Abstract
Background:
Eosinophilic asthma and nasal polyposis are hallmarks of aspirin-exacerbated respiratory disease (AERD), and IL-5 inhibition has been shown to provide therapeutic benefit. However, IL-5Rα is expressed on many cells in addition to eosinophils, and the mechanisms by which IL-5 inhibition leads to clinical benefit in eosinophilic asthma and nasal polyposis are unlikely to be due exclusively to anti-eosinophil effects.
Objective:
We sought to identify the mechanisms by which anti-IL-5 treatment with mepolizumab improves respiratory inflammation in AERD.
Methods:
Clinical characteristics, circulating granulocytes, nasal scraping transcripts, eosinophilic cationic protein, tryptase, antibody levels, and urinary and nasal eicosanoid levels were measured for 18 AERD subjects on mepolizumab and were compared to 18 matched AERD subjects not on mepolizumab.
Results:
Subjects on mepolizumab had significantly fewer peripheral blood eosinophils and basophils, and those cells that remained had higher surface CRTH2 expression than did those from subjects not on mepolizumab. Nasal prostaglandin (PG)F2α, PGD2 metabolites, leukotriene (LT)B4, and thromboxane levels were lower in subjects on mepolizumab, as were urinary levels of tetranor-PGD2 and LTE4. Nasal epithelial cell transcripts overexpressed among AERD subjects on mepolizumab were enriched for genes involved in tight junction formation and cilium organization. Nasal and urinary PGE2, tryptase, and antibody levels were not different between the two groups.
Conclusion:
IL-5 inhibition in AERD decreases production of inflammatory eicosanoids and upregulates tight junction-associated nasal epithelial cell transcripts, likely due to decreased IL-5 signaling on tissue mast cells, eosinophils, and epithelial cells. These direct effects on multiple relevant immune cells contributes to the mechanism of benefit afforded by mepolizumab.
Keywords: Aspirin-exacerbated respiratory disease, interleukin 5, nasal polyp, mepolizumab, prostaglandin F2α, prostaglandin D2, CRTH2, chronic rhinosinusitis, leukotriene
Capsule Summary:
Subjects with aspirin-exacerbated respiratory disease (AERD) treated with mepolizumab had decreased inflammatory eicosanoids and upregulation of nasal epithelial cell transcripts involved in tight junction pathways when compared to matched subjects with AERD not treated with mepolizumab.
Graphical Abstract
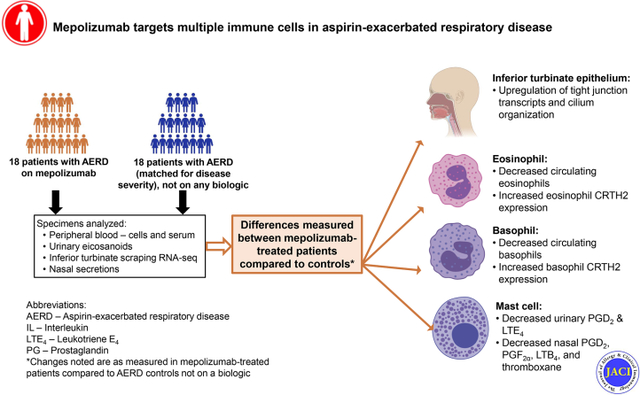
Introduction:
Aspirin-exacerbated respiratory disease (AERD) is characterized by chronic eosinophilic Type 2 inflammation of the upper and lower airways, marked by chronic rhinosinusitis with nasal polyps (CRSwNP), difficult-to-control asthma, and pathognomonic respiratory reactions to medications that inhibit cyclooxygenase-1. Nasal polyps are associated with nasal obstruction and anosmia, significant impairment in quality of life, and substantial medical resource consumption (1, 2), and are remarkably severe and recalcitrant in patients with AERD (3).
Mechanisms underlying the severe nasal polyposis and difficult-to-control asthma in patients with AERD are complex. Tissue eosinophilia is more pronounced in patients with AERD compared to aspirin-tolerant CRSwNP (4), but the role of eosinophils in disease pathogenesis is unclear. A recent study of dexpramipexole, an experimental drug that nearly completely depletes all eosinophils from within the blood and nasal polyp tissue, failed to show any significant symptomatic improvement or reduction in nasal polyp size in patients with CRSwNP (5). This ‘negative’ study suggests that although the tissue eosinophilia in most patients with CRSwNP and AERD is substantial, eosinophils are not the main effector cells that drive ongoing inflammation in the disease.
Mast cell-derived mediators, epithelial barrier dysfunction, and locally produced antibodies are also thought to contribute to tissue inflammation in CRSwNP. Activated tissue mast cells play an important role in AERD pathophysiology, with ongoing release of inflammatory mediators including cysteinyl leukotrienes and prostaglandin D2 (PGD2) (6–8). PGD2 can then amplify respiratory inflammation by binding the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) receptor, expressed on eosinophils, basophils, type 2 innate lymphoid cells, and Th2 cells. Defects in epithelial barrier integrity and epithelial tight junction expression have been noted in CRSwNP and AERD, leading to increased permeability and compromised host defense responses within the upper airway (9, 10). Further, elevated levels of several antibody classes have been noted within nasal polyp tissue (11). We recently found elevated levels of local IgE within the nasal polyps in AERD and described a role for local nasal tissue IgE in relationship to the rapidity of nasal polyp regrowth. This study also identified elevated interleukin (IL)-5Rα transcript and surface expression in plasma cells from subjects with AERD, and found that IL-5 signaling on plasma cells may play a role in facilitating their survival (12). A more complete understanding of the role of IL-5/IL-5Rα signaling in inflammatory disorders has both mechanistic and therapeutic implications.
Humanized monoclonal antibodies against IL-5 or IL-5Rα demonstrate efficacy in the treatment of eosinophilic asthma and studies suggest improvement in some patients with nasal polyposis (13, 14). A Phase 2 trial of IL-5 inhibition with mepolizumab in patients with CRSwNP showed that 60% of the subjects in the mepolizumab treatment arm experienced a therapeutic response with a corresponding decrease in total polyp score (15) and we reported that mepolizumab can improve both upper and lower airway symptoms in some subjects with AERD (16). While the mechanism of response in the subset of patients for whom mepolizumab is efficacious has been attributed to the effects of inhibiting IL-5 on eosinophils, IL-5Rα is expressed on many relevant cells including mast cells, basophils, B cells, plasma cells, some T cells, and ciliated epithelial cells (17–20). Further, it was recently shown that human airway epithelial cells express functional IL-5Rα, and that IL-5 signaling leads to downregulation of adhesion molecules, suggesting that IL-5 may reduce strength of the epithelial barrier through weakening cell-to-cell adhesions (19).
Given our recent findings that IL-5Rα expression is increased in nasal polyp cells from subjects with AERD (12), and the negative results of the clinical trial of eosinophil depletion with dexpramipexole in CRSwNP, we suspect that targeting IL-5 or IL-5Rα may work through multiple cellular mechanisms, in addition to inhibiting eosinophils. In this study, we sought to explore the effect of treatment with mepolizumab, a monoclonal antibody targeting IL-5, on mast cell activation, antibody and eicosanoid production, and nasal epithelial transcript expression in subjects with AERD.
Methods:
Patient characterization
Subjects between the ages of 18 and 75 years were recruited from the Brigham and Women’s Hospital (Boston, MA) Allergy and Immunology Clinics between October 2018 and October 2019 (Table 1). The Mass General Brigham Institutional Review Board approved the study and all subjects provided written informed consent. Patients with AERD all had asthma, nasal polyposis, and a diagnosis of AERD confirmed via a physician-observed graded oral challenge to aspirin which induced objectively-defined upper and/or lower respiratory symptoms including nasal congestion, rhinorrhea, sneezing, ocular pruritus, conjunctival injection, wheezing, dyspnea, and/or fall in FEV1.
TABLE 1.
Patient Characteristics.
| Mepolizumab (N = 18) | Control (N = 18) | P-value | |
|---|---|---|---|
| Age (Mean ± SEM) | 53.9 ± 3.1 | 47.2 ± 2.7 | 0.11@ |
| Gender (% Female) | 50% | 61% | 0.74# |
| FEV1 % Predicted (Mean ± SEM) | 74.8 ± 3.9 | 83.7 ± 3.5 | 0.10@ |
| SNOT-22 Score (Mean ± SEM) | 34.7 ± 5.1 | 38.3 ± 6.3 | 0.66@ |
| ACQ-6 (Mean ± SEM) | 1.0 ± 0.2 | 1.1 ± 0.3 | 0.70@ |
| Daily aspirin use (% on aspirin) | 33% | 22% | 0.71# |
| Zileuton (% on zileuton) | 28% | 11% | 0.40# |
| Budesonide sinonasal irrigation use (% on budesonide irrigations) | 89% | 83% | >0.99# |
| Months of mepolizumab use (Mean, range) | 15.9, 5 – 32 | N/A |
t test
Fisher’s exact test
SEM: Standard error of the mean
Subjects with AERD who met clinical criteria for the FDA-approved use of monthly mepolizumab (100 mg subcutaneously) for treatment of severe, uncontrolled, eosinophilic asthma and who had been on treatment with mepolizumab for at least three months were recruited. Subjects had been started on mepolizumab by their pulmonologist or allergist/immunologist as part of their usual clinical care. Subjects on mepolizumab were compared to an age, sex, and disease severity-matched control population of AERD patients who elected not to use mepolizumab either due to insurance rejection or to patient desire to avoid use of a biologic agent. Subjects were excluded if they were on any other biologic therapy within six months of enrollment.
Clinical Procedures
All subjects had a single study visit at Brigham and Women’s Hospital. Biological specimens including urine, blood, nasal fluid, and nasal cells from the inferior turbinate were collected, as were clinical parameters, FEV1, and patient-reported outcome measures including sinonasal outcome test (SNOT)-22, and Asthma Control Questionnaire-6 (ACQ-6) scores.
Specimen Procurement
Peripheral blood was drawn into heparinized tubes and processed or assayed within 1 hour of collection. Serum was obtained from the top layer after a 15-minute centrifuge at 1500g at 4°C. Nasal secretions were sampled separately from both nostrils with Nasosorption™ FX-R (Hunt Developments Ltd, Midhurst, UK), a non-invasive upper airway sampling method that uses a synthetic absorptive matrix to collect nasal mucosal lining fluid directly from the nasal mucosal surface. Nasal secretions were placed in either 300 μL of 0.5% BSA (two samples from one nostril) or 300 μL of 100% methanol (from the other nostril) and were stored in 75 μL, 150 μL, or 200 μL aliquots at −80°C until analysis (21). Nasal epithelial tissue was collected from the inferior turbinate using the Rhino-Pro Curette, a sterile, disposable, mucosal collection device, as described (22). One sample was taken from the right or left mid-inferior portion of the inferior turbinate using a gentle scraping motion and was placed directly in RNAprotect Tissue Reagent (Qiagen, Germantown, MD). Urine was collected and stored at −80°C until further analysis.
Flow Cytometry
Peripheral blood was kept at room temperature and 50 μL of whole blood was used per staining condition. Red blood cells were lysed within 30 minutes of staining, and cells were fixed in 1% paraformaldehyde. Eosinophils were identified as CD45+/CCR3+ cells within the granulocyte gate on FSC/SSC; basophils were identified as CD45low/CCR3+ cells within the SSClow/lymphocyte gate; neutrophils were identified as CD45+/CD16+ cells within the SSChigh/FSChigh granulocyte gate (see Supplemental Figure E1 for gating strategy). Surface CRTH2 expression was measured on eosinophils and basophils as compared to an isotype control. All antibodies were commercially available and from either BioLegend (San Diego, CA) or BD Biosciences (San Jose, CA), the specific details of vendor, clone and fluorophore are included in Supplemental Table E1. As cells were stained immediately after collection, no live-dead stain was used.
Mediator Quantification
Nasal secretions and serum were measured for levels of total IgG (Invitrogen, Waltham MA), IgA (Invitrogen), IgE (Invitrogen for serum, Abcam, Cambridge, MA for nasal secretions) and IgG4 (eBioscience, San Diego CA) by ELISA, according to the manufacturer’s instructions. Serum was further analyzed for IL-5 by ELISA (R&D Systems, Minneapolis, MN) and for total tryptase at Virginia Commonwealth University. Nasal secretions were further analyzed for eosinophilic cationic protein (ECP) by ELISA (Lifespan Biosciences, Seattle, WA). Urinary eicosanoids (Vanderbilt University) and nasal eicosanoids (University of California San Diego) were measured by using gas chromatography-mass spectrometry (23).
Inferior Nasal Turbinate Epithelial Cell Bulk RNA-sequencing
For bulk RNA sequencing, epithelial cells were sampled from the inferior turbinate via nasal curettage as described above. RNA was normalized to 10 ng as the input amount for a 2.2X SPRI ratio cleanup using Agencourt RNAClean XP beads (Beckman Coulter, A63987). After oligo-dT priming, Maxima H Minus Reverse Transcriptase (ThermoFisher EP0753) was used to synthesize cDNA with an elongation step at 52 °C before PCR amplification (15 cycles) using KAPA HiFi PCR Mastermix (Kapa Biosystems KK2602). Sequencing libraries were prepared using the Nextera XT DNA tagmentation kit (Illumina FC-131-1096) with 250 pg input for each sample. Libraries were pooled post-Nextera and cleaned using Agencourt AMPure SPRI beads with successive 0.7X and 0.8X ratio SPRIs and sequenced with an Illumina 75 Cycle NextSeq500/550v2.5 kit (Illumina FC-404-2005) with loading density at 2.2 pM, with paired end 35 cycle read structure. Samples were sequenced at an average read depth of 9.7 million reads per sample. Samples were aligned to the Hg19 genome and transcriptome using STAR and RSEM (24, 25). Samples with alignment rate <20% were not further analyzed. After concatenating read counts for technical replicates, differential expression analysis was conducted using the DESeq2 package for R taking patient origin into account (26). Genes with Benjamini-Hochberg adjusted p-values corresponding to a false discovery rate (FDR) <0.05 were regarded as differentially expressed. Pre-ranked genes with unadjusted P-value ≤0.05 were used for enrichment analysis based on gene ontology (GO Biological Process) and KEGG pathways, using GEne SeT AnaLysis Toolkit (27). Expression levels of genes enriched for tight junction pathway and hierarchical clustering analysis was performed with the R tool pheatmap (Version 1.0.12).
See the article’s Online Repository for additional details regarding single-cell RNA-sequencing analysis of surgically excised sinus tissue to identify sinus tissue cells expressing IL5RA.
Statistical Analysis
Data are expressed as mean ± standard error of the mean unless otherwise noted. Two-sided unpaired Student’s t test, Mann-Whitney test, and Fisher’s exact test assessed differences in patients with AERD on mepolizumab versus controls. Correlation between biomarkers was assessed using Pearson correlation coefficient. Analysis was performed using GraphPad Prism version 7.0d (GraphPad, La Jolla, CA).
Results:
Study population and demographics
Eighteen subjects with physician-diagnosed AERD and severe, uncontrolled, eosinophilic asthma on mepolizumab as an add-on asthma maintenance therapy and 18 subjects with AERD not on mepolizumab participated in the study (Table 1). There were no statistically significant differences in age, gender, FEV1, ACQ-6 or SNOT-22 between groups, although there was a trend toward lower FEV1 in the mepolizumab group. There were no differences in high-dose daily aspirin therapy and zileuton (a 5-lipoxygenase inhibitor) use between groups; no patients were on oral corticosteroids. Sixteen of 18 patients in the mepolizumab group and 15 of 18 patients in the control group used nasal budesonide irrigations.
Blood eosinophil and basophil levels are reduced, and their CRTH2 expression increased, in subjects on mepolizumab
Peripheral blood eosinophils and basophils, reported as a percentage of CD45+ cells, were lower in the patients with AERD treated with mepolizumab compared to controls (Figure 1 A and B, P < 0.0001 and P = 0.0083, respectively). There was no difference in numbers of neutrophils, measured as a percentage of CD45+ cells (Figure 1 C).
Figure 1. Peripheral blood granulocyte levels and their CRTH2 expression in subjects on mepolizumab compared to controls.
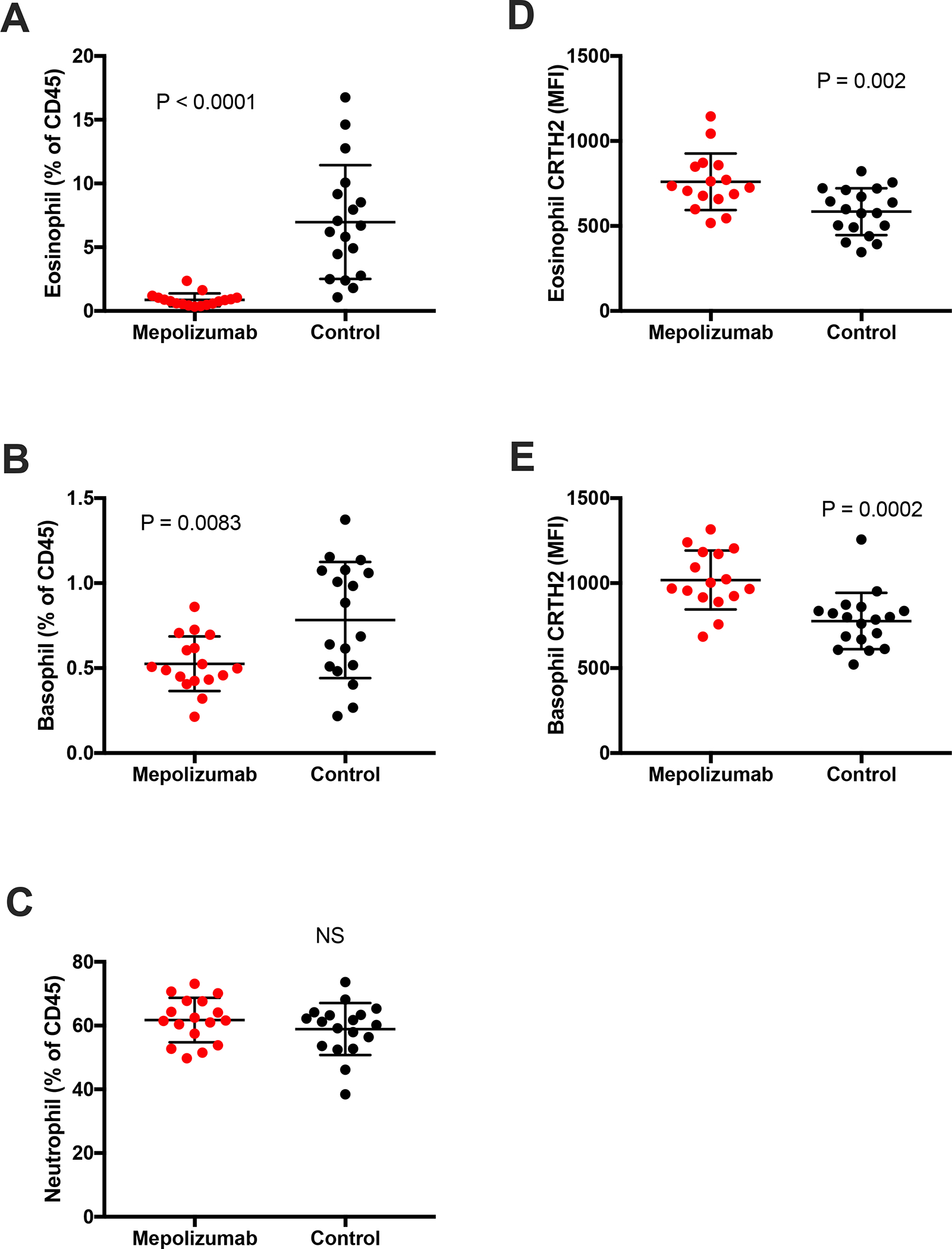
Circulating eosinophil (A), basophil (B) and neutrophil (C) levels were measured by flow cytometry and expressed as a percentage of all CD45+ cells in AERD subjects treated (Mepolizumab) or not (Control) with mepolizumab. Surface CRTH2 expression of eosinophils (D) and basophils (E) was calculated for the same subjects, expressed as Median Fluorescence Intensity (MFI) of CRTH2 with isotype subtracted.
The surface expression of CRTH2 on both blood eosinophils and basophils, calculated as a median fluorescence intensity, was higher in subjects treated with mepolizumab compared to controls (Figure 1 D and E, P = 0.002 and 0.0002, respectively).
Effects of mepolizumab treatment on nasal and urinary eicosanoid levels
Nasal fluid levels of PGF2α metabolites (PGF2α + tetranor PGFM) were significantly decreased in subjects with AERD treated with mepolizumab, compared to controls (Figure 2B). Although not statistically significant, nasal levels of the PGD2 metabolite DHKPGD2 were on average lower in subjects treated with mepolizumab, and the 8 highest DHKPGD2 levels were all in untreated subjects (Figure 2A). Both nasal thromboxane B2 (TXB2) and LTB4 levels were also decreased in mepolizumab-treated patients (Figure 2C and 2D, P=0.002 and P=0.03, respectively). Urinary levels of tetranor PGDM trended to be lower in subjects on mepolizumab, and urinary levels of LTE4 were significantly decreased (Figure 2E and F). Further, the nasal levels of both DHKPGD2 and the PGF2α metabolites were inversely correlated with surface CRTH2 expression of both circulating eosinophils and basophils (Figure 3A–D).
Figure 2. Nasal and urinary eicosanoid levels in subjects on mepolizumab compared to controls.
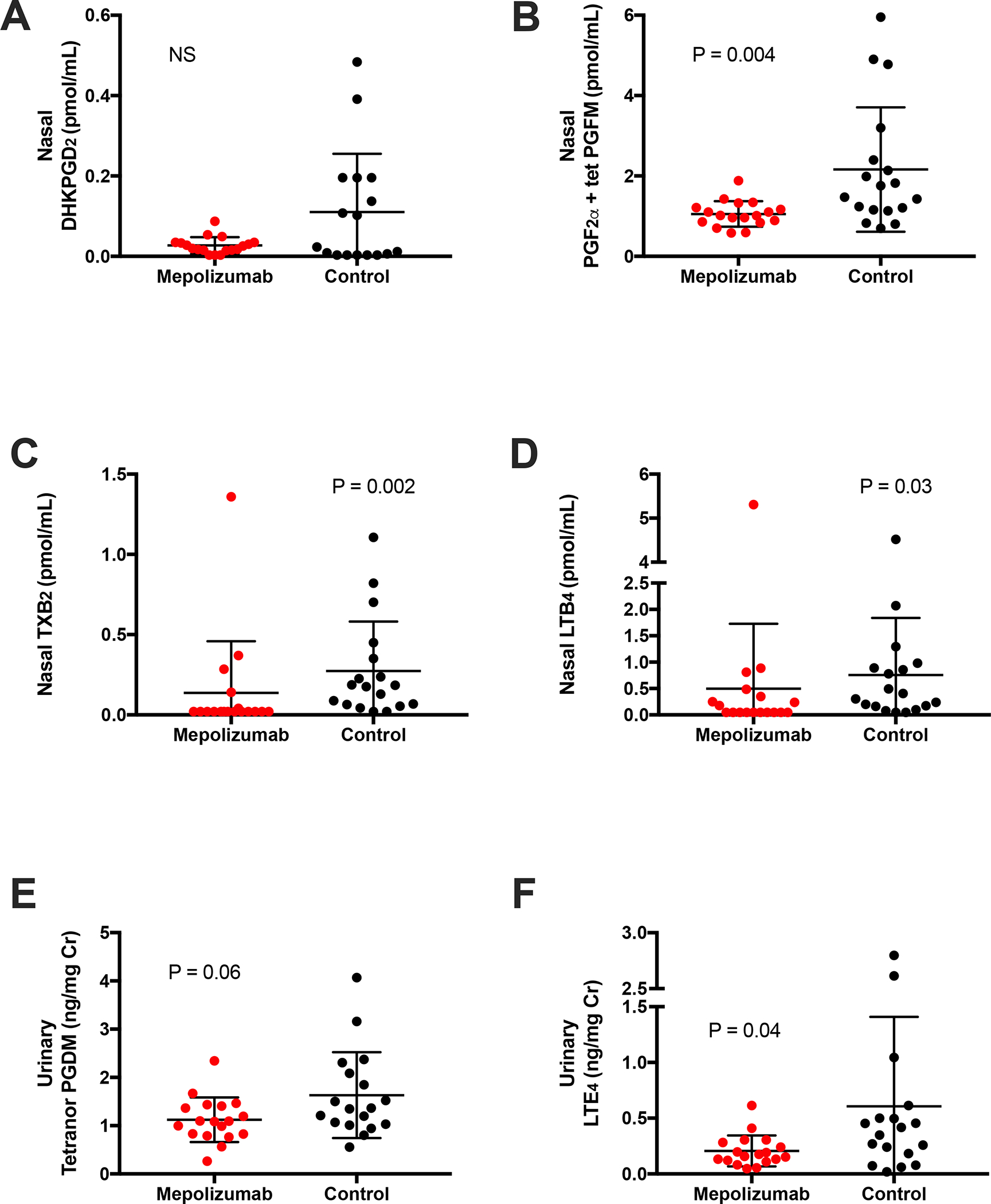
Nasal fluid levels of (A) DHKPGD2 (B) PGF2α + tetranor PGFM (C) TXB2 and (D) LTB4. Urinary levels of (E) tetranor PGDM and (F) LTE4.
Figure 3. Relationship of nasal eicosanoids to granulocyte expression of CRTH2 in subjects on mepolizumab and controls.
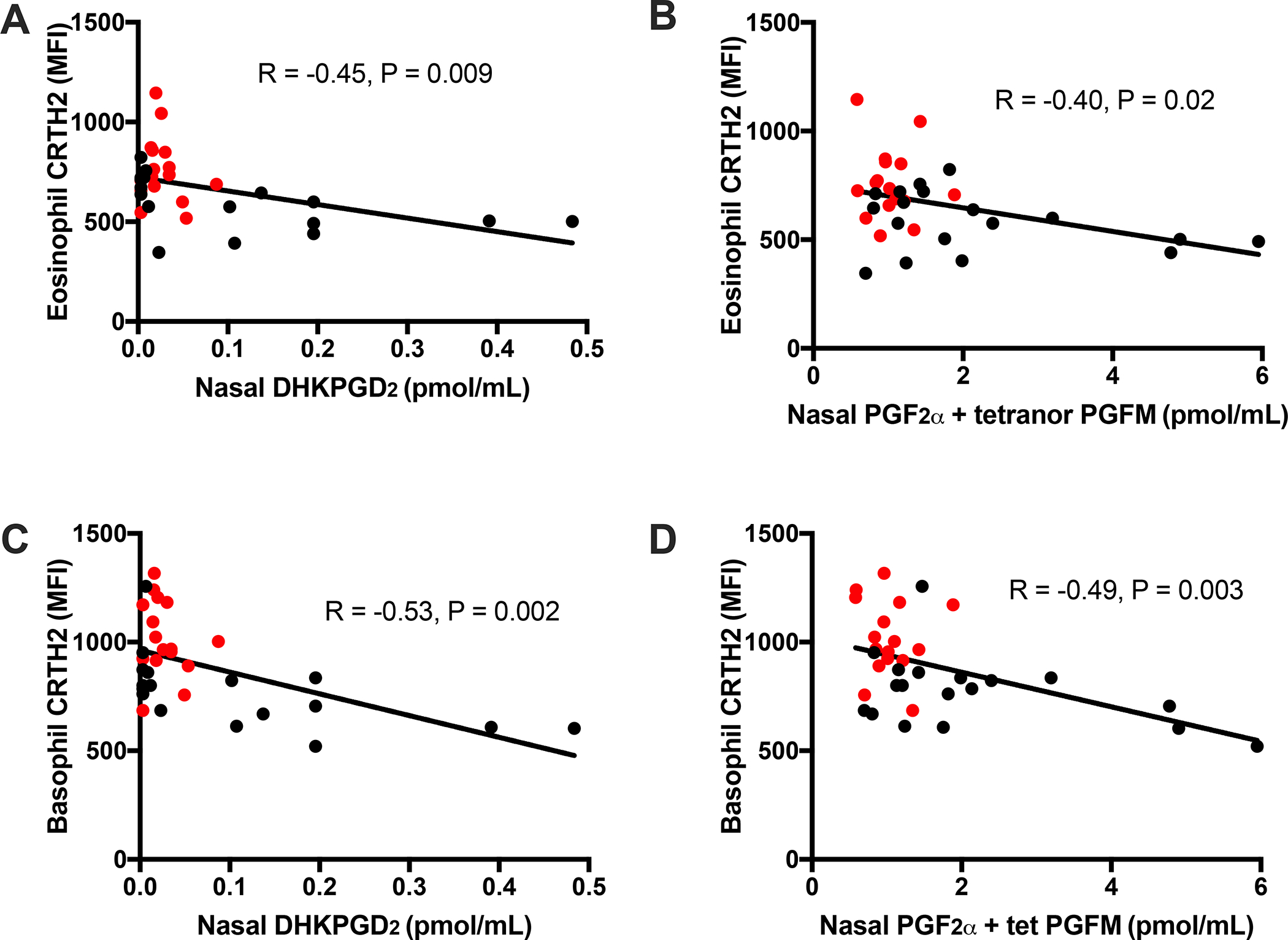
Correlation of nasal DHKPGD2 with surface CRTH2 expression of eosinophils (A) and basophils (B). Correlation of PGF2α + tetranor PGFM with surface CRTH2 expression of eosinophils (C) and basophils (D). Subjects treated with mepolizumab represented by red circles, controls represented by black circles.
We did not detect differences in nasal PGE2, nasal tetranor PGEM, and urinary PGEM between subjects treated with mepolizumab and controls (data not shown). There were no differences in nasal or urinary eicosanoid levels in the patients on daily high-dose aspirin therapy vs not on daily aspirin in the mepolizumab group or the control group.
Nasal eosinophilic cationic protein and serum IL-5 levels
Nasal fluid ECP levels did not differ between patients on and off mepolizumab (2316 pg/mL ±729 and 3171 pg/mL ±1193, respectively; P=0.71, data not shown). Serum IL-5 levels were significantly higher in subjects treated with mepolizumab than in those not on mepolizumab (34.1 pg/mL ±33.3 and 2.3 pg/mL ±3.1, respectively; P < 0.0001, data not shown).
Nasal and serum antibody levels and tryptase are unchanged in subjects on mepolizumab
There was no difference in levels of IgE and IgG4 in the nasal secretions or serum of patients on or off mepolizumab (Figure 4 A – D). Serum tryptase levels also did not differ between patients on and off mepolizumab (5.6 ng/mL ±0.6, and 6.5 ng/mL ±0.8, respectively; P=0.26). We could not detect nasal tryptase levels in an adequate number of subjects in each group to compare nasal tryptase levels between groups.
Figure 4. IgE and IgG4 levels in subjects on mepolizumab compared to controls.
