Abstract
Exosomes are extracellular vesicles found in various tissues, blood circulation, and tissue fluids, secreted into the extracellular environment by fusing a multivesicular body with a plasma membrane. Various cell types release these vesicles to contribute to many cellular functions, including intercellular communication, cell proliferation, differentiation, angiogenesis, response to stress, and immune system signaling. These natural nanoparticles have therapeutic effects in various diseases and exhibit a behavior similar to the cell from which they originated. In the meantime, exosomes derived from mesenchymal stem cells have attracted the attention of many researchers and physicians due to their unique ability to modulate the immune system, repair tissue and reduce inflammation. Numerous clinical and preclinical studies have examined the effect of MSC-derived exosomes in various diseases, and their results have been published in prestigious journals. This review article discusses the biogenesis and sources of exosomes, MSC-derived exosomes, the use of these exosomes in regenerative medicine, and treatments based on exosomes derived from stem cells in respiratory diseases.
Keywords: Cell therapy, Exosome, MSC-derived exosomes, Lung diseases
Introduction
Mesenchymal stem cells (MSCs) have unique biological properties due to their stem cell nature. These cells can regenerate themselves and differentiate into multiple cells [1]. Mesenchymal stem cells are isolated from various tissues and are widely distributed throughout the body, including bone marrow and adipose tissue [2]. Identification features of human mesenchymal stem cells, including adhesion capability in traditional culture, expression of CD105, CD73, and CD90, non-expression of CD45, CD34, CD14, CD11b, CD79a, CD19, and HLA-DR, differentiation into osteoblasts, adipocytes, and chondrocytes in vitro, have been expressed by International Association of Cell Therapy [3]. The significant capability of MSCs to proliferate in vitro and differentiate into different cells introduces these cells as therapeutic agents for regenerating necrotic cells or for connective tissue apoptosis. Mesenchymal stem cells can be differentiated into several classes, including adipocytes, endothelial cells, cardiomyocytes, chondrocytes, osteoblasts, and various cells like hepatocytes and neuron-like cells [4, 5]. Mesenchymal stem cells have low immunogenicity because of the lowly expression of MHC-I and the expression of a small number of MHC-II molecules [6, 7]. MSCs have also shown immune system modulation and regeneration capacity in various disease models [8–11]. At present, significant advances have been made in stem cell technology with good therapeutic prospects for treating different diseases such as respiratory diseases [12]. Many studies indicate that MSCs can affect activation, proliferation, and differentiation of cells acting on natural killer cells (NK), dendritic cells (DCS), macrophages, B lymphocytes, and T lymphocytes [13–16]. MSC migrate to the inflammation site through adhesion molecules and integrins such as VCAM-1 and VLA-4, affecting the damaged tissue through cell–cell contact and secretion of various trophic factors [17]. In a clinical trial on patients with cirrhosis, it was shown that mesenchymal stem cells were trapped in the lungs in the early hours after injection into peripheral blood and that they left the lungs after 48 h, migrated to the liver and spleen, and remained in these tissues for several days [18]. Other investigations have indicated that most injected MSCs are commonly trapped in the liver, spleen, and lungs and that a small number of these cells reach the damaged site. Therefore, focusing on cell-free therapies is of high importance [19]. MSCs secrete soluble factors such as growth factors, cytokines, and chemokines, and they also release extracellular vesicles (EVs), leading to therapeutic consequences through the exchange of cytoplasm and genetic material [20, 21]. The therapeutic ability of MSCs may depend on paracrine factors in the vesicles [22]. This paper will examine exosomes (especially MSC-derived ones) and their application in diagnosing and treating lung diseases.
Biogenesis and sources of exosomes
Extracellular vesicles (EVs) are released from various cellular sources and have been known as messengers of cellular transmission through the delivery of lipids, proteins, and biologically active RNAs. EVs are separated into three subtypes: exosomes, microvesicles, and apoptotic bodies [23]. Among these extracellular vesicles, exosomes play an essential role in modulating the immune system, as well as in cellular communication [24]. Exosomes from MSCs show similar natural activity to these cells by encompassing and transporting functional biomolecules such as peptides, proteins, and RNA species to damaged tissues and cells [25]. In terms of mechanisms and cellular composition, exosomes resemble the cells derived from them [26]. Exosomes can be classified according to their cell or tissue of origin based on cellular elements and protein content. Proteins of cell-derived exosomes typically include integrins, adhesive molecules, MHC I and II, transferrin receptors, and other cell surface exosomes. Nonspecific proteins of exosomes restricted to plasma membranes, cytosols, and endosomal elements, including fusion and transporter proteins, and, cytoskeletal proteins, heat shock proteins, contribute to multivesicular and other cellular processes [27]. Studies have indicated that exosomes derived from endosomes recreate a necessary function in cell-to-cell communication. Exosomes are discovered in most biological fluids, including serum, breast milk, saliva, urine, synovial fluid, amniotic fluid, lymph, bile, gastric acid, tears, and cerebrospinal fluid (CSF) produced in a variety of cells. In addition to cellular communication, exosomes are involved in tumor progression, myelin formation, and cell maintenance [28].
