Abstract
Background:
Pivotal trials of percutaneous left atrial appendage occlusion (LAAO) used specific post-procedure treatment protocols.
Objectives:
This study sought to evaluate patterns of post-procedure care after LAAO with the Watchman device in clinical practice and compare the risk of adverse events for different discharge antithrombotic strategies.
Methods:
We evaluated patients in the NCDR LAAO Registry who underwent LAAO with this device between 2016 and 2018. We assessed adherence to the full post-procedure trial protocol including standardized follow-up, imaging and antithrombotic agents and then evaluated the most commonly used antithrombotic strategies and compared the rates and odds of adverse events at 45 days and 6 months using multivariable logistic regression.
Results:
Among 31,994 patients undergoing successful LAAO, only 12.2% received the full post-procedure treatment protocol studied in pivotal trials; the most common protocol deviations were with discharge antithrombotic medications. The most common discharge medication strategies were warfarin and aspirin (36.9%), DOAC and aspirin (20.8%), warfarin only (13.5%), DOAC only (12.3%), and DAPT (5.0%). In multivariable logistic regression, the adjusted odds of any adverse event through the 45-day follow-up visit were significantly lower for discharge on warfarin alone (odds ratio [OR]: 0.637, 95% CI: 0.525–0.774) and DOAC alone (OR: 0.734; 95% CI: 0.579–0.931) compared with warfarin and aspirin. Warfarin alone remained lower risk at the 6-month follow-up.
Conclusions:
In contemporary US practice, practitioners rarely used the full Food and Drug Administration–approved post-procedure treatment protocols studied in pivotal trials of this LAAO device. Discharge post-implantation on warfarin or a DOAC without concomitant aspirin was associated with lower risk of adverse outcomes.
Keywords: anticoagulation, antiplatelet, stroke
Condensed Abstract
Among 31,994 Watchman patients in the LAAO Registry, only 12.2% received the full post-procedure treatment protocol studied in pivotal trials; the most common deviations were with discharge antithrombotic medications. The most common discharge medication strategies were warfarin and aspirin (36.9%), direct oral anticoagulants (DOACs) and aspirin (20.8%), warfarin only (13.5%), DOAC only (12.3%), and dual antiplatelet therapy (5.0%). In multivariable logistic regression, the adjusted odds of any adverse event through the 45-day follow-up visit were significantly lower for discharge on warfarin alone and DOACs alone compared with warfarin and aspirin. Warfarin alone remained lower risk at the 6-month follow-up.
INTRODUCTION
Patients with atrial fibrillation (AF) are at increased risk of thromboembolic stroke, predominantly due to the formation and embolization of clots from within the left atrial appendage. Percutaneous left atrial appendage occlusion (LAAO) lowers the risk of AF-related stroke by mechanically excluding the LAA from the systemic circulation (1–8). In March 2015, the US Food and Drug Administration (FDA) approved the Watchman LAAO device (Boston Scientific Corporation), which consists of a self-expanding nitinol frame with fixation barbs and a permeable polyester fabric cover, after 2 pivotal randomized controlled clinical trials (9–12). Both trials stipulated the use of standardized post-procedure protocols, including follow-up, imaging, and antithrombotic medications that were designed to minimize the risk of device-related thrombosis and associated thromboembolism and stroke; accordingly, this regimen was incorporated into the FDA’s device approval. Specifically, patients randomized to LAAO were discharged on warfarin and aspirin (81–325 mg) for 45 days post-implantation. At the follow up visit (45 days ± 2 weeks) patients underwent transesophageal echocardiography (TEE) and if there was a peridevice leak >5 mm, they were maintained on warfarin and aspirin; if there was no leak or a leak ≤5 mm, patients were transitioned to dual antiplatelet therapy (DAPT) with clopidogrel 75 mg daily and aspirin daily until 6 months post-implantation, and then aspirin thereafter (11,13).
Several factors may lead clinicians to deviate from the standardized FDA-approved treatment protocols. Data from the first 3 years of the National Cardiovascular Data Registry (NCDR) LAAO Registry showed that patients undergoing LAAO in the United States are substantially older, and a higher proportion have had clinically-significant bleeding events compared with patients enrolled in the randomized trials (14,15). In addition, most individuals with AF receive direct oral anticoagulants (DOACs), which were not approved for use at the time that the randomized trials of LAAO were designed. DOACs are favored for stroke prevention in AF due to better safety and effectiveness relative to warfarin (16–19); recent data suggest that transitioning to warfarin after LAAO in an individual already treated with a DOAC may be unnecessary (20). Finally, small studies suggest that DAPT alone may be sufficient to prevent device-related thrombus and stroke, and this practice is common in Europe (21,22).
The presence and extent of variation from the full post-procedure treatment protocols used in the pivotal trials has not been described. Furthermore, practice variation in post-procedural antithrombotic treatment offers the opportunity for a natural experiment to examine whether different approaches to treatment are associated with differences in outcomes. To address these gaps in knowledge, we analyzed data from patients enrolled the NCDR LAAO Registry between 2016 and 2018 who received an LAAO device with a self-expanding nitinol frame with fixation barbs and a permeable polyester fabric cover to evaluate deviations from the full post-procedure treatment protocols studied in pivotal LAAO trials, including standardized follow-up, imaging, and antithrombotic treatment. We then focused on the anticoagulation and antiplatelet treatment patterns and compared the rates and adjusted odds of adverse events at 45 days and 6 months associated with different antithrombotic strategies.
METHODS
DATA SOURCES AND DATA COLLECTION.
The NCDR LAAO Registry serves as the formal post-market surveillance vehicle required by the FDA for the Watchman device, and it is the only registry approved by the Centers for Medicare & Medicaid Services to satisfy the coverage decision data submission requirements (14). As of April 2016, US hospitals were required to submit data for all procedures using the LAAO device with a self-expanding nitinol frame with fixation barbs and a permeable polyester fabric cover to the LAAO Registry to qualify for Medicare reimbursement. Hospitals are encouraged to submit data on all device recipients regardless of insurance status (23). The NCDR LAAO Registry v1.1 data collection form was used to establish comparison groups and covariates. The Yale University Human Investigation Committee approved analysis of a limited dataset derived from the LAAO Registry with waiver of informed consent.
LAAO Registry data collection methods have been detailed previously (4). In brief, the LAAO Registry collects approximately 220 data elements from the implantation hospitalization, 60 for each follow-up visit, and 15 data elements to support the adjudication of adverse events (4). Data are collected at discharge, and follow-up visits over the first year occur at 45 days (±14 days), 180 days (−30 days ± 60 days), and 365 days (±60 days). The NCDR Data Quality Reporting process is designed to ensure that submissions are complete, valid, and accurate. This involves an annual audit of about 5% of sites that are randomly selected; during the audit, submitted data are compared with source documentation and billing data (24). The LAAO Registry developed and validated a novel process to adjudicate adverse clinical events over follow-up. A computer-based algorithm uses discrete combinations of registry data elements based on standard event definitions to adjudicate adverse events (15). Cases are manually adjudicated when registry data elements are incomplete or conflicting. Adjudicated adverse events in the registry include ischemic stroke, hemorrhagic stroke, undetermined stroke, transient ischemic attack (TIA), intracranial hemorrhage, systemic arterial embolism (other than stroke), major bleeding, and major vascular complication.
STUDY POPULATION AND OUTCOMES.
This analysis included patients enrolled in the LAAO Registry who underwent LAAO using the self-expanding nitinol frame with fixation barbs and a permeable polyester fabric cover between January 1, 2016 and November 30, 2018. Because of the time frame of this study, it only includes patients with the older-generation device, rather than the newest-generation Watchman FLX device which was commercially released in late 2020 (25). We excluded patients who died during their index hospitalization, who did not have a device successfully implanted, or who were treated with rarely used anticoagulant and antiplatelet agents, including Aggrenox (aspirin/dipyridamole), vorapaxar, and Durlaza (aspirin capsules).
The full post-procedure treatment protocol, including standardized follow-up, imaging, and antithrombotic therapy, was defined according to the protocols used in the PROTECT-AF (WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients With Atrial Fibrillation) and PREVAIL (Evaluation of the WATCHMAN Left Atrial Appendage (LAA) Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy) trials and subsequently codified in the device approval by the FDA (9,10). Per-protocol was defined as 1) discharged on warfarin and aspirin (81–325 mg daily), 2) first follow-up assessment at 45 days ± 14 days post-procedure, 3) TEE performed in follow-up window, 4) discontinuation of warfarin if residual leak 0–5 mm and no atrial thrombus detected on TEE OR continuation of warfarin if residual leak ≥5 mm or atrial thrombus detected on TEE, 5) second follow-up assessment at 6 months +60 days/−30 days post-procedure, 6) taking clopidogrel 75 mg and aspirin until the second follow-up visit or warfarin and aspirin if previously maintained on that. A patient must have met all of these criteria to be considered per-protocol.
After defining the rate of deviation from the full post-procedure protocols used in the trials, all subsequent analyses evaluated discharge antithrombotic treatment patterns and associated outcomes. Anticoagulant and antiplatelet medications captured on discharge included the following: aspirin, warfarin, P2Y12 inhibitor (clopidogrel, prasugrel, ticlopidine, or ticagrelor), DAPT (aspirin and P2Y12 inhibitor), DOAC (apixaban, dabigatran, rivaroxaban, or edoxaban), bridging anticoagulation therapy (unfractionated heparin, fondaparinux, low molecular weight heparin, or heparin derivative). Discharge medications were grouped into the 5 most common and clinically-relevant mutually-exclusive discharge medication strategies: 1) warfarin and aspirin; 2) warfarin only; 3) DOAC and aspirin; 4) DOAC only; and 5) DAPT (aspirin and P2Y12 inhibitor) without an anticoagulant; these mutually-exclusive medication groupings were used for all analyses of outcomes according to antithrombotic treatment.
The primary outcomes included: 1) any adverse event, 2) major adverse events, 3) any stroke or TIA, and 4) any readmission through the follow-up visit at 45 days ± 14 days. Secondary outcomes included these same adverse events through the follow-up visit at 6 months +60 days/−30 days. Additional secondary outcomes included unadjusted rates of device-related thrombus and peridevice leak >5 mm through 45 days ± 14 days follow-up. Because our data is derived from clinical practice, TEE was available in 91% of patients within the 45-day ± 14-day time window, but TEE imaging after this timepoint was substantially less frequently performed, limiting our ability to assess secondary endpoints at 6 months. Any adverse events consisted of cardiovascular, systemic, gastrointestinal/genitourinary, device, peripheral vascular, neurologic, bleeding, and pulmonary events (Supplemental Table 1). A link to the full current data collection forms for the index hospitalization and all follow-up visits is publicly available (26). A major adverse event was defined as death, cardiac arrest, myocardial infarction, pericardial effusion requiring intervention, systemic embolism, device embolization, major vascular complication, hemorrhagic stroke, ischemic stroke, undetermined stroke, TIA, intracranial hemorrhage, or major bleeding (Supplemental Table 2).
STATISTICAL ANALYSIS.
Statistical analyses were performed using SAS v9.4 (SAS institute). The rate of adherence to the full post-procedure protocols, including follow-up, imaging, and antithrombotic therapy, were described as numbers and percentages. Discharge medication use rates were first described as numbers and percentages of patients discharged on a given medication in non–mutually-exclusive categories. Numbers and percentages were then calculated for the most common and clinically relevant mutually-exclusive discharge medication strategies. Baseline patient characteristics according to use of per-protocol anticoagulation versus non–per-protocol were compared using Pearson’s chi-square test for categorical data and the Wilcoxon rank-sum test for continuous data and reported as number (%) and median (interquartile range [IQR]) or mean ± SD, with associated P values. Similarly, baseline characteristics were reported for the 5 most common and clinically-relevant discharge medication strategies.
Numbers and rates of adverse events were compared across the 5 mutually exclusive discharge medication groupings at 2 discrete follow-up visit intervals: 45 days ± 2 weeks and 6 months +60 days/−30 days. The risk of adverse events was evaluated between these groups using time-to-event analysis with Cox frailty models using warfarin and aspirin as the reference group (trial per-protocol discharge medications). The risk of adverse events was assessed at 2 follow-up visit intervals: 45 days ± 2 weeks and 6 months +60 days/−30 days. HRs and the corresponding 95% CIs for any adverse event, major adverse event, any stroke or TIA, and any readmission were calculated. Clinically-relevant covariates were evaluated, and all covariates with an unadjusted HR P value <0.2 were included in the models. Candidate variables included in the adjusted models are listed in Supplemental Table 3. Age, female sex, race, CHA2DS2-VASC score components (congestive heart failure/ left ventricular dysfunction, hypertension, diabetes mellitus, stroke, transient ischemic attack, thromboembolic event, vascular disease), HAS-BLED score components (uncontrolled hypertension, abnormal renal function, abnormal liver function, prior bleeding, labile international normalized ratio (INR), alcohol use, antiplatelet drug, nonsteroidal anti-inflammatory drug, increased risk of falls), valvular atrial fibrillation, prior atrial flutter, cardiomyopathy, chronic lung disease, sleep apnea, prior cardiac structural intervention, coronary artery disease, height, weight, blood pressure, hemoglobin, prothrombin time/INR, serum creatinine, platelet count, baseline medications (warfarin, DOAC, P2Y12 inhibitor, DAPT), bridging anticoagulant therapy, transesophageal echocardiogram (TEE) performed, indication for LAAO (increased thromboembolic stroke risk, history of major bleed, high fall risk, labile INR, patient preference, noncompliance with anticoagulation therapy), procedure canceled, device margin residual leak. Missing values were imputed before modeling. Dichotomous (yes/no) variables were assumed to be no, whereas all other variables were imputed using fully conditional specification (27). For our analysis, all variables were missing <1% of the time. Sensitivity analyses were then performed using multivariable logistic regression models for adverse events at the 2 follow-up visit intervals, 45 days ± 2 weeks and 6 months +60 days/−30 days.
RESULTS
BASELINE CHARACTERISTICS.
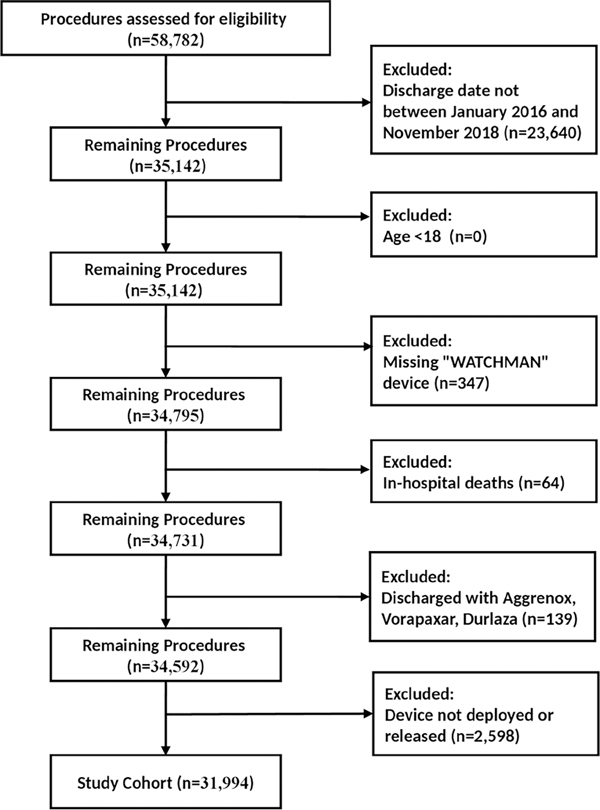
A total of 35,142 patients who underwent LAAO with the device with the self-expanding nitinol frame with fixation barbs and a permeable polyester fabric cover were enrolled in the NCDR LAAO Registry between January 1, 2016 and November 31, 2018. After applying exclusions, 31,994 patients remained in the cohort (Figure 1). The mean age of the overall cohort was 76 years, and 41% were female. The mean CHA2DS2-VASC score was 4.6, and the mean HAS-BLED score was 3 (Supplemental Table 4). The median follow-up time for patients at the first follow-up visit was 47 days (interquartile range [IQR]: 43–51), and the median follow-up time for patients at the second follow-up visit was 183 days (IQR: 162–202).
Figure 1. Cohort Assembly Diagram.
A total of 35,142 LAAO patients who underwent implantation of the LAAO device with the self-expanding nitinol frame with fixation barbs and a permeable polyester fabric cover were enrolled in the NCDR LAAO Registry between January 1, 2016 and November 31, 2018. After applying exclusions, 31,994 patients remained in the cohort.
LAAO = left atrial appendage occlusion; NCDR = National Cardiovascular Data Registry
ADHERENCE TO FDA-APPROVED POST-PROCEDURE PROTOCOLS.
Only 12.2% of patients were treated with the full FDA-approved discharge and follow-up protocols, which included discharge medications, standardized follow-up visits, imaging, and medication transitions that were defined in the pivotal trials (Supplemental Figure 1). The most common deviations from the FDA-approved protocol were discharge on antithrombotic medications other than warfarin and aspirin (61.5%) or without antithrombotic agents (1.8%). Other deviations were far less common, including failure to perform a TEE within the 45-day ± 14-day time window (9.1%), failure to follow-up in the 45-day time window, and failure to institute clopidogrel and aspirin within the 6-month time window.
DISCHARGE MEDICATIONS.
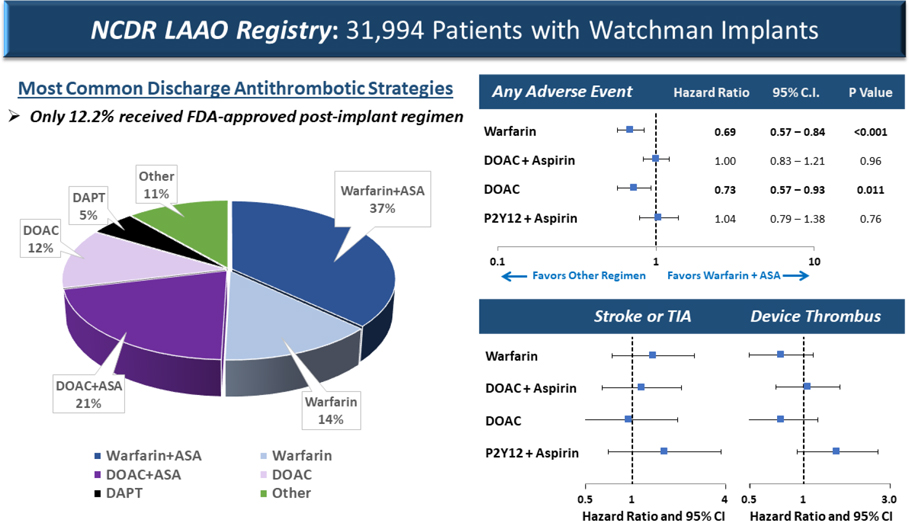
Among 31,944 patients who underwent successful LAAO, were enrolled in the NCDR LAAO Registry, and were included in our cohort between January 1, 2016 and November 31, 2018, almost all (98.2%) were discharged on an anticoagulant and/or antiplatelet medication (Table 1). The most common discharge medications were aspirin (67.9%), warfarin (55.6%), DOAC (36.4%), and P2Y12 inhibitors (13.2%). The most common mutually-exclusive discharge medication strategies were warfarin and aspirin (36.9%), DOAC and aspirin (20.8%), warfarin only (13.5%), DOAC only (12.3%), and DAPT (5.0%) (Central Illustration).
Table 1.
Discharge Medications Among 31,994 Patients in the LAAO Registry Between January 2016 and November 2018.
| Discharge Medications (Not Mutually Exclusive) | Number | (%) |
|---|---|---|
| Aspirin | 21,714 | (67.87) |
| Warfarin | 17,774 | (55.55) |
| DOAC* | 11,658 | (36.44) |
| P2Y12 inhibitor† | 4,229 | (13.22) |
| Bridging anticoagulant therapy‡ | 335 | (1.05) |
| None of anticoagulation or antiplatelet therapy or P2Y12 or bridging | 583 | (1.82) |
|
| ||
| Most Common Discharge Medication Strategies (Mutually-Exclusive Groupings) | ||
| Warfarin and aspirin | 11,811 | (36.92) |
| Warfarin only | 4,330 | (13.53) |
| DOAC and aspirin | 6,649 | (20.78) |
| DOAC only | 3,948 | (12.34) |
| DAPT (aspirin and P2Y12 inhibitor) | 1,614 | (5.04) |
| Other | 3,642 | (11.38) |
Includes apixaban, dabigatran, rivaroxaban or edoxaban
P2Y12 inhibitor includes clopidogrel, prasugrel, ticlopidine, ticagrelor
Bridging anticoagulation therapy includes unfractionated heparin, fondaparinux, low molecular weight heparin, heparin derivatives
DAPT = dual antiplatelet therapy; DOAC = direct oral anticoagulant; LAOO = left atrial appendage occlusion
Central Illustration. Post-procedure Antithrombotic Strategies and Associated Odds of Adverse Outcomes.
Among 31,944 patients in the NCDR LAAO Registry who underwent implantation with the LAOO device with the self-expanding nitinol frame with fixation barbs and a permeable polyester fabric cover between 2016 and 2018, the most common post-procedure antithrombotic strategies were warfarin plus aspirin, DOAC plus aspirin, warfarin alone, DOAC alone, and DAPT. Warfarin and DOAC alone without aspirin were associated with a lower risk of any adverse event compared with warfarin and aspirin, largely driven by lower risk of bleeding. There were no differences in the risk of ischemic stroke/transient ischemic attack or device related thrombus between the groups.
ASA = aspirin; DAPT = dual antiplatelet therapy; DOAC = direct oral anticoagulant.
BASELINE CHARACTERISTICS AND RATES OF ADVERSE EVENTS BY DISCHARGE MEDICATION STRATEGY.
Although there were statistically-significant differences between patients treated with the 5 mutually exclusive discharge medication regimens, due to the size of the cohort, the absolute differences were modest except for patients treated with DAPT, who were generally older and had higher rates of comorbidities, particularly prior bleeding (Table 2).
Table 2.
Baseline Characteristics of LAAO Registry Patients Between 2016–2018 in Mutually-Exclusive Discharge Medication Groupings.
| Characteristic | Warfarin and Aspirin | Warfarin Only | DOAC and Aspirin | DOAC Only | DAPT | P Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| N | % | N | % | N | % | N | % | N | % | ||
|
| |||||||||||
| Overall | 11,811 | 4,330 | 6,649 | 3,948 | 1,614 | ||||||
| Demographics | |||||||||||
| Age, mean (SD), y | 75.95 | (7.84) | 76.03 | (8.17) | 75.85 | (8.10) | 75.92 | (8.31 | 76.63 | (8.45) | 0.0118 |
| Age categories | |||||||||||
| <55 y | 147 | (1.24) | 61 | (1.41) | 84 | (1.26) | 67 | (1.70) | 21 | (1.30) | 0.0029 |
| 55 to 64 y | 704 | (5.96) | 266 | (6.14) | 416 | (6.26) | 241 | (6.10) | 92 | (5.70) | |
| 65 to 74 y | 3,829 | (32.42) | 1,400 | (32.33) | 2,146 | (32.28) | 1,265 | (32.04) | 478 | (29.62) | |
| 75 to 84 y | 5,620 | (47.58) | 1,965 | (45.38) | 3,080 | (46.32) | 1,827 | (46.28) | 751 | (46.53) | |
| ≥85 y | 1,511 | (12.79) | 638 | (14.73) | 923 | (13.88) | 548 | (13.88) | 272 | (16.85) | |
| Sex | |||||||||||
| Male | 7,127 | (60.34) | 2,379 | (54.94) | 3,845 | (57.83) | 2,167 | (54.89) | 969 | (60.04) | <0.0001 |
| Female | 4,680 | (39.62) | 1,950 | (45.03) | 2,802 | (42.14) | 1,776 | (44.98) | 645 | (39.96) | |
| Race | |||||||||||
| White | 10987 | (93.02) | 3985 | (92.03) | 6172 | (92.83) | 3666 | (92.86) | 1450 | (89.84) | <0.0001 |
| Black | 538 | (4.56) | 227 | (5.24) | 283 | (4.26) | 154 | (3.90) | 107 | (6.63) | |
| Hispanic | 40 | (0.34) | 10 | (0.23) | 33 | (0.50) | 10 | (0.25) | 10 | (0.62) | |
| Asian | 157 | (1.33) | 80 | (1.85) | 110 | (1.65) | 82 | (2.08) | 35 | (2.17) | |
| American Indian/Alaskan Native | 31 | (0.26) | 15 | (0.35) | 18 | (0.27) | 7 | (0.18) | 3 | (0.19) | |
| Native Hawaiian/Pacific Islander | 17 | (0.14) | 5 | (0.12) | 6 | (0.09) | 7 | (0.18) | 4 | (0.25) | |
| Other | 41 | (0.35) | 8 | (0.18) | 27 | (0.41) | 22 | (0.56) | 5 | (0.31) | |
| Primary insurance payer | |||||||||||
| Medicare/Medicaid | 10374 | (87.83) | 3738 | (86.33) | 5762 | (86.66) | 3383 | (85.69) | 1427 | (88.41) | 0.0031 |
| Private health insurance | 1281 | (10.85) | 526 | (12.15) | 808 | (12.15) | 499 | (12.64) | 163 | (10.10) | |
| Other | 156 | (1.32) | 66 | (1.52) | 79 | (1.19) | 66 | (1.67) | 24 | (1.49) | |
| CHA2DS2- VASC score, mean ± SD | 4.57 | (1.45) | 4.50 | (1.47) | 4.49 | (1.46) | 4.45 | (1.45) | 4.67 | (1.49) | <.0001 |
| Congestive heart failure | 4504 | (38.13) | 1682 | (38.85) | 2253 | (33.88) | 1278 | (32.37) | 594 | (36.80) | <0.0001 |
| Congestive heart failure class | |||||||||||
| NYHA class I | 1022 | (8.65) | 418 | (9.65) | 601 | (9.04) | 342 | (8.66) | 132 | (8.18) | <0.0001 |
| NYHA class II | 2152 | (18.22) | 770 | (17.78) | 1046 | (15.73) | 558 | (14.13) | 254 | (15.74) | |
| NYHA class III | 976 | (8.26) | 343 | (7.92) | 445 | (6.69) | 258 | (6.53) | 144 | (8.92) | |
| NYHA class IV | 50 | (0.42) | 32 | (0.74) | 27 | (0.41) | 22 | (0.56) | 9 | (0.56) | |
| Hypertension | 10963 | (92.82) | 3952 | (91.27) | 6132 | (92.22) | 3619 | (91.67) | 1470 | (91.08) | 0.0022 |
| Diabetes mellitus | 4672 | (39.56) | 1636 | (37.78) | 2357 | (35.45) | 1354 | (34.30) | 602 | (37.30) | <0.0001 |
| Prior transient ischemic attack | 1685 | (14.27) | 604 | (13.95) | 955 | (14.36) | 624 | (15.81) | 233 | (14.44) | <0.0001 |
| Prior thromboembolic event | 2131 | (18.04) | 733 | (16.93) | 1300 | (19.55) | 748 | (18.95) | 319 | (19.76) | <0.0001 |
| Vascular disease | 5084 | (43.04) | 1572 | (36.30) | 2773 | (41.71) | 1457 | (36.90) | 734 | (45.48) | <0.0001 |
| Prior myocardial infarction | 2527 | (21.40) | 632 | (14.60) | 1316 | (19.79) | 544 | (13.78) | 355 | (22.00) | <0.0001 |
| Peripheral arterial disease | 1760 | (14.90) | 561 | (12.96) | 935 | (14.06) | 432 | (10.94) | 227 | (14.06) | <0.0001 |
| Known aortic plaque | 538 | (4.56) | 184 | (4.25) | 261 | (3.93) | 151 | (3.82) | 63 | (3.90) | <0.0001 |
| HAS-BLED score, mean (SD) | 2.99 | (1.13) | 3.13 | (1.12) | 2.85 | (1.13) | 2.93 | (1.12) | 2.75 | (1.14) | <0.0001 |
| Uncontrolled hypertension | 2882 | (24.40) | 1204 | (27.81) | 1778 | (26.74) | 1106 | (28.01) | 386 | (23.92) | 0.0003 |
| Abnormal renal function | 1714 | (14.51) | 593 | (13.70) | 695 | (10.45) | 467 | (11.83) | 283 | (17.53) | <0.0001 |
| Abnormal liver function | 374 | (3.17) | 165 | (3.81) | 203 | (3.05) | 114 | (2.89) | 48 | (2.97) | 0.0073 |
| Prior stroke | 3210 | (27.18) | 1114 | (25.73) | 1793 | (26.97) | 1096 | (27.76) | 511 | (31.66) | 0.0014 |
| Ischemic | 1760 | (14.90) | 559 | (12.91) | 1082 | (16.27) | 631 | (15.98) | 252 | (15.61) | <0.0001 |
| Hemorrhagic | 884 | (7.48) | 340 | (7.85) | 409 | (6.15) | 303 | (7.67) | 195 | (12.08) | <0.0001 |
| Undetermined | 740 | (6.27) | 256 | (5.91) | 395 | (5.94) | 236 | (5.98) | 87 | (5.39) | 0.0005 |
| Prior bleeding | 8522 | (72.15) | 3045 | (70.32) | 4329 | (65.11) | 2684 | (67.98) | 1291 | (79.99) | <0.0001 |
| Labile INR | 1849 | (15.65) | 706 | (16.30) | 507 | (7.63) | 246 | (6.23) | 98 | (6.07) | <0.0001 |
| Alcohol use | 626 | (5.30) | 238 | (5.50) | 395 | (5.94) | 269 | (6.81) | 74 | (4.58) | <0.0001 |
| Antiplatelet medication use | 3265 | (27.64) | 800 | (18.48) | 1709 | (25.70) | 715 | (18.11) | 596 | (36.93) | <0.0001 |
| Nonsteroidal anti-inflammatory drug use | 4279 | (36.23) | 645 | (14.90) | 2358 | (35.46) | 654 | (16.57) | 632 | (39.16) | <0.0001 |
| Other history and risk factors | |||||||||||
| Clinically-relevant prior bleeding | 8461 | (71.64) | 3063 | (70.74) | 4219 | (63.45) | 2674 | (67.73) | 1328 | (82.28) | <0.0001 |
| Intracranial | 1440 | (12.19) | 518 | (11.96) | 699 | (10.51) | 487 | (12.34) | 311 | (19.27) | <0.0001 |
| Epistaxis | 747 | (6.32) | 269 | (6.21) | 423 | (6.36) | 241 | (6.10) | 90 | (5.58) | <0.0001 |
| Gastrointestinal | 5186 | (43.91) | 1910 | (44.11) | 2438 | (36.67) | 1548 | (39.21) | 748 | (46.34) | <0.0001 |
| Other | 1809 | (15.32) | 599 | (13.83) | 998 | (15.01) | 604 | (15.30) | 279 | (17.29) | <0.0001 |
| Fall risk | 4515 | (38.23) | 1757 | (40.58) | 2632 | (39.58) | 1626 | (41.19) | 641 | (39.71) | <0.0001 |
| Genetic coagulopathy | 92 | (0.78) | 41 | (0.95) | 36 | (0.54) | 33 | (0.84) | 13 | (0.81) | <0.0001 |
| Cardiomyopathy | 2574 | (21.79) | 907 | (20.95) | 1350 | (20.30) | 685 | (17.35) | 321 | (19.89) | <0.0001 |
| Ischemic | 1321 | (11.18) | 382 | (8.82) | 655 | (9.85) | 281 | (7.12) | 196 | (12.14) | <0.0001 |
| Nonischemic | 907 | (7.68) | 374 | (8.64) | 484 | (7.28) | 298 | (7.55) | 96 | (5.95) | <0.0001 |
| Chronic lung disease | 2522 | (21.35) | 902 | (20.83) | 1320 | (19.85) | 745 | (18.87) | 388 | (24.04) | <0.0001 |
| Coronary artery disease | 5738 | (48.58) | 1668 | (38.52) | 3009 | (45.25) | 1470 | (37.23) | 866 | (53.66) | <0.0001 |
| Sleep apnea | 3261 | (27.61) | 1124 | (25.96) | 1689 | (25.40) | 931 | (23.58) | 365 | (22.61) | <0.0001 |
| Arrhythmia history | |||||||||||
| Atrial fibrillation type | |||||||||||
| Paroxysmal | 6044 | (51.17) | 2140 | (49.42) | 3635 | (54.67) | 2016 | (51.06) | 836 | (51.80) | <0.0001 |
| Persistent (>7 d) | 2317 | (19.62) | 907 | (20.95) | 1468 | (22.08) | 964 | (24.42) | 334 | (20.69) | |
| Long-standing persistent (>1 year) | 1206 | (10.21) | 515 | (11.89) | 541 | (8.14) | 365 | (9.25) | 152 | (9.42) | |
| Permanent | 2205 | (18.67) | 755 | (17.44) | 988 | (14.86) | 582 | (14.74) | 281 | (17.41) | |
| Atrial flutter | 1615 | (13.67) | 593 | (13.70) | 1011 | (15.21) | 561 | (14.21) | 178 | (11.03) | 0.0005 |
INR = International Normalized Ratio; NYHA = New York Heart Association. Other abbreviations as in Table 1.
The unadjusted rate of any adverse event within the 45-day follow-up window was highest among those treated with warfarin and aspirin (5.7%), followed by DAPT (5.6%), DOAC and aspirin (5.3%), warfarin (4.0%), and DOAC (3.8%) (Table 3). The rate of any major adverse event followed a similar pattern and was highest among those discharged on warfarin and aspirin (4.4%), followed by DOAC and aspirin (4.3%), DAPT (4.3%), warfarin (3.3%), and DOAC (3.2%). These differences in adverse events were largely driven by differences in rates of bleeding. There were no significant differences in rates of readmission or any stroke or TIA. There were no differences in the rate of peridevice leak >5 mm (among those with a TEE), but DAPT was associated with a significantly higher unadjusted rate of device-related thrombus (among those with a TEE) compared with the other treatment groups.
Table 3.
Adverse Event Rates from Discharge Through 45 ± 14 Days in Mutually-Exclusive Discharge Medication Groupings.
| Adverse Event | Warfarin and Aspirin | Warfarin Only | DOAC and Aspirin | DOAC Only | DAPT | P Value* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | ||
|
| |||||||||||
| Any adverse event | 626 | (5.72) | 157 | (4.01) | 321 | (5.27) | 134 | (3.83) | 78 | (5.56) | <0.0001 |
| Any major adverse event † | 483 | (4.41) | 130 | (3.32) | 262 | (4.30) | 111 | (3.17) | 60 | (4.28) | 0.0017 |
| Death | 88 | (0.80) | 32 | (0.82) | 52 | (0.85) | 27 | (0.77) | 14 | (1.00) | 0.0205 |
| Ischemic stroke | 16 | (0.15) | 9 | (0.23) | 20 | (0.33) | 7 | (0.20) | 6 | (0.43) | <0.0001 |
| Hemorrhagic stroke | 15 | (0.14) | 1 | (0.03) | 10 | (0.16) | 2 | (0.06) | 2 | (0.14) | <0.0001 |
| Undetermined stroke | 2 | (0.02) | 0 | (0.00) | 0 | (0.00) | 2 | (0.06) | 1 | (0.07) | <0.0001 |
| TIA | 11 | (0.10) | 8 | (0.20) | 6 | (0.10) | 4 | (0.11) | 0 | (0.00) | <0.0001 |
| Intracranial hemorrhage | 9 | (0.08) | 2 | (0.05) | 5 | (0.08) | 2 | (0.06) | 1 | (0.07) | 0.0001 |
| Systemic arterial embolism | 4 | (0.04) | 1 | (0.03) | 0 | (0.00) | 3 | (0.09) | 2 | (0.14) | <0.0001 |
| Major bleeding | 336 | (3.07) | 72 | (1.84) | 172 | (2.83) | 60 | (1.71) | 31 | (2.21) | <0.0001 |
| Major vascular complication | 17 | (0.16) | 9 | (0.23) | 12 | (0.20) | 3 | (0.09) | 1 | (0.07) | <0.0001 |
| Myocardial infarction | 18 | (0.16) | 6 | (0.15) | 7 | (0.11) | 2 | (0.06) | 4 | (0.29) | 0.0022 |
| Pericardial effusion requiring intervention | 24 | (0.22) | 1 | (0.03) | 12 | (0.20) | 6 | (0.17) | 5 | (0.36) | 0.0006 |
| Device embolization | 3 | (0.03) | 3 | (0.08) | 0 | (0.00) | 2 | (0.06) | 0 | (0.00) | 0.0006 |
| Readmission | 616 | (5.62) | 209 | (5.33) | 355 | (5.83) | 176 | (5.03) | 77 | (5.49) | 0.5183 |
| Any stroke or TIA | 43 | (0.39) | 18 | (0.46) | 36 | (0.59) | 15 | (0.43) | 9 | (0.64) | 0.3589 |
| Atrial/device-related thrombus among those with TEE | 187 | (1.85) | 53 | (1.48) | 96 | (1.73) | 58 | (1.82) | 37 | (3.31) | <0.0001 |
| Peri-device Leak >5 mm among those with TEE | 86 | (0.85) | 20 | (0.56) | 34 | (0.61) | 26 | (0.82) | 5 | (0.45) | 0.1940 |
P values for comparisons across all groups.
Major adverse events were defined as death, myocardial infarction, pericardial effusion requiring intervention, systemic embolism, device embolization, major vascular complication, hemorrhagic stroke, ischemic stroke, undetermined stroke, TIA, intracranial hemorrhage, or major bleeding
TEE = transesophageal echocardiography; TIA = transient ischemic attack. Other abbreviations as in Table 1.
The rate of any adverse event from discharge through the 6-month follow-up window was highest among those treated with warfarin and aspirin (10.3%), followed by DAPT (9.1%), DOAC and aspirin (9.1%), warfarin (8.5%), and DOAC (8.3%) (Table 4). The rate of any major adverse event followed a similar pattern and was highest among those discharged on warfarin and aspirin (9.78%), followed by DAPT (9.1%), DOAC and aspirin (9.1%), warfarin (8.5%), and DOAC (8.3%). These differences in adverse events were again largely accounted for by differences in rates of bleeding. There were no significant differences in the rates of readmission or any stroke or TIA.
Table 4.
Adverse Event Rates from Discharge Through 6 Months in Mutually Exclusive Discharge Medication Groupings.
| Adverse Event | Warfarin and Aspirin Only | Warfarin Only | DOAC and Aspirin Only | DOAC Only | DAPT Only | P Value* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | ||
|
| |||||||||||
| Any adverse event | 1157 | (10.27) | 346 | (8.54) | 567 | (9.07) | 300 | (8.29) | 134 | (9.14) | 0.0006 |
| Any major adverse event † | 1090 | (9.67) | 347 | (8.57) | 554 | (8.87) | 290 | (8.02) | 137 | (9.35) | 0.0211 |
| Death | 384 | (3.41) | 143 | (3.53) | 200 | (3.20) | 106 | (2.93) | 63 | (4.30) | 0.1383 |
| Ischemic stroke | 59 | (0.52) | 28 | (0.69) | 42 | (0.67 | 24 | (0.66) | 10 | (0.68) | 0.0002 |
| Hemorrhagic stroke | 29 | (0.26) | 8 | (0.20) | 18 | (0.29) | 9 | (0.25) | 3 | (0.20) | 0.0037 |
| Undetermined stroke | 4 | (0.04) | 0 | (0.00) | 1 | (0.02) | 3 | (0.08) | 1 | (0.07) | 0.0006 |
| TiA | 33 | (0.29) | 16 | (0.39) | 29 | (0.46) | 13 | (0.36) | 5 | (0.34) | 0.0004 |
| Intracranial hemorrhage | 29 | (0.26) | 3 | (0.07) | 17 | (0.27) | 9 | (0.25) | 4 | (0.27) | 0.0008 |
| Systemic arterial embolism | 21 | (0.19) | 9 | (0.22) | 12 | (0.19) | 15 | (0.41) | 6 | (0.41) | <.0001 |
| Major bleeding | 560 | (4.97) | 154 | (3.80) | 272 | (4.35) | 120 | (3.32) | 49 | (3.34) | 0.0005 |
| Major vascular complication | 26 | (0.23) | 10 | (0.25) | 16 | (0.26) | 3 | (0.08) | 2 | (0.14) | 0.0029 |
| Myocardial infarction | 49 | (0.43) | 11 | (0.27) | 17 | (0.27) | 12 | (0.33) | 11 | (0.75) | 0.0052 |
| Pericardial effusion requiring intervention | 38 | (0.34) | 2 | (0.05) | 16 | (0.26) | 8 | (0.22) | 5 | (0.34) | 0.0068 |
| Device embolization | 3 | (0.03) | 3 | (0.07) | 0 | (0.00) | 2 | (0.06) | 0 | (0.00) | 0.0159 |
| Readmission | 1,608 | (14.27) | 520 | (12.84) | 901 | (14.42) | 498 | (13.76) | 194 | (13.23) | 0.1355 |
| Any stroke or TIA | 122 | (1.08) | 51 | (1.26) | 88 | (1.41) | 47 | (1.30) | 19 | (1.30) | 0.4226 |
P values for comparisons across all groups.
Major adverse events were defined as death, myocardial infarction, pericardial effusion requiring intervention, systemic embolism, device embolization, major vascular complication, hemorrhagic stroke, ischemic stroke, undetermined stroke, TIA, intracranial hemorrhage, or major bleeding
ADJUSTED RISK OF ADVERSE EVENTS BY DISCHARGE MEDICATION STRATEGY.
In adjusted Cox regression analyses, the risk of any adverse event within the 45-day time window was statistically significantly lower for warfarin alone (HR: 0.692; 95% CI: 0.569–0.841) and DOAC alone (HR: 0.731; 95% CI 0.574–0.930) relative to warfarin and aspirin (Table 5, Central Illustration). There were no differences among the other groups. The hazard of any major adverse event was significantly lower for those treated with warfarin alone (HR: 0.658; 95% CI: 0.536–0.808) and DOAC alone (HR: 0.767; 95% CI: 0.597–0.985) compared with warfarin and aspirin. The risk of any readmission was higher for those treated with warfarin alone (HR: 1.405; 95% CI: 1.167–1.692) and DOAC alone (HR: 1.275: 95% CI: 1.004–1.617) compared with warfarin and aspirin. There were no significant differences in the risk of any stroke or TIA, peridevice leak >5 mm (among those with a TEE), or atrial/device-related thrombus (among those with a TEE) (Table 5 and Central Illustration).
Table 5.
Risk of Adverse Events from Discharge Through 45 ± 14 Mutually-Exclusive Discharge Medication Groupings.
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
|
| ||||||
| Any adverse event | ||||||
| Warfarin and aspirin | Reference | |||||
| Warfarin | 0.779 | 0.649–0.936 | 0.008 | 0.692 | 0.569–0.841 | 0<0.001 |
| DOAC and aspirin | 0.953 | 0.824–1.103 | 0.519 | 1.005 | 0.833–1.213 | 0.956 |
| DOAC | 0.772 | 0.630–0.945 | 0.012 | 0.731 | 0.574–0.930 | 0.011 |
| DAPT (P2Y12 and aspirin) | 1.038 | 0.805–1.339 | 0.772 | 1.044 | 0.790–1.378 |
0.763 |
| Any major adverse event | ||||||
| Warfarin and aspirin | Reference | |||||
| Warfarin | 0.765 | 0.629–0.932 | 0.008 | 0.658 | 0.536–0.808 | <0.001 |
| DOAC and aspirin | 0.979 | 0.838–1.145 | 0.794 | 1.198 | 0.982–1.460 | 0.075 |
| DOAC | 0.739 | 0.597–0.914 | 0.005 | 0.767 | 0.597–0.985 | 0.038 |
| DAPT (P2Y12 and aspirin) | 1.026 | 0.778–1.353 | 0.8577 | 0.841 | 0.622–1.137 | 0.261 |
| Any readmissions | ||||||
| Warfarin and aspirin | Reference | |||||
| Warfarin | 1.325 | 1.117–1.572 | 0.001 | 1.405 | 1.167–1.692 | <0.001 |
| DOAC and aspirin | 1.117 | 0.966–1.293 | 0.136 | 1.028 | 0.847–1.248 | 0.779 |
| DOAC | 1.331 | 1.100–1.612 | 0.003 | 1.275 | 1.004–1.617 | 0.046 |
| DAPT (P2Y12 and aspirin) | 1.062 | 0.818–1.380 | 0.650 | 1.042 | 0.785–1.383 | 0.777 |
| Any stroke or TIA | ||||||
| Warfarin and aspirin | Reference | |||||
| Warfarin | 1.243 | 0.729–2.118 | 0.424 | 1.358 | 0.738–2.500 | 0.325 |
| DOAC and aspirin | 1.465 | 0.963–1.230 | 0.075 | 1.155 | 0.641–2.081 | 0.632 |
| DOAC | 1.285 | 0.732–2.256 | 0.382 | 0.947 | 0.460–1.948 | 0.882 |
| DAPT (P2Y12 and aspirin) | 1.418 | 0.688–2.921 | 0.344 | 1.615 | 0.704–3.705 | 0.258 |
| Device-related thrombus (among those with TEE) | ||||||
| Warfarin and aspirin | Reference | |||||
| Warfarin | 0.825 | 0.599–1.136 | 0.239 | 0.754 | 0.502–1.133 | 0.174 |
| DOAC and aspirin | 0.997 | 0.768–1.295 | 0.985 | 1.057 | 0.702–1.592 | 0.791 |
| DOAC | 0.753 | 0.550–1.029 | 0.075 | 0.746 | 0.463–1.201 | 0.227 |
| DAPT (P2Y12 and aspirin) | 1.160 | 0.800–1.682 | 0.433 | 1.532 | 0.915–2.566 | 0.105 |
| Peridevice leak >5 mm (among those with TEE) | ||||||
| Warfarin and aspirin | Reference | |||||
| Warfarin | 0.675 | 0.412–1.106 | 0.118 | 0.671 | 0.401–1.124 | 0.129 |
| DOAC and aspirin | 0.721 | 0.480–1.084 | 0.116 | 0.795 | 0.474–1.333 | 0.384 |
| DOAC | 0.890 | 0.563–1.408 | 0.620 | 0.991 | 0.558–1.761 | 0.975 |
| DAPT (P2Y12 and aspirin) | 0.533 | 0.214–1.328 | 0.177 | 0.628 | 0.240–1.644 | 0.343 |
In adjusted Cox regression analyses, the risk of any adverse event within the 6-month time window was statistically significantly lower for warfarin alone (HR: 0.814; 95% CI: 0.712–0.931) compared with warfarin and aspirin; there were no differences among the other medication groups (Table 6). The risk of any major adverse event were also significantly lower for those treated with warfarin alone (HR: 0.840: 95% CI: 0.737–0.958); there were no differences among the other medication groups. There were no significant differences in the risk of any readmission or any stroke or TIA.
Table 6.
Risk of Adverse Events from Discharge Through 6 Months In Mutually-Exclusive Discharge Medication Groupings.
| Adverse event | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
|
| ||||||
| Any adverse event | ||||||
| Warfarin and aspirin | Reference | |||||
| Warfarin | 0.902 | 0.795–1.024 | 0.111 | 0.814 | 0.712–0.931 | 0.003 |
| DOAC and aspirin | 0.928 | 0.831–1.037 | 0.187 | 1.048 | 0.910–1.206 | 0.519 |
| DOAC | 0.826 | 0.719–0.949 | 0.007 | 0.863 | 0.730–1.021 | 0.085 |
| DAPT (P2Y12 and aspirin) | 1.017 | 0.836–1.236 | 0.868 | 0.979 | 0.791–1.212 | 0.847 |
| Any major adverse event | ||||||
| Warfarin and aspirin | Reference | |||||
| Warfarin | 0.915 | 0.807–1.037 | 0.164 | 0.840 | 0.737–0.958 | 0.010 |
| DOAC and aspirin | 0.918 | 0.823–1.023 | 0.121 | 1.098 | 0.956–1.260 | 0.187 |
| DOAC | 0.844 | 0.736–0.969 | 0.016 | 0.918 | 0.777–1.084 | 0.314 |
| DAPT (P2Y12 and aspirin) | 1.018 | 0.843–1.229 | 0.854 | 0.834 | 0.679–1.025 | 0.085 |
| Any readmission | ||||||
| Warfarin and aspirin | Reference | |||||
| Warfarin | 1.064 | 0.957–1.183 | 0.251 | 1.081 | 0.966–1.210 | 0.175 |
| DOAC and aspirin | 1.105 | 1.009–1.211 | 0.032 | 1.066 | 0.944–1.205 | 0.303 |
| DOAC | 1.155 | 1.030–1.295 | 0.014 | 1.146 | 0.994–1.321 | 0.061 |
| DAPT (P2Y12 and aspirin) | 1.067 | 0.906–1.257 | 0.435 | 1.061 | 0.887–1.269 | 0.517 |
| Any stroke or TIA | ||||||
| Warfarin and aspirin | Reference | |||||
| Warfarin | 1.108 | 0.807–1.521 | 0.527 | 1.129 | 0.801–1.591 | 0.489 |
| DOAC and aspirin | 1.298 | 1.003–1.680 | 0.048 | 1.051 | 0.751–1.470 | 0.772 |
| DOAC | 1.241 | 0.903–1.705 | 0.183 | 0.920 | 0.618–1.369 | 0.680 |
| DAPT (P2Y12 and aspirin) | 1.230 | 0.778–1.942 | 0.376 | 1.086 | 0.654–1.803 | 0.750 |
Abbreviations as in Table 1.
In sensitivity analyses performed using multivariable logistic regression, the results were qualitatively extremely similar, with a lower risk for any adverse events and major adverse events for both warfarin alone and DOAC alone within the 45-day time window (Supplemental Table 5); warfarin alone remained associated with a lower hazard for any adverse events and major adverse events within the 6-month time window (Supplemental Table 6). There were no differences with regards to readmission for any of the groups at either time point.
DISCUSSION
Our study of post-procedural management for 31,944 patients who underwent successful implantation of the device with the self-expanding nitinol frame with fixation barbs and a permeable polyester fabric cover, captured in the LAAO Registry between January 1, 2016 and November 31, 2018, demonstrated substantial deviation from the treatment protocols used in the pivotal randomized trials. Only 1 in 10 patients undergoing implantation with this device were treated with the full FDA-approved protocol for post-procedural pharmacologic therapy and monitoring used in these trials, with discharge on antithrombotic strategies other than warfarin plus aspirin being the most common deviation. The most commonly used discharge medication strategies included warfarin and aspirin, which was studied in pivotal trials, followed by DOAC and aspirin, warfarin only, DOAC only, and DAPT. The risk of any adverse event and any major adverse event at the first follow-up visit at 45 days were significantly lower for warfarin alone and DOAC alone compared with warfarin and aspirin. Warfarin alone remained lower risk for any adverse event or any major adverse event through the second follow-up at 6 months. There were no differences between DAPT and warfarin plus aspirin at either follow-up.
Our study is the first to comprehensively assess post-discharge medication treatment patterns in contemporary practice and to definitively demonstrate how rarely patients are treated using the standardized discharge medication protocol delineated in the PROTECT AF and PREVAIL trials (11,13). Although these trials enrolled AF patients who were candidates for long-term oral anticoagulation, Centers for Medicare & Medicaid Services reimbursement mandates that patients are deemed unable to tolerate long-term oral anticoagulation (14). In fact, 70% of the patients in our study had a history of prior bleeding, and the median HAS BLED score was 3, which correlates to a 5.8% major bleeding event rate (15,28). As such, it is not surprising that clinicians did not always discharge patients on the combination of warfarin and aspirin. In addition, the rapid onset and offset, reliable pharmacokinetics, and avoidance of laboratory testing with the DOAC medications makes them an attractive alternative to warfarin, particularly for short-term periprocedural use (16–19). Based on this, the newest-generation device was evaluated in the single-arm PINNACLE FLX (Investigational Device Evaluation of the WATCHMAN FLX LAA Closure Technology) study, with post-procedure treatment with DOAC and aspirin therapy, and the device was approved by the FDA in 2020 (after the time frame for our study) using this post-procedure treatment paradigm (25).
Our study demonstrates an association between increased risk of adverse events, particularly bleeding events, associated with adding aspirin to anticoagulation upon discharge after LAAO device implantation. This finding parallels results from studies of antithrombotic agents following transcatheter aortic valve replacement (29). Discharge on warfarin or DOAC anticoagulation alone was associated with a similar reduction in major adverse events through the 45-day follow-up window with no increase in ischemic events. Indeed, the recent 400-person single-arm PINNACLE FLX FDA study of the newest-generation device demonstrated the relative safety of discharge on DOAC and aspirin (as compared with event rates from the pivotal trials); accordingly, this regimen was recently approved by the FDA (30). Although we showed an increased risk of readmission at 45 days with warfarin and DOAC alone, this finding did not persist at 6 months, and it was not significant at 45 days or at 6 months in sensitivity analyses using logistic regression. Thus, our data show that avoiding discharge on aspirin in addition to anticoagulation may offer an opportunity to substantially improve patient outcomes. As such, a randomized clinical trial that removes aspirin from the list of recommended post-procedure treatments may be warranted.
Only about 5% of patients in this US-based registry were discharged on DAPT. Although the unadjusted rates of adverse events of atrial/device related thrombus were higher in this group, these patients were at extremely high risk of both stroke and bleeding. Indeed, in the registry, patients receiving a DAPT discharge regimen were older, had the highest CHA2DS2-VASc and HAS BLED scores, and the highest rates of prior intracranial bleeding and hemorrhagic strokes. After adjustment for these differences in risk profile, the risk of adverse events associated with DAPT did not differ significantly through either the 45-day follow-up window or the 6-month follow-up window compared with discharge on warfarin and aspirin, including no difference in ischemic events or device-related thrombus. Discharge treatment with DAPT after implantation of the LAOO device is common outside of the United States, including in Europe and Canada, but the regimen has only been evaluated with relatively small studies to date and without randomization against other antithrombotic regimens (22,31). The results of Amulet IDE (AMPLATZER Amulet LAA Occluder Trial) were recently reported, comparing the Amulet self-expanding, double-disc device (Abbott) and discharge treatment with DAPT for most patients versus Watchman and discharge treatment with warfarin and aspirin for most patient, showing comparable safety and effectiveness (32); accordingly the self-expanding double-disc device was subsequently approved in August 2021 for commercial use in the United States with DAPT as a post-procedure treatment option. Thus, our data provide support that a DAPT discharge regimen may be an acceptable alternative to the use of anticoagulation and aspirin following implantation of the LAOO device with the self-expanding nitinol frame with fixation barbs and a permeable polyester fabric cover, particularly in patients for whom the risks of-short term anticoagulants are prohibitively high. However, it is worth noting that there was a trend toward a higher rate of device-related thrombus at 45 days of follow up (HR: 1.53; P = 0.11) (Table 5) with DAPT.
Finally, this study demonstrates the value of large, national, post-market registries to systematically evaluate how novel technologies are adopted into real-world clinical practice and how they may inform shared decision-making and post-procedural management.
LIMITATIONS.
As in any observational cohort study, our findings may reflect some degree of residual confounding. However, the baseline characteristics between discharge medication groups were overall very similar except for patients treated with DAPT, who were generally older, with a higher prevalence of comorbidities, particularly prior bleeding, and lower rates of ischemic heart and vascular disease for those treated without aspirin. In addition, the LAAO Registry collects data on most recognized prognostic factors, and our analyses were fully adjusted for all major clinical risk factors. A randomized trial may be warranted to better define the risks and benefits of different antithrombotic regimens. Adverse event rates were captured through site-reported data, and as such, under-reporting of adverse events is possible. However, the NCDR uses a rigorous Data Quality Reporting process to ensure that submissions are complete, valid, and accurate. This process involves an annual audit of a random selection of about 5% of sites, during which submitted data are compared with source documentation and billing data. In addition, under-reporting is unlikely to vary differentially as a function of the discharge medication strategy used. Our study compared adverse event rates by discharge anticoagulation and/or antiplatelet medications, although the subsequent follow-up medication changes and the timing of those transitions may clearly have an impact on outcomes. There were many other medication combinations used over the extended follow-up; however, our approach focused on the most clinically-relevant combinations of discharge medications. Finally, because of the time frame of this study, it only includes patients who received the older-generation device, rather than the newest-generation device, which was commercially released in late 2020 (25). Rates of procedural success, device-related thrombus, peridevice leakage, and clinical outcomes may differ for the new device, which will be evaluated when more data has accrued in the LAAO Registry on those patients (33–35).
CONCLUSIONS
Our study showed that in real-world contemporary practice, strict adherence to the full FDA-approved post-procedure protocols studied in pivotal trials, including discharge medications, standardized follow-up visits, imaging, and medication transitions, was rare, and the most common deviations were discharge on unstudied antithrombotic regimens. Compared with the trial-studied regimen of warfarin and aspirin, discharge on anticoagulation without aspirin was associated with a lower risk of adverse events, particularly bleeding events, without evidence of increased risk of stroke/TIA or atrial-/device-related thrombus. As LAAO devices iterate and new devices are approved, ongoing study of post-procedure antithrombotic therapy will be vital to optimize patient outcomes.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
In a registry of patients with atrial fibrillation (AF) undergoing left atrial appendage occlusion (LAAO) with the device with the self-expanding nitinol frame with fixation barbs and a permeable polyester fabric cover, post-procedural anticoagulation with warfarin or a target-specific oral agent (DOAC) without concomitant aspirin was associated with lower risk of adverse outcomes.
TRANSLATIONAL OUTLOOK:
Further research is necessary to establish the optimum type and duration of antithrombotic therapy in relation to patient, device, and procedural characteristics for patients with AF undergoing LAAO for stroke prevention.
Acknowledgments
Disclosures This study was funded by the American College of Cardiology (ACC) National Cardiovascular Data Registry (NCDR) and the National Heart, Lung and Blood Institute (NHLBI) grants R56HL142765 and R01HL142765. Dr. Freeman has reported salary support from the ACC NCDR and the NHLBI and consulting/advisory board fees from Boston Scientific, Medtronic, Janssen Pharmaceuticals, and Biosense Webster. Dr Price has reported consulting/advisory board fees from Boston Scientific, Medtronic, Abbott Vascular, W.L. Gore Medical, and Conformal Medical; speaker’s fees from Chiesi USA; and research grants (to institution) from Daiichi Sankyo. Dr Masoudi has reported an institutional contract with the American College of Cardiology for his role as Chief Scientific Advisor of the NCDR. Dr Curtis has reported an institutional contract with the American College of Cardiology for his role as Senior Scientific Advisor of the NCDR. Dr Doshi has reported research grants (to institution) and consulting/advisory board fees from Boston Scientific, Biosense Webster, Conformal Medical, Terumo, and Abbott Vascular. Dr Goldsweig has reported grant support from the National Institute of General Medical Sciences (1U54GM115458) and the UNMC Center for Heart and Vascular Research. Dr Friedman has reported receiving educational grants from Boston Scientific, Medtronic, and Abbott; research grants from the National Cardiovascular Data Registry, Boston Scientific, Abbott, Medtronic, and Biosense Webster; and consulting fees from Abbott and AtriCure. Dr Gibson has reports consulting for Boston Scientific, Abbott, and Biosense Webster. Dr Reddy is an unpaid consultant to, and has received grant support from, Boston Scientific; he also has other disclosures unrelated to this paper as follows: Abbott (Consultant), Ablacon (Consultant, Equity), Acutus Medical (Consultant, Equity), Affera (Consultant, Equity), Apama Medical (Consultant, Equity), Aquaheart (Consultant, Equity), Atacor (Consultant, Equity), Autonomix (Consultant, Equity), Axon Therapeutics (Consultant, Equity), Backbeat (Consultant, Equity), BioSig (Consultant, Equity), Biosense-Webster (Consultant), Biotronik (Consultant), Cardiac Implants (Consultant, Equity), CardiaCare (Consultant, Equity), Cardiofocus (Consultant), Cardionomic (Consultant), CardioNXT / AFTx (Consultant), Circa Scientific (Consultant, Equity), Corvia Medical (Consultant, Equity), Dinova-Hangzhou Nuomao Medtech Co, Ltd (Consultant, Equity), East End Medical (Consultant, Equity), EBR (Consultant), EPD (Consultant, Equity), Epix Therapeutics (Consultant, Equity), EpiEP (Consultant, Equity), Eximo (Consultant, Equity), Farapulse (Consultant, Equity), Fire1 (Consultant), HRT (Consultant, Equity), Impulse Dynamics (Consultant), Intershunt (Consultant, Equity), Javelin (Consultant, Equity), Kardium (Consultant, Equity), Keystone Heart (Consultant, Equity), LuxMed (Consultant, Equity), Manual Surgical Sciences (Equity), Medlumics (Consultant), Medtronic (Consultant), Middlepeak (Consultant, Equity), Newpace (Equity), Nuvera (Consultant, Equity), Philips (Consultant), Pulse Biosciences (Consultant), Sirona Medical (Consultant, Equity), Surecor (Equity), Thermedical (Consultant), Valcare (Consultant, Equity) and Vizaramed (Equity). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- AF
atrial fibrillation
- DAPT
dual antiplatelet therapy
- DOAC
direct oral anticoagulant
- IQR
interquartile range
- LAAO
left atrial appendage occlusion
- OR
odds ratio
- TEE
transesophageal echocardiogram
- TIA
transient ischemic attack
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755–759. [DOI] [PubMed] [Google Scholar]
- 2.Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol 1995;25:452–9. [DOI] [PubMed] [Google Scholar]
- 3.Jain AK, Gallagher S. Percutaneous occlusion of the left atrial appendage in non-valvular atrial fibrillation for the prevention of thromboembolism: NICE guidance. Heart 2011;97:762–765. [DOI] [PubMed] [Google Scholar]
- 4.Bartus K, Bednarek J, Myc J, et al. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm 2011;8:188–193. [DOI] [PubMed] [Google Scholar]
- 5.Fountain R, Holmes DR Jr, Hodgson PK, Chandrasekaran K, Van Tassel R, Sick P. Potential applicability and utilization of left atrial appendage occlusion devices in patients with atrial fibrillation. Am Heart J 2006;152:720–723. [DOI] [PubMed] [Google Scholar]
- 6.Block PC, Burstein S, Casale PN, et al. Percutaneous left atrial appendage occlusion for patients in atrial fibrillation suboptimal for warfarin therapy: 5-year results of the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) Study. J Am Coll Cardiol Intv 2009;2:594–600. [DOI] [PubMed] [Google Scholar]
- 7.Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62:108–118. [DOI] [PubMed] [Google Scholar]
- 8.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132. [DOI] [PubMed] [Google Scholar]
- 9.Holmes DR, Reddy VY, Turi ZG, et al. ; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534–542. [DOI] [PubMed] [Google Scholar]
- 10.Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 11.Reddy VY, Sievert H, Halperin J, et al. ; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014;312:1988–1998. [DOI] [PubMed] [Google Scholar]
- 12.Reddy VY, Doshi SK, Kar S, et al. ; PREVAIL and PROTECT AF Investigators. 5-Year outcomes after left atrial appendage closure: From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol 2017;70:2964–2975. [DOI] [PubMed] [Google Scholar]
- 13.Holmes DR, Reddy VY, Turi ZG, et al. ; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534–542. [DOI] [PubMed] [Google Scholar]
- 14.Decision Memo for Percutaneous Left Atrial Appendage (LAA) Closure Therapy (CAG-00445N). Centers for Medicare & Medicaid Services, 2016. Available at: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=281. Accessed March 7, 2022.
- 15.Freeman JV, Varosy P, Price MJ, et al. The NCDR Left Atrial Appendage Occlusion Registry. J Am Coll Cardiol 2020;75:1503–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Mahaffey KW, Garg J et al. ; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 18.Granger CB, Alexander JH, McMurray JJ, et al. ; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 19.Giugliano RP, Ruff CT, Braunwald E, et al. ; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto Y, Gadiyaram VK, Gianni C, et al. Use of non-warfarin oral anticoagulants instead of warfarin during left atrial appendage closure with the Watchman device. Heart Rhythm 2017;14:19–24. [DOI] [PubMed] [Google Scholar]
- 21.Reddy VY, Gibson DN, Kar S, et al. Post-approval U.S. experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2017;69:253–261. [DOI] [PubMed] [Google Scholar]
- 22.Boersma LV, Ince H, Kische S, et al. ; following investigators and institutions participated in the EWOLUTION study. Evaluating real-world clinical outcomes in atrial fibrillation patients receiving the WATCHMAN left atrial appendage closure technology: Final 2-year outcome data of the EWOLUTION trial focusing on history of stroke and hemorrhage. Circ Arrhythm Electrophysiol 2019;12:e006841. [DOI] [PubMed] [Google Scholar]
- 23.Masoudi FA, Ponirakis A, de Lemos JA, et al. Trends in U.S. cardiovascular care: 2016 report from 4 ACC National Cardiovascular Data Registries. J Am Coll Cardiol 2017;69:1427–1450. [DOI] [PubMed] [Google Scholar]
- 24.Messenger JC, Ho KK, Young CH, et al. ; NCDR Science and Quality Oversight Committee Data Quality Workgroup. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol 2012;60:1484–1488. [DOI] [PubMed] [Google Scholar]
- 25.Kar S, Doshi SK, Sadhu A, et al. ; PINNACLE FLX Investigators. Primary outcome evaluation of a next-generation left atrial appendage closure device: Results from the PINNACLE FLX trial. Circulation 2021;143:1754–1762. [DOI] [PubMed] [Google Scholar]
- 26.Data Collection Form v1.3, LAAO Registry. NCDR. Accessed March 7, 2022. https://cvquality.acc.org/docs/default-source/ncdr/data-collection/laao_v1-3_data-collection-form.pdf.
- 27.van Buuren S Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–242. [DOI] [PubMed] [Google Scholar]
- 28.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011;57:173–180. [DOI] [PubMed] [Google Scholar]
- 29.Nijenhuis VJ, Brouwer J, Delewi R, et al. Anticoagulation with or without clopidogrel after transcatheter aortic-valve implantation. N Engl J Med 2020;382:1696–1707. [DOI] [PubMed] [Google Scholar]
- 30.Doshi S Primary outcome evaluation of a next generation LAAC device: The PINNACLE FLX Trial. Paper presented at: Heart Rhythm Society Annual Scientific Sessions 2020, May 6–9, 2020; San Diego, CA. [Google Scholar]
- 31.Osmancik P, Herman D, Neuzil P, et al. ; PRAGUE-17 Trial Investigators. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol 2020;75:3122–3135. [DOI] [PubMed] [Google Scholar]
- 32.Lakkireddy D, Thaler D, Ellis CR, et al. Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE): A randomized controlled trial. Circulation 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dukkipati SR, Kar S, Holmes DR, et al. Device-related thrombus after left atrial appendage closure: Incidence, predictors, and outcomes. Circulation 2018;138:874–885. [DOI] [PubMed] [Google Scholar]
- 34.Boersma LV, Schmidt B, Betts TR, et al. ; EWOLUTION investigators. Implant success and safety of left atrial appendage closure with the WATCHMAN device: periprocedural outcomes from the EWOLUTION registry. Eur Heart J 2016;37:2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turagam MK, Reddy VY, Dukkipati SR. EWOLUTION of Watchman left atrial appendage closure to patients with contraindication to oral anticoagulation. Circ Arrhythm Electrophysiol 2019;12:e007257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.