 0.12 |
| Yeo et al. [60] | 1987 | 2, 4 | patients | 38.40 | 34.00 | reported | performance | WAIS performance | 41 | 0.06 |
| Willerman et al. [5] | 1991 | 1, 2, 3, 4 | healthy | 18.90 | 0.00 | reported | FSIQ | WAIS-R: voc, sim, bd, pc | 20 | 0.33 |
| Willerman et al. [5] | 1991 | 1, 2, 3, 4 | healthy | 18.90 | 100.00 | reported | FSIQ | WAIS-R: voc, sim, bd, pc | 20 | 0.51 |
| Andreasen et al. [61] | 1993 | 1, 2, 3, 4 | healthy | 38.00 | 0.00 | reported | FSIQ | WAIS-R | 30 | 0.44 |
| Andreasen et al. [61] | 1993 | 1, 2, 3, 4 | healthy | 38.00 | 100.00 | reported | FSIQ | WAIS-R | 37 | 0.40 |
| Andreasen et al. [61] | 1993 | 2, 4 | healthy | 38.00 | 0.00 | reported | verbal | WAIS-R verbal | 30 | 0.43 |
| Andreasen et al. [61] | 1993 | 2,4 | healthy | 38.00 | 100.00 | reported | verbal | WAIS-R verbal | 37 | 0.33 |
| Andreasen et al. [61] | 1993 | 2, 4 | healthy | 38.00 | 0.00 | reported | performance | WAIS-R performance | 30 | 0.30 |
| Andreasen et al. [61] | 1993 | 2, 4 | healthy | 38.00 | 100.00 | reported | performance | WAIS-R performance | 37 | 0.43 |
| Raz et al. [62] | 1993 | 1, 2, 3, 4 | healthy | 43.80 | 59.00 | reported | fluid | CFIT | 29 | 0.43 |
| Raz et al. [62] | 1993 | 2, 4 | healthy | 43.80 | 59.00 | reported | verbal | Extended Vocabulary (V3) | 29 | 0.10 |
| Castellanos et al. [63] | 1994 | 1, 2, 4 | healthy | 12.10 | 100.00 | reported | verbal | WISC-R: voc | 46 | 0.33 |
| Harvey et al. [64] | 1994 | 2, 4 | patients | 35.60 | 38.00 | reported | verbal | NART | 26 | 0.38 |
| Harvey et al. [64] | 1994 | 2, 4 | patients | 31.10 | 77.00 | reported | verbal | NART | 48 | 0.24 |
| Harvey et al. [64] | 1994 | 2, 4 | healthy | 31.60 | 55.00 | reported | verbal | NART | 34 | 0.69 |
| Jones et al. [65] | 1994 | 2, 4 | healthy | 31.70 | 64.00 | reported | verbal | NART or WAIS-R verbal | 67 | 0.30 |
| Wickett et al. [66] | 1994 | 1, 2, 3, 4 | healthy | 25.00 | 0.00 | reported | FSIQ | MAB | 40 | 0.40 |
| Wickett et al. [66] | 1994 | 2, 4 | healthy | 25.00 | 0.00 | reported | verbal | MAB verbal | 40 | 0.44 |
| Wickett et al. [66] | 1994 | 2, 4 | healthy | 25.00 | 0.00 | reported | performance | MAB performance | 40 | 0.28 |
| Bigler [67] | 1995 | 2, 4 | patients | 29.40 | 71.00 | reported | FSIQ | WAIS-R | 72 | −0.03 |
| Egan et al. [68] | 1995 | 1, 2, 3, 4 | healthy | 22.50 | 100.00 | reported | FSIQ | WAIS-R | 40 | 0.31 |
| Egan et al. [68] | 1995 | 2, 4 | healthy | 22.50 | 100.00 | reported | verbal | WAIS-R verbal | 40 | 0.21 |
| Egan et al. [68] | 1995 | 2, 4 | healthy | 22.50 | 100.00 | reported | performance | WAIS-R performance | 40 | 0.22 |
| Haier et al. [69] | 1995 | 2, 4 | patients | 26.39 | 54.00 | reported | FSIQ | WAIS-R | 28 | 0.65 |
| Kareken et al. [70] | 1995 | 1, 2, 4 | healthy | 27.66 | 63.00 | PC | FSIQ | WAIS-R | 68 | 0.30 |
| Kareken et al. [70] | 1995 | 4 | patients | 29.75 | 63.00 | reported | verbal | COWA, Animal Naming, Boston Naming, Token Test, WRAT: Reading | 68 | 0.36 |
| Kareken et al. [70] | 1995 | 4 | healthy | 27.66 | 63.00 | reported | verbal | COWA, Animal Naming, Boston Naming, Token Test, WRAT: Reading | 68 | 0.24 |
| Kareken et al. [70] | 1995 | 4 | patients | 29.75 | 63.00 | reported | performance | WAIS-R: bd; Benton Line Orientation, Geometric Figure Drawings | 68 | 0.18 |
| Kareken et al. [70] | 1995 | 4 | healthy | 27.66 | 63.00 | reported | performance | WAIS-R: bd; Benton Line Orientation, Geometric Figure Drawings | 68 | 0.26 |
| Raz et al. [71] | 1995 | 2, 4 | patients | 35.20 | 77.00 | reported | FSIQ | WPPSI-R + BCS | 11 | −0.24 |
| Reiss et al. [72] | 1995 | 2, 4 | healthy | 11.28 | 42.00 | PC | FSIQ | WISC-R or SB or BSID | 87 | 0.00 |
| Reiss et al. [72] | 1995 | 2, 4 | patients | 10.80 | 35.00 | reported | FSIQ | WISC-R or SB or BSID | 51 | 0.25 |
| Reiss et al. [73] | 1996 | 1, 2, 4 | healthy | 10.60 | 0.00 | PC | FSIQ | unknown FS | 57 | 0.37 |
| Reiss et al. [73] | 1996 | 2, 4 | healthy | 10.10 | 100.00 | PC | FSIQ | unknown FS | 12 | 0.52 |
| Blatter et al. [74] | 1997 | 2, 4 | patients | NA | NA | reported | verbal | WAIS-R verbal | 22 | 0.57 |
| Blatter et al. [74] | 1997 | 2, 4 | patients | NA | NA | reported | performance | WAIS-R performance | 21 | 0.47 |
| Mori et al. [75] | 1997 | 2, 4 | patients | 70.20 | 38.00 | reported | FSIQ | WAIS-R | 60 | 0.40 |
| Mori et al. [75] | 1997 | 2, 4 | patients | 70.20 | 38.00 | reported | verbal | WAIS-R verbal | 60 | 0.37 |
| Mori et al. [75] | 1997 | 2, 4 | patients | 70.20 | 38.00 | reported | performance | WAIS-R performance | 60 | 0.37 |
| Paradiso et al. [76] | 1997 | 2, 3, 4 | healthy | 24.80 | 53.00 | reported | FSIQ | WAIS-R | 62 | 0.38 |
| Paradiso et al. [76] | 1997 | 2, 4 | healthy | 24.80 | 53.00 | reported | verbal | WAIS-R: voc | 62 | 0.27 |
| Paradiso et al. [76] | 1997 | 2, 4 | healthy | 24.80 | 53.00 | reported | performance | WAIS-R: bd | 62 | 0.32 |
| Paradiso et al. [76] | 1997 | 4 | healthy | 24.80 | 53.00 | reported | verbal | WAIS-R: span | 62 | 0.11 |
| Flashman et al. [77] | 1998 | 1, 2, 4 | healthy | 27.00 | 53.00 | reported | FSIQ | WAIS-R | 90 | 0.25 |
| Flashman et al. [77] | 1998 | 2, 4 | healthy | 27.00 | 53.00 | reported | verbal | WAIS-R verbal | 90 | 0.16 |
| Flashman et al. [77] | 1998 | 2, 4 | healthy | 27.00 | 53.00 | reported | performance | WAIS-R performance | 90 | 0.26 |
| Gur et al. [78] | 1999 | 1, 2, 3, 4 | healthy | 25.00 | 0.00 | reported | FSIQ | WAIS-R: voc, bd; CVLT, JLO | 40 | 0.40 |
| Gur et al. [78] | 1999 | 1, 2, 3, 4 | healthy | 27.00 | 100.00 | reported | FSIQ | WAIS-R: voc, bd; CVLT, JLO | 40 | 0.39 |
| Gur et al. [78] | 1999 | 2, 4 | healthy | 25.00 | 0.00 | reported | verbal | WAIS-R: voc; CVLT | 40 | 0.40 |
| Gur et al. [78] | 1999 | 2, 4 | healthy | 27.00 | 100.00 | PC | verbal | WAIS-R: voc; CVLT | 40 | 0.00 |
| Gur et al. [78] | 1999 | 4 | healthy | 25.00 | 0.00 | reported | performance | WAIS-R: bd; JLO | 40 | 0.57 |
| Gur et al. [78] | 1999 | 4 | healthy | 27.00 | 100.00 | reported | performance | WAIS-R: bd; JLO | 40 | 0.35 |
| Leonard et al. [79] | 1999 | 2, 4 | patients | 43.00 | 100.00 | PC | verbal | WAIS-R verbal | 37 | 0.00 |
| Leonard et al. [79] | 1999 | 2, 4 | healthy | 42.00 | 100.00 | PC | verbal | WAIS-R verbal | 33 | 0.00 |
| Leonard et al. [79] | 1999 | 2, 4 | patients | 43.00 | 100.00 | PC | performance | WAIS-R performance | 37 | 0.00 |
| Leonard et al. [79] | 1999 | 2, 4 | healthy | 42.00 | 100.00 | PC | performance | WAIS-R performance | 33 | 0.00 |
| Tan et al. [80] | 1999 | 1, 2, 3, 4 | healthy | 22.00 | 0.00 | reported | fluid | CFIT | 54 | 0.62 |
| Tan et al. [80] | 1999 | 1, 2, 3, 4 | healthy | 22.00 | 100.00 | reported | fluid | CFIT | 49 | 0.28 |
| Warwick et al. [81] | 1999 | 2, 4 | patients | 21.60 | 0.00 | PC | verbal | Quick IQ Test [82] | 11 | 0.00 |
| Warwick et al. [81] | 1999 | 2, 4 | healthy | 21.50 | 0.00 | PC | verbal | Quick IQ Test [82] | 13 | 0.00 |
| Warwick et al. [81] | 1999 | 2, 4 | patients | 21.80 | 100.00 | PC | verbal | Quick IQ Test [82] | 10 | 0.00 |
| Warwick et al. [81] | 1999 | 2, 4 | patients | 21.80 | 100.00 | PC | verbal | Quick IQ Test [82] | 10 | 0.00 |
| Warwick et al. [81] | 1999 | 2, 4 | healthy | 21.50 | 100.00 | PC | verbal | Quick IQ Test [82] | 25 | 0.00 |
| Warwick et al. [81] | 1999 | 2, 4 | patients | 21.63 | 100.00 | reported | verbal | Quick IQ Test [82] | 45 | 0.31 |
| Warwick et al. [81] | 1999 | 2, 4 | patients | 21.55 | 0.00 | reported | verbal | Quick IQ Test [82] | 24 | 0.53 |
| Garde et al. [83] | 2000 | 1, 2, 3, 4 | healthy | 80.70 | 0.00 | PC | FSIQ | WAIS | 22 | 0.22 |
| Garde et al. [83] | 2000 | 1, 2, 3, 4 | healthy | 80.70 | 100.00 | PC | FSIQ | WAIS | 46 | 0.07 |
| Isaacs et al. [84] | 2000 | 2, 4 | healthy | 7.75 | 73.00 | PC | FSIQ | WISC-III | 11 | −0.03 |
| Isaacs et al. [84] | 2000 | 2, 4 | healthy | 7.75 | 38.00 | PC | FSIQ | WISC-III | 8 | 0.55 |
| Isaacs et al. [84] | 2000 | 2, 4 | healthy | 7.75 | 73.00 | PC | verbal | WISC-III verbal | 11 | −0.04 |
| Isaacs et al. [84] | 2000 | 2, 4 | healthy | 7.75 | 38.00 | PC | verbal | WISC-III verbal | 8 | 0.57 |
| Isaacs et al. [84] | 2000 | 2, 4 | healthy | 7.75 | 73.00 | PC | performance | WISC-III performance | 11 | −0.18 |
| Isaacs et al. [84] | 2000 | 2, 4 | healthy | 7.75 | 38.00 | PC | performance | WISC-III performance | 8 | 0.35 |
| Kumra et al. [85] | 2000 | 2, 4 | patients | 12.30 | 81.00 | PC | FSIQ | WISC-III or WISC-R or WAIS: voc, bd | 27 | 0.00 |
| Kumra et al. [85] | 2000 | 2, 4 | patients | 14.40 | 57.00 | PC | FSIQ | WISC-III or WISC-R or WAIS: voc, bd | 44 | 0.00 |
| Lawson et al. [86] | 2000 | 2, 4 | patients | NA | NA | reported | FSIQ | WISC-III or WPPSI-R or DAS or SB or GMDS | 47 | 0.43 |
| Pennington et al. [87] | 2000 | 1, 2, 4 | healthy | 19.06 | 44.00 | reported | FSIQ | WISC-R or WAIS-R FS | 36 | 0.31 |
| Pennington et al. [87] | 2000 | 2, 4 | healthy | 16.97 | 58.00 | reported | FSIQ | WISC-R or WAIS-R FS | 96 | 0.42 |
| Schoenemann et al. [88] | 2000 | 1, 2, 3, 4 | healthy | 23.20 | 0.00 | PC | fluid | RSPM | 72 | 0.21 |
| Schoenemann et al. [88] | 2000 | 2, 4 | healthy | 23.20 | 0.00 | reported | verbal | MAB vocabulary | 36 | 0.12 |
| Wickett et al. [89] | 2000 | 1, 2, 3, 4 | healthy | 24.97 | 100.00 | reported | FSIQ | MAB | 68 | 0.35 |
| Wickett et al. [89] | 2000 | 2, 4 | healthy | 24.97 | 100.00 | reported | verbal | MAB verbal | 68 | 0.33 |
| Wickett et al. [89] | 2000 | 2, 4 | healthy | 24.97 | 100.00 | reported | performance | MAB performance | 68 | 0.31 |
| Castellanos et al. [90] | 2001 | 2, 4 | patients | 9.70 | 0.00 | reported | FSIQ | WISC-R or WISC-III: voc, bd | 40 | 0.36 |
| Coffey et al. [91] | 2001 | 2, 4 | healthy | 74.85 | 38.00 | reported | verbal | Verbal fluecy Task | 319 | −0.06 |
| Coffey et al. [91] | 2001 | 2, 4 | healthy | 74.85 | 38.00 | reported | performance | WAIS-R: bd | 318 | 0.06 |
| Aylward et al. [92] | 2002 | 1, 2, 4 | healthy | NA | 100.00 | PC | FSIQ | unknown FS | 46 | −0.13 |
| Aylward et al. [92] | 2002 | 1, 2, 4 | healthy | NA | NA | PC | FSIQ | unknown FS | 30 | 0.08 |
| Aylward et al. [92] | 2002 | 2, 4 | patients | 18.80 | 87.00 | reported | FSIQ | unknown FS | 67 | 0.10 |
| Aylward et al. [92] | 2002 | 2, 4 | patients | 18.80 | 87.00 | reported | verbal | unknown verbal | 67 | 0.08 |
| Aylward et al. [92] | 2002 | 2, 4 | healthy | 18.90 | 92.00 | reported | verbal | unknown verbal | 83 | −0.01 |
| Aylward et al. [92] | 2002 | 2, 4 | patients | 18.80 | 87.00 | reported | performance | unknown performance | 67 | 0.10 |
| Aylward et al. [92] | 2002 | 2, 4 | healthy | 18.90 | 92.00 | reported | performance | unknown performance | 83 | 0.09 |
| MacLullich et al. [93] | 2002 | 1, 2, 3, 4 | healthy | 67.80 | 100.00 | reported | fluid | RSPM | 95 | 0.39 |
| MacLullich et al. [93] | 2002 | 2, 4 | healthy | 67.80 | 100.00 | reported | verbal | NART | 97 | 0.30 |
| Nosarti et al. [94] | 2002 | 1, 2, 4 | healthy | 14.90 | 65.00 | PC | FSIQ | unknown FS | 42 | 0.37 |
| Shapleske et al. [95] | 2002 | 1, 2, 3, 4 | healthy | 33.30 | 100.00 | PC | FSIQ | unknown FS | 23 | 0.13 |
| Collinson et al. [96] | 2003 | 2, 4 | healthy | 16.40 | 60.00 | PC | FSIQ | WISC-R or WAIS-R | 22 | −0.13 |
| Collinson et al. [96] | 2003 | 2, 4 | patients | 16.80 | 67.00 | PC | FSIQ | WISC-R or WAIS-R | 32 | −0.27 |
| Collinson et al. [96] | 2003 | 2, 4 | patients | 16.80 | 67.00 | PC | verbal | WISC-R or WAIS-R verbal | 32 | −0.28 |
| Collinson et al. [96] | 2003 | 2, 4 | healthy | 16.40 | 60.00 | PC | verbal | WISC-R or WAIS-R verbal | 22 | −0.09 |
| Collinson et al. [96] | 2003 | 2, 4 | patients | 16.80 | 67.00 | PC | performance | WISC-R or WAIS-R performance | 32 | −0.19 |
| Collinson et al. [96] | 2003 | 2, 4 | healthy | 16.40 | 60.00 | PC | performance | WISC-R or WAIS-R performance | 22 | −0.17 |
| Giedd [97] | 2003 | 1, 2, 4 | healthy | NA | 0.00 | PC | FSIQ | unknown FS | 8 | 0.46 |
| Giedd [97] | 2003 | 1, 2, 4 | healthy | NA | 100.00 | PC | FSIQ | unknown FS | 7 | 0.17 |
| Giedd [97] | 2003 | 1, 2, 4 | healthy | NA | 0.00 | PC | FSIQ | unknown FS | 7 | −0.67 |
| Giedd [97] | 2003 | 1, 2, 4 | healthy | NA | 100.00 | PC | FSIQ | unknown FS | 7 | 0.67 |
| Giedd [97] | 2003 | 1, 2, 4 | healthy | NA | 0.00 | PC | FSIQ | unknown FS | 39 | 0.34 |
| Giedd [97] | 2003 | 1, 2, 4 | healthy | NA | 100.00 | PC | FSIQ | unknown FS | 63 | 0.27 |
| Kesler et al. [98] | 2003 | 2, 4 | patients | 26.16 | 52.00 | reported | FSIQ | WAIS-R | 25 | 0.47 |
| Kesler et al. [98] | 2003 | 2, 4 | patients | 26.16 | 52.00 | reported | verbal | WAIS-R verbal | 25 | 0.57 |
| Yurgelun-Todd et al. [99] | 2003 | 2, 4 | healthy | 14.60 | 0.00 | reported | FSIQ | Shipley total | 24 | 0.20 |
| Yurgelun-Todd et al. [99] | 2003 | 2, 4 | healthy | 14.50 | 100.00 | reported | FSIQ | Shipley total | 13 | 0.26 |
| Yurgelun-Todd et al. [99] | 2003 | 2, 4 | healthy | 14.60 | 0.00 | reported | verbal | Shipley verbal | 24 | 0.17 |
| Yurgelun-Todd et al. [99] | 2003 | 2, 4 | healthy | 14.50 | 100.00 | reported | verbal | Shipley verbal | 13 | 0.19 |
| Yurgelun-Todd et al. [99] | 2003 | 4 | healthy | 14.60 | 0.00 | reported | verbal | WAIS-III: span | 24 | 0.19 |
| Yurgelun-Todd et al. [99] | 2003 | 4 | healthy | 14.50 | 100.00 | reported | verbal | WAIS-III: span | 13 | 0.55 |
| Yurgelun-Todd et al. [99] | 2003 | 4 | healthy | 14.60 | 0.00 | reported | performance | WAIS-III: ds | 24 | 0.07 |
| Yurgelun-Todd et al. [99] | 2003 | 4 | healthy | 14.50 | 100.00 | reported | performance | WAIS-III: ds | 13 | 0.48 |
| Frangou et al. [100] | 2004 | 1, 2, 4 | healthy | 15.05 | 50.00 | reported | FSIQ | WISC-III or WAIS-III | 40 | 0.41 |
| Isaacs et al. [101] | 2004 | 2, 4 | healthy | 15.90 | 0.00 | PC | FSIQ | Wechsler FS | 38 | 0.24 |
| Isaacs et al. [101] | 2004 | 2, 4 | healthy | 15.90 | 100.00 | PC | FSIQ | Wechsler FS | 38 | 0.27 |
| Isaacs et al. [101] | 2004 | 2, 4 | healthy | 14.86 | 50.00 | PC | FSIQ | Wechsler FS | 16 | 0.49 |
| Isaacs et al. [101] | 2004 | 2, 4 | healthy | 15.60 | 0.00 | PC | verbal | Wechsler verbal | 38 | 0.20 |
| Isaacs et al. [101] | 2004 | 2, 4 | healthy | 15.90 | 0.00 | PC | performance | Wechsler performance | 38 | 0.21 |
| Isaacs et al. [101] | 2004 | 2, 4 | healthy | 15.90 | 100.00 | PC | performance | Wechsler performance | 38 | 0.15 |
| Ivanovic et al. [102,103] | 2004 | 1, 2, 4 | healthy | 18.00 | 0.00 | reported | FSIQ | WAIS-R | 49 | 0.37 |
| Ivanovic et al. [102,103] | 2004 | 1, 2, 4 | healthy | 18.00 | 100.00 | reported | FSIQ | WAIS-R | 47 | 0.55 |
| Ivanovic et al. [102,103] | 2004 | 2, 4 | healthy | 18.00 | 0.00 | reported | verbal | WAIS-R verbal | 49 | 0.33 |
| Ivanovic et al. [102,103] | 2004 | 2, 4 | healthy | 18.00 | 100.00 | reported | verbal | WAIS-R verbal | 47 | 0.55 |
| Ivanovic et al. [102,103] | 2004 | 2, 4 | healthy | 18.00 | 0.00 | reported | performance | WAIS-R performance | 49 | 0.38 |
| Ivanovic et al. [102,103] | 2004 | 2, 4 | healthy | 18.00 | 100.00 | reported | performance | WAIS-R performance | 47 | 0.52 |
| Rojas et al. [104] | 2004 | 2, 3, 4 | healthy | 43.62 | 47.00 | PC | FSIQ | WAIS-R or WAIS-III | 17 | 0.31 |
| Rojas et al. [104] | 2004 | 2, 4 | patients | 30.30 | 87.00 | PC | FSIQ | WAIS-R or WAIS-III | 15 | 0.07 |
| Rojas et al. [104] | 2004 | 2, 4 | patients | 30.30 | 87.00 | PC | verbal | WAIS-R or WAIS-III verbal | 15 | 0.30 |
| Rojas et al. [104] | 2004 | 2, 4 | healthy | 43.62 | 47.00 | PC | verbal | WAIS-R or WAIS-III verbal | 17 | 0.19 |
| Rojas et al. [104] | 2004 | 2, 4 | patients | 30.30 | 87.00 | PC | performance | WAIS-R or WAIS-III performance | 15 | 0.15 |
| Rojas et al. [104] | 2004 | 2, 4 | healthy | 43.62 | 47.00 | PC | performance | WAIS-R or WAIS-III performance | 17 | 0.27 |
| Toulopoulou et al. [105] | 2004 | 2, 4 | patients | 42.23 | 50.00 | reported | FSIQ | WAIS-R | 201 | 0.28 |
| Toulopoulou et al. [105] | 2004 | 2, 4 | patients | 42.23 | 50.00 | reported | verbal | WAIS-R verbal | 201 | 0.28 |
| Waiter et al. [106] | 2004 | 2, 4 | healthy | 15.50 | 100.00 | PC | FSIQ | WISC-III-R or WAIS-IV | 16 | 0.13 |
| Waiter et al. [106] | 2004 | 2, 4 | patients | 15.40 | 100.00 | PC | FSIQ | WISC-III-R or WAIS-IV | 16 | −0.06 |
| Waiter et al. [106] | 2004 | 2, 4 | patients | 15.40 | 100.00 | PC | verbal | WISC-III-R or WAIS-IV verbal | 16 | −0.17 |