Exosomes originated from mesenchymal stem cells
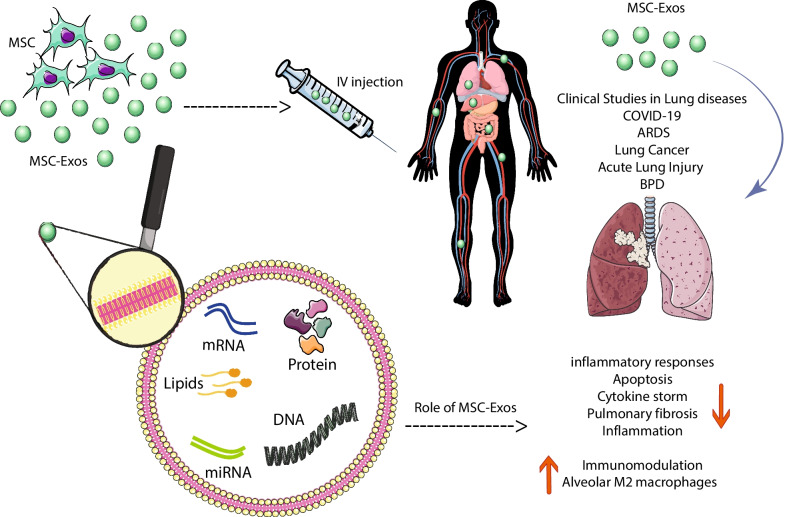
Mesenchymal stem cells from different sources have received significant attention as an alternative treatment for various rare diseases due to their ability to stimulate tissue regeneration and modulate active immune cells [29, 30]. These cells act in various diseases through cell–cell contact and environmental changes induced by the release of soluble factors [31]. MSC-derived exosomes have a crucial role in the function of mesenchymal stem cells as stromal support cells responding to external stimuli and maintaining tissue homeostasis. At injury or disease, tissue homeostasis is impaired, and exosomes’ key role becomes apparent. MSC-derived exosomes are affluent in biologically active molecules like proteins and RNAs and can adequately play their role [32]. Exosomes contain large amounts of membrane and cytoplasmic proteins such as extracellular matrix proteins, receptors, enzymes, transcription factors, nucleic acids, and lipids [33] (Fig. 1). MSC exosomes express CD markers such as CD73, CD44, CD29, and CD105 [34] and include proteins, mRNAs, and microRNAs transported to receptor cells and change the manners of neighboring cells [35]. MSC-derived exosomes represent adhesion molecules (FN1, EZR, IQGAP1, CD47, integrin, and LGALS1/LGALS3), receptors (PDGFRB, EGFR, and PLAUR), signaling molecules (RRAS/NRAS, MAPK1, GNA13/GNG12, CDC42, and VAV2), and antigens related to MSCs (CD63, CD63, CD81, CD109, CD151, CD248, and CD276) [36]. MSC exosomes contribute to cellular functions, including proliferation, adhesion, transcription, migration, and differentiation [35]. MSC-derived exosomes inhibit inflammation, induce angiogenesis, prevent fibrosis, increase neuronal survival and differentiation, stimulate ECM regeneration and modulate immune cells [37].
Fig. 1.
The schematic role of MSC exosomes in respiratory diseases
Studies have shown that MSC-derived exosomes comprise more than 850 and 150 gene products and miRNAs, respectively [38, 39], which are applied in biological processes such as organism growth, immune modulation (miR-155 and miR-146), epigenetic regulation, tumorigenesis, and tumor advance (miR-23b, 451miR-, miR-223, miR-24, miR-125b, miR-31, miR-214, and miR-122) [40, 41]. These exosomes contain growth factors and cytokines including IL-10, TGFβ1, IL-6, and hepatic growth factor (HGF), which are involved in immune system modulation [42]. In addition, studies have demonstrated that MSC-derived exosomes have a pivotal role in promoting angiogenesis and tissue repair through factors such as vascular endothelial growth factor (VEGF), extracellular matrix metalloproteinase inhibitor (EMMPRIN), and matrix metallopeptidase 9 (MMP-9) [43].
Utilization of MSC-derived exosomes in regenerative medicine
MSCs have the fantastic possibility to sustain tissue homeostasis because these cells can enter into damaged tissue and regulate the immune system and tissue regeneration through cellular and molecular processes [44–46]. Further studies have revealed that the beneficial effects of MSCs in repair are related to paracrine signaling, including secreted vesicles such as exosomes [47–50]. Various studies show that exosomes secreted by MSCs can replace stem cell-based therapies in different models of injury and disease [51, 52]. The therapeutic effects of MSC exosomes have been demonstrated in preclinical studies in various diseases such as CVD, renal, hepatic, and neurological diseases, healing of wounds, and other diseases [53]. We briefly review some of these investigations.
