Abstract
There is now considerable evidence supporting the role of a subpopulation of neurons in the arcuate nucleus of the hypothalamus that co-express kisspeptin, neurokinin B, and dynorphin (abbreviated as KNDy neurons) as the long sought-after GnRH pulse generator. The “KNDy hypothesis” of pulse generation has largely been based on findings in rodents and ruminants, and there is considerably less information about the anatomical and functional organization of the KNDy subpopulation in the primate hypothalamus. In this review, we focus on the applicability of this hypothesis, and the roles of kisspeptin, neurokinin B and dynorphin in reproduction, to humans and non-human primates, reviewing available data and pointing out important gaps in our current knowledge. With recent application of drugs that target KNDy peptides and their receptors to therapeutic treatments for reproductive disorders, it is imperative we fully understand the primate KNDy network and its role in the control of GnRH secretion, as well as species differences in this system that may exist between humans, non-human primates and other mammals.
Keywords: kisspeptin, neurokinin B, dynorphin, gonadotropins, estradiol
Introduction
Over the last decade considerable evidence was developed based on work in rodents and ruminants supporting the hypothesis that a group of arcuate (ARC) neurons that contain kisspeptin, neurokinin B (NKB), and dynorphin (known as KNDy neurons) play a key role in synchronizing GnRH neural activity during episodic GnRH secretion (1,2). The original form of this hypothesis proposed that during generation of GnRH pulses, kisspeptin was the output signal from KNDy neurons to GnRH cells, with NKB and dynorphin acting as start and stop signals within the KNDy neural circuitry (3–5). Because estradiol (E2) and testosterone inhibited kisspeptin expression in the ARC (6,7) it was also proposed that these neurons mediate the negative feedback actions of gonadal steroids on episodic GnRH/LH secretion (8). Subsequent tests of various aspects of this hypothesis, while generally supporting it, have also pointed to species differences among rodents and ruminants in the operation of this system (9) and led to some controversy as to their role in steroid negative feedback (10,11). In light of the recent reviews of this topic that emphasized work in rodents and ruminants (2,9), here we will focus on the evidence for, and against, the applicability of this model to humans and non-human primates. We will first briefly summarize the species differences among rodents and ruminants and then review the available data on the roles of kisspeptin, NKB, and dynorphin in controlling GnRH/LH pulses and mediating steroid negative feedback in monkeys and humans.
Comparison of data in rodents and ruminants
The strongest evidence that synchronous activity of KNDy neurons drives episodic GnRH/LH secretion comes from a recent optogenetic study in mice (12). Moreover, several lines of evidence from both rodents and ruminants support the following proposed roles for kisspeptin and NKB: 1) kisspeptin is the output signal from KNDy neurons that drives GnRH secretion during a pulse (13,14), 2) kisspeptin may also act on non-KNDy neurons in the ARC to stimulate GnRH/LH pulse frequency (15,16), and 3) NKB stimulates GnRH release primarily by increasing kisspeptin release from KNDy neurons (17).
On the other hand, there appear to be two major differences in the functioning of this system between rodents and ruminants. First, there is considerable redundancy among the three major tachykinins-receptor signaling complexes (NKB-NK3R, neurokinin A [NKA]-NK2R, and substance P [SP]-NK1R) in rodents so that NKA or SP can substitute for the loss of NKB to maintain LH pulses (18). Thus the combined action of antagonists to NK1R, NK2R, and NK3R are needed to inhibit LH pulses in ovariectomized (OVX) rats (19) and the stimulatory actions of NKB on the electrical activity of KNDy neurons in murine slice preparations (20). In contrast, selective NK3R antagonists readily inhibit LH pulses in OVX ewes (16,21) and selective agonists for NK1R and NK2R produce little, if any, stimulatory effect in sheep (22) and goats (23). Second, there appears to be a major difference in the role of dynorphin. The stimulatory actions of nor-binaltorphimine (nor-BNI), a selective antagonist to the dynorphin receptor (kappa opioid receptor, KOR) on pulse frequency in sheep (16) and goats (4), and the internalization of KOR in KNDy neurons during a pulse (24) provides strong evidence for the proposed inhibitory actions of this endogenous opioid peptide (EOP). In contrast, although there is evidence that nor-BNI can depolarize KNDy neurons after their optogenetic stimulation in vitro (25), this antagonist had no effect on either LH pulses in OVX rats (26,27) or the basal electrical activity of KNDy neurons in murine slices (20,28).
Initial studies demonstrating the inhibitory effects of testosterone and E2 on kisspeptin expression in the ARC of rodents led to the hypothesis that these actions could account for the negative feedback actions of gonadal steroids (8), but subsequent studies questioned this conclusion. Specifically in male mice, castration decreased the acute in vitro electrical activity of KNDy neurons (28,29). However, more long-term electrical recordings in vitro (30) and chronic monitoring of calcium transients in vivo (31) clearly support a negative feedback action of the testes on KNDy neural activity. Moreover, changes in NKB and dynorphin might contribute to these actions. Castration increased NKB expression in KNDy neurons (28,32) and gonadal steroids suppressed the in vitro response of these neurons to an NK3R agonist (33). These steroids also enhanced the inhibitory actions of a KOR agonist (33), but since castration decreases dynorphin expression in rodents (28), the role of this peptide in males remains unclear. There is similar controversy about the role of KNDy neurons in mediating E2 negative feedback because knockout of ERα in kisspeptin neurons (KERKO mice) did not affect the ability of E2 to inhibit LH secretion, but did block the inhibitory actions of this steroid on kisspeptin expression (10). On the other hand, the report (11) of fewer LH pulses on the day of estrus in normal mice than in KERKO mice (which are in constant estrus) suggested that ERα in KNDy neurons is necessary for estrogen negative feedback. However, this difference could reflect the absence of a progesterone peak on the day before sampling in KERKO mice (that do not have an LH surge) since progesterone secretion on proestrus in rodents is responsible for the slow pulse frequency on estrus (34,35). Finally, there is some pharmacological evidence supporting a role for dynorphin in estrogen negative feedback in rats (27,36), but as noted above this is inconsistent with the inhibitory effects of steroids on dynorphin expression in rodents (3).