| Waiter et al. [106] | 2004 | 2, 4 | healthy | 15.50 | 100.00 | PC | verbal | WISC-III-R or WAIS-IV verbal | 16 | 0.20 |
| Waiter et al. [106] | 2004 | 2, 4 | patients | 15.40 | 100.00 | PC | performance | WISC-III-R or WAIS-IV performance | 16 | 0.10 |
| Waiter et al. [106] | 2004 | 2, 4 | healthy | 15.50 | 100.00 | PC | performance | WISC-III-R or WAIS-IV performance | 16 | 0.23 |
| Antonova et al. [107] | 2005 | 2, 4 | patients | 40.49 | 60.00 | PC | verbal | WAIS-III: voc | 44 | 0.16 |
| Antonova et al. [107] | 2005 | 2, 4 | healthy | 33.72 | 58.00 | PC | verbal | WAIS-III: voc | 43 | 0.24 |
| Lodygensky et al. [108] | 2005 | 2, 4 | healthy | 8.42 | 57.00 | PC | FSIQ | WISC-R | 21 | 0.46 |
| Lodygensky et al. [108] | 2005 | 2, 4 | patients | 8.58 | 53.00 | PC | FSIQ | WISC-R | 60 | 0.35 |
| Thoma et al. [109] | 2005 | 2, 3, 4 | healthy | 23.50 | 100.00 | reported | FSIQ | RPM, TrailsAB, WAIS-R: voc, bd, ds; VMRT, COWA | 19 | 0.27 |
| Debbané et al. [110] | 2006 | 2, 4 | healthy | 15.10 | 43.00 | PC | FSIQ | WISC-III or WAIS-III | 41 | 0.16 |
| Debbané et al. [110] | 2006 | 2, 4 | patients | 16.70 | 37.00 | PC | FSIQ | WISC-III or WAIS-III | 43 | 0.16 |
| Rojas et al. [111] | 2006 | 2, 4 | healthy | 21.41 | 100.00 | PC | FSIQ | WAIS-III or WISC-III | 23 | 0.46 |
| Rojas et al. [111] | 2006 | 2, 4 | patients | 20.79 | 100.00 | PC | FSIQ | WAIS-III or WISC-III | 24 | 0.30 |
| Rojas et al. [111] | 2006 | 2, 4 | patients | 20.79 | 100.00 | PC | verbal | WAIS-III or WISC-III verbal | 24 | 0.28 |
| Rojas et al. [111] | 2006 | 2, 4 | healthy | 21.41 | 100.00 | PC | verbal | WAIS-III or WISC-III verbal | 23 | 0.55 |
| Rojas et al. [111] | 2006 | 2, 4 | patients | 20.79 | 100.00 | PC | performance | WAIS-III or WISC-III performance | 24 | 0.31 |
| Rojas et al. [111] | 2006 | 2, 4 | healthy | 21.41 | 100.00 | PC | performance | WAIS-III or WISC-III performance | 23 | 0.09 |
| Staff et al. [112] | 2006 | 1, 2, 4 | healthy | 79.50 | 61.00 | PC | fluid | RSPM | 102 | −0.10 |
| Staff et al. [112] | 2006 | 2, 4 | healthy | 79.50 | 61.00 | PC | verbal | NART | 102 | −0.14 |
| Voelbel et al. [113] | 2006 | 2, 4 | healthy | 10.77 | 100.00 | PC | FSIQ | WISC-III | 13 | −0.11 |
| Voelbel et al. [113] | 2006 | 2, 4 | patients | 10.16 | 100.00 | PC | FSIQ | WISC-III | 38 | 0.02 |
| Voelbel et al. [113] | 2006 | 2, 4 | patients | 10.08 | 100.00 | PC | FSIQ | WISC-III | 12 | −0.14 |
| Voelbel et al. [113] | 2006 | 2, 4 | patients | 10.16 | 100.00 | PC | verbal | WISC-III verbal | 38 | 0.08 |
| Voelbel et al. [113] | 2006 | 2, 4 | patients | 10.08 | 100.00 | PC | verbal | WISC-III verbal | 12 | 0.23 |
| Voelbel et al. [113] | 2006 | 2, 4 | healthy | 10.77 | 100.00 | PC | verbal | WISC-III verbal | 13 | −0.15 |
| Voelbel et al. [113] | 2006 | 2, 4 | healthy | 10.77 | 100.00 | PC | performance | WISC-III performance | 13 | 0.06 |
| Voelbel et al. [113] | 2006 | 2, 4 | patients | 10.08 | 100.00 | PC | performance | WISC-III performance | 12 | −0.48 |
| Voelbel et al. [113] | 2006 | 2, 4 | patients | 10.16 | 100.00 | PC | performance | WISC-III performance | 38 | −0.02 |
| Wozniak et al. [114] | 2006 | 2, 4 | healthy | 12.40 | 46.20 | PC | FSIQ | WISC-III or WISC-IV | 13 | 0.59 |
| Wozniak et al. [114] | 2006 | 2, 4 | patients | 12.30 | 50.00 | PC | FSIQ | WISC-III or WISC-IV | 14 | 0.41 |
| Chiang et al. [115] | 2007 | 2, 4 | patients | 29.20 | 45.00 | reported | verbal | WAIS verbal | 39 | −0.02 |
| Chiang et al. [115] | 2007 | 2, 4 | healthy | NA | NA | reported | verbal | WAIS verbal | 16 | −0.44 |
| Chiang et al. [115] | 2007 | 2, 4 | patients | 29.20 | 45.00 | reported | performance | WAIS performance | 39 | 0.10 |
| Chiang et al. [115] | 2007 | 2, 4 | healthy | NA | NA | reported | performance | WAIS performance | 16 | 0.41 |
| DeBoer et al. [116] | 2007 | 2, 4 | healthy | 10.50 | NA | PC | FSIQ | WISC-III or WISC-IV | 20 | −0.55 |
| DeBoer et al. [116] | 2007 | 2, 4 | patients | 10.75 | NA | PC | FSIQ | WISC-III or WISC-IV | 21 | 0.25 |
| DeBoer et al. [116] | 2007 | 2, 4 | patients | 10.75 | NA | PC | verbal | WISC-III or WISC-IV: VCI | 21 | 0.30 |
| DeBoer et al. [116] | 2007 | 2, 4 | healthy | 10.50 | NA | PC | verbal | WISC-III or WISC-IV: VCI | 20 | −0.20 |
| DeBoer et al. [116] | 2007 | 2, 4 | patients | 10.75 | NA | PC | performance | WISC-III or WISC-IV: POI | 21 | 0.38 |
| DeBoer et al. [116] | 2007 | 2, 4 | healthy | 10.50 | NA | PC | performance | WISC-III or WISC-IV: POI | 20 | −0.22 |
| Doernte [117] | 2007 | 4 | healthy | 58.50 | 0.00 | grey | FSIQ | HAWIE-R: sim, info, bd, pc | 18 | −0.23 |
| Doernte [117] | 2007 | 4 | healthy | 58.50 | 100.00 | grey | FSIQ | HAWIE-R: sim, info, bd, pc | 17 | 0.18 |
| Doernte [117] | 2007 | 4 | patients | 59.10 | 0.00 | grey | FSIQ | HAWIE-R: sim, info, bd, pc | 12 | −0.02 |
| Doernte [117] | 2007 | 4 | patients | 59.10 | 100.00 | grey | FSIQ | HAWIE-R: sim, info, bd, pc | 23 | −0.01 |
| Fine et al. [118] | 2007 | 2, 4 | healthy | 40.10 | 45.00 | PC | FSIQ | WASI | 44 | −0.11 |
| Fine et al. [118] | 2007 | 2, 4 | healthy | 10.47 | 63.00 | PC | FSIQ | WASI | 24 | 0.23 |
| Luders et al. [119] | 2007 | 2, 3, 4 | healthy | 28.48 | 45.00 | reported | FSIQ | WAIS-R | 62 | 0.28 |
| Nakamura et al. [120] | 2007 | 2, 3, 4 | healthy | 40.80 | 90.00 | PC | FSIQ | WAIS-III | 44 | 0.38 |
| Nakamura et al. [120] | 2007 | 2, 4 | patients | 40.60 | 90.00 | PC | FSIQ | WAIS-III | 43 | 0.32 |
| Nakamura et al. [120] | 2007 | 2, 4 | patients | 40.60 | 90.00 | PC | verbal | WAIS-III verbal | 44 | 0.26 |
| Nakamura et al. [120] | 2007 | 2, 4 | healthy | 40.80 | 90.00 | PC | verbal | WAIS-III verbal | 44 | 0.40 |
| Nakamura et al. [120] | 2007 | 2, 4 | patients | 40.60 | 90.00 | PC | performance | WAIS-III performance | 44 | 0.34 |
| Nakamura et al. [120] | 2007 | 2, 4 | healthy | 40.80 | 90.00 | PC | performance | WAIS-III performance | 43 | 0.29 |
| Narr et al. [121] | 2007 | 4 | healthy | 28.24 | 46.20 | reported | FSIQ | WAIS | 63 | 0.36 |
| Schottenbauer et al. [122] | 2007 | 2, 3, 4 | healthy | 34.32 | 0.00 | PC | FSIQ | WAIS-R | 22 | 0.60 |
| Schottenbauer et al. [122] | 2007 | 2, 3, 4 | healthy | 37.77 | 100.00 | PC | FSIQ | WAIS-R | 35 | 0.33 |
| Schottenbauer et al. [122] | 2007 | 2, 4 | patients | 40.96 | 0.00 | PC | FSIQ | WAIS-R | 69 | 0.34 |
| Schottenbauer et al. [122] | 2007 | 2, 4 | patients | 39.64 | 100.00 | PC | FSIQ | WAIS-R | 205 | 0.28 |
| Schottenbauer et al. [122] | 2007 | 2, 4 | patients | 40.90 | 0.00 | PC | verbal | WAIS-R: voc | 68 | 0.43 |
| Schottenbauer et al. [122] | 2007 | 2, 4 | healthy | 34.32 | 0.00 | PC | verbal | WAIS-R: voc | 22 | 0.54 |
| Schottenbauer et al. [122] | 2007 | 2, 4 | patients | 39.66 | 100.00 | PC | verbal | WAIS-R: voc | 202 | 0.28 |
| Schottenbauer et al. [122] | 2007 | 2, 4 | healthy | 37.77 | 100.00 | PC | verbal | WAIS-R: voc | 35 | 0.38 |
| Schottenbauer et al. [122] | 2007 | 2, 4 | patients | 40.90 | 0.00 | PC | performance | WAIS-R: bd | 68 | 0.29 |
| Schottenbauer et al. [122] | 2007 | 2, 4 | healthy | 34.32 | 0.00 | PC | performance | WAIS-R: bd | 22 | 0.30 |
| Schottenbauer et al. [122] | 2007 | 2, 4 | patients | 39.65 | 100.00 | PC | performance | WAIS-R: bd | 203 | 0.17 |
| Schottenbauer et al. [122] | 2007 | 2, 4 | healthy | 37.77 | 100.00 | PC | performance | WAIS-R: bd | 35 | 0.17 |
| Schumann et al. [123] | 2007 | 2, 4 | healthy | 13.10 | 100.00 | reported | FSIQ | WASI | 22 | 0.41 |
| Schumann et al. [123] | 2007 | 2, 4 | healthy | 13.10 | 100.00 | reported | verbal | WASI verbal | 22 | 0.38 |
| Schumann et al. [123] | 2007 | 2, 4 | healthy | 13.10 | 100.00 | reported | performance | WASI performance | 22 | 0.25 |
| Amat et al. [124] | 2008 | 2, 3, 4 | healthy | 31.50 | 56.00 | PC | FSIQ | WAIS-R | 27 | −0.11 |
| Amat et al. [124] | 2008 | 2, 4 | healthy | 31.50 | 56.00 | PC | verbal | WAIS-R verbal | 27 | −0.29 |
| Amat et al. [124] | 2008 | 2, 4 | healthy | 31.50 | 56.00 | PC | performance | WAIS-R performance | 27 | 0.18 |
| Choi et al. [125] | 2008 | 4 | healthy | 21.60 | 54.30 | reported | FSIQ | WAIS-R | 164 | 0.35 |
| Ebner et al. [126] | 2008 | 2, 4 | patients | 34.52 | 68.00 | PC | verbal | MWT-B | 44 | 0.15 |
| Ebner et al. [126] | 2008 | 2, 4 | healthy | 32.45 | 51.00 | PC | verbal | MWT-B | 37 | −0.13 |
| Raz et al. [127] | 2008 | 2, 4 | healthy | 51.11 | 43.00 | PC | fluid | CFIT (form 2) | 55 | 0.18 |
| Raz et al. [127] | 2008 | 2, 4 | patients | 59.75 | 25.00 | PC | fluid | CFIT (form 2) | 32 | −0.02 |
| Raz et al. [127] | 2008 | 2, 4 | patients | 59.75 | 25.00 | PC | verbal | Vocabulary Test (V2 & V3) | 31 | 0.15 |
| Raz et al. [127] | 2008 | 2, 4 | healthy | 51.11 | 43.00 | PC | verbal | Vocabulary Test (V2 & V3) | 55 | 0.13 |
| Castro-Fornieles et al. [128] | 2009 | 2, 4 | patients | 14.50 | 8.00 | PC | verbal | WISC-R: voc | 12 | 0.11 |
| Castro-Fornieles et al. [128] | 2009 | 2, 4 | healthy | 14.60 | 11.00 | PC | verbal | WISC-R: voc | 9 | 0.43 |
| Castro-Fornieles et al. [128] | 2009 | 2, 4 | patients | 14.50 | 8.00 | PC | performance | WISC-R: bd | 12 | 0.38 |
| Castro-Fornieles et al. [128] | 2009 | 2, 4 | healthy | 14.60 | 11.00 | PC | performance | WISC-R: bd | 9 | 0.55 |
| Miller et al. [129] | 2009 | 2, 4 | healthy | 9.25 | 33.00 | reported | FSIQ | WJIII (GIA) | 12 | 0.23 |
| Miller et al. [129] | 2009 | 2, 4 | healthy | 12.08 | NA | reported | fluid | WJIII: thinking ability | 11 | −0.11 |
| Miller et al. [129] | 2009 | 2, 4 | patients | 16.53 | 63.00 | reported | FSIQ | WJIII (GIA) | 16 | −0.30 |
| Miller et al. [129] | 2009 | 2, 4 | healthy | 12.08 | NA | reported | verbal | WJIII: Cog verbal ability | 11 | −0.65 |
| Miller et al. [129] | 2009 | 2, 4 | healthy | 9.25 | NA | reported | verbal | WJIII: Cog verbal ability | 5 | 0.84 |
| Miller et al. [129] | 2009 | 2, 4 | patients | 16.53 | NA | reported | verbal | WJIII: Cog verbal ability | 6 | 0.76 |
| Qiu et al. [130] | 2009 | 2, 4 | healthy | 10.50 | 53.00 | PC | FSIQ | WISC-III or WISC-IV | 66 | 0.26 |
| Qiu et al. [130] | 2009 | 2, 4 | patients | 10.40 | 57.00 | PC | FSIQ | WISC-III or WISC-IV | 47 | 0.26 |
| Qiu et al. [130] | 2009 | 2, 4 | patients | 10.40 | 57.00 | PC | verbal | WISC-III or WISC-IV: VCI | 47 | 0.21 |
| Qiu et al. [130] | 2009 | 2, 4 | healthy | 10.50 | 53.00 | PC | verbal | WISC-III or WISC-IV: VCI | 66 | 0.35 |
| Qiu et al. [130] | 2009 | 2, 4 | patients | 10.40 | 57.00 | PC | performance | WISC-III or WISC-IV: POI | 47 | 0.20 |
| Qiu et al. [130] | 2009 | 2, 4 | healthy | 10.50 | 53.00 | PC | performance | WISC-III or WISC-IV: PRI | 66 | 0.12 |
| Shenkin et al. [131] | 2009 | 2, 3, 4 | healthy | 78.40 | 29.00 | reported | FSIQ | MHT, RSPM, Verbal fluency, lm | 99 | 0.21 |
| Shenkin et al. [131] | 2009 | 2, 4 | healthy | 78.40 | 29.00 | reported | verbal | COWA (verbal fluency) | 107 | 0.13 |
| Van Leeuwen et al. [132] | 2009 | 2, 4 | healthy | 9.10 | 50.00 | reported | fluid | RSPM | 209 | 0.20 |
| Van Leeuwen et al. [132] | 2009 | 2, 4 | healthy | 9.10 | 50.00 | reported | verbal | WISC-III: comp | 209 | 0.33 |
| Van Leeuwen et al. [132] | 2009 | 2, 4 | healthy | 9.10 | 50.00 | reported | performance | WISC-III: POI | 209 | 0.28 |
| Van Leeuwen et al. [132] | 2009 | 4 | healthy | 9.10 | 50.00 | reported | performance | WISC-III: PSI | 209 | 0.12 |
| Weniger et al. [133] | 2009 | 2, 4 | patients | 32.00 | 0.00 | PC | FSIQ | HAWIE-R | 10 | 0.02 |
| Weniger et al. [133] | 2009 | 2, 3, 4 | healthy | 33.00 | 0.00 | PC | FSIQ | HAWIE-R | 25 | 0.15 |
| Weniger et al. [133] | 2009 | 2, 4 | patients | 32.00 | 0.00 | PC | FSIQ | HAWIE-R | 13 | 0.27 |
| Weniger et al. [133] | 2009 | 2, 4 | patients | 32.00 | 0.00 | PC | verbal | HAWIE-R verbal | 13 | 0.35 |
| Weniger et al. [133] | 2009 | 2, 4 | healthy | 33.00 | 0.00 | PC | verbal | HAWIE-R verbal | 25 | 0.00 |
| Weniger et al. [133] | 2009 | 2, 4 | patients | 32.00 | 0.00 | PC | verbal | HAWIE-R verbal | 10 | −0.17 |
| Weniger et al. [133] | 2009 | 2, 4 | patients | 32.00 | 0.00 | PC | performance | HAWIE-R performance | 10 | 0.23 |
| Weniger et al. [133] | 2009 | 2, 4 | healthy | 33.00 | 0.00 | PC | performance | HAWIE-R performance | 25 | 0.24 |
| Weniger et al. [133] | 2009 | 2, 4 | patients | 32.00 | 0.00 | PC | performance | HAWIE-R performance | 13 | 0.16 |
| Zeegers et al. [134] | 2009 | 2, 4 | patients | 3.72 | 91.00 | reported | FSIQ | unknown FS | 21 | 0.06 |
| Zeegers et al. [134] | 2009 | 2, 4 | patients | 3.44 | 92.00 | reported | FSIQ | unknown FS | 10 | 0.73 |
| Betjemann et al. [135] | 2010 | 2, 4 | healthy | 11.40 | 52.00 | reported | verbal | WISC-R verbal | 142 | 0.14 |
| Betjemann et al. [135] | 2010 | 2, 4 | healthy | 11.40 | 52.00 | reported | performance | WISC-R performance | 142 | 0.42 |
| Hermann [136] | 2010 | 2, 3, 4 | healthy | 33.34 | 42.00 | PC | FSIQ | Wechsler FS | 67 | 0.31 |
| Hermann [136] | 2010 | 2, 4 | patients | 36.09 | 35.00 | PC | FSIQ | Wechsler FS | 77 | 0.21 |
| Hermann [136] | 2010 | 2, 4 | patients | 36.09 | 35.00 | PC | verbal | Wechsler verbal | 77 | 0.28 |
| Hermann [136] | 2010 | 2, 4 | healthy | 33.34 | 42.00 | PC | verbal | Wechsler verbal | 67 | 0.23 |
| Hermann [136] | 2010 | 2, 4 | patients | 36.09 | 35.00 | PC | performance | Wechsler performance | 77 | 0.09 |
| Hermann [136] | 2010 | 2, 4 | healthy | 33.34 | 42.00 | PC | performance | Wechsler performance | 67 | 0.33 |
| Hogan et al. [137] | 2010 | 2, 4 | healthy | 68.69 | 53.00 | PC | fluid | RSPM | 234 | 0.11 |
| Hogan et al. [137] | 2010 | 2, 4 | healthy | 68.69 | 53.00 | PC | verbal | NART | 235 | 0.00 |
| Isaacs et al. [138] | 2010 | 2, 4 | healthy | 15.75 | 0.00 | PC | FSIQ | WISC-III or WAIS-III | 24 | 0.00 |
| Isaacs et al. [138] | 2010 | 2, 4 | healthy | 15.75 | 100.00 | reported | FSIQ | WISC-III or WAIS-III | 26 | 0.36 |
| Isaacs et al. [138] | 2010 | 2, 4 | healthy | 15.75 | 0.00 | PC | verbal | WISC-III or WAIS-III verbal | 24 | 0.00 |
| Isaacs et al. [138] | 2010 | 2, 4 | healthy | 15.75 | 100.00 | reported | verbal | WISC-III or WAIS-III verbal | 26 | 0.48 |
| Isaacs et al. [138] | 2010 | 2, 4 | healthy | 15.75 | 0.00 | PC | performance | WISC-III or WAIS-III performance | 24 | 0.00 |
| Isaacs et al. [138] | 2010 | 2, 4 | healthy | 15.75 | 100.00 | reported | performance | WISC-III or WAIS-III performance | 26 | 0.19 |
| Lange et al. [139] | 2010 | 2, 4 | healthy | 10.88 | 0.00 | reported | FSIQ | WASI | 166 | 0.22 |
| Lange et al. [139] | 2010 | 2, 4 | healthy | 10.95 | 100.00 | reported | FSIQ | WASI | 143 | 0.23 |
| Wallace et al. [140] | 2010 | 2, 4 | healthy | 11.80 | 48.00 | reported | FSIQ | WASI | 649 | 0.14 |
| Wallace et al. [140] | 2010 | 2, 4 | healthy | 11.80 | 48.00 | reported | verbal | WASI verbal | 649 | 0.13 |
| Wallace et al. [140] | 2010 | 2, 4 | healthy | 11.80 | 48.00 | reported | performance | WASI performance | 649 | 0.14 |
| Ashtari et al. [141] | 2011 | 2, 3, 4 | healthy | 18.50 | 100.00 | reported | FSIQ | WRAT-III | 14 | 0.57 |
| Ashtari et al. [141] | 2011 | 2, 4 | patients | 19.30 | 100.00 | reported | FSIQ | WRAT-III | 14 | 0.29 |
| Chen et al. [142] | 2011 | 4 | healthy | 22.56 | 44.00 | reported | FSIQ | WASI | 27 | 0.02 |
| Chen et al. [142] | 2011 | 4 | patients | 23.07 | 46.70 | reported | FSIQ | WASI | 30 | 0.68 |
| Chen et al. [142] | 2011 | 4 | patients | 23.02 | 27.00 | reported | FSIQ | WASI | 37 | 0.41 |
| Kievit et al. [143] | 2011 | 2, 3, 4 | healthy | 21.10 | 36.00 | PC | FSIQ | WAIS-III | 80 | 0.29 |
| Kievit et al. [143] | 2011 | 2, 4 | healthy | 21.10 | 36.00 | PC | verbal | WAIS-III verbal | 80 | 0.23 |
| Tate et al. [144] | 2011 | 2, 4 | patients | 81.70 | 43.00 | PC | FSIQ | Shipley | 194 | 0.00 |
| Aydin et al. [145] | 2012 | 2, 4 | healthy | 15.10 | 100.00 | reported | FSIQ | WISC-R | 30 | 0.40 |
| Aydin et al. [145] | 2012 | 2, 4 | healthy | 15.10 | 100.00 | reported | verbal | WISC-R verbal | 30 | 0.26 |
| Aydin et al. [145] | 2012 | 2, 4 | healthy | 15.10 | 100.00 | reported | performance | WISC-R performance | 30 | 0.34 |
| Burgaleta et al. [146] | 2012 | 2, 3, 4 | healthy | 19.88 | 44.00 | reported | FSIQ | 9 tests from APM, DAT-AR-5, PMR-R | 100 | 0.17 |
| Bigler et al. [147] | 2013 | 4 | patients | 10.66 | 58.00 | reported | performance | WISC-IV: PSI | 47 | 0.00 |
