Abstract
Our understanding of the immune basis of food allergy has grown rapidly in parallel with the development of new immune-targeted interventions for the treatment of food allergy. Local tissue factors, including the composition of skin and gastrointestinal microbiota and production of Th2-inducing cytokines (TSLP, IL-33, and IL-25) from barrier sites, have been shown not only to contribute to the development of food allergy, but also to act as effective targets for treatment in mice. Ongoing clinical trials are testing the targeting of these factors in human disease. There is a growing understanding of the contribution of IL-13 to the induction of high-affinity IgE and the need for continual T-cell help in the maintenance of long-lived IgE. This provides a strong rationale to test biologics targeting both IL-4 and IL-13 in the treatment of established food allergy. Various forms of allergen immunotherapy for food allergy have clearly shown that low specific IgE and elevated specific IgG4 are predictive of sustained treatment effect. Treatments that mimic that immune response, for example, lowering IgE, with monoclonal antibodies such as omalizumab, or administering allergen-specific IgG, are in various stages of investigation. As we gain more opportunities to use immune-modifying treatments for the treatment of food allergy, studies of the immune and clinical response to those interventions will continue to rapidly advance our understanding of the immune basis of food allergy and tolerance.
Keywords: epicutaneous immunotherapy, food immunotherapy, IgE, IgG4, oral immunotherapy, regulatory T cell, sublingual immunotherapy, Tfh13, Th2, Th2a
1 |. INTRODUCTION
The evolution of our understanding of food allergy pathogenesis parallels the mechanisms of action of emerging targeted therapies in the field. The goals of treatment for food allergy include desensitization and ultimately sustained unresponsiveness to the allergenic food(s), thereby resulting in a decrease in the risk of life-threatening hypersensitivity reactions. The first Food and Drug Administration-approved therapy for a food allergy in the United States, Palforzia®, is a peanut flour-based oral immunotherapy that was approved in early 2020.1 Targeted biologic immunomodulators and probiotics are also being studied to treat food allergy as stand-alone approaches or as adjuvants to oral or epicutaneous immunotherapies/dietary preventions.2–5(p1),6–8
Mechanistic studies performed in conjunction with clinical trials or in mouse models have provided new insights into the pathogenesis of food allergy and the induction of desensitization and tolerance. Cellular and molecular biomarkers predictive of treatment response have been identified in both the innate and adaptive immune system for food allergy.9,10(p4),11(p4) In this review, our objective is to discuss future immunotherapies for food allergy and the evidence for their potential utility in food allergy.
2 |. MECHANISMS OF PATHOGENESIS WITH RELEVANCE TO IMMUNOTHERAPY
Factors contributing to food allergy development include the skin and mucosal barrier and their associated exposomes, including microbiomes, allergens, and pollutants (Figure 1).12–22
FIGURE 1.
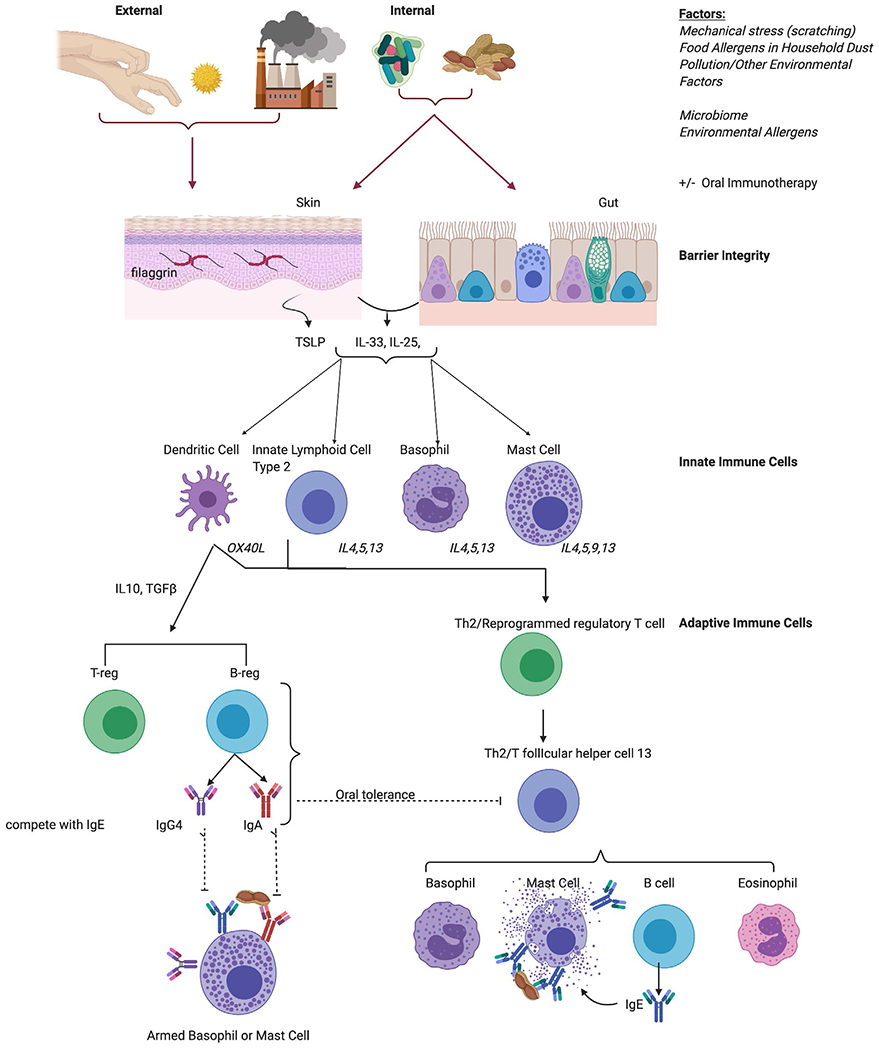
Cellular Mechanisms of Food Allergy Pathogenesis and Tolerance. Both external and internal factors affect the skin and gut mucosal barriers to produce barrier cytokines, which stimulate innate immune cells to produce Th2 or regulatory cytokines to therefore induce tolerance or allergic cell degranulation
Microbiome:
Specific skin and gut microbiome signatures have been correlated with atopic condition development, resolution, and protection particularly for food allergy in human and mouse studies.13–18,21,22 Non-lesional skin abundance of S aureus is increased in children with food allergy and atopic dermatitis when compared to non-allergic children.13 In the same study, skin barrier, characterized by transepidermal water loss, filaggrin, and ceramide content, was also found to be more compromised in food-allergic children with atopic dermatitis than in children with atopic dermatitis alone. Gastrointestinal microbiome patterns as measured in stool have been characterized for specific food allergen sensitizations, such as milk and egg.14,16 In a study of milk-allergic children, a specific microbiome composition consisting of enrichment for Clostridia and Firmicutes was associated with resolution of allergy. Metagenomic functional prediction of the signature associated with resolution supported decreased fatty acid metabolism.22
Mouse models have provided more definitive evidence for microbial differences driving allergy susceptibility. As in humans, mice that are genetically susceptible to allergy through an overactive IL-4 receptor (Il4raF709 mice) demonstrated a distinct microbiome signature after sensitization to egg.21 This allergy-associated microbiota transmitted the allergy phenotype to germ-free mice. In an adjuvant-driven mouse model of food allergy, a high-fiber diet promoted short-chain fatty acid (acetate and butyrate) release from gut microbiota and protected against food allergy.15 Likewise, mice that were lacking SCFA receptors had exacerbated food allergy responses. Two recent studies were able to extend these findings by linking human microbial differences to food allergy susceptibility in mice. Human food allergy-associated microbiota transferred susceptibility to mice. More importantly from a therapeutic perspective, microbiota from food-tolerant individuals could be used to protect mice from the development of food allergy.2,17 These data on the effect of the microbiome on the development and resolution of food allergy portend potential treatment mechanisms, which will be discussed in the next section.