The data on steroid negative feedback in ruminants is less extensive, but also more consistent. There is little information in males, but kisspeptin and NKB expression in the ARC do increase in castrated sheep (37). Data that a KISS1R agonist increased LH secretion in the presence, but not the absence, of testosterone in castrated goats is consistent with maximal endogenous kisspeptin signaling following castration (38), but no other pharmacological tests are available in male ruminants. In contrast, there have been a number of studies in ewes (described in several recent reviews), which are largely consistent with the hypothesis that E2 inhibits GnRH/LH pulse amplitude by suppressing kisspeptin release from KNDy neurons, while progesterone inhibits GnRH/LH pulse frequency by increasing dynorphin release from the same cells (9,39,40).
Evidence for the proposed role of kisspeptin in control of GnRH/LH pulses in humans and other primates
Roles for kisspeptin and KISS1R in the regulation of the reproductive axis were first established by observations made in humans, where loss-of-function mutations in KISS1R were shown to result in normosmic congenital hypogonadotropic hypogonadism. These seminal studies revealed that inactivating mutations in the KISS1R gene resulted in partial or complete failure of puberty and infertility (41,42). Infusion of kisspeptin into human male and female subjects was shown shortly thereafter to significantly increase plasma LH, FSH, and gonadal steroids (43,44). That the KISS1R and its cognate ligand, kisspeptin, are obligatory elements of neuroendocrine circuits controlling GnRH release, puberty, and adult fertility was confirmed in a variety of mouse models, including studies of reproductive deficits in Kiss1R−/− and Kiss−/− mice (41,45).
Reduced basal serum LH levels and altered pulsatile LH secretory profiles have been consistent findings in individuals with loss-of-function KISS1R mutations, with loss of signaling rigorously documented in cell expression systems. Surprisingly, LH pulsatility appears to persist in these individuals at a relatively normal frequency, albeit greatly reduced pulse amplitude (41,46). By contrast, an inactivating mutation in KISS1 was described in a large consanguineous family that was found to result in failure of puberty associated with a complete absence of detectable LH in serial blood samples (47). It is difficult to conclude from the latter study that the absence of LH pulses reflects an inoperative GnRH pulse generator, since residual pulsatility may occur at levels below the detectable limits of the assay. Nevertheless, the observations of severely reduced LH pulse amplitude and mean LH in the absence of either kisspeptin or its cognate receptor are consistent with the idea that kisspeptin signals through the KISS1R to mediate most of the synchronized pulse initiation signal to GnRH neurons. It is possible that low-amplitude pulses that persist in the absence of kisspeptin or KISS1R signaling reflect the capability of GnRH neurons to maintain an intrinsic pulsatile secretory output, as suggested in early experiments using cultured immortalized GnRH neurons (48). The ability of continuous kisspeptin infusions to restore pulsatile LH secretion in patients with NKB signaling deficiencies (49) may be explained by the emergence of an intrinsic GnRH neuronal pulsatility in the absence of endogenous kisspeptin-KISS1R signaling, and the amplification of that pulsatile output by kisspeptin. Alternatively, the KNDy cell pulse generator model derived from mouse studies may require modification as it pertains to humans, where a GnRH neuron-autonomous pulse generating mechanism may play a more predominant role.
It also remains unknown whether low-amplitude LH pulses that are observed in some of the foregoing affected patients reflect engagement of compensatory kisspeptin- or KISS1R-independent mechanisms during development. Residual GnRH secretory activity has been documented in Kiss1R−/− and Kiss−/− mice (50), and indeed appears to underlie the maintenance of fertility in animals in which kisspeptin neurons have been ablated early in development, attesting to the remarkable plasticity of neural systems governing pulsatile GnRH release. The induction of kisspeptin cell-specific gene ablation in adult animals will hopefully be possible in the foreseeable future, thereby allowing analyses that circumvent the confounding effects of compensatory developmental mechanisms.
Compelling evidence has been derived from studies of non-human primates that kisspeptin-expressing cells of the ARC comprise an integral component of the GnRH pulse generator. Early studies by Knobil and colleagues (51) used multiunit recording methods to characterize the electrophysiological correlates of GnRH pulsatility in the mediobasal hypothalamus (MBH) of rhesus macaques. While it has yet to be determined that these activity patterns arise from intermittent, synchronized activation of kisspeptin neurons, recent studies using optical imaging methods in mice make this scenario extremely likely (12). Microdialysis experiments in female rhesus macaques have revealed that kisspeptin is released in a pulsatile manner in the ARC-median eminence, and that fully 75% of kisspeptin pulses are temporally associated with GnRH pulse (52). Pulsatile injections of kisspeptin-10 induce corresponding GnRH-dependent LH pulses in juvenile male monkeys, where spontaneous GnRH release is virtually absent (53). Structural evidence for kisspeptin subserving GnRH pulsatility in the monkey is derived from the observations that kisspeptin and GnRH fibers are intimately associated in the median eminence (54). It is not yet known if GnRH neurons in the monkey extend “dendron”-like processes into the median eminence where they are synaptically innervated by arcuate kisspeptin neurons, as has been shown to be the case in the mouse (55).
Additional support for kisspeptin-mediated pulsatility is provided by studies of kisspeptin release throughout puberty in the rhesus macaque. Puberty is initiated in monkeys by an activation of pulsatile GnRH release consisting of increased pulse frequency, amplitude and mean release level (56). Microdialysis experiments have revealed that kisspeptin pulsatility is increased in a parallel manner, viz. increased pulse frequency, amplitude, and mean kisspeptin level, throughout puberty in female rhesus monkeys (57). These findings are consistent with the foregoing model for the GnRH pulse generator in which kisspeptin neurons – likely KNDy neurons – comprise a pulse generating network that delivers kisspeptin intermittently to synaptic contacts with GnRH nerve fibers in the median eminence. During the quiescent juvenile period, the activity of the kisspeptin pulse generating network is suppressed, as is the downstream GnRH releasing terminal network in the median eminence. Upon activation by unknown pubertal initiation signals, kisspeptin pulsatility is increased and at least in part leads to the activation of the GnRH pulse generator. Additional evidence for the contribution of NKB release in this process is discussed below. It has been proposed that KNDy neurons play an integral role in the stimulation of GnRH pulses, but they are also passive with respect to the timing of pubertal onset (58).