| Bigler et al. [147] | 2013 | 4 | patients | 10.67 | 68.00 | reported | performance | WISC-IV: PSI | 32 | 0.00 |
| Bigler et al. [147] | 2013 | 4 | patients | 10.14 | 58.00 | reported | performance | WISC-IV: PSI | 27 | 0.00 |
| Royle et al. [148] | 2013 | 2, 3, 4 | healthy | 72.47 | 100.00 | reported | FSIQ | WAIS-III: ins, span, mr, bd, ss, ds | 293 | 0.26 |
| Royle et al. [148] | 2013 | 2, 3, 4 | healthy | 72.60 | 0.00 | reported | FSIQ | WAIS-III: ins, span, mr, bd, ss, ds | 327 | 0.27 |
| Royle et al. [148] | 2013 | 4 | healthy | 72.47 | 100.00 | reported | verbal | WAIS-III: lns | 293 | 0.10 |
| Royle et al. [148] | 2013 | 4 | healthy | 72.60 | 0.00 | reported | verbal | WAIS-III: lns | 327 | 0.22 |
| Royle et al. [148] | 2013 | 4 | healthy | 72.47 | 100.00 | reported | verbal | WAIS-III: span b | 293 | 0.11 |
| Royle et al. [148] | 2013 | 4 | healthy | 72.60 | 0.00 | reported | verbal | WAIS-III: span b | 327 | 0.23 |
| Royle et al. [148] | 2013 | 4 | healthy | 72.47 | 100.00 | reported | performance | WAIS-III: bd | 293 | 0.25 |
| Royle et al. [148] | 2013 | 4 | healthy | 72.60 | 0.00 | reported | performance | WAIS-III: bd | 327 | 0.25 |
| Royle et al. [148] | 2013 | 4 | healthy | 72.47 | 100.00 | reported | performance | WAIS-III: mr | 293 | 0.14 |
| Royle et al. [148] | 2013 | 4 | healthy | 72.60 | 0.00 | reported | performance | WAIS-III: mr | 327 | 0.18 |
| Royle et al. [148] | 2013 | 4 | healthy | 72.47 | 100.00 | reported | performance | WAIS-III: ds | 293 | 0.22 |
| Royle et al. [148] | 2013 | 4 | healthy | 72.60 | 0.00 | reported | performance | WAIS-III: ds | 327 | 0.33 |
| Royle et al. [148] | 2013 | 4 | healthy | 72.47 | 100.00 | reported | performance | WAIS-III: ss | 293 | 0.17 |
| Royle et al. [148] | 2013 | 4 | healthy | 72.60 | 0.00 | reported | performance | WAIS-III: ss | 327 | 0.34 |
| Zelko et al. [149] | 2013 | 4 | healthy | 14.90 | 53.00 | reported | FSIQ | WAIS or WISC: voc, sim, pc, bd, arith, ds, ss | 36 | 0.25 |
| Zelko et al. [149] | 2013 | 4 | patients | 14.60 | 49.00 | reported | FSIQ | WAIS or WISC: voc, sim, pc, bd, arith, ds, ss | 108 | 0.23 |
| Zelko et al. [149] | 2013 | 4 | patients | 14.60 | 49.00 | reported | verbal | WAIS or WISC: voc, sim | 108 | 0.23 |
| Zelko et al. [149] | 2013 | 4 | healthy | 14.90 | 53.00 | reported | verbal | WAIS or WISC: voc, sim | 36 | 0.04 |
| Zelko et al. [149] | 2013 | 4 | patients | 14.60 | 49.00 | reported | verbal | WAIS or WISC: arith, span | 108 | 0.26 |
| Zelko et al. [149] | 2013 | 4 | healthy | 14.90 | 53.00 | reported | verbal | WAIS or WISC: arith, span | 36 | 0.33 |
| Zelko et al. [149] | 2013 | 4 | patients | 14.60 | 49.00 | reported | performance | WAIS or WISC: pc, bd | 108 | 0.21 |
| Zelko et al. [149] | 2013 | 4 | healthy | 14.90 | 53.00 | reported | performance | WAIS or WISC: pc, bd | 36 | 0.30 |
| Zelko et al. [149] | 2013 | 4 | patients | 14.60 | 49.00 | reported | performance | WAIS or WISC: cod, sym | 108 | 0.09 |
| Zelko et al. [149] | 2013 | 4 | healthy | 14.90 | 53.00 | reported | performance | WAIS or WISC: cod, sym | 36 | −0.12 |
| Bjuland et al. [150] | 2014 | 4 | healthy | 20.30 | 42.00 | reported | FSIQ | WAIS-III | 60 | 0.36 |
| Bjuland et al. [150] | 2014 | 4 | patients | 20.10 | 41.00 | reported | FSIQ | WAIS-III | 43 | 0.56 |
| Bjuland et al. [150] | 2014 | 4 | patients | 20.30 | 41.00 | reported | verbal | WAIS-III: VCI | 42 | 0.44 |
| Bjuland et al. [150] | 2014 | 4 | patients | 20.30 | 41.00 | reported | verbal | WAIS-III: WMI | 42 | 0.54 |
| Bjuland et al. [150] | 2014 | 4 | patients | 20.30 | 41.00 | reported | performance | WAIS-II: POI | 43 | 0.48 |
| Bjuland et al. [150] | 2014 | 4 | patients | 20.30 | 41.00 | reported | performance | WAIS-II: PSI | 43 | 0.48 |
| Grunewaldt et al. [151] | 2014 | 4 | patients | 10.17 | 34.80 | reported | FSIQ | WISC-III | 21 | 0.00 |
| Grunewaldt et al. [151] | 2014 | 4 | patients | 10.17 | 34.80 | reported | verbal | WISC-III: WMI | 21 | 0.00 |
| Jenkins et al. [152] | 2014 | 4 | healthy | 11.70 | 41.70 | reported | FSIQ | WASI, WISC-III or WPPSI-R: voc, mr | 102 | 0.19 |
| MacDonald et al. [153] | 2014 | 4 | healthy | 11.60 | 100.00 | reported | FSIQ | WASI | 142 | 0.23 |
| MacDonald et al. [153] | 2014 | 4 | healthy | 11.30 | 0.00 | reported | FSIQ | WASI | 161 | 0.22 |
| MacDonald et al. [153] | 2014 | 4 | healthy | 11.60 | 100.00 | reported | verbal | WASI verbal | 142 | 0.13 |
| MacDonald et al. [153] | 2014 | 4 | healthy | 11.30 | 0.00 | reported | verbal | WASI verbal | 161 | 0.18 |
| MacDonald et al. [153] | 2014 | 4 | healthy | 11.60 | 100.00 | reported | performance | WASI performance | 142 | 0.29 |
| MacDonald et al. [153] | 2014 | 4 | healthy | 11.30 | 0.00 | reported | performance | WASI performance | 161 | 0.19 |
| McCoy et al. [154] | 2014 | 4 | patients | 13.00 | 100.00 | reported | FSIQ | WISC-IV (GAI) | 10 | 0.59 |
| McCoy et al. [154] | 2014 | 4 | patients | 13.00 | 0.00 | reported | FSIQ | WISC-IV (GAI) | 16 | 0.62 |
| Zhu et al. [155] | 2014 | 4 | healthy | 20.41 | 41.00 | reported | FSIQ | WAIS-R (Chinese) | 316 | 0.10 |
| Boberg et al. [156] | 2015 | 4 | healthy | 8.00 | 54.00 | grey | FSIQ | WISC-IV (Swedish) | 10 | 0.69 |
| Boberg et al. [156] | 2015 | 4 | healthy | 8.20 | 35.80 | grey | FSIQ | WISC-IV (Swedish) | 9 | 0.00 |
| Boberg et al. [156] | 2015 | 4 | healthy | 8.30 | 50.00 | grey | FSIQ | WISC-IV | 21 | 0.00 |
| Grazioplene et al. [157] | 2015 | 4 | healthy | 26.20 | 51.00 | reported | FSIQ | WAIS-IV: voc, sim, mr, bd | 285 | 0.28 |
| Grazioplene et al. [157] | 2015 | 4 | healthy | 23.50 | 100.00 | reported | FSIQ | WAIS-IV: voc, sim, mr, bd | 107 | 0.08 |
| Grazioplene et al. [157] | 2015 | 4 | healthy | 21.70 | 54.00 | reported | FSIQ | WASI | 125 | 0.04 |
| Grazioplene et al. [157] | 2015 | 4 | healthy | 26.20 | 51.00 | reported | verbal | WAIS-IV: voc, sim | 285 | 0.18 |
| Grazioplene et al. [157] | 2015 | 4 | healthy | 21.70 | 54.00 | reported | verbal | WASI verbal | 125 | 0.04 |
| Grazioplene et al. [157] | 2015 | 4 | healthy | 23.50 | 100.00 | reported | verbal | WAIS-III: voc, sim | 107 | 0.10 |
| Grazioplene et al. [157] | 2015 | 4 | healthy | 26.20 | 51.00 | reported | performance | WAIS-IV: mr, bd | 285 | 0.30 |
| Grazioplene et al. [157] | 2015 | 4 | healthy | 21.70 | 54.00 | reported | performance | WASI performance | 125 | 0.04 |
| Grazioplene et al. [157] | 2015 | 4 | healthy | 23.50 | 100.00 | reported | performance | WAIS-III: mr, bd | 107 | 0.04 |
| Lefebvre et al. [158] | 2015 | 4 | healthy | 17.00 | 83.00 | reported | FSIQ | Wechsler | 366 | 0.23 |
| Lefebvre et al. [158] | 2015 | 4 | patients | 16.60 | 88.00 | reported | FSIQ | Wechsler | 328 | 0.04 |
| Lefebvre et al. [158] | 2015 | 4 | patients | 16.60 | 88.00 | reported | verbal | unknown verbal | 241 | 0.08 |
| Lefebvre et al. [158] | 2015 | 4 | healthy | 17.00 | 83.00 | reported | verbal | unknown verbal | 297 | 0.22 |
| Lefebvre et al. [158] | 2015 | 4 | patients | 16.60 | 88.00 | reported | performance | unknown performance | 241 | 0.17 |
| Lefebvre et al. [158] | 2015 | 4 | healthy | 17.00 | 83.00 | reported | performance | unknown performance | 297 | 0.18 |
| Paul et al. [159] | 2015 | 4 | healthy | 24.57 | 0.00 | reported | verbal | Reading Span, Rotation Span, Symmetrie Span | 90 | 0.25 |
| Paul et al. [159] | 2015 | 4 | healthy | 24.07 | 100.00 | reported | verbal | Reading Span, Rotation Span, Symmetrie Span | 121 | 0.18 |
| Walters et al. [160] | 2015 | 4 | patients | 17.32 | 100.00 | reported | FSIQ | WAIS or WISC: voc, mr | 178 | 0.19 |
| Ballester-Plane et al. [161] | 2016 | 4 | patients | 25.10 | 67.00 | reported | FSIQ | RCPM | 30 | 0.73 |
| Ballester-Plane et al. [161] | 2016 | 4 | patients | 25.10 | 67.00 | reported | verbal | PPVT-III | 30 | 0.71 |
| Ballester-Plane et al. [161] | 2016 | 4 | patients | 25.10 | 67.00 | reported | performance | WASI performance | 30 | 0.72 |
| Bohlken et al. [162] | 2016 | 4 | healthy | 32.69 | 42.00 | reported | FSIQ | WAIS-III: ds, bd, arith., span, inf | 164 | 0.26 |
| Bohlken et al. [162] | 2016 | 4 | healthy | 32.70 | 42.00 | reported | verbal | WAIS-III: inf | 164 | 0.18 |
| Bohlken et al. [162] | 2016 | 4 | healthy | 32.70 | 42.00 | reported | verbal | WAIS-III: arith | 164 | 0.26 |
| Bohlken et al. [162] | 2016 | 4 | healthy | 32.70 | 42.00 | reported | verbal | WAIS-III: span | 164 | 0.00 |
| Bohlken et al. [162] | 2016 | 4 | healthy | 32.70 | 42.00 | reported | performance | WAIS-III: bd | 164 | 0.31 |
| Bohlken et al. [162] | 2016 | 4 | healthy | 32.70 | 42.00 | reported | performance | WAIS-III: ds | 164 | 0.12 |
| Ferreira et al. [163] | 2016 | 4 | healthy | 45.10 | 49.00 | reported | verbal | WAIS-III: voc | 73 | 0.36 |
| Ferreira et al. [163] | 2016 | 4 | healthy | 45.10 | 49.00 | reported | verbal | WAIS-III: inf | 73 | 0.50 |
| Ferreira et al. [163] | 2016 | 4 | healthy | 45.10 | 49.00 | reported | performance | WAIS-III: bd | 73 | 0.33 |
| Gregory et al. [164] | 2016 | 4 | healthy | 14.70 | 57.00 | reported | FSIQ | Conditional Exclusion, Emotional Differentiation, Emotional Identification, Face Memory, Letter N-Back, Line Orientation, Matrix Reasoning, Verbal Reasoning, Visual Object Learning, Word Memory; WRAT: Reading | 662 | 0.24 |
| Monson et al. [165] | 2016 | 4 | patients | 7.50 | 50.00 | reported | FSIQ | WASI | 134 | 0.26 |
| Gregory et al. [164] | 2016 | 4 | patients | 7.50 | 50.00 | reported | verbal | WASI verbal | 134 | 0.11 |
| Gregory et al. [164] | 2016 | 4 | patients | 7.50 | 50.00 | reported | performance | WASI performance | 134 | 0.31 |
| Nikolaidis et al. [166] | 2016 | 4 | healthy | 21.15 | 34.00 | reported | fluid | RAPM, Shipley Abstraction, Letter Sets, Spatial Relations, Paper Folding, Form Boards | 71 | 0.44 |
| Nikolaidis et al. [166] | 2016 | 4 | healthy | 21.15 | 34.00 | reported | verbal | Visual Short-Term Memory, Spatial Working Memory, Running Span | 71 | 0.13 |
| Paul et al. [159] | 2016 | 4 | healthy | 24.57 | 0.00 | reported | fluid | BOMAT, Number Series, Letter Sets | 90 | 0.14 |
| Paul et al. [159] | 2016 | 4 | healthy | 24.07 | 100.00 | reported | fluid | BOMAT, Number Series, Letter Sets | 121 | 0.13 |
| Treit et al. [167] | 2016 | 4 | patients | 12.50 | 53.00 | reported | FSIQ | WRIT or WISC | 50 | 0.21 |
| Treit et al. [167] | 2016 | 4 | healthy | 11.90 | 48.00 | reported | FSIQ | WRIT or WISC | 66 | 0.09 |
| Amaral et al. [168] | 2017 | 4 | healthy | 3.00 | 100.00 | reported | FSIQ | MSEL | 49 | 0.35 |
| Amaral et al. [168] | 2017 | 4 | patients | 3.08 | 100.00 | reported | FSIQ | MSEL | 19 | −0.18 |
| Amaral et al. [168] | 2017 | 4 | patients | 3.13 | 100.00 | reported | FSIQ | MSEL | 110 | 0.01 |
| Arhan et al. [169] | 2017 | 4 | healthy | 9.20 | 46.00 | reported | FSIQ | WISC-R (Turkish) | 46 | 0.51 |
| Arhan et al. [169] | 2017 | 4 | healthy | 9.20 | 46.00 | reported | verbal | WISC-R: voc | 46 | 0.71 |
| Arhan et al. [169] | 2017 | 4 | healthy | 9.20 | 46.00 | reported | verbal | WISC-R: sim | 46 | 0.54 |
| Arhan et al. [169] | 2017 | 4 | healthy | 9.20 | 46.00 | reported | verbal | WISC-R: inf | 46 | 0.38 |
| Arhan et al. [169] | 2017 | 4 | healthy | 9.20 | 46.00 | reported | verbal | WISC-R: comp | 46 | 0.45 |
| Arhan et al. [169] | 2017 | 4 | healthy | 9.20 | 46.00 | reported | verbal | WISC-R: arith | 46 | 0.24 |
| Arhan et al. [169] | 2017 | 4 | healthy | 9.20 | 46.00 | reported | verbal | WISC-R: span | 46 | 0.31 |
| Arhan et al. [169] | 2017 | 4 | healthy | 9.20 | 46.00 | reported | performance | WISC-R: pc | 46 | 0.77 |
| Arhan et al. [169] | 2017 | 4 | healthy | 9.20 | 46.00 | reported | performance | WISC-R: pic | 46 | 0.24 |
| Arhan et al. [169] | 2017 | 4 | healthy | 9.20 | 46.00 | reported | performance | WISC-R: bd | 46 | 0.04 |
| Arhan et al. [169] | 2017 | 4 | healthy | 9.20 | 46.00 | reported | performance | WISC-R: obj | 46 | 0.04 |
| Arhan et al. [169] | 2017 | 4 | healthy | 9.20 | 46.00 | reported | performance | WISC-R: ds | 46 | 0.27 |
| Martinez et al. [170] | 2017 | 4 | healthy | 19.60 | 0.00 | reported | verbal | DAT-VR, PMA-V, Reading Span, Letter Memory, Keep Track, Flanker (verbal + numerical), Simple Recognition verbal task | 40 | 0.28 |
| Martinez et al. [170] | 2017 | 4 | healthy | 20.20 | 100.00 | reported | verbal | DAT-VR, PMA-V, Reading Span, Letter Memory, Keep Track, Flanker (verbal + numerical), Simple Recognition verbal task | 40 | −0.04 |
| Martinez et al. [170] | 2017 | 4 | healthy | 19.60 | 0.00 | reported | spatial | DAT-SR, PMA-S, Rotation of Solid Figures, Dot Matrix, 2-Backl task, spatial Simon task, spatial Simple Recognition | 40 | 0.39 |
| Martinez et al. [170] | 2017 | 4 | healthy | 20.20 | 100.00 | reported | spatial | DAT-SR, PMA-S, Rotation of Solid Figures, Dot Matrix, 2-Backl task, spatial Simon task, spatial Simple Recognition | 40 | 0.24 |
| Ritchie et al. [171] | 2017 | 4 | healthy | 92.10 | 45.00 | reported | fluid | WAIS-III: ds, WMS-R: Logical Memory Story A, Phonemic Verbal Fluency [172] | 34 | 0.23 |
| Ritchie et al. [171] | 2017 | 4 | healthy | 92.10 | 45.00 | reported | performance | WAIS-III: ds | 34 | 0.19 |
| van der Linden et al. [173] | 2017 | 4 | healthy | 28.82 | 0.00 | reported | FSIQ | Penn Progressive Matrices, Peabody Vocabulary, Oral Reading Recognition, List Sorting, Picture Sequence Memory, Penn Line Orientation, Dimensional Change Card Sorting, Word Memory, Salthouse Pattern Comparison, Flanker Task | 503 | 0.26 |
| van der Linden et al. [173] | 2017 | 4 | healthy | 28.82 | 100.00 | reported | FSIQ | Penn Progressive Matrices, Peabody Vocabulary, Oral Reading Recognition, List Sorting, Picture Sequence Memory, Penn Line Orientation, Dimensional Change Card Sorting, Word Memory, Salthouse Pattern Comparison, Flanker Task | 393 | 0.25 |
| Vreeker et al. [174] | 2017 | 4 | healthy | 44.60 | 49.00 | reported | FSIQ | WAIS-III (Dutch): info, srith., bf, ds | 160 | 0.28 |
| Annink et al. [175] | 2018 | 4 | patients | 9.79 | 48.00 | reported | FSIQ | WISC-III (Dutch) | 52 | 0.43 |
| Jensen et al. [176] | 2018 | 4 | healthy | 24.91 | 59.00 | PC | FSIQ | WAIS-III (Danish): voc, sim, bd, mr | 56 | 0.30 |
| Jensen et al. [176] | 2018 | 4 | patients | 24.69 | 57.40 | PC | FSIQ | WAIS-III (Danish): voc, sim, bd, mr | 54 | 0.14 |
| Jensen et al. [176] | 2018 | 4 | healthy | 24.91 | 59.00 | PC | verbal | WAIS-III (Danish): voc, sim | 56 | 0.30 |
| Jensen et al. [176] | 2018 | 4 | patients | 24.69 | 57.40 | PC | verbal | WAIS-III (Danish): voc, sim | 54 | 0.09 |
| Jensen et al. [176] | 2018 | 4 | healthy | 24.91 | 59.00 | PC | performance | WAIS-III (Danish): bd, mr | 56 | 0.19 |
| Jensen et al. [176] | 2018 | 4 | patients | 24.69 | 57.40 | PC | performance | WAIS-III (Danish): bd, mr | 54 | 0.20 |
| Lammers et al. [177] | 2018 | 4 | patients | 72.00 | 61.00 | reported | FSIQ | Paired Associate Learning, Verbal Recognition Memory, Spatial Span Length, Simple Reaction Time, TrailAB, Grooved Pegboard task | 243 | 0.18 |
| Mankovsky et al. [178] | 2018 | 4 | patients | 62.30 | 33.00 | reported | verbal | RAVLT: immediate recall, delayed recall, delayed recognition + WAIS-III: span | 93 | 0.02 |
| Mankovsky et al. [178] | 2018 | 4 | patients | 62.30 | 33.00 | reported | performance | Trails (A), SCWT (I + II), WAIS-II: ds | 93 | 0.08 |
| Nygaard et al. [179] | 2018 | 4 | patients | 18.96 | 60.00 | reported | FSIQ | WASI: voc, mr | 82 | 0.30 |
| Sreedharan et al. [180] | 2018 | 4 | patients | 10.80 | 66.00 | reported | FSIQ | WISC (Malayalam translation) | 30 | 0.00 |
| Takeuchi et al. [181] | 2018 | 4 | healthy | 20.80 | 58.00 | reported | FSIQ | Tanaka B Intelligence Scale | 1319 | 0.07 |
| Tozer et al. [182] | 2018 | 4 | patients | 70.01 | 65.00 | reported | FSIQ | span, lm, Visual Reproduction, BIRT Memory and Information Processing Battery, Speed of Information Processing, ds, Grooved Pegboard, trails, Verbal Fluency, WCST | 118 | 0.23 |
| Tozer et al. [182] | 2018 | 4 | patients | 70.01 | 65.00 | reported | performance | unknown | 115 | 0.28 |
| Ahn et al. [183] | 2019 | 4 | patients | 32.97 | 42.00 | reported | FSIQ | K-WAIS-R | 38 | 0.00 |
| Ahn et al. [183] | 2019 | 4 | patients | 32.97 | 42.00 | reported | verbal | WAIS-R verbal | 38 | 0.00 |
