Abstract
The co-combustion of biomass and coal has both environmental and economic benefits in terms of pollutants and greenhouse gas emissions. However, one of the key factors affecting the feasibility of this technology is the ash deposition and corrosion caused by the high alkali metal content of biomass, especially K. After the addition of elemental S to corn stalk/Xiaolongtan lignite blended fuel and Al2O3 to corn stalk/Datong lignite, combustion experiments were carried out in a tubular furnace to explore the effects of S and Al in coal on K migration and transformation. The experimental results show that when S/K < 6, an increase in the S/K ratio inhibited the release of K. When S/K > 6, the sulfation become saturated, and an increase in S promoted the release of K. When S/K = 6, the higher the temperature was, and the more obvious the inhibitory effect on the release of K was. Increasing the S/K ratio not only increased the CaSO4 content of the ash but also increased the content of water-soluble K compounds, such as K2SO4, and decreased the contents of acid-soluble K compounds and insoluble K compounds, such as KAlSi3O8. After Al2O3 was added, as the Al/K ratio increased, the K release rate gradually decreased. When the sample with Al/K = 2.5 and the original samples were burned at 600–700 °C, the difference in the K release rates of the two samples was relatively small. When the temperature was higher than 700 °C, the higher the temperature was, and the greater the difference in the K release rates of the samples was, which indicates that a high temperature promotes the formation of aluminosilicates containing K.
1. Introduction
The co-combustion of biomass and coal has both environmental and economic benefits in terms of pollutants and greenhouse gas emissions.1 Not only can it reduce the combustion of traditional fossil fuels and save energy, but it also effectively improves the combustion performance of the fuel compared with pure biomass combustion, which is conducive to improving boiler efficiency.2 Moreover, the cost of converting existing coal-fired power plants into biomass-coal cofired power plants is lower than the cost of establishing new biomass power plants.3 In addition, in order to minimize the fluctuating supply of some secondary fuels and ensure the safety of power generation, a flexible method (i.e., different proportions of secondary fuels) can be adopted for co-combustion, which is certain to produce huge economic benefits.4 Compared with coal combustion, the mixed combustion of coal and biomass can significantly reduce the release of pollutants such as SOx and NOx, which makes this a clean combustion technology with low carbon emissions.5
However, one of the key factors affecting the feasibility of this technology is the ash deposition and corrosion caused by the high alkali metal content of biomass.6 Because of the relatively high K content of biomass (especially herbs), during the process of mixed combustion, alkaline substances (e.g., KCl) may be transformed to the gas phase due to their high chemical activity, resulting in ash accumulation, slagging, and corrosion on the boiler heating surface, which leads to a reduced heat transfer capacity, increased heat loss, deviation from the designed operation of the boiler, and a greatly reduced boiler operation efficiency.7−9 During the co-combustion process, the alkali metals in the biomass will react with the inorganic minerals in the coal to produce low-temperature melts and volatile alkali metal compounds, making the ash composition more complicated.10−12 Therefore, it is particularly important to study the migration, transformation, and deposition behaviors of alkali metals during the process of biomass-coal combustion to ensure the safe and efficient operation of the boiler.
Regarding the occurrence forms of alkali metals, the current research method mainly involves chemical step-by-step extraction of samples, sampling, and classification of samples at different stages.10−13 This method has a wide range of applications in experimental research and industry. Generally, the solid-phase occurrence of alkali metals can be divided into four categories via extraction separation.14−16 (1) Water-soluble alkali metals: alkali metals that are soluble in water, such as KCl, K2SO4, and K2CO3. (2) Ammonium acetate soluble alkali metals: alkali metals bonded with oxygen-containing functional groups such as a carboxyl group, which can be plasma exchanged with NH4+. (3) Hydrochloric acid soluble alkali metals: including other forms of alkali metals in addition to aluminosilicates and those wrapped by a carbon matrix, such as silicates containing alkali metals. (4) Insoluble alkali metals: alkali metals that are insoluble in hydrochloric acid, sulfuric acid, and other general acids but are soluble in hydrofluoric acid, mainly including aluminosilicates containing alkali metals.
Because the fuel characteristics of coal and biomass are very different, and their ash compositions are not the same, the alkali metals and alkaline earth metals in biomass fuel easily combine with the inorganic minerals in coal during their co-combustion, resulting in volatile alkali metals and a low-temperature eutectic, which seriously affects the safe and efficient operation of the currently available thermal equipment.
Wei et al.17 analyzed the influence of other elements on alkali metal release during the co-combustion of straw and coal, and they found that Si, Al, Ca, Mg, and S can greatly affect the behavior of Cl, K, and Na, while other minor elements (e.g., Fe, Ti, and Mn) have little influence. During co-combustion, most of the potassium is released as KCl(g), KOH(g), and K2SO4(g) since aluminum preferentially combines with Ca and Mg to form corresponding compounds, such as Ca3Al2O6, CaAl2O4, and MgAl2O4. When there are more aluminum and silicon in the mixed fuel, most of the potassium exists as solid KAlSi2O6, and less than 10% of the potassium precipitates in the form of gaseous KC1(g) and KOH(g). Gaseous KC1(g), KOH(g), K2SO4(g), and molten K2Si4O9 are easily deposited on the heat exchange surface, resulting in serious ash deposition. Glazer et al.18 studied the migration and transformation of alkali metals during the co-combustion of coal and biomass in a circulating fluidized bed, and they found that the ash forming elements in coal and biomass have a great impact on the release of alkali metals. The release of alkali metals is mainly affected by the Cl content rather than the contents of the alkali metals. In addition, the silicon and aluminum in the fuel will inhibit the release of alkali metals, but it will form alkali metal silicates with low melting points, resulting in ash deposition and agglomeration problems. The use of biomass and coal with complementary compositions significantly reduces the release of alkali metals.
Yang et al.19 studied the effect of the sulfur in coal on the behaviors of alkali metal during the co-combustion of biomass and coal. Their results revealed that when the S/K molar ratio was >2, the production of potassium sulfate increased greatly with the addition of FeS2. In addition, increasing the dosage of FeS2 can reduce the formation of KCl(g) and KOH(g) and promote the release of HCl(g). Johansen et al.20 studied the release of potassium, chlorine, and sulfur during the combustion of high chlorine biomass in small and pilot-scale test benches and found that the release of K increased during combustion in larger fuel beds, while the release of Cl significantly decreased in the temperature range of 900–1000 °C. As the proportion of low chlorine sawdust increased, the form of Cl changed from a high concentration of HCl to KCl. Li et al.21 studied the influence of temperature on the release and transformation of alkali metals during the co-combustion of coal and sulfur-rich wheat straw. Their results revealed that the presence of Fe, Ti, S, Si, and Al in the mixture can reduce the release of K and Na during co-combustion. Theis et al.22 found that when peat/bark and peat/straw mixtures were burned, the deposition rate began to increase only when the Cl/S molar ratio of the feed ash exceeded 0.15. During the co-combustion of peat and bark, sulfur can sulfate alkali metals, reduce the content of KCl in ash, and inhibit ash deposition. Wang et al.23 found that high contents of potassium silicate and phosphate in the ash led to serious coking problems, and adding Ca to reduce the K/Ca ratio promoted the production of calcium silicate and calcium phosphate and reduced the coking problems.
The above-mentioned research indicates that the inorganic elements in coal have an important influence on the release of K and the ash deposition and slagging caused by the release of K during co-combustion. However, quantitative analysis of the influences of the inorganic elements in coal on the release of K, the transformations between the occurrence forms of K, and the influence of the crystalline phase of the ash is still lacking. These analyses are of great significance to reducing the ash deposition and slagging caused by alkali metals during co-combustion, to further increasing the biomass blending ratio, and to further development of biomass-coal co-combustion technology.
Compared with biomass, the Al and S contents of coal are much higher, and these elements affect the migration and transformation of K. Coal has a much higher sulfur content than biomass. In the co-combustion of biomass and coal, the volatile alkali metals in the biomass are retained in the solid phase through reactions with the sulfur in the coal in the form of alkali metal sulfates, thus reducing the content of alkali metal chlorides in the sediments with low melting points, alleviating the problems caused by slagging, corrosion, and bed material agglomeration, and reducing the release of SO2. The reduction of both alkali metal mitigation and acid gas emissions can be achieved in an economical and environmentally friendly manner.
The sulfur in coal exists in three forms: inorganic sulfur, organic sulfur, and elemental sulfur.24 Elemental sulfur is one of the most important forms of sulfur in coal. Furthermore, pyrite is the most abundant sulfur mineral in coal, but several other sulfide minerals can be present. During coal combustion, first, the pyrite is decomposed into elemental sulfur and ferrous sulfide, and then, the S can react with H and CO to produce H2S and COS, or it can be directly polymerized to Sn.25 Therefore, elemental S and Al2O3 were added to the raw materials in this study to explore the effects of S and Al in coal on the migration and transformation of K during co-combustion in order to determine the optimum S/K and Al/K ratios for actual combustion.
2. Materials and Methods
2.1. Materials
Corn stalks (CS) from Donghai, Jiangsu Province, were chosen as a representative form of biomass. Lignite from Xiaolongtan, Yunnan Province (XL), and lignite from Datong, Shanxi Province (DL), were selected as representative coal samples. All of the materials were crushed and sieved, and the <0.18 mm fraction was selected for the experimental study. The proximate analysis of the samples was carried out according to standards ASTM D 3173-03 (M), ASTM D 3174-04 (Ash), ASTM D 3175-07 (VM), and the FC (fixed carbon) value was determined by subtracting the sum of M (moisture), Ash, and VM (volatile matter) from 100%. The ultimate analysis of the samples was conducted using an Elementar Unicube elemental analyzer. The results of the proximate analysis and ultimate analysis of the materials are presented in Table 1. An X-ray fluorescence spectrometer (XRF, Shimadzu XRF1800, Japan) was used to analyze the ash content of the materials, and the results are presented in Table 2. As can be seen from Table 1, the volatile content of the biomass was much higher than that of the coal, while the S content of the coal was much higher than that of the biomass, and the S content of DL was the highest. Table 2 shows that the K content of the corn stalk ash was higher, the Ca content of the XL ash was the highest, the Fe content of the DL ash was higher, and the Al content of XL was slightly higher than that of DL. Since the maximum biomass blending mass ratio in most power plants is about 20–30%,26 the biomass blending ratio in this experimental study was set as 25%.
Table 1. Results of Proximate and Ultimate Analyses of Samples (wt %, ad).
| ultimate analysis |
proximate analysis |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| samples | C | H | O | N | S | M | ash | VM | FC |
| CS | 42.13 | 6.29 | 36.72 | 1.27 | 0.15 | 4.26 | 9.17 | 72.57 | 14.00 |
| XL | 46.56 | 3.72 | 17.90 | 1.38 | 1.46 | 13.90 | 15.08 | 39.53 | 31.49 |
| DL | 60.87 | 4.57 | 7.79 | 1.10 | 2.71 | 5.94 | 17.03 | 31.09 | 45.94 |
Table 2. Ash Compositions of the Samples.
| samples | K2O | Na2O | MgO | CaO | Fe2O3 | SiO2 | Al2O3 | P2O5 |
|---|---|---|---|---|---|---|---|---|
| CS | 31.01 | 0.41 | 1.46 | 4.02 | 0.35 | 31.48 | 5.34 | 8.35 |
| XL | 0.96 | 0.27 | 2.34 | 39.16 | 9.58 | 21.13 | 13.32 | 0.07 |
| DL | 0.05 | 0.23 | 1.86 | 9.70 | 27.71 | 28.72 | 12.57 | 0.08 |
2.2. Analytical Methods
The chemical step-by-step extraction method is widely used to measure the contents of alkali metals (K, Na), and the specific method used in this study was as follows.27 A 200 mg sample (raw material or ash sample) was dissolved in 50 mL of deionized water at 60 °C and stirred for 24 h. After filtration, the content of the water-soluble K in the filtrate was measured via inductively coupled plasma-optical emission spectrometry (ICP-OES, iCap-6300, America). Then, the filtrate was successively dissolved in 1 M NH4Ac solution and 1 M HCl solution. The above steps were repeated to obtain the NH4Ac-soluble K content and HCl-soluble K content, respectively. Finally, the filter residue was digested using a microwave digestion instrument (Mars, America), and the insoluble K content was determined via ICP-OES. The Al content of the raw material was directly digested in the digester and was measured via ICP-OES. The K and Al contents of the raw materials are presented in Table 3. The Al content of the coal was much higher than that of the biomass, and the Al content of XL was the highest. The K content of the biomass was much higher than that of the coal, and it mainly existed in the form of water-soluble K. The crystalline phases in the samples were measured using an X-ray diffractometer (XRD, Bruker D8 Advance, Germany), with a Cu K-α target, a scanning speed of 4°/min, and a scanning range of 5–90°.
Table 3. Al and K Contents in the Raw Materials.
| samples | Al (wt %) | total K (mg/kg) | water-soluble K (wt %) | NH4Ac-soluble K (wt %) | HCl-soluble K (wt %) | insoluble K (wt %) |
|---|---|---|---|---|---|---|
| CS | 0.001 | 16531.22 | 84.61 | 5.22 | 3.21 | 6.96 |
| XL | 0.171 | 370.26 | 5.27 | 21.73 | 67.84 | 5.16 |
| DL | 0.165 | 41.31 | 8.41 | 35.16 | 30.46 | 25.97 |
2.3. Experimental Apparatus and Methods
The combustion experiment was carried out in an electric heating tube furnace (Figure 1), with a maximum heating temperature of 1050 °C. The tube was composed of quartz, with an inner diameter of 54 mm and a length of 1 m. There was a cold-water sleeve at the left end of the tube furnace to cool the sample. Air was supplied through high-pressure steel cylinders. In each run, 4 g of fuel was evenly spread in a quartz boat and was placed in the cooling section at the left end of the instrument. The air flow rate was 1 L/min. The tube furnace was heated to the specified temperature, and then, the quartz boat was pushed into the heating section of the furnace using a push rod, and the exhaust gas was connected to the flue gas analyzer to determine whether the reaction was over. The reaction time was controlled at 70 min, at which time the reaction was over under all of the operating conditions. Finally, the ash samples were removed from the furnace and analyzed.
Figure 1.
Schematic diagram of the combustion device used in the experiments.
Because the S content of XL was lower, the Al content of DL was lower, and the biomass blending ratio was 25%, the S/K molar ratio of the CS/XL raw material was 3.13, and the Al/K molar ratio of the CS/DL raw material was 0.43. Therefore, elemental S was added to the CS/XL to achieve S/K molar ratios of 4, 5, 6, and 7. The effects of the different S/K molar ratios on the K migration and transformation were investigated, and the combustion temperature was changed while S/K = 6 was maintained to explore the effects of S on the migration and transformation of K at different temperatures. Al2O3 was added to the CS/DL material to make mixtures with Al/K molar ratios of 1, 1.5, 2, and 2.5 in order to explore the effect of the Al/K molar ratio on the K migration and transformation. The combustion temperature was changed while maintaining Al/K = 2.5 to explore the effect of Al on the migration and transformation of K at different temperatures.
3. Results and Discussion
3.1. Influences of S and Al in Coal on the Ash Yield
After the combustion of the raw materials, the mass of the ash sample was weighed, and the ash rate was calculated using eq 1.
| 1 |
where A is the ash yield, mfuel is the mass of the raw material, and mash is the mass of the ash sample after combustion. The ash yield can initially reflect the release of the inorganic substances in the ash, and it can be used to calculate the K release rate. In addition, the theoretical ash yield was calculated based on the assumption that there was no interaction between the coal and biomass during the combustion and that the S was converted into SO2 and released.
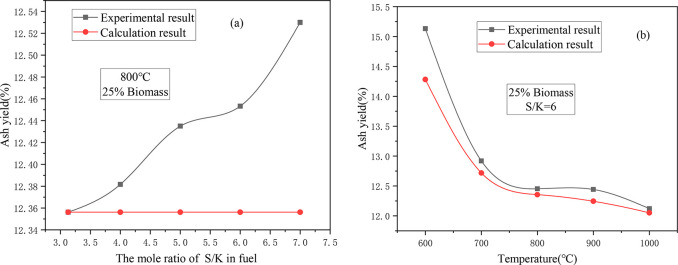
The ash yield of CS/XL after adding S is shown in Figure 2. The original S/K of the raw material was 3.13. After the addition of S, theoretically S should be converted into SO2 and released, and the ash yield should not change as the S/K ratio increases. However, it was found that in the experiments, as the S increased, the ash yield gradually increased, indicating that the added S was not completely released, and it reacted with the raw materials and was fixed in the ash. The actual ash yield increased at a variable rate as the S/K ratio increased. When the S/K ratio was 3.13–5 and 6–7, the ash yield increased rapidly with the addition of S, but when the S/K ratio was 5–6, the ash yield did not change significantly. This indicates that different reactions will occur under different S/K ratios, resulting in different amounts of S being fixed in the ash.
Figure 2.
Effects of adding S on the ash yield of CS/XL: (a) ash yield under different S/K mole ratios; and (b) ash yield at different temperatures.
Figure 2b shows the curve of the ash yield versus temperature for a sample with S/K = 6. It can be seen that as the temperature increased, both the actual ash yield and the theoretical ash yield gradually decreased, and the trends of both were very similar to that of the change in temperature. However, there were some differences between the actual ash yield and the theoretical ash yield in the different temperature ranges. When the temperature was between 600 and 800 °C, the actual ash yield was always higher than the theoretical ash yield, and the difference between them decreased gradually with increasing temperature. This is mainly because the increase in temperature promoted the release of SO2. However, when the temperature was between 800 and 1000 °C, the difference between the actual yield and the theoretical ash yield initially increased and then decreased with increasing temperature, which may have been caused by the melting of ash samples when the temperature was higher than 800 °C. The melting of the ash samples increased the resistance to the diffusion of SO2 from inside the fuel to the surface and inhibited the release of SO2.
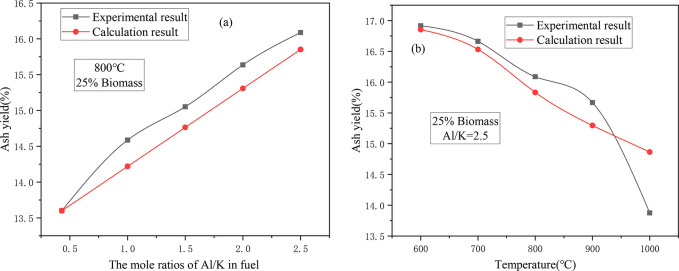
Figure 3 shows the ash yield of CS/DL after adding Al2O3. Figure 3a shows the ash yield curves for different Al/K molar ratios. For the theoretical ash yield, the reaction between Al2O3 and the fuel is not considered after the Al2O3 is added. After the addition of Al2O3, the actual ash yield was always higher than the theoretical ash yield. It was found that for the various Al/K ratios, the actual ash yield was always higher than the theoretical ash yield, but the difference was not large, and it is much smaller than the difference between the theoretical and actual ash yields after adding S. This indicates that Al2O3 may be less reactive and less effective in fixing the gas phase substances.
Figure 3.
Effect of adding Al2O3 on the ash yield of CS/DL: (a) ash yield under different Al/K molar ratios; and (b) ash yield at different temperatures.
Figure 3b shows the variation of the ash yield with temperature for Al/K = 2.5. In the different temperature ranges, the variations in theoretical and actual ash yields were also not the same. At 600–800 °C, the actual ash yield was higher than the theoretical ash yield, but the difference between the two was not large, indicating that within this temperature range, Al2O3 had a certain fixation effect on the release of the gas phase substances. When the temperature was about 800–900 °C, the difference between the actual and theoretical ash yields increased, indicating that within this temperature range, the reaction activity of Al2O3 was stronger, and more gas-phase substances were fixed in the ash. When the temperature was higher than 900 °C, the difference between the theoretical and the actual ash yields gradually decreased. In particular, when the combustion temperature was 1000 °C, the actual ash yield was much lower than the theoretical ash yield. It is preliminarily speculated that a high temperature may promote the reaction of the Ca in ash with Si and Al to form aluminosilicates, which results in less Ca binding to S and promotes the release of SO2.
3.2. Influence of S and Al in Coal on the Release and Transformation of K during Co-Combustion
Combined with the ash yield, the release rate of K under various operating conditions was calculated using eq 2:
| 2 |
where R is the release rate of K, and A is the ash yield. Cash is the total concentration of K in the ash sample, and Cfuel is the total concentration of K in the raw material. The release rate of K can reflect the proportion of the K in the raw material that is released into the gas phase.
In addition, after combustion, the ash samples were chemically extracted step-by-step to investigate the conversions between the different occurrence forms of the K. For the convenience of comparison, the results of the alkali metal occurrences were converted into the alkali metal contents retained in the initial 1 g of fuel using eq 3:
| 3 |
where M is the K concentration of the different occurrence forms in the raw material, Nash is the concentration of the different occurrence forms of K in the ash, and A is the ash yield.
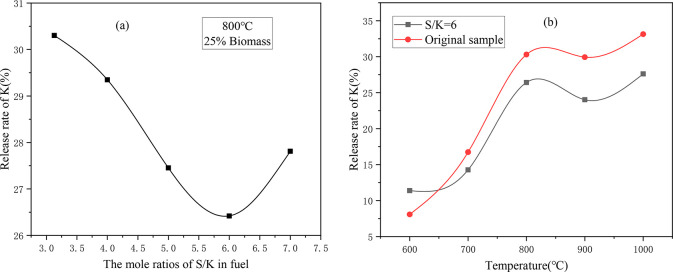
The K release rate of CS/XL under different S/K molar ratios after adding S is shown in Figure 4. When S/K < 6, the release rate of K gradually decreased with increasing addition of S, indicating that the addition of S cause more K to be fixed in the ash sample. The main reason for this phenomenon may be that the addition of S promotes Reaction (R1),7,28 and thus, a large amount of KCl is converted into K2SO4, reducing the release rate of K. When S/K > 6, the release rate of K increased with increasing addition of S. The release rate of K initially decreased and then increased as the S/K ratio increased, which has also been reported in previous studies. The possible reason for this is that when the S/K ratio increased to six, the sulfation of the KCl became saturated.19 In addition, when the K2SO4 content was sufficiently high, KCl and K2SO4 were still below the eutectic at low temperatures, making the melting point of the eutectic compound lower than that of KCl, thus promoting the release of water-soluble K.29
| R1 |
Figure 4.
Effect of adding S on the K release rate of CS/XL: (a) K release rates for different S/K mole ratios; and (b) K release rates at different temperatures.
Figure 4b shows the K release rates of the sample with S/K = 6 and the original sample at different temperatures. The trend of the K release rate with temperature is similar before and after the addition of S, and the K release rate decreased as the temperature increased from 800 to 900 °C. Generally, the release of K is a process in which gaseous potassium compounds move from the interior of the ash particles to the surfaces of the ash particles, resulting in a higher release of K from finer fuel particles than from coarser fuel particles. In addition, the higher the temperature is, the more easily the gaseous potassium compounds can migrate from the interior to the surface, thus increasing the release rate.30,31 From the physical and chemical properties of the ash, it is known that the ash samples melt at high temperatures. This can enhance the fixation reaction between the potassium and silicon aluminum minerals. In addition, it can also increase the resistance of the release of the K in the sample into the gas phase, thus reducing the ability to release K in this temperature range.32 When the temperature was between 900 and 1000 °C, the release rate of K began to increase again with increasing temperature, which indicates that the release of K was affected by two factors. The increase in temperature increased the power of the gaseous potassium compounds to move from the inside to the surface of the ash sample, but the increase in temperature caused the ash sample to melt, which increased the resistance to the internal potassium being released to the gas phase. When the temperature was higher than 900 °C, the effect of the temperature on the release of K was stronger. When the temperature was between 650 and 1000 °C, the K release rate after the addition of S was always lower than that of the original sample, and the difference between the two increased with increasing temperature. This is because after the addition of S, more of the K was sulfated and fixed in the ash sample. Since the compounds such as KCl in the original sample were more easily released at high temperatures, the effect of the K being fixed in the ash due to sulfation was more obvious at higher temperatures. It should be noted that when the temperature was lower than 650 °C, the release rate of K from the sample with S/K = 6 was lower than that of the original sample. This special phenomenon may have been caused by the fact that the K-containing compounds were not released in large quantities at low temperatures, but the addition of S promoted the combustion of the sample, which increased the release of K.
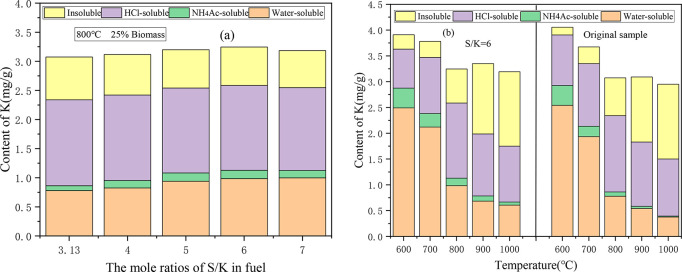
The solid retention of the different occurrence forms of K in the ash for the co-combustion of CS/DL after the addition of S is shown in Figure 5. Figure 5a shows the change in the occurrence form of the K in the ash under different S/K molar ratios. As the S/K ratio increased, the content of water-soluble K in the ash continuously increased, while the contents of HCl-soluble K and insoluble K decreased. This indicates that the addition of S resulted in the sulfation of the K, so the content of water-soluble K in the ash increased, and certain quantities of the HCl-soluble K and insoluble K were converted into water-soluble K. On the basis of Figure 4a, when S/K > 6, the addition of S increased the release rate of K. In contrast, in Figure 5a, when S/K > 6, the increase in the content of the water-soluble K was no longer obvious, but the contents of HCl-soluble K and insoluble K still decreased, indicating that the K fixation effect of S had become saturated. It is generally believed that when coal and biomass are cocombusted, the S in the coal will generate a large amount of SO2, which will be trapped by the solid phase33 and will mainly react with inorganic alkali metal salts in the sample space to form alkali metal sulfate. The sulfur will compete with the silicates and aluminosilicates for the alkali metals.34 A low temperature and sufficiently high sulfur content can support the formation of alkali metal sulfates.35 In addition, it was found that as the S/K ratio increased, the contents of the NH4Ac-soluble K and water-soluble K gradually increased. This occurred because the water-soluble K will react with the SiO2 in the ash to promote the formation of NH4Ac-soluble K.36
Figure 5.
Effect of adding S on the retention of the different occurrence forms of K in the CS/XL ash: (a) the occurrence forms of K under different S/K mole ratios; and (b) the occurrence forms of K at different temperatures.
Figure 5b shows the different occurrence forms of K in the ash of the sample with S/K = 6 and the original sample at different temperatures. After the addition of S, the content of the water-soluble K in the ash increased at all of the tested temperatures. In addition, as the temperature increased, compared with the original sample, the increase in the content of the water-soluble K in the ash was greater for the sample with S/K = 6. The reason for this phenomenon is that the increase in S led to an increase in the SO2 concentration, which promoted the occurrence of Reaction (R1) and sulfated part of the KCl since the melting point of K2SO4 is higher than that of KCl. At low temperatures, less KCl was released, and the effect of sulfation on the release of K was not obvious. At high temperatures, more K2SO4 was produced in the combustion of the sample with S/K = 6, so more water-soluble K was fixed in the ash. In addition, the contents of the HCl-soluble K and insoluble K of the ash from sample with S/K = 6 were lower than those of the ash from the original sample at all of the tested temperatures, and the difference was more obvious at lower temperatures.
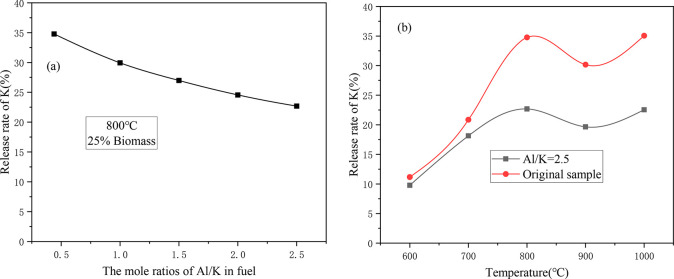
Figure 6 shows the change in the K release rate of sample CS/DL after the addition of Al2O3. Figure 6a shows the change in the K release rate under different Al/K molar ratios. As the Al/K ratio increased, the release rate of K gradually decreased, and the rate of decreases became smaller and smaller, which indicates that the addition of Al2O3 had an inhibitory effect on the release of K. According to Figure 3a, when Al/K > 1, the difference between the actual and the theoretical ash yields remained almost unchanged, which indicates that the addition of Al2O3 caused the K to become fixed in the ash and also promoted the release of other gaseous substances.
Figure 6.
Effect of adding Al2O3 on the release rate of K for CS/DL: (a) K release rates for different Al/K molar ratios; and (b) K release rates at different temperatures.
Figure 6b shows the K release rates of the sample with Al/K = 2.5 and the original sample under different combustion temperatures. When the temperature was between 600 and 700 °C, the difference in the release rate of K between the sample with Al/K = 2.5 and the original sample was small, signifying that the K retention capacity with the addition of Al2O3 was limited within this temperature range. When the temperature was higher than 700 °C, the difference between the two increased gradually, and this shows that the K retention capacity of Al2O3 increased gradually. When the temperature was higher than 800 °C, the difference remained almost the same, which indicates that the K retention capacity of Al2O3 tended to be saturated when the temperature was higher than 800 °C.
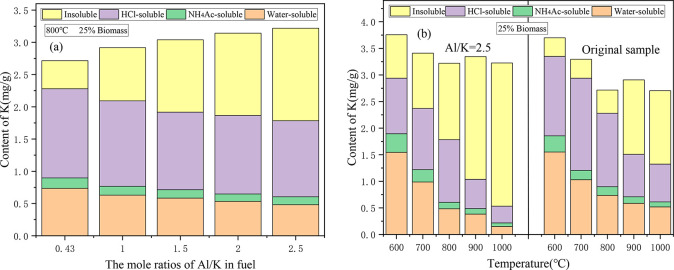
Figure 7 shows the retentions of the different occurrence forms of K in the CS/DL ash after the addition of Al2O3. It can be seen from Figure 7a that as the Al/K molar ratio increased, the contents of both the water-soluble K and HCl-soluble K decreased, while the content of insoluble K increased significantly, which demonstrates that the addition of Al2O3 not only converted the water-soluble K into insoluble K, but also partially converted the HCl-soluble K into insoluble K, leaving more K in the ash.
Figure 7.
Effect of adding Al2O3 on the retentions of the different occurrence forms of K in the CS/DL ash: (a) the occurrence forms of K under different Al/K mole ratios; and (b) the occurrence forms of K at different temperatures.
Figure 7b shows the different forms of K in the ash of the CS/DL sample with Al/K = 2.5 and the ash of the original sample at different temperatures. When the temperature was between 600 and 700 °C, the contents of the water-soluble K in the ash of the sample with Al/K = 2.5 and the original sample were not significantly different, while the content of HCl-soluble K in the ash of the sample with Al/K = 2.5 decreased, while the content of insoluble K increased. Therefore, when the temperature was within the range of 600–700 °C, the Al2O3 was mainly used to convert some of the HCl-soluble K into insoluble K. When the temperature was higher than 800 °C, for the ash sample with Al/K = 2.5, the content of water-soluble K was significantly lower than that of the original sample, the content of HCl-soluble K was lower than that of the original sample, and the insoluble K content was significantly higher than that of the original sample. Therefore, when the temperature was higher than 800 °C, the addition of Al2O3 resulted in the conversion of the water-soluble K and HCl-soluble K into insoluble K during the combustion process. In addition to retaining more K in the ash, it also improved the ash fusion.
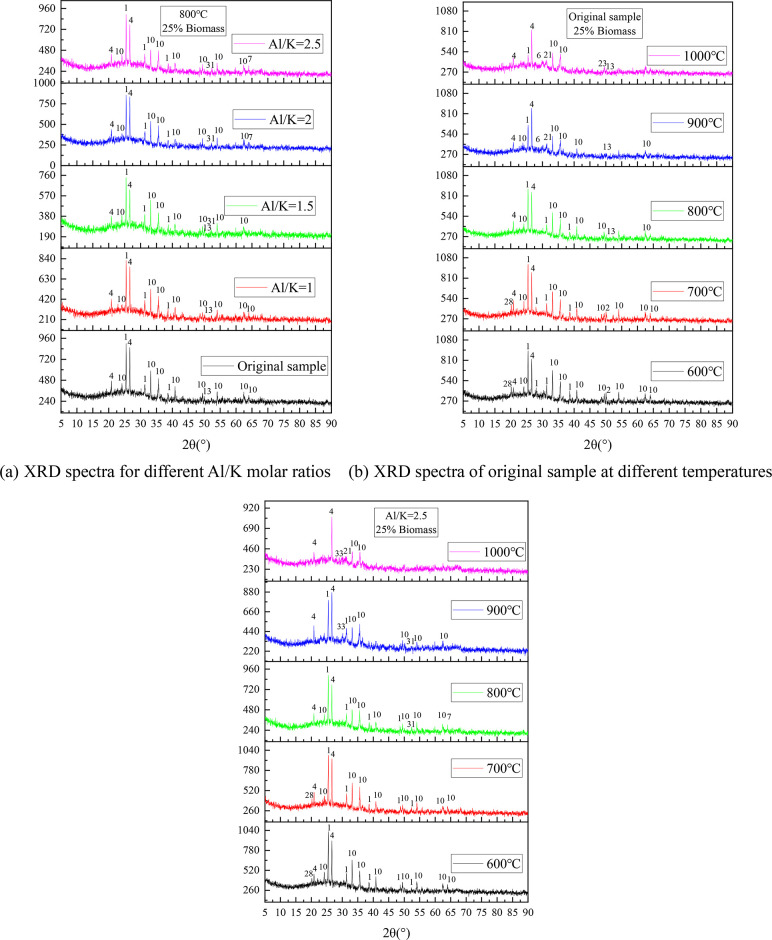
3.3. Effects of S and Al in Coal on Crystal Phase of Ash after Co-Combustion
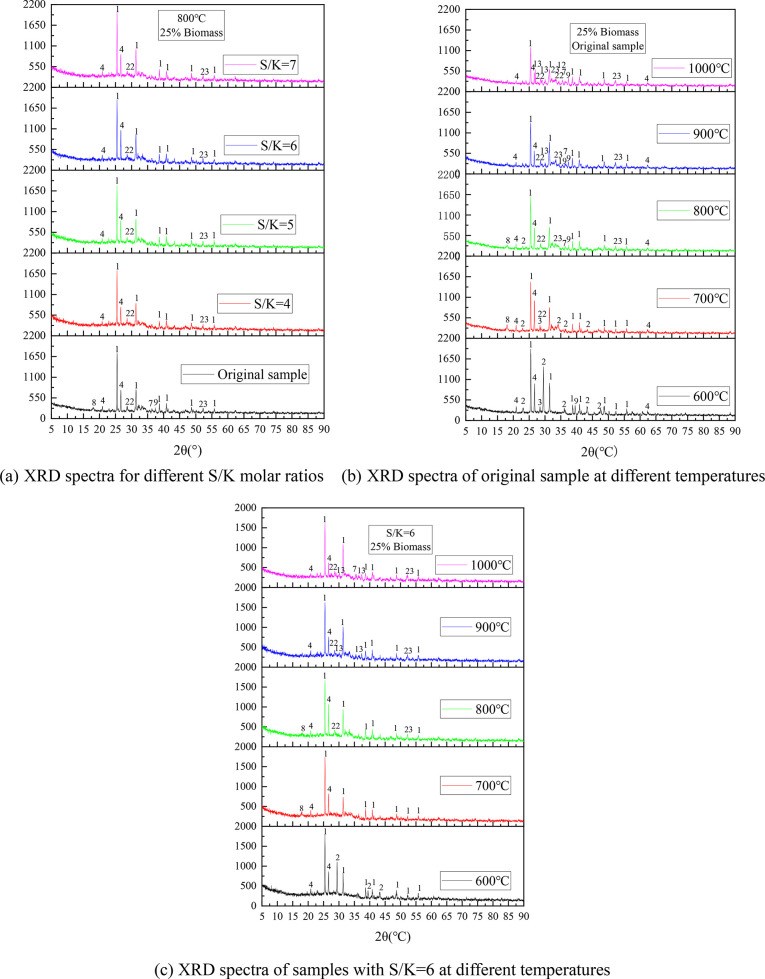
Figure 8 shows the XRD spectra of the CS/XL ash samples after the addition of S. Figure 8a shows the crystalline phase of the ash samples after combustion for different S/K mole ratios. There is no new K2SO4 peak in the ash samples after the addition of S, which is mainly due to the low K content of the raw material and the difficulty of detecting K2SO4 compared with KCl. However, as the addition of S increased, the CaSO4 peak at 2θ = 25.14° gradually strengthened, while the CaO peak at 2θ = 37.4° disappeared. In addition, the KAlSi3O8 peak at 2θ = 36.3° disappeared, which also corresponds to the decrease in the content of insoluble K in the ash with increasing addition of S (Figure 5a).
Figure 8.
(a–c) XRD spectra of CS/XL ash after the addition of S.
Figure 8b shows the XRD spectra of the ash of the CS/DL samples at different temperatures. The K in the ash sample was mainly in the form of KCl and potassium aluminosilicates. As the temperature increased, the intensity of the KCl peak gradually decreased, while the intensity of the potassium aluminosilicate peak gradually increased. In addition, as the temperature increased, the CaSO4 and SiO2 peaks decreased, and new peaks of various silicates appeared. This shows that the increase in temperature also promoted the decomposition of CaSO4 and promoted the reaction between Ca and SiO2. When the temperature was 900 °C, there was an Fe3O4 peak at 2θ = 35.5°, and when the temperature was increased to 1000 °C, this peak disappeared and an MgFe2O4 peak appeared. Thus, it is speculated that Reaction (R3) may have occurred.
| R2 |
| R3 |
Figure 8c shows the XRD spectra of ash samples with S/K = 6 at different combustion temperatures. By comparing these spectra with that of the original sample, it was found that the spectra of the two samples combusted at 600 °C still contained a large number of CaCO3 peaks, but the peak intensity of CaCO3 weakened with the addition of S. The spectrum of the original sample combusted at 700 °C still contained a small number of CaCO3 peaks, but CaCO3 no peaks were observed after the addition of S. This indicates that the addition of S inhibited the formation of CaCO3 and resulted in more Ca combining with the S to form CaSO4. In addition, when the original sample was combusted at 600–700 °C, there was a KCl peak at 2θ = 28.7°, but it disappeared when S was added, suggesting that the addition of S promoted Reaction (R1) and caused the KCl to be sulfated into K2SO4. When the temperature was higher than 800 °C, the number of Ca2SiO4 and KAlSi3O8 peaks in the spectrum of the ash sample decreased after the addition of S, which indicates that at high temperatures, the S competed with the silicates and aluminosilicates for K and Ca.
Figure 9 shows the XRD spectra of the CS/DL sample after the addition of Al2O3. It can be seen from Figure 9a, when Al/K = 1.5, a new Al2O3·3CaO·SiO2 peak appeared at 2θ = 52.3°. This may be because the addition of Al2O3 promoted Reaction (R4). In addition, when Al/K ≥ 2, a new KAlSi3O8 peak appears at 2θ = 64.1°, which may be because the addition of Al2O3 promoted Reactions (R5) and (R6).32 Therefore, increasing the Al content of the mixed fuel will generally promote the formation of more aluminosilicates and will increase the content of insoluble K in the ash.
| R4 |
| R5 |
| R6 |
Figure 9.
(a–c) XRD spectra of CS/DL ash samples after the addition of Al2O3.
The XRD spectra of the CS/DL ash samples obtained at different temperatures are shown in Figure 9b. Fe2O3 was common in the ash samples for all of the tested temperatures because the Fe content of DL was higher (Table 2). The Fe in the coal mainly existed in the form of FeS2, so Reaction (R7) occurred during the combustion process. Because there was less Ca in DL than XL, a small CaCO3 peak only appeared at 2θ = 50.1° when 600 °C. When the combustion temperature was increased to 800 °C, a large amount of CaCO3 decomposed, CaSiO3 began to form, and a large amount of Al2O3 disappeared and began to transform into aluminosilicates. As the temperature increased to 900 and 1000 °C, two new peaks (K2Fe2O4 and K6Fe2O5) appeared at 2θ = 30.1° and 31.4°. Because of the high content of Fe2O3 in the ash, it can be inferred that Reactions (R8–R10) occurred at 900 and 1000 °C.36,37
| R7 |
| R8 |
| R9 |
| R10 |
Figure 9c shows the XRD spectra of the ash samples with Al/K = 2.5 formed at different combustion temperatures. Through comparison with the spectra of the original samples, it was found that they exhibited several common features. As the temperature increased, the intensities of the CaSO4 and Fe2O3 peaks gradually decreased, and a new F6FeO5 peak appeared at 1000 °C, which indicates that when the Al content was high, Reaction (R10) was still promoted at high temperatures. Although the intensity of the CaSO4 peak decreased, calcium containing silicates such as CaSiO3 and Ca2SiO4 appeared in the original sample. However, when Al/K = 2.5, these peaks were not observed, indicating that the addition of Al may transform these silicates into aluminosilicates. When the temperature was higher than 900 °C, a new K2Al2Si2O8 peak appeared at 2θ = 30°, and the KAlSi3O8 peak at 2θ = 64° disappeared. This may be because high temperatures and high Al contents promote Reaction (R11).
| R11 |
4. Conclusions
In order to explore the influences of the S and Al in coal on the migration and transformation of K in the co-combustion of biomass and coal, elemental S and Al2O3 were added to blended fuel samples. The ash yield, release rate of K, occurrence forms of K, and the change in the crystal phase of the ash samples were studied under different combustion temperatures, S/K molar ratios, and Al/K molar ratios.
The S in coal is not completely released in the form of SO2, and it is partially retained in the ash. When S/K < 6, the release rate of K decreases with increasing S content. When S/K > 6, sulfation becomes saturated, and the addition of S will promote the release of K. The release rate of K for the sample with S/K = 6 was always lower than that of the original sample at 650–1000 °C, and the higher the temperature was, the greater the difference was. As the addition of S increased, the CaSO4 content increased, the content of water-soluble K compounds such as K2SO4 increased, and the content of insoluble K compounds such as KAlSi3O8 decreased. Therefore, the S/K ratio of biomass and coal co-combustion fuel should be less than or equal to 6 in order to control the release of K and reduce the emission of SO2.
As the Al/K ratio increased, more water-soluble K was converted into insoluble K, and the release rate of K decreased. In addition, the HCl-soluble K was converted into insoluble K. When the sample with Al/K = 2.5 and the original sample were combusted at 600–700 °C, the difference in the K release rates was relatively small. When the temperature was higher than 700 °C, the higher the temperature was, and the greater the difference in the K release rates was. This is because at high temperatures, the increase in the Al/K ratio promoted the formation of aluminosilicates containing K. Therefore, when the K content of the biomass is very high and the K release rate is also very high, the co-combustion of coal with a high Al content should be considered.
Acknowledgments
This research was supported by the National Natural Science Foundations of China [Grant No. 51976035] and the Open Foundation of the Key Laboratory for Thermal Science and Power Engineering of the Ministry of Education, Tsinghua University, China.
Author Contributions
Q.L. conceptualization, writing of the original draft. W.Z.: funding acquisition. J.Z. and Z.Y. experiments and writing. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Sami M.; Annamalai K.; Wooldridge M. Co-firing of coal and biomass fuel blends. Prog. Energy Combust. 2001, 27 (2), 171–214. 10.1016/S0360-1285(00)00020-4. [DOI] [Google Scholar]
- Baxter L. Biomass-coal co-combustion: opportunity for affordable renewable energy. Fuel 2005, 84 (10), 1295–1302. 10.1016/j.fuel.2004.09.023. [DOI] [Google Scholar]
- Spliethoff H.; Hein K. R. G. Effect of co-combustion of biomass on emissions in pulverized fuel furnaces. Fuel Process. Technol. 1998, 54 (1), 189–205. 10.1016/S0378-3820(97)00069-6. [DOI] [Google Scholar]
- Leckner B. Co-combustion - A summary of technology. Therm. Sci. 2007, 11 (4), 5–40. 10.2298/TSCI0704005L. [DOI] [Google Scholar]
- Sahu S. G.; Chakraborty N.; Sarkar P. Coal-biomass co-combustion: An overview. Renew. Sust. Energy Rev. 2014, 39, 575–586. 10.1016/j.rser.2014.07.106. [DOI] [Google Scholar]
- Niu Y.; Tan H.; Hui S. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. 2016, 52, 1–61. 10.1016/j.pecs.2015.09.003. [DOI] [Google Scholar]
- Lupianez C.; Mayoral M. C.; Guedea I.; Espatolero S.; Diez L. I.; Laguarta S.; Andres J. M. Effect of co-firing on emissions and deposition during fluidized bed oxy-combustion. Fuel 2016, 184, 261–268. 10.1016/j.fuel.2016.07.027. [DOI] [Google Scholar]
- Pronobis M. Evaluation of the influence of biomass co-combustion on boiler furnace slagging by means of fusibility correlations. Biomass Bioenergy 2005, 28 (4), 375–383. 10.1016/j.biombioe.2004.11.003. [DOI] [Google Scholar]
- Mlonka-Medrala A.; Magdziarz A.; Kalemba-Rec I.; Nowak W. The influence of potassium-rich biomass ashes on steel corrosion above 550 degrees C. Energy Convers. Manage. 2019, 187, 15–28. 10.1016/j.enconman.2019.02.074. [DOI] [Google Scholar]
- Gogebakan Z.; Gogebakan Y.; Selcuk N.; Selcuk E. Investigation of ash deposition in a pilot-scale fluidized bed combustor co-firing biomass with lignite. Bioresour. Technol. 2009, 100 (2), 1033–1036. 10.1016/j.biortech.2008.07.037. [DOI] [PubMed] [Google Scholar]
- Chen X.; Tang J.; Tian X.; Wang L. Influence of biomass addition on Jincheng coal ash fusion temperatures. Fuel 2015, 160, 614–620. 10.1016/j.fuel.2015.08.024. [DOI] [Google Scholar]
- Xing P.; Darvell L. I.; Jones J. M.; Ma L.; Pourkashanian M.; Williams A. Experimental and theoretical methods for evaluating ash properties of pine and El Cerrejon coal used in co-firing. Fuel 2016, 183, 39–54. 10.1016/j.fuel.2016.06.036. [DOI] [Google Scholar]
- Zhang J.; Han C.-L.; Yan Z.; Liu K.; Xu Y.; Sheng C.-D.; Pan W.-P. The varying characterization of alkali metals (Na, K) from coal during the initial stage of coal combustion. Energy Fuel 2001, 15 (4), 786–793. 10.1021/ef000140u. [DOI] [Google Scholar]
- Andrea Jordan C.; Akay G. Speciation and distribution of alkali, alkali earth metals and major ash forming elements during gasification of fuel cane bagasse. Fuel 2012, 91 (1), 253–263. 10.1016/j.fuel.2011.05.031. [DOI] [Google Scholar]
- Werkelin J.; Skrifvars B. J.; Zevenhoven M.; Holmbom B.; Hupa M. Chemical forms of ash-forming elements in woody biomass fuels. Fuel 2010, 89 (2), 481–493. 10.1016/j.fuel.2009.09.005. [DOI] [Google Scholar]
- Zevenhoven M.; Yrjas P.; Skrifvars B. J.; Hupa M. Characterization of Ash-Forming Matter in Various Solid Fuels by Selective Leaching and Its Implications for Fluidized-Bed Combustion. Energy Fuel 2012, 26 (10), 6366–6386. 10.1021/ef300621j. [DOI] [Google Scholar]
- Wei X.; Lopez C.; Von Puttkamer T.; Schnell U.; Unterberger S.; Hein K. R. G. Assessment of chlorine-alkali-mineral interactions during co-combustion of coal and straw. Energ. Fuel 2002, 16 (5), 1095–1108. 10.1021/ef0102925. [DOI] [Google Scholar]
- Glazer M. P.; Khan N. A.; De Jong W.; Spliethoff H.; Schürmann H.; Monkhouse P. Alkali metals in circulating fluidized bed combustion of biomass and coal: Measurements and chemical equilibrium analysis. Energy Fuel 2005, 19 (5), 1889–1897. 10.1021/ef0500336. [DOI] [Google Scholar]
- Yang T.; Kai X.; Sun Y.; He Y.; Li R. The effect of coal sulfur on the behavior of alkali metals during co-firing biomass and coal. Fuel 2011, 90 (7), 2454–2460. 10.1016/j.fuel.2011.02.031. [DOI] [Google Scholar]
- Johansen J. M.; Aho M.; Paakkinen K.; Taipale R.; Egsgaard H.; Jakobsen J. G.; Frandsen F. J.; Glarborg P. Release of K, Cl, and S during combustion and co-combustion with wood of high-chlorine biomass in bench and pilot scale fuel beds. P. Combust. Inst. 2013, 34 (2), 2363–2372. 10.1016/j.proci.2012.07.025. [DOI] [Google Scholar]
- Li R.; Kai X.; Yang T.; Sun Y.; He Y.; Shen S. Release and transformation of alkali metals during co-combustion of coal and sulfur-rich wheat straw. Energy Convers. Manage. 2014, 83, 197–202. 10.1016/j.enconman.2014.02.059. [DOI] [Google Scholar]
- Theis M.; Skrifvars B. J.; Zevenhoven M.; Hupa M.; Tran H. Fouling tendency of ash resulting from burning mixtures of biofuels. Part 2: Deposit chemistry. Fuel 2006, 85 (14–15), 1992–2001. 10.1016/j.fuel.2006.03.015. [DOI] [Google Scholar]
- Wang L.; Skreiberg O.; Becidan M.; Li H. Investigation of rye straw ash sintering characteristics and the effect of additives. Appl. Energy 2016, 162, 1195–1204. 10.1016/j.apenergy.2015.05.027. [DOI] [Google Scholar]
- Shen Y.; Hu Y.; Wang M.; Bao W.; Chang L.; Xie K. Speciation and thermal transformation of sulfur forms in high-sulfur coal and its utilization in coal-blending coking process: A review. Chin. J. Chem. Eng. 2021, 35, 70–82. 10.1016/j.cjche.2021.04.007. [DOI] [Google Scholar]
- Tian L.; Yang W.; Chen Z.; Wang X.; Yang H.; Chen H. Sulfur behavior during coal combustion in oxy-fuel circulating fluidized bed condition by using TG-FTIR. J. Energy Inst. 2016, 89, 264–270. 10.1016/j.joei.2015.01.020. [DOI] [Google Scholar]
- De S.; Assadi M. Impact of cofiring biomass with coal in power plants – A techno-economic assessment. Biomass Bioenergy 2009, 33 (2), 283–293. 10.1016/j.biombioe.2008.07.005. [DOI] [Google Scholar]
- Liu Y.; Cheng L.; Zhao Y.; Ji J.; Wang Q.; Luo Z.; Bai Y. Transformation behavior of alkali metals in high-alkali coals. Fuel. Process Technol. 2018, 169, 288–294. 10.1016/j.fuproc.2017.09.013. [DOI] [Google Scholar]
- Aho M.; Ferrer E. Importance of coal ash composition in protecting the boiler against chlorine deposition during combustion of chlorine-rich biomass. Fuel 2005, 84 (2), 201–212. 10.1016/j.fuel.2004.08.022. [DOI] [Google Scholar]
- Arvelakis S.; Jensen P. A.; Dam-Johansen M. Simultaneous thermal analysis (STA) on ash from high-alkali biomass. Energy Fuel 2004, 18 (4), 1066–1076. 10.1021/ef034065+. [DOI] [Google Scholar]
- Mason P. E.; Darvell L. I.; Jones J. M.; Williams A. Observations on the release of gas-phase potassium during the combustion of single particles of biomass. Fuel 2016, 182, 110–117. 10.1016/j.fuel.2016.05.077. [DOI] [Google Scholar]
- Yang Y. B.; Sharifi V. N.; Swithenbank J.; Ma L.; Darvell L. I.; Jones J. M.; Pourkashanian M.; Williams A. Combustion of a Single Particle of Biomass. Energy Fuel 2008, 22 (1), 306–316. 10.1021/ef700305r. [DOI] [Google Scholar]
- Xue Z.; Zhong Z.; Zhang B.; Zhang J.; Xie X. Potassium transfer characteristics during co-combustion of rice straw and coal. Appl. Therm. Eng. 2017, 124, 1418–1424. 10.1016/j.applthermaleng.2017.06.116. [DOI] [Google Scholar]
- Davidsson K. O.; Amand L. E.; Leckner B.; Kovacevik B.; Svane M.; Hagstrom M.; Pettersson J. B. C.; Pettersson J.; Asteman H.; Svensson J. E.; Johansson L. G. Potassium, chlorine, and sulfur in ash, particles, deposits, and corrosion during wood combustion in a circulating fluidized-bed boiler. Energy Fuel 2007, 21 (1), 71–81. 10.1021/ef060306c. [DOI] [Google Scholar]
- Shah K. V.; Cieplik M. K.; Betrand C. I.; van de Kamp W. L.; Vuthaluru H. B. Correlating the effects of ash elements and their association in the fuel matrix with the ash release during pulverized fuel combustion. Fuel Process. Technol. 2010, 91 (5), 531–545. 10.1016/j.fuproc.2009.12.016. [DOI] [Google Scholar]
- Schuermann H.; Monkhouse P. B.; Unterberger S.; Hein K. R. G. In situ parametric study of alkali release in pulverized coal combustion: Effects of operating conditions and gas composition. P. Combust. Inst. 2007, 31 (2), 1913–1920. 10.1016/j.proci.2006.07.040. [DOI] [Google Scholar]
- Lin W. G.; Dam-Johansen K.; Frandsen F. Agglomeration in bio-fuel fired fluidized bed combustors. Chem. Eng. J. 2003, 96 (1–3), 171–185. 10.1016/j.cej.2003.08.008. [DOI] [Google Scholar]
- Chi H.; Pans M. A.; Sun C.; Liu H. An investigation of lime addition to fuel as a countermeasure to bed agglomeration for the combustion of non-woody biomass fuels in a 20kWth bubbling fluidised bed combustor. Fuel 2019, 240, 349–361. 10.1016/j.fuel.2018.11.122. [DOI] [Google Scholar]