A study by Cui et al. on exosomes derived from adipose tissue MSCs in disease showed that these exosomes caused a significant increase in survival of H9C2 cell line under hypoxia/re-oxygenation (H/R) conditions in vitro. In this study, administration of Ad-MSC-derived exosomes via the Wnt/β-catenin signaling pathway is protected against myocardial ischemia in vivo [54]. In another research on a model of myocardial ischemic injury, exosomes from BM-derived mesenchymal stem cells reduced apoptosis and the size of myocardial infarction and, after that, recovered heart function by persuading cardiac autophagy via two pathways such as AMPK/mTOR and Akt/mTOR routes [55]. MSCs have revealed promising results in acute and chronic kidney damage. A study on a rat model of renal damage revealed that intravascular injection of human umbilical cord-derived mesenchymal stem cells (huMSCs) in a mouse model with ischemia–reperfusion injury (IRI) of the kidney increases renal vein density, reducing renal fibrosis by direct transfer of vascular endothelial growth factor, a process in which mRNAs are involved [56]. Another study showed that an intrarenal injection of adipose tissue-derived EVs in a swine model with renal artery stenosis reduced the level of proinflammatory factors, including TNF-α, IL-6, and IL-1β, increased IL-10 levels in renal vein and decreased kidney inflammation, indicating the immune-modulating capacity of EVs by changing proinflammatory to tubular repair macrophages [57]. Effects such as protecting the liver tissue of exosomes separated from human embryonic stem cell-derived mesenchymal stem cells (hESC-MSCs) were investigated in a model of acute liver injury [58].
Moreover, it was found that these exosomes contribute to the regeneration of damaged liver tissue through positive regulation of PCNA expression, cyclin D1 cell cycle regulator, and Bcl-xL anti-apoptotic gene [58]. In another research, Li et al. assessed exosomes derived from human umbilical cord mesenchymal stem cells (hUMSC) and showed that these exosomes improve liver fibrosis by inhibiting EMT of liver cells and producing collagen, as well as recovering aspartate aminotransferase activity in serum and inactivating TGF-β1/Smad2 pathway [59]. One of the most prominent outcomes of MSC-derived exosomes is their ability to transit the blood–brain barrier (BBB) and reach the brain parenchyma. Therapeutic advantages of MSC-derived exosomes in the cure of neurodegenerative disorders have been demonstrated in different investigations. Exosomes targeted against α-synuclein reduce mRNA and alpha-synuclein protein levels in the brain [60, 61]. Studies on BM-MSC exosomes have indicated that these exosomes contain miR-133b, which leads to neurite regeneration and improves stroke in mouse models [62]. Intravenous injection of BM-MSC exosomes in a mouse stroke model increased neurovascular flexibility and improved axon density in the ischemic margin region of the brain [63]. Exosomes derived from Wharton Jelly mesenchymal stem cells lead to angiogenesis in vivo and enhance wound healing via the Wnt4 pathway and activation of β-catenin [64]. MiRNAs such as miR-21, miR-23a, miR-125b, and miR-145 originating from WJ-MSC exosomes contribute to wound healing by inhibiting scar formation and myofibroblast accumulation and reducing collagen deposition [65]. Another study found that exosomes derived from BM-MSC via Akt, ERK, and STAT3 signaling pathways could raise fibroblast proliferation, migration and increase HGF, IGF1, NGF, and SDF1 levels [66]. Exosomes derived from Wharton Jelly-MSC showed significant medicinal effects by improving bronchopulmonary dysplasia, pneumonia, pulmonary hypertension, fibrosis, and regulating the phenotype of pulmonary macrophages in the lung tissue [67].
Studies have shown that microRNAs from exosomes derived from mesenchymal stem cells, such as miR-125a-3p, improve Treg survival and prevent T cells from differentiating into effector cells [68]. These microRNAs, including miR-146a, function in inflammatory responses using the NF-κB signaling pathway [69]. They have debilitating effects on dendritic cells through miR-21-5p, miR-142-3p, miR-223-3p, and miR 126-3p [70]. In addition, they inhibit the production of cytokines such as IL-6 through miR-142-3p [70].
MSC-derived exosomes-based therapies in respiratory disease
Clinical trials based on anti-inflammatory medications combined with glucocorticoids have failed to treat lung diseases [71, 72]. Preclinical studies indicate that MSC-derived exosomes have a significant healing prospect in the regeneration and rehabilitation of several lung diseases via various molecular pathways and by influencing lung tissue target cells like immune cells, endothelial and epithelial cells [73, 74]. MSC-derived exosomes are promising for the cure of diverse lung diseases, including idiopathic pulmonary fibrosis (IPF), ALI, ARDS, pulmonary artery hypertension, asthma, pneumonia, inflammatory lung disease, silicosis, chronic obstructive pulmonary disease (COPD), and Bronchopulmonary dysplasia [75]. While the production of exosomes is practically and economically tricky compared to that of MSCs, they are not trapped in the lungs as MSCs injected intravenously do, have advantages such as small size (approximately 100 nm), and can be favorable as aerosol inhalation for the treatment of airborne diseases [76] (Fig. 1). Proinflammatory mechanisms are inhibited by MSC-derived exosomes and are associated with remodeling of inflammatory lung disease and reduction in oxidative stress and pulmonary fibrosis [77].
One study used exosomes derived from human BM mesenchymal stem cells in a bleomycin-induced pulmonary fibrosis model. This study demonstrated the use of exosomes as an exciting and innovative approach to treating fibrotic lung disease. Exosomes improve pulmonary fibrosis by modulating the monocyte phenotype [78]. In a study by Ahn et al., VEGF was shown to be highly important in protecting exosomes in pulmonary hyperoxia damages. Exosomes derived from umbilical cord mesenchymal stem cells have significant efficacy in improving impaired alveolar function through angiogenic effects, reducing apoptosis, and limiting macrophages and inflammatory responses in a mouse model of lung injury [74]. In another study, mitochondrial MSC exosomes increased the production of alveolar M2 macrophages to reduce acute lung damage and inhibit inflammatory cytokines [79]. Intravenous injection of MSC exosomes exhibits immunomodulatory impacts in bacterial pneumonia lesions by increasing monocytes’ phagocytic capacity and decreasing the secretion of inflammatory cytokines. Exosomes restore the metabolism of alveolar type 2 epithelial cells by raising intracellular ATP levels [80]. MSC-MVs stopped endothelial cell apoptosis via rising IL-10 levels and decreasing IL-6 expression in endothelial cell culture medium through HGF factor in vitro [81]. In an animal model of chronic obstructive pulmonary disease (COPD) study, a comparison was made between the healing capacity of hUC-MSCEVs and hUC-MSCs in remedy.
Both MSCs and exosomes derived from them improved peribronchial and vascular inflammation, thereby reducing the thickening of the alveolar septum in COPD via reducing the production of zeta C kinase protein and NF-κB subunits of p50 and p65 subunits [82]. MSC-derived exosomes are effective, promising treatments for ALI/ARDS. In one study, human BM was injected by chips using MSCs into an ALI model. This research observed a significant decrease in macrophage-2 inflammatory protein levels in Bronchoalveolar lavage fluid (BALF), pneumonia, neutrophil infiltration, and protein penetrance [83]. In addition, a study by Abreu et al. [84] found that exosomes derived from HBM-MSCs could help reduce inflammatory responses in ARDS through mitochondrial transmission. Studies have shown that the injection of BM-MSC-derived exosomes can prevent myofibroblastic differentiation associated with TGF-β1 in pulmonary fibrosis [85]. Despite the COVID-19 pandemic, MSC-derived exosomes can be good as a therapeutic agent in this disease and its associated difficulties, such as acute lung injury and ARDS.
As a therapeutic strategy for severe COVID-19, exosomes are used with convalescent plasma since it contains acquired immune antibodies that require consideration that it contains trillions of exosomes. These exosomes are produced by immunomodulatory cells that transmit miRNAs [86]. MSC-derived exosomes have remarkable characteristics, including antiviral properties, immune system regulation, and tissue repair. A present investigation showed that MSC-derived exosomes could replace MSCs because they are similar to mesenchymal stem cells in COVID-19 [87]. In a study of severe COVID-19 disease, exosomes derived from allogeneic BM-MSCs were used in 24 patients, which showed that MSC exosomes could be a potent therapeutic candidate for treating severe COVID-19 [88]. Because the pathogenesis of SARS-CoV-2 is equivalent to many viruses and results in complications such as ARDS and lung damage, treatment approaches launched on MSCs or MSC-derived exosomes were examined in SARS-CoV-2. In previous studies, MSC-derived exosomes have been shown a favorable reaction to ARDS and suppress cytokine storms by transmitting mRNA and miRNA to lung tissues [23, 89]. The schematic role of MSC exosomes in respiratory diseases is depicted in Fig. 1.
In recent years, clinical studies on the use of exosomes in lung diseases such as COVID-19, ARDS, Early-staged Lung Cancer, Acute Lung Injury and BPD have been documented, as shown in Table 1.
Table 1.
Clinical trials in Exosomes Therapy In Respiratory Diseases
| No | Title and sponsor | Trial ID | Location | Design | Primary outcome | Recruitment status | Phase |
|---|---|---|---|---|---|---|---|
| 1. |
The use of exosomes for the treatment of acute respiratory distress syndrome or novel coronavirus pneumonia caused by COVID-19 (ARDOXSO) Sponsor: AVEM HealthCare |
NCT04798716 | United States, California |
Open label, interventional, mesenchymal stem cell exosomes for the treatment of COVID-19 positive patients with acute respiratory distress syndrome and/or novel coronavirus pneumonia N:55 |
Measure and report the number of participants with treatment-related-adverse events Tabulate and report the number of IMV days for patients receiving ARDOXSO™ perinatal MSC-derived exosome therapy |
Not yet recruiting July 21, 2021 |
Phase 1 Phase 2 |
| 2. |
Safety and efficiency of method of exosome inhalation in COVID-19 associated pneumonia (COVID-19EXO2) Sponsor: Olga Tyumina |
NCT04602442 | Russian, Samara |
Randomized, interventional, the extended protocol of evaluation of safety and efficiency of method of exosome inhalation in COVID-19 associated two-sided pneumonia N: 90 |
Number of participants with non-serious and serious adverse events during trial Number of participants with non-serious and serious adverse during inhalation procedure |
Enrolling by invitation October 26, 2020 |
Phase 2 |
| 3. |
Evaluation of safety and efficiency of method of exosome inhalation in SARS-CoV-2 associated pneumonia. (COVID-19EXO) Sponsor: State-Financed Health Facility “Samara Regional Medical Center Dinasty” |
NCT04491240 | Russian, Samara |