2.1 |. Barrier immunity and Th2 cytokines
The immune milieu of the site of initial allergen exposure determines the outcome of allergy or tolerance. In humans, mutations in the skin structural protein filaggrin have been noted to confer risk for allergic diseases, including food allergy.12 This was comprehensively summarized in a systematic review of 24 studies. In mice, mechanical skin injury is used to break tolerance to antigens and model the development of food allergy.23 In addition to driving local cytokine and immune cell changes in the skin, skin tape stripping induces distal changes in the gastrointestinal tract including an expansion of mast cells. In oral sensitization models, adjuvants such as cholera toxin or Staphylococcal enterotoxin B are used to break tolerance. Skin tape stripping and adjuvants act to drive the production of a trio of Th2-inducing cytokines that are released from barrier sites. IL-33, IL-25, and thymic stromal lymphopoietin (TSLP) are secreted by epithelial cells in the skin and gastrointestinal tract, leading to production of Th2-associated cytokines (IL-4, IL-5, IL-9, and IL-13) from innate lymphoid cell type 2 (ILC2) and basophils. ILC2 have been linked to food allergy pathogenesis in mice through production of Th2-associated cytokines.24,25 TSLP and IL-33 also act on dendritic cells (DCs) to change their phenotype including upregulation of the co-stimulatory molecule OX40 ligand that promotes Th2 polarization.26–29 Presentation of food allergen by these modified DCs together with Th2 cytokine production by ILC2 drives the generation and amplification of Th2-skewed CD4+ T-helper cells in draining lymph nodes. Basophils and mast cells, in addition to their roles driving type I hypersensitivity reactions, can also function as innate sources of Th2 cytokines similar to the role of ILC2s and contribute to the sensitization phase of food allergy,30.31 Through their production of IL-4, IL-13, and IL-9, these cells promote the generation of CD4+ Th2 cells, expand mucosal mast cells, and suppress the generation of Tregs. Studies in humans with food allergy and Il4raF709 mice have shown that food allergy is associated with Th2 reprogramming of regulatory T cells.32 These Th2 reprogrammed Tregs have diminished suppressive capacity and in fact contribute to food allergy pathogenesis by producing IL-4.
The key mechanism by which CD4+ T cells producing Th2 cytokines contribute to food allergy is through B-cell help and support for IgE class-switching. Cross-linking of allergen-specific IgE bound to mast cells and basophils leads to activation of these effector cells and release of stored granule contents and rapidly synthesized lipid mediators that contribute to acute allergic symptoms. Although IgE has long been understood to be dependent on Th2 cytokines, recent studies have identified a unique cell subset critical to the development of high-affinity IgE. T follicular helper cells (Tfh cells) express the chemokine receptor CXCR5 that is essential for guiding these cells to the B-cell follicle to provide B-cell support. A subset of Tfh cells that co-express IL-4 and IL-13, and express the transcription factors BCL6 and GATA3 were found to be essential for the production of high-affinity IgE in mice that could promote anaphylaxis. These cells, termed Tfh13 cells, were identified in peanut-allergic humans.33 Human food allergy is also associated with highly differentiated Th2 cells, variously termed Th2A cells, or pathogenic effector Th2 cells.34–36 Their contribution to disease pathogenesis is not clear.
Targeting of multiple cytokines in the Th2 pathway has been shown to effectively suppress the development of food allergy in mice. Neutralization of IL-4, IL-13, IL-9, IL-25, TSLP, and IL-33 have all demonstrated roles during the sensitization phase of food allergy. Interestingly, simultaneous neutralization of all three barrier cytokines (IL-33, IL-25, and TSLP) was able to suppress food-allergic reactions in established disease.37
2.2 |. IgE production and maintenance
Downstream of the allergen-specific CD4+ T-cell response are B cells that produce IgE. The maintenance of allergen-specific IgE over a lifetime of careful allergen avoidance has been a poorly understood phenomenon. Plasma cells producing IgE have been characterized as short-lived compared with other immunoglobulin isotypes. Studies in mouse models have shown that B cells require continual CD4+ T-cell help in order to maintain IgE production and that IgE plasma cells are continually renewed from IgG1 + memory B cells in a STAT6-dependent manner, again highlighting the continual need for Th2 cytokines.38 In contrast, Asrat et al reported that chronic antigen exposure was necessary before IgE plasma cells could be identified in the bone marrow, where they were maintained over a period of 8 months.39 Recent work in humans with peanut allergy showed that the gastrointestinal tract, particularly the stomach and duodenum, was a reservoir of IgE plasma cells.40 Furthermore, the co-localization of shared IgG and IgE clones within the gastrointestinal tract supports the concept of sequential class-switching from IgG to IgE in the maintenance of IgE, and suggests that this may occur at relevant sites outside of lymphoid tissues or bone marrow.
