This cohort study compares overall survival in patients with multifocal intrahepatic cholangiocarcinoma who underwent hepatic arterial infusion pump chemotherapy vs resection.
Key Points
Question
Can hepatic arterial infusion pump (HAIP) floxuridine chemotherapy be considered as an alternative treatment to resection in patients with multifocal intrahepatic cholangiocarcinoma?
Findings
In this cohort study of 319 patients with multifocal intrahepatic cholangiocarcinoma, those treated with HAIP floxuridine chemotherapy had similar overall survival to those who underwent resection.
Meaning
Results of this study suggest that resection of multifocal intrahepatic cholangiocarcinoma needs to be considered carefully given the complication rate of major liver resection, and HAIP floxuridine chemotherapy may be an effective alternative treatment option.
Abstract
Importance
Intrahepatic cholangiocarcinoma (iCCA) is often multifocal (ie, satellites or intrahepatic metastases) at presentation.
Objective
To compare the overall survival (OS) of patients with multifocal iCCA after hepatic arterial infusion pump (HAIP) floxuridine chemotherapy vs resection.
Design, Setting, and Participants
In this cohort study, patients with histologically confirmed, multifocal iCCA were eligible. The HAIP group consisted of consecutive patients from a single center who underwent HAIP floxuridine chemotherapy for unresectable multifocal iCCA between January 1, 2001, and December 31, 2018. The resection group consisted of consecutive patients from 12 centers who underwent a curative-intent resection for multifocal iCCA between January 1, 1990, and December 31, 2017. Resectable metastatic disease to regional lymph nodes and previous systemic therapy were permitted. Patients with distant metastatic disease (ie, stage IV), those who underwent resection before starting HAIP floxuridine chemotherapy, and those who received a liver transplant were excluded. Data were analyzed on September 1, 2021.
Main Outcomes and Measures
Overall survival in the 2 treatment groups was compared using the Kaplan-Meier method and log-rank test.
Results
A total of 319 patients with multifocal iCCA were included: 141 in the HAIP group (median [IQR] age, 62 [53-70] years; 79 [56.0%] women) and 178 in the resection group (median [IQR] age, 60 [50-69] years; 91 [51.1%] men). The HAIP group was characterized by a higher percentage of bilobar disease (88.0% [n = 124] vs 34.3% [n = 61]), larger tumors (median, 8.4 cm vs 7.0 cm), and a higher proportion of patients with 4 or more lesions (66.7% [94] vs 24.2% [43]). Postoperative mortality after 30 days was 0.8% (95% CI, 0.0%-2.1%) in the HAIP group vs 6.2% (95% CI, 2.3%-9.7%) in the resection group (P = .01). The median OS for HAIP was 20.3 months vs 18.9 months for resection (P = .32). Five-year OS in patients with 2 or 3 lesions was 23.7% (95% CI, 12.3%-45.7%) in the HAIP group vs 25.7% (95% CI, 17.9%-37.0%) in the resection group. Five-year OS in patients with 4 or more lesions was 5.0% (95% CI, 1.7%-14.3%) in the HAIP group vs 6.8% (95% CI, 1.8%-25.3%) in the resection group. After adjustment for tumor diameter, number of tumors, and lymph node metastases, the hazard ratio of HAIP vs resection was 0.75 (95% CI, 0.55-1.03; P = .07).
Conclusions and Relevance
This cohort study found that patients with multifocal iCCA had similar OS after HAIP floxuridine chemotherapy vs resection. Resection of multifocal intrahepatic cholangiocarcinoma needs to be considered carefully given the complication rate of major liver resection; HAIP floxuridine chemotherapy may be an effective alternative option.
Introduction
Intrahepatic cholangiocarcinoma (iCCA) is the second most common primary liver malignant neoplasm after hepatocellular carcinoma. Intrahepatic cholangiocarcinoma arises from the epithelial cells of the intrahepatic bile ducts.1,2 The incidence of iCCA appears to be increasing and may be as high as 2.1 cases per 100 000 person-years in Western countries.3 Intrahepatic cholangiocarcinoma occurs in the periphery of the liver, proximal to the second-degree bile ducts.
Intrahepatic cholangiocarcinoma is often multifocal (ie, satellites or intrahepatic metastases) at presentation, which is staged as T2 (ie, there are 2 or more tumors).4,5 Multifocal iCCA is associated, however, with a poor prognosis, and most guidelines recommend palliative chemotherapy rather than surgical resection.5,6,7,8,9,10,11 Only in select patients with limited multifocal disease can resection be considered.10,11 In a subgroup analysis of the Advanced Biliary Cancer (ABC) trials, patients who received gemcitabine with cisplatin for advanced iCCA without distant metastases had a median survival of 16.7 months, and almost all patients had died after 2.5 years.12 These survival outcomes of systemic treatment have become the benchmark for local treatments, including surgery.
A hepatic arterial infusion pump (HAIP) enables the delivery of high-dose chemotherapy directly into the liver. The liver’s dual blood supply preferentially delivers high doses of chemotherapeutic agents to the hepatic artery. Cancer cells derive most of their blood supply from the artery, whereas blood delivered by the portal vein maintains the health of the nonneoplastic liver parenchyma.13,14 Because the liver metabolizes the chemotherapy (first-pass effect), intra-arterial delivery diminishes systemic toxic effects. The most effective agent is floxuridine, a precursor of fluorouracil, which has a first-pass effect of 95%. With this approach, approximately 200-fold higher tumor drug levels are reached compared with systemic administration.15,16 Three phase 2 clinical trials that evaluated the use of HAIP chemotherapy for unresectable iCCA found a median overall survival (OS) ranging from 25.0 to 29.5 months.17,18,19 The aim of the current study was to compare OS of patients with multifocal iCCA treated with HAIP floxuridine chemotherapy vs surgical resection.
Methods
Study Population
In this cohort study, all consecutive patients who were treated with HAIP floxuridine chemotherapy between January 1, 2001, and December 31, 2018, were included in a prospectively maintained database at Memorial Sloan Kettering Cancer Center in New York. Patients were eligible for HAIP floxuridine chemotherapy if they had unresectable iCCA, as ascertained at the multidisciplinary team review. Unresectable disease was defined as the inability to achieve an R0 resection with an adequate functional liver remnant or poor tumor biology as reflected by multiple lesions. For the present study, only patients with multiple lesions were eligible. Past systemic therapy was permitted. Patients who did undergo a resection before starting with HAIP chemotherapy with floxuridine and patients with distant metastatic disease (ie, stage IV) were excluded.
All consecutive patients undergoing resection for iCCA between January 1, 1990, and December 31, 2017, were identified from 12 major hepatobiliary institutions in the US, Asia, Australia, and Europe (Johns Hopkins University, Baltimore, Maryland; Emory University, Atlanta, Georgia; Stanford University Medical Center, Stanford, California; University of Virginia Health System, Charlottesville, Virginia; Fundeni Clinical Institute, Bucharest, Romania; Beaujon Hospital, Clichy, France; Curry Cabral Hospital, Lisbon, Portugal; Eastern Hepatobiliary Surgery Hospital, Shanghai, China; Ottawa General Hospital, Ottawa, Canada; Royal Prince Alfred Hospital, Sydney, Australia; San Raffaele Hospital, Milan, Italy; and Erasmus MC, University Medical Centre Rotterdam, Rotterdam, the Netherlands).20 Patients who did not undergo resection, patients who had a macroscopically positive resection margin (ie, R2 resection), patients who received a liver transplant, and patients with distant metastatic disease (ie, stage IV) were excluded. Only patients with histologically confirmed iCCA and multiple lesions on imaging or at the time of surgery were included. Institutional review boards at each participating institution approved this study and waived the requirement for patient informed consent because only deidentified data were used. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Acquisition
Demographic and clinical data were retrieved from hospital medical records and included age, sex, and body mass index (calculated as weight in kilograms divided by height in meters squared). Data on race and ethnicity were not collected. Pathological data such as tumor number, tumor size, grade of differentiation, presence of nodal metastases, and final resection margin were also retrieved. Margin status was categorized as R0 for tumor-negative resection margins and R1 for microscopically positive margins. Tumor size was recorded differently in the 2 cohorts; the maximum diameter of the largest lesion on cross-sectional imaging was documented in the HAIP group, and the cumulative sum of the size of multiple tumors was documented in the resection group. Multiple lesions were categorized as 2 lesions, 3 lesions, and 4 or more lesions. The date and vital status of last follow-up were collected for all patients. Overall survival was calculated from the date of HAIP placement or surgical resection. A prespecified subgroup analysis was performed for the number of intrahepatic lesions.
Statistical Analysis
All data were analyzed on September 1, 2021. Summary statistics were provided as whole numbers and percentages for categorical variables and medians with IQR for continuous variables. The distribution of categorical variables was tested using the χ2 test or Fisher exact test, as appropriate. The distribution of continuous variables was tested using the Mann-Whitney U test. The primary outcome of interest was OS, defined as the time interval between the date of surgery and the date of death or last follow-up. Estimates for OS were calculated using the Kaplan-Meier method. Differences in OS were assessed using the log-rank test.
Univariable and multivariable Cox proportional hazards models were used to identify potential risk factors for survival time. In the multivariable regression, risk factors including age, grade of differentiation, lymph node metastases, tumor size, and number of tumors were included. Backward selection was performed. Two-sided P < .05 was considered to be statistically significant. Analyses were performed using SPSS, version 24 (IBM Corp) and RStudio, version 1.0.153 (RStudio).
Results
Cohort Description
In total, 319 patients with multifocal iCCA were included: 141 in the HAIP group (median [IQR] age, 62 [53-70] years; 62 [44.0%] men and 79 [56.0%] women) and 178 in the resection group (median [IQR] age, 60 [50-69] years; 91 [51.1%] men and 87 [48.9%] women). Baseline characteristics are noted in Table 1. The HAIP group was characterized by a higher percentage of bilobar disease (88.0% [124] vs 34.3% [61]), larger tumor size (median [IQR], 8.4 [5.9-11.3] cm vs 7.0 [5.0-9.5] cm), and a higher proportion of patients with 4 or more lesions (66.7% [94] vs 24.2% [43]). In the HAIP group, the median (IQR) number of floxuridine cycles administered was 8 (4-12). In 46 patients (32.6%) in the HAIP group, systemic chemotherapy was administered before HAIP floxuridine chemotherapy, and 109 patients (77.3%) received concurrent systemic chemotherapy. In the resection group, 14 patients (7.9%) received preoperative chemotherapy and 74 patients (41.6%) adjuvant chemotherapy.
Table 1. Baseline Characteristics.
| Characteristic | Patients, No. (%) | P value | |
|---|---|---|---|
| HAIP (n = 141) | Resection (n = 178) | ||
| Sex | |||
| Male | 62 (44.0) | 91 (51.1) | .17 |
| Female | 79 (56.0) | 87 (48.9) | |
| Age, median (IQR), y | 62 (53-70) | 60 (50-69) | .23 |
| BMI, median (IQR) | 27.0 (23.6-30.4) | 25.6 (22.7-27.9) | .006 |
| Bilobar tumor distribution | 124 (88.0) | 61 (34.3) | <.001 |
| Largest tumor diameter on imaging, median (IQR), cm | 8.4 (5.9-11.3) | 7.0 (5.0-9.5)a | .005 |
| No. of lesions | |||
| 2 | 31 (22.0) | 104 (58.4) | <.001 |
| 3 | 16 (11.3) | 31 (17.4) | |
| ≥4 | 94 (66.7) | 43 (24.2) | |
| Regional nodal disease | 72 (51.1) | 44 (24.7) | <.001 |
| Grade of differentiation | |||
| Well differentiated | 6 (4.3) | 15 (8.4) | .09 |
| Moderately differentiated | 64 (45.4) | 107 (60.1) | |
| Poorly differentiated | 42 (29.8) | 43 (24.2) | |
| Not specified | 29 (20.6) | 13 (7.3) | |
| R1 margin | NA | 26 (14.6) | |
| Systemic chemotherapy | |||
| Before HAIP or resection | 46 (32.6) | 14 (7.9) | <.001 |
| During HAIP | 109 (77.3)b | NA | |
| Adjuvant | NA | 74 (41.6) | |
| Radioembolization | 4 (2.8) | 16 (9.0) | .06 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HAIP, hepatic arterial infusion pump; NA, not applicable.
In the resection group, the sum of tumor diameters is reported.
Some patients received systemic chemotherapy both before and during HAIP chemotherapy.
Surgery and Postoperative Complications
The median (IQR) postoperative length of hospital stay was 6 (5-7) days after HAIP placement and 12 (7-17) days after resection. Postoperative complications of Clavien-Dindo grade 3A or higher occurred in 9 patients (6.4%) after HAIP placement and in 45 patients (25.3%) after resection (P = .04).21 Postoperative 30-day mortality was 0.8% (95% CI, 0.0%-2.1%) in the HAIP group vs 6.2% (95% CI, 2.3%-9.7%) in the resection group (P = .01); postoperative 90-day mortality was 5.0% (95% CI, 1.3%-8.6%) in the HAIP group vs 7.9% (95% CI, 3.9%-11.8%) in the resection group (P = .37). In the HAIP group, 10 patients (7.1%) underwent a resection after initial HAIP chemotherapy. In the resection group, major liver resection was performed in 126 patients (70.8%), extended hemihepatectomy in 54 (30.3%), hemihepatectomy in 67 (37.6%), and central hepatectomy in 5 (2.8%). A bisegmentectomy was performed in 24 patients (13.5%) and a single segmentectomy or a nonanatomic wedge resection in 23 (12.9%).
Survival
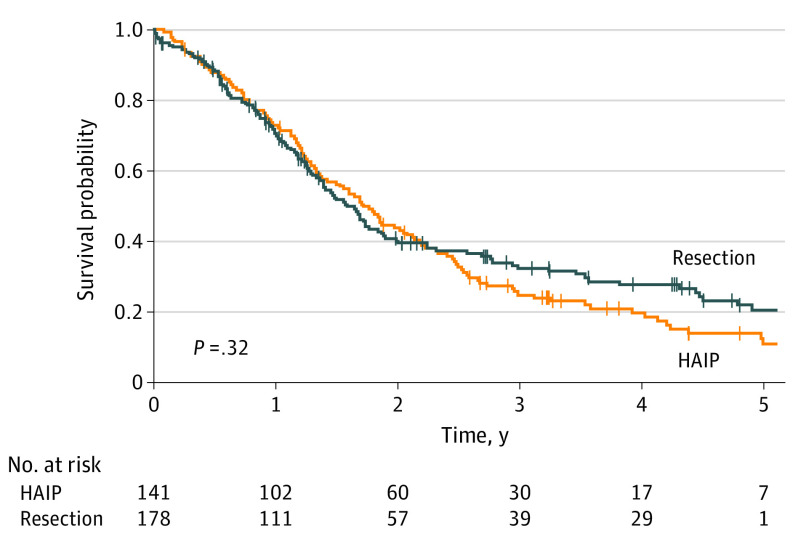
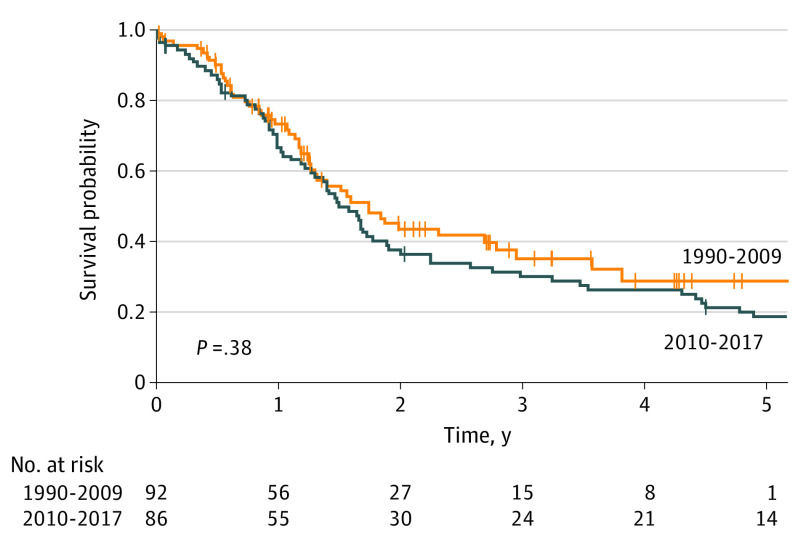
Median OS for HAIP floxuridine chemotherapy was 20.3 months vs 18.9 months for resection (P = .32) (Figure 1). Five-year OS for HAIP floxuridine chemotherapy was 12.5% (95% CI, 7.4%-21.1%) vs 20.7% (95% CI, 14.4%-29.7%) for resection. Median OS for patients with 2 or 3 lesions was 29.5 months in the HAIP group vs 20.4 months in the resection group (P = .46) (Figure 2A). Five-year OS for patients with 2 or 3 lesions was 23.7% (95% CI, 12.3%-45.7%) in the HAIP group vs 25.7% (95% CI, 17.9%-37.0%) in the resection group. Median OS for patients with 4 or more lesions was 18.0 months in the HAIP group vs 15.6 months in the resection group (P = .86) (Figure 2B). Five-year OS for patients with 4 or more lesions was 5.0% (95% CI, 1.7%-14.3%) in the HAIP group vs 6.8% (95% CI, 1.8%-25.3%) in the resection group. We performed a subgroup analysis in the resection group. No difference in survival outcomes of surgical patients from the most recent decade was found (Figure 3).
Figure 1. Overall Survival for Hepatic Arterial Infusion Pump vs Resection.
HAIP indicates hepatic arterial infusion pump.
Figure 2. Overall Survival per Number of Lesions.
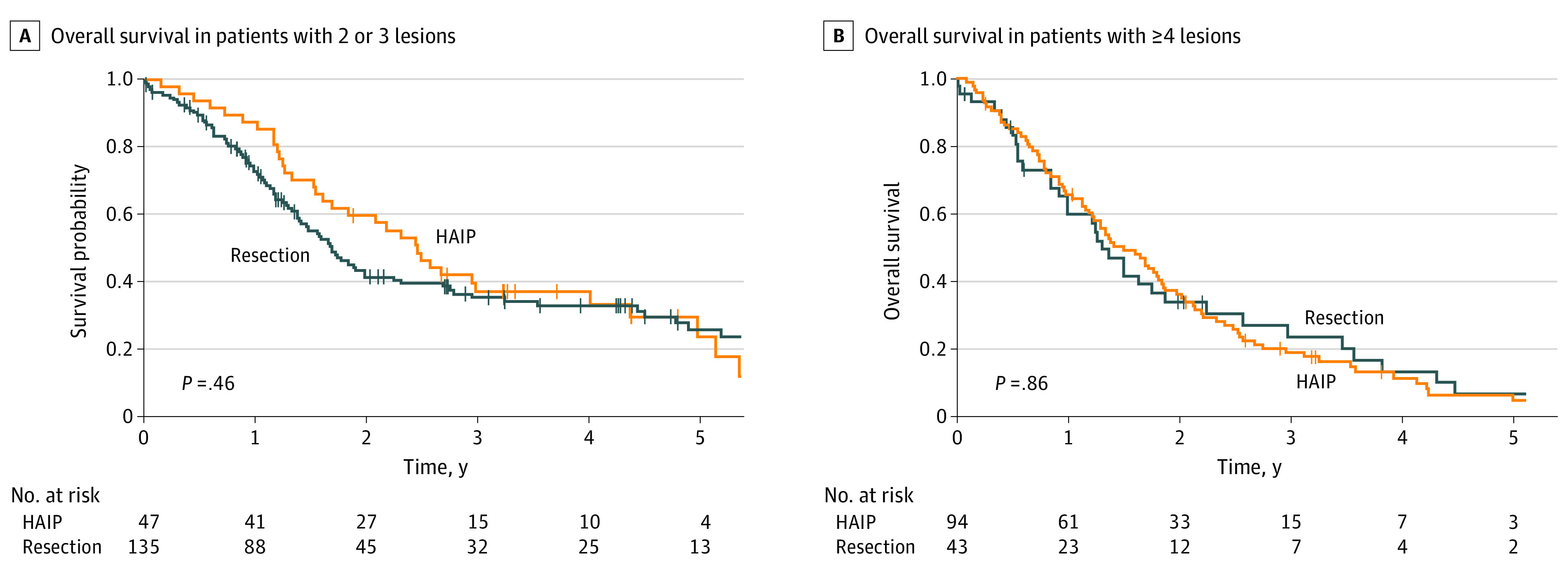
HAIP indicates hepatic arterial infusion pump.
Figure 3. Overall Survival in Year of Resection.
Multivariable Analysis
Risk factors for decreased OS in patients with multifocal iCCA after HAIP chemotherapy or resection were tumor diameter (hazard ratio [HR], 1.74; 95% CI, 1.20-2.52; P < .01), number of lesions (HR, 1.84; 95% CI, 1.39-2.44; P < .01), and regional nodal disease (HR, 1.51; 95% CI, 1.17-1.96; P < .01) (Table 2). All 3 factors were also independent risk factors for decreased OS. The HR of HAIP vs resection after adjustment for tumor diameter, number of tumors, and lymph node metastases was 0.75 (95% CI, 0.55-1.03; P = .07).
Table 2. Univariable and Multivariable Survival Analysis.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 0.99 (0.98-1.01) | .37 | NA | NA |
| Tumor diameter ≥5 cm | 1.74 (1.20-2.52) | <.01 | 1.58 (1.06-2.15) | .02 |
| No. of lesions | ||||
| 2 | 1 [Reference] | NA | 1 [Reference] | NA |
| 3 | 1.59 (1.08-2.35) | .03 | 1.41 (0.94-2.12) | .09 |
| ≥4 | 1.84 (1.39-2.44) | <.01 | 1.71 (1.27-2.30) | <.001 |
| Regional nodal disease | 1.51 (1.17-1.96) | <.01 | 1.44 (1.06-1.94) | .02 |
| Grade of differentiation | ||||
| Well differentiated | 1 [Reference] | NA | NA | NA |
| Moderately differentiated | 1.05 (0.61-1.80) | .87 | NA | NA |
| Poorly differentiated | 0.79 (0.47-1.34) | .39 | NA | NA |
| Treatment | ||||
| Resection | 1 [Reference] | NA | 1 [Reference] | NA |
| HAIP | 0.88 (0.68-1.13) | .32 | 0.75 (0.55-1.03) | .07 |
Abbreviations: HAIP, hepatic arterial infusion pump; HR, hazard ratio; NA, not applicable.
Discussion
In this study of 319 patients, we found that those with multifocal iCCA had similar OS after HAIP chemotherapy or resection. Overall survival curves were overlapping for patients with 2 to 3 lesions as well as those with 4 or more lesions. Hepatic arterial infusion pump chemotherapy had an HR of 0.75 (95% CI, 0.55-1.03) compared with resection after adjusting for tumor diameter, number of lesions, and nodal disease. The number of tumors was associated with OS after resection; median OS for 2 or 3 tumors was 20.4 months compared with 15.6 months for 4 or more tumors.
Other studies have reported a similar OS after resection in patients with multifocal iCCA.22,23,24,25,26,27 An international study of 449 patients included 120 patients with multifocal iCCA who underwent a resection between 1973 and 2010.22 The median OS was 19.0 months, which is similar to the 18.9 months in the resection group (n = 178) of the present study.
Most guidelines recommend palliative chemotherapy rather than resection for multifocal iCCA.5,6,7,8,9,11 Patients who received gemcitabine with cisplatin for advanced iCCA without distant metastases in the ABC trials, however, had a median survival of only 16.7 months, with no survivors beyond 2.5 years.28 The main rationale for locoregional treatment for advanced iCCA is that most patients (approximately 70%) die from progressive disease in the liver with biliary obstruction and liver failure.29 Surgical resection can control disease in the liver in patients with multifocal iCCA, although most patients will eventually die from distant metastases.11 Patients who underwent a resection for multifocal iCCA in the present study had a median OS of 18.9 months and a 5-year survival of 20.7%. Although the median OS after palliative systemic chemotherapy and resection was similar, the difference in 5-year survival suggests that select patients with multifocal iCCA can benefit from resection in the long term. Median OS outcomes in the 2 groups analyzed in this study are not entirely dissimilar from reported median OS outcomes for patients treated with systemic chemotherapy alone (17 months).12 The present study shows the limitation of reporting only the median OS. The median OS is only 1 point of the survival curve, and patients and physicians care about the entire curve.
Three phase 2 trials investigated the combination of systemic and HAIP chemotherapy for locally advanced iCCA.17,18,19 Approximately 62% of these patients had multifocal disease. These trials consistently reported a partial response rate of approximately 50%, a median OS of approximately 25 months, and a 3-year OS rate of approximately 35%. Most patients who received HAIP chemotherapy also underwent systemic chemotherapy before or during HAIP chemotherapy. It is unlikely, however, that systemic chemotherapy alone was responsible for the favorable survival outcomes of the HAIP group, considering that 3-year OS was not observed with systemic chemotherapy alone in the ABC trials.12 In the resection group, only 41.6% of patients received adjuvant systemic chemotherapy. The use of adjuvant chemotherapy has increased since the publication of the BILCAP (Capecitabine or Observation After Surgery in Treating Patients With Biliary Tract Cancer) trial in 2019, which found superior OS with adjuvant capecitabine in the per-protocol analysis.30 Only 7.9% of patients received chemotherapy before resection. Future studies should identify the role of preoperative treatment with systemic chemotherapy and targeted agents in patients with iCCA. Overall survival of the resection group may have been somewhat better if more patients had received perioperative systemic chemotherapy.
The present study found that, in patients with multifocal iCCA, OS was similar for HAIP chemotherapy and resection. Disease control in the liver with HAIP chemotherapy appears to be equally effective as surgical resection in patients with multifocal disease. Long-term follow-up found that almost all patients with multifocal iCCA will eventually die from distant metastatic disease.11 This also explains the limited role of resection after HAIP chemotherapy (7.1% [10] of patients in the present study). Hepatic arterial infusion pump chemotherapy can make surgical resection technically feasible because of the 50% partial response rate. However, tumor biology (in particular, progression of distant metastases) and surgical risk mostly prevail over what is technically feasible.
Liver-directed therapies are recommended by international guidelines for multifocal or locally advanced (ie, unresectable) iCCA.5,6,9,11 Intrahepatic cholangiocarcinoma lesions are often too large for percutaneous ablation with radiofrequency ablation or microwave ablation. Several percutaneous intra-arterial approaches have been investigated, including transarterial chemoembolization and selective internal radiotherapy. To our knowledge, no randomized clinical trials comparing hepatic intra-arterial therapies have been published. A systematic review compared HAIP chemotherapy, transarterial chemoembolization, and radioembolization.31 In this review, 20 studies were analyzed, and the longest median survival was associated with HAIP (22.8 months), followed by radioembolization (13.9 months) and transarterial chemoembolization (12.4 months). Unlike other locoregional approaches, HAIP chemotherapy is not limited by tumor size, number, or distribution across the liver.32,33,34
Limitations
This study has limitations. First, the comparison between the 2 treatment groups was not randomized, and patients were included over a long period of time. We adjusted for known risk factors for decreased survival, but unmeasured risk factors could be unbalanced across treatment groups and may partly explain the difference in OS. Second, details about how the multifocal iCCA was spread across the liver were not available. Therefore, multifocal iCCA could not be further divided into intrahepatic metastases (ie, several lesions spread across the liver) and satellite lesions (small lesions surrounding a large lesion).9 Third, genomic alterations are of increasing importance for targeted therapy (ie, isocitrate dehydrogenase and fibroblast growth factor receptor alterations) and prognosis. However, data on genomic alterations were not available for most patients in the present study.35 Fourth, no data were collected on HAIP-related complications in this cohort. Several large studies of patients who underwent HAIP chemotherapy for colorectal liver metastases provide the most precise estimate of these complications.36,37 Fifth, HAIP chemotherapy is currently offered in only approximately 30 centers worldwide. It is a complex treatment requiring close collaboration of a multidisciplinary team. But in experienced hands, the mortality is much lower than for an extended hemihepatectomy, and the complication rates (eg, biliary sclerosis and pump pocket infection) are low.36
Conclusions
The findings of this cohort study suggest that select patients with multifocal iCCA may benefit from surgical resection. Shared decision-making involves a careful tradeoff between individual surgical risk and long-term oncological benefit. A minor liver resection or even a major resection in a patient with a good performance score may be justified in select patients with multifocal iCCA, particularly those with only 2 or 3 lesions. Hepatic arterial infusion pump chemotherapy may be considered for patients with an increased surgical risk or more advanced disease, as reflected by the tumor diameter, number of tumors (4 or more), and nodal disease. Resection can be considered after HAIP chemotherapy in patients with a good response and a good performance score.
References
- 1.Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford). 2008;10(2):77-82. doi: 10.1080/13651820801992641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahnemai-Azar AA, Weisbrod A, Dillhoff M, Schmidt C, Pawlik TM. Intrahepatic cholangiocarcinoma: molecular markers for diagnosis and prognosis. Surg Oncol. 2017;26(2):125-137. doi: 10.1016/j.suronc.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 3.Yang JD, Kim B, Sanderson SO, et al. Biliary tract cancers in Olmsted County, Minnesota, 1976-2008. Am J Gastroenterol. 2012;107(8):1256-1262. doi: 10.1038/ajg.2012.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyder O, Marsh JW, Salem R, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol. 2013;20(12):3779-3786. doi: 10.1245/s10434-013-3127-y [DOI] [PubMed] [Google Scholar]
- 5.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 6.Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268-1289. doi: 10.1016/j.jhep.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 7.Kang SH, Hwang S, Lee YJ, et al. Prognostic comparison of the 7th and 8th editions of the American Joint Committee on Cancer staging system for intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2018;25(4):240-248. doi: 10.1002/jhbp.543 [DOI] [PubMed] [Google Scholar]
- 8.Spolverato G, Bagante F, Weiss M, et al. Comparative performances of the 7th and the 8th editions of the American Joint Committee on Cancer staging systems for intrahepatic cholangiocarcinoma. J Surg Oncol. 2017;115(6):696-703. doi: 10.1002/jso.24569 [DOI] [PubMed] [Google Scholar]
- 9.Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17(8):669-680. doi: 10.1111/hpb.12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massani M, Nistri C, Ruffolo C, et al. Intrahepatic chemotherapy for unresectable cholangiocarcinoma: review of literature and personal experience. Updates Surg. 2015;67(4):389-400. doi: 10.1007/s13304-015-0330-3 [DOI] [PubMed] [Google Scholar]
- 11.Konstantinidis IT, Groot Koerkamp B, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122(5):758-765. doi: 10.1002/cncr.29824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamarca A, Ross P, Wasan HS, et al. Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst. 2020;112(2):200-210. doi: 10.1093/jnci/djz071 [DOI] [PubMed] [Google Scholar]
- 13.Kemeny N, Daly J, Oderman P, et al. Hepatic artery pump infusion: toxicity and results in patients with metastatic colorectal carcinoma. J Clin Oncol. 1984;2(6):595-600. doi: 10.1200/JCO.1984.2.6.595 [DOI] [PubMed] [Google Scholar]
- 14.McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol. 2007;16(1):3-5. doi: 10.1016/j.suronc.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 15.Cohen AD, Kemeny NE. An update on hepatic arterial infusion chemotherapy for colorectal cancer. Oncologist. 2003;8(6):553-566. doi: 10.1634/theoncologist.8-6-553 [DOI] [PubMed] [Google Scholar]
- 16.Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol. 1983;10(2):176-182. [PubMed] [Google Scholar]
- 17.Andrea C, Nancy EK, Thomas B, et al. A bi-institutional phase II study of hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone (Dex) combined with systemic gemcitabine and oxaliplatin (GemOx) for unresectable intrahepatic cholangiocarcinoma (ICC). J Clin Oncol. 2018;36(15)(suppl):4092-4092. doi: 10.1200/JCO.2018.36.15_suppl.4092 [DOI] [Google Scholar]
- 18.Jarnagin WR, Schwartz LH, Gultekin DH, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20(9):1589-1595. doi: 10.1093/annonc/mdp029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemeny NE, Schwartz L, Gönen M, et al. Treating primary liver cancer with hepatic arterial infusion of floxuridine and dexamethasone: does the addition of systemic bevacizumab improve results? Oncology. 2011;80(3-4):153-159. doi: 10.1159/000324704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buettner S, Ten Cate DWG, Bagante F, et al. Survival after resection of multiple tumor foci of intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2019;23(11):2239-2246. doi: 10.1007/s11605-019-04184-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140-3145. doi: 10.1200/JCO.2011.35.6519 [DOI] [PubMed] [Google Scholar]
- 23.Ribero D, Pinna AD, Guglielmi A, et al. ; Italian Intrahepatic Cholangiocarcinoma Study Group . Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg. 2012;147(12):1107-1113. doi: 10.1001/archsurg.2012.1962 [DOI] [PubMed] [Google Scholar]
- 24.Jiang W, Zeng ZC, Tang ZY, et al. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol. 2011;22(7):1644-1652. doi: 10.1093/annonc/mdq650 [DOI] [PubMed] [Google Scholar]
- 25.Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254(5):824-829. doi: 10.1097/SLA.0b013e318236c21d [DOI] [PubMed] [Google Scholar]
- 26.Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149(6):565-574. doi: 10.1001/jamasurg.2013.5137 [DOI] [PubMed] [Google Scholar]
- 27.Palen A, Garnier J, Hobeika C, et al. Oncological relevance of major hepatectomy with inferior vena cava resection for intrahepatic cholangiocarcinoma. HPB (Oxford). 2021;23(9):1439-1447. doi: 10.1016/j.hpb.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 28.Valle J, Wasan H, Palmer DH, et al. ; ABC-02 Trial Investigators . Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281. doi: 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 29.Yamashita S, Koay EJ, Passot G, et al. Local therapy reduces the risk of liver failure and improves survival in patients with intrahepatic cholangiocarcinoma: a comprehensive analysis of 362 consecutive patients. Cancer. 2017;123(8):1354-1362. doi: 10.1002/cncr.30488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Primrose JN, Fox RP, Palmer DH, et al. ; BILCAP study group . Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663-673. doi: 10.1016/S1470-2045(18)30915-X [DOI] [PubMed] [Google Scholar]
- 31.Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111(2):213-220. doi: 10.1002/jso.23781 [DOI] [PubMed] [Google Scholar]
- 32.Akinwande O, Dendy M, Ludwig JM, Kim HS. Hepatic intra-arterial injection of irinotecan drug eluting beads (DEBIRI) for patients with unresectable colorectal liver metastases: a systematic review. Surg Oncol. 2017;26(3):268-275. doi: 10.1016/j.suronc.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 33.Elganainy D, Holliday EB, Taniguchi CM, et al. Dose escalation of radiotherapy in unresectable extrahepatic cholangiocarcinoma. Cancer Med. 2018;7(10):4880-4892. doi: 10.1002/cam4.1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong TS, Wo JY, Yeap BY, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016;34(5):460-468. doi: 10.1200/JCO.2015.64.2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boerner T, Drill E, Pak LM, et al. Genetic determinants of outcome in intrahepatic cholangiocarcinoma. Hepatology. 2021;74(3):1429-1444. doi: 10.1002/hep.31829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg. 2005;201(1):57-65. doi: 10.1016/j.jamcollsurg.2005.03.019 [DOI] [PubMed] [Google Scholar]
- 37.Ito K, Ito H, Kemeny NE, et al. Biliary sclerosis after hepatic arterial infusion pump chemotherapy for patients with colorectal cancer liver metastasis: incidence, clinical features, and risk factors. Ann Surg Oncol. 2012;19(5):1609-1617. doi: 10.1245/s10434-011-2102-8 [DOI] [PubMed] [Google Scholar]