Abstract
Background: The current treatments for chronic knee osteoarthritis (OA), a disabling and costly healthcare condition in the United States, vary in their level of supporting evidence. Although total knee replacement is one of the best-supported interventions, its associated risks should not be taken lightly, especially in older patients with comorbidities. Genicular nerve block with subsequent genicular nerve radiofrequency neurotomy (GN-RFN) has emerged as a promising intervention for refractory pain in knee OA. Purposes/Questions: We sought to assess the pain and functional outcomes of genicular nerve bipolar radiofrequency neurotomy (B-RFN) for the treatment of chronic pain due to knee OA. Methods: A total of 21 patients who underwent unilateral genicular nerve B-RFN after positive diagnostic genicular nerve block (50% or greater pain relief) treated between July 2018 to December 2018 were included. Pain numeric rating scale (NRS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores were collected at baseline, 3 months, and 6 months post-B-RFN. Changes at each time point were compared to baseline scores using paired sample t tests. Results: At 3 months, 62% of patients had a greater than 50% improvement in NRS scores and 57% of patients had a greater than 50% improvement in WOMAC scores. At 6 months, 81% of patients had a greater than 50% improvement in NRS scores and 67% had a greater than 50% improvement in WOMAC scores. The absolute change in mean NRS (± standard deviation) at 6 months went from 7.5 ± 1.9 to 2.5 ± 1.2. The absolute change in mean WOMAC scores at 6 months went from 46.9 ± 8.0 to 19.0 ± 6.2. Conclusion: Of 21 patients, 14 (67%) saw greater than 50% improvements in both NRS and WOMAC scores at 6 months after genicular nerve B-RFN. Further prospective studies are needed to determine the selection criteria of patients most likely to benefit from this procedure.
Keywords: radiofrequency ablation, pain medicine, interventional pain management
Introduction
Chronic knee osteoarthritis (OA) is a disabling and costly healthcare condition in the United States. One study estimated the average lifetime costs for persons diagnosed with knee OA to be as high as $140,300, $129,600 attributed to the medical cost alone [10]. Furthermore, the prevalence of knee OA is increasing. A 2017 study of cadaver-derived skeletons of people 50 years or older (n = 2516) and the Framingham Osteoarthritis Study showed that the prevalence of knee OA doubled since the mid-20th century, controlling for age, body mass index (BMI), and other variables [12,15].
Unfortunately, the current treatments for knee OA vary in their level of supporting evidence. There were as many as 23 guidelines for recommendations on the management of hip and knee OA in a systematic review of the available literature that found the overall quality of evidence is limited [16]. Although total knee replacement is one of the best-supported interventions, the risks associated with procedure should not be taken lightly, especially for older patients with comorbidities. In a cohort study of 83,756 procedures performed between 1997 and 2011, 3% of patients who underwent total joint replacements experienced a venous thromboembolism (VTE), myocardial infarction (MI), stroke, or bleeding [13].
Genicular nerve block and subsequent genicular nerve radiofrequency neurotomy (GN-RFN), a novel intervention for refractory pain in knee OA, has shown promise in short- and long-term follow-ups. In a 2011 double-blind randomized trial of 38 patients, Choi et al demonstrated the effectiveness of GN-RFN in short-term pain relief at 4 and 12 weeks [3]. Similarly, in 2017 a non-controlled longitudinal study, Pineda et al demonstrated the effectiveness of GN-RFN in long-term pain relief (greater than 50% improvement) at 6 months based on visual analogue pain scale and disability scores (Western Ontario and McMaster Universities Osteoarthritis) [14]. Even more promising, a systemic review of GN-RFN concluded that the procedure provided optimal results for up to 1 year with minimal complications [7].
There are different techniques for performing GN-RFN: conventional, pulsed, cooled, and bipolar. Each method varies on the number of RFN electrodes, gauge of the needle, duration of the burn, maximal temperature, size of the burn diameter, and cost. One downside of the conventional GN-RFN, which is the oldest and more commonly performed technique, is that the burn diameter is limited. The minimal burn diameter of the largest size needle available commercially (16 gauge) is only 2.4 mm, requiring extreme precision with each needle placement [1]. Hence, techniques such as cooled and bipolar GN-RFN (B-GN-RFN) have emerged to increase the size of the burn diameter and ultimately the success of GN-RFN.