In addition to advances elucidating mechanisms of long-lived IgE memory, recent work has uncovered new mechanisms that help to explain why individuals with the same level of specific IgE can have differing clinical presentations of tolerance or allergy. Shade et al studied glycosylation patterns of IgE comparing non-allergic individuals with those with oral food challenge–confirmed peanut allergy and found that IgE from allergic individuals had higher levels of sialylation.41 Removal of sialic acid content from IgE suppressed the ability to induce anaphylaxis in mice, and removal of sialic acid from human IgE reduced the ability to induce degranulation of the mast cell line LAD2. Targeting IgE sialylation in vivo with a novel fusion protein of IgE constant regions with neuraminidase to remove sialic acid could suppress anaphylaxis in vivo.
At each stage in the above review of food allergy pathogenesis, summarized in Figure 1, there are opportunities for immunotherapeutic approaches, which will be described in the next section. These are also listed by mechanism and phase of development in Table 1.
TABLE 1.
Status of immunotherapies for food allergy
| Class of therapy | Target | Therapy | Stage of development |
|---|---|---|---|
| Gut-targeted (microbiome, oral immunotherapy) | Gut dendritic cells | Palforzia* | FDA-approved, commercially available |
| Gut microbiome | Fecal microbial transplant | Phase I studies in adults with peanut allergy5 | |
| Gut microbiome | VE416 (commensal bacteria ± vancomycin and peanut OIT) | Phase I/II studies in adolescents and adults with peanut allergy50 | |
| Gut microbiome and dendritic cells | PRT100 (probiotic Lactobacillus rhamnosus in combination with peanut OIT) | Phase IIb studies in children are underway, comparing whether superior to peanut OIT alone. Phase IIa study showed that 80% achieved SU.4 | |
|
| |||
| Skin-targeted | Cutaneous dendritic cells | Viaskin patch (egg/milk*/peanut*) | Egg: preclinical studies are ongoing Milk: completed phase II for ages 2-1745 Peanut: FDA application pending for ages 4-11; phase III ongoing for ages 1-3; phase II ongoing for adolescents and adults |
|
| |||
| Th2 pathway | TSLP | Tezepelumab | Preclinical study in mice showed decreased reaction when TSLP is blocked at the same time as IL-33 and IL-25.37 |
| IL-33 | REGN3500 | Preclinical study in mice showed decreased reaction when IL-33 is blocked at the same time as TSLP and IL-25.37 | |
| Etokimab | Phase I study showed effective, short-lived results in peanut-allergic adults.47 | ||
|
| |||
| Blocking antibody | Mast cell/basophil/IgE | Allergen IgG | No food allergen therapeutics available currently. Studies with cat and birch allergy were promising in phase I and phase II, respectively.73 |
|
| |||
| Anti-IgE | FCERI | Omalizumab* | Ongoing phase III trial for monotherapy and as adjunct to OIT in multi-food–allergic children and adults. |
| Ligelizumab | No studies available in food allergy models or humans. | ||
|
| |||
| Tyrosine Kinase Inhibitor | BTK | Ibrutinib | Phase 1 study in peanut and tree nut–allergic adults completed. No OFCs were done.74 Suppresses anaphylaxis in humanized mouse. |
|
| |||
| Th2 pathway | OX40 | Anti-OX40 | OX40L mediates Th2 skewing in mouse model of egg allergy.28 |
|
| |||
| Allergen | Dendritic cells | ARA-LAMP-VAX | Phase I study ongoing in peanut-allergic adolescents.6 |
|
| |||
| Th2 cytokines | IL-4Ralpha | Dupilumab | Phase I studies ongoing in peanut-allergic children as monotherapy and as OIT adjunct.7,8 |
| IL-13 | Lebrikizumab | No studies available in food allergy models or humans. | |
|
| |||
| JAK inhibitor | JAK1/2 | Ruxolitinib | Preclinical study showed a decrease in symptoms in a mouse model of egg allergy.61 |
| Baricitinib | No studies available in food allergy models or humans. | ||
| Upadacitinib | |||
| Abrocitinib | |||
| ASN002 | |||
Indicates therapies granted breakthrough status by the FDA.
3 |. FUTURE TREATMENT OPPORTUNITIES BASED ON FOOD ALLERGY PATHOGENESIS
There are a number of stages within the food allergy pathogenesis landscape to target with new and developing therapeutic or prevention strategies (summarized in Figure 2 and Table 1). This section will focus on the therapeutic strategies in development or discovery phases, which target immunologic features of food allergy.
FIGURE 2.
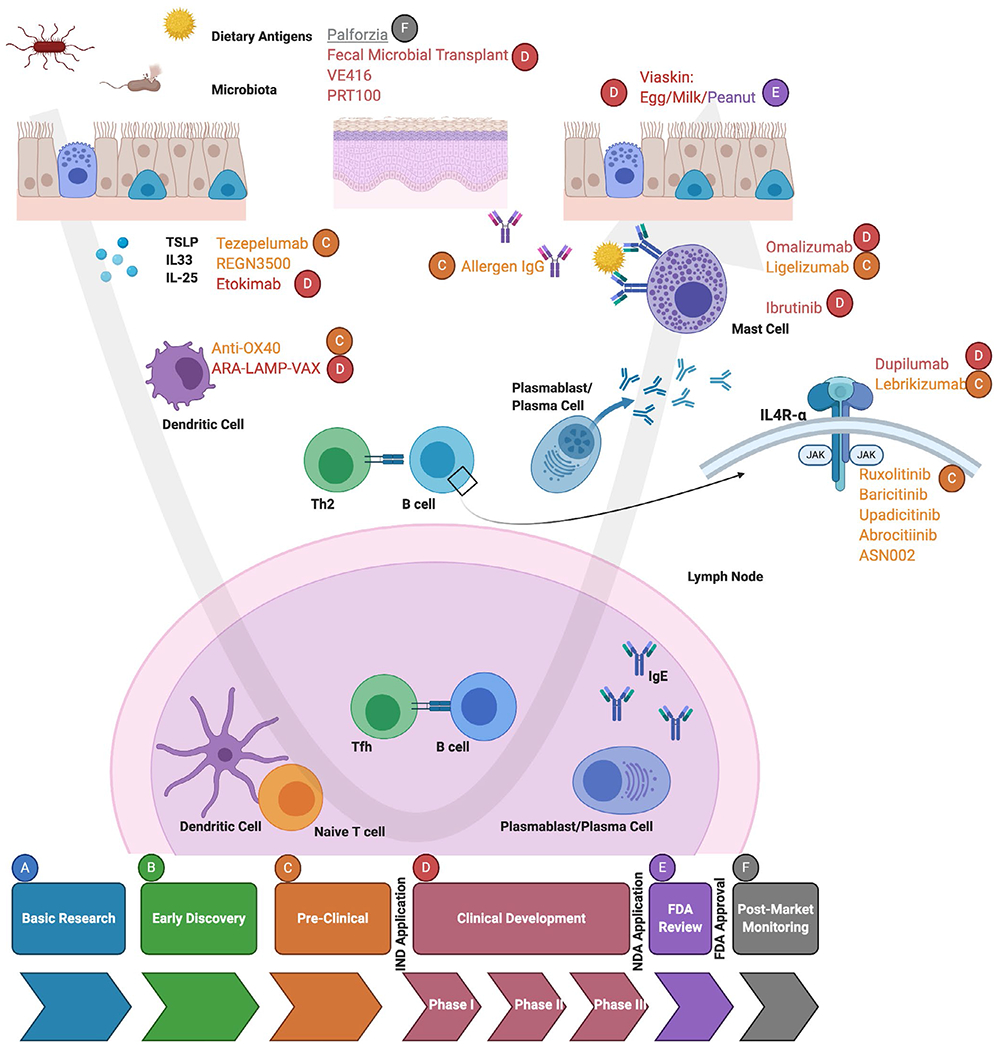
Mechanisms of Action for Treatment Candidates in the Various Stages of Drug Development for Food Allergy. Treatment candidates exist in food allergy from preclinical, clinical development, FDA review, and FDA approval
3.1 |. Allergen immunotherapy
The most advanced therapeutic approach is based on controlled allergen exposure (allergen immunotherapy), given by different routes. Oral immunotherapy (OIT) for peanut allergy has recently been approved by the FDA,1 while epicutaneous immunotherapy (EPIT) has been granted breakthrough status by the FDA,42 and sublingual immunotherapy (SLIT) is currently under investigation. Subcutaneous immunotherapy with a chemically altered peanut extract has completed a phase I, double-blind, placebo-controlled, multicenter trial in 42 patients; results are still pending.43 Food immunotherapy (OIT and SLIT) can lead to sustained unresponsiveness (SU) in a subset of treated patients; EPIT has only achieved SU in animal models.44 Although natural skin exposure to peanut allergen is a risk factor for development of peanut allergy (in those with eczema), EPIT as treatment induces tolerance in mice and increases threshold of reactivity to peanut in children. Studies are ongoing for milk and egg EPIT in clinical trials.45
Although allergen immunotherapy does not specifically target an immune pathway, many studies have attempted to parse out the immune mechanism of treatment response by studying the impact of treatment in clinical responders and non-responders. Theoretically, administration of allergen without co-stimulation (adjuvant) is thought to induce production of blocking antibodies (IgG4, IgA) and Tregs (producing IL-10 and TGF-β). The subgroup of patients, in both OIT and SLIT, that achieve sustained unresponsiveness, have greater levels of peanut sIgG4 antibodies with treatment.9,46 Furthermore, low baseline-specific IgE levels are the best predictor of sustained unresponsiveness.47 These differences in IgE and IgG4 antibodies are reflected in differences in basophil activation, a functional readout that integrates changes in IgE, IgG4, and inherent basophil desensitization. Patients who do not achieve sustained unresponsiveness to OIT demonstrate a rebound in their basophil activation after treatment is stopped.9 Food allergen immunotherapy is associated with a reduction in allergen-specific Th2 cells, including reduced Th2A cells.34,48 EPIT induces unique hypermethylation patterns of the transcription factor Gata4 in Th2 cells, according to a recent mouse study.44 However, there is currently a lack of information on the relationship between immunotherapy-associated T-cell responses and treatment outcome. It has been described that suppression of allergen-specific Th2 responses with OIT is transient.49
3.2 |. Microbial therapeutics
Based on studies showing that ex vivo transfer of stool from food-allergic patients and healthy patients could confer susceptibility and protection from food allergy in mouse models,5,17 fecal microbiota transplant (FMT) is being studied in food-allergic patients.5 Other microbial therapies are being combined with oral immunotherapy to promote a tolerogenic mucosal immune environment while providing allergen. Probiotic therapy with Lactobacillus rhamnosus in combination with peanut OIT (PRT100) for peanut allergy induced sustained unresponsiveness in 80% of patients after 2-5 weeks of discontinuation of therapy.4 Studies comparing PRT100 with peanut OIT are pending to determine whether there is a significant improvement with the addition of probiotic. Another approach is testing a combination of commensal bacteria provided in a capsule, together with the antibiotic vancomycin to help the administered bacteria to colonize, together with peanut OIT. This is an ongoing phase 1, randomized, double-blind trial of 40 peanut-allergic patients.50
3.3 |. Biologies targeting Th2 pathways
Cytokines that drive Th2 pathways including TSLP, IL-33, and IL-25 are secreted by epithelial cells of the skin or gastrointestinal tract, and neutralization inhibits food allergy in mice.37 TSLP and IL-33 can be directly targeted by monoclonal antibodies, tezepelumab (TSLP), etokimab (IL-33), and REGN3500 (IL-33). Inhibition of IL-33 with etokimab showed promising initial but unfortunately short-lived results in a small human study.47 Seventy-three percent of patients treated with a single dose of etokimab could tolerate 275 mg of peanut protein after 15 days and 57% could at 45 days, in comparison with 0% of 5 patients given placebo. Etokimab led to a transient decrease in peanut-specific IgE and peanut-specific IL-4 and IL-5, with a more sustained suppression of peanut-specific IL-9, IL-13, and ST2. REGN3500 has not been studied in food-allergic patients yet, but given its promising results in phase 2 studies of asthma, it has similar potential as etokimab.51 As indicated by studies in mice, combined neutralization of multiple pro-Th2 cytokines may be required for treatment efficacy. Alternatively, targeting of these cytokines may be required together with allergen administration to effectively reprogram the adaptive immune response.
TSLP and IL-33 upregulate OX40L on dendritic cells that drives Th2 skewing. There are two human anti-OX40 antibodies in phase 2 trials for atopic dermatitis and asthma.52(p40),53 There is also an anti-OX40L antibody that was tested in asthma and did not prove efficacious in decreasing asthma symptoms, but did significantly lower total IgE.54 Given the role of OX40L in Th2 cytokine response in mouse models of food allergy, these OX40 and OX40L antibodies may be effective as an adjuvant to food immunotherapy protocols.28
Inhibition of Th2 cytokines is currently being tested as a monotherapy for peanut allergy and together with oral immunotherapy.7,8,55,56 Dupilumab is a neutralizing antibody for IL-4 receptor alpha, thereby inhibiting both IL-4 and IL-13 signaling. Studies in chronic sinusitis with nasal polyposis and allergic asthma showed a corresponding decrease in total IgE with dupilumab treatment.57,58 Combined with results in mice showing that maintenance of IgE requires continual Th2 help, this provides the rationale for the use of dupilumab for the treatment of food allergy. Monoclonal antibodies have also been developed that target IL-13 (lebrikizumab, anrukinzumab, and tralokinumab) but have not yet been tested in food allergy.59
Cytokines mediate their activity through receptors that signal through Janus kinases (JAKs). Inhibitors of Jak (jakinibs) are currently being studied in atopic dermatitis with favorable results in phase 2 studies and breakthrough status designation from the FDA.60 An early jakinib, ruxolitinib, was tested in a mouse model of food allergy. Treatment was associated with decreased allergic response, mast cell activation, and IL-13 release.61 These preclinical results suggest that JAKs are a potential target for food allergy treatment, particularly given the development of newer and safer Jak inhibitors that have shown efficacy and safety in other atopic conditions. As with dupilumab, jakinibs may be effective as monotherapy or immunotherapy-adjuvant treatment for food allergy.
3.4 |. IgE targeting
Targeting of Th2 pathways is hypothesized to be effective by having an end effect on allergen-specific IgE. IgE has also been targeted directly. Omalizumab and the newer higher-affinity monoclonal antibody, ligelizumab, bind free IgE and decrease its binding to high-affinity and low-affinity IgE receptors, thereby abrogating the effector response to food allergens in tissues.62 Omalizumab has been studied as both a monotherapy and an adjuvant to food immunotherapy.63–71 A benefit of targeting IgE is the antigen non-specificity of the approach. Reduction in IgE on the surface of cells should reduce allergic reactions to any food. As an adjuvant to immunotherapy, anti-IgE therapy improves the safety profile of immunotherapy, and it is hypothesized that the immunotherapy would have potentially sustained immune-modifying activity such that treatment could potentially be stopped. Omalizumab has been designated with breakthrough status by the FDA for food allergy, given the promising results in human trials with increased threshold and tolerance of avoided foods.72
In addition to blocking IgE with an IgE-specific antibody, another approach is to compete with IgE binding and cross-linking with an allergen. This conceivably could be achieved with allergen-specific IgG, which is shown to be predictive of clinical response in immunotherapy trials. There is currently no food allergen–specific IgG in the therapeutic pipeline; however, Regeneron® is currently developing subcutaneously administered cat and birch allergen–specific IgG therapeutics, which suppress immediate allergic reactions in humans in phase I and II trials, respectively.73
3.5 |. Suppressing effector cells
Food-allergic reactions are mediated by IgE signaling through the high-affinity FcεRI receptor on mast cells and basophils. A cascade of kinases, including Lyn, Syk, and Bruton’s tyrosine kinase (BTK), regulate calcium signaling and control cell degranulation. Medications that inhibit BTK are currently being used to treat chronic lymphocytic leukemia and non-Hodgkin lymphoma.74 After a pilot in aeroallergen-sensitized patients showed abrogation of skin test reactivity and basophil activation, a follow-up study in peanut- and tree nut-allergic adults showed promising results after a short course (7 days) of treatment, with a rapid rebound in reactivity.75 There are safety concerns with the use of ibrutinib, and the challenge remains to identify signaling components downstream of the IgE receptor that are not also essential for other key elements of the host immune response. However, as a proof of principle, this study demonstrates that inhibition of a single kinase can suppress allergic reactions in a rapid and reversible manner, which may be useful for short-term treatment (eg, to provide safety during immunotherapy, or where risk of allergen exposure may be unavoidably increased). Inhibition of BTK could also completely suppress anaphylaxis mediated by human IgE in humanized mice.76
3.6 |. Duration of treatment effect
The ideal treatment for food allergy would result in a long-lasting immune tolerance. Allergen immunotherapy has allowed some individuals to incorporate the food into the diet ad libitum,48,77 but generally, the greater the duration of allergen avoidance after treatment, the greater the rebound of clinical reactivity.78 Targeting of IL-4 and IL-13 does not eliminate the source of Th2 cytokines and therefore would not be expected to result in sustained effects. Similarly, targeting of IgE does not eliminate the cells producing the IgE. It is likely that the reservoir of IgE memory (IgG+ memory B cells) and Th2 memory would need to be extinguished to have disease-modifying effects. Our growing understanding of the mechanisms involved in IgE maintenance may reveal new targets for durable treatment of food allergy, as summarized in Table 1.
4 |. CONCLUSIONS
This review article summarizes the latest additions to the understanding of the immune basis of food allergy pathogenesis and implications for future therapies. The testing of new biologics for the treatment of food allergy, and the opportunity to study the immune basis of treatment response, will accelerate our understanding of immune mechanisms of disease. As illustrated in Figure 2, there are currently treatments at every phase of development, from preclinical to FDA approval, with a variety of routes of administration. The landscape of food allergy therapeutics is vast, and there are many new developments on the horizon to potentially transform the quality of life for food allergy patients worldwide.
Key Message.
Our growing understanding of the immune mechanisms of allergy and tolerance to foods supports the testing of immunomodulators in the treatment of food allergy, including IgE targeting, type 2 cytokine targeting, and microbial therapeutics. In this review article, we summarize recent advances in our immune understanding of food allergy and the implications for the use of novel therapeutics.
Funding information
Dr Ramsey is supported by the AAAAI/Jaffe Food Allergy Institute Fellowship Program. Dr Berin is supported in part by the National Institutes of Health grants AI151707 and AI136053.
Footnotes
CONFLICTS OF INTEREST
Dr Ramsey has no conflicts of interest to declare. Dr Berin is on the advisory board at Prota Therapeutics and has served as a speaker for DBV Technologies.
ETHICAL APPROVAL
Not applicable, not original research.
REFERENCES
- 1.Peanut allergen powder (Palforzia). Med Lett Drugs Ther. 2020;62(1593):33–34. [PubMed] [Google Scholar]
- 2.Abdel-Gadir A, Schneider L, Casini A, et al. Oral immunotherapy with omalizumab reverses the Th2 cell-like programme of regulatory T cells and restores their function. Clin Exp Allergy. 2018;48(7):825–836. 10.1111/cea.13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berni Canani R, Di Costanzo M, Bedogni G, et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J Allergy Clin Immunol. 2017;139(6):1906–1913.e4. 10.1016/j.jaci.2016.10.050 [DOI] [PubMed] [Google Scholar]
- 4.Tang MLK, Ponsonby A-L, Orsini F, et al. Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol. 2015;135(3):737–744.e8. 10.1016/j.jaci.2014.11.034 [DOI] [PubMed] [Google Scholar]
- 5.Rachid R A Phase I Open Label Trial to Evaluate the Safety and Efficacy of Oral Encapsulated Fecal Microbiota Transplantation in Peanut Allergic Patients. Clinicaltrials.gov; 2020. Accessed July 9, 2020. https://clinicaltrials.gov/ct2/show/NCT02960074
- 6.Astellas Pharma Global Development, Inc. A Phase 1, Randomized, Placebo-Controlled Study to Evaluate Safety, Tolerability and Immune Response in Adolescents Allergic to Peanut After Receiving Intradermal Administration of ASP0892 (ARA-LAMP-Vax), a Single Multivalent Peanut (Ara H1, H2, H3) Lysosomal Associated Membrane Protein DNA Plasmid Vaccine. Clinicaltrials.gov; 2020. Accessed July 9, 2020. https://clinicaltrials.gov/ct2/show/NCT03755713
- 7.Study in Pediatric Subjects With Peanut Allergy to Evaluate Efficacy and Safety of Dupilumab as Adjunct to AR101 (Peanut Oral Immunotherapy) - Full Text View - ClinicalTrials.gov. Accessed July 9, 2020. https://clinicaltrials.gov/ct2/show/NCT03682770
- 8.Study to Evaluate Dupilumab Monotherapy in Pediatric Patients With Peanut Allergy - Full Text View - ClinicalTrials.gov. Accessed July 9, 2020. https://clinicaltrials.gov/ct2/show/NCT03793608
- 9.Patil SU, Steinbrecher J, Calatroni A, et al. Early decrease in basophil sensitivity to Ara h 2 precedes sustained unresponsiveness after peanut oral immunotherapy. J Allergy Clin Immunol. 2019;144(5):1310–1319.e4. 10.1016/j.jaci.2019.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton OT, Logsdon SL, Zhou JS, et al. Oral immunotherapy induces IgG antibodies that act through FcγRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. 2014;134(6):1310–1317.e6. 10.1016/j.jaci.2014.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton OT, Tamayo JM, Stranks AJ, Koleoglou KJ, Oettgen HC. Allergen-specific IgG antibody signaling through FcγRIIb promotes food tolerance. J Allergy Clin Immunol. 2018;141(1):189–201.e3. 10.1016/j.jaci.2017.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Oord RAHM, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ. 2009;339:b2433. 10.1136/bmj.b2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung DYM, Calatroni A, Zaramela LS, et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Transl Med. 2019;11(480):eaav2685. 10.1126/scitranslmed.aav2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage JH, Lee-Sarwar KA, Sordillo J, et al. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy. 2018;73(1):145–152. 10.1111/all.13232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan J, McKenzie C, Vuillermin PJ, et al. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep. 2016;15(12):2809–2824. 10.1016/j.celrep.2016.05.047 [DOI] [PubMed] [Google Scholar]
- 16.Fazlollahi M, Chun Y, Grishin A, et al. Early-life gut microbiome and egg allergy. Allergy. 2018;73(7):1515–1524. 10.1111/all.13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feehley T, Plunkett CH, Bao R, et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med. 2019;25(3):448–453. 10.1038/s41591-018-0324-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saulyte J, Regueira C, Montes-Martínez A, Khudyakov P, Takkouche B. Active or passive exposure to tobacco smoking and allergic rhinitis, allergic dermatitis, and food allergy in adults and children: a systematic review and meta-analysis. PLoS Medicine. 2014;11(3):e1001611. 10.1371/journal.pmed.1001611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brough HA, Liu AH, Sicherer S, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 2015;135(1):164–170. 10.1016/j.jaci.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marrs T, Logan K, Craven J, et al. Dog ownership at three months of age is associated with protection against food allergy. Allergy. 2019;74(11):2212–2219. 10.1111/all.13868 [DOI] [PubMed] [Google Scholar]
- 21.Noval Rivas M, Burton OT, Wise P, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131(1):201–212. 10.1016/j.jaci.2012.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunyavanich S, Shen N, Grishin A, et al. Early-life gut microbiome composition and milk allergy resolution. J Allergy Clin Immunol. 2016;138(4):1122–1130. 10.1016/j.jaci.2016.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leyva-Castillo J-M, Galand C, Kam C, et al. Mechanical skin injury promotes food anaphylaxis by driving intestinal mast cell expansion. Immunity. 2019;50(5):1262–1275.e4. 10.1016/j.immuni.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton OT, Medina Tamayo J, Stranks AJ, et al. IgE promotes type 2 innate lymphoid cells in murine food allergy. Clin Exp Allergy. 2018;48(3):288–296. 10.1111/cea.13075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016;138(3):801–811.e9. 10.1016/j.jaci.2016.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito T, Wang Y-H, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202(9):1213–1223. 10.1084/jem.20051135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33–activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123(5):1047–1054. 10.1016/j.jaci.2009.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blázquez AB, Berin MC. Gastrointestinal dendritic cells promote Th2 skewing via OX40L. J Immunol. 2008;180(7):4441–4450. [DOI] [PubMed] [Google Scholar]
- 29.Chu DK, Llop-Guevara A, Walker TD, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013;131(1):187–200.e8. 10.1016/j.jaci.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 30.Muto T, Fukuoka A, Kabashima K, et al. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int Immunol. 2014;26(10):539–549. 10.1093/intimm/dxu058 [DOI] [PubMed] [Google Scholar]
- 31.Chen C-Y, Lee J-B, Liu B, et al. Induction of interleukin-9-producing mucosal mast cells promotes susceptibility to IgE-mediated experimental food allergy. Immunity. 2015;43(4):788–802. 10.1016/j.immuni.2015.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noval Rivas M, Burton OT, Wise P, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42(3):512–523. 10.1016/j.immuni.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gowthaman U, Chen JS, Zhang B, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science. 2019;365(6456):eaaw6433. 10.1126/science.aaw6433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wambre E, Bajzik V, DeLong JH, et al. A phenotypically and functionally distinct human T H 2 cell subpopulation is associated with allergic disorders. Sci Transl Med. 2017;9(401):eaam9171. 10.1126/scitranslmed.aam9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiang D, Chen X, Jones SM, et al. Single-cell profiling of peanut-responsive T cells in patients with peanut allergy reveals heterogeneous effector TH2 subsets. J Allergy Clin Immunol. 2018;141(6):2107–2120. 10.1016/j.jaci.2017.11.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiter B, Smith NP, Monian B, et al. Expansion of the CD4+ effector T-cell repertoire characterizes peanut-allergic patients with heightened clinical sensitivity. J Allergy Clin Immunol. 2020;145(1):270–282. 10.1016/j.jaci.2019.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khodoun MV, Tomar S, Tocker JE, Wang YH, Finkelman FD. Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25, and IL-33. J Allergy Clin Immunol. 2018;141(1):171–179.e1. 10.1016/j.jaci.2017.02.046 [DOI] [PubMed] [Google Scholar]
- 38.Jiménez-Saiz R, Chu DK, Mandur TS, et al. Lifelong memory responses perpetuate humoral T H 2 immunity and anaphylaxis in patients with food allergy. J Allergy Clin Immunol. Published online February 2017. doi: 10.1016/j.jaci.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asrat S, Kaur N, Liu X, et al. Chronic allergen exposure drives accumulation of long-lived IgE plasma cells in the bone marrow, giving rise to serological memory. Sci Immunol. 2020;5(43):eaav8402. 10.1126/sciimmunol.aav8402 [DOI] [PubMed] [Google Scholar]
- 40.Hoh RA, Joshi SA, Lee J-Y, et al. Origins and clonal convergence of gastrointestinal IgE+ B cells in human peanut allergy. Sci Immunol. 2020;5(45):eaay4209. 10.1126/sciimmunol.aay4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shade K-TC, Platzer B, Washburn N, et al. A single glycan on IgE is indispensable for initiation of anaphylaxis. J Exp Med. 2015;212(4):457–467. 10.1084/jem.20142182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donne N DBV Technologies Receives FDA Breakthrough Therapy Designation for Viaskin Peanut for the Treatment of Peanut Allergy in Children. Published April 9, 2015. Accessed November 3, 2020. https://www.dbv-technologies.com/wp-content/uploads/2017/09/1875pr-viaskin-peanut-breakthrough-desi.pdf
- 43.HAL-MPE1 Safety and Tolerability Study - Full Text View - ClinicalTrials.gov. Accessed July 29, 2020. https://clinicaltrials.gov/ct2/show/NCT02991885
- 44.Mondoulet L, Dioszeghy V, Busato F, et al. Gata3 hypermethylation and Foxp3 hypomethylation are associated with sustained protection and bystander effect following epicutaneous immunotherapy in peanut-sensitized mice. Allergy. 2019;74(1):152–164. 10.1111/all.13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Technologies DBV, Double-Blind A. Placebo-Controlled Randomized Trial to Study the Viaskin Milk Efficacy and Safety for Treating IgE-Mediated Cow’s Milk Allergy in Children. clinicaltrials.gov; 2019. Accessed July 22, 2020. https://clinicaltrials.gov/ct2/show/NCT02223182
- 46.Orgel K, Burk C, Smeekens J, et al. Blocking antibodies induced by peanut oral and sublingual immunotherapy suppress basophil activation and are associated with sustained unresponsiveness. Clin Exp Allergy. 2019;49(4):461–470. 10.1111/cea.13305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chinthrajah S, Cao S, Liu C, et al. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight. 2019;4(22): 10.1172/jci.insight.131347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones SM, Sicherer SH, Burks AW, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol. 2017;139(4):1242–1252.e9. 10.1016/j.jaci.2016.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorelik M, Narisety SD, Guerrerio AL, et al. Suppression of the immunologic response to peanut during immunotherapy is often transient. J Allergy Clin Immunol. 2015;135(5):1283–1292. 10.1016/j.jaci.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.VE416 for Treatment of Food Allergy - Full Text View - ClinicalTrials.gov. Accessed July 29, 2020. https://clinicaltrials.gov/ct2/show/NCT03936998?cond=ve-416&draw=2&rank=1
- 51.Study of Safety, Tolerability, and Pharmacokinetics of Multiple Ascending Doses of REGN3500 in Adults With Moderate Asthma - Full Text View - ClinicalTrials.gov. Accessed July 29, 2020. https://clinicaltrials.gov/ct2/show/NCT02999711?cond=regn3500&draw=2&rank=5
- 52.Study of an Anti-OX40 Monoclonal Antibody (KHK4083) in Subjects With Moderate to Severe Atopic Dermatitis - Full Text View - ClinicalTrials.gov. Accessed July 24, 2020. https://clinicaltrials.gov/ct2/show/NCT03703102?cond=ox40&draw=2&rank=10
- 53.Phase 2b Study to Evaluate the Efficacy and Safety of ISB 830 in Adults With Moderate to Severe Atopic Dermatitis - Full Text View - ClinicalTrials.gov. Accessed July 24, 2020. https://clinicaltrials.gov/ct2/show/NCT03568162?cond=ox40&draw=4&rank=28
- 54.Gauvreau GM, Boulet L-P, Cockcroft DW, et al. OX40L blockade and allergen-induced airway responses in subjects with mild asthma. Clin Exp Allergy. 2014;44(1):29–37. 10.1111/cea.12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Bruin-Weller DMS. Effectiveness of Dupilumab inn Food Allergic Patients With Moderate to Severe Atopic Dermatitis, clinicaltrials.gov; 2020. Accessed July 27, 2020. https://clinicaltrials.gov/ct2/show/NCT04462055
- 56.Chinthrajah RS. Phase 2 Randomized Controlled Trial Using Biologics to Improve Multi OIT Outcomes, clinicaltrials.gov; 2020. Accessed July 27, 2020. https://clinicaltrials.gov/ct2/show/NCT03679676
- 57.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 58.Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous Dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315(5):469. 10.1001/jama.2015.19330 [DOI] [PubMed] [Google Scholar]
- 59.Ultsch M, Bevers J, Nakamura G, et al. Structural basis of signaling blockade by anti-IL-13 antibody Lebrikizumab. J Mol Biol. 2013;425(8):1330–1339. 10.1016/j.jmb.2013.01.024 [DOI] [PubMed] [Google Scholar]
- 60.AbbVie. A Phase 2b Multicenter, Randomized, Placebo-Controlled, Double-Blind Dose-Ranging Study to Evaluate ABT-494 (Upadacitinib) in Adult Subjects With Moderate to Severe Atopic Dermatitis, clinicaltrials.gov; 2020. Accessed July 27, 2020. https://clinicaltrials.gov/ct2/show/results/NCT02925117
- 61.Yamaki K, Yoshino S. A new, rapid in vivo method to evaluate allergic responses through distinctive distribution of a fluorescent-labeled immune complex: Potential to investigate anti-allergic effects of compounds administered either systemically or topically to the skin. J Immunol Methods. 2016;428:58–68. 10.1016/j.jim.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 62.Arm JP, Bottoli I, Skerjanec A, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy. 2014;44(11):1371–1385. 10.1111/cea.12400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sampson HA, Leung DYM, Burks AW, et al. A phase II, randomized, double-blind, parallel-group, placebo-controlled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011;127(5):1309–1310.e1. 10.1016/j.jaci.2011.01.051 [DOI] [PubMed] [Google Scholar]
- 64.Savage JH, Courneya J-P, Sterba PM, Macglashan DW, Saini SS, Wood RA. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol. 2012;130(5):1123–1129.e2. 10.1016/j.jaci.2012.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brandström J, Vetander M, Lilja G, et al. Individually dosed omalizumab: an effective treatment for severe peanut allergy. Clin Exp Allergy. 2017;47(4):540–550. 10.1111/cea.12862 [DOI] [PubMed] [Google Scholar]
- 66.Fiocchi A, Artesani MC, Riccardi C, et al. Impact of Omalizumab on Food Allergy in Patients Treated for Asthma: A Real-Life Study. J Allergy Clin Immunol Pract. 2019;7(6):1901–1909.e5. 10.1016/j.jaip.2019.01.023 [DOI] [PubMed] [Google Scholar]
- 67.MacGinnitie AJ, Rachid R, Gragg H, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol. 2017;139(3):873–881.e8. 10.1016/j.jaci.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wood RA, Kim JS, Lindblad R, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol. 2016;137(4):1103–1110.e11. 10.1016/j.jaci.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andorf S, Purington N, Block WM, et al. Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2018;3(2):85–94. 10.1016/S2468-1253(17)30392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andorf S, Manohar M, Dominguez T, et al. Observational long-term follow-up study of rapid food oral immunotherapy with omalizumab. Allergy Asthma Clin Immunol. 2017;13:51. 10.1186/s13223-017-0223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andorf S, Purington N, Kumar D, et al. A phase 2 randomized controlled multisite study using Omalizumab-facilitated rapid desensitization to test continued vs discontinued dosing in multifood allergic individuals. EClinicalMedicine. 2019;7:27–38. 10.1016/j.eclinm.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.FDA Grants Breakthrough Designation to Omalizumab for Food Allergies. Pharmacy Times. Accessed July 27, 2020. https://www.pharmacytimes.com/news/fda-grants-breakthrough-designation-to-omalizumab-for-food-allergies
- 73.Orengo JM, Radin AR, Kamat V, et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat Commun. 2018;9(1):1421. 10.1038/s41467-018-03636-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Regan JA, Cao Y, Dispenza MC, et al. Ibrutinib, a Bruton’s tyrosine kinase inhibitor used for treatment of lymphoproliferative disorders, eliminates both aeroallergen skin test and basophil activation test reactivity. J Allergy Clin Immunol. 2017;140(3):875–879.e1. 10.1016/j.jaci.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dispenza MC, Pongracic JA, Singh AM, Bochner BS. Short-term ibrutinib therapy suppresses skin test responses and eliminates IgE-mediated basophil activation in adults with peanut or tree nut allergy. J Allergy Clin Immunol. 2018;141(5):1914–1916.e7. 10.1016/j.jaci.2017.12.987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dispenza MC, Krier-Burris RA, Chhiba KD, Undem BJ, Robida PA, Bochner BS. Bruton’s tyrosine kinase inhibition effectively protects against human IgE-mediated anaphylaxis. J Clin Invest. 2020;130(9):4759–4770. 10.1172/JCI138448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim EH, Jones SM, Burks AW, et al. A 5-year summary of real-life dietary egg consumption after completion of a 4-year egg powder oral immunotherapy (eOIT) protocol. J Allergy Clin Immunol. 2020;145(4):1292–1295.e1. 10.1016/j.jaci.2019.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chinthrajah RS, Purington N, Andorf S, et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2019;394(10207):1437–1449. 10.1016/S0140-6736(19)31793-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