Randomized, interventional, the protocol of evaluation of safety and efficiency of method of exosome inhalation in SARS-CoV-2 associated two-sided pneumonia N: 30 |
Number of participants with non-serious and serious adverse events during trial Number of participants with non-serious and serious adverse during inhalation procedure |
Completed November 4, 2020 |
Phase 1 Phase 2 |
| 4. |
A safety study of IV stem cell-derived extracellular vesicles (UNEX-42) in preterm neonates at high risk for BPD Sponsor: United Therapeutics |
NCT03857841 | United States, Colorado, Massachusetts, Mississippi, Missouri |
Randomized, interventional, a safety study of intravenous infusion of bone marrow mesenchymal stem cell-derived extracellular vesicles (UNEX-42) in preterm neonates at high risk for bronchopulmonary dysplasia N: 3 |
Number of subjects with treatment-emergent adverse events during the post-treatment phase |
Terminated October 12, 2021 |
Phase 1 |
| 5. |
A clinical study of mesenchymal stem cell exosomes nebulizer for the treatment of ARDS Sponsor: Ruijin Hospital |
NCT04602104 | China, Shanghai |
Randomized, double-blinded, controlled clinical study of allogeneic human mesenchymal stem cell exosomes (hMSC-Exos) nebulized inhalation in the treatment of acute respiratory distress syndrome N:169 |
Incidence of adverse reaction Time to clinical improvement 28-day mortality |
Recruiting November 2, 2021 |
Phase 1 Phase 2 |
| 6. |
A tolerance clinical study on aerosol inhalation of mesenchymal stem cells exosomes in healthy volunteers Sponsor: Ruijin Hospital |
NCT04313647 | China, Shanghai |
Open label, non randomized, interventional, a tolerance clinical study on aerosol inhalation of mesenchymal stem cells exosomes in healthy volunteers N: 24 |
Number of Participants With Adverse Reaction (AE) and severe adverse reaction (SAE) |
Completed August 4, 2021 |
Phase 1 |
| 7. |
Omics sequencing of exosomes in body fluids of patients with acute lung injury Sponsor: Nanfang Hospital of Southern Medical University |
NCT05058768 | China, Guangdong |
Observational, case–control, exosomes in urine, blood, and alveolar lavage fluid from patients with acute respiratory distress syndrome (ADRS) were sequenced by omics N:180 |
Compare the omics differences of blood samples between the experimental and control groups Compare the omics differences of urine samples between the experimental and control groups |
Recruiting September 28, 2021 |
Case–Control |
| 8. |
Exosomes derived from placental mesenchymal stem cells as treatment for severe COVID-19: Phase 1 and 2 clinical trials Sponsor: Omid Cell and Tissue center |
IRCT20200413047063N2 | Tehran, Iran |
Participants were randomly divided into two equal groups using a randomized double AB blocking method based on a random number table. Patients allocated randomly to two groups: (1) Intervention 1, Patients will receive Six doses of Exosomes. (2) Control, Patients will receive conventional therapy N:50 |
Adverse events assessment |
Recruiting July 8, 2021 |
Phase 1 Phase 2 |
| 9. |
Molecular profiling of exosomes in tumor-draining vein of early-staged lung cancer (ExOnSite-Pro) Sponsor: University Hospital, Limoges |
NCT04939324 | France, Limoges |
Open label, single group assignment, Analyse du Profil moléculaire Des Exosomes de la Veine Pulmonaire Dans le Cancer Bronchique de Stade précoce N:30 |
Evaluate size distribution, concentration and molecular profiling of pulmonary vein exosomes at inclusion |
Recruiting November 11, 2021 |
Not applicable |
| 10. |
A pilot clinical study on inhalation of mesenchymal stem cells exosomes treating severe novel coronavirus pneumonia Sponsor: Ruijin Hospital |
NCT04276987 | China, Shanghai |
Open label, single group assignment, a pilot clinical study on aerosol inhalation of the exosomes derived from allogenic adipose mesenchymal stem cells in the treatment of severe patients with novel coronavirus pneumonia N:24 |
Adverse reaction (AE) and severe adverse reaction (SAE) Time to clinical improvement (TTIC) |
Completed September 7, 2020 |
Phase 1 |
| 11. |
Extracellular vesicle infusion treatment for COVID-19 Associated ARDS (EXIT-COVID19) Sponsor: Direct Biologics, LLC |
NCT04493242 | United States, Alabama, California, Pennsylvania, Texas |
Randomized, double-blinded, bone marrow mesenchymal stem cell derived extracellular vesicles infusion treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a phase II clinical trial N: 120 |
7 day change in partial pressure of arterial oxygen to fraction of inspired oxygen ratio |
Completed December 6, 2021 |
Phase 2 |
| 12. |
Safety and efficacy of exosomes overexpressing CD24 in two doses for patients with moderate or severe COVID-19 Sponsor: Athens Medical Society |
NCT04902183 | Greece, Athens, Attica |
Randomized, single, a Phase II randomized, single-blind dose study to evaluate the safety and efficacy of exosomes overexpressing CD24 in 10^9 dose versus 10^10 dose, for the prevention of clinical deterioration in patients with moderate or severe COVID-19 N:90 |
Collection of serious adverse events Proportion of patients related with Respiratory rate and SpO2 saturation |
Recruiting June 15, 2021 |
Phase 2 |
Conclusion
Clinical and preclinical studies have shown advantageous effects of MSC-derived exosomes. Since stem cell therapy is associated with clinical challenges such as high cell count, selective dose, cell injection routes, cell safety, exosomes derived from these cells have become highly important in various diseases. Exosomes have received much attention in biomarker research today and are even regarded as an alternative strategy for stem cell-based regenerative therapies. For this purpose, the use of separation methods and optimization of these exosomes can be promising in clinical studies of various diseases, including lung diseases.
Acknowledgements
Not applicable.
Abbreviations
- PF
Pulmonary fibrosis
- IPF
Idiopathic pulmonary fibrosis
- BALF
Bronchoalveolar lavage fluid
- COPD
Chronic obstructive pulmonary disease
- ATII
Alveolar cell type 2
- MSCs
Mesenchymal stem cells
- PD-MSCs
Placenta-derived mesenchymal stem cells
- HBM-MSC
Human bone marrow-derived mesenchymal stem cell
- hUMSC
Human umbilical cord mesenchymal stem cells
- huMSCs
Human umbilical cord-derived mesenchymal stem cells
- WJ-MSC
Wharton’s jelly-derived mesenchymal stem cells
- AD-MSC
Adipose tissue-derived mesenchymal stem cell
- hESC-MSCs
Human embryonic stem cell-derived mesenchymal stem cells
- EVs
Extracellular vesicles
- HLA
Human leukocyte antigen
- BBB
Blood–brain barrier
- CSF
Cerebrospinal fluid
- VEGF
Vascular endothelial growth factor
- TGF-b1
Transforming growth beta-1
- IL-10
Interleukin 10
- IL-6
Interleukin 6
- IL-1β
Interleukin 1β
- IGF-1
Insulin-like growth factor 1
- NGF
Nerve growth factor
- EMMPRIN
Extracellular matrix metalloproteinase inhibitor
- HGF
Hepatocyte growth factor
- NK cell
Natural killer cells
- DCS
Dendritic cells
- PD-L1
Programmed death ligand 1
- MMPs
Matrix metalloproteases
- H/R
Hypoxia/Re-oxygenation
- CVD
Cardiovascular disease
Author contributions
MJZ, SK, and MS contributed to the concept of the review. MJZ, KK, and MS were responsible for the reference selection and writing of the manuscript. MJZ, KK, MV, MSV, and HSK contributed to the critical review of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Tarbiat Modares University.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Saeid Kaviani and Masoud Soleimani contributed equally to this work
Contributor Information
Saeid Kaviani, Email: kavianis@modares.ac.ir.
Masoud Soleimani, Email: Soleim_m@modares.ac.ir.
References
- 1.Meirelles LDS, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 2.Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 4.Crapnell K, Blaesius R, Hastings A, Lennon DP, Caplan AI, Bruder SP. Growth, differentiation capacity, and function of mesenchymal stem cells expanded in serum-free medium developed via combinatorial screening. Exp Cell Res. 2013;319(10):1409–1418. doi: 10.1016/j.yexcr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105(3):369–377. doi: 10.1016/S0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 6.Lee M, Jeong SY, Ha J, Kim M, Jin HJ, Kwon S-J, et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun. 2014;446(4):983–989. doi: 10.1016/j.bbrc.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 7.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9(1):1–14. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 9.Le Blanc K, Davies LC. Mesenchymal stromal cells and the innate immune response. Immunol Lett. 2015;168(2):140–146. doi: 10.1016/j.imlet.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22(12):2267–2277. doi: 10.3727/096368911X582769c. [DOI] [PubMed] [Google Scholar]
- 11.Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12(1):64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Fatima F, Nawaz M. Stem cell-derived exosomes: roles in stromal remodeling, tumor progression, and cancer immunotherapy. Chin J Cancer. 2015;34(3):1–13. doi: 10.1186/s40880-015-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. doi: 10.1155/2015/394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Castro LL, Lopes-Pacheco M, Weiss DJ, Cruz FF, Rocco PRM. Current understanding of the immunosuppressive properties of mesenchymal stromal cells. J Mol Med. 2019;97(5):605–618. doi: 10.1007/s00109-019-01776-y. [DOI] [PubMed] [Google Scholar]
- 15.Shokri M-R, Bozorgmehr M, Ghanavatinejad A, Falak R, Aleahmad M, Kazemnejad S, et al. Human menstrual blood-derived stromal/stem cells modulate functional features of natural killer cells. Sci Rep. 2019;9(1):1–13. doi: 10.1038/s41598-019-46316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asari S, Itakura S, Ferreri K, Liu C-P, Kuroda Y, Kandeel F, et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009;37(5):604–615. doi: 10.1016/j.exphem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleh M, Taher M, Sohrabpour AA, Vaezi AA, Nasiri Toosi M, Kavianpour M, et al. Perspective of placenta derived mesenchymal stem cells in acute liver failure. Cell Biosci. 2020;10:1–11. doi: 10.1186/s13578-020-00433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gholamrezanezhad A, Mirpour S, Bagheri M, Mohamadnejad M, Alimoghaddam K, Abdolahzadeh L, et al. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol. 2011;38(7):961–967. doi: 10.1016/j.nucmedbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25(11):2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 20.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis A. The origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5(11):899–904. doi: 10.1038/nrc1740. [DOI] [PubMed] [Google Scholar]
- 22.Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev Rep. 2015;11(1):150–160. doi: 10.1007/s12015-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 23.Andaloussi SE, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J Clin Med. 2019;8(7):1025. doi: 10.3390/jcm8071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Niel G, d'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 27.Mashouri L, Yousefi H, Aref A, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makalakshmi M, Jain MS, Ganesan H, Duttaroy AK, Pathak S, Banerjee A. Current understanding of the mesenchymal stem cell-derived exosomes in cancer and aging. Biotechnol Rep. 2021;31:e00658. doi: 10.1016/j.btre.2021.e00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleh M, Vaezi AA, Aliannejad R, Sohrabpour AA, Kiaei SZF, Shadnoush M, et al. Cell therapy in patients with COVID-19 using Wharton’s jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Res Ther. 2021;12(1):410. doi: 10.1186/s13287-021-02483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashemian S-MR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini S-E, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12(1):1–12. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, et al. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6–7):667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maumus M, Jorgensen C, Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie. 2013;95(12):2229–2234. doi: 10.1016/j.biochi.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16(4):415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Ramos TL, Sánchez-Abarca LI, Muntión S, Preciado S, Puig N, López-Ruano G, et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal. 2016;14(1):1–14. doi: 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9(1):1–9. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H-S, Choi D-Y, Yun SJ, Choi S-M, Kang JW, Jung JW, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11(2):839–849. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 37.Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, et al. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10(1):1–11. doi: 10.1186/s13287-019-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen TS, Lai RC, Lee MM, Choo ABH, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38(1):215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, De Kleijn DP, et al. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteom. 2012;2012. [DOI] [PMC free article] [PubMed]
- 40.Di Trapani M, Bassi G, Midolo M, Gatti A, Kamga PT, Cassaro A, et al. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep. 2016;6(1):1–13. doi: 10.1038/srep24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi R-U, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63-ra. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 42.Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G. Stem cell-derived extracellular vesicles and immune-modulation. Front Cell Dev Biol. 2016;4:83. doi: 10.3389/fcell.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vrijsen KR, Maring JA, Chamuleau SA, Verhage V, Mol EA, Deddens JC, et al. Exosomes from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via EMMPRIN. Adv Healthc Mater. 2016;5(19):2555–2565. doi: 10.1002/adhm.201600308. [DOI] [PubMed] [Google Scholar]
- 44.Lai RC, Yeo RWY, Lim SK, editors. Mesenchymal stem cell exosomes. Seminars in cell and developmental biology. Amsterdam: Elsevier; 2015. [DOI] [PubMed] [Google Scholar]
- 45.Wei X, Yang X, Han Z-P, Qu F-F, Shao L, Shi Y-F. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34(6):747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baba MA, Bouchriti Y, Achbani A, Kharbach A, Sine H, Naciri A. Risk of COVID-19 for patients with cancer: a narrative overview. Eur J Med Educ Technol. 2020;13(3):2008. doi: 10.30935/ejmets/8257. [DOI] [Google Scholar]
- 47.Barreca MM, Cancemi P, Geraci F. Mesenchymal and induced pluripotent stem cells-derived extracellular vesicles: the new frontier for regenerative medicine? Cells. 2020;9(5):1163. doi: 10.3390/cells9051163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drommelschmidt K, Serdar M, Bendix I, Herz J, Bertling F, Prager S, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun. 2017;60:220–232. doi: 10.1016/j.bbi.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 49.Nakano M, Nagaishi K, Konari N, Saito Y, Chikenji T, Mizue Y, et al. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci Rep. 2016;6(1):1–14. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Ye Y, Su X, He J, Bai W, He X. MSCs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front Cell Neurosci. 2017;11:55. doi: 10.3389/fncel.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjørge I, Kim S, Mano J, Kalionis B, Chrzanowski W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine–a new paradigm for tissue repair. Biomater Sci. 2018;6(1):60–78. doi: 10.1039/C7BM00479F. [DOI] [PubMed] [Google Scholar]
- 52.Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7(1):1–8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18(1):1–21. doi: 10.1186/s12967-020-02622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui X, He Z, Liang Z, Chen Z, Wang H, Zhang J. Exosomes from adipose-derived mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through Wnt/β-catenin signaling pathway. J Cardiovasc Pharmacol. 2017;70(4):225. doi: 10.1097/FJC.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L, Jin X, Hu C-F, Li R, Shen C-X. Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways. Cell Physiol Biochem. 2017;43(1):52–68. doi: 10.1159/000480317. [DOI] [PubMed] [Google Scholar]
- 56.Zou X, Gu D, Xing X, Cheng Z, Gong D, Zhang G, et al. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am J Transl Res. 2016;8(10):4289. [PMC free article] [PubMed] [Google Scholar]
- 57.Eirin A, Zhu X-Y, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, et al. Mesenchymal stem cell–derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92(1):114–124. doi: 10.1016/j.kint.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan CY, Lai RC, Wong W, Dan YY, Lim S-K, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5(3):1–14. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22(6):845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luarte A, Bátiz LF, Wyneken U, Lafourcade C. Potential therapies by stem cell-derived exosomes in CNS diseases: focusing on the neurogenic niche. Stem Cells Int. 2016;2016:5736059. doi: 10.1155/2016/5736059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooper JM, Wiklander PO, Nordin JZ, Al-Shawi R, Wood MJ, Vithlani M, et al. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord. 2014;29(12):1476–1485. doi: 10.1002/mds.25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem cells. 2013;31(12):2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33(11):1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015;4(5):513–522. doi: 10.5966/sctm.2014-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med. 2016;5(10):1425–1439. doi: 10.5966/sctm.2015-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Badiavas EV. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24(14):1635–1647. doi: 10.1089/scd.2014.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197(1):104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fujii S, Miura Y, Fujishiro A, Shindo T, Shimazu Y, Hirai H, et al. Graft-versus-host disease amelioration by human bone marrow mesenchymal stromal/stem cell-derived extracellular vesicles is associated with peripheral preservation of naive T cell populations. Stem cells. 2018;36(3):434–445. doi: 10.1002/stem.2759. [DOI] [PubMed] [Google Scholar]
- 69.Wu H, Fan H, Shou Z, Xu M, Chen Q, Ai C, et al. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int Immunopharmacol. 2019;68:204–212. doi: 10.1016/j.intimp.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 70.Reis M, Mavin E, Nicholson L, Green K, Dickinson AM, Wang X-N. Mesenchymal stromal cell-derived extracellular vesicles attenuate dendritic cell maturation and function. Front Immunol. 2018;9:2538. doi: 10.3389/fimmu.2018.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suissa S, Barnes P. Inhaled corticosteroids in COPD: the case against. Eur Respiratory Soc. 2009;34:13–16. doi: 10.1183/09031936.00190908. [DOI] [PubMed] [Google Scholar]
- 72.Barnes PJ. Inhaled corticosteroids in COPD: a controversy. Respiration. 2010;80(2):89–95. doi: 10.1159/000315416. [DOI] [PubMed] [Google Scholar]
- 73.Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9(1):1–13. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahn SY, Park WS, Kim YE, Sung DK, Sung SI, Ahn JY, et al. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp Mol Med. 2018;50(4):1–12. doi: 10.1038/s12276-018-0055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raghav A, Khan ZA, Upadhayay VK, Tripathi P, Gautam KA, Mishra BK, et al. Mesenchymal stem cell-derived exosomes exhibit promising potential for treating SARS-CoV-2-infected patients. Cells. 2021;10(3):587. doi: 10.3390/cells10030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsuchiya A, Takeuchi S, Iwasawa T, Kumagai M, Sato T, Motegi S, et al. Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID-19) cases. Inflamm Regen. 2020;40(1):1–6. doi: 10.1186/s41232-020-00121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujita Y, Kadota T, Araya J, Ochiya T, Kuwano K. Clinical application of mesenchymal stem cell-derived extracellular vesicle-based therapeutics for inflammatory lung diseases. J Clin Med. 2018;7(10):355. doi: 10.3390/jcm7100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mansouri N, Willis GR, Fernandez-Gonzalez A, Reis M, Nassiri S, Mitsialis SA, et al. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. 2019;4(21):e128060. doi: 10.1172/jci.insight.128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Monsel A, Zhu Y-G, Gennai S, Hao Q, Hu S, Rouby J-J, et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192(3):324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang H, Zheng R, Chen Q, Shao J, Yu J, Hu S. Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF) Stem Cell Res Ther. 2017;8(1):1–10. doi: 10.1186/s13287-016-0461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ridzuan N, Zakaria N, Widera D, Sheard J, Morimoto M, Kiyokawa H, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles ameliorate airway inflammation in a rat model of chronic obstructive pulmonary disease (COPD) Stem Cell Res Ther. 2021;12(1):1–21. doi: 10.1186/s13287-020-02088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32(1):116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abreu SC, Lopes-Pacheco M, Weiss DJ, Rocco PR. Mesenchymal stromal cell-derived extracellular vesicles in lung diseases: current status and perspectives. Front Cell Dev Biol. 2021;9:97. doi: 10.3389/fcell.2021.600711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shentu T-P, Huang T-S, Cernelc-Kohan M, Chan J, Wong SS, Espinoza CR, et al. Thy-1 dependent uptake of mesenchymal stem cell-derived extracellular vesicles blocks myofibroblastic differentiation. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-18288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Askenase PW. COVID-19 therapy with mesenchymal stromal cells (MSC) and convalescent plasma must consider exosome involvement: do the exosomes in convalescent plasma antagonize the weak immune antibodies? J Extracell Vesicles. 2020;10(1):e12004. doi: 10.1002/jev2.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taghavi-Farahabadi M, Mahmoudi M, Soudi S, Hashemi SM. Hypothesis for the management and treatment of the COVID-19-induced acute respiratory distress syndrome and lung injury using mesenchymal stem cell-derived exosomes. Med Hypotheses. 2020;144:109865. doi: 10.1016/j.mehy.2020.109865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mahmoudi M, Taghavi Farahabadi M, Hashemi SM. Exosomes: mediators of immune regulation. Immunoregulation. 2019;2(1):3–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.