| Ahn et al. [183] | 2019 | 4 | patients | 32.97 | 42.00 | reported | performance | WAIS-R performance | 38 | 0.00 |
| Bathelt et al. [184] | 2019 | 4 | healthy | 9.93 | 54.00 | reported | FSIQ | WASI-II: Reasoning; AWMA: Digit Recall, Backward Digit Recall, Dot Matrix, Mr X | 63 | 0.07 |
| Bathelt et al. [184] | 2019 | 4 | patients | 9.35 | 64.70 | reported | FSIQ | WASI-II: Reasoning; AWMA: Digit Recall, Backward Digit Recall, Dot Matrix, Mr X | 139 | 0.02 |
| Cox et al. [185] | 2019 | 4 | healthy | 63.13 | 100.00 | reported | FSIQ | composite of 4 tests | 3900 | 0.21 |
| Cox et al. [185] | 2019 | 4 | healthy | 63.13 | 0.00 | reported | FSIQ | composite of 4 tests | 4192 | 0.26 |
| de Zwarte et al. [186] | 2019 | 4 | patients | 27.49 | 60.00 | reported | FSIQ | WAIS-III (Dutch): inf, arith, bd, ds | 516 | 0.29 |
| de Zwarte et al. [186] | 2019 | 4 | patients | 52.85 | 32.00 | reported | FSIQ | GIT (short) | 85 | 0.06 |
| Elliott et al. [187] | 2019 | 4 | healthy | 45.00 | 48.00 | reported | FSIQ | WAIS-IV | 596 | 0.35 |
| Elliott et al. [187] | 2019 | 4 | healthy | 22.23 | 47.00 | reported | FSIQ | Shipley | 1163 | 0.12 |
| Elliott et al. [187] | 2019 | 4 | healthy | 20.26 | 47.00 | reported | FSIQ | WASI: voc, mr | 515 | 0.16 |
| Hiraiwa et al. [188] | 2019 | 4 | patients | 9.43 | 52.00 | reported | FSIQ | WISC-IV | 27 | 0.34 |
| van Haren et al. [189] | 2019 | 4 | healthy | 12.74 | 53.00 | reported | FSIQ | WISC-III or WAIS-III: voc, inf, bd, pic | 40 | 0.34 |
| van Haren et al. [189] | 2019 | 4 | patients | 13.77 | 30.00 | reported | FSIQ | WISC-III or WAIS-III: voc, inf, bd, pic | 40 | 0.53 |
| van Haren et al. [189] | 2019 | 4 | patients | 14.52 | 56.00 | reported | FSIQ | WISC-III or WAIS-III: voc, inf, bd, pic | 63 | 0.39 |
| Cadenas-Sanchez et al. [190] | 2020 | 4 | patients | 10.00 | 60.00 | reported | FSIQ | K-BIT | 100 | −0.03 |
| Cadenas-Sanchez et al. [190] | 2020 | 4 | patients | 10.00 | 60.00 | reported | verbal | Delayed non-Match to Sample Task | 100 | 0.06 |
| Corley et al. [191] | 2020 | 4 | healthy | 79.40 | 53.10 | reported | FSIQ | WAIS-III: mr, bd, ss, ds; WMS-III: spatial span f + b, vpa, lm; Four-Choice Reaction Time, Visual Inspection Time, NART, WTAR, PVF | 358 | 0.19 |
| Corley et al. [191] | 2020 | 4 | healthy | 79.40 | 53.10 | reported | verbal | NART, WTAR, PVF | 358 | 0.08 |
| Corley et al. [191] | 2020 | 4 | healthy | 79.40 | 53.10 | reported | verbal | WMS-III: vpa, lm; WAIS-III: ds (b) | 358 | 0.16 |
| Corley et al. [191] | 2020 | 4 | healthy | 79.40 | 53.10 | reported | performance | WAIS-III: mr, bd; WMS-III: spatial span f + b | 358 | 0.12 |
| Corley et al. [191] | 2020 | 4 | healthy | 79.40 | 53.10 | reported | performance | WAIS-III: ss, ds; Four Choice Reaction Time, Visual Inspection Time | 358 | 0.29 |
| de Zwarte et al. [186] | 2020 | 4 | patients | 32.14 | 46.00 | reported | FSIQ | mostly abbreviated Wechsler Scales | 968 | 0.20 |
| de Zwarte et al. [186] | 2020 | 4 | patients | 27.48 | 41.00 | reported | FSIQ | mostly abbreviated Wechsler Scales | 507 | 0.20 |
| Elias [192] | 2020 | 4 | patients | 69.02 | 100.00 | grey | verbal | WTAR | 49 | 0.35 |
| Laliberté Durish [193] | 2020 | 4 | patients | 12.30 | 62.40 | grey | FSIQ | WASI-II | 304 | 0.19 |
| Laliberté Durish [193] | 2020 | 4 | patients | 12.50 | 51.90 | grey | FSIQ | WASI-II | 161 | −0.07 |
| Laliberté Durish [193] | 2020 | 4 | healthy | 39.60 | 43.00 | reported | FSIQ | Verbal Learning, ds, Conditional Exclusion, Spatial Working Memory, Facial Memory, TrailAB, Continuous Performance, Letter-Number Seq, Balloon Analog, Oral Word Association, Emotional Recognition | 1216 | 0.12 |
| Mitchell et al. [194] | 2020 | 4 | healthy | 22.30 | 38.00 | reported | FSIQ | MAB (5 subtests); WAIS-R: ds | 1097 | 0.25 |
| Williams et al. [195] | 2020 | 4 | patients | 14.54 | 87.74 | reported | FSIQ | Wechsler | 302 | 0.08 |
| Williams et al. [195] | 2020 | 4 | healthy | 14.54 | 80.40 | reported | FSIQ | Wechsler | 352 | 0.31 |
| Yankowitz et al. [196] | 2020 | 4 | healthy | 13.10 | 72.70 | reported | FSIQ | DAS (GCA) or WISC-IV or WASI (I or II) | 216 | 0.38 |
| Yankowitz et al. [196] | 2020 | 4 | patients | 13.00 | 82.10 | reported | FSIQ | DAS (GCA) or WISC-IV or WASI (I or II) | 240 | 0.05 |
| Yankowitz et al. [196] | 2020 | 4 | healthy | 20.60 | 100.00 | reported | FSIQ | WISC-III or WASI-I or WAIS (R or III) | 89 | 0.35 |
| Yankowitz et al. [196] | 2020 | 4 | patients | 16.60 | 100.00 | reported | FSIQ | WISC-III or WASI-I or WAIS (R or III) | 86 | 0.25 |
| Hedderich et al. [197] | 2021 | 4 | patients | 26.70 | 57.40 | reported | FSIQ | abb. WAIS-III (German) | 97 | 0.38 |
| Naef et al. [198] | 2021 | 4 | patients | 26.71 | 38.60 | reported | FSIQ | short form of WAIS-IV | 44 | 0.14 |
3. Results
A summary effect of r = 0.23 for full-scale IQ was observed (k = 194; I2 = 60.70; 95% CI [0.21; 0.26]), when all available independent effect sizes were synthesized by means of the Hedges & Olkin approach. Summary effects were somewhat smaller when associations were limited to verbal (r = 0.20; k = 115; I2 = 43.84; 95% CI [0.16; 0.23]) or performance IQ domains (r = 0.20; k = 82; I2 = 27.66; 95% CI [0.17; 0.24]).
The Hunter & Schmidt-typed synthesis of artefact-corrected coefficients was broadly consistent with the results of the Hedges & Olkin approach, but unsurprisingly yielded somewhat larger effects for full-scale (r = 0.26; k = 116; I2 = 61.28; 95% CI [0.21; 0.031]), verbal (r = 0.22; k = 50; I2 = 50.28; 95% CI [0.15; 0.29]) and performance IQ (r = 0.25; k = 45; I2 = 30.42; 95% CI [0.20; 0.31]). Of note, these analyses were based on fewer observations compared to the other approaches, because necessary information for corrections (e.g. within-sample standard deviations of IQ scores) had not been reported. Results from the robust variance estimation-based approach were virtually identical with the Hedges & Olkin analyses showing small-to-moderate associations for full-scale (r = 0.23; k = 203; I2 = 54.85; 95% CI [0.20; 0.25]), verbal (r = 0.21; k = 141; I2 = 48.34; 95% CI [0.18; 0.24]) and performance IQ (r = 0.21; k = 110; I2 = 32.41; 95% CI [0.18; 0.24]).
This pattern of results remained virtually identical when analyses were limited to healthy (neurotypical) samples, although effect sizes were somewhat larger in this subgroup ranging from r = 0.19 to 0.24 for the Hedges & Olkin, 0.23 to 0.29 for the Hunter & Schmidt, and 0.20 to 0.24 for the RVE method (left side of table 2; figure 2 for a forest plot of the Hedges & Olkin analysis). Patient samples also showed non-trivial small-to-moderate effects, although they were slightly weaker than those of healthy samples (excepting Hedges & Olkin, as well as RVE analyses for verbal IQ; right part of table 2; figure 3 for a forest plot of the Hedges & Olkin-typed analysis).
Table 2.
Summary effects of healthy and patient samples according to three different analysis approaches. Note. In the RVE approach, the number of synthesized effect sizes is followed by the number of independent samples in parentheses; I2 = percentage of variability due to variability of true effects; LCI = lower bound of 95% confidence interval; UCI = upper bound of 95% confidence interval.
| healthy samples |
patient samples |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | n | I2 | r | LCI | UCI | k | n | I2 | r | LCI | UCI | |
| full-scale IQ | ||||||||||||
| Hedges & Olkin approach | 123 | 23 403 | 55.75 | 0.24 | 0.22 | 0.27 | 71 | 5361 | 60.43 | 0.21 | 0.16 | 0.26 |
| psychometric meta-analysis | 69 | 9057 | 32.76 | 0.29 | 0.24 | 0.33 | 47 | 3773 | 72.11 | 0.20 | 0.11 | 0.29 |
| robust variance estimation (RVE) | 128 (121) | 24 543 | 53.04 | 0.24 | 0.22 | 0.27 | 75 (69) | 7185 | 56.34 | 0.20 | 0.15 | 0.27 |
| verbal IQ | ||||||||||||
| Hedges & Olkin approach | 70 | 5440 | 47.47 | 0.19 | 0.14 | 0.23 | 45 | 2237 | 35.95 | 0.22 | 0.16 | 0.28 |
| psychometric meta-analysis | 31 | 2349 | 44.66 | 0.23 | 0.15 | 0.31 | 19 | 948 | 52.33 | 0.19 | 0.06 | 0.32 |
| robust variance estimation (RVE) | 93 (75) | 8262 | 52.65 | 0.20 | 0.16 | 0.25 | 48 (46) | 2550 | 39.32 | 0.22 | 0.16 | 0.28 |
| performance IQ | ||||||||||||
| Hedges & Olkin approach | 49 | 4162 | 31.96 | 0.21 | 0.17 | 0.25 | 33 | 1858 | 23.66 | 0.19 | 0.12 | 0.25 |
| psychometric meta-analysis | 28 | 2192 | 15.23 | 0.28 | 0.22 | 0.33 | 17 | 879 | 35.30 | 0.20 | 0.08 | 0.32 |
| robust variance estimation (RVE) | 74 (59) | 8095 | 31.69 | 0.22 | 0.19 | 0.26 | 36 (32) | 2078 | 33.90 | 0.19 | 0.13 | 0.25 |
Figure 2.
Forest plot of healthy samples (Hedges & Olkin model).
Figure 3.
Forest plot of patient samples (Hedges & Olkin model).
Results of leave-one-out analyses did not show any meaningful influences of single leverage points on effect-size estimations for all three IQ domains in overall analyses or subsets. Outlier analyses revealed a maximum of three potential leverage points in the datasets (see, electronic supplementary material, S1). Because recalculations of effect estimates when omitting these data points did not lead to meaningful changes in summary effect calculations (the largest observed influence of any of these outliers led to changes in the third decimal place of the summary effect in either domain), all subsequent analyses were performed without excluding these data points.
3.1. Subgroup analyses
No significant group differences were observed between healthy and patient-based samples in full-scale (k = 194; Q(1) = 1.06; p = 0.304), verbal (k = 115; Q(1) = 0.75; p = 0.385) or performance IQ effects (k = 82; Q(1) = 0.41; p = 0.520). However, we assessed healthy (neurotypical) and patient-based samples separately in the subsequent subgroup analyses to allow meaningful comparisons with the results of previous accounts (i.e. [23–25]).
Positive meaningful associations between IQ and brain volume were observable within all investigated subsets (i.e. regardless of sample type: healthy versus patient samples or IQ domain: full-scale versus verbal versus performance IQ; excepting a trivial positive full-scale IQ and brain volume associations for grey literature findings in patient; note that another three out of a total of 65 summary effects failed to reach nominal significance), thus generalizing across publication status, sample age, volumetric measurement type (i.e. total brain versus intracranial volume), sex, and g-ness (see, table 3). Somewhat numerically larger effects were consistently observed in healthy compared to patient samples for full-scale and performance, but not for verbal IQ.
Table 3.
Subgroup analyses for full-scale, verbal, and performance IQ. Note. Grey literature results were excluded from subgroup calculations for verbal and performance IQ because of low cell frequencies; TBV = Total brain volume; ICV = intracranial volume.
| healthy samples |
patient samples |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | n | I2 | r | LCI | UCI | k | n | I2 | r | LCI | UCI | |
| full-scale IQ | ||||||||||||
| total | 123 | 23 403 | 55.75 | 0.24 | 0.22 | 0.27 | 71 | 5361 | 60.43 | 0.21 | 0.16 | 0.26 |
| publication status | k = 123; Q(2) = 2.08; p = 0.353 | k = 71; Q(2) = 6.72; p = 0.035 | ||||||||||
| reported | 74 | 21 826 | 66.78 | 0.25 | 0.22 | 0.28 | 47 | 3842 | 67.97 | 0.24 | 0.18 | 0.31 |
| grey literature | 5 | 75 | 24.26 | 0.10 | −0.34 | 0.50 | 4 | 500 | 58.89 | 0.05 | −0.17 | 0.27 |
| personal communication | 44 | 1502 | 22.54 | 0.21 | 0.14 | 0.28 | 20 | 1019 | 30.04 | 0.17 | 0.09 | 0.25 |
| age | k = 123; Q(1) < 0.01; p = 0.969 | k = 71; Q(1) = 2.50; p = 0.114 | ||||||||||
| children/adolescents | 56 | 4326 | 33.30 | 0.24 | 0.19 | 0.29 | 36 | 2569 | 54.46 | 0.17 | 0.10 | 0.24 |
| adults | 67 | 19 077 | 65.72 | 0.24 | 0.21 | 0.28 | 35 | 2792 | 61.47 | 0.25 | 0.18 | 0.32 |
| volumetric measurement type | k = 94; Q(1) = 0.91; p = 0.339 | k = 62; Q(1) = 3.34; p = 0.068 | ||||||||||
| TBV | 78 | 17 891 | 48.86 | 0.24 | 0.21 | 0.27 | 45 | 3913 | 58.16 | 0.19 | 0.13 | 0.25 |
| ICV | 16 | 4448 | 80.08 | 0.28 | 0.19 | 0.36 | 17 | 1077 | 68.40 | 0.31 | 0.18 | 0.43 |
| sex | k = 60; Q(1) = 0.10; p = 0.751 | k = 18; Q(1) = 4.44; p = 0.035 | ||||||||||
| men | 36 | 6137 | 12.91 | 0.26 | 0.22 | 0.29 | 12 | 735 | 22.15 | 0.16 | 0.06 | 0.26 |
| women | 24 | 5994 | 0.02 | 0.26 | 0.23 | 0.29 | 6 | 160 | <0.01 | 0.33 | 0.15 | 0.49 |
| g-ness | k = 106; Q(2) = 23.69; p < 0.001 | k = 63; Q(2) = 0.38; p = 0.829 | ||||||||||
| fair g-ness | 7 | 563 | 59.00 | 0.33 | 0.16 | 0.47 | 2 | 62 | 91.94 | 0.42 | −1.00 | 1.00 |
| good g-ness | 48 | 18 309 | 56.70 | 0.19 | 0.16 | 0.22 | 30 | 3045 | 62.32 | 0.20 | 0.13 | 0.27 |
| excellent g-ness | 51 | 3364 | 4.07 | 0.31 | 0.27 | 0.34 | 31 | 1404 | 45.86 | 0.22 | 0.14 | 0.30 |
| verbal IQ | ||||||||||||
| total | 70 | 5440 | 47.47 | 0.19 | 0.14 | 0.23 | 45 | 2237 | 35.95 | 0.22 | 0.15 | 0.28 |
| publication status | k = 70; Q(1) = 0.85; p = 0.358 | k = 44; Q(1) = 2.39; p = 0.302 | ||||||||||
| reported | 42 | 4336 | 54.48 | 0.20 | 0.14 | 0.25 | 21 | 1316 | 62.36 | 0.26 | 0.15 | 0.37 |
| grey literature | — | — | — | — | — | — | 1 | 49 | — | 0.35 | 0.08 | 0.58 |
| personal communication | 28 | 1104 | 33.79 | 0.15 | 0.07 | .24 | 23 | 872 | 14.68 | 0.19 | 0.11 | 0.26 |
| age | k = 70; Q(1) = 0.49; p = 0.484 | k = 45; Q(1) = 7.11; p = 0.008 | ||||||||||
| children/adolescents | 26 | 2131 | 28.06 | 0.21 | 0.14 | 0.28 | 13 | 693 | 0.01 | 0.11 | 0.02 | 0.20 |
| adults | 44 | 3309 | 51.64 | 0.18 | 0.12 | 0.23 | 32 | 1544 | 33.84 | 0.25 | 0.18 | 0.32 |
| volumetric measurement type | k = 58; Q(1) = 3.47; p = 0.062 | k = 36; Q(1) = 4.19; p = 0.004 | ||||||||||
| TBV | 45 | 4296 | 40.38 | 0.17 | 0.12 | 0.21 | 23 | 1213 | 27.72 | 0.17 | 0.08 | 0.25 |
| ICV | 13 | 765 | 64.64 | 0.30 | 0.15 | 0.43 | 13 | 756 | 54.60 | 0.31 | 0.19 | 0.43 |
| sex | k = 35; Q(1) = 0.08; p = 0.772 | k = 15; Q(1) = 2.36; p = 0.125 | ||||||||||
| men | 21 | 1021 | 28.08 | 0.23 | 0.15 | 0.31 | 5 | 126 | <0.01 | 0.38 | 0.12 | 0.60 |
| women | 14 | 632 | <0.01 | 0.25 | 0.17 | 0.32 | 10 | 443 | <0.01 | 0.23 | 0.13 | 0.32 |
| Performance IQ | ||||||||||||
| total | 49 | 4162 | 31.96 | 0.21 | 0.17 | 0.25 | 33 | 1858 | 23.66 | 0.19 | 0.12 | 0.25 |
| publication status | k = 49; Q(1) = 2.65; p = 0.104 | k = 33; Q(1) = 0.81; p = 0.370 | ||||||||||
| reported | 28 | 3549 | 50.32 | 0.23 | 0.18 | 0.28 | 16 | 1135 | 54.52 | 0.21 | 0.10 | 0.32 |
| grey literature | — | — | — | — | — | — | — | — | — | — | — | — |
| personal communication | 21 | 613 | <0.01 | 0.17 | 0.10 | 0.23 | 17 | 1206 | <0.01 | 0.16 | 0.08 | 0.23 |
| age | k = 49; Q(1) = 0.53; p = 0.469 | k = 33; Q(1) = 1.84; p = 0.175 | ||||||||||
| children/adolescents | 23 | 2075 | 32.43 | 0.23 | 0.16 | 0.29 | 13 | 767 | 24.61 | 0.14 | 0.03 | 0.24 |
| adults | 26 | 2087 | 29.68 | 0.20 | 0.15 | 0.25 | 20 | 1091 | 36.90 | 0.22 | 0.13 | 0.31 |
| volumetric measurement type | k = 38; Q(1) = 2.33; p = 0.127 | k = 25; Q(1) = 0.708; p = 0.400 | ||||||||||
| TBV | 32 | 3700 | 43.73 | 0.21 | 0.16 | 0.26 | 16 | 1012 | <0.01 | 0.16 | 0.09 | 0.23 |
| ICV | 6 | 180 | <0.01 | 0.29 | 0.17 | 0.40 | 9 | 609 | 79.25 | 0.26 | <0.01 | 0.48 |
| sex | k = 25; Q(1) = 0.05; p = 0.820 | k = 9; Q(1) = 5.01; p = 0.025 | ||||||||||
| men | 16 | 717 | 17.44 | 0.24 | 0.16 | 0.31 | 6 | 330 | 6.21 | 0.11 | −0.07 | 0.28 |
| women | 9 | 429 | <0.01 | 0.25 | 0.17 | 0.32 | 3 | 91 | <0.01 | 0.27 | 0.13 | 0.40 |
There was only one significant difference between subset summary effects in healthy participants, indicating stronger associations with tests that were deemed to possess excellent compared to those that possess good g-ness. For patient samples, publication type in full-scale IQ, volumetric measurement in verbal IQ, and sex in performance IQ showed significantly larger effects for published, intracranial volume and female samples. However, these patient-based findings should be taken with a grain of salt because of low sample numbers (for details, see rightmost columns of table 3). Of note, between-studies variation remained moderate-to-large according to widely accepted classifications (0–25% suggest trivial, 25–50% small, 50–75% moderate and 75–100% large heterogeneity; see [199]), indicating potential further sources for explaining unobserved heterogeneity.