There is even less data on the role of kisspeptin from KNDy neurons in mediating steroid negative feedback in humans and monkeys, but most of the available information supports this hypothesis. Thus kisspeptin expression in the MBH increases in older men (59), post-menopausal women (60) and peri-menopausal rhesus monkeys (61) when gonadal steroid levels are low. More directly, OVX increased and E2 inhibited KISS1 mRNA expression in monkeys (61) and both and E2 and progesterone treatments decreased the number of KISS1-positive cells in the ARC in these animals (62). Similarly, testosterone replacement at the time of castration suppressed KISS1 mRNA expression in the ARC, but not the preoptic area, of male monkeys (63). It should be noted, however, that exogenous estrogen increases the response of young (64) and post-menopausal (65) women to kisspeptin infusions, although this may relate more to the positive, than negative, feedback actions of this steroid (64).
In summary, a wealth of observations in humans and non-human primates supports the basic contention that a kisspeptin neuronal network in the ARC functions as integral components of the GnRH pulse generator and likely plays a role in steroid negative feedback. It remains to be determined whether 1) KNDy neurons serve as the entirety of the GnRH pulse generating mechanism, or some portion thereof, 2) non-kisspeptin cells systems contribute to pulse generation, 3) KNDy neurons solely innervate GnRH fibers in the median eminence or act in part via interneurons, and 4) GnRH neurons are capable of intrinsic pulsatility that may be amplified and/or entrained by kisspeptin signals.
Evidence for the proposed role of NKB in control of GnRH/LH pulses in humans and other primates
Perhaps the strongest evidence that NKB plays a key role in control of GnRH/LH pulses in humans was the discovery that mutations in NKB-NK3R signaling caused infertility in humans due to inadequate GnRH secretion (66). This observation also served as an important catalyst for the development of the KNDy hypothesis for GnRH pulse generation (1) and a number of subsequent studies tested this proposed role for NKB in humans and non-human primates. In contrast, there is almost no information in these species on the possible role of NKB in steroid negative feedback. Consequently, this section will focus on three questions relevant to the proposed role of NKB in generation of GnRH pulses: 1) What is the degree of overlap of NKB and kisspeptin expression in neuronal cell bodies? 2) Is there redundancy in tachykinin signaling similar to that seen in rodents? and 3) Do the stimulatory actions of NKB occur via, or independent, of kisspeptin release?
Co-localization of kisspeptin and NKB.
Although there is indirect evidence using in situ hybridization (ISH) that mRNAs for NKB and kisspeptin are found in the same neurons in post-menopausal women (60,67), there are no dual ISH studies using primate tissue, so this discussion will be limited to reports using dual immunohistochemistry (IHC). One important caveat applicable to interpretation of any study of co-localization is that expression of kisspeptin and NKB varies with hormonal status, and under some circumstances there can be changes in expression of only one of these peptides (68). It is thus important to examine co-localization under a variety of endocrine milieus whenever possible.
Two reports have described co-localization of kisspeptin and NKB in the rhesus monkey, both in males. In castrated adults 40–60% of kisspeptin perikarya in the ARC also contained NKB, while no cell bodies containing NKB alone were found (69). A similar percentage of kisspeptin cells containing NKB were observed in castrated infant (59%) and juvenile (61%) monkeys, although serum LH concentrations and the number of kisspeptin-positive cells were much lower in the latter (70). Cells containing only NKB were “seldom observed”. Thus, it appears that in the ARC of male monkeys there are approximately equal numbers of kisspeptin-only and kisspeptin+NKB neurons, while few, if any, cells contain only NKB. Whether this is also true in females is an important unresolved question.
There is considerably more data on co-localization of kisspeptin and NKB in humans (59,71–76), and several of these reports examined both cell bodies and kisspeptin or NKB-containing close contacts onto GnRH neurons (59,72–74). The latter, however, are difficult to interpret because the source of the input is not known (e.g. they could be from kisspeptin-only or NKB-only neurons with cell bodies outside the ARC). Although there are both sex-dependent (72) and age-dependent (59) changes in the number of kisspeptin-ir and NKB-ir neurons in the ARC, there is much less variation in co-localization of NKB in kisspeptin neurons (74) with a range from 73% (young men) (59,73) to 78% (old men and post-menopausal women) (59,75). In contrast the percent of NKB neurons containing kisspeptin varies from 33–36% in young men (59,73) to 68% in old men (59) and 67–84% in post-menopausal women (74,75). These data indicate that there are likely three populations of kisspeptin and NKB-containing neurons in the ARC of humans and that there is a selective increase in kisspeptin expression (and hence higher co-localization with NKB) in older individuals.
Possible redundancy in tachykinin signaling.
The strongest evidence for redundancy among the three major tachykinins comes from reports that some patients with mutations in TAC3 or TACR3 show spontaneous recovery of reproductive function, and in some cases fertility, after hormonal treatments to induce development of the reproductive tracks and secondary sexual characteristics (77–79). Similarly, when examined, clear instances of episodic LH secretion have been reported after hormonal treatments, although pulse frequency is slower than in normal individuals (49,77,79). However, non-episodic LH patterns after treatment have also been reported (77,80,81) and these have always been observed before treatments (77). Although they have not been directly compared, there is suggestive evidence that the deficits in LH secretion may be more severe with TACR3, than TAC3, mutations. For example in one of the first reports, the maximum LH concentrations in three patients with TACR3 mutations ranged from 0.15 to 0.2 IU/L compared to 2.4 to 3.8 U/L in three with mutations of TAC3 (80); this group subsequently reported more frequent LH pulses in two patients with TAC3 mutations than in two with them in TACR3 (49). On the other hand, several males with mutations in TACR3 had clear evidence of spontaneous improvements in reproductive function, although only 3 of 13 were fertile (77). Interestingly, this same study found no evidence of spontaneous recovery in four females with TACR3 probands, while 3 of 4 females with TAC3 probands recovered normal function (77). This difference, if confirmed in future work, points to a redundancy in tachykinin ligands, rather than receptors, as a likely explanation for this spontaneous recovery. If this is the case, then SP is the most likely candidate because it can stimulate LH secretion in men (82), is co-localized with kisspeptin and NKB in the human ARC (83) and expression of the mRNA for SP increases in post-menopausal women (67).
While genetic studies argue for redundancy in tachykinin signaling in humans, pharmacological work argues against it. Thus, two different antagonists that are specific for NK3R inhibit tonic LH secretion in normal men and women (84–86) as well as post-menopausal women (87) and patients with polycystic ovarian syndrome (PCOS) (88). This contrast with the data in rats, in which a specific NK3R antagonist had no effect on LH pulses in OVX animals (19). Similarly, most data in non-human primates point to a lack of redundancy in tachykinin signaling. There are very few SP-containing neurons in the primate ARC, although the number did increase with castration, and exogenous SP failed to stimulate LH secretion in male monkeys (89). More importantly, a NK3R antagonist inhibited LH secretion in castrated monkeys and suppressed estradiol levels and delayed (or blocked) the LH surge during the menstrual cycle of cynomolgus monkeys (90).
In summary, strong genetic data point to some redundancy in tachykinin signaling in humans, with SP likely able to substitute for NKB in some of these patients. In contrast, pharmacological data in both humans and non-human primates indicates that little, if any, redundancy exists. Based on analogous situations in mice, the simplest explanation for this apparent paradox is that in normal individuals NKB-NK3R signaling is critical for pulsatile LH secretion, but when this signaling is disrupted redundant systems come on-line during in utero or post-natal development.
Interactions of kisspeptin and NKB in stimulating GnRH secretion:
Not surprisingly, there is little direct information on the relationship between kisspeptin and NKB in humans. There is one report that co-infusion of NKB decreased the stimulatory effects of kisspeptin on mean LH concentrations in men by about 20%, but NKB had no significant effect on LH pulse patterns in that study (91). The ability of exogenous kisspeptin to stimulate LH secretion in patients with mutations in TAC or TACR3 (49,79) supports the hypothesis that NKB action “is proximal to kisspeptin” (49) in stimulating GnRH secretion. However, as discussed above, the possibility of redundancy in tachykinin signaling in these patients argues for caution in interpreting these data.
There is more direct evidence that NKB acts via kisspeptin in rhesus monkeys. In an early test of this relationship, down regulation of KISS1R in castrated juvenile males blocked the ability of senktide, a NK3R agonist, to increase LH, but suppression of NK3R had no effect on the stimulatory actions of kisspeptin (92). Furthermore, senktide increased kisspeptin concentrations in microdialysates of the MBH of both male and female monkeys (93,94). More recent work using reverse microdialysis to deliver agonists and antagonists to the MBH of rhesus monkeys has suggested a more complex relationship between kisspeptin and NKB (93,94). Infusion of p234, a KISS1R antagonist, blocked the stimulatory actions of senktide, on GnRH release in prepubertal male and pubertal male and female (post menarche, but before first ovulation) monkeys. In contrast, in prepubertal female monkeys this antagonist delayed, but did not completely block, the actions of senktide so that GnRH release increased shortly after termination of treatment with both senktide and p234. While these results are largely consistent with the hypothesis that NKB acts via kisspeptin, the experiments with the NK3R antagonist, SB222200, were not. This antagonist blocked the stimulatory action of kisspeptin in prepubertal males and pubertal females, but not in pubertal males and prepubertal females. Thus kisspeptin may act via NKB at some stages of pubertal development, but not at others. These results led to the proposal that there are interactions between kisspeptin-only and NKB-only neurons in the MBH of monkeys, both of which project to GnRH cells, and that these change over development (93), but there is currently no evidence for neurons that contain only NKB in monkeys. An alternative explanation is that in prepubertal males and pubertal females kisspeptin or NKB input alone to GnRH neurons is insufficient to stimulate GnRH release, so that both are required to increase GnRH secretion. It should be noted, however, that it is unclear whether GnRH neurons in primates contain NK3R (or KISS1R). Finally, there are two reports in humans that an NK3R antagonist had no effect on the acute response to kisspeptin administration, one in men (85) and the other in women (64).
Key gaps in knowledge.
In conclusion, there are some important gaps in our knowledge on the roles of NKB and kisspeptin in humans and non-human primates. Specifically, three critical questions arise from this review: 1) Are there NKB-only neurons in the ARC of female rhesus monkeys? 2) Do GnRH neurons contain NK3R in humans or non-human primates? 3) Do the differences in effects of NK3R antagonists on the stimulatory actions of kisspeptin between humans and monkeys reflect species differences or are they due to the lack of studies in adult monkeys to compare with humans?
Evidence for proposed role for dynorphin in GnRH pulse secretion in humans and other primates
Early evidence in humans linked endogenous opioid peptides (EOPs) to an inhibitory role in the control of GnRH/LH secretion (95,96). In mammals, the EOP system consists of three ligand families: the endorphins, the enkephalins and the dynorphins (97). There are three main classes of EOP receptors that mediate the function of EOPs in the brain: δ, μ and κ, all of which are Gi/Go protein-coupled seven-transmembrane receptors (97,98). The main EOPs that are involved in the hypothalamic control of LH secretion are dynorphin and β-endorphin. Compelling evidence on the role of KNDy neurons in regulation of pulsatile LH secretion in rodents (3) and sheep (16) led to an interest in the inhibitory role of one EOP, dynorphin, and its κ-opioid receptor (KOR) on termination of the activity of the KNDy network-induced GnRH/LH pulse. Another EOP, β-endorphin which mainly binds to δ and μ receptors, is also believed to inhibit pulsatile GnRH secretion from the hypothalamus. Therefore, in the current section, we will discuss EOPs as inhibitory modulators of GnRH secretion in humans and non-human primates through: 1) anatomical distribution of dynorphin in relation to GnRH regulation; 2) the role of EOPs in the regulation of pulsatile secretion of GnRH/LH; and 3) the role of EOPs in mediating steroid negative feedback.
Colocalization of kisspeptin and dynorphin.
Early anatomical evidence described the distribution of dynorphin-immunoreactive (dyn-ir) cells in the human ARC (99). The hypothesis that KNDy neurons are the GnRH pulse generator (69,100,101), has raised the interest in the role of dynorphin in the ARC in regulation of LH pulses in mammals, and most importantly, in humans. However, current evidence indicates that the degree of colocalization of dynorphin with kisspeptin and NKB in non-humans primates and humans is limited (60,102,103). It is likely that dynorphin is co-expressed with kisspeptin in the ARC of non-human primates, but with lower prevalence (103). Evidence has shown a high co-expression of dynorphin and kisspeptin of 92% in the murine ARC (3) and 94% in the ovine ARC (101). There is only one study of this co-localization in non-human primates and it found, using IHC, that only 7% of kisspeptin-ir cells and 54% of kisspeptin-ir fibers in the ARC of female rhesus monkeys also co-localized dynorphin (103). The former is relatively low compared with over 40% NKB co-expression in kisspeptin cells in the ARC of the male primate (69). When interpreting these data, it should be noted that the much higher co-localization in kisspeptin-ir fibers may provide a more reliable index of co-localization if kisspeptin is rapidly transported out of the soma. Whether the low degree of co-localization is due to technical limitations of IHC in primates remains to be addressed.
Evidence from human studies has at times appeared to be conflicting on the co-expression of dynorphin and kisspeptin depending on sex and age. Low prevalence in dynorphin-ir cell bodies colocalized with kisspeptin was found in the ARC of young men (73), and post-menopausal women (76). However, the latter may be due to the loss of steroid negative feedback in older women. In contrast, using ISH, dynorphin neurons were later reported to be present in pre- and post-menopausal women, with prodynorphin (the precursor for dynorphin) mRNA detected in the ARC, and these cells showing a hypertrophy post menopause similar to kisspeptin and NKB neurons in the ARC (102). Unlike the elevation of NKB and kisspeptin gene expression, dynorphin mRNA expression decreased in the human ARC after menopause (102). Consistent with this, lower dynorphin expression levels in ovariectomized (OVX) ewes (104) and monkeys (105) have been reported. Thus, the rare colocalization in older women may reflect the loss of the stimulatory effects of sex steroids in dynorphin expression. Taken together, it appears that in both humans and other primates dynorphin is expressed in the KNDy population, but at fairly low levels. Whether the reported rare colocalization of dynorphin and kisspeptin negates the model of KNDy neurons in regulation of GnRH in primates should be interpreted with caution, because reproductive neuropeptide regulation varies between sexes, at different ages and under different hormone milieus.
Interactions of kisspeptin and EOP in control of GnRH secretion.
The inhibitory role of EOPs on LH secretion was reported long before the role of KNDy neurons as a upstream regulator of GnRH was discovered (106). Yet, there is no direct evidence in humans and non-human primates on the role of specific EOPs, especially dynorphin, in this action.
The opioid receptor antagonist, naloxone, may provide some evidence of the involvement of this pathway. Compelling clinical evidence has shown the lack of effects of naloxone on LH secretion in post-menopausal women (107–110) with absent ovarian steroid feedback. Consistent with this, in oophorectomized women (111), a 4 hr naloxone infusion had no effect on the LH secretion. These data contrast with work in younger women and men. In young women, administration of naloxone in the late follicular phase and luteal phase, but not early follicular phase, increased pulsatile LH release and LH pulse frequency (106). Similarly, in normal men, administration of naloxone (112) or naltrexone (91) increased the average LH level. In the latter study, co-infusion of naltrexone did not increase the effects of kisspeptin on mean LH concentrations, so that there may be separate mechanisms regulating LH secretion by EOP and kisspeptin in men. It should be noted that the effects of naloxone in young women and men may be confounded by EOP mediation of steroid negative feedback, but the recent report that naloxone increased LH pulse frequency in women with hypogonadotropic hypogonadism (79) indicates that naloxone can stimulate LH in the absence of steroid negative feedback. In summary, there is no direct evidence in primates that an EOP terminates GnRH/LH pulses. However, this may reflect use of relatively non-specific EOP antagonists such as naloxone. Evidence in sheep that naloxone doesn’t affect LH pulses in OVX animals (113), but nor-BNI, a specific KOR antagonist (114), increases the frequency of pulses in OVX sheep (16) and of MUA in OVX goats (4) indicates that more work, using a specific KOR antagonist, is needed in primates.
Role of EOP in steroid negative feedback.
Numerous lines of evidence suggest that EOP are involved in the negative feedback effects of sex steroids in animals as well as humans. In normal cycling women, EOP are strongly involved in the regulation of the menstrual cycle and the reproductive axis via inhibitory effects on hypothalamic pulsatile GnRH secretion (115,116). Moreover, the proposal that dynorphin is the EOP that mediates the negative feedback effect of progesterone is supported by compelling data from ewes. This is based on the following observations: 1) almost all dynorphin cells in the ARC express progesterone receptors (117); 2) prodynorphin mRNA expression decreased after OVX (104); and 3) nor-BNI increased LH pulse frequency in luteal phase ewes (118). Thus, a key question is whether dynorphin, or another EOP, conveys steroid negative feedback in monkeys, and more importantly in humans, but evidence in these species is not as abundant as in the ewe.
As mentioned above, dynorphin is co-expressed with kisspeptin in the ARC of primates, even though at low abundance (76,103). The most important early evidence implicating dynorphin in steroid negative feedback in humans is the ISH study showing dynorphin expressing cells have a hypertrophy (102) similar to that of kisspeptin and NKB cells in the human ARC after menopause (60). Moreover, in post-menopausal women, the number of neurons expressing prodynorphin mRNA in the ARC is reduced by 73% compared to pre-menopausal women (102); this contrasts with the elevated kisspeptin (60) and NKB mRNA (67) expression in these women. This reduction of inhibitory dynorphin mRNA in the absence of steroid negative feedback in post-menopausal women, pointed to a link between dynorphin and steroid negative feedback effects.
Studies using naloxone have also provided functional support for this proposal. In the early follicular phase of the normal menstrual cycle, when sex steroid levels are low, administration of naloxone did not alter pulsatile LH secretion (96,106). Consistent with this are the reports of lack of effects of naloxone treatments in post-menopausal women (110) and oophorectomized young women (111). In contrast, in women with a high progesterone milieu, whether under exogenous progesterone treatment (111) or endogenous progesterone secretion during the luteal phase (119), infusion of naloxone increased LH levels. Similar stimulatory effects of naloxone have been observed during the luteal phase of the monkey menstrual cycle (120–122) and in OVX animals with progesterone supplementation, but not OVX animals (113). These data strongly support the proposal that an inhibitory EOP mediates the negative feedback effects of progesterone. However, we should note that naloxone is a classic opioid receptor antagonist, which can bind to all three opioid receptors (123) so these data do not point to a specific EOP.
As noted above, changes in prodynorphin after menopause point to dynorphin, but that is also true for β-endorphin. Thus, based on ISH data, pro-opiomelanocortin (the precursor for β-endorphin) mRNA decreases in the human ARC after menopause (124). Moreover, progesterone administration increases β-endorphin in the hypophyseal portal blood of monkeys (125). In contrast, infusion of β-endorphin had no effect on LH secretion in post-menopausal women (107). This might be explained by data from female rats that steroids strongly upregulate the expression of the µ-opioid receptor (126), but this may not be the case in humans because no difference was found in µ-opioid binding potentials using positron emission tomography during the menstrual cycle in women (127). In ewes, MBH microimplants of nor-BNI, but not aδ or μ-opioid receptor antagonist, increased LH pulse frequency in the luteal phase (118). Moreover, several lines of evidence in sheep (118) and rats (128) using nor-BNI, have indicated that dynorphin and its receptor KOR mediate this feedback effect but there is only modest support for this in primates. Taken together, although it appears that an EOP, possibly dynorphin, plays an important role in the progesterone negative feedback in primates, more evidence using specific EOP receptor antagonists may help answer the question.
There is also some limited data that EOPs may mediate the negative feedback actions of other steroids in humans. The possibility that an EOP is involved in estradiol inhibitory effects on LH secretion has been proposed based on the early findings that naloxone had no effect on LH secretion in post-menopausal women, but increased LH secretion when these women received estrogen treatment (109,111). Moreover, in normal cycling women, naloxone increased LH secretion in the late follicular phase under a high estrogen milieu, but not in the early follicular phase when estrogen levels are low (96,106). Similarly, naltrexone was able to overcome the inhibitory effects of estradiol treatment in normal men (112). As mentioned above, expression of precursor mRNAs for both dynorphin and β-endorphin decrease after menopause in women, which could reflect the low estrogen levels in these women. It thus seems reasonable to propose that an EOP may be involved in the negative feedback effects of estrogen in humans. There is evidence that dynorphin plays a role in estrogen negative feedback in rats (27), but EOPs do not seem to be involved in monkeys (120,122) or ewes (129). However, the role of dynorphin in this effect in women is unclear because of lack of evidence using specific EOP receptor antagonists. There is even less data on the possible role of EOP in androgen negative feedback in primates. As noted earlier, the stimulatory effects of naloxone (112) and naltrexone (91) in normal men support this role. In the only report directly testing this hypothesis in men, administration of dihydrotestosterone strongly inhibited LH secretion, and infusion of naloxone abolished the inhibitory effects of this androgen on LH levels (112). Therefore, the limited data available suggest that an EOP might at least be partly involved in the androgen negative feedback in men.
In summary, it is generally accepted that EOP have inhibitory effects on pulsatile GnRH/LH secretion in humans and non-human primates and, in particular, mediate progesterone negative feedback. Although some evidence suggests that dynorphin may possibly be the EOP mediating progesterone negative feedback, it is too early for a clear conclusion in primates before more work on the anatomical and physiological functions of dynorphin and KOR is done.
Key gaps in knowledge.
We know that a variety of endocrinological clinical conditions indicate that, in females, an EOP serves as a “brake” to GnRH/LH pulsatile secretion, the disturbance of which can result in diseases and infertility, such as PCOS. Moreover, it seems likely that dynorphin may be involved in the progesterone negative feedback in humans and other primates. However, there are several major gaps in our current knowledge on the role of EOP in humans and non-human primates. These include: 1) Is KOR expressed in either KNDy and/or GnRH neurons? Preliminary evidence suggests that KOR is co-localized in NKB neurons of the female monkey ARC (Fig. 1), but this needs to be confirmed with co-localization of kisspeptin and dynorphin. 2) What is the extent of dynorphin colocalization with both kisspeptin and NKB in the monkey and human ARC? Is the reported low co-localization of dynorphin and kisspeptin due to technical reasons? 2) Does nor-BNI, or other specific KOR inhibitors, overcome the inhibitory effects of progesterone, or other gonadal steroids, in humans and non-human primates? 3) Does an EOP act through the KNDy network to produce the inhibitory effects on pulsatile LH secretion seen in humans and non-human primates?
Figure 1.
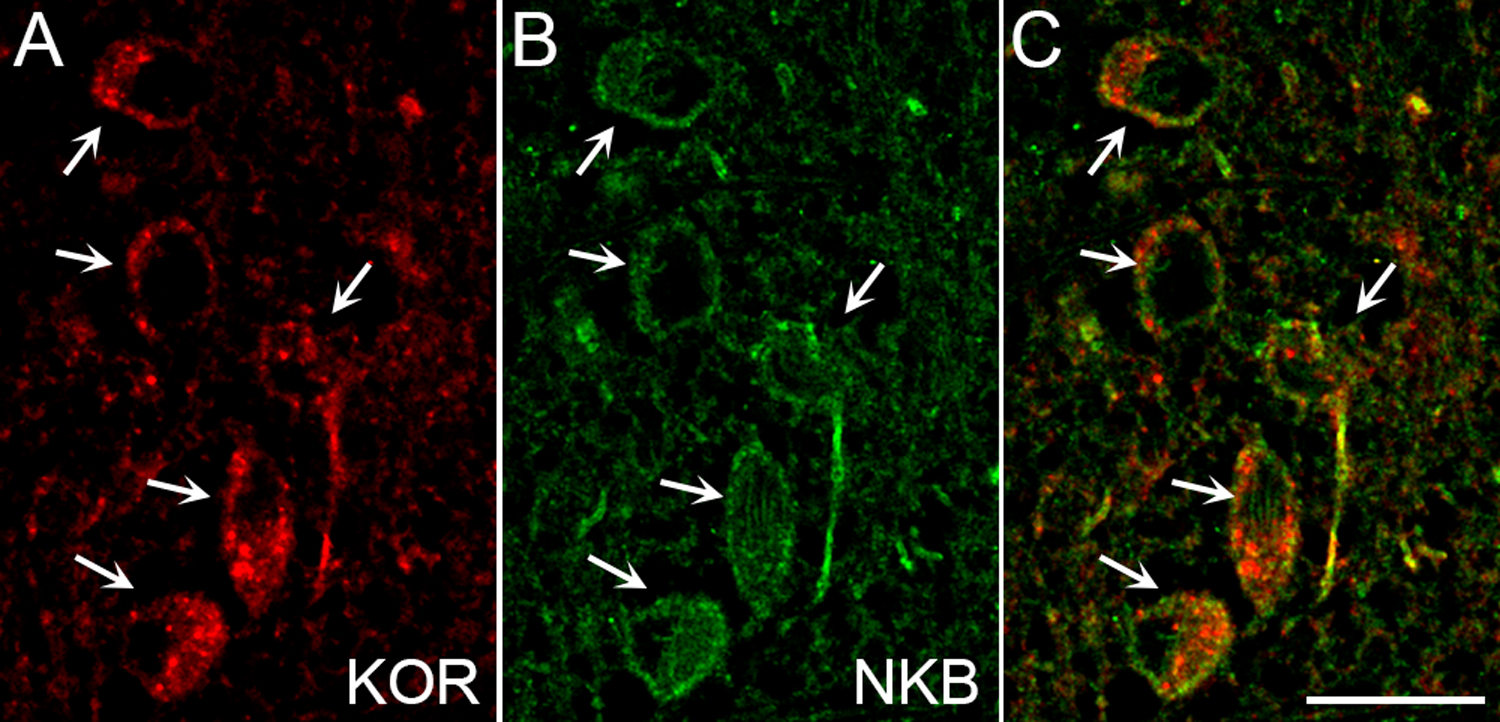
Colocalization of KOR and NKB in the non-human primate ARC. Confocal image (1 µm optical section) of neurons immunolabelled for KOR (A, red), NKB (B, green), and the merged image (C) in an adult female Rhesus macaque. Arrows indicate dual-labeled cells.
Sex differences in KNDy neurons in humans and other primates
There is clear evidence for anatomical sexual dimorphism in the KNDy cell population in humans, with men having fewer kisspeptin and NKB-containing neurons than women (72). A similar sexual dimorphism in NKB, kisspeptin, and dynorphin expression is seen in KNDy neurons of sheep (130,131), but the situation in rodents is more complex. There is no sexual dimorphism in ARC kisspeptin expression in mice (132); in rats, IHC (133–136), but not ISH (137–140), studies found more kisspeptin neurons in the ARC, and NKB neurons in the caudal ARC (136), in females than in males. There is currently a lack of evidence as to whether the sex differences in KNDy peptides observed in humans are present in non-human primates, and this is a critical gap in the literature. It should be noted that sex differences in numbers of adult neurons could result from either a long-term change in gene/peptide expression and/or developmental effects on cell differentiation or survival. Hence, sexual dimorphism in humans could reflect differences in steroid negative feedback because the tissue was from older individuals so the women would have been post-menopausal and have increased expression of kisspeptin and NKB (60,67). The sexual dimorphism observed in KNDy neurons in sheep most likely reflects the organizational actions of androgens during pregnancy (130,131), and it remains to be seen whether prenatal steroids exert similar influences on the expression of KNDy peptides in the adult primate brain.
The functional significance of this sexual dimorphism is unclear, in part because most studies testing the KNDy hypothesis have been done in females (2), and there are few direct comparisons between male and female rats, although the LH response to senktide is lower in male than female rats (141). Interestingly, the limited available evidence for functional sex differences in this system comes largely from primates. For example, a NK3R antagonist (SB222200) blocked kisspeptin stimulation of GnRH release in female (93), but not male (94), post-pubertal rhesus monkeys. There are also sex differences in response to senktide and kisspeptin: the senktide-induced increase in kisspeptin and the kisspeptin-induced increase in GnRH are greater in female monkeys (93,94). In contrast, men are more responsive than women to kisspeptin-10 (44). One intriguing sexual dimorphism in humans is that kisspeptin-10 delays the next LH pulse in men (142), but not women (143), which raises the possibility of important sex differences in the effects of kisspeptin on the GnRH pulse generator. The mechanisms by which kisspeptin resets the pulse generator in the primate are unknown, although one possibility is that that this effect is mediated by non-KNDy, KISS1R-expressing neurons of the ARC (144) which in the rat and sheep have been shown to stimulate GnRH/LH pulse frequency (15,16)
Conclusions
While evidence from anatomical, pharmacological, electrophysiological, and optogenetic studies in rodents and ruminants have strongly supported the “KNDy hypothesis” of pulse generation, important questions remain regarding the anatomical organization and physiological roles of this ARC subpopulation in the human and non-human primate brain. At a start, these include significant gaps in knowledge and potential differences between primates and other species in the co-localization/presence of the three KNDy peptides and receptors (Table 1). For example, the co-localization of NK3R and KOR within KNDy cells underlies one of the core elements of the KNDy hypothesis, namely the ability of NKB and dynorphin to act within a reciprocally interconnected network of KNDy cells as start and stop signals, respectively, for each GnRH pulse. NK3R and KOR colocalization has been reported in ARC kisspeptin cells in both mouse and sheep, albeit with different percentages (perhaps due to the use of IHC vs ISH, see NK3R in sheep, Table 1), but the co-expression of either receptor in monkey or human KNDy cells has yet to be examined. It would also be important to assess the co-expression of these two receptors in GnRH neurons. In addition, as mentioned above, differences in reported low co-localization of dynorphin and kisspeptin in the monkey ARC, and particularly in humans, may be due to technical reasons or attributable to sex, steroid or age differences; this needs to be rigorously re-examined with the same techniques and controls for those variables.
Table 1.
KNDy peptide/receptor co-localization in rodents, sheep and primates
| % ARC kisspeptin (KP) cells co-localizing: | Rodents | Sheep | Monkeys | Human |
|---|---|---|---|---|
| NKB | Mouse: 90%, OVX and OVX+E (3), 94%, castrated and castrated+T males (32); Rat: 97% (147) | 80%, OVX+E, breeding season (80); 91%, OVX+E breeding season and anestrus (148) | 40–60%, castrated adult males (45); 60%, infant and juvenile agonadal males (46) | 71–78%, young and aged men and women (47), postmenopausal women (83) |
| Dynorphin | Mouse, 92%, OVX and OVX+E (3), 86%, castrated males (32) | 94%, OVX+E, breeding season (80) | 7% in KP cells but 54% in KP fibers, OVX adult female (82) | “Very rare”, young men (49), postmenopausal women (53) |
| NK3R | Mouse, 96%, OVX (3), 76%, castrated males (32) | 47% (IHC), artificial follicular phase(149), 94% (ISH), luteal phase (He et al., SfN abstract, 2019) | N/A | N/A |
| KOR | Mouse: 20%, OVX and OVX+E females (3); 6%, castrated males (32) | 98%, luteal phase, estrous cycle (150) | N/A | N/A |
Beyond gaps to be filled with respect to the anatomical features of KNDy cells in primates, there are a number of questions regarding the physiological roles of KNDy peptides and receptors in the control of GnRH pulses. Some of these, such as whether kisspeptin drives, or is just permissive for, episodic GnRH release have yet to be completely addressed in any species. Others relate to possible species differences in the roles of these peptides. One of the latter is whether dynorphin (or another EOP) acts through the KNDy network to inhibit GnRH pulses in the human or non-human primate brain, as it has been shown to do in ruminants. Studies to explore whether nor-BNI, or other specific KOR inhibitors, overcome the inhibitory effects of progesterone or other gonadal steroids would be particularly worthwhile in testing the role of dynorphin as a stop signal for pulses in primates, regardless of whether that action is via KNDy or other cells. Finally, it is important to know whether the reported differences in effects of NK3R antagonists on the stimulatory actions of kisspeptin between humans and monkeys reflect species differences or age differences in the subjects.
Overall, there are a number of key questions regarding KNDy neurons in the primate hypothalamus that need answering in order to determine whether the KNDy model for control of GnRH pulses, as currently framed, can be applied to monkeys and humans. Given significant species differences between rodents and ruminants in the organization and function of this system reviewed above and elsewhere (9), it is entirely possible that the GnRH pulse generator in the primate is comprised of slightly different neurons and/or and neuropeptide/receptor components than other mammals. On the other hand, evidence to date on the central roles of kisspeptin and NKB controlling GnRH secretion in primates suggests that the major elements may well be the same. With recent translation of findings stemming from the discovery of KNDy neurons to new therapeutic treatments for infertility (145), PCOS (88) and hot flushes (87,146), it is critical that we fully understand the KNDy network and its role in pulse generation in primates so that future clinical interventions are based on the most solid and rigorous scientific foundation possible.
Acknowledgments
This work was supported by NIH R01 HD39916 to M.N.L. and R.L.G.
References
- 1.Goodman RL, Coolen LM, Lehman MN. Unraveling the mechanism of action of the GnRH pulse generator: A possible role for kisspeptin/neurokinin B/Dynorphin (KNDy) neurons. In: Conn MD, ed. Cellular Endocrinology in Health and Disease. Amsterdam: Elsevier; 2014:133–152. [Google Scholar]
- 2.Herbison AE. The Gonadotropin-Releasing Hormone Pulse Generator. Endocrinology 2018; 159:3723–3736 [DOI] [PubMed] [Google Scholar]
- 3.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009; 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. The Journal of neuroscience : the official journal of the Society for Neuroscience 2010; 30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010; 151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005; 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 7.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 2005; 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 8.Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology 2006; 147:1154–1158 [DOI] [PubMed] [Google Scholar]
- 9.Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy cells revisited. Endocrinology 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois SL, Acosta-Martinez M, DeJoseph MR, Wolfe A, Radovick S, Boehm U, Urban JH, Levine JE. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor alpha in kisspeptin neurons. Endocrinology 2015; 156:1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Burger LL, Greenwald-Yarnell ML, Myers MG Jr., Moenter SM. Glutamatergic Transmission to Hypothalamic Kisspeptin Neurons Is Differentially Regulated by Estradiol through Estrogen Receptor alpha in Adult Female Mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 2018; 38:1061–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proceedings of the National Academy of Sciences of the United States of America 2017; 114:E10216–E10223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology 2011; 152:1001–1012 [DOI] [PubMed] [Google Scholar]
- 14.Higo S, Iijima N, Ozawa H. Characterization of Kiss1r (Gpr54)-expressing neurons in the arcuate nucleus of the female rat hypothalamus. Journal of neuroendocrinology 2016; [DOI] [PubMed] [Google Scholar]
- 15.Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O’Byrne KT. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PloS one 2009; 4:e8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology 2013; 154:4259–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenrohr M, Tena-Sempere M. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology 2012; 153:316–328 [DOI] [PubMed] [Google Scholar]
- 18.Fergani C, Navarro VM. Expanding the Role of Tachykinins in the Neuroendocrine Control of Reproduction. Reproduction 2016; 153:R1–R14 [DOI] [PubMed] [Google Scholar]
- 19.Noritake K, Matsuoka T, Ohsawa T, Shimomura K, Sanbuissho A, