Abstract
Background
The cardiovascular effects of glucagon-like peptide-1 (GLP-1) receptor agonists are still controversial in the treatment of type 2 diabetes mellitus (T2DM) patients. The purpose of this study was to evaluate the risk of cardiovascular events of GLP-1 (albiglutide, exenatide, liraglutide, semaglutide, lixisenatide and dulaglutide) receptor agonists in T2DM patients.
Methods
PubMed and Embase were searched to find relevant randomized controlled trials (RCTs) from inception to June 2019 that evaluated the effect of GLP-1 receptor agonists on cardiovascular events in patients with T2DM. The T2DM patients of all the eligible trials received either GLP-1 therapy or placebo, and the cardiovascular outcomes included death from cardiovascular causes, fatal or non-fatal myocardial infarction and fatal or non-fatal stroke.
Results
We included 6 multinational double-blind randomized placebo-control trials that included a total of 52821 T2DM patients. The results indicated that GLP-1 receptor agonists reduced the risk of death from cardiovascular causes (RR: 0.90; 95% CI: 0.83–0.97; P = 0.004) and fatal or non-fatal stroke (RR: 0.85; 95% CI: 0.77–0.94; P = 0.001) compared with the placebo controls. But GLP-1 receptor agonists did not significantly alter the fatal or non-fatal myocardial infarction compared with the placebo (RR: 0.91; 95% CI: 0.82 – 1.01; P = 0.06).
Conclusion
We concluded that GLP-1 receptor agonist therapy could reduce the risk of death from cardiovascular causes and fatal or non-fatal stroke compared with the placebo in the treatment of T2DM patients in trials with cardiovascular outcomes.
Keywords: Glucagon-like peptide-1, Type 2 diabetes mellitus, Cardiovascular events
Background
Type 2 diabetes mellitus patients have a very high risk of cardiovascular events, including death from cardiovascular causes, fatal or non-fatal myocardial infarction and fatal or non-fatal stroke [1]. The rates of cardiovascular death are 2- to 4-fold higher for patients with diabetes compared with the rates for those without diabetes [2]. GLP-1 receptor agonists, which as the glucose-lowering therapeutic agents in the treatment of type 2 diabetes mellitus, have been shown to affect the incidence of cardiovascular outcomes in patients with type 2 diabetes mellitus, although the results regarding GLP-1 receptor agonists remain inconsistent [3, 4]. It is well known that GLP-1, as a peptide hormone, stimulates insulin secretion and inhibits glucagon secretion in a glucose-dependent manner [5]. An increasing number of studies have shown that glucagon-like peptide-1 (GLP-1) may improve endothelial functioning and may have direct effects in protecting the vascular system [6]. There are several GLP-1 receptor agonists that are used as therapeutic agents for treating type 2 diabetes mellitus patients in clinical fields. Recently, the GLP-1 receptor was believed to have an effect on individual cardiovascular outcomes in the treatment of diabetes, but not all GLP-1 receptor agonists showed the effect of reducing cardiovascular outcomes because of the varied effectiveness of the different GLP-1 drugs. Just like some multinational randomized controlled trials elaborated that the use of GLP-1 receptor agonists to reduce the rate of cardiovascular events in T2DM patients [7–10]. While other clinical studies concluded that the GLP-1 receptor agonists did not significantly alter the major cardiovascular outcomes in patients with type 2 diabetes [3, 4]. Moreover several meta-analysis had concentrated on the cardiovascular effects and the safety in GLP-1-treated T2DM patients [11, 12], but the conclusion were inconsistent.
Therefore, we performed a meta-analysis of double-blind randomized placebo-controlled clinical trials to investigate the cardiovascular complications of GLP-1 receptor agonists in T2DM patients. The cardiovascular outcomes included death from cardiovascular causes, fatal or non-fatal myocardial infarction and fatal or non-fatal stroke.
Methods
Data sources and search strategy
We comprehensively searched PubMed and Embase to find relevant randomized controlled trials (RCTs) from inception to June 2019 that evaluated the effect of GLP-1 receptor agonists on cardiovascular events in patients with T2DM. This meta-analysis was conducted and reported in accordance with the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statements [13]. We adhere to the PRISMA guidelines in this meta-analysis [13]. The language was confined to English. The search terms were as follows: “incretin” OR “GLP-1” OR “glucagon-like peptide-1 analogue” OR “Liraglutide” OR “exenatide” OR “liraglutide” OR “lixisenatide” OR “albiglutide” OR “dulaglutide” OR “semaglutide” OR “taspoglutide” AND “type 2 diabetes mellitus” OR “T2DM” AND “randomized controlled trials” OR “RCT”. We also comprehensively screened the references of reviews and articles in order to find more eligible articles.
Data selection criteria
The literature search was screened independently by two authors; if there were some inconsistencies, we discussed within the group until a consensus was reached. The research titles and abstracts were initially screened, and then we screened the study design, interventions, control, and outcomes in detail to determine the included trials.
The criteria for including eligible studies were as follows: (1) the studies were double-blind, randomized placebo-controlled trials; (2) the RCTs were evaluating GLP-1 versus placebo in T2DM patients; (3) there was a comparison of cardiovascular risk between GLP-1 receptor agonists and placebo in T2DM patients with or without cardiovascular diseases; and (4) a risk ratio (RR) with corresponding 95% confidence intervals (CIs) or data was reported.
Regarding the exclusion criteria, we excluded studies with the following criteria: (1) the case and control patients; (2) studies with irrelevant data and the small sample size trials which contain less than 3000 T2DM patients; and (3) duplicate publications, animal experimental studies, reviews, conference abstracts, or meta-analyses.
Data extraction and quality assessment
The following data were extracted from the included RCTs by two authors independently: first author’s name; publication year, country, sample size, study design, intervention, glycated haemoglobin, duration of diabetes and the follow-up periods. The quality of the included studies was assessed by the Cochrane Collaboration tool for the risk of bias [14]. Each studies was identified as “low,” “high” or “unclear” risk of bias based on the following items: the statement of randomization, blinding, details of withdrawals and dropouts, generation of random numbers, and concealment of allocation. The quality of each results was assessed according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system which classifies the quality of evidence as high, moderate, low or very low [15].
Data synthesis and statistical analysis
This meta-analysis was performed using Review Manager 5.3 software (RevMan), The Cochrane Collaboration, Copenhagen. For the cardiovascular events in our study, we calculated the risk ratio (RR) with 95% confidence intervals (CIs) to standardize the differences between the GLP-1 receptor agonist and placebo. The forest plots were conducted using a fixed-effect model if there was no obvious heterogeneity or using a random-effect model when heterogeneity of the included studies was obvious [16, 17]. Additionally, the chi-squared (χ2) test and the I2 test were used to assess the heterogeneity between studies. When P ≤ 0.10 and I2 > 50%, the heterogeneity between those included studies was defined as obvious heterogeneity [18]. Moreover, if the I2 test value was 25–50%, it was defined as mild heterogeneity, 50–75% as moderate heterogeneity, and 75% as severe heterogeneity. To measure publication bias, we performed a funnel plot and Egger’s tests. A funnel plot was used to qualitatively measure the publication bias [19, 20], and P ≤ 0.05 was considered significant publication bias in this meta-analysis.
Results
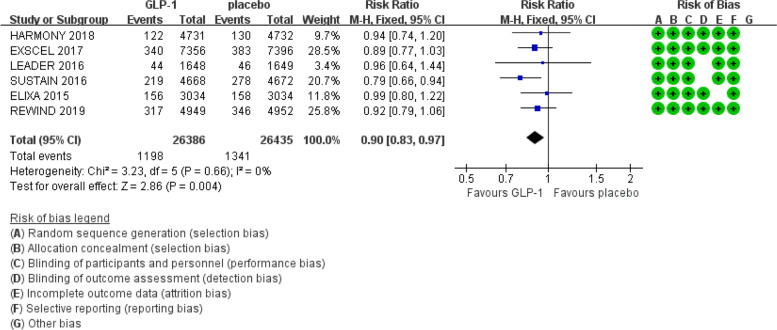
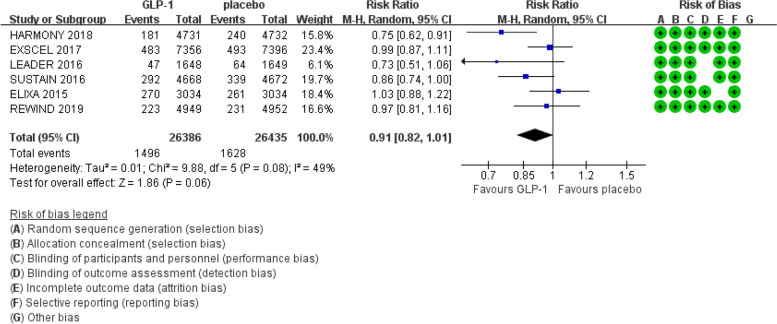
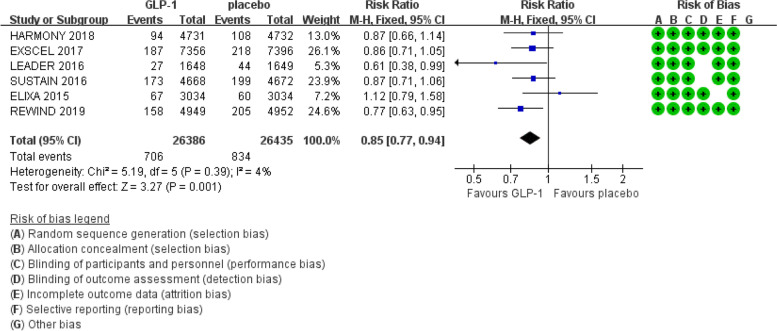
We obtained 1930 articles after searching PubMed and Embase from 2011 to June 2019. Then, we screened the titles and abstracts and removed the duplicate articles, reviews and conference abstracts, and 11 articles remained for evaluating the details of the full text to determine whether they met the inclusion criteria. Finally, 6 trials were included in this meta-analysis (Fig. 1) [3, 4, 7–10]. The Cochrane Collaboration tool was applied to evaluate the quality of the included trials. The results regarding the individual quality of the included trials are shown in Fig. 2,3,4.
Fig. 1.
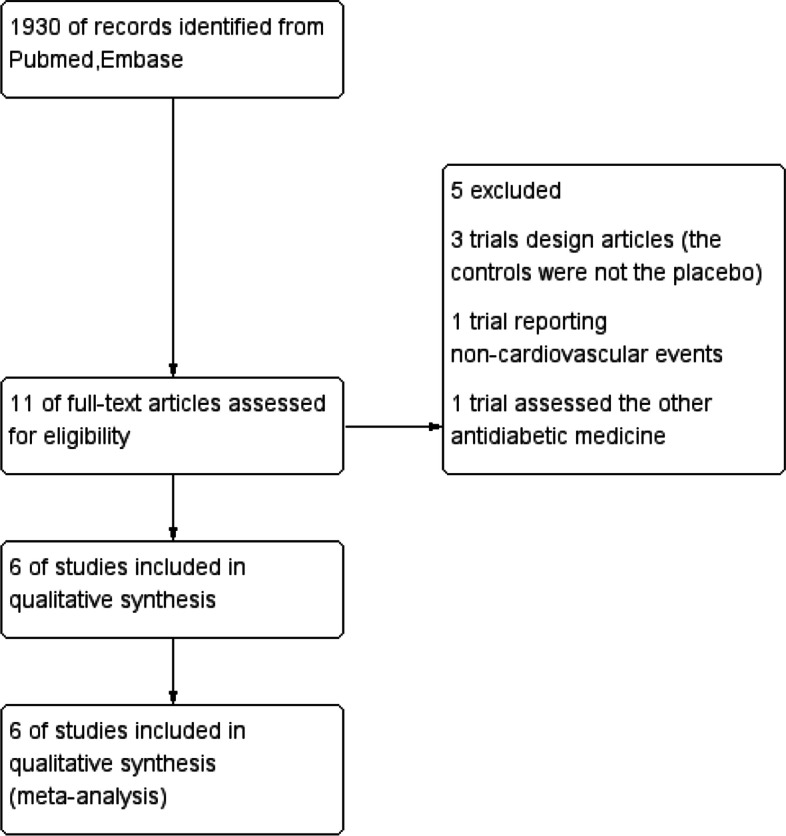
The flow diagram of the included studies
Fig. 2.
Effect of GLP-1 versus placebo on death from cardiovascular causes
Fig. 3.
Effect of GLP-1 versus placebo on fatal or non-fatal myocardial infarction
Fig. 4.
Effect of GLP-1 versus placebo on fatal or non-fatal stroke
The selected studies were published between 2015 and 2019. The GLP-1 receptor agonist arms included 26386 patients, and the placebo control arms included 26435 patients. The main characteristics of the included trials are presented in Table 1. The control treatment in the included trials was placebo according to the experimental trial treatment. The cardiovascular outcomes included death from cardiovascular causes, fatal or non-fatal myocardial infarction and fatal or non-fatal stroke.
Table 1.
The characteristics of included trials
| Study | Country of publication | Country of patients | Experimental Sample size | Control Sample Size | Age | Duration of diabetes | Intensive Therapy | Control therapy | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Adrian F Hernandez 2018 [10] | USA |
Western Europe Eastern and central Europe North America Latin America Asia Pacific |
4731 | 4732 |
64.2/ 64.2 |
4.1/ 4.2 |
Albiglutide (30-50mg) once a week |
Placebo once a week | 1.5 years |
| Rury R. Holman 2017 [4] | United Kingdom |
Europe, Latin America Europen subcategories, Asia-pacific, Eastern Europe, Western Europe, North America |
7356 | 7396 |
<65y 8813 ≥65y 5939 |
12.0 (7.0-18.0) |
exenatide at a dose of 2 mg once weekly | Placebo once a week | 2.3 years |
| Steven P2016 [7] | USA |
North AmericaEurope Asia Rest of the world |
4668 | 4672 |
<60y 2321 ≥60y 7019 |
12.8 years | liraglutide 1.8 mg (or the maximum tolerated dose) once daily | placebo once daily | 3.8 years |
| Steven P. Marso2016 [8] | United Kingdom | 230 sites in 20 countries. | 1648 | 1649 |
64.6± 7.4 |
13.9± 8.1 |
semaglutide (0.5mg or 1.0mg) once a week |
Placebo once a week | 109 weeks |
| Preffer MA 2015 [3] | Thailand | 49 countrirs | 3034 | 3034 | 60.3 | 9.3 | lixisenatide | placebo | 2.1 years |
| Gerstein HC 2019 [9] | UK |
371 sites 24 countries |
4949 | 4952 | 66.2 |
10.5/ 9.5 |
dulaglutide 1.5mg | placebo | 5.4 years |
There was no obvious heterogeneity in the six included studies (I2 = 0%, Cochran Q test P = 0.66) (Fig. 2) regarding the risk of death from cardiovascular causes. Therefore, we used the fixed effect model in the RevMan software. GLP-1 receptor agonists reduced the risk of death from cardiovascular causes compared with the placebo (RR: 0.90; 95% CI: 0.83 – 0.97; P = 0.004) according to the results of the meta-analysis.
There was mild heterogeneity (I2 = 49%, Cochran Q test P = 0.08) (Fig. 3) regarding the risk of fatal or non-fatal myocardial infarction in the included studies. Therefore, we used the random effect model in the RevMan software. No significant effect of GLP-1 receptor agonists identified on the risk of fatal or non-fatal myocardial infarction compared with the placebo controls (RR: 0.91; 95% CI: 0.82 – 1.01; P = 0.06).
There was also no evidence of heterogeneity observed across the included trials regarding fatal or non-fatal stroke (I2 = 4%, Cochran Q test P = 0.39) (see Fig. 4). The fixed effect model was applied in the RevMan software. The results of the meta-analysis indicated that GLP-1 receptor agonists reduced the risk of fatal or non-fatal stroke compared with the placebo (RR: 0.85; 95% CI: 0.77 – 0.94; P = 0.001).
Because only six trials were included, which is less than ten, we have no evidence of publication bias in this meta-analysis by the funnel plot. No individual study had a significant effect on the pooled effect size according to the results of the sensitivity analysis at all end points.
Discussion
The objective of this meta-analysis was to explore the effect of GLP-1 receptor agonists on cardiovascular outcomes in type 2 diabetes mellitus patients. The results of this meta-analysis suggest that GLP-1 therapy has a significant impact on the incidence of death from cardiovascular causes and fatal or non-fatal stroke in T2DM patients. There was no heterogeneity in these six included studies in their assessment of the effect of GLP-1 receptor agonists on the risk of death from cardiovascular causes and fatal or non-fatal stroke; however, there was mild heterogeneity regarding the risk of fatal or non-fatal myocardial infarction in the included studies, and the reasons may be the specific medicine molecule and GLP-1 receptor agonist dose tested, differences in the randomized patients (such as medical history and baseline characteristics), duration of follow-up years and adherence to treatment. As our findings are based on good-quality studies that were all multinational double-blind randomized placebo-control trials and our meta-analysis was based on a mean follow-up of 3.45 years (minimum 1.5 year – maximum 5.4 years), the confounding and attrition bias are controlled, so the risk of unreliable results is diminished.
Similar to our results, some studies [21, 22] also indicated that GLP-1 receptor agonists had a positive effect on the heart and enhanced cardiac function. While some previous studies [23] found a lower incidence of cardiovascular disease (CVD) events when GLP-1 receptor agonists were compared with placebo. Furthermore, a comparison meta-analysis [24–26] indicated that there was no significant reduction in CVD events by GLP-1 receptor agonists. Similarly, Inzucchi et al [27] believed that the evidence of GLP-1 receptor agonist cardiovascular protection was still limited, and the cardiovascular system benefits related to GLP-1 receptor agonists may be independent of its glucose, lipid, or energy metabolism effects. In order to better define the cardiovascular effects of GLP-1 receptor agonist in type 2 diabetes, we perform this meta-analysis. The trials included in the meta-analysis are the EXSCEL study (exenatide), LEADER study (liraglutide), SUSTAIN 6 study (semaglutide) and HARMONY outcomes study (albiglutide), ELIXA study (lixisenatide) and REWIND study (dulaglutide). Not all GLP-1 receptor agonist may have the same effect on cardiovascular events, since the classification of Glucagon-like peptide-1 (GLP-1) receptor agonists included the short acting and long acting. But there were no hand-to-hand cardiovascular outcomes studies focused on GLP-1 receptor agonist class, limiting the investigation of cardiovascular events differences between GLP-1 receptor agonists of different mechanical structure or drug potency.
GLP-1 receptor agonists have had cardiovascular protection effects in cardiovascular trials, the cardiovascular protection mechanisms of GLP-1 RAs contain direct and indirect effects which have been well discussed in preclinical and clinical studies [28]. The cardiovascular indirect effects of GLP-1 receptor agonists might be mediated via improve the common cardiovascular metabolic risk factors such as HbA1c, systolic blood pressure, and body weight, anti-inflammatory pathways, ischaemic conditioning and endothelial function [12, 28–30]. In addition, the direct effects of GLP-1 RAs to the cardiovascular system included enhancing the endothelial function, cardiac output, and myocardial glucose uptake, since the abundant GLP-1 receptor expressed in cardiac and vascular tissues [28, 30].
This meta-analysis has several strengths: (1) Only RCTs were included, so this meta-analysis eliminated the potential control group biases; (2) The large sample size of the 6 included trials allowed us to quantitatively evaluate the GLP-1 receptor agonist effects in T2DM patients; (3) A wide range of patient characteristics was represented, which ensured a comprehensive assessment of the effect of GLP-1 receptor agonists in the treatment of patients with T2DM.
This study has some limitations. First, the publication bias is an inevitable problem in any meta-analysis. Second, T2DM patients who received various GLP-1 receptor agonist drugs, such as albiglutide, exenatide, liraglutide, semaglutide, lixisenatide and dulaglutide might have biased the meta-analysis results. Third, a more detailed analysis was restricted because the meta-analysis used pooled data.
Conclusion
The findings of this study indicated that GLP-1 receptor agonist therapy reduced the incidence of death from cardiovascular causes and fatal or non-fatal stroke in the treatment of T2DM patients. We need additional large RCTs in the future to evaluate the treatment effects of GLP-1 receptor agonists in T2DM.
Acknowledgements
Not applicable.
Abbreviations
- GLP-1 RAs
Glucagon-like peptide 1 receptor agonists
- T2DM
type 2 diabetes mellitus
- RCTs
randomized controlled trials
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluation
- CVD
cardiovascular diseases
- RR
risk ratio
- Cis
confidence intervals
- HbA1c
glycosylated hemoglobin
Authors’ contributions
LS is the corresponding author of this study. She designed this study, collected and analyzed data, wrote the manuscript and made the decision to submit and publish the manuscript. JQ is the first author for the meta-analysis, including collected and analyzed data, and wrote the manuscript. The author(s) read and approved the final manuscript.
Funding
No funding.
Availability of data and materials
The datasets generated or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare that there are no conflict of interests or special relationships with industry in this meta-analysis.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407–18. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SJ, Cassells H. Hyperglycemia as a cardiovascular risk factor. Am J Me. 2003;1013115(Suppl. 8A):6S–11S. doi: 10.1016/j.amjmed.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 4.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–39. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocrine Rev. 2012;33:187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motta AJ, Koska J, Reaven P, Migrino RQ. Vascular protective effects of diabetes medications that mimic or increase glucagon-like peptide-1 activity. Recent Pat. Cardiovasc. Drug Discov. 2012;7:2–9. doi: 10.2174/157489012799362368. [DOI] [PubMed] [Google Scholar]
- 7.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;19:1834–44. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 9.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Sr, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 11.Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials, Diabetes. Obes Metab. 2014;16:38–47. doi: 10.1111/dom.12175. [DOI] [PubMed] [Google Scholar]
- 12.Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, Pagidipati NJ, Chan JC, Gustavson SM, Iqbal N, Maggioni AP, Ohman P, Poulter NR, Ramachandran A, Zinman B, Hernandez AF, Holman RR EXSCEL Study Group Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105–113. doi: 10.1016/S2213-8587(17)30412-6. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyatt GH, et al. Incorporating considerations of resources use into grading recommendations. BMJ. 2008;336:1170–1173. doi: 10.1136/bmj.39504.506319.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–54. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–45. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 21.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 22.Zhao T, Parikh P, Bhashyam S, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 23.Sun F, Yu K, Wu S, et al. Cardiovascular safety and glycemic control of glucagon- like peptide-1 receptor agonists for type 2 diabetes mellitus: a pairwise and network meta-analysis. Diabetes Res Clin Pract. 2012;98:386–395. doi: 10.1016/j.diabres.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Monami M, Cremasco F, Lamanna C, et al. Glucagon-like peptide-1 receptor agonists and cardiovascular events: a meta-analysis of randomized clinical trials. Exp Diabetes Res. 2011;2011:215764. doi: 10.1155/2011/215764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratner R, Han J, Nicewarner D, Yushmanova I, Hoogwerf BJ, Shen L. Cardiovascu- lar safety of exenatide BID: an integrated analysis from controlled clinical trials in participants with type 2 diabetes. Cardiovasc Diabetol. 2011;10:22. doi: 10.1186/1475-2840-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marso SP, Lindsey JB, Stolker JM, et al. Cardiovascular safety of liraglutide assessed in a patient-level pooled analysis of phase 2:3 liraglutide clinical development studies. Diab Vasc Dis Res. 2011;8:237–240. doi: 10.1177/1479164111408937. [DOI] [PubMed] [Google Scholar]
- 27.Inzucchi SE, McGuire DK. New drugs for the treatment of diabetes: part II: Incretin-based therapy and beyond. Circulation. 2008;117:574–584. doi: 10.1161/CIRCULATIONAHA.107.735795. [DOI] [PubMed] [Google Scholar]
- 28.Nauck MA, Meier JJ, Cavender MA, El AbdAziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–870. doi: 10.1161/CIRCULATIONAHA.117.028136. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-Villalobos NA, Trevino-Alvarez AM, Gonzalez-Gonzalez JG. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:1797–1798. doi: 10.1056/NEJMc1611289. [DOI] [PubMed] [Google Scholar]
- 30.Kang YM, Jung CH. Cardiovascular effects of glucagon-like peptide-1 receptor agonists. Endocrinol Metab (Seoul) 2016;31:258–274. doi: 10.3803/EnM.2016.31.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study available from the corresponding author on reasonable request.