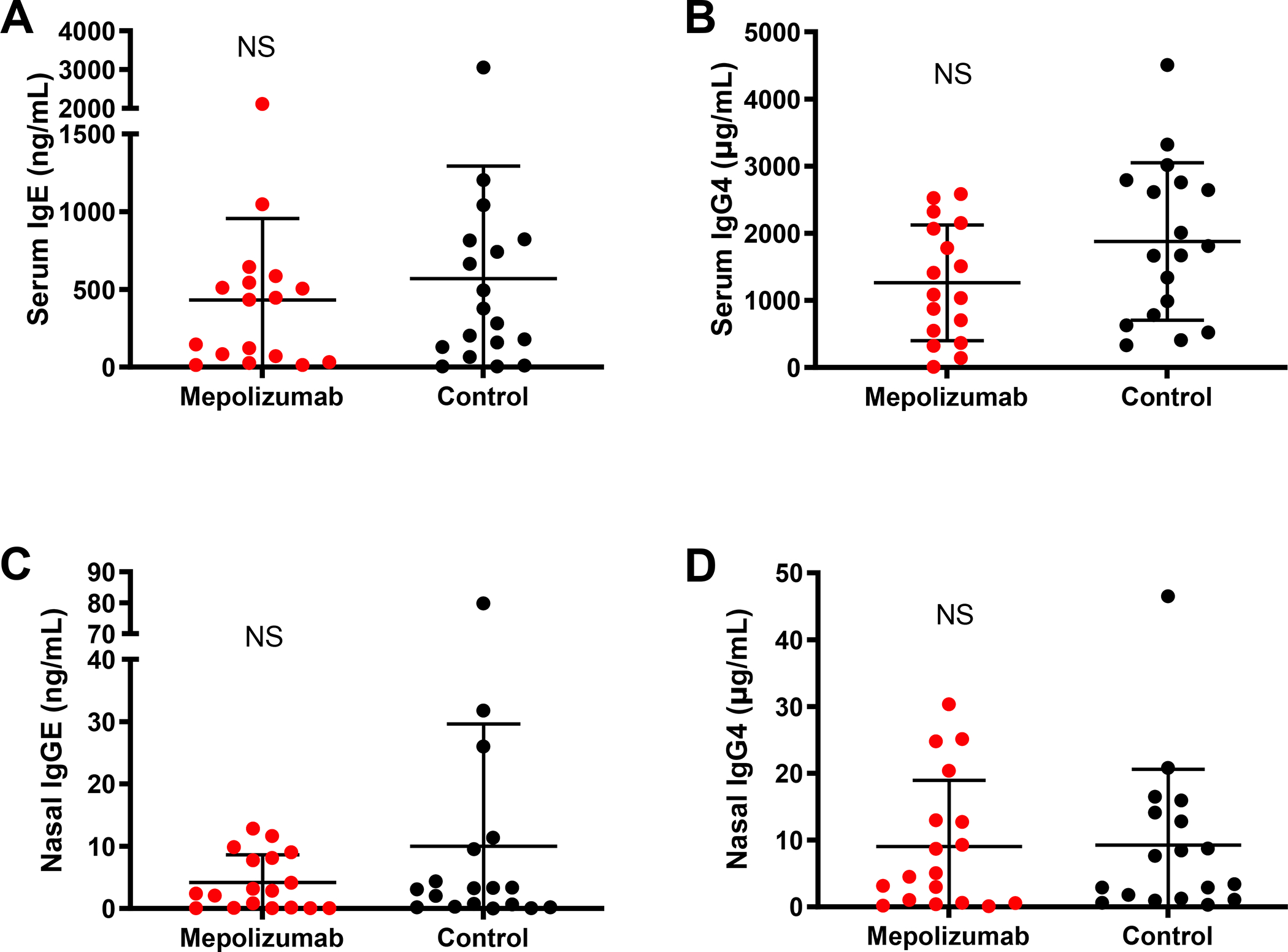
Serum (A-B) and nasal fluid (C-D) levels of IgE and IgG4 in AERD subjects treated (Mepolizumab, red circles) or not (Control, black circles) with mepolizumab.
Inferior Turbinate RNA-sequencing
We utilized a previously generated single-cell RNA-seq (scRNA-seq) dataset to identify sinus tissue cells that express IL5RA and found the highest expression of IL5RA in sinus tissue plasma cells, ciliated epithelial cells, and mast cells (Figure 5A). Analysis of the inferior turbinate scraping samples (14 from subjects treated with mepolizumab and 11 from patients not on treatment had sufficient quality for inclusion) revealed that 242 genes were differentially regulated, including 94 upregulated genes and 148 downregulated genes in subjects treated with mepolizumab, that passed the false discovery rate with a Padj value of <0.05 (Table E2). Based on prior scRNA-seq, we recover T cells, eosinophils, mast cells, neutrophils, myeloid cells, and basal, secretory and ciliated epithelial cells from inferior turbinate scrapings. Approximately 75% of cells recovered from the inferior turbinate scrapings are epithelial cells (28). We enriched these DEGs in the KEGG database and noted that the “Tight Junction hsa04530” pathway was enriched. GO biological process enrichment analysis additionally revealed induction of GO term GO cilium organization in mepolizmab treated group. Out of 169 genes from the Tight Junction gene set, 19 were present in our DEGs; of these 19, 15 were upregulated and 4 were downregulated. Upregulated genes included TJP3 (tight junction protein 3) (29), ACTN4 (actinin-4 protein, involved in tight junction assembly in epithelial cells) (30), and AMOT (angiomotin, part of a tight-junction-associated protein complex) (31). However, several genes involved in tight-junction formation, such as CLDN17 (Claudin 17) (32), were also downregulated (Figure 5B).
Figure 5. Sinus tissue single-cell RNA-sequencing and hierarchical clustering analysis of nasal inferior turbinate scraping transcriptomic changes in mepolizumab-treated patients.
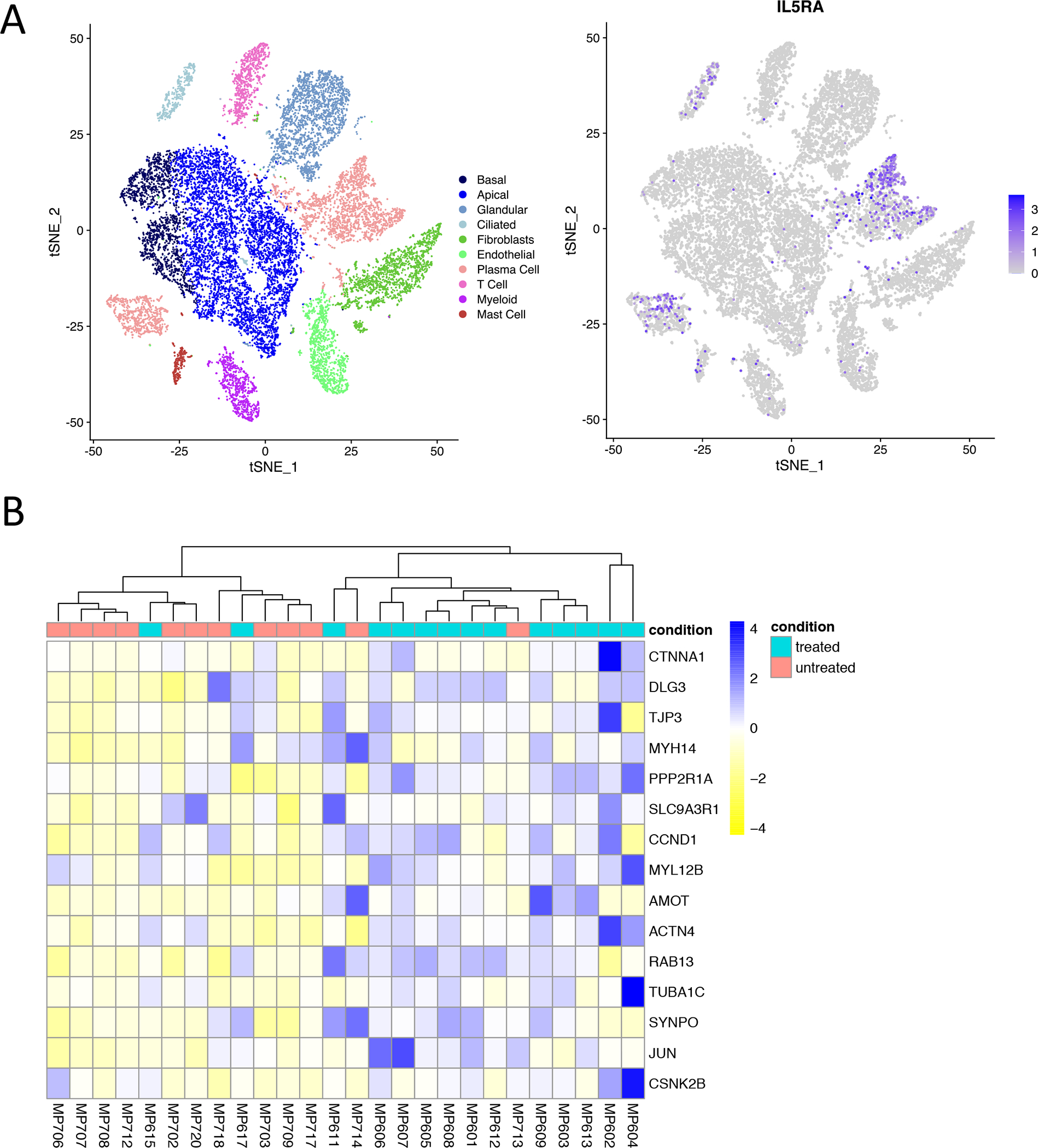
(A) T-stochastic neighbor embedding (t-SNE) plot of 18,036 surgically excised sinus tissue cells from subjects with AERD (n=3 samples), CRSwNP (n=3 samples), and CRS without nasal polyps (n=5 samples) colored by cell type (left) and IL5RA expression (right). (B) Unsupervised hierarchical clustering analysis of tight junction-related genes shows clustering of mepolizumab-treated subjects and untreated control subjects, with induction of tight junction related genes in mepolizumab-treated group (row-normalized gene expression values), P < 0.05 for all genes.
Discussion:
Overall our results comparing differences in a variety of inflammatory mediators and cellular readouts in AERD patients show that IL-5 inhibition induces a wide array of disease-relevant immunologic changes. Although the effects of IL-5 inhibition on eosinophils are important, we suspect that many of the mepolizumab-induced differences found in this study are due to the consequences of decreased IL-5 signaling on other immune cells, including mast cells, basophils, and epithelial cells within the respiratory system.
Considering the role of IL-5 on both eosinophils and basophils, the mepolizumab-induced decrease in circulating eosinophils we found is expected, and the reduction in basophils follows as well (33). However, although eosinophilic infiltration into both the upper and lower respiratory tissues is a hallmark of AERD, complete pharmacologic depletion of tissue eosinophils does not provide therapeutic benefit – therefore, other effector cells must be playing a key role in this disease (5). Nasal fluid ECP correlates strongly with nasal eosinophilia, and thus can serve as a surrogate biomarker for local eosinophil numbers (34). As was shown in the Phase 2 study of mepolizumab in CRSwNP (14), we too found that nasal ECP was not significantly decreased in the mepolizumab-treated patients compared to the untreated patients. The lack of a mepolizumab-induced decrease in nasal ECP suggests that nasal eosinophil numbers may not be dramatically altered by the treatment, though most subjects with CRSwNP who are treated with mepolizumab experience a therapeutic response and a decrease in polyp burden (14). Given this, we suspect that the mechanisms by which mepolizumab provides therapeutic improvement for the responding subset of patients with CRSwNP is largely unrelated to a decrease in nasal polyp eosinophils.
A decrease in PGD2, LTE4, and PGF2α (Figure 2, A, B, E, F) may well underlie some of the mechanism of benefit afforded by mepolizumab. These three eicosanoids are all known to be pro-inflammatory in AERD (6, 23, 35). Elevated tissue levels of PGD2 in AERD can lead to both nasal edema through vasodilation, mediated through the DP1 receptor (36), and activation and recruitment of eosinophils, basophils, and ILC2s, mediated through the DP2/CRTH2 receptor (37, 38). LTE4, the end-product of cysteinyl leukotriene metabolism is a major mediator of both the chronic disease in AERD, and also of the acute aspirin-induced reactions (39, 40). PGF2α has been less thoroughly studied in AERD, though its levels do rise during aspirin-induced reactions (41), and PGF2α can induce bronchoconstriction and bronchial hyperreactivity (42, 43). Furthermore, PGF2α, like PGD2, is a CRTH2 agonist (44). As both PGD2 and PGF2α are full agonists of the CRTH2 receptor and can lead to activation, mobilization, and degranulation of eosinophils and basophils (44–46), their reduction would allow for decreased activation of these granulocytes. Further corroborating this is our finding that the circulating eosinophils and basophils remaining in the blood of patients treated with mepolizumab had significantly higher surface expression of CRTH2 (Figure 1, D and E). The likely explanation for this novel finding is that CRTH2 stimulation by either PGD2 or PGF2α leads to receptor internalization and reduced surface expression; upon removing the eicosanoid stimuli, an increase in CRTH2 expression would be expected (46, 47). Nasal polyp tissue mast cells also express CRTH2, and CRTH2 signaling on mast cells may lead to intracellular calcium mobilization and cellular migration, suggesting that decreased local levels of PGD2 may also lead to less mast cell activation and accumulation (48, 49).
The mechanism by which mepolizumab reduces PGD2, LTE4, and PGF2α is likely through direct inhibition of IL-5Rα signaling on eosinophils, basophils, and mast cells. PGD2 and LTE4 are produced by eosinophils, basophils, and mast cells (50–54). PGF2α is produced by eosinophils and mast cells (41, 55, 56), and possibly by basophils and respiratory epithelial cells as well (57–59). For all three of the granulocytes, production of LTE4 is upregulated following stimulation with IL-5 (50, 53, 54, 60), suggesting that inhibition of IL-5 signaling with mepolizumab would decrease cellular release of LTE4. Although IL-5 stimulation has not been directly linked to increased release of PGD2, there is cross-talk between the stimulatory roles of PGD2 and cysteinyl leukotrienes; as cysteinyl leukotrienes can stimulate PGD2 production, mepolizumab-induced decreases in LTE4 may in turn reduce granulocyte production of PGD2 (41, 61). IL-5 stimulation of human bronchial epithelial cells also leads to upregulation of the enzymes required to make PGF2α, suggesting that mepolizumab-induced inhibition of that pathway could decrease PGF2α release from the respiratory epithelium (19).
Two additional eicosanoids, TXB2 and LTB4, were also lower in the nasal fluid of the patients treated with mepolizumab compared to those off treatment, indicating a broad effect of IL-5 inhibition on eicosanoid metabolism (Figure 2 C and D). A decrease in local TXB2 may be of particular therapeutic importance in AERD, as platelet activation and platelet-dependent inflammation play a role in the chronic respiratory inflammation and the acute aspirin-induced reactions (62, 63). Although the direct effect of IL-5 on TXB2 production by immune cells is not known, both sinus tissue mast cells and eosinophils have the capacity to produce it (64). The inflammatory role of LTB4 in respiratory inflammation and asthma has also been well documented (65), with high levels of LTB4 also noted within nasal polyp tissue (66). Neutrophils are a primary source of LTB4, and although human lung neutrophils do have functional IL-5Rα (67), it is not known whether IL-5 stimulation of neutrophils affects their LTB4 release. Both eosinophils and basophils can also produce LTB4 (68), and IL-5 priming of rat basophilic leukemia-1 cells does increase their production of LTB4 (69), suggesting a mechanism by which IL-5 inhibition could lead to decreased local LTB4 in the sinuses.
Our finding of variable mepolizumab-induced differential expression of tight-junction-related transcripts in the inferior turbinate scrapings is of unclear clinical significance. A number of the transcripts found to be upregulated in patients on mepolizumab, including ACTN4 and AMOT, were also noted to be downregulated in human bronchial epithelial cells stimulated ex vivo with IL-5 (19). Therefore, the epithelial cell transcript differences noted in our study could indeed be a result of in vivo inhibition of IL-5. The variability of the differences between subjects, and the finding that some tight-junction associated transcripts were actually downregulated on mepolizumab, suggests that these changes are unlikely to be the sole driving mechanism that underlies the therapeutic benefit of mepolizumab experienced by patients with CRSwNP. Additionally, we see that ciliated epithelial cells express IL-5Rα (Figure 5A) and there is enrichment of genes related to cilium organization in the mepolizumab treated group, suggesting that inhibition of IL-5 may also impact ciliated epithelial cells in the nasal tissue.
The treatment-induced increase in serum IL-5 found in this study is consistent with the known effects of antil-IL-5 treatment (70). The serum IL-5 detected during treatment with mepolizumab may be part of a bound immunoglobulin complex that prolongs the half-life of IL-5, leading to detection of increased levels (71). One major limitation of this study is that the case-control design captured clinical and mechanistic data from only a single visit, without any longitudinal data available to gauge each patient’s response to mepolizumab. Therefore, the treatment-related immunologic differences seen in this study are presumed to be directly mediated by mepolizumab, but without repeat measures, this cannot be fully confirmed. Additionally, we are not able to determine the extent of clinical response to mepolizumab or relate any of the mepolizumab-related immunologic differences to a responder/non-responder analysis. Despite these shortcomings, our findings clearly show that there are immunologic changes that occur following treatment with mepolizumab, and which extend beyond just the predicted effects of IL-5 inhibition on eosinophils. We conclude that IL-5 inhibition with mepolizumab in patients with AERD leads to decreased production of relevant inflammatory eicosanoids, including PGD2, PGF2α, and cysteinyl leukotrienes, and upregulation of nasal epithelial cell transcripts involved in tight junction pathways and cilium organization. These changes are likely due to the combined effects of decreased IL-5 signaling on local respiratory tissue eosinophils, basophils, mast cells, and epithelial cells, all of which have functional IL-5Rα.
Supplementary Material
Key Messages:
Subjects with AERD treated with mepolizumab had decreased production of inflammatory eicosanoids, and upregulation of nasal epithelial cell transcripts involved in tight junction pathways and cilium organization, compared to AERD patients not treated with mepolizumab.
These effects of mepolizumab are likely due to decreased signaling of IL-5 on local respiratory tissue eosinophils, basophils, mast cells, and epithelial cells.
The mechanism by which IL-5 inhibition provides therapeutic benefit in respiratory inflammation is not due exclusively to anti-eosinophil effects
Funding:
This work was supported by GlaxoSmithKline, the National Institutes of Health (NIH grants U19AI095219, K23AI139352, R01HL128241), and by generous contributions from the Vinik and Kaye Families. J.O.M is a New York Stem Cell Foundation – Robertson Investigator. J.O.M was supported by the Richard and Susan Smith Family Foundation, the HHMI Damon Runyon Cancer Research Foundation Fellowship (DRG-2274-16), the AGA Research Foundation’s AGA-Takeda Pharmaceuticals Research Scholar Award in IBD – AGA2020-13-01, the Food Allergy Science Initiative, and The New York Stem Cell Foundation. A.K.S. was support by the Beckman Young Investigator Program, a Sloan Fellowship in Chemistry, and NIH 5U24AI118672.
Abbreviations:
- ACQ-6
Asthma Control Questionnaire-6
- AERD
aspirin-exacerbated respiratory disease
- CRSwNP
chronic rhinosinusitis with nasal polyps
- IL
interleukin
- ILC2
type 2 innate lymphoid cells
- LT
leukotriene
- PG
prostaglandin
- SNOT
Sinonasal Outcome Test
- CRTH2
chemoattractant receptor-homologous molecule expressed on Th2 cells
- TXB2
thromboxane B2
Footnotes
Conflict of Interest: T Laidlaw has served on scientific advisory boards for GlaxoSmithKline and Sanofi-Genzyme, Optinose, and Regeneron. K Buchheit has served on scientific advisory boards for AstraZeneca and GlaxoSmithKline. J Bensko has served on scientific advisory boards for GlaxoSmithKline. J Ordovas-Montanes reports compensation for consulting services with Cellarity and Hovione. A Shalek reports compensation for consulting and/or SAB membership from Merck, Honeycomb Biotechnologies, Cellarity, Repertoire Immune Medicines, Hovione, Third Rock Ventures, Ochre Bio, Relation Therapeutics, and Dahlia Biosciences. A Shalek has received research support from Merck, Novartis, Leo Pharma, Janssen, the Bill and Melinda Gates Foundation, the Moore Foundation, the Pew-Stewart Trust, Fondation MIT, the Chan Zuckerberg Initiative, Novo Nordisk and the FDA unrelated to this work. The rest of the authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Bhattacharyya N Assessing the additional disease burden of polyps in chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2009;118(3):185–9. [DOI] [PubMed] [Google Scholar]
- 2.Campbell AP, Phillips KM, Hoehle LP, Feng AL, Bergmark RW, Caradonna DS, et al. Depression symptoms and lost productivity in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2017;118(3):286–9. [DOI] [PubMed] [Google Scholar]
- 3.McMains KC, Kountakis SE. Medical and surgical considerations in patients with Samter’s triad. American journal of rhinology. 2006;20(6):573–6. [DOI] [PubMed] [Google Scholar]
- 4.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. Am J Respir Crit Care Med. 2015;192(6):682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laidlaw TM, Prussin C, Panettieri RA, Lee S, Ferguson BJ, Adappa ND, et al. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope. 2019;129(2):E61–E6. [DOI] [PubMed] [Google Scholar]
- 6.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015;135(1):245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kowalski ML, Sliwinska-Kowalska M, Igarashi Y, White MV, Wojciechowska B, Brayton P, et al. Nasal secretions in response to acetylsalicylic acid. J Allergy Clin Immunol. 1993;91(2):580–98. [DOI] [PubMed] [Google Scholar]
- 8.Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. J Allergy Clin Immunol. 2003;111(4):743–9. [DOI] [PubMed] [Google Scholar]
- 9.Tieu DD, Kern RC, Schleimer RP. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. The Journal of allergy and clinical immunology. 2009;124(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. The Journal of allergy and clinical immunology. 2012;130(5):1087–96 e10. [DOI] [PubMed] [Google Scholar]
- 11.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131(4):1075–83, 83 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchheit KM, Dwyer DF, Ordovas-Montanes J, Katz HR, Lewis E, Vukovic M, et al. IL-5Ralpha marks nasal polyp IgG4- and IgE-expressing cells in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2020;145(6):1574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. The New England journal of medicine. 2009;360(10):973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. The Journal of allergy and clinical immunology. 2011;128(5):989–95 e1–8. [DOI] [PubMed] [Google Scholar]
- 15.Bachert C, Sousa AR, Lund VJ, Scadding GK, Gevaert P, Nasser S, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J Allergy Clin Immunol. 2017;140(4):1024–31 e14. [DOI] [PubMed] [Google Scholar]
- 16.Tuttle KL, Buchheit KM, Laidlaw TM, Cahill KN. A retrospective analysis of mepolizumab in subjects with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takatsu K Interleukin-5 and IL-5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(8):463–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takatsu K Interleukin 5 and B cell differentiation. Cytokine Growth Factor Rev. 1998;9(1):25–35. [DOI] [PubMed] [Google Scholar]
- 19.Barretto KT, Brockman-Schneider RA, Kuipers I, Basnet S, Bochkov YA, Altman MC, et al. Human airway epithelial cells express a functional IL-5 receptor. Allergy. 2020;75(8):2127–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochi H, De Jesus NH, Hsieh FH, Austen KF, Boyce JA. IL-4 and −5 prime human mast cells for different profiles of IgE-dependent cytokine production. Proc Natl Acad Sci U S A. 2000;97(19):10509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thwaites RS, Jarvis HC, Singh N, Jha A, Pritchard A, Fan H, et al. Absorption of Nasal and Bronchial Fluids: Precision Sampling of the Human Respiratory Mucosa and Laboratory Processing of Samples. J Vis Exp. 2018(131). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders SP, Siekierski ES, Richards SM, Porter JD, Imani F, Proud D. Rhinovirus infection induces expression of type 2 nitric oxide synthase in human respiratory epithelial cells in vitro and in vivo. The Journal of allergy and clinical immunology. 2001;107(2):235–43. [DOI] [PubMed] [Google Scholar]
- 23.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119(16):3790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019;47(W1):W199–W205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ordovas-Montanes J, Dwyer DF, Nyquist SK, Buchheit KM, Vukovic M, Deb C, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560(7720):649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol. 2000;11(4):315–24. [DOI] [PubMed] [Google Scholar]
- 30.Nakatsuji H, Nishimura N, Yamamura R, Kanayama HO, Sasaki T. Involvement of actinin-4 in the recruitment of JRAB/MICAL-L2 to cell-cell junctions and the formation of functional tight junctions. Mol Cell Biol. 2008;28(10):3324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, An J, Zhang P, Xu C, Gao K, Wu D, et al. The Nedd4-like ubiquitin E3 ligases target angiomotin/p130 to ubiquitin-dependent degradation. Biochem J. 2012;444(2):279–89. [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Feng L, Cui J. Increased expression of claudin-17 promotes a malignant phenotype in hepatocyte via Tyk2/Stat3 signaling and is associated with poor prognosis in patients with hepatocellular carcinoma. Diagn Pathol. 2018;13(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright AKA, Diver S, McCarthy J, Marvin A, Soares M, Thornton T, et al. Mepolizumab does not alter the blood basophil count in severe asthma. Allergy. 2019;74(12):2488–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasp G, Thomas PA, Bujia J. Eosinophil inflammation of the nasal mucosa in allergic and non-allergic rhinitis measured by eosinophil cationic protein levels in native nasal fluid and serum. Clin Exp Allergy. 1994;24(12):1151–6. [DOI] [PubMed] [Google Scholar]
- 35.Ban GY, Cho K, Kim SH, Yoon MK, Kim JH, Lee HY, et al. Metabolomic analysis identifies potential diagnostic biomarkers for aspirin-exacerbated respiratory disease. Clin Exp Allergy. 2017;47(1):37–47. [DOI] [PubMed] [Google Scholar]
- 36.Cheng K, Wu TJ, Wu KK, Sturino C, Metters K, Gottesdiener K, et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci U S A. 2006;103(17):6682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. The Journal of allergy and clinical immunology. 2014;133(4):1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193(2):255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christie PE, Tagari P, Ford-Hutchinson AW, Charlesson S, Chee P, Arm JP, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143(5 Pt 1):1025–9. [DOI] [PubMed] [Google Scholar]
- 40.Sestini P, Armetti L, Gambaro G, Pieroni MG, Refini RM, Sala A, et al. Inhaled PGE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthma. Am J Respir Crit Care Med. 1996;153(2):572–5. [DOI] [PubMed] [Google Scholar]
- 41.Lazarinis N, Bood J, Gomez C, Kolmert J, Lantz AS, Gyllfors P, et al. Leukotriene E4 induces airflow obstruction and mast cell activation through the cysteinyl leukotriene type 1 receptor. The Journal of allergy and clinical immunology. 2018;142(4):1080–9. [DOI] [PubMed] [Google Scholar]
- 42.Mathe AA, Hedqvist P, Holmgren A, Svanborg N. Bronchial hyperreactivity to prostaglandin F 2 and histamine in patients with asthma. Br Med J. 1973;1(5847):193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith AP, Cuthbert MF, Dunlop LS. Effects of inhaled prostaglandins E1, E2, and F2alpha on the airway resistance of healthy and asthmatic man. Clin Sci Mol Med. 1975;48(5):421–30. [DOI] [PubMed] [Google Scholar]
- 44.Sandig H, Andrew D, Barnes AA, Sabroe I, Pease J. 9alpha,11beta-PGF2 and its stereoisomer PGF2alpha are novel agonists of the chemoattractant receptor, CRTH2. FEBS Lett. 2006;580(2):373–9. [DOI] [PubMed] [Google Scholar]
- 45.Peinhaupt M, Sturm EM, Heinemann A. Prostaglandins and Their Receptors in Eosinophil Function and As Therapeutic Targets. Front Med (Lausanne). 2017;4:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshimura-Uchiyama C, Iikura M, Yamaguchi M, Nagase H, Ishii A, Matsushima K, et al. Differential modulation of human basophil functions through prostaglandin D2 receptors DP and chemoattractant receptor-homologous molecule expressed on Th2 cells/DP2. Clin Exp Allergy. 2004;34(8):1283–90. [DOI] [PubMed] [Google Scholar]
- 47.Hamada K, Yamada Y, Kamada Y, Ueki S, Yamaguchi K, Oyamada H, et al. Prostaglandin D2 and Interleukin-5 Reduce Crth2 Surface Expression on Human Eosinophils. Allergology International. 2004;53(2):179–84. [Google Scholar]
- 48.Boehme SA, Franz-Bacon K, Chen EP, Ly TW, Kawakami Y, Bacon KB. Murine bone marrow-derived mast cells express chemoattractant receptor-homologous molecule expressed on T-helper class 2 cells (CRTh2). Int Immunol. 2009;21(6):621–32. [DOI] [PubMed] [Google Scholar]
- 49.Moon TC, Campos-Alberto E, Yoshimura T, Bredo G, Rieger AM, Puttagunta L, et al. Expression of DP2 (CRTh2), a prostaglandin D(2) receptor, in human mast cells. PLoS One. 2014;9(9):e108595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bischoff SC, Brunner T, De Weck AL, Dahinden CA. Interleukin 5 modifies histamine release and leukotriene generation by human basophils in response to diverse agonists. J Exp Med. 1990;172(6):1577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ugajin T, Satoh T, Kanamori T, Aritake K, Urade Y, Yokozeki H. FcepsilonRI, but not FcgammaR, signals induce prostaglandin D2 and E2 production from basophils. Am J Pathol. 2011;179(2):775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luna-Gomes T, Magalhaes KG, Mesquita-Santos FP, Bakker-Abreu I, Samico RF, Molinaro R, et al. Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation. J Immunol. 2011;187(12):6518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kajita T, Yui Y, Mita H, Taniguchi N, Saito H, Mishima T, et al. Release of leukotriene C4 from human eosinophils and its relation to the cell density. Int Arch Allergy Appl Immunol. 1985;78(4):406–10. [DOI] [PubMed] [Google Scholar]
- 54.Hsieh FH, Lam BK, Penrose JF, Austen KF, Boyce JA. T helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells: profound induction of leukotriene C(4) synthase expression by interleukin 4. J Exp Med. 2001;193(1):123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kroegel C, Matthys H. Platelet-activating factor-induced human eosinophil activation. Generation and release of cyclo-oxygenase metabolites in human blood eosinophils from asthmatics. Immunology. 1993;78(2):279–85. [PMC free article] [PubMed] [Google Scholar]
- 56.Schleimer RP, Schulman ES, MacGlashan DW Jr., Peters SP, Hayes EC, Adams GK 3rd, et al. Effects of dexamethasone on mediator release from human lung fragments and purified human lung mast cells. J Clin Invest. 1983;71(6):1830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monaco G, Lee B, Xu W, Mustafah S, Hwang YY, Carre C, et al. RNA-Seq Signatures Normalized by mRNA Abundance Allow Absolute Deconvolution of Human Immune Cell Types. Cell Rep. 2019;26(6):1627–40 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.The Human Protein Atlas; AKR1C3 Blood Atlas. 2020. [Available from: https://www.proteinatlas.org/ENSG00000196139-AKR1C3/blood.
- 59.The Human Protein Atlas; AKR1C3 Cell Atlas. 2020. [Available from: https://www.proteinatlas.org/ENSG00000196139-AKR1C3/cell.
- 60.Rothenberg ME, Petersen J, Stevens RL, Silberstein DS, McKenzie DT, Austen KF, et al. IL-5-dependent conversion of normodense human eosinophils to the hypodense phenotype uses 3T3 fibroblasts for enhanced viability, accelerated hypodensity, and sustained antibody-dependent cytotoxicity. J Immunol. 1989;143(7):2311–6. [PubMed] [Google Scholar]
- 61.Mesquita-Santos FP, Vieira-de-Abreu A, Calheiros AS, Figueiredo IH, Castro-Faria-Neto HC, Weller PF, et al. Cutting edge: prostaglandin D2 enhances leukotriene C4 synthesis by eosinophils during allergic inflammation: synergistic in vivo role of endogenous eotaxin. J Immunol. 2006;176(3):1326–30. [DOI] [PubMed] [Google Scholar]
- 62.Laidlaw TM, Boyce JA. Platelets in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015;135(6):1407–14; quiz 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc Natl Acad Sci U S A. 2013;110(42):16987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mita H, Ishii T, Akiyama K. Generation of thromboxane A2 from highly purified human sinus mast cells after immunological stimulation. Prostaglandins Leukot Essent Fatty Acids. 1999;60(3):175–80. [DOI] [PubMed] [Google Scholar]
- 65.Busse WW. Leukotrienes and inflammation. Am J Respir Crit Care Med. 1998;157(6 Pt 1):S210–3. [PubMed] [Google Scholar]
- 66.Pinto S, Gallo O, Polli G, Boccuzzi S, Paniccia R, Brunelli T, et al. Cyclooxygenase and lipoxygenase metabolite generation in nasal polyps. Prostaglandins Leukot Essent Fatty Acids. 1997;57(6):533–7. [DOI] [PubMed] [Google Scholar]
- 67.Gorski SA, Lawrence MG, Hinkelman A, Spano MM, Steinke JW, Borish L, et al. Expression of IL-5 receptor alpha by murine and human lung neutrophils. PLoS One. 2019;14(8):e0221113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pal K, Feng X, Steinke JW, Burdick MD, Shim YM, Sung SS, et al. Leukotriene A4 Hydrolase Activation and Leukotriene B4 Production by Eosinophils in Severe Asthma. Am J Respir Cell Mol Biol. 2019;60(4):413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsumoto S, Hamasaki Y, Ichimaru T, Miyazaki S. IL-3 and IL-5 enhance the production of LTB4 stimulated by calcium ionophore in rat basophilic leukemia cells. Prostaglandins Leukot Essent Fatty Acids. 1995;52(6):417–22. [DOI] [PubMed] [Google Scholar]
- 70.Tsukamoto N, Takahashi N, Itoh H, Pouliquen I. Pharmacokinetics and pharmacodynamics of mepolizumab, an anti-interleukin 5 monoclonal antibody, in healthy Japanese male subjects. Clin Pharmacol Drug Dev. 2016;5(2):102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skrgat S, Sušanj PG, Stojkovič UB, Korošec P. Increase in systemic IL-5 is associated with mepolizumab treatment failure in patients with severe asthma. Eur Respir J. 2018;52:1132. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


