Abstract
Trichoderma harzianum biotypes Th1, Th2, and Th3 produced volatile metabolites in vitro which had similar fungistatic effects on the growth of Agaricus bisporus. Metabolites present in agar colonized by these strains also inhibited mycelial growth of A. bisporus, although the reduction in growth was less in the presence of metabolites produced by biotype Th2 than that in the presence of metabolites produced by Th1 or Th3. A. bisporus produced metabolites in liquid culture that inhibited the growth of Th1 and Th3 but stimulated the growth of Th2. A compound(s) responsible for the inhibition and stimulation was extracted from A. bisporus culture filtrate and from compost-grown fruit bodies with n-butanol, but the identity of the compound(s) was not determined. We suggest that the stimulation of Th2 by metabolites produced by A. bisporus and the relatively low level of inhibition of A. bisporus by Th2 facilitate colonization of compost by both fungi. However, as compost colonization reaches a maximum, a change in the competitive balance in favor of Th2 results in the inhibition of fruit body production by A. bisporus and the devastating green mold epidemics affecting mushroom production.
One of three biological forms of Trichoderma harzianum Rifai, group 2 described by Muthumeenakshi et al. (12) and designated biotype Th2 in this paper, is the main agent responsible for green mold epidemics affecting the commercial mushroom (Agaricus bisporus) in the British Isles (13, 17). Two other forms of T. harzianum, biotypes Th1 and Th3, also have been identified but are excluded during compost pasteurization (13). A fourth T. harzianum biotype, which is similar to but genetically distinct from biotype Th2 and was designated group 4 by Muthumeenakshi et al. (11), has caused serious compost infestation in North America (15).
Of the three T. harzianum biotypes found in the British Isles, biotype Th2, although rarely isolated from compost raw materials, once introduced, often on infected spawn, colonizes compost effectively (13, 14). During severe green mold epidemics, when no mushrooms are produced, T. harzianum Th2 predominates in affected compost. In contrast, biotypes Th1 and Th3, which are commonly found in compost raw materials but are infrequently found in pasteurized or colonized compost, rarely cause problems during mushroom production. If introduced, however, Th1 and Th3 will colonize a restricted area of compost from which A. bisporus is excluded (13). The relative abilities of the three biotypes of T. harzianum to colonize compost in competition with A. bisporus and their influence on A. bisporus growth may be associated with secondary metabolite production. The present study was made to determine, in vitro, the biological activity of compound(s) produced by T. harzianum and A. bisporus, separately, and to determine their possible relationship to green mold disease.
Fungal cultures.
Three strains of A. bisporus (commercial spawn strains 130, 229, and 280), all U3 types, and three isolates each of T. harzianum biotypes Th1, Th2, and Th3 were used in the study. One isolate each of Th1 (IMI 359823), Th2 (IMI 359824), and Th3 (IMI 359825) were deposited in the International Mycological Institute (IMI) culture collection; the other two isolates of each biotype were identical at the molecular level (10) to those deposited. Subsequent to the completion of our study, Th3 was confirmed to be Trichoderma atroviride (11). These Trichoderma isolates have been tested for their aggressiveness in compost trials (16), and the Th2 isolates continue to cause green mold disease.
Data analysis.
Data were subjected to analysis of variance; arc sine transformation was employed for percentage data. Least significant difference (LSD) values are calculated from the standard errors of the means (SEM) × √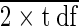 .
.
Volatile T. harzianum metabolites are toxic to A. bisporus.
Trichoderma spp. produce both volatile and nonvolatile metabolites that adversely affect growth of different fungi (1, 3, 5, 6, 9). To determine the effects of volatile metabolites released by the three T. harzianum biotypes on the growth of A. bisporus, discs (4-mm diameter) excised (with a no. 2 cork borer) from the leading edge of a 12-day-old colony of each of the three strains of A. bisporus were placed at the center of 2% malt extract agar (MEA) in petri dishes (90-mm diameter) and incubated for 10 days prior to the interaction study. Discs from three isolates each of T. harzianum biotypes Th1, Th2, and Th3, excised from the leading edge of cultures grown on potato dextrose agar (Oxoid, Basingstoke, United Kingdom), were placed on 2% MEA immediately before the interaction took place. The petri dish lids were removed, and a plate containing A. bisporus was placed over a plate containing T. harzianum. The two plates were held together with adhesive tape. For each strain of A. bisporus, three replicate plates for each T. harzianum isolate and two sets of controls were used. One control set comprised A. bisporus cultures paired with plates of uninoculated MEA, and the second set comprised A. bisporus paired with Rhizoctonia solani which was inoculated and grown on 2% MEA in the same manner as T. harzianum. R. solani was included as a control since Hutchinson and Cowan (7) found that growth of Aspergillus niger, Pestalotia rhododendri, and several bacteria in the headspace gases from cultures of a strain of T. harzianum could be accounted for solely by the presence of CO2 and ethanol produced by the T. harzianum cultures. R. solani has a growth rate similar to T. harzianum and presumably releases similar levels of CO2. All treatment and control plates were randomized and incubated at 25°C in the dark.
Colony size was measured daily for 4 days after pairing of the cultures. We found that, in the presence of T. harzianum, A. bisporus mycelial growth ceased rapidly. The volatile compound(s) produced by Th1, Th2, and Th3 significantly reduced the radial growth of all three A. bisporus strains (P < 0.05) from day 2 onward (Table 1). The mean effects of volatiles produced by the three T. harzianum biotypes over the 4-day incubation period were not significantly different. The reduced suppression of A. bisporus growth in the R. solani controls (Table 1) suggests that CO2 alone is unlikely to be responsible for the observed level of inhibition of A. bisporus by T. harzianum. When A. bisporus from these pairings was transferred to fresh 2% MEA, it regrew after 2 to 3 days from inoculation, demonstrating that the effect of the volatiles was fungistatic.
TABLE 1.
Growth of A. bisporus strains in the presence of volatiles released by T. harzianum biotypes Th1, Th2, and Th3
| Treatment | Radial growth (mm per day) of A. bisporus strains (130, 229, and 280) on daya
|
Mean daily radial growth | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
3
|
4
|
||||||||||
| 130 | 229 | 280 | 130 | 229 | 280 | 130 | 229 | 280 | 130 | 229 | 280 | ||
| Th1 | 1.32 | 3.77 | 1.06 | 0.80 | 0.89 | 0.37 | 0.35 | 0.08 | 0.0 | 0.0 | 0.0 | 0.0 | 0.50 |
| Th2 | 1.46 | 0.92 | 0.89 | 1.05 | 0.39 | 0.47 | 0.69 | 0.04 | 0.18 | 0.0 | 0.0 | 0.0 | 0.52 |
| Th3 | 1.42 | 1.01 | 0.71 | 0.83 | 0.46 | 0.29 | 0.16 | 0.12 | 0.0 | 0.0 | 0.0 | 0.0 | 0.42 |
| R. solani | 1.68 | 1.29 | 1.10 | 1.36 | 0.93 | 0.88 | 1.03 | 0.31 | 0.74 | 1.01 | 0.30 | 0.62 | 0.92 |
| Uninoculated control (SEM [df, 10])b | 1.96 (0.174, NS) | 1.06 (0.28, NS) | 1.39 (0.10**) | 1.85 (0.0189*) | 1.08 (0.15*) | 1.20 (0.10**) | 1.46 (0.023*) | 1.08 (0.06*) | 1.18 (0.06**) | 1.37 (0.025**) | 1.03 (0.05**) | 1.17 (0.09**) | 1.36 (0.09**) |
Data are the averages for three replicate sets.
NS denotes no significant difference, ∗ indicates significant difference at P level of <0.05, and ∗∗ indicates significant difference at P level of <0.01. LSD is SEM × √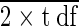 .
.
Diffusible T. harzianum metabolites are toxic to A. bisporus.
To assess the toxic effects on A. bisporus of substances released by the T. harzianum strains in the agar, we overlaid 2% MEA with a sterilized cellophane membrane (Courtauld Films; 50-μm thick). The plates were left overnight to allow excess water to evaporate. A disc (4-mm diameter) excised (with a no. 2 cork borer) from the leading edge of an actively growing T. harzianum culture was placed on the cellophane at the center of each agar plate and incubated at 25°C in darkness for 2 days. The cellophane and adhering fungus were removed, and a disc (4-mm diameter) of A. bisporus, excised from the leading edge of a 12-day-old culture, was placed at the center of the plate. The plates were randomized and incubated at 25°C for 12 days, and the diameters of the A. bisporus cultures were measured. Three replicate plates were established for each treatment, and the experiment was repeated twice.
All three T. harzianum strains released metabolites that diffused through the cellophane membrane into the agar medium and inhibited the growth of A. bisporus. The growth of A. bisporus was suppressed by more than 90% by metabolites secreted by Th1 and Th3 (Table 2), while significantly (P < 0.05) less suppression of the growth occurred in the presence of metabolites secreted by Th2 (Table 2).
TABLE 2.
Growth of A. bisporus strains 12 days after inoculation onto agar containing diffusible substances produced by T. harzianum biotypes Th1, Th2 and Th3
| Treatment | Radial growth (mm in 12 days) of A. bisporus straina
|
||
|---|---|---|---|
| 130 | 229 | 280 | |
| Th1 | 0.24 | 0.18 | 0.18 |
| Th2 | 6.96 | 7.70 | 8.34 |
| Th3 | 0.16 | 0.38 | 0.90 |
| Controlb | 12.1 | 14.2 | 13.9 |
The data are the averages for three replicate sets.
A. bisporus was grown on fresh 2% MEA. The SEM (df, 18) for the results for A. bisporus multiplied by the SEM for the results for T. harzianum was 1.356, which revealed a significant difference at a P level of <0.05; LSD is SEM × 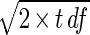 .
.
No attempt was made to characterize either the volatile or agar-diffusible substances, and these experiments are not sufficient to prove that both nonvolatile and volatile inhibitors were produced by T. harzianum. For example, it is possible that a substance could have been partitioned between the airspace and the agar and have been present in the agar at concentrations sufficient to inhibit A. bisporus. Thus, while the three T. harzianum biotypes may produce different substances with varying toxicities to A. bisporus, our results also could be explained if Th2 consistently produced less of a single marginally volatile substance than did Th1 and Th3.
Metabolites produced by A. bisporus.
The ability of the slow-growing mycelium of A. bisporus to colonize and dominate a nonsterile mushroom compost has prompted speculation that it may produce antibiotic(s). Certain metabolites of A. bisporus have been isolated, and antibiotic potential has been attributed to their derivative or analogues (2). We assessed the effect of a metabolite(s) produced by A. bisporus in fruit bodies and in liquid culture on the growth of T. harzianum.
A. bisporus mycelia from 7-day-old stationary liquid cultures (each, 25 ml) in yeast extract-glucose medium (YEG medium) (containing 0.1 g of yeast extract, 10 g of glucose, 0.14 g of sodium glutamate, 0.2 g of potassium chloride, 0.2 g of magnesium sulfate, and 0.2 g of calcium chloride per 1 liter of distilled water) were transferred to 100 ml of fresh YEG medium in 250-ml conical flasks and incubated in an orbital incubator (40 rpm, 25°C). Culture filtrate (100 ml) was removed from different flasks each day (on days 9, 12, 15, 18, and 21) and filter sterilized (0.2-μm-pore-size filters), and 20 ml from each of three replicate flasks was stored at −20°C until used. The effect of the culture filtrates on the growth of T. harzianum was assessed by cutting a well (6-mm diameter) with a no. 3 cork borer at the center of 2% MEA (25 ml) in petri dishes (90-mm diameter). A. bisporus culture filtrate (150 μl) was pipetted into each well and immediately covered by a disc (8-mm diameter, mycelium-side down) cut with a no. 4 cork borer from the leading edge of an actively growing T. harzianum colony. Three replicate plates were established for each treatment. The plates were then placed in an incubator at 25°C. Filter-sterilized, uninoculated medium (150 μl) was used in control plates. Growth radii were measured twice at right angles, 24 h after inoculation. The experiment was repeated twice, and all treated plates were compared with the controls to calculate the percentage stimulation or inhibition of growth of T. harzianum.
Filtrates of 9-day-old cultures of A. bisporus suppressed the growth of T. harzianum biotypes Th1 and Th3 by 6% and 5%, respectively, but stimulated the growth of biotype Th2 by ca. 7%. These results varied little with the strain or age of the culture (data not shown).
A water-soluble component(s) was extracted with n-butanol from the culture filtrate and from A. bisporus fruit bodies as follows. A. bisporus (strain 280) was grown in YEG medium as described above for 12 days. Pooled culture filtrate (200 ml) was clarified by vacuum filtration through Whatman no. 3 paper and extracted three times with 200 ml of n-butanol. The n-butanol extracts (600 ml) were pooled, dried over anhydrous sodium sulfate, and evaporated in vacuo almost to dryness. Three replicate sets of culture filtrates were extracted in this manner. Mushroom caps (100 g), which had just begun to open, were homogenized (Waring blender) in 200 ml of methanol. The methanol extract was clarified by filtration through Whatman no. 3 paper, and the methanol was vacuum evaporated. The aqueous residue was extracted three times with 50 ml of n-butanol each time. The pooled n-butanol extract was dried over anhydrous sodium sulfate and then vacuum evaporated to dryness.
To determine the biological activity of the n-butanol extracts, the residues were dissolved, each in 10 ml of sterile distilled water, and from these solutions a 1:5 dilution series was made to give 2 × 10−1, 4 × 10−2, 8 × 10−3, 1.6 × 10−3, 3.2 × 10−4, 6.4 × 10−5, and 1.3 × 10−5 dilutions of the extracts. Aqueous dilutions of the n-butanol extracts were pipetted into wells cut in agar plates and bioassayed with isolates of Th1, Th2, and Th3. Sterile distilled water was used in control plates. Three replicate plates were used for each treatment; the plates were placed in an incubator at 25°C in a randomized manner. The experiment was repeated three times, and all treated plates were compared with the controls to calculate the percentage of inhibition or stimulation of the T. harzianum biotypes.
The more-concentrated solutions of the extracts from both the culture filtrates and the fruit bodies suppressed growth of all three T. harzianum biotypes. As the extracts were diluted, the growth of Th1 and Th3 continued to be suppressed while that of Th2 was enhanced (Table 3). No investigations have been made to determine the chemical composition of the n-butanol extract or the properties of the components. However, as n-butanol will dissolve a number of water-soluble compounds from biological material, the extract is unlikely to have been homogeneous. We do not know, therefore, if the same compound(s) inhibited Th1 and Th3 as stimulated Th2. The fact that the active compound(s) was extracted from the fruit bodies does, however, suggest that it is a constituent component of the fungus. Phenylhydrazines (4, 8) and phenolic compounds (18) with antibiotic activity have been isolated from fruit bodies of A. bisporus, but their extraction or synthesis for bioassay against T. harzianum was beyond the scope of this study.
TABLE 3.
Effects of compounds extracted with n-butanol from liquid cultures and fruit bodies of A. bisporus on the growth of T. harzianum biotypes
| Extract source | Dilution series | Mean % inhibition (−) or stimulation (+) of growth (actual radial growth [mm/24 h]) of T. harzianum biotypea
|
||
|---|---|---|---|---|
| Th1 | Th2 | Th3 | ||
| 12-day-old liquid culturesbd | 1 | −11.8 (19.3) | −11.2 (17.8) | −14.6 (15.8) |
| 2 | −6.8 (20.4) | −2.9 (19.4) | −7.0 (17.2) | |
| 3 | −4.8 (20.8) | −0.1 (20.0) | −3.4 (17.9) | |
| 4 | −4.2 (21.0) | +6.8 (21.4) | −3.0 (17.9) | |
| 5 | −1.1 (21.70) | +11.0 (22.2) | −4.8 (17.6) | |
| Control | (21.9) | (20.0) | (18.5) | |
| Fruit bodiescd | 1 | −20.6 (16.8) | −8.8 (17.8) | −29.8 (12.8) |
| 2 | −9.9 (19.1) | −2.0 (19.1) | −17.2 (15.2) | |
| 3 | −6.0 (19.9) | +0.7 (19.6) | −5.7 (17.3) | |
| 4 | −5.9 (19.9) | +6.0 (20.7) | −8.3 (16.8) | |
| 5 | −5.0 (20.1) | +5.4 (20.6) | −3.9 (17.6) | |
| Control | (21.2) | (19.5) | (18.3) | |
Inhibition and stimulation values were calculated as percentages of the growth of T. harzianum on control plates.
SEM (df, 46) for T. harzianum was 0.76, and that for extract dilutions was 1.25. Dilution 1 denotes n-butanol extract of 200 ml of 12-day-old A. bisporus liquid culture redissolved in 10 ml of distilled water; dilutions 2, 3, 4, and 5 were sequential 1:5 dilutions of dilution 1.
The SEM (df, 46) for T. harzianum was 0.82, and that for extract dilutions was 1.46. Dilution 1 denotes n-butanol extract from 100 g of mushroom fruit bodies redissolved in 10 ml of distilled water; dilutions 2, 3, 4, and 5 were sequential 1:5 dilutions of dilution 1.
There was a significant difference at a P value of <0.01 for percent inhibition or stimulation for T. harzianum and that for the extract dilutions for both the liquid cultures and the fruit bodies. LSD is 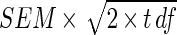 .
.
T. harzianum biotype Th2, as the causal agent of green mold disease, might have been expected to produce the greatest toxic effect on A. bisporus. Our study, however, has shown that this is not so and that greater levels of toxicity were produced by Th1 and Th3. Biotypes Th1 and Th3, which our study also showed to be more strongly inhibited than Th2 by a compound(s) produced by A. bisporus, are usually confined to small localized areas in the compost from which A. bisporus is excluded (13). These findings might suggest mutual inhibition by A. bisporus and Th1 and Th3. The stimulation of Th2 by a compound(s) produced by A. bisporus and the relative tolerance of A. bisporus for toxins produced by Th2 may allow the simultaneous growth of the two fungi in compost. We suggest that this relationship holds until compost colonization reaches a maximum, nutrients become limited, and a change in the competitive balance occurs in favor of Th2. Fruit body production by A. bisporus is then suppressed, and the green mold disease occurs. Our observations suggest that a very specific relationship exists between A. bisporus and Th2, a form of T. harzianum which has not been isolated from anywhere other than mushroom compost (10).
Acknowledgments
We thank Sally Watson for statistical analysis.
A. Mumpuni thanks the British Council for financial support. This study was partially supported by the International Fund for Ireland through the Centre for Innovation in Biotechnology.
REFERENCES
- 1.Bruce A, Austin W J, King B. Control of growth of Lentinus lepideus by volatiles from Trichoderma. Trans Br Mycol Soc. 1984;82:423–428. [Google Scholar]
- 2.Claydon N. Secondary metabolic products of selected agarics. In: Moore D, Casselton L A, Wood D A, Frankland J A, editors. Symposium of the British Mycological Society. Cambridge, England: Cambridge University Press; 1985. [Google Scholar]
- 3.Corley D G, Miller-Wideman M, Durley R C. Isolation and structure of harzianum A: a new trichothecene from Trichoderma harzianum. J Nat Prod. 1994;57:422–425. doi: 10.1021/np50105a019. [DOI] [PubMed] [Google Scholar]
- 4.Daniels E G, Kelly R B, Hinman J W. Agaritine: an improved isolation procedure and confirmation of structure by synthesis. J Am Chem Soc. 1961;83:3333–3334. [Google Scholar]
- 5.Dennis C, Webster J. Antagonistic properties of species-group of Trichoderma. II. Production of volatile antibiotics. Trans Br Mycol Soc. 1971;57:41–48. [Google Scholar]
- 6.Horvarth E M, Burgel J L, Messner K. The production of soluble antifungal metabolites by the biocontrol fungus Trichoderma harzianum in connection with the formation of conidiospores. Mat Org. 1995;29:1–4. [Google Scholar]
- 7.Hutchinson S A, Cowan M E. Identification and biological effects of volatile metabolites from cultures of Trichoderma harzianum. Trans Br Mycol Soc. 1972;59:71–77. [Google Scholar]
- 8.Levenberg B. Structure and enzymatic cleavage of agaritine, a phenylhydrazide of l-glutamic acid isolated from Agaricaceae. J Am Chem Soc. 1961;83:503–504. [Google Scholar]
- 9.Moss M O, Jackson R M, Rodgers D. The characterization of 6-pent-1-enyl)pyrone from Trichoderma viride. Phytochemistry. 1975;14:2706–2708. [Google Scholar]
- 10.Muthumeenakshi S. Molecular taxonomy of the genus Trichoderma. Ph.D. thesis. Belfast, United Kingdom: The Queen’s University of Belfast; 1996. [Google Scholar]
- 11.Muthumeenakshi S, Brown A E, Mills P R. Genetic comparison of the aggressive weed mould strains of Trichoderma harzianum from mushroom compost in North America and the British Isles. Mycol Res. 1998;102:385–390. [Google Scholar]
- 12.Muthumeenakshi S, Mills P R, Brown A E, Seaby D A. Intraspecific molecular variation among Trichoderma harzianum S isolates colonizing mushroom compost in the British Isles. Microbiology. 1994;140:769–777. doi: 10.1099/00221287-140-4-769. [DOI] [PubMed] [Google Scholar]
- 13.Seaby D. Infection of mushroom compost by Trichoderma species. Mushroom J. 1987;179:355–361. [Google Scholar]
- 14.Seaby D. Further observation on Trichoderma. Mushroom J. 1989;197:147–151. [Google Scholar]
- 15.Seaby D. Differentiation of Trichoderma taxa associated with mushroom production. Plant Pathol. 1996;45:905–912. [Google Scholar]
- 16.Sharma, H. S. S., M. Kilpatrick, F. Ward, G. Lyons, and L. Burns. Submitted for publication.
- 17.Staunton L. Trichoderma green mold in mushroom compost. Mushroom J. 1987;179:362–363. [Google Scholar]
- 18.Stussi H, Rast D M. The biosynthesis and possible function of glutaminyl-4-hydroxybenzene in Agaricus bisporus. Phytochemistry. 1981;20:2347–2352. [Google Scholar]