3.2. Meta-regressions
3.2.1. Single regressions
Primary study publication years negatively predicted effect sizes of brain size associations with full-scale IQ in the total and healthy samples (bs = −0.004 and −0.005; ps = 0.028 and 0.006, respectively), but not in patient samples (b < 0.001; p = 0.979). In the verbal and performance IQ domains, publication years again showed consistently negative influences on associations in total and healthy samples, although associations failed to reach nominal statistical significance (bs range: −0.006 to ≥0.001; p range: 0.055 to 0.883; effect declines for healthy samples by domain are illustrated in the electronic supplementary material, S1). Regression coefficients for patient samples were inconsistent in signs (ps > 0.475).
In our RVE-based comparisons of intelligence domains for our total samples (i.e. full-scale versus verbal versus performance IQ), no significant differences were observable between associations (all ps > 0.05, when referencing to full-scale IQ). When removing the intercept from the model, regression coefficients yielded similar values for full-scale, performance and verbal IQ (rs = 0.22, 0.21 and 0.23, respectively; all ps < 0.001). These outcomes were similar when running regressions on either healthy or patient samples only. No significant domain differences in association strength were observed in either group (all ps > 0.05) and once more full-scale, verbal and performance IQ showed consistently significant coefficients for healthy and patient samples (rs = 0.24 and 0.20, 0.20 and 0.23, 0.24 and 0.18, respectively).
3.2.2. Multiple regressions
In our hierarchical multiple meta-regressions, g-ness, primary study publication years and primary study publication status emerged as the most meaningful predictors of associations with full-scale IQ in healthy samples in our first block, explaining 46.18% of variance. Positive associations with g-ness indicated stronger effects for more highly g-loaded tests, thus corroborating our results from the subgroup analyses. Negative associations with publication years pointed towards declining effect sizes over time and effects were bigger when they had been published than when they had been obtained from the grey literature or personal communications. Neither addition of male ratio and mean age in our second nor study goal and number of corrections within studies in our third block led to improved model fit (top left of table 4).
Table 4.
Theory-guided multiple hierarchical meta-regressions by sample type and IQ domain. Note. SE b = standard error of regression coefficient; g-ness: 0 = fair/good, 1 = excellent; NoC = Number of controlled variables; Publication status: 0 = published, 1 = unpublished (i.e. obtained from grey literature or personal communications); Study goal: 0 = report of correlation was not focus of study, 1 = report of correlation was focus of study; if likelihood ratio tests of block 2 versus block 1 did not yield significant results, block 3 was compared with block 1; all VIFs < 1.88.
| healthy samples |
patient samples |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | LCI | UCI | SE b | p | B | LCI | UCI | SE b | p | |
| full-scale IQ | ||||||||||
| block 1 | k = 104; R2 = 46.18 | k = 62; R2 = 15.72 | ||||||||
| g-ness | 0.064 | 0.020 | 0.109 | 0.023 | 0.005 | 0.027 | −0.104 | 0.158 | 0.065 | 0.680 |
| publication year | −0.008 | −0.012 | −0.005 | 0.002 | <0.001 | 0.001 | −0.008 | 0.010 | 0.004 | 0.781 |
| publication status | −0.096 | −0.172 | −0.020 | 0.038 | 0.014 | −0.124 | −0.250 | 0.003 | 0.063 | 0.055 |
| block 2 | k = 104; R2 = 58.94; χ2(2) = 2.179; p = 0.336 | k = 62; R2 = 25.09; χ2(2)=4.013; p = 0.135 | ||||||||
| g-ness | 0.069 | 0.024 | 0.113 | 0.022 | 0.003 | 0.028 | −0.103 | 0.159 | 0.065 | 0.666 |
| publication year | −0.008 | −0.012 | −0.005 | 0.002 | <0.001 | 0.001 | −0.007 | 0.010 | 0.004 | 0.753 |
| publication status | −0.098 | −0.175 | −0.021 | 0.039 | 0.013 | −0.115 | −0.240 | 0.010 | 0.062 | 0.070 |
| male ratio | −0.016 | −0.086 | 0.053 | 0.035 | 0.647 | −0.212 | −0.446 | 0.023 | 0.117 | 0.076 |
| mean age | 0.001 | ≥0.001 | 0.002 | 0.001 | 0.121 | −0.001 | −0.004 | 0.002 | 0.002 | 0.650 |
| block 3 | k = 104; R2 = 58.73; χ2(4)=4.791; p = 0.310 | k = 62; R2 = 36.67; χ2(4)=9.894; p = 0.042 | ||||||||
| g-ness | 0.066 | 0.022 | 0.110 | 0.022 | 0.004 | 0.058 | −0.079 | 0.196 | 0.069 | 0.399 |
| publication year | −0.007 | −0.011 | −0.003 | 0.002 | 0.001 | 0.001 | −0.008 | 0.010 | 0.004 | 0.797 |
| publication status | −0.092 | −0.178 | −0.006 | 0.043 | 0.037 | −0.107 | −0.232 | 0.018 | 0.062 | 0.091 |
| male ratio | −0.014 | −0.083 | 0.056 | 0.035 | 0.694 | −0.267 | −0.502 | −0.032 | 0.117 | 0.027 |
| mean age | 0.001 | −0.001 | 0.002 | 0.001 | 0.299 | ≥0.001 | −0.004 | 0.003 | 0.002 | 0.908 |
| study goal | −0.019 | −0.074 | 0.037 | 0.028 | 0.509 | −0.117 | −0.247 | 0.013 | 0.065 | 0.078 |
| NoC | −0.014 | −0.034 | 0.006 | 0.010 | 0.162 | 0.020 | −0.031 | 0.071 | 0.025 | 0.443 |
| verbal IQ | ||||||||||
| block 1 | k = 67; R2 = 10.30 | k = 41; R2 = <0.01 | ||||||||
| publication year | −0.006 | −0.012 | ≥0.001 | 0.003 | 0.046 | −0.002 | −0.010 | 0.007 | 0.004 | 0.639 |
| publication status | −0.049 | −0.144 | 0.047 | 0.048 | 0.313 | −0.038 | −0.166 | 0.090 | 0.063 | 0.552 |
| block 2 | k = 67; R2 = 43.72; χ2(2) = 5.024; p = 0.081 | k = 41; R2 = 26.81; χ2(2) = 2.173; p = 0.337 | ||||||||
| publication year | −0.006 | −0.011 | ≥0.001 | 0.003 | 0.039 | −0.002 | −0.010 | 0.007 | 0.004 | 0.658 |
| publication status | −0.036 | −0.129 | 0.057 | 0.047 | 0.443 | −0.031 | −0.162 | 0.100 | 0.065 | 0.629 |
| male ratio | −0.008 | −0.131 | 0.116 | 0.062 | 0.901 | −0.070 | −0.293 | 0.153 | 0.110 | 0.527 |
| mean age | −0.002 | −0.004 | ≥0.001 | 0.001 | 0.025 | 0.002 | −0.002 | 0.006 | 0.002 | 0.291 |
| block 3 | k = 67; R2 = 74.19; χ²(4)=13.649; p = 0.009 | k = 41; R2 = 39.13; χ²(4)=4.083; p = 0. 395 | ||||||||
| publication year | −0.006 | −0.011 | −0.001 | 0.003 | 0.022 | −0.004 | −0.014 | 0.006 | 0.005 | 0.466 |
| publication status | −0.086 | −0.185 | 0.014 | 0.050 | 0.091 | 0.029 | −0.133 | 0.191 | 0.080 | 0.718 |
| male ratio | −0.027 | −0.145 | 0.091 | 0.059 | 0.651 | −0.061 | −0.287 | 0.166 | 0.111 | 0.590 |
| mean age | −0.001 | −0.003 | <0.001 | 0.001 | 0.120 | 0.002 | −0.002 | 0.006 | 0.002 | 0.315 |
| study goal | 0.045 | −0.042 | 0.132 | 0.044 | 0.308 | −0.066 | −0.246 | 0.114 | 0.089 | 0.463 |
| noc | −0.051 | −0.087 | −0.015 | 0.018 | 0.006 | 0.032 | −0.028 | 0.092 | 0.030 | 0.290 |
| performance IQ | ||||||||||
| block 1 | k = 47; R2 < 0.01 | k = 31; R2 = 41.92 | ||||||||
| publication year | −0.003 | −0.008 | 0.003 | 0.003 | 0.331 | 0.004 | −0.006 | 0.014 | 0.005 | 0.449 |
| publication status | −0.057 | −0.153 | 0.040 | 0.048 | 0.245 | −0.043 | −0.184 | 0.098 | 0.069 | 0.539 |
| block 2 | k = 47; R2 = 33.65; χ²(2)=3.800; p = 0.150 | k = 31; R2 = 61.61; χ²(2)=1.579; p = 0.454 | ||||||||
| publication year | −0.003 | −0.008 | 0.003 | 0.003 | 0.321 | 0.004 | −0.006 | 0.014 | 0.005 | 0.434 |
| publication status | −0.055 | −0.151 | 0.041 | 0.048 | 0.255 | −0.035 | −0.180 | 0.110 | 0.070 | 0.624 |
| male ratio | −0.032 | −0.153 | 0.089 | 0.060 | 0.602 | −0.074 | −0.322 | 0.173 | 0.120 | 0.542 |
| mean age | −0.002 | −0.004 | ≥0.001 | 0.001 | 0.031 | 0.001 | −0.002 | 0.005 | 0.002 | 0.510 |
| block 3 | k = 47; R2 = 26.54; χ2(4) =7.162; p = 0.128 | k = 31; R2 = 99.86; χ2(4) = 14.259; p = 0.007 | ||||||||
| publication year | −0.001 | −0.007 | 0.005 | 0.003 | 0.665 | <0.001 | −0.009 | 0.010 | 0.005 | 0.937 |
| publication status | −0.030 | −0.141 | 0.080 | 0.055 | 0.581 | 0.093 | −0.058 | 0.243 | 0.073 | 0.216 |
| male ratio | −0.038 | −0.156 | 0.081 | 0.059 | 0.524 | −0.080 | −0.289 | 0.129 | 0.101 | 0.438 |
| mean age | −0.001 | −0.003 | 0.001 | 0.001 | 0.480 | −0.001 | −0.004 | 0.003 | 0.002 | 0.745 |
| study goal | −0.076 | −0.174 | 0.023 | 0.049 | 0.128 | −0.111 | −0.265 | 0.044 | 0.075 | 0.152 |
| NoC | −0.016 | −0.049 | 0.018 | 0.017 | 0.359 | 0.086 | 0.026 | 0.145 | 0.029 | 0.006 |
Effects on full-scale IQ in patient samples were on the whole weaker and showed a single nominally significant effect of male ratio in block three, indicating smaller associations in men than in women (top right of table 4). However, this finding should be interpreted with caution, because male ratio did not emerge as a significant predictor in any other of our analyses and may be driven by untypically small full-scale IQ correlations of exclusively male patient samples, as evident from our subgroup estimates (table 3).
For verbal IQ, publication year once more negatively predicted effect sizes, but was only significant for healthy samples. In healthy samples, model fit significantly improved when number of corrections within primary studies was included, indicating larger associations when fewer corrections were used. In patient samples, no included predictor yielded significant associations in any block of the model (centre rows of table 4).
For performance IQ, neither variables in the first nor in the subsequent blocks showed significant influences on effect sizes in healthy samples. A similar pattern emerged for patient samples, showing no significant influences in the initial two blocks. Block 3 showed significantly improved model fit compared to Block 1, which was driven by a positive association between the number of corrections within primary studies, indicating smaller associations when fewer corrections were used (bottom of table 4).
3.3. Dissemination bias
First, a power-enhanced funnel plot [44] was used to visually inspect full-scale IQ effects of healthy samples. The funnel plot appeared to be fairly symmetrical, but figure 4 shows that most studies had lower power than desirable, yielding a median power estimate of 60.7%. The TES did not provide evidence for confounding dissemination bias (p = 0.152), but the replicability index was low (52.5%), thus indicating the presence of inflated results. Median power for verbal and performance IQ was suboptimal as well, yielding 31.2% and 45.9%. Similar to full-scale IQ, TES-results did not indicate dissemination bias in either verbal (p = 0.431) or performance IQ (p = 0.931), but replicability indices were once again low (13.9% and 38.3%, respectively).
Figure 4.
Power-enhanced funnel plot of published healthy sample effect sizes for full-scale IQ.
Second, Sterne & Egger's regressions showed significant evidence for funnel-plot asymmetry in full-scale IQ (p < 0.001), thus pointing toward confounding dissemination bias. Although there was no bias evidence for verbal IQ (p = 0.265), results for performance IQ were once again significant (p = 0.011).
Third, trim-and-fill analyses for full-scale IQ indicated 20 missing studies on the left side of the summary effect, leading to an adjusted effect estimate of r = 0.21. Although this adjusted estimate should not be used as a correction of the observed summary effect, it serves as a sensitivity analysis that is presently indicative of summary effect inflation. Similar results were observed for verbal and performance IQ were 7 and 11 effect sizes were detected to be missing on the left-hand side, thus yielding adjusted effects of 0.16 and 0.17, respectively (see electronic supplementary material, S1).
Fourth, p-curve did not indicate any evidence for confounding p-hacking (no evidence for right skew in binomial or continuous tests for full and half p-curves; all ps < 0.001), and primary studies were on average sufficiently powered to indicate evidential value of the observed non-null effect (all ps > 0.999 for binomial and continuous tests against 33% power). p-curve-based effect estimations yielded a similar effect to the above reported traditional estimate (r = 0.24). Results from p-uniform analyses did not indicate confounding bias either (p = 0.975), but yielded a somewhat larger summary effect (r = 0.26; 95% CI [0.22; 0.28]). The estimate based on p-uniform* showed a somewhat smaller summary effect than the other two p-value-based estimation methods (r = 0.22; 95% CI [0.18; 0.26]).
Both verbal and performance IQ showed no evidence for p-hacking (all ps < 0.001), and the available primary studies appeared to provide evidential value (all ps > 0.748). Effect estimates were similar to traditional calculations yielding r = 0.21 and 0.24, respectively. Results from p-uniform did not indicate bias in either domain (ps > 0.768) and effect estimates were similar to p-curve-based values for verbal (r = 0.22; 95% CI [0.15; 0.29]) and performance IQ (r = 0.23; 95% CI [0.16; 0.31]). Once more, p-uniform*-based estimates were lower than those of p-uniform yielding r = 0.18 (95% CI [0.11; 0.25]) for verbal and r = 0.23 (95% CI [0.15; 0.29]) for performance IQ.
Fifth, full-scale IQ-adjusted parameters (i.e. based on the four different weight functions from [200]) did not vary considerably (ranging from r = 0.23 to 0.25) and were broadly consistent with the observed unadjusted r = 0.25 in.this subset. Results for verbal and performance IQ conformed to this observation, showing rather small variations between different weight function estimates (r range for verbal IQ: 0.15–0.19, r range for performance IQ: 0.20–0.22) and corresponding broadly to the unadjusted rs = 0.20 and 0.23.
By contrast, the selection model of Copas & Shi [54] provided some evidence for dissemination bias in full-scale IQ, indicating 44 missing studies on the left side of the observed summary effect and suggesting an adjusted summary effect of r = 0.20. No missing effect sizes were detected for verbal IQ, leading to a virtually identical summary effect of r = 0.20, but 11 effects were estimated to be missing on the left side of the performance IQ summary effect, thus leading to an adjustment to r = 0.18.
Sixth, confidence intervals estimates for full-scale IQ of the Henmi & Copas [57] approach differed somewhat from the conventional DerSimonian-Laird estimation (95% CIs [0.17; 0.27] versus [0.22; 0.28]). For verbal and performance IQ, confidence intervals showed similar patterns (95% CIs [0.10; 0.23] versus [0.14; 0.26] and [0.14; 0.26] versus [0.18; 0.28], for conventional and Henmi-Copas estimates, respectively).
Seventh, in a subgroup analysis published summary effects did not differ significantly from unpublished summary effects (i.e. effect sizes from grey literature or personal communications) for full-scale IQ (Q(1) = 1.720; p = 0.190). However, as expected, published effects were larger than unpublished ones (rs = 0.25 versus 0.21, respectively). Results for verbal and performance IQ domains were virtually identical, yielding no nominally significant differences, but consistently larger published summary effects (table 3 for parameters).
Finally, visual inspections of cumulative forest plots of full-scale IQ associations show a clear pattern of systematically decreasing effect sizes over time, thus conforming to our findings of significant negative influences of study years on effect sizes (figure 5). When cumulating results according to sample sizes, a less unequivocal picture emerged, although larger samples appeared to show weaker effects than smaller samples, thus conforming to the above evidence that suggests the presence of some confounding bias in the present data (figure 6). Virtual identical pictures for both publication years and sample sizes result from cumulating verbal and performance IQ data (see electronic supplementary material, S1).
Figure 5.
Cumulative forest plot of healthy sample effects according to publication year for full-scale IQ.
Figure 6.
Cumulative forest plot of healthy sample effects according to sample size for full-scale IQ.
3.4. Multiverse meta-analyses
3.4.1. Combinatorial meta-analyses
Results of combinatorial meta-analyses did not show evidence for substantial deviations of the majority of possible combinations from the estimated summary effects. For healthy samples, the interquartile range of the summary-effect distribution for full-scale IQ analyses merely amounted to a difference of 0.02 in r values (Q1 = 0.24; Q3 = 0.26; figure 7). Visual inspection of GOSH-plots for the verbal and performance IQ analyses did not indicate systematic patterns either, although interquartile ranges were somewhat larger than for full-scale IQ, most likely owing to the smaller number of included effect sizes (interquartile ranges = 0.03 and 0.03; Q1 = 0.17 and 0.20; Q3 = 0.20 and 0.23; figures 8 and 9). Results of combinatorial meta-analyses for patient samples showed similar patterns and are included in electronic supplementary material, S1.
Figure 7.
GOSH-plot of 100 000 randomly sampled healthy subsets of all possible combinations for full-scale IQ.
Figure 8.
GOSH-plot of 100 000 randomly sampled healthy subsets of all possible combinations for verbal IQ.
Figure 9.
GOSH-plot of 100 000 randomly sampled healthy subsets of all possible combinations for performance IQ.
3.4.2. Specification-curve meta-analyses
These indicated that virtually any reasonable specification in any domain consistently leads to a positive small-to-moderate association between brain volume and IQ. For full-scale IQ, summary effects ranged from a minimum of r = 0.10 to a maximum effect of r = 0.37. As can be seen in figure 10, most specifications yielded values in the r = 0.20 range. These specifications tended to provide comparatively precise estimates (i.e. effects with narrow confidence intervals), while estimates at the lower and upper end of the effect distribution were less precise. Verbal and performance IQ specification curves were consistent with these observations, almost invariably yielding positive small-to-moderate effects, mostly in the r = 0.20 range (figures 11 and 12; minimum rs = 0.11 and <0.01; maximum rs = 0.33 and 0.32, respectively).
Figure 10.
Descriptive meta-analytic specification-curve plot (see [14]) of summary effects from all reasonable specifications for full-scale IQ. Note. The top panel shows summary effects with 95% confidence intervals according to effect strength. The middle panel indicates the number of samples within respective subsets. The bottom panel indicates the respective ‘which’ and ‘how’ factors that were used for the respective effect estimation. Warmer colours indicate lower effect precision (i.e. larger confidence intervals).
Figure 11.
Descriptive meta-analytic specification-curve plot (see [14]) of summary effects from all reasonable specifications for verbal IQ. Note. The top panel shows summary effects with 95% confidence intervals according to effect strength. The middle panel indicates the number of samples within respective subsets. The bottom panel indicates the respective ‘which’ and ‘how’ factors that were used for the respective effect estimation. Warmer colours indicate lower effect precision (i.e. larger confidence intervals).
Figure 12.
Descriptive meta-analytic specification-curve plot (see [14]) of summary effects from all reasonable specifications for performance IQ. Note. The top panel shows summary effects with 95% confidence intervals according to effect strength. The middle panel indicates the number of samples within respective subsets. The bottom panel indicates the respective ‘which’ and ‘how’ factors that were used for the respective effect estimation. Warmer colours indicate lower effect precision (i.e. larger confidence intervals).
4. Discussion
In this quantitative research synthesis, we show that positive associations of in vivo brain volume with IQ are highly reproducible. This link is consistently observable regardless of which empirical studies are included in a formal meta-analysis and how they are analysed. Results of our analyses convergently indicate that the effect strength must be assumed to be small-to-moderate in size, with the best available estimates for healthy participants in full-scale IQ ranging from r = 0.24 (uncorrected; approximately 6% explained variance) to 0.29 (corrected approximately 8% explained variance). Effects for full-scale IQ appear to be stronger and more systematically related to moderators compared to verbal and performance IQ. However, these three intelligence domains are highly intercorrelated and their correlation with IQ test results are to be seen as manifestations of a largely similar true effect across domains. We, therefore, focus on full-scale IQ findings of healthy samples in our discussion, unless indicated otherwise.
4.1. Comparisons with previous meta-analyses
The strengths of the observed summary effects in the present meta-analysis correspond closely to those identified by Pietschnig et al. [24], although the number of participants in this updated analysis is more than three times larger. The observed association for full-scale IQ in healthy samples (i.e. corresponding to selection criteria of the meta-analyses from [25], and [23]) resulted in an estimate of r = 0.24 (95% CI [0.22; 0.27]), thus indicating considerably lower associations than those reported by Gignac & Bates [25]) and McDaniel [23]). Key characteristics of the available meta-analyses are summarized in table 5.
Table 5.
Characteristics of available meta-analyses on the in vivo brain volume and intelligence link. Note. k = number of independent samples in analysis; summary effect = best estimate according to authors of meta-analysis; when both Hedges & Olkin- as well as Hunter & Schmidt-typed analyses were performed, both estimates are provided, respectively.
| McDaniel [23] | Pietschnig et al. [24] | Gignac & Bates [25] | present study | |
|---|---|---|---|---|
| sample type | healthy samples | healthy and patient samples | healthy samples | healthy and patient samples |
| K | 37 | 148 | 32 | 194 |
| N | 1530 | 8034 | 2305 | 26 764 |
| meta-analytic approach | Hunter & Schmidt-typed | Hedges & Olkin-typed | Hunter & Schmidt-typed | Both |
| summary effect (r) | 0.33 | 0.24 (healthy r = 0.24; patient r = 0.20) | 0.40 | 0.23/0.26 (healthy r = 0.24/0.29; patient r = 0.21/0.20) |
| meta-analysis overlap | all studies of McDaniel [23] included | subset of Pietschnig et al. [24] | all studies of Pietschnig et al. [24] included |
It could be argued that these inconsistencies are to a certain extent due to the differing methodological focus of the used analyses because both meta-analyses of Gignac & Bates [25] and McDaniel [23] reported values that were corrected for direct range restriction. However, when we respecified our analyses to apply identical methods, full-scale IQ associations for healthy samples once more led to a lower estimate, yielding r = 0.29. This indicates that the reported estimates of prior Hunter & Schmidt-based syntheses were inflated (i.e. even before accounting for dissemination bias).
This idea is supported by our analyses of individual data subsets that used the very same specifications as these prior studies. For instance, Gignac & Bates [25] showed that IQ assessments with higher g-ness (i.e. reflecting abilities that are more closely related to psychometric g, thus providing a better representation of cognitive abilities) yielded larger associations than less g-loaded assessments. They concluded that the most salient estimate of the brain volume and IQ association averages r = 0.40 (i.e. corresponding to about 16% of explained variance), based on a specific subset of effect sizes that should provide the most credible results (i.e. using healthy samples, tests with excellent g-ness and attenuation-corrected effect sizes only).
None of the reasonable specifications that were included in our specification curve analysis yielded a summary effect that was larger than r = 0.37. Importantly, this most extreme upper value of all possible specifications was based on the very same inclusion criteria as the specification that is supposed to represent the best operationalization of this association according to Gignac & Bates [25], healthy samples, excellent g-ness, range departure corrected, Hunter & Schmidt estimator), excepting sample age (this uppermost value was based on children/adolescents only; the same specification with all ages yielded r = 0.34, corresponding to 11% of explained variance). This is important for a number of reasons.
First, it shows that the specification that was chosen by Gignac & Bates [25] leads to estimates in the extreme upper tail of the distribution of reasonable summary effects. Besides yielding uncharacteristically large values, these estimates have large confidence intervals (i.e. representing higher effect volatility), because they are based on comparatively small sample numbers. Results from our combinatorial meta-analyses showed that at least 75% (i.e. the bottom three quartiles) of results yielded values below r = 0.26.
Second, these findings suggest that the estimate reported in Gignac & Bates [25] must be considered to have been inflated, even when one was to assume that this extreme specification yields the most salient estimate for the brain volume and IQ association (i.e. the summary effect in [25], exceeds the upper threshold of any estimate of the present summary effect distribution). Third, the lower summary effects in the present analyses compared to the earlier estimate of Gignac & Bates [25], when identical specifications were used, indicate that the studies that were added in the present update of the literature reported lower correlations, thus conforming to a decline effect [21,22].
Consistent with this interpretation, publication years of primary studies predicted brain volume and IQ associations negatively, indicating decreasing effect sizes over time. Cross-temporally declining effect sizes have been demonstrated to be prevalent in psychological science in general and intelligence research in particular, especially when initial study sample sizes are small [22]. This means that early and small n (=imprecise) primary study reports represent more often than not overestimates of the brain size and IQ association, thus having led to inflated meta-analytic summary effects. The presently observed effect declines and comparatively large effect estimates of early small-n studies (e.g. [5]) are consistent with the decline effect and its assumed drivers.
4.2. Moderators
It is unsurprising that effects were typically stronger in healthy than in patient samples because the included patients suffered from different conditions that are likely to impair cognitive functioning (e.g. autism, brain traumas, schizophrenia) which is bound to introduce statistical noise into the data. Therefore, effects of moderators were substantially weaker and less unequivocal for patients than for healthy samples.
Consistent with Gignac & Bates [25], there were stronger associations with highly g-loaded tests compared to fairly g-loaded ones in healthy participants (uncorrected rs = 0.31 versus 0.19; Q(2) = 23.69; p < 0.001), but not in patient samples. These results were supported by the findings from our regression analyses where larger g-ness positively predicted effect sizes of healthy participants.
Within any examined subgroup, correlations that had been reported within publications were numerically larger than those that had been obtained through personal communications or from the grey literature. This suggests that correlations were selectively reported in the published literature although only differences in full-scale IQ associations of healthy samples reached nominal significance. This observation is consistent with effect inflation because larger associations are more likely than smaller ones to be numerically reported in the literature (numerically stronger effects are more likely to become significant—depending on sample sizes and accuracy—and therefore more likely to be published), thus potentially leading to inadequate assumptions of the readers about the effect strength. This finding is supported by results from our regression analyses that showed weaker effects of unpublished than published effect sizes. This suggests that the reported effects in the brain size and intelligence literature are more often inflated than not, thus conforming to results from Pietschnig et al. [24].
In a similar vein, publication years were negatively related to effect sizes, thus indicating a confounding decline effect [21] and conforming to cross-temporally decreasing effect sizes as reported in an earlier meta-analysis [24].
The only further moderator with consistent directions in terms of the observed association appeared to be measurement type which consistently yielded larger estimates for intracranial than for total brain volume, although these differences did not reach nominal significance (except for verbal IQ in patient samples). There were no consistent patterns in regard to age or sex in subgroup or regression analyses, thus conforming to a previous account that indicated that brain volume and IQ associations generalize over participant age bands and sex ([24]; but see [23], for conflicting findings).
4.3. Dissemination bias
Three of our formal methods for detecting dissemination yielded significant bias indications for both full-scale and performance IQ (Sterne & Egger's regression, Trim-and-Fill analysis, Copas & Shi's method), while only one method (Trim-and-Fill analysis) indicated bias in verbal IQ. The evidence for bias was stronger for full-scale than performance IQ. It should be noted, that both Sterne and Egger's regression, as well as the Trim-and-Fill analysis, are funnel plot asymmetry-based methods and consequently particularly sensitive for the detection of small-sample effects. This means that the detected bias seems to be rooted in the correspondingly large error variance of underpowered (i.e. small sample size) studies and is consistent with previously raised concerns about suboptimal power in neuroscientific research [201]. Viewed from this perspective, declining effect sizes over time appear to be somewhat reconciliatory, because this may well mean that average study power has increased in this field (or at least in studies addressing this research question).
The low observed replicability indices for all three domains further corroborate the evidence for effect inflation. Similarly, results of our effect estimations by means of p-value-based methods support the evidence for confounding dissemination bias, as previously observed in regard to this research question [24]. This interpretation is consistent with larger effects from published sources than from those that were obtained from the grey literature or personal communications, although these differences only reached nominal significance in meta-regressions, but not subgroup analyses.
The present findings contrast the conclusions of Gignac & Bates [25] who did not identify bias evidence in their analysis. This discrepancy may be due to two different causes.
On the one hand, Gignac & Bates [25] included unpublished results in the publication bias detection analyses (i.e. results that [24], had obtained from the grey literature or through personal communications with authors), which (i) prevent potential bias from detection and (ii) are conceptually unsuitable to be used in p-curve and p-uniform analyses [50,51]. On the other hand, different methods of dissemination bias detection are not equally sensitive for different forms of bias, thus necessitating a triangulation of methods for bias estimation according to current recommendations [42]. Relying on comparatively few and conceptually similar detection methods (i.e. publication bias tests of two p-value-based methods; p-curve and p-uniform; Henmi-Copas approach) may have contributed to the non-detection of bias evidence in this past meta-analysis [25], particularly because these methods are not suitable to detect small-sample effects.
Although the present findings indicate a presence of confounding publication bias, this should not be interpreted as evidence against a brain volume and IQ link. As pointed out above, these associations appear to generalize across numerous potential moderators and replicate well in terms of the identified direction. However, confounding dissemination bias suggests that the obtained summary effects in many primary studies (and even some meta-analyses) represent inflated estimates of the true association. However, it needs to be acknowledged that the future development of more reliable methods for assessing IQ on the one or in vivo brain volume on the other hand may lead to larger correlation estimates in primary studies. Nonetheless, the strength of the brain volume and IQ association must be considered to be small-to-medium-sized at best.
4.4. Significance of the observed effect
On the one hand, the strength of the observed summary effect suggests that effects of mere neuron numbers, glial cells, or brain reserve are unlikely candidates for the explanation of between-individuals intelligence differences. On the other hand, the effect is clearly non-trivial and has turned out to be remarkably reproducible in terms of its positive direction across a large number of primary studies. Consequently, brain volume should not be seen as a supervenient (i.e. one-to-one) but rather an isomorphic (i.e. many-to-one) proxy of human intelligence. This may mean that brain volume in its own right is too coarse of a measure to reliably predict intelligence differences. It seems likely that examining the role of functional aspects (e.g. white matter integrity) and more fine-grained structural elements (e.g. cortical thickness; see [2]) may help in further clarifying the neurobiological bases of human intelligence.
5. Conclusion
In the present meta-analysis, we show evidence for modestly sized associations between in vivo brain volume and IQ. The effect appears to be stronger when more g-loaded test instruments are used and when full-scale IQ, rather than verbal or performance IQ domains, are assessed. This link appears to be remarkably robust in terms of other potentially moderating variables, generalizing across age, sex or primary study properties, such as study goal and number of variables, that have been controlled for. Importantly, examination of all possible reasonable specifications in regard to how meta-analytical calculations were performed and which studies had been included in the analysis showed consistently positive associations that ranged from r = 0.10 to 0.37, with a majority of specifications yielding values around r = 0.24. Although in the available literature this link replicated well across studies, some allowance must be made for overestimations of summary effects due to confounding dissemination biases. This indicates that the observed summary effect estimates must be considered to be inflated, thus representing an upper threshold of the true brain size and IQ association.
Data accessibility
All data are available at OSF: https://osf.io/y6msp.
Authors' contributions
J.P.: conceptualization, formal analysis, methodology, project administration, supervision, validation, writing—original draft, writing—review and editing; D.G.: data curation, formal analysis, investigation, validation, visualization, writing—original draft, writing—review and editing; M.Z.: data curation, investigation, validation, writing—review and editing; M.V.: conceptualization, methodology, software, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare no conflicts of interest.
Funding
We received no funding for this study.
References
References marked with an asterisk indicates studies included in the meta-analysis.
- 1.Tiedemann F. 1836. XXIII. On the brain of the negro, compared with that of the European and the orang-outang. Phil. Trans. R. Soc. 126, 497-527. ( 10.1098/rstl.1836.0025) [DOI] [Google Scholar]
- 2.Ritchie SJ, et al. 2015. Beyond a bigger brain: multivariable structural brain imaging and intelligence. Intelligence 51, 47-56. ( 10.1016/j.intell.2015.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern Y, Barnes CA, Grady C, Jones RN, Raz N. 2019. Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiol. Aging 83, 124-129. ( 10.1016/j.neurobiolaging.2019.03.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galton F. 1889. Head growth in students at the University of Cambridge. Nature 40, 318. ( 10.1038/040318a0) [DOI] [Google Scholar]
- 5.Willerman L, Schulz R, Rutledge JN, Bigler ED. 1991. In vivo brain size and intelligence. Intelligence 15, 223-228. ( 10.1016/0160-2896(91)90031-8) [DOI] [Google Scholar]
- 6.Cohen J. 1988. Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 7.Gignac G, Vernon PA, Wickett JC. 2003. Factors influencing the relationship between brain size and intelligence. In The scientific study of general intelligence, pp. 93-106. Oxford, UK: Pergamon. [Google Scholar]
- 8.Miller GF, Penke L. 2007. The evolution of human intelligence and the coefficient of additive genetic variance in human brain size. Intelligence 35, 97-114. [Google Scholar]
- 9.Rushton JP, Ankney CD. 1996. Brain size and cognitive ability: correlations with age, sex, social class, and race. Psychon. Bull. Rev. 3, 21-36. ( 10.3758/BF03210739) [DOI] [PubMed] [Google Scholar]
- 10.Rushton JP, Ankney CD. 2000. Size matters: a review and new analyses of racial differences in cranial capacity and intelligence that refute Kamin and Omari. Pers. Individ. Dif. 29, 591-620. ( 10.1016/S0191-8869(99)00256-1) [DOI] [Google Scholar]
- 11.Rushton JP, Ankney CD. 2009. Whole brain size and general mental ability: a review. Int. J. Neurosci. 119, 692-732. ( 10.1080/00207450802325843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vernon PA, Wickett JC, Bazana PG, Stelmack RM. 2000. The neuropsychology and psychophysiology of human intelligence. In, Handbook of intelligence (ed. Sternberg RJ), pp. 245-266. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 13.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. 2009. Introduction to meta-analysis. Chichester, UK: Wiley. [Google Scholar]
- 14.Voracek M, Kossmeier M, Tran US. 2019. Which data to meta-analyze, and how? A specification-curve and multiverse-analysis approach to meta-analysis. Zeitschrift für Psychologie 227, 64-82. ( 10.1027/2151-2604/a000357) [DOI] [Google Scholar]
- 15.Steegen S, Tuerlinckx F, Gelman A, Vanpaemel W. 2016. Increasing transparency through a multiverse analysis. Perspect. Psychol. Sci. 11, 702-712. ( 10.1177/1745691616658637) [DOI] [PubMed] [Google Scholar]
- 16.Simonsohon U, Simmons JP, Nelson LD. 2015. Specification curve: Descriptive and inferential statistics on all reasonable specifications. Retrieved from http://sticerd.lse.ac.uk/seminarpapers/psyc16022016.pdf.
- 17.Simonsohn U, Simmons JP, Nelson LD. 2020. Specification curve analysis. Nat. Hum. Behav. 4, 1208-1214. ( 10.1038/s41562-020-0912-z) [DOI] [PubMed] [Google Scholar]
- 18.Wicherts JM, Veldkamp CLS, Augeusteijn HEM, Bakker M, van Aert RCM, van Assen MALM. 2016. Degrees of freedom in planning, running, and reporting psychological studies: a checklist to avoid p-hacking. Front. Psychol. 7, 1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung K, Shavitt S, Viswanathan M, Hilbe JM. 2014. Female hurricanes are deadlier than male hurricanes. Proc. Natl Acad. Sci. USA 111, 8782-8787. ( 10.1073/pnas.1402786111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olkin I, Dahabreh IJ, Trikalinos TA. 2012. GOSH–a graphical display of study heterogeneity. Res. Synth. Methods 3, 214-223. ( 10.1002/jrsm.1053) [DOI] [PubMed] [Google Scholar]
- 21.Schooler J. 2011. Unpublished results hide the decline effect. Nature 470, 437. ( 10.1038/470437a) [DOI] [PubMed] [Google Scholar]
- 22.Pietschnig J, Siegel M, Eder JSN, Gittler G. 2019. Effect declines are systematic, strong and ubiquitous: a meta-meta-analysis of the decline effect in intelligence research. Front. Psychol. 10, 2874. ( 10.3389/fpsyg.2019.02874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDaniel MA. 2005. Big-brained people are smarter: a meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence 33, 337-346. ( 10.1016/j.intell.2004.11.005) [DOI] [Google Scholar]
- 24.Pietschnig J, Penke L, Wicherts JM, Zeiler M, Voracek M. 2015. Meta-analysis of associations between human brain volume and intelligence differences: how strong are they and what do they mean? Neurosci. Biobehav. Rev. 57, 411-432. ( 10.1016/j.neubiorev.2015.09.017) [DOI] [PubMed] [Google Scholar]
- 25.Gignac GE, Bates TC. 2017. Brain volume and intelligence: the moderating role of intelligence measurement quality. Intelligence 64, 18-29. ( 10.1016/j.intell.2017.06.004) [DOI] [Google Scholar]
- 26.Hunter JE, Schmidt FL. 2015. Methods of meta-analysis: correcting error and bias in research findings, 3rd edn. Newbury Park, CA: Sage. [Google Scholar]
- 27.Hedges LV, Olkin I. 1985. Statistical methods for meta-analysis. New York, NY: Academic Press. [Google Scholar]
- 28.Dürlinger F, Pietschnig J. 2022. Meta-analyzing intelligence and religiosity associations: evidence from the multiverse. PLoS ONE 17, e0262699. ( 10.1371/journal.pone.0262699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedges LV, Tipton E, Johnson MC. 2010. Robust variance estimation in meta-regression with dependent effect size estimates. Res. Synth. Methods 1, 39-65. ( 10.1002/jrsm.5) [DOI] [PubMed] [Google Scholar]
- 30.Pigott TD. 2009. Handling missing data. In The handbook of research synthesis and meta-analysis (eds Cooper HM, Hedges LV, Valentine JC), pp. 399-416, 2nd edn. Sage. [Google Scholar]
- 31.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1-48. [Google Scholar]
- 32.Olkin I, Pratt JW. 1958. Unbiased estimation of certain correlation coefficients. Ann. Math. Stat. 29, 201-211. ( 10.1214/aoms/1177706717) [DOI] [Google Scholar]
- 33.Knapp G, Hartung J. 2003. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 22, 2693-2710. ( 10.1002/sim.1482) [DOI] [PubMed] [Google Scholar]
- 34.Sidik K, Jonkman JN. 2002. A simple confidence interval for meta-analysis. Stat. Med. 21, 3153-3159. ( 10.1002/sim.1262) [DOI] [PubMed] [Google Scholar]
- 35.Viechtbauer W, Cheung MW-L. 2010. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 1, 112-125. ( 10.1002/jrsm.11) [DOI] [PubMed] [Google Scholar]
- 36.Thorndike RL. 1949. Personnel selection: test and measurement techniques. New York, NY: Wiley. [Google Scholar]
- 37.Kelley TL. 1923. Statistical methods. New York, NY: Macmillan. [Google Scholar]
- 38.Duan B, Dunlap WP. 1997. The accuracy of different methods for estimating the standard error of correlations corrected for range restriction. Educ. Psychol. Meas. 57, 254-265. (doi:10.1177%2F0013164497057002005) [Google Scholar]
- 39.Pigott TD, Polanin JR. 2020. Methodological guidance paper: high-quality meta-analysis in a systematic review. Rev. Educ. Res. 90, 24-46. (doi:10.3102%2F0034654319877153) [Google Scholar]
- 40.Hedges LV. 2019. Stochastically dependent effect sizes. In The handbook of research synthesis and meta-analysis (eds Cooper H, Hedges LV, Valentine JC), pp. 281-297, 3rd edn. Russel Sage Foundation. [Google Scholar]
- 41.Tanner-Smith EE, Tipton E, Polanin JR. 2016. Handling complex meta-analytic data structures using robust variance estimation: a tutorial in R. J. Dev. Life-Course Criminol. 5, 614-615. ( 10.1007/s40865-019-00127-2) [DOI] [Google Scholar]
- 42.Carter EC, Schönbrodt FD, Gervais WM, Hilgard J. 2019. Correcting for bias in psychology: a comparison of meta-analytic methods. Adv. Methods Pract. Psychol. Sci. 2, 115-144. (doi:10.1177%2F2515245919847196) [Google Scholar]
- 43.Renkewitz F, Keiner M. 2019. How to detect publication bias in psychological pesearch: a comparative evaluation of six statistical methods. Zeitschrift Für Psychologie/Journal of Psychology 227, 261-279. ( 10.1027/2151-2604/a000386) [DOI] [Google Scholar]
- 44.Kossmeier M, Tran US, Voracek M. 2020. Power-enhanced funnel plots for meta-analysis. Zeitschrift für Psychologie 228, 43-49. ( 10.1027/2151-2604/a000392) [DOI] [Google Scholar]
- 45.Kossmeier M, Tran US, Voracek M. 2020. Metaviz: forest plots, funnel plots, and visual funnel plot inference for meta-analysis (version 0.3.1). See https://cran.r-project.org/package=metaviz.
- 46.Ioannidis JP, Trikalinos TA. 2007. An exploratory test for an excess of significant findings. Clin. Trials 4, 245-253. (doi:10.1177%2F1740774507079441) [DOI] [PubMed] [Google Scholar]
- 47.Schimmack U. 2016. The replicability-index: Quantifying statistical research integrity. See https://replicationindex.wordpress.com/2016/01/31/a-revised-introduction-to-the-r-index/.
- 48.Sterne JAC, Egger M. 2005. Regression methods to detect publication and other bias in meta-analysis. In Publication bias in meta-analysis: prevention, assessment, and adjustments (eds Rothstein HR, Sutton AJ, Borenstein M), pp. 99-110. Wiley. [Google Scholar]
- 49.Duval SJ, Tweedie RL. 2000. A nonparametric ‘trim and fill’ method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association 95, 89-98. ( 10.1080/01621459.2000.10473905) [DOI] [Google Scholar]
- 50.Simonsohn U, Nelson LD, Simmons JP. 2014. P-curve: a key to the file-drawer. J. Exp. Psychol. 143, 534-547. ( 10.1037/a0033242) [DOI] [PubMed] [Google Scholar]
- 51.van Assen MALM, van Aert RCM, Wicherts JM. 2015. Meta-analysis using effect size distributions of only statistically significant studies. Psychol. Methods 20, 293-309. ( 10.1037/met0000025) [DOI] [PubMed] [Google Scholar]
- 52.van Aert RCM, van Assen MALM. 2018. p-uniform*. doi:10.31222/osf.io/zqjr9
- 53.van Aert RCM. 2020. puniform: meta-analysis methods correcting for publication bias. R package version 0.2.2. See https://CRAN.R-project.org/package=puniform.
- 54.Copas JB, Shi JQ. 2001. A sensitivity analysis for publication bias in systematic reviews. Stat. Methods Med. Res. 10, 251-265. (doi:10.1177%2F096228020101000402) [DOI] [PubMed] [Google Scholar]
- 55.Hedges LV, Vevea JL. 2005. Selection method approaches. In Publication bias in meta-analysis: prevention, assessment, and adjustments (eds Rothstein HR, Sutton AJ, Borenstein M), pp. 145-174. New York, NY: Wiley. [Google Scholar]
- 56.Schwarzer G, Carpenter JR, Rücker G. 2020. metasens: advanced statistical methods to model and adjust for bias in meta-analysis. R package version 0.5-0. See https://CRAN.R-project.org/package=metasens.
- 57.Henmi M, Copas JB. 2010. Confidence intervals for random effects meta-analysis and robustness to publication bias. Stat. Med. 29, 2969-2983. ( 10.1002/sim.4029) [DOI] [PubMed] [Google Scholar]
- 58.Egan V, Chiswick A, Santosh C, Naidu K, Rimmington JE, Best JJ. 1994. Size isn't everything: A study of brain volume, intelligence and auditory evoked potentials. Pers. Individ. Differ. 17, 357-367. ( 10.1016/0191-8869(94)90283-6) [DOI] [Google Scholar]
- 59.Witelson SF, Beresh H, Kigar DL. 2006. Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors. Brain 129, 386-398. ( 10.1093/brain/awh696) [DOI] [PubMed] [Google Scholar]
- 60.(*)Yeo RA, Turkheimer E, Raz N, Bigler ED. 1987. Volumetric asymmetries of the human brain: intellectual correlates. Brain Cogn. 6, 15-23. ( 10.1016/0278-2626(87)90043-1) [DOI] [PubMed] [Google Scholar]
- 61.(*)Andreasen NC, Flaum M, Swayze II V, O'Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WTC. 1993. Intelligence and brain structure in normal individuals. Am. J. Psychiatry 150, 130-1134. [DOI] [PubMed] [Google Scholar]
- 62.(*)Raz N, Torres IJ, Spencer WD, Millman D, Baertschi JC, Sarpel Gm1993. Neuroanatomical correlates of age-sensitive and age-invariant cognitive abilities: an in vivo MRI investigation. Intelligence 17, 407-422. ( 10.1016/0160-2896(93)90008-S) [DOI] [Google Scholar]
- 63.(*)Castellanos FX, Giedd JN, Eckburg P, Marsh WL, Vaittuzis AC, Kaysen D, Hamburger SD, Rapoport JL. 1994. Quantitative morphology of the caudate nucleus in attention deficit hyperactivity disorder. Am. J. Psychiatry 151, 1791-1796. ( 10.1176/ajp.151.12.1791) [DOI] [PubMed] [Google Scholar]
- 64.(*)Harvey I, Persaud R, Ron MA, Baker G, Murray RM. 1994. Volumetric MRI measurements in bipolars compared with schizophrenics and healthy controls. Psychol. Med. 24, 689-699. ( 10.1017/S0033291700027847) [DOI] [PubMed] [Google Scholar]
- 65.(*)Jones PB, Harvey I, Lewis SW, Toone BK, Van Os J, Williams M, Murray RM. 1994. Cerebral ventricle dimensions as risk factors for schizophrenia and affective psychosis: an epidemiological approach to analysis. Psychol. Med. 24, 995-1011. ( 10.1017/S0033291700029081) [DOI] [PubMed] [Google Scholar]
- 66.(*)Wickett J, Vernon PA, Lee DH. 1994. In vivo brain size, head perimeter, and intelligence in a sample of healthy adult females. Pers. Individ. Differ. 16, 831-838. ( 10.1016/0191-8869(94)90227-5) [DOI] [Google Scholar]
- 67.(*)Bigler ED. 1995. Brain morphology and intelligence. Dev. Neuropsychol. 11, 377-403. ( 10.1080/87565649509540628) [DOI] [Google Scholar]
- 68.(*)Egan V, Wickett JC, Vernon PA. 1995. Brain size and intelligence: erratum, addendum, and correction. Pers. Individ. Differ. 19, 113-115. ( 10.1016/0191-8869(95)00043-6) [DOI] [Google Scholar]
- 69.(*)Haier RJ, Chueh D, Touchette P, Lott I, Buchsbaum MS, MacMillan D, Sandman C, LaCasse L, Sosa E. 1995. Brain size and cerebral glucose metabolic rate in nonspecific mental retardation and Down syndrome. Intelligence 20, 191-210. ( 10.1016/0160-2896(95)90032-2) [DOI] [Google Scholar]
- 70.(*)Kareken DA, Gur RC, Mozley PD, Mozley LH, Saykin AJ, Shtasel DL, Gur RE. 1995. Cognitive functioning and neuroanatomic Vol. measures in schizophrenia. Neuropsychology 9, 211-219. ( 10.1037/0894-4105.9.2.211) [DOI] [Google Scholar]
- 71.(*)Raz N, et al. 1995. Selective neuroanatornic abnormalities in Down's syndrome and their cognitive correlates: evidence from MRI morphometry. Neurology 45, 356-366. ( 10.1212/WNL.45.2.356) [DOI] [PubMed] [Google Scholar]
- 72.(*)Reiss AL, Abrams MT, Greenlaw R, Freund L, Denckla MB. 1995. Neurodevelopmental effects of the FMR-1 full mutation in humans. Nat. Med. 1, 159-167. ( 10.1038/nm0295-159) [DOI] [PubMed] [Google Scholar]
- 73.(*)Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. 1996. Brain development, gender and IQ in children. Brain 119, 1763-1774. ( 10.1093/brain/119.5.1763) [DOI] [PubMed] [Google Scholar]
- 74.(*)Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Ryser D, Macnamara SE, Bailey BJ. 1997. MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: correlation with neuropsychological outcome. Am. J. Neuroradiol. 18, 1-10. [PMC free article] [PubMed] [Google Scholar]
- 75.(*)Mori E, Hirono N, Yamashita H, Imamura T, Ikejiri Y, Ikeda M, Kitagaki H, Shimomura T, Yoneda Y. 1997. Premorbid brain size as a determinant of reserve capacity against intellectual decline in Alzheimer's disease. Am. J. Psychiatry 154, 18-24. [DOI] [PubMed] [Google Scholar]
- 76.(*)Paradiso S, Andreasen NC, O'Leary DS, Arndt S, Robinson RG. 1997. Cerebellar size and cognition: correlations with IQ, verbal memory and motor dexterity. Cogn. Behav. Neurol. 10, 1-8. [PubMed] [Google Scholar]
- 77.(*)Flashman LA, Andreasen NC, Flaum M, Swayze VW. 1998. Intelligence and regional brain volumes in normal controls. Intelligence 25, 149-160. ( 10.1016/S0160-2896(97)90039-8) [DOI] [Google Scholar]
- 78.(*)Gur RC, Turetsky BI, Matsui M, Lan M, Bilker W, Hughett P, Gur RE. 1999. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J. Neurosci. 19, 4065-4072. ( 10.1523/JNEUROSCI.19-10-04065.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.(*)Leonard CM, et al. 1999. Cumulative effect of anatomical risk factors for schizophrenia: an MRI study. Biol. Psychiatry 46, 374-382. ( 10.1016/S0006-3223(99)00052-9) [DOI] [PubMed] [Google Scholar]
- 80.(*)Tan U, Tan M, Polat P, Ceylan Y, Suma S, Okur A. 1999. Magnetic resonance imaging brain size/IQ relations in Turkish university students. Intelligence 27, 83-92. ( 10.1016/S0160-2896(99)00015-X) [DOI] [Google Scholar]
- 81.(*)Warwick MM, Doody GA, Lawrie SM, Kestelman JN, Best JJ, Johnstone EC. 1999. Volumetric magnetic resonance imaging study of the brain in subjects with sex chromosome aneuploidies. J. Neurol. Neurosurg. Psychiatry 66, 628-632. ( 10.1136/jnnp.66.5.628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ammons RB, Ammons CH.. 1962. The Quick Test (QT): Provisional manual. Psych. Rep. 11, 111-161. ( 10.1177/003329416201100106) [DOI] [Google Scholar]
- 83.(*)Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HBW. 2000. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 356, 628-634. ( 10.1016/S0140-6736(00)02604-0) [DOI] [PubMed] [Google Scholar]
- 84.(*)Isaacs EBet al. 2000. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr. Res. 47, 713-720. ( 10.1203/00006450-200006000-00006) [DOI] [PubMed] [Google Scholar]
- 85.(*)Kumra S, et al. 2000. Childhood-onset psychotic disorders: magnetic resonance imaging of volumetric differences in brain structure. Am. J. Psychiatry 157, 1467-1474. ( 10.1176/appi.ajp.157.9.1467) [DOI] [PubMed] [Google Scholar]
- 86.(*)Lawson JA, Vogrin S, Bleasel AF, Cook MJ, Bye AM. 2000. Cerebral and cerebellar volume reduction in children with intractable epilepsy. Epilepsia 41, 1456-1462. ( 10.1111/j.1528-1157.2000.tb00122.x) [DOI] [PubMed] [Google Scholar]
- 87.(*)Pennington BF, Filipek PA, Lefly D, Chhabildas N, Kennedy DN, Simon JH, Filley CM, Galaburda A, DeFries JC. 2000. A twin MRI study of size variations in the human brain. J. Cogn. Neurosci. 12, 223-232. ( 10.1162/089892900561850) [DOI] [PubMed] [Google Scholar]
- 88.(*)Schoenemann PT, Budinger TF, Sarich VM, Wang WSY. 2000. Brain size does not predict general cognitive ability within families. Proc. Natl Acad. Sci. USA 97, 4932-4937. ( 10.1073/pnas.97.9.4932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.(*)Wickett J, Vernon PA, Lee DH. 2000. Relationships between factors of intelligence and brain volume. Pers. Individ. Differ. 29, 1095-1122. ( 10.1016/S0191-8869(99)00258-5) [DOI] [Google Scholar]
- 90.(*)Castellanos FX, et al. 2001. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 58, 289-295. ( 10.1001/archpsyc.58.3.289) [DOI] [PubMed] [Google Scholar]
- 91.(*)Coffey CE, Ratcliff G, Saxton JA, Bryan RN, Fried LP, Lucke JF. 2001. Cognitive correlates of human brain aging: a quantitative magnetic resonance imaging investigation. J. Neuropsychiatry Clin. Neurosci. 13, 471-485. ( 10.1176/jnp.13.4.471) [DOI] [PubMed] [Google Scholar]
- 92.(*)Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. 2002. Effects of age on brain volume and head circumference in autism. Neurology 59, 175-183. ( 10.1212/WNL.59.2.175). [DOI] [PubMed] [Google Scholar]
- 93.(*)MacLullich AMJ, Ferguson KL, Deary IJ, Seckl JR, Starr JM, Wardlaw JM. 2002. Intracranial capacity and brain volumes are associated with cognition in elderly men. Neurology 59, 169-174. ( 10.1212/WNL.59.2.169) [DOI] [PubMed] [Google Scholar]
- 94.(*)Nosarti C, Mazin HS, Al-Asady SF, Stewart AL, Rifkin L, Murray RM. 2002. Adolescents who were born very preterm have decreased brain volumes. Brain 125, 1616-1623. ( 10.1093/brain/awf157) [DOI] [PubMed] [Google Scholar]
- 95.(*)Shapleske J, Rossell SL, Chitnis XA, Suckling J, Simmons A, Bullmore ET, Woodruff PWR, David AS. 2002. A computational morphometric MRI study pf schizophrenia: effects of hallucinations. Cereb. Cortex 12, 1331-1341. ( 10.1093/cercor/12.12.1331) [DOI] [PubMed] [Google Scholar]
- 96.(*)Collinson SL, Mackay CE, James AC, Quested DJ, Phillips T, Roberts N, Crow TJ. 2003. Brain volume, asymmetry and intellectual impairment in relation to sex in early-onset schizophrenia. Br. J. Psychiatry 183, 114-120. ( 10.1192/bjp.183.2.114) [DOI] [PubMed] [Google Scholar]
- 97.(*)Giedd JN. 2003. Personal communication to M.A. McDaniel, obtained through the metaanalysis of McDaniel.
- 98.(*)Kesler SR, Adams HF, Blasey CM, Bigler ED. 2003. Premorbid intellectual functioning, education, and brain size in traumatic brain injury: an investigation of the cognitive reserve hypothesis. Appl. Neuropsychol. 10, 153-162. ( 10.1207/S15324826AN1003_04) [DOI] [PubMed] [Google Scholar]
- 99.(*)Yurgelun-Todd DA, Killgore WD, Cintron CB. 2003. Cognitive correlates of medial temporal lobe development across adolescence: a magnetic resonance imaging study. Percept. Mot. Skills 96, 3-17. ( 10.2466/pms.2003.96.1.3) [DOI] [PubMed] [Google Scholar]
- 100.(*)Frangou S, Chitins X, Williams SCR. 2004. Mapping IQ and gray matter density in healthy young people. Neuroimage 23, 800-805. ( 10.1016/j.neuroimage.2004.05.027) [DOI] [PubMed] [Google Scholar]
- 101.(*)Isaacs EB , Edmonds CJ, Chong WK, Lucas A, Morley R, Gadian DG. 2004. Brain morphometry and IQ measurements in preterm children. Brain 127, 2595-2607. (doi:10.1093/brain/awh300) [DOI] [PubMed] [Google Scholar]
- 102.(*)Ivanovic DM, et al. 2004. Brain development parameters and intelligence in Chilean high school graduates. Intelligence 32, 461-479. ( 10.1016/j.intell.2004.07.001) [DOI] [Google Scholar]
- 103.(*)Ivanovic DM, et al. 2004. Head size and intelligence, learning, nutritional status and brain development: head, IQ, learning, nutrition and brain. Neuropsychologia 42, 1118-1131. ( 10.1016/j.neuropsychologia.2003.11.022m) [DOI] [PubMed] [Google Scholar]
- 104.(*)Rojas DC, Smith JA, Benkers TL, Camou SL, Reite ML, Rogers SJ. 2004. Hippocampus and amygdala volumes in parents of children with autistic disorder. Am. J. Psychiatry 161, 2038-2044. [DOI] [PubMed] [Google Scholar]
- 105.(*)Toulopoulou T, Grech A, Morris RG, Schulze K, McDonald C, Chapple B, Rabe-Hesketh S, Murray RM. 2004. The relationship between volumetric brain changes and cognitive function: a family study on schizophrenia. Biol. Psychiatry 56, 447-453. ( 10.1016/j.biopsych.2004.06.026) [DOI] [PubMed] [Google Scholar]
- 106.(*)Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. 2004. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage 22, 619-625. ( 10.1016/j.neuroimage.2004.02.029) [DOI] [PubMed] [Google Scholar]
- 107.(*)Antonova E, Kumari V, Morris R, Halari R, Anilkumar A, Mehrotra R, Sharma T. 2005. The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol. Psychiatry 58, 457-467. ( 10.1016/j.biopsych.2005.04.036) [DOI] [PubMed] [Google Scholar]
- 108.(*)Lodygensky GA, Rademaker K, Zimine S, Gex-Fabry M, Lieftink AF, Lazeyras F, Groenendaal F, de Vries LS, Huppi PS. 2005. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics 116, 1-7. ( 10.1542/peds.2004-1275) [DOI] [PubMed] [Google Scholar]
- 109.(*)Thoma RJ, Yeo RA, Gangestad SW, Halgren E, Sanchez NM, Lewine JD. 2005. Cortical volume and developmental instability are independent predictors of general intellectual ability. Intelligence 33, 27-38. ( 10.1016/j.intell.2004.08.004) [DOI] [Google Scholar]
- 110.(*)Debbané M, Schaer M, Farhoumand R, Glaser B, Eliez S. 2006. Hippocampal volume reduction in 22q11. 2 deletion syndrome. Neuropsychologia 44, 2360-2365. ( 10.1016/j.neuropsychologia.2006.05.006) [DOI] [PubMed] [Google Scholar]
- 111.(*)Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, Tregellas JR. 2006. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry 6, 56. ( 10.1186/1471-244X-6-56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.(*)Staff RT, Murray AD, Deary IJ, Whalley LJ. 2006. Generality and specificity in cognitive aging: a volumetric brain analysis. Neuroimage 30, 1433-1440. ( 10.1016/j.neuroimage.2005.11.004) [DOI] [PubMed] [Google Scholar]
- 113.(*)Voelbel GT, Bates ME, Buckman JF, Pandina G, Hendren RL. 2006. Caudate nucleus volume and cognitive performance: are they related in childhood psychopathology? Biol. Psychiatry 60, 942-950. ( 10.1016/j.biopsych.2006.03.071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.(*)Wozniak JR, Mueller BA, Chang PN, Muetzel RL, Caros L, Lim KO. 2006. Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol.: Clin. Exp. Res. 30, 1799-1806. ( 10.1111/j.1530-0277.2006.00213.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.(*)Chiang MC, Reiss AL, Lee AD, Bellugi U, Galaburda AM, Korenberg JR, Mills DL, Toga AW, Thompson PM. 2007. 3D pattern of brain abnormalities in Williams syndrome visualized using tensor-based morphometry. Neuroimage 36, 1096-1109. ( 10.1016/j.neuroimage.2007.04.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.(*)DeBoer T, Wu Z, Lee A, Simon TJ. 2007. Hippocampal volume reduction in children with chromosome 22q11.2 deletion syndrome is associated with cognitive impairment. Behav. Brain Funct. 3, 54. ( 10.1186/1744-9081-3-54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.(*)Dörnte J. 2007. Intrakranielle Volumenänderungen im Magnetresonanztomogramm (MRT) und neuropsychologische Veränderungen bei Patienten mit Mild Cognitive Impairment (MCI), University of Göttingen. Deutsche Nationalbibliothek. https://d-nb.info/989315606/34. [Google Scholar]
- 118.(*)Fine JG, Semrud-Clikeman M, Keith TZ, Stapleton LM, Hynd GW. 2007. Reading and the corpus callosum: an MRI family study of Vol. and area. Neuropsychology 21, 235-241. ( 10.1037/0894-4105.21.2.235) [DOI] [PubMed] [Google Scholar]
- 119.(*)Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, Hamilton L, Toga AW. 2007. Positive correlations between corpus callosum thickness and intelligence. Neuroimage 37, 1457-1464. ( 10.1016/j.neuroimage.2007.06.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.(*)Nakamura M, Nestor PG, McCarley RW, Levitt JJ, Hsu L, Kawashima T, Niznikiewicz M, Shenton ME. 2007. Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain 130, 693-707. ( 10.1093/brain/awm007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.(*)Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. 2007. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb. Cortex 17, 2163-2171. ( 10.1093/cercor/bhl125) [DOI] [PubMed] [Google Scholar]
- 122.(*)Schottenbauer MA, Momenan R, Kerick M, Hommer DW. 2007. Relationships among aging, IQ, and intracranial Vol. in alcoholics and control subjects. Neuropsychology 21, 337-345. ( 10.1037/0894-4105.21.3.337) [DOI] [PubMed] [Google Scholar]
- 123.(*)Schumann CM, Hamstra J, Goodlin-Jones BL, Kwon H, Reiss AL, Amaral DG. 2007. Hippocampal size positively correlates with verbal IQ in male children. Hippocampus 17, 486-493. ( 10.1002/hipo.20282) [DOI] [PubMed] [Google Scholar]
- 124.(*)Amat JA, Bansal R, Whiteman R, Haggerty R, Royal J, Peterson BS. 2008. Correlates of intellectual ability with morphology of the hippocampus and amygdala in healthy adults. Brain Cogn. 66, 105-114. ( 10.1016/j.bandc.2007.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.(*)Choi YY, et al. 2008. Multiple bases of human intelligence revealed by cortical thickness and neural activation. J. Neurosci. 28, 10 323-10 329. ( 10.1523/JNEUROSCI.3259-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.(*)Ebner F, et al. 2008. The hippocampus in families with schizophrenia in relation to obstetric complications. Schizophr. Res. 104, 71-78. ( 10.1016/j.schres.2008.06.007) [DOI] [PubMed] [Google Scholar]
- 127.(*)Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. 2008. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cereb. Cortex 18, 718-726. ( 10.1093/cercor/bhm108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.(*)Castro-Fornieles J, Bargalló N, Lázaro L, Andrés S, Falcon C, Plana MT, Junqué C. 2009. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. J. Psychiatr. Res. 43, 331-340. ( 10.1016/j.jpsychires.2008.03.013) [DOI] [PubMed] [Google Scholar]
- 129.(*)Miller JL, et al. 2009. Early childhood obesity is associated with compromised cerebellar development. Dev. Neuropsychol. 34, 272-283. ( 10.1080/87565640802530961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.(*)Qiu A, Crocetti D, Adler M, Mahone EM, Denckla MB, Miller MI, Mostofsky SH. 2009. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am. J. Psychiatry 166, 74-82. ( 10.1176/appi.ajp.2008.08030426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.(*)Shenkin SD, Rivers CS, Deary IJ, Starr JM, Wardlaw JM. 2009. Maximum (prior) brain size, not atrophy, correlates with cognition in community-dwelling older people: a cross-sectional neuroimaging study. BMC Geriatr. 9, 12. ( 10.1186/1471-2318-9-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.(*)van Leeuwen M, Peper JS, van den Berg SM, Brouwer RM, Hulshoff Pol HE, Kahn RS, Boomsma DI. 2009. A genetic analysis of brain volumes and IQ in children. Intelligence 37, 181-191. ( 10.1016/j.intell.2008.10.005) [DOI] [Google Scholar]
- 133.(*)Weniger G, Lange C, Sachsse U, Irle E. 2009. Reduced amygdala and hippocampus size in trauma-exposed women with borderline personality disorder and without posttraumatic stress disorder. J. Psychiatry Neurosci. 34, 383-388. [PMC free article] [PubMed] [Google Scholar]
- 134.(*)Zeegers M, Pol HH, Durston S, Nederveen H, Schnack H, van Daalen E, Dietz C, van Engeland H, Buitelaar J. 2009. No differences in MR-based volumetry between 2-and 7-year-old children with autism spectrum disorder and developmental delay. Brain Dev. 31, 725-730. ( 10.1016/j.braindev.2008.11.002) [DOI] [PubMed] [Google Scholar]
- 135.(*)Betjemann RS, Johnson EP, Barnard H, Boada R, Filley CM, Filipek PA, Willcutt EG, DeFries JC, Pennington BF. 2010. Genetic covariation between brain volumes and IQ, reading performance, and processing speed. Behav. Genet. 40, 135-145. ( 10.1007/s10519-009-9328-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.(*)Hermann BP 2010. IQ and brain volume data. Unpublished.
- 137.(*)Hogan MJ, Staff RT, Bunting BP, Murray AD, Ahearn TS, Deary IJ, Whalley LJ. 2011. Cerebellar brain volume accounts for variance in cognitive performance in older adults. Cortex 47, 441-450. ( 10.1016/j.cortex.2010.01.001) [DOI] [PubMed] [Google Scholar]
- 138.(*)Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. 2010. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr. Res. 67, 357-362. ( 10.1203/PDR.0b013e3181d026da) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.(*)Lange N, Froimowitz MP, Bigler ED, Lainhart JE, Brain Development Cooperative Group. 2010. Associations between IQ, total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Dev. Neuropsychol. 35, 296-317. ( 10.1080/87565641003696833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.(*)Wallace GL, Lee NR, Prom-Wormley EC, Medland SE, Lenroot RK, Clasen LS, Schmitt JE, Neale MC, Giedd JN. 2010. A bivariate twin study of regional brain volumes and verbal and nonverbal intellectual skills during childhood and adolescence. Behav. Genet. 40, 125-134. ( 10.1007/s10519-009-9329-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.(*)Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, Gee J, Sevy S, Kumra S. 2011. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J. Psychiatr. Res. 45, 1055-1066. ( 10.1016/j.jpsychires.2011.01.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.(*)Chen X, Coles CD, Lynch ME, Hu X. 2012. Understanding specific effects of prenatal alcohol exposure on brain structure in young adults. Hum. Brain Mapp. 33, 1663-1676. ( 10.1002/hbm.21313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.(*)Kievit RA, Romeijn JW, Waldorp LJ, Wicherts JM, Scholte HS, Borsboom D. 2011. Mind the gap: a psychometric approach to the reduction problem. Psychol. Inq. 22, 67-87. ( 10.1080/1047840X.2011.550181) [DOI] [Google Scholar]
- 144.(*)Tate DF, et al. 2011. Intracranial volume and dementia: some evidence in support of the cerebral reserve hypothesis. Brain Res. 1385, 151-162. ( 10.1016/j.brainres.2010.12.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.(*)Aydin K, Uysal S, Yakut A, Emiroglu B, Yılmaz F. 2012. N-acetylaspartate concentration in corpus callosum is positively correlated with intelligence in adolescents. Neuroimage 59, 1058-1064. ( 10.1016/j.neuroimage.2011.08.114) [DOI] [PubMed] [Google Scholar]
- 146.(*)Burgaleta M, Head K, Álvarez-Linera J, Martínez K, Escorial S, Haier R, Colom R. 2012. Sex differences in brain volume are related to specific skills, not to general intelligence. Intelligence 40, 60-68. ( 10.1016/j.intell.2011.10.006) [DOI] [Google Scholar]
- 147.(*)Bigler ED, et al. 2013. Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology 27, 438-451. ( 10.1037/a0032837) [DOI] [PubMed] [Google Scholar]
- 148.(*)Royle NA, et al. 2013. Estimated maximal and current brain volume predict cognitive ability in old age. Neurobiol. Aging 34, 2726-2733. ( 10.1016/j.neurobiolaging.2013.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.(*)Zelko FA, Pardoe HR, Blackstone SR, Jackson GD, Berg AT. 2014. Regional brain volumes and cognition in childhood epilepsy: does size really matter? Epilepsy Res. 108, 692-700. ( 10.1016/j.eplepsyres.2014.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.(*)Bjuland KJ, Rimol LM, Løhaugen GC, Skranes J. 2014. Brain volumes and cognitive function in very-low-birth-weight (VLBW) young adults. Eur. J. Paediatr. Neurol. 18, 578-590. ( 10.1016/j.ejpn.2014.04.004) [DOI] [PubMed] [Google Scholar]
- 151.(*)Grunewaldt KH, Fjørtoft T, Bjuland KJ, Brubakk AM, Eikenes L, Håberg AK, Løhaugen GCC, Skranes J. 2014. Follow-up at age 10 years in ELBW children - functional outcome, brain morphology and results from motor assessments in infancy. Early Hum. Dev. 90, 571-578. ( 10.1016/j.earlhumdev.2014.07.005) [DOI] [PubMed] [Google Scholar]
- 152.(*)Jenkins JVM, Woolley DP, Hooper SR, De Bellis MD. 2013. Direct and indirect effects of brain volume, socioeconomic status and family stress on child IQ. J. Child Adolesc. Behav. 1, 1000107. ( 10.4172/2375-4494.1000107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.(*)MacDonald PA, Ganjavi H, Collins DL, Evans AC, Karama S. 2014. Investigating the relation between striatal volume and IQ. Brain Imaging Behav. 8, 52-59. ( 10.1007/s11682-013-9242-3) [DOI] [PubMed] [Google Scholar]
- 154.(*)McCoy TE, Conrad AL, Richman LC, Brumbaugh JE, Magnotta VA, Bell EF, Nopoulos PC. 2014. The relationship between brain structure and cognition in transfused preterm children at school age. Dev. Neuropsychol. 39, 226-232. ( 10.1080/87565641.2013.874428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.(*)Zhu B, Chen C, Xue G, Lei X, Li J, Moyzis RK, Dong Q, Lin C. 2014. The GABRB1 gene is associated with thalamus volume and modulates the association between thalamus volume and intelligence. Neuroimage 102, 756-763. ( 10.1016/j.neuroimage.2014.08.048) [DOI] [PubMed] [Google Scholar]
- 156.(*)Boberg R, Wallström S. 2015. A study of twins born preterm: Functional lateralization, cognition, and brain volumes in twin and single-born children at early school ages, , Umea University. [Google Scholar]
- 157.(*)Grazioplene RG, Ryman S G, Gray JR, Rustichini A, Jung RE, DeYoung CG. 2015. Subcortical intelligence: caudate volume predicts IQ in healthy adults. Hum. Brain Mapp. 36, 1407-1416. ( 10.1002/hbm.22710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.(*)Lefebvre A, Beggiato A, Bourgeron T, Toro Rm2015. Neuroanatomical diversity of corpus callosum and brain volume in autism: meta-analysis, analysis of the autism brain imaging data exchange project, and simulation. Biol. Psychiatry 78, 126-134. ( 10.1016/j.biopsych.2015.02.010) [DOI] [PubMed] [Google Scholar]
- 159.(*)Paul EJ, Larsen RJ, Nikolaidis A, Ward N, Hillman CH, Cohen NJ, Kramer AF, Barbey AK, Barbey AK. 2016. Dissociable brain biomarkers of fluid intelligence. Neuroimage 137, 201-211. ( 10.1016/j.neuroimage.2016.05.037) [DOI] [PubMed] [Google Scholar]
- 160.(*)Walters GD, Kiehl KA. 2015. Limbic correlates of fearlessness and disinhibition in incarcerated youth: exploring the brain–behavior relationship with the Hare Psychopathy Checklist: Youth Version. Psychiatry Res. 230, 205-210. ( 10.1016/j.psychres.2015.08.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.(*)Ballester-Plané J, et al. 2016. Measuring intellectual ability in cerebral palsy: the comparison of three tests and their neuroimaging correlates. Res. Dev. Disabil. 56, 83-98. ( 10.1016/j.ridd.2016.04.009) [DOI] [PubMed] [Google Scholar]
- 162.(*)Bohlken MM, Brouwer RM, Mandl RC, Hedman AM, van den Heuvel MP, van Haren NE, Kahn RS, Pol HEH. 2016. Topology of genetic associations between regional gray matter volume and intellectual ability: evidence for a high capacity network. Neuroimage 124, 1044-1053. ( 10.1016/j.neuroimage.2015.09.046) [DOI] [PubMed] [Google Scholar]
- 163.(*)Ferreira D, Bartrés-Faz D, Nygren L, Rundkvist LJ, Molina Y, Machado A, Jungué C, Barroso J, Westman E. 2016. Different reserve proxies confer overlapping and unique endurance to cortical thinning in healthy middle-aged adults. Behav. Brain Res. 311, 375-383. ( 10.1016/j.bbr.2016.05.061) [DOI] [PubMed] [Google Scholar]
- 164.(*)Gregory MD, Kippenhan JS, Dickinson D, Carrasco J, Mattay VS, Weinberger DR, Berman KF. 2016. Regional variations in brain gyrification are associated with general cognitive ability in humans. Curr. Biol. 26, 1301-1305. ( 10.1016/j.cub.2016.03.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.(*)Monson BB, Anderson PJ, Matthews LG, Neil JJ, Kapur K, Cheong JL, Doyle LW, Thompson TK, Inder TE. 2016. Examination of the pattern of growth of cerebral tissue volumes from hospital discharge to early childhood in very preterm infants. JAMA Pediatr. 170, 772-779. ( 10.1001/jamapediatrics.2016.0781) [DOI] [PubMed] [Google Scholar]
- 166.(*)Nikolaidis A, Baniqued PL, Kranz MB, Scavuzzo CJ, Barbey AK, Kramer AF, Larsen RJ. 2017. Multivariate associations of fluid intelligence and NAA. Cereb. Cortex 27, 2607-2616. ( 10.1093/cercor/bhw070) [DOI] [PubMed] [Google Scholar]
- 167.(*)Treit S, et al. 2016. Relationships between head circumference, brain volume and cognition in children with prenatal alcohol exposure. PLoS ONE 11, e0150370. ( 10.1371/journal.pone.0150370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.(*)Amaral DG, Li D, Libero L, Solomon M, Van de Water J, Mastergeorge A, Naigles L, Rogers S, Wu Nordahl C. 2017. In pursuit of neurophenotypes: the consequences of having autism and a big brain. Autism Res. 10, 711-722. ( 10.1002/aur.1755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.(*)Arhan E, et al. 2017. Regional brain volume reduction and cognitive outcomes in preterm children at low risk at 9 years of age. Childs Nerv. Syst. 33, 1317-1325. ( 10.1007/s00381-017-3421-2) [DOI] [PubMed] [Google Scholar]
- 170.(*)Martínez K, et al. 2017. Individual differences in the dominance of interhemispheric connections predict cognitive ability beyond sex and brain size. Neuroimage 155, 234-244. ( 10.1016/j.neuroimage.2017.04.029) [DOI] [PubMed] [Google Scholar]
- 171.(*)Ritchie SJ, et al. 2018. Brain structural differences between 73-and 92-year olds matched for childhood intelligence, social background, and intracranial volume. Neurobiol. Aging 62, 146-158. ( 10.1016/j.neurobiolaging.2017.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lezak. 2004. Neuropsychological testing. Oxford, UK: Oxford University Press.
- 173.(*)van der Linden D, Dunkel CS, Madison G. 2017. Sex differences in brain size and general intelligence (g). Intelligence 63, 78-88. ( 10.1016/j.intell.2017.04.007) [DOI] [Google Scholar]
- 174.(*)Vreeker A, Abramovic L, Boks MP, Verkooijen S, van Bergen AH, Ophoff RA, Kahn RS, van Haren NEM. 2017. The relationship between brain volumes and intelligence in bipolar disorder. J. Affect. Disord. 223, 59-64. ( 10.1016/j.jad.2017.07.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.(*)Annink KV, et al. 2019. The long-term effect of perinatal asphyxia on hippocampal volumes. Pediatr. Res. 85, 43-49. ( 10.1038/s41390-018-0115-8) [DOI] [PubMed] [Google Scholar]
- 176.(*)Jensen MH, Bak N, Rostrup E, Nielsen MØ, Pantelis C, Glenthøj BY, Ebdrup BH, Fagerlund B. 2019. The impact of schizophrenia and intelligence on the relationship between age and brain volume. Schizophr. Res.: Cogn. 15, 1-6. ( 10.1016/j.scog.2018.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.(*)Lammers F, et al. 2018. Basal forebrain cholinergic system volume is associated with general cognitive ability in the elderly. Neuropsychologia 119, 145-156. ( 10.1016/j.neuropsychologia.2018.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.(*)Mankovsky B, Zherdova N, van den Berg E, Biessels GJ, de Bresser J. . 2018. Cognitive functioning and structural brain abnormalities in people with Type 2 diabetes mellitus. Diabet Med. 35, 1663-1670. ( 10.1111/dme.13800) [DOI] [PubMed] [Google Scholar]
- 179.(*)Nygaard E, Slinning K, Moe V, Due-Tønnessen P, Fjell A, Walhovd KB. 2018. Neuroanatomical characteristics of youths with prenatal opioid and poly-drug exposure. Neurotoxicol. Teratol. 68, 13-26. ( 10.1016/j.ntt.2018.04.004) [DOI] [PubMed] [Google Scholar]
- 180.(*)Sreedharan RM, Sheelakumari R, Anila KM, Kesavadas C, Thomas SV. 2018. Reduced brain volumes in children of women with epilepsy: a neuropsychological and voxel based morphometric analysis in pre-adolescent children. J. Neuroradiol. 45, 380-385. ( 10.1016/j.neurad.2018.02.005) [DOI] [PubMed] [Google Scholar]
- 181.(*)Takeuchi H, et al. 2018. Refractive error is associated with intracranial volume. Sci. Rep. 8, 1-11. ( 10.1038/s41598-017-18669-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.(*)Tozer DJ, Zeestraten E, Lawrence AJ, Barrick TR, Markus HS. 2018. Texture analysis of T1-weighted and fluid-attenuated inversion recovery images detects abnormalities that correlate with cognitive decline in small vessel disease. Stroke 49, 1656-1661. ( 10.1161/STROKEAHA.117.019970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.(*)Ahn JI, Yu ST, Sung G, Choi TK, Lee KS, Bang M, Lee SH. 2019. Intra-individual variability in neurocognitive function in schizophrenia: Relationships with the corpus callosum. Psychiatry Res.: Neuroimaging 283, 1-6. ( 10.1016/j.pscychresns.2018.11.005) [DOI] [PubMed] [Google Scholar]
- 184.(*)Bathelt J, Scerif G, Nobre AC, Astle DE. 2019. Whole-brain white matter organization, intelligence, and educational attainment. Trends Neurosci. Educ. 15, 38-47. ( 10.1016/j.tine.2019.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.(*)Cox SR, Ritchie SJ, Fawns-Ritchie C, Tucker-Drob EM, Deary IJ. 2019. Structural brain imaging correlates of general intelligence in UK Biobank. Intelligence 76, 101376. ( 10.1016/j.intell.2019.101376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.de Zwarte SM et al. 2019. Running in the family? Structural brain abnormalities and IQ in offspring, siblings, parents, and co-twins of patients with schizophrenia. Schizophr. Bull. 45, 1209-1217. ( 10.1093/schbul/sby182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.(*)Elliott ML, et al. 2019. A polygenic score for higher educational attainment is associated with larger brains. Cereb. Cortex 29, 3496-3504. ( 10.1093/cercor/bhy219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.(*)Hiraiwa A, Kawasaki Y, Ibuki K, Hirono K, Matsui M, Yoshimura N, Origasa H, Oishi K, Ichida F. 2019. Brain development of children with single ventricle physiology or transposition of the great arteries: a longitudinal observation study. Semin. Thorac. Cardiovasc. Surg. 32, 936-944. ( 10.1053/j.semtcvs.2019.06.013) [DOI] [PubMed] [Google Scholar]
- 189.(*)van Haren NEM, Setiaman N, Koevoets MGJC, Baalbergen H, Kahn RS, Hillegers MHJ. 2020. Brain structure, IQ, and psychopathology in young offspring of patients with schizophrenia or bipolar disorder. Eur. Psychiatry 63, e5. ( 10.1192/j.eurpsy.2019.19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cadenas-Sanchez C, Migueles JH, Erickson KI, Esteban-Cornejo I, Catena A, Ortega FB. 2020. Do fitter kids have bigger brains? J. Med. Sci. Sports 30, 2498-2502. ( 10.1111/sms.13824) [DOI] [PubMed] [Google Scholar]
- 191.Corley, et al. 2020. Dietary patterns, cognitive function, and structural neuroimaging measures of brain aging. Exp. Gerontol. 142, 111117. ( 10.1016/j.exger.2020.111117) [DOI] [PubMed] [Google Scholar]
- 192.Elias. 2020. The Australian imaging, biomarkers and lifestyle study of ageing (AIBL) Veterans study - post traumatic stress disorder and risk of Alzheimer's disease. Unpublished doctoral dissertation, Melbourne, Australia: University of Melbourne.
- 193.(*)Mathias SR, et al. 2020. Minimal relationship between local gyrification and general cognitive ability in humans. Cereb. Cortex 30, 3439-3450. ( 10.1093/cercor/bhz319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.(*)Mitchell BL, et al. 2020. Educational attainment polygenic scores are associated with cortical total surface area and regions important for language and memory. Neuroimage 212, 116691. ( 10.1016/j.neuroimage.2020.116691) [DOI] [PubMed] [Google Scholar]
- 195.Williams, Peyre H, Toro R, Beggiato A, Ramus F. 2020. Adjusting for allometric scaling in ABIDEI challenges subcortical volume differences in autism spectrum disorder. Hum. Brain Mapp. 41, 4610-4629. ( 10.1002/hbm.25145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yankowitz, Herrington JD, Yerys BE, Pereira JA, Pandey J, Schultz RT. 2020. Evidence against the "normaliziation" prediction of the early brain overgrowth hypothesis of autism. Mol. Autism 11, 51. ( 10.1186/s13229-020-00353-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hedderich, et al. 2021. Increased brain age gap estimate (BrainAGE) in young adults after premature birth. Front. Aging. Neurosci. 13, 653365. ( 10.3389/fnagi.2021.653365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Naef, et al. 2021. Brain volumes in adults with congenitual heart disease correlate with executive function abilities. Brain Imaging Behav. 15, 2308–2316. ( 10.1007/s11682-020-00424-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. 2003. Measuring inconsistency in meta-analyses. BMJ 327, 557-560. ( 10.1136/bmj.327.7414.557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vevea JL, Woods CM. 2005. Publication bias in research synthesis: sensitivity analysis using a priori weight functions. Psychol. Methods 10, 428-443. [DOI] [PubMed] [Google Scholar]
- 201.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR. 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365-376. ( 10.1038/nrn3475) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available at OSF: https://osf.io/y6msp.