Abstract
The introduction of targeted therapy has revolutionized cancer treatment. Nonetheless, for this approach to succeed, it is crucial to identify the targets, particularly when activated, in tumor tissues. Phosphorylation is a posttranslational modification that causes activation of numerous oncogenic protein kinases and transcription regulators. Hence, phosphoproteins is a class of biomarkers that has therapeutic and prognostic implications directly relevant to cancer patients’ management. Despite the progress in histopathology methodology, analysis of the expression of phosphoproteins in tumor tissues still represents a challenge owing to preanalytical and analytical factors that include antigen retrieval strategies. In this study, we tested the hypothesis that optimizing antigen retrieval methods will improve phosphoproteins unmasking and enhance their immunohistochemical staining signal. We screened 4 antigen retrieval methods by using antibodies specific for 3 oncogenic phosphoproteins to stain human lymphoma tumors that were developed in SCID mice and subsequently fixed in formalin for 2 years. Then, we used antibodies specific for 15 survival phosphoproteins to compare the most effective method identified from our screening experiment to the antigen retrieval method that is most commonly utilized. Using the antigen retrieval buffer Tris–EDTA at pH 9.0 and heating for 45 minutes at 97°C unmasked and significantly enhanced the staining of 9 of the 15 phosphoproteins (P<0.0001). Our antigen retrieval approach is cost effective and feasible for clinical and research settings. We anticipate that combining this approach with the newly proposed methods to improve tissue fixation will further improve unmasking of phosphoproteins in human and animal tissues.
Keywords: Immunohistochemical staining, formalin fixation, antigen retrieval, phosphoproteins, epitope unmasking
INTRODUCTION
Phosphorylation is a posttranslational modification that occurs in tyrosine, serine, threonine or histidine amino acid residues to induce important functional effects in biologically active proteins. For instance, ligand binding to interleukins, interferons, and growth factors receptors induces phosphorylation of tyrosine residues located within the kinase domains of the receptor-associated Janus kinase (JAK) family of tyrosine kinases; a process that activates JAKs.1–3 Subsequently, phosphorylated/activated JAKs induce the phosphorylation of tyrosine residues located within the SH2 domains of the signal transducers and activators of transcription (STATs) family of proteins causing their activation, which leads to STATs’ translocation to the nucleus where they induce or suppress the transcription of genes that play critical roles in cell survival, apoptosis, proliferation, and cell cycle regulation.4 In contrast, AKT-induced serine/threonine phosphorylation deactivates the Forkhead box O (FOXO) family of transcription factors via sequestration and subsequent degradation in the cytoplasm.5
Using basic and translational experimental approaches, animal and human tissue-based detection of phosphoproteins has served for many years as a valuable tool to dissect signaling pathways and study the contribution of these phosphoproteins to the resistance to experimental therapies. With the advent of clinical trials aimed at identifying novel targeted therapies based on biomarkers that are relevant to specific types of cancer, qualitative and quantitative evaluations of the expression of tissue signaling phosphoproteins has become a valuable approach not only in basic and translational experimental research but also in the clinical laboratory setting. In line with these developments, over the past few years, immunohistochemical (IHC) staining of an increasing number of phosphoproteins has been performed according to the Clinical Laboratory Improvements Amendments (CLIA) approved protocols, and used as a prospective or retrospective biomarker testing in clinical trials.
Formalin is the most widely used fixative in histopathology. Tissues are routinely fixed in 10% neutral-buffered formalin, i.e., 4% formaldehyde solution buffered to a neutral pH, that crosslinks peptides and reacts with nucleotides and unsaturated fatty acids.6,7 Formalin preserves cellular structure and tissues architecture, which allows the use of archival paraffin-embedded tissues for clinical diagnosis and for research purposes as well. Despite that formalin is useful in preservation of morphological detail, a major disadvantage is that it masks antigenic epitopes recognized by antibodies, which is more pronounced with extended fixation times.8,9 For instance, increasing formalin fixation time from 24 to 48 hours decreased significantly Ki-67 staining intensity in colorectal cancer xenografts and clinical leiomyosarcoma samples.10 Heating in citrate buffer at pH 6.0 for 20 min is the most widely used method for antigen retrieval, however, this method often fails to reverse the effects of prolonged formalin fixation.11,12
Studies evaluating staining of phosphoproteins in formalin-fixed and paraffin-embedded (FFPE) tissues are limited. The aim of this study was to identify an optimal, yet practical and cost effective, method to unmask phosphoproteins in FFPE tissues, including tissues that were fixed in formalin for extended periods. To achieve our aim, we tested the hypothesis that optimizing the currently utilized antigen retrieval approach could successfully lead to unmasking of important signaling phosphoproteins in tissues that were fixed in formalin for extended time. Hence, we compared several antigen retrieval methods and identified one that reversed the masking of phosphoproteins more effectively than the conventional method without introducing background staining.
METHODS
Antibodies –
To determine the optimal method of antigen retrieval, we tested the effects of different antigen retrieval methods on IHC of 15 phosphoproteins that are commonly studied because of their contributions to cell survival and/or because of their relevance to the response to investigational therapies (Table 1). Vendor-recommended dilutions were used for all the antibodies.
Table 1.
Antibodies used in the study.
| Antibody | Source | Catalogue Number | Dilution |
|---|---|---|---|
| p-4E-BP1Ser65 | Santa Cruz Biotechnology (Dallas, TX) | sc293124 | 1:500 |
| p-AKTSer473 | Cell Signaling Technology (Danvers, MA) | 4060 | 1:100 |
| p-AxlTyr779 | R&D Systems (Minneapolis, MN) | AF2228 | 1:40 |
| p-cdc25CSer216 | Cell Signaling Technology | 4901 | 1:100 |
| p-c-JunSer73 | Cell Signaling Technology | 3270 | 1:200 |
| p-ERK1/2Thr202/Tyr204 | Cell Signaling Technology | 4370 | 1:400 |
| p-Histone H2A.XSer139 | Cell Signaling Technology | 9718 | 1:480 |
| p-IGF-IRTyr1161 | Abcam (Cambridge, MA) | Ab39398 | 1:75 |
| p-JNKThr183/Tyr185 | Santa Cruz Biotechnology | sc6254 | 1:500 |
| p-MetTyr1234/1235 | Cell Signaling Technology | 3077 | 1:320 |
| p-p70 S6 kinase-αSer434 | Santa Cruz Biotechnology | sc8416 | 1:100 |
| p-RbThr821/826 | Santa Cruz Biotechnology | sc271930 | 1:500 |
| p-SMC1Ser957 | Santa Cruz Biotechnology | sc56746 | 1:100 |
| p-STAT3Tyr705 | Cell Signaling Technology | 9145 | 1:100 |
| p-STAT5Tyr694 | Cell Signaling Technology | 9314 | 1:400 |
Formalin fixation and paraffin-embedding of tissues –
Tissues included in this study were residual xenograft tissues from previously performed basic/translational research projects related to anaplastic lymphoma kinase-positive (ALK+) T-cell anaplastic large-cell lymphoma (ALCL).13 The lymphoma was systemically established in female C.B-17 SCID mice (6–8 weeks old, Taconic, Cambridge City, IN) using Karpas 299 cell line, a commonly utilized human ALK+ T-cell ALCL cell line.14 Studies in mice were approved by our Institutional Animal Care and Use Committee. Systemic lymphoma tumors were collected at necropsy and fixed in 10% neutral-buffered formalin for 2 years. Tumor tissues were then embedded in paraffin using routine methods, and sections of 3.0 μM were mounted on “plus” glass slides.
IHC staining –
Prior to staining, tissues were manually deparaffinized with xylene, rehydrated in graded ethanol, and rinsed in water. Tissues were then subjected to antigen retrieval. The retrieval reagent was Dako Target Retrieval Solution, citrate buffer, pH 6.0 (catalogue number S1699; Agilent, Santa Clara, CA) or Dako Target Retrieval Solution, Tris–EDTA buffer, pH 9.0 (S2367; Agilent). Tissue sections were placed in a steamer with preheated retrieval solution and then maintained at 97°C for either 20 min for the tissues processed in citrate buffer or 45 min for the tissues processed in Tris-EDTA buffer. Tissue sections were left for 20 min to cool down to room temperature, washed, and incubated in 3% H2O2 for 15 min to block endogenous peroxidase activity. Tissue sections were then blocked for 30 min at room temperature in Dako serum-free blocking solution (X0909; Agilent). The primary antibody, diluted in blocking buffer, was added to the tissues, which were incubated overnight at 4°C. Thereafter, tissue sections were washed 3 times in 1× phosphate-buffered saline with Tween20 and incubated for 30 min with the secondary antibody Dako EnVision+ Link System-HRP (K4063, Agilent). Signals were developed using 3,3´-diaminobenzidine tetrachloride substrate and haematoxylin was used for counterstaining. The stains were scored using a 4-tier system: 0, 1+, 2+ or 3+ when there was no detectable stain, or there was a weak, intermediate or strong stain intensity, respectively.
Imaging –
Photomicrographs were captured using an Olympus BX41 microscope (Olympus Scientific Solutions Americas Corp., Waltham, MA), an Infinity 3 camera (Teledyne Lumenera, Ottawa, ON, Canada), and Infinity Capture Mac software (version 6.3.2, Teledyne Lumenera).
Statistical analysis –
Statistical significance between the methods used for antigen retrieval was determined by using a 2-way t-test for paired data (version 8.2.1, Prism 8 for macOS, GraphPad Software, San Diego, CA). P<0.05 was considered statistically significant.
RESULTS
To determine the antigen retrieval buffer and heating duration that are optimal for unmasking phosphoproteins, we first utilized IHC to stain xenograft ALK+ T-cell ALCL tissues using combinations of citrate buffer at pH 6.0 or Tris–EDTA buffer at pH 9.0, and 20 min or 45 min of heating at 97°C. The 3 phosphoproteins p-IGF-IRTyr1161, p-STAT3Tyr705, and p-STAT5Tyr694 that we selected for initial evaluation were known to be highly expressed and play important roles in survival signaling in several types of cancer including ALK+ T-cell ALCL.15–18 Two different specimens were used to ensure reproducibility. IHC staining results (Table 2) showed that heating in Tris–EDTA buffer at pH 9.0 for 45 min was the most effective in unmasking the 3 phosphoproteins, whereas heating in citrate buffer at pH 6.0 for 45 min or in Tris–EDTA buffer at pH 9.0 for 20 min showed moderate immunoreactivity. The weakest immunoreactivity resulted from heating in citrate buffer at pH 6.0 for 20 min. Next, to compare heating in Tris–EDTA buffer at pH 9.0 for 45 min vs. citrate buffer at pH 6.0 for 20 min, we set to stain additional tumor tissues using antibodies specific for 15 phosphoproteins (Table 1).
Table 2.
Staining scores of 3 phosphoproteins using 4 different antigen retrieval methods.
| Antibody | Citrate buffer (pH 6.0 + 20 minutes of heating) | Citrate buffer (pH 6.0 + 45 minutes of heating) | Tris–EDTA buffer (pH 9.0 + 20 minutes of heating) | Tris–EDTA buffer (pH 9.0 + 45 minutes of heating) |
|---|---|---|---|---|
| p-IGF-IRTyr1161 | 1+ | 2+ | 2+ | 3+ |
| p-STAT3Tyr705 | 1+ | 2+ | 2+ | 3+ |
| p-STAT5Tyr694 | 1+ | 2+ | 2+ | 3+ |
Nine of the 15 phosphoproteins (60%) showed improved unmasking after using the Tris–EDTA buffer at pH 9.0 and 45 min of heating vs. the citrate buffer at pH 6.0 and 20 min of heating. Improved unmasking was documented by microscopic evaluation of the stained sections, which demonstrated gains of the IHC staining intensity of the 9 phosphoproteins (Fig. 1). Moreover, this improvement was further established when the intensity of the IHC staining was scored (Fig. 2). Four of these phosphoantibodies – p-IGF-IRTyr1161, p-STAT3Tyr705, p-STAT5Tyr694, and p-SMC1Ser957 – showed weak (1+) IHC with the citrate buffer and strong (3+) IHC with the Tris–EDTA buffer. No IHC was noted for p-4E-BP1Ser65, and p-AxlTyr779 when the citrate buffer was used, but antigen retrieval with the Tris–EDTA buffer unmasked these phosphoproteins and improved the IHC scores to 2+ for p-4E-BP1Ser65 and 1+ for p-AxlTyr779. IHC score for p-p70 S6 kinase-αSer434 and p-JNKThr183/Tyr185 was enhanced from 2+ with the citrate buffer to 3+ with the Tris–EDTA buffer, and IHC score for p-RbThr821/826 was enhanced from 1+ with the Tris–EDTA to 2+ with the Tris–EDTA buffer. Six phosphoproteins –p-AKTSer473, p-cdc25CSer216, p-c-JunSer73, p-ERK1/2Thr202/Tyr204, p-Histone H2A.XSer139, and p-MetTyr1234/1235 – lacked immunoreactivities with the citrate buffer and failed to unmask with the Tris–EDTA buffer. Fig. 3A illustrates that the mean of the 15 phosphoproteins IHC scores significantly increased from 0.6 ± 0.2 (mean ± SE) when the citrate buffer at pH 6.0 and heating for 20 min were used to 1.5 ± 0.4 when the Tris–EDTA buffer at pH 9.0 and heating for 45 min were used (P=0.001). Fig. 3B shows that statistical analysis that included only the 9 phosphoproteins that were successfully unmasked demonstrated that the mean of the IHC scores increased from 1.0 ± 0.2 with the citrate buffer at pH 6.0 and heating for 20 min to 2.6 ± 0.2 with the Tris–EDTA buffer at pH 9.0 and heating for 45 min were used (P<0.0001).
Figure 1. Immunoreactivity of phosphoproteins in formalin-fixed archival xenograft ALK+ ALCL tissues after using 2 different antigen retrieval methods –
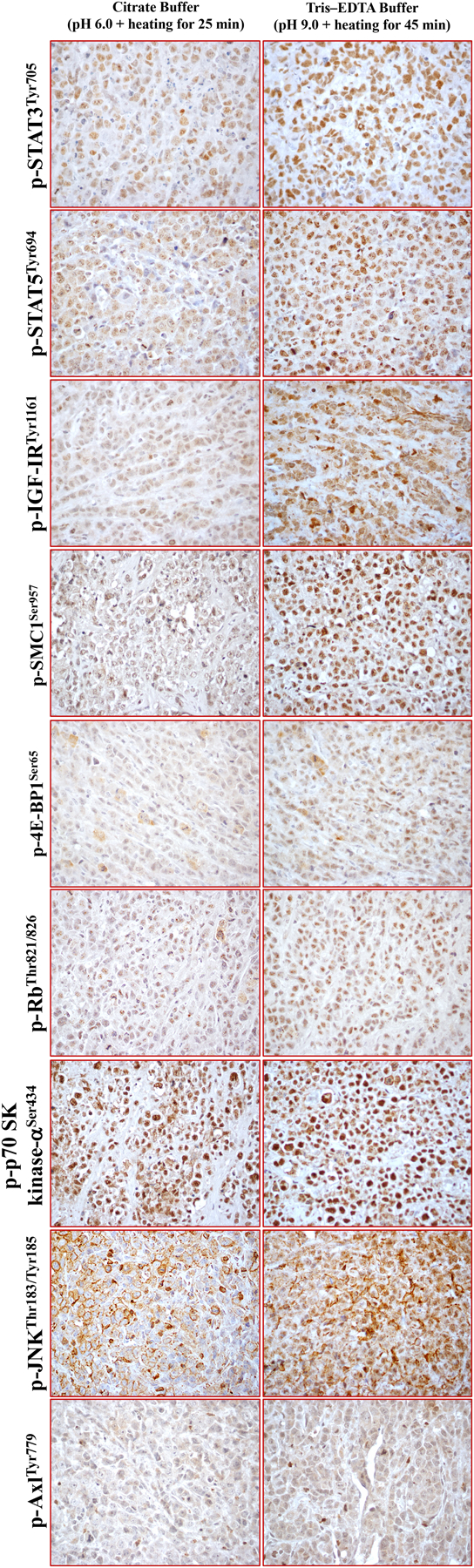
Photomicrograph illustrating the 9 phosphoproteins that were unmasked and showed enhancement of their antigenicity using the optimized method of antigen retravel by heating the tissues in TRIS–EDTA buffer at 97°C and pH 9.0 for 45 min compared to using citrate buffer at 97°C and pH 6.0 for 25 min. Original magnification is ×400.
Figure 2. IHC staining scores of the 15 phosphoproteins –
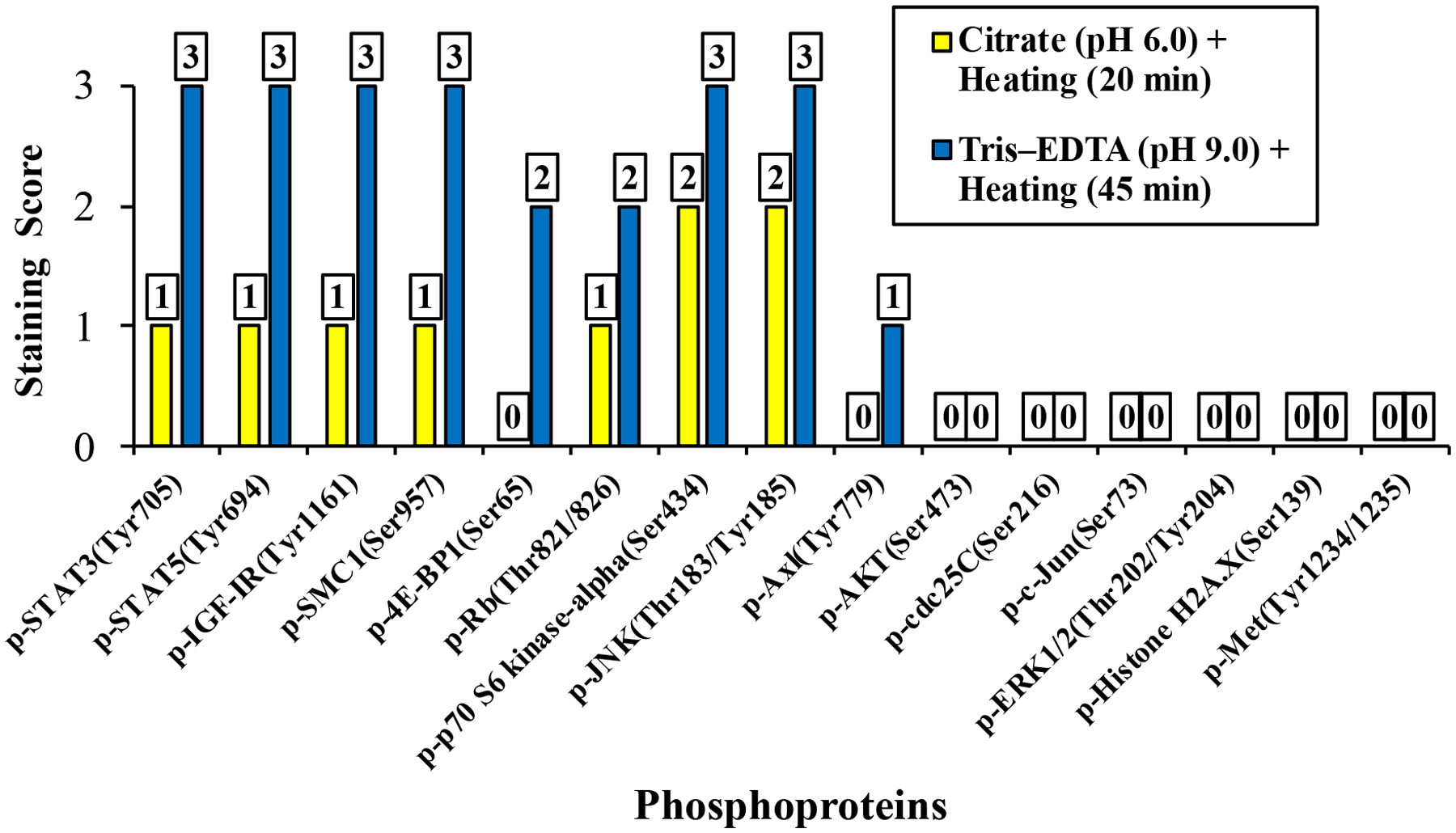
Antigen retrieval using the optimized method of heating the tissues in TRIS–EDTA buffer at 97°C and pH 9.0 for 45 min successfully unmasked and improved the immunoreactivity of 9 phosphoproteins including p-4E-BP1Ser65, p-AxlTyr779, p-IGF-IRTyr1161, p-JNKThr183/Tyr185, p-p70 6S kinase-αSer434, p-RBThr821/826, p-SMC1Ser957, pSTAT3Tyr705, and pSTAT5Tyr694.
Figure 3. Statistical analysis of the IHC staining scores –
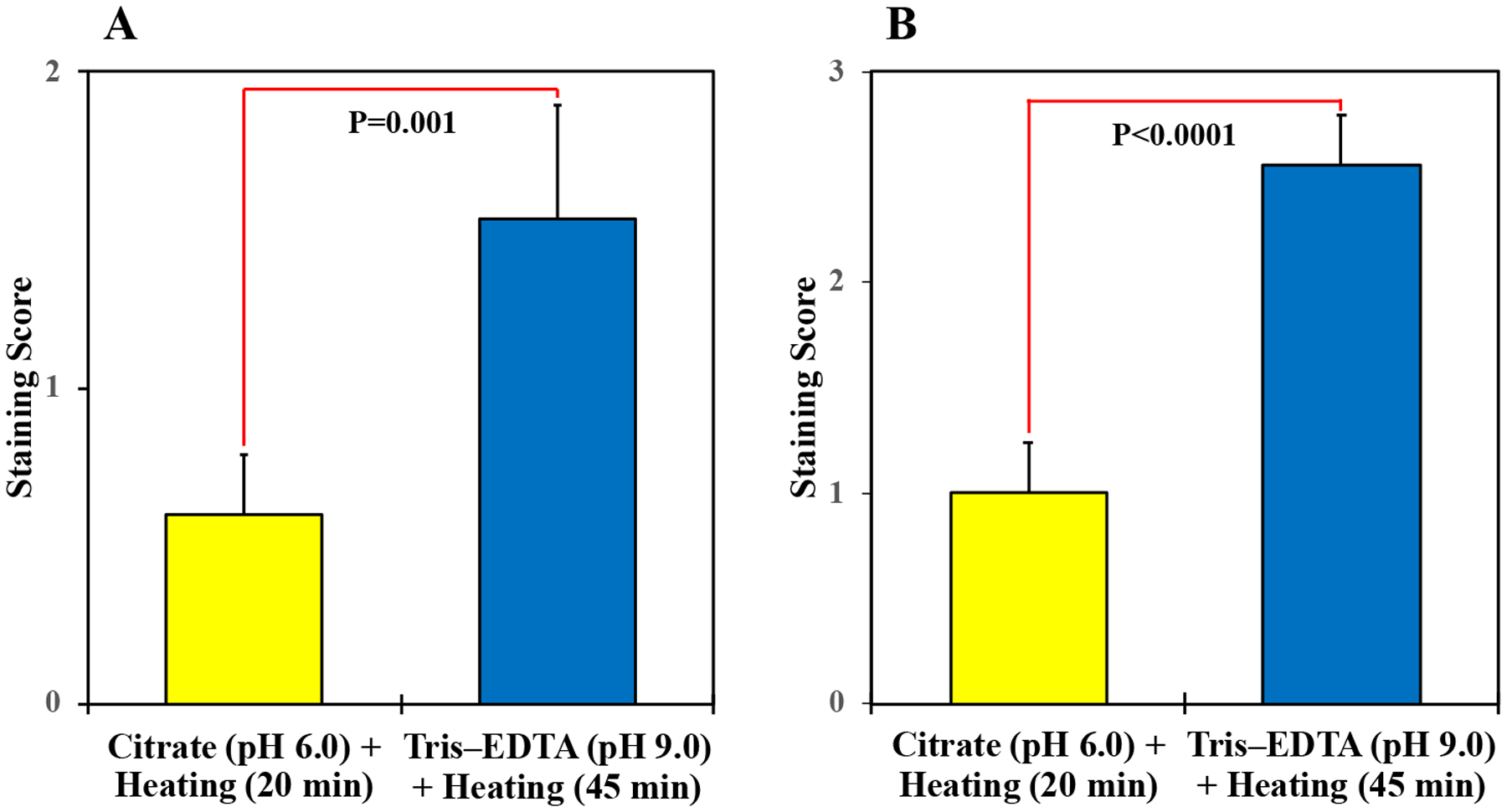
Compared with antigen retrieval by heating the tissues in citrate buffer at 97°C and pH 6.0 for 25 min, using the TRIS–EDTA buffer at 97°C and pH 9.0 for 45 min led to significant increase in the IHC scores (A, P=0.001 when all of the 15 phosphoproteins were included in the statistical analysis; B, P<0.0001 when only the 9 unmasked phosphoproteins were included). Results are shown as means ± SE.
DISCUSSION
In the current paper we studied the unmasking of 15 signaling phosphoproteins known to play important roles in cell survival and drug resistance by comparing 2 different antigen retrieval methods: 1) heating in Tris–EDTA buffer at 97°C and pH 9.0 for 45 min vs. 2) the commonly utilized method for antigen retrieval by heating in citrate buffer at 97°C and pH 6.0 for 20 min. The xenograft lymphoma tissues used in the study were fixed in formalin for 2 years. Extended formalin fixation for 2 years masked most of the phosphoproteins that we examined resulting in a weak or total lack of IHC signal with citrate buffer at pH 6.0 and 20 min of heating at 97°C. In contrast, our optimized antigen retrieval method in which we used Tris–EDTA buffer at pH 9.0 with prolonged heating for 45 min at 97°C reversed the masking of 9 of 15 phosphoproteins.
A large number of clinical trials are currently investigating the effects of targeted therapies that aim at specific oncogenic proteins such as kinases and transcription factors. Therefore, analyzing the expression of the activated/phosphorylated forms of these proteins in the tumors and corresponding noncancerous tissues has gained significant attention because of critical therapeutic and prognostic implications. Therefore, the number of antibodies that have been approved for the detection of the expression of phosphoproteins in the clinical setting is on the rise. We have recently introduced a CLIA-approved IHC staining method to detect p-IGF-IRTyr1161 in FFPE tissues to the clinical IHC laboratory at our institution based on data from multicenter clinical trials that showed that the low expression of p-IGF-IRTyr1161 in Ewing sarcoma pre-treatment biopsies was associated with a therapeutic response to anti-IGF-IR antibodies.19 In addition to pIGF-IRTyr1161, there are other antibodies specific for phosphoproteins, such as p-ERK1/2Thr202/Tyr204, p-STAT1Tyr701, and p-STAT3Tyr705, that are routinely used in our clinical IHC laboratory under CLIA-approved staining protocols.
It is estimated that 100–200 million proteins in each cell are involved in signaling pathways, and approximately one-third of these proteins are phosphorylated.20,21 Whereas analyzing the expression of phosphoproteins in animal and human tissues by using Western blotting or IHC is a routinely utilized approach in experimental medicine, this approach is still evolving in the clinical setting. Despite the availability of numerous phospho-specific antibodies, analyzing the expression of phosphoproteins by IHC remains challenging owing to important preanalytical and analytical considerations. Cold ischemia is a major preanalytical factor that influences the stability of phosphoproteins.22–24 Tissues become oxygen deficient within only 30 minutes after procurement, which accelerates the activation of kinase inhibitors and phosphatases that induce rapid protein dephosphorylation.25–27 Recent studies concluded that tissues must be fixed in less than 17 minutes after procurement in order to preserve phosphorylation of signaling proteins.28
Another important preanalytical factor that affects the stability of phosphoproteins is how the tissues are fixed.29 Ten percent neutral-buffered formalin is the most commonly used fixative in the clinical and research laboratories. Formalin cross-links peptides by formation of hydroxymethyl groups on reactive amino acids, and this process provides excellent preservation of tissue architecture. Nonetheless, formalin decreases antigenicity by inducing epitope masking. Indeed, several approaches have been already proposed to improve formalin fixation and preserve phosphoprotein expression in surgical specimens.28,30–34 Temperature, time, penetration rate, specimen dimensions, and pH are some of the other preanalytical factors that influence formalin fixation of surgical specimens.
Antigen retrieval is an important analytical step that affects IHC outcome. The mechanisms of antigen retrieval are not fully understood. It is believed that antigen retrieval breaks the methylation bridges developed during formalin fixation, which exposes the antigen sites and allows the antibodies to bind.35 Formalin-fixed tissues must be subjected to antigen retrieval to unmask the antigens, and heat is the most commonly utilized approach.36 The effectiveness of heat-induced antigen retrieval is markedly influenced by heating duration as well as pH and ionic strength of the antigen retrieval buffers.37,38 In general, appropriate antigen retrieval needs to be optimized for every antibody because it is dependent on the antibody and the target protein.
Heating in citrate buffer at pH 6.0 for 20 min is the most widely used heat-induced antigen retrieval method. However, this method often fails to reverse the effects of prolonged formalin fixation.11,12 A previous study found that heating tissues at 97°C for 45 min in Tris–EDTA–SDS buffer at pH 8.5 was superior to using citrate buffer at pH 6.0.12 In the current study we investigated epitope unmasking under 2 technically challenging conditions that were not examined in previous studies: 1) IHC staining of phosphoproteins, which are known to degrade substantially quicker than basal unphosphorylated proteins;22–24 and 2) staining of tissues after an extended period of formalin fixation. Few studies have investigated the unmasking of over fixed tissues. The antigen retrieval method of heating tissues at 98°C for 45 min in 0.05% citraconic anhydride solution at pH 7.4 was previously described as more effective in reversing the effects of formalin fixation.39 However, because of the toxicity of citraconic anhydride, this procedure has to be performed in a fume hood. It has been previously shown that increasing antigen retrieval time to 60 min at pH 6.0 unmasked a few but not all of the antigens from tissues fixed for 8 days.40 For most of these tissues, raising the retrieval temperature to 121°C resulted in significantly stronger staining.40 However, an antigen retrieval temperature of more than 97°C is not advisable because it can cause non-specific staining through the introduction of heat artifacts into the nuclei and connective tissues. Our antigen retrieval method of heating tissues at 97°C for 45 min at pH 9.0 successfully retrieved most of the antigens in tissues fixed in formalin for 2 years and did not introduce notable background staining.
A few antigens retain their antigenicity even after prolonged fixation. In our study, p-p70 S6 kinase-αSer434 and p-JNKThr183/Tyr185 retained a moderate antigenicity after 2 years of fixation. A recent study found that prolonged formalin fixation of human brain tissues up to 20 years did not affect the antigenicity of CD68 and caspase-3.41 According to Webster et al. (2009), amylin, calponin, calretinin, CD3, CD10, CD18, E-cadherin, insulin, MHC II, myoglobulin, and progesterone receptor retained their antigenicity after 7 weeks of fixation.11 From our own experience, if extended formalin fixation does not affect immunoreactivity and conventional method can be used to retrieve antigens, caution should be followed when using antigen retrieval methods with increased pH and prolonged heating durations because such conditions might introduce background staining. Therefore, same IHC conditions should be adopted for control and treated samples to avoid the effects of heterogeneity.
In summary, we screened 4 different antigen retrieval methods to unmask 3 phosphoproteins with known oncogenic effects in tissues that were fixed in formalin for an extended period of time that lasted 2 years. Based on our screening data, we utilized 15 phosphoantibodies to further compare the most effective antigen retrieval method to the most commonly used one. Our data showed that using the antigen retrieval buffer Tris–EDTA at pH 9.0 and heating for 45 min at 97°C enhanced significantly the staining of 9 of the 15 phosphoproteins. Our method is easy to perform, cost effective, and feasible for utilization in the clinical and research settings. We anticipate that combining our antigen retrieval strategy with the newly proposed methods to enhance tissue fixation28,30–34 will further enhance unmasking of phosphoproteins in human and animal tissues.
Funding:
This work was supported in part by the National Institutes of Health (NIH) grants R01CA151533 and R01CA260872, and by a Bridge Funding Grant from the University of Texas MD Anderson Cancer Center to HMA.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests related to this work.
REFERENCES
- 1.Witthuhn BA, Quelle FW, Silvennoinen O, et al. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74(2):227–236. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson SE, Oates AC, Harpur AG, et al. Tyrosine kinase JAK1 is associated with the granulocyte-colony-stimulating factor receptor and both become tyrosine-phosphorylated after receptor activation. Proc Natl Acad Sci USA. 1994;91(8):2985–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston JA, Kawamura M, Kirken RA, et al. Phosphorylation and activation of Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370(6485):151–153. [DOI] [PubMed] [Google Scholar]
- 4.Stark GR, Darnell JE Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36(4):503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(2):857–868. [DOI] [PubMed] [Google Scholar]
- 6.Fox CH, Johnson FB, Whiting J, et al. Formaldehyde fixation. J Histochem Cytochem. 1985;33(8):845–853. [DOI] [PubMed] [Google Scholar]
- 7.Thavarajah R, Mudimbaimannar VK, Elizabeth J, et al. Chemical and physical basics of routine formaldehyde fixation. J Oral Maxillofac Pathol. 2012;16(3):400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold MM, Srivastava S, Fredenburgh J, et al. Effects of fixation and tissue processing on immunohistochemical demonstration of specific antigens. Biotech Histochem. 1996;71(5):224–230. [DOI] [PubMed] [Google Scholar]
- 9.Ramos-Vara JA. Technical aspects of immunohistochemistry. Vet Pathol. 2005;42(4):405–426. [DOI] [PubMed] [Google Scholar]
- 10.Hitchman E, Hodgkinson C, Roberts D, et al. Effect of prolonged formalin fixation on immunohistochemical staining for the proliferation marker Ki67. Histopathology. 2011;59(6): 1261–1263. [DOI] [PubMed] [Google Scholar]
- 11.Webster JD, Miller MA, Dusold D, et al. Effects of prolonged formalin fixation on diagnostic immunohistochemistry in domestic animals. J Histochem Cytochem. 2009;57(8):753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syrbu SI, Cohen MB. An enhanced antigen-retrieval protocol for immunohistochemical staining of formalin-fixed, paraffin-embedded tissues. In: Kalyuzhny A, ed. Signal Transduction Immunohistochemistry. Methods in Molecular Biology (Methods and Protocols) , vol 717. Totowa: Humana Press, 2011:101–110. [DOI] [PubMed] [Google Scholar]
- 13.George SK, Vishwamitra D, Manshouri R, et al. The ALK inhibitor ASP3026 eradicates NPM-ALK+ T-cell anaplastic large-cell lymphoma in vitro and in a systemic xenograft lymphoma model. Oncotarget. 2014;5(14):5750–5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer P, Nacheva E, Mason DY, et al. A Ki-1 (CD30)-positive human cell line (Karpas 299) established from a high-grade non-Hodgkin’s lymphoma, showing a 2;5 translocation and rearrangement of the T-cell receptor β-chain gene. Blood. 1988;72(1):234–240. [PubMed] [Google Scholar]
- 15.Nieborowska-Skorska M, Slupianek A, Xue L, et al. Role of signal transducer and activator of transcription 5 in nucleophosmin/anaplastic lymphoma kinase-mediated malignant transformation in lymphoid cells. Cancer Res. 2001;61(17):6517–6523. [PubMed] [Google Scholar]
- 16.Amin HM, McDonnell TJ, Ma Y, et al. Selective inhibition of STAT3 induces apoptosis and G1 cell cycle arrest in ALK-positive anaplastic large cell lymphoma. Oncogene. 2004;23(32):5426–5434. [DOI] [PubMed] [Google Scholar]
- 17.Shi P, Lai R, Lin Q, et al. IGF-IR tyrosine kinase interacts with NPM-ALK oncogene to induce survival of T-cell ALK+ anaplastic large-cell lymphoma cells. Blood. 2009;114(2):360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George B, George SK, Shi W, et al. Dual inhibition of IGF-IR and ALK as an effective strategy to eradicate NPM-ALK+ T-cell lymphoma. J Hematol Oncol. 2019;12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amin HM, Morani AC, Daw NC, et al. IGF-1R/mTOR targeted therapy for Ewing sarcoma: a meta-analysis of five IGF-1R-related trials matched to proteomic and radiologic predictive biomarkers. Cancers (Basels). 2020;12(7):1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milo R, Jorgensen P, Moran U, et al. BioNumbers–the database of key numbers in molecular and cell biology. Nucleic Acid Res. 2010;38(Database issue):D750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332(6030):680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinhel IF, Macneill FA, Hills MJ, et al. Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer. Breast Cancer Res. 2010;12:R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holzer TR, Fulford AD, Arkins AM, et al. Ischemic time impacts biological integrity of phospho-proteins in PI3K/Akt, Erk/MAPK, and p38 MAPK signaling networks. Anticancer Res. 2011;31(6):2073–2081. [PubMed] [Google Scholar]
- 24.Vassilakopoulou M, Parisi F, Siddiqui S, et al. Preanalytical variables and phosphoepitope expression in FFPE tissue: quantitative epitope assessment after variable cold ischemic time. Lab Invest. 2015;95:334–341. [DOI] [PubMed] [Google Scholar]
- 25.Baker AF, Dragovich T, Ihle NT, et al. Stability of phosphoprotein as a biological marker of tumor signaling. Clin Cancer Res. 2005;11(12):4338–4340. [DOI] [PubMed] [Google Scholar]
- 26.Blow N Tissue preparation: tissue issues. Nature. 2007;448(7156):959–963. [DOI] [PubMed] [Google Scholar]
- 27.Espina V, Edmiston KH, Heiby M, et al. A portrait of tissue phosphoprotein stability in the clinical tissue procurement process. Mol Cell Proteomics. 2008;7(10):1998–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theiss AP, Chafin D, Bauer DR, et al. Immunohistochemistry of colorectal cancer biomarker phosphorylation requires controlled tissue fixation. PLoS One. 2014;9(11):e113608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Hurley G, Sjöstedt E, Rahman A, et al. Garbage in, garbage out: a critical evaluation of strategies used for validation of immunohistochemical biomarkers. Mol Oncol. 2014;(4):783–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burns JA, Li Y, Cheney CA, et al. Choice of fixative is crucial to successful immunohistochemical detection of phosphoproteins in paraffin-embedded tumor tissues. J Histochem Cytochem. 2009;57(3):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller C, Edmiston KH, Carpenter C, et al. One-step preservation of phosphoproteins and tissue morphology at room temperature for diagnostic and research specimen. PLoS One. 2011;6(8):e23780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chafin D, Theiss A, Roberts E, et al. Rapid two-temperature formalin fixation. PLoS One. 2013;8(1):e54138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathieson W, Marcon N, Antunes L, et al. A critical evaluation of the PAXgene tissue fixation system: morphology, immunohistochemistry, molecular biology, and proteomics. Am J Clin Pathol. 2016;146(1):25–40. [DOI] [PubMed] [Google Scholar]
- 34.Lerch ML, Kenerson HL, Chafin D, et al. Effect of immediate cold formalin fixation on phosphoprotein IHC tumor biomarker signal in liver tumors using a cold transport device. Sci Rep. 2020;10(1):2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fowler CB, Evers DL, O’Leary TJ, et al. Antigen retrieval causes protein unfolding: evidence for a linear epitope model for recovered immunoreactivity. J Histochem Cytochem. 2011;59(4):366–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissues sections. J Histochem Cytochem. 1991;39(6):741–748. [DOI] [PubMed] [Google Scholar]
- 37.Emoto K, Yamashita S, Okada Y. Mechanisms of heat-induced antigen retrieval: does pH or ionic strength of the solution play a role for refolding antigens? J Histochem Cytochem. 2005;53(11):1311–1321. [DOI] [PubMed] [Google Scholar]
- 38.Leong TY, Leong AS. How does antigen retrieval work? Adv Anat Pathol. 2007;14(2):129–131. [DOI] [PubMed] [Google Scholar]
- 39.Namimatsu S, Ghazizadeh M, Sugisaki Y. Reversing the effects of formalin fixation with citraconic anhydride and heat: a universal antigen retrieval method. J Histochem Cytochem. 2005;53(1):3–11. [DOI] [PubMed] [Google Scholar]
- 40.Boenisch T Effect of heat-induced antigen retrieval following inconsistent formalin fixation. Appl Immunohistochem Mol Morphol. 2005;13(3):283–286. [DOI] [PubMed] [Google Scholar]
- 41.Alrafiah A, Alshali R. The effect of prolonged formalin fixation on the staining characteristics of archival human brain tissue. Folia Morphol (Warsz). 2019;78(2):230–236. [DOI] [PubMed] [Google Scholar]