Our study aimed to evaluate patient reported functional outcomes and improvement in pain after B-GN-RFN for the treatment of chronic knee pain due to OA.
Methods
This retrospective chart review conducted at an urban academic outpatient physiatry clinic was approved by our institutional review board. Patients treated between July 2018 to December 2018 were included. Inclusion criteria were (1) ages 18 to 99 years; (2) radiographic evidence of knee OA, using a Kellgren-Lawrence classification; (3) completion of a physical therapy program; (4) 50% or greater concordant pain relief of typical knee pain during walking and weight bearing following a set of diagnostic superomedial (SM), superolateral (SL), and inferomedial (IM) genicular nerve blocks with 1 mL of 2% lidocaine at each location; (5) B-GN-RFN of the SM, SL, and IM genicular nerves; and (6) at least 6 months of follow-up after the B-GN-RFN procedure.
Patients were positioned supine, with the affected knee placed in 30° to 40° flexion. Local anesthesia was provided superficially with 1 to 2 mL 1% lidocaine using a 25-G needle. We targeted the SM, SL, and IM genicular nerves with 2 20 G 100 mm with 10 mm active tip introducer needle cannulas (EPIMED, Dallas, Texas). The 2 cannulas were separated by approximately 1 cm. The SM genicular nerve site was identified at the confluence of the medial femoral shaft and medial femoral condyle on anteroposterior (AP) view and at the midpoint of the femur on the lateral view. The SL genicular nerve site was identified at the confluence of the lateral femoral shaft and the lateral femoral condyle on AP view and at the midpoint of the femur on the lateral view. The IM genicular nerve site was identified at the confluence of the medial tibial shaft and the tibial flare on AP view and at the midpoint of the tibia on lateral view (Fig. 1). The femoral condyle was superimposed by adjusting the fluoroscope obliquity on all lateral views. In addition, the tibial plateau was squared off by adjusting the fluoroscope “wig-wag.” With the introducer needles in place, a radiofrequency electrode was placed into each introducer. Needle positioning was confirmed using AP and lateral fluoroscopic views (Fig. 2 and Fig. 3). Motor nerve activity was ruled out with 2 Hz stimulation at 1 mA. Then, 1 mL 2% lidocaine was injected through all 6 introducer needles at each of the 3 sites. Each nerve then underwent a bipolar lesion for 150 seconds at a temperature of 80°C, which imparts a tissue temperature of 77°C to 80°C surrounding the electrode [6].
Fig. 1.
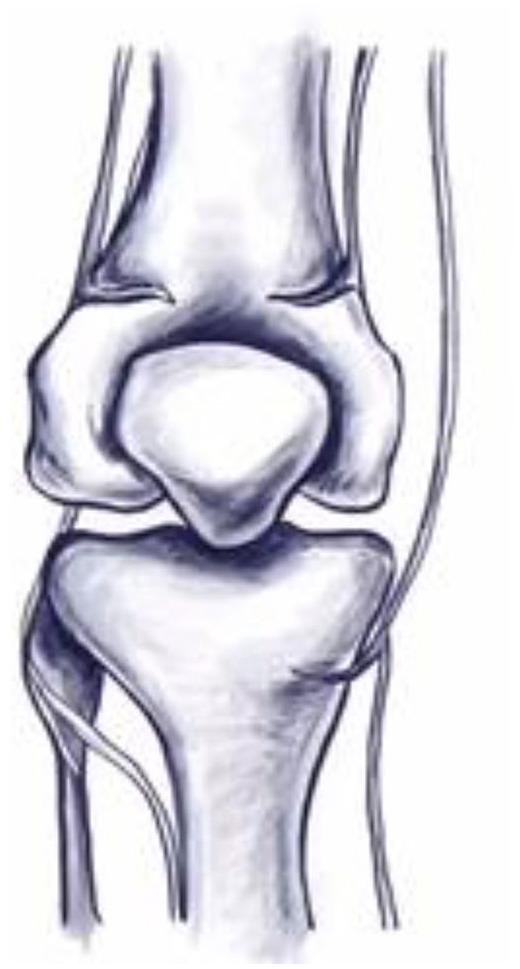
Illustration of the genicular nerve trajectory (superior lateral, superior medial, and inferior medial branches). Illustration by Susie S. Kwon, MD.
Fig. 2.
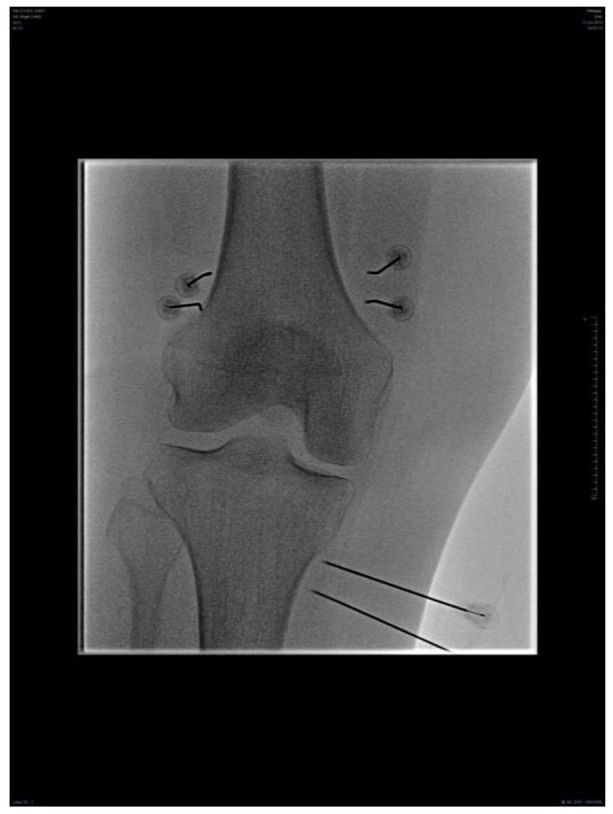
Anterior-posterior view: fluoroscopic image of a right-sided bipolar genicular nerve radiofrequency neurotomy.
Fig. 3.

Lateral view: fluoroscopic image of a right-sided bipolar genicular nerve radiofrequency neurotomy.
We retrospectively reviewed and collected the following data from charts: demographics (age, sex), weight, BMI, numeric rating scale (NRS) pain score prior to B-GN-RFN, WOMAC Physical Function score (WOMAC score) prior to B-GN-RFN, and laterality of B-GN-RFN procedure. All participants completed patient reported outcomes that included their current NRS score for knee pain, and their WOMAC score, at 3 months and 6 months following B-GN-RFN.
The primary outcome, defined as complete responders, were patients reporting a combination of greater than 50% improvement in the NRS score and greater than 50% improvement in the WOMAC score. Secondary measures, defined as partial responders, included a greater than 50% improvement in the NRS score or a greater than 50% improvement in the WOMAC score, as well as absolute changes in the NRS and WOMAC scores.
Statistical Analysis
Normality of these data was assessed using the Shapiro–Wilk normality test. Binomial confidence intervals were calculated using the classic Clopper–Pearson method. P value of less than 0.05 was required to reject the null hypothesis. Data analysis was performed using Microsoft Excel for Mac 2011 Version 14.0.0 (Microsoft, Redmond, Washington)
Results
A total of 21 patients met the inclusion criteria. Table 1 reflects the baseline demographic, clinical, and procedural characteristics of the patients included in the study, as well as their baseline NRS and WOMAC scores: 7.5 ± 1.9 and 46.9 ± 8.0, respectively.
Table 1.
Demographic data.
| N = 21 | |
|---|---|
| Age | 67.5 ± 9.0 a |
| Gender (Female) | 9 (43%) |
| BMI | 31.1 ± 4.6 |
| Left | 10 (47%) |
| Right | 8 (36%) |
| Bilateral | 3 (14% |
| Baseline NRS | 7.5 ± 1.9 a |
| Baseline WOMAC | 46.9 ± 8.0 a |
BMI body mass index, NRS numeric rating scale, WOMAC Western Ontario and McMaster Universities Osteoarthritis Index.
Mean ± standard deviation.
In addition, 14 of the 21 patients (67%) were complete responders at 6 months post-procedure. There were no reported serious adverse events. Partial responders (those who achieved >50% improvement in either NRS or WOMAC scores) at 3 and 6 months post-procedure are listed in Table 2.
Table 2.
Partial responders (>50% improvement in either NRS or WOMAC).
| N = 21 | 3 months | 6 months |
|---|---|---|
| >50% improvement in NRS | 13 (62%) | 17 (81%) |
| >50% improvement in WOMAC | 12 (57%) | 14 (67%) |
NRS numeric rating scale, WOMAC Western Ontario and McMaster Universities Osteoarthritis Index.
At 3 months, 62% of patients showed >50% improvement in NRS and 57% of patients showed >50% improvement in WOMAC score. At 6 months, 81% of patients showed >50% improvement in NRS and 67% of patients showed >50% improvement in WOMAC scores. The number of partial responders increased from 3 to 6 months by 19% in NRS and 10% in WOMAC scores, suggesting durability of the results.
The overall change in NRS scores at 3 and 6 months post-procedure (Table 3) documented that patients improved from 7.5 ± 1.9 at baseline to 3.3 ± 2.0 at 3 months and then 2.5 ± 1.2 at 6 months showing no significant change at 3 months (P = .14) but significant improvement at 6 months (P = .03). The overall change in WOMAC scores at baseline, 3, and 6 months post-procedure went from 46.9 ± 8.0 to 24.6 ± 8.6 to 19.0 ± 6.2, respectively (Table 4). The change in WOMAC scores progressively improved from baseline to 6 months. Based on previous work, the minimal clinically important differences for changes in WOMAC pain and function scores was set at 20%. We used 50% improvement in function to determine responders for categorical analysis.
Table 3.
Numerical Rating Scale Scores.
| N = 21 | P value (CI) | |
|---|---|---|
| Baseline | 7.5 ± 1.9 | |
| 3 months | 3.3 ± 2.0 | P = .14 (-1.4 to 9.8) |
| 6 months | 2.5 ± 1.2 | P = .03 (0.5 to 9.5) |
CI confidence interval.
Paired t test to calculate statistical significance (P < .05).
Data reported as mean ± standard deviation.
Table 4.
Change in WOMAC.
| N = 21 | P value (CI) | |
|---|---|---|
| Baseline | 46.9 ± 8.0 | |
| 3 months | 24.6 ± 8.6 | P = .06 (-1.4 to 46.0) |
| 6 months | 19.0 ± 6.2 | P = .01 (7.4 to 48.4) |
WOMAC Western Ontario and McMaster Universities Osteoarthritis Index, CI confidence interval.
Paired t test to calculate statistical significance (P < .05).
Data reported as mean ± standard deviation.
The primary outcome, a greater than 50% improvement in both the NRS AND WOMAC scores, was achieved in 9 of 21 patients (43%) at 3 months post-procedure (Table 5).
Table 5.
Complete responders (>50% improvement in both NRS or WOMAC).
| N = 21 | 3 months | 6 months |
|---|---|---|
| >50% improvement in NRS and WOMAC | 9 (43%) | 14 (67%) |
NRS numeric rating scale, WOMAC Western Ontario and McMaster Universities Osteoarthritis Index.
Discussion
This study investigated pain and functional outcomes of patients who underwent B-GN-RFN to address chronic knee pain secondary to OA. The success rate (when success was defined as greater than 50% improvement in both NRS and WOMAC scores) at 3 and 6 months was 43 and 67%, respectively. Of note, 3 patients had 100% pain relief (NRS score of 0) at 3 months post-procedure. In 2 of these 3 patients, the 100% pain relief was sustained through 6 months. Most important, none of the 21 patients underwent knee arthroplasty by the 6-month follow-up.
The current study is limited by its retrospective design and lack of a control/comparison group. Given the natural history of the knee and progression of knee OA, 6-month follow-up is short and therefore a limitation of this study. Furthermore, other factors shown to affect patient pain outcomes (including anxiety, depression, other psychological comorbidities, and level of function) were not assessed or controlled for. Future trials will aim to include a prospective design, use sham groups, and control for factors shown to affect patient pain outcomes.
Other nonoperative treatments for knee OA such as intraarticular cortisone injections have not been shown to have long term clinical benefit. A 2015 Cochrane review found that corticosteroid injections into the knee had no clear benefit at 1 to 6 weeks and no evidence of benefit up to 6 months. The authors graded this quality of evidence as “low” [9].
Also, a systematic review performed to determine the clinical significance of injectable hyaluronic acid (HA) for knee OA did not show clinically important differences of HA treatment over placebo [8].
It is notable to mention that this B-GN-RFN study found 62% of patients had greater than 50% improvement in NRS score alone at 3 months post-procedure. However, this study’s long-term benefit at 6 months is notable for 67% success rate, which again, is defined as greater than 50% improvement in both NRS and WOMAC scores. This is higher than that of 35% success rate mentioned in the McCormick et al study, which defined their success rate as greater than 50% improvement in NRS and reduction of 3.4 or more points in Medication Quantification Scale III, utilizing cooled genicular nerve RFN (C-GN-RFN) [11]. This finding may suggest the superiority of B-GN-RFN over C-GN-RFN in terms of pain control and functional outcome over long-term.
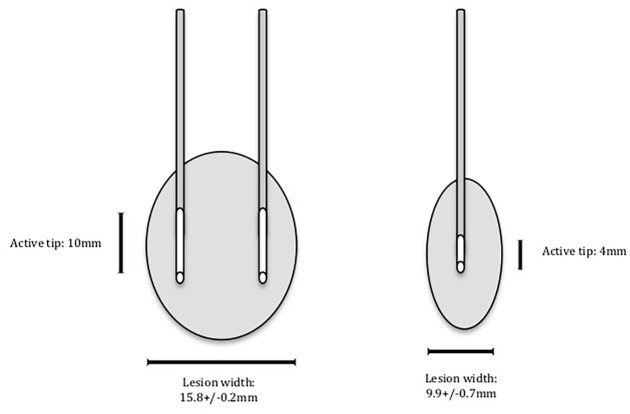
Current literature lacks direct comparison of B-GN-RFN to C-GN-RFN. However, the Cheng et al study successfully showed the superiority of B-RFN over C-RFN in targeting sacroiliac joint. It showed that the percentage of patients with greater than 50% reduction in pain was higher in B-RFN compared to C-RFN in sacroiliac joint at 3 months, 6 months, and 12 months follow-up [2]. This significant difference may be attributable to the larger lesion size bipolar technique can produce compared to that of the C-RFN. Cosman et al quantified lesion volume stratified by various metrics for different RFN’s. They found that a 20G bipolar probe, 10 mm tip length, with 10 mm spacing at a 150 second burn duration resulted in a 15.8 ± 0.2 mm lesion width, which is much larger than the 9.9 ± 0.7 mm lesion width produced by C-RFN using 18G, 4 mm tip length for the same duration [4] (Fig. 4). This study suggested that the bigger lesion using B-RFN compared to C-RFN technique may increase the likelihood of a more effective neurolysis and improved pain outcomes.
Fig. 4.
Illustration of lesion size utilizing bipolar radiofrequency neurotomy (RFN) done with 20-gauge probe compared to that of cooled RFN done with an 18-gauge probe.
In addition, B-RFN may offer its superiority to C-RFN in time and cost efficiency. Cheng et al found a 50% reduction in operating room time, 80% less X-ray/fluoroscopy time, and at least $1,000 in cost savings when performing B-RFN compared to C-RFN for the sacroiliac joint [2].
In terms of time efficiency, our B-GN-RFN treatment time using the conventional radiofrequency generator was 300 seconds total as compared to 450 seconds total required for C-RFN. For our B-GN-RFN, 4 lesions surrounding the SL and SM genicular nerves can be performed simultaneously for 150 seconds followed by 2 lesions surrounding IM genicular nerve for additional 150 seconds. On the other hand, C-RFN requires each genicular nerve to be lesioned for 150 seconds, for a total of 450 seconds.
The setting (office vs. ambulatory/hospital surgery center) the procedure is performed at also bears consideration when recommending GN-RFN to patients. Due to regulatory and compliance issues, C-RFN is currently only performed in ambulatory/hospital surgery centers, while B-RFN can be performed in office-based setting [5]. This directly impacts healthcare costs and in some cases out of pocket expenses to the patient. Looking at costs according to the Centers for Medicare and Medicaid Services fee-for-service payments (https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched), performing an office based bipolar genicular ablation costs on average $136.00 per nerve for a total of $408.00 compared to a total cost of $1719.00 if done in the hospital outpatient or ambulatory surgery center settings. Further, the cost of other conventional treatments such as viscosupplementation injections average $1500.00 for a series of 3 injections.
In conclusion, this retrospective, cross-sectional survey of patients who underwent B-GN-RFN to address chronic knee pain due to primary OA documented that 14 of 21 patients (67%) saw more than 50% improvements in both NRS and WOMAC scores 6 months after genicular B-RFN. The data suggest a clinically important long-term improvement in pain scores at 6 months, comparable to and possibly superior to outcomes after conventional and C-RFN of the genicular nerves. Nearly 10% of patients experienced 100% pain relief at 6 months. Furthermore, B-GN-RFN can be performed in an office setting, which can decrease healthcare costs as well as improve accessibility for patients. Prospective studies are needed to determine the selection criteria of patients most likely to benefit from this procedure.
Supplemental Material
Supplemental material, sj-docx-1-hss-10.1177_15563316211040416 for Bipolar Radiofrequency Neurotomy of the Genicular Nerves for Chronic Pain Due to Knee Osteoarthritis by Jaspal R. Singh, Susie S. Kwon, Frank V. Schirripa, Behnum A. Habibi and Ethan Rand in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Supplemental material, sj-docx-2-hss-10.1177_15563316211040416 for Bipolar Radiofrequency Neurotomy of the Genicular Nerves for Chronic Pain Due to Knee Osteoarthritis by Jaspal R. Singh, Susie S. Kwon, Frank V. Schirripa, Behnum A. Habibi and Ethan Rand in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Supplemental material, sj-docx-3-hss-10.1177_15563316211040416 for Bipolar Radiofrequency Neurotomy of the Genicular Nerves for Chronic Pain Due to Knee Osteoarthritis by Jaspal R. Singh, Susie S. Kwon, Frank V. Schirripa, Behnum A. Habibi and Ethan Rand in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Supplemental material, sj-docx-4-hss-10.1177_15563316211040416 for Bipolar Radiofrequency Neurotomy of the Genicular Nerves for Chronic Pain Due to Knee Osteoarthritis by Jaspal R. Singh, Susie S. Kwon, Frank V. Schirripa, Behnum A. Habibi and Ethan Rand in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Supplemental material, sj-docx-5-hss-10.1177_15563316211040416 for Bipolar Radiofrequency Neurotomy of the Genicular Nerves for Chronic Pain Due to Knee Osteoarthritis by Jaspal R. Singh, Susie S. Kwon, Frank V. Schirripa, Behnum A. Habibi and Ethan Rand in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Acknowledgments
Susie S. Kwon, MD, illustrated Figs 1 and 4.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Human/Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent: Informed consent was waived from all patients included in this study.
Level of Evidence: Level IV: Retrospective Therapeutic Study.
Required Author Forms: Disclosure forms provided by the authors are available with the online version of this article as supplemental material.
References
- 1. Bogduk N. Practice Guidelines for Spinal Diagnostic and Treatment Procedures. 2nd ed. San. Francisco, CA: International Spine Intervention Society; 2013. [Google Scholar]
- 2. Cheng J, Chen SL, Zimmerman N, Dalton JE, LaSalle G, Rosenquist R. A new radiofrequency ablation procedure to treat sacroiliac joint pain. Pain Physician. 2016;19(8):603–615. [PubMed] [Google Scholar]
- 3. Choi WJ, Hwang SJ, Song JG, et al. Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double-blind randomized controlled trial. Pain. 2011;1523:481–487. [DOI] [PubMed] [Google Scholar]
- 4. Cosman ER, Dolensky JR, Hoffman RA. Factors that affect radiofrequency heat lesion size. Pain Med. 2014;15(12):2020–2036. [DOI] [PubMed] [Google Scholar]
- 5. Desai M, Bentley A, Keck WA, Haag T, Taylor RS, Dakin H. Cooled radiofrequency ablation of the genicular nerves for chronic pain due to osteoarthritis of the knee: a cost-effectiveness analysis based on trial data. BMC Musculoskelet Disord. 2019;20:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Franco C, Buvanendran A, Petersohn J, Menzies RD, Menzies LP. Innervation of the anterior capsule of the human knee. Implications for radiofrequency ablation. Reg Anesth Pain Med. 2015;54:363–368. [DOI] [PubMed] [Google Scholar]
- 7. Gupta A, Huettner DP, Dukewich M. Comparative effectiveness review of cooled versus pulsed radiofrequency ablation for the treatment of knee osteoarthritis: a systematic review. Pain Physician. 2017;20(3):155–171. [PubMed] [Google Scholar]
- 8. Jevsevar D, Donnelly P, Brown GA, Cummins DS. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J Bone Joint Surg Am. 2015;97(24):2047–2060. 10.2106/JBJS.N.00743. [DOI] [PubMed] [Google Scholar]
- 9. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;10:CD005328. 10.1002/14651858.CD005328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of knee osteoarthritis management in the united states: impact of extending indications for total knee arthroplasty. Arthritis Care Res. 2015;67(2):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mccormick ZL, Korn M, Reddy R, et al. Cooled radiofrequency ablation of the genicular nerves for chronic pain due to knee osteoarthritis: six-month outcomes. Pain Med. 2017;18(9):1631–1641. [DOI] [PubMed] [Google Scholar]
- 12. Nguyen UD, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155(11):725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J. 2014;96-B4:479–485. [DOI] [PubMed] [Google Scholar]
- 14. Pineda MM, Vanlinthout LE, Martín AM, van Zundert J, Huertas FR, Novalbos Ruiz JP. Analgesic effect and functional improvement caused by radiofrequency treatment of genicular nerves in patients with advanced osteoarthritis of the knee until 1 year following treatment. Reg Anesth Pain Med. 2017;42(1):62–68. [DOI] [PubMed] [Google Scholar]
- 15. Wallace IJ, Worthington S, Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci U S A. 2017;114(35):9332–9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang W, Moskowitz R, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15(9):981–1000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-hss-10.1177_15563316211040416 for Bipolar Radiofrequency Neurotomy of the Genicular Nerves for Chronic Pain Due to Knee Osteoarthritis by Jaspal R. Singh, Susie S. Kwon, Frank V. Schirripa, Behnum A. Habibi and Ethan Rand in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Supplemental material, sj-docx-2-hss-10.1177_15563316211040416 for Bipolar Radiofrequency Neurotomy of the Genicular Nerves for Chronic Pain Due to Knee Osteoarthritis by Jaspal R. Singh, Susie S. Kwon, Frank V. Schirripa, Behnum A. Habibi and Ethan Rand in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Supplemental material, sj-docx-3-hss-10.1177_15563316211040416 for Bipolar Radiofrequency Neurotomy of the Genicular Nerves for Chronic Pain Due to Knee Osteoarthritis by Jaspal R. Singh, Susie S. Kwon, Frank V. Schirripa, Behnum A. Habibi and Ethan Rand in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Supplemental material, sj-docx-4-hss-10.1177_15563316211040416 for Bipolar Radiofrequency Neurotomy of the Genicular Nerves for Chronic Pain Due to Knee Osteoarthritis by Jaspal R. Singh, Susie S. Kwon, Frank V. Schirripa, Behnum A. Habibi and Ethan Rand in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Supplemental material, sj-docx-5-hss-10.1177_15563316211040416 for Bipolar Radiofrequency Neurotomy of the Genicular Nerves for Chronic Pain Due to Knee Osteoarthritis by Jaspal R. Singh, Susie S. Kwon, Frank V. Schirripa, Behnum A. Habibi and Ethan Rand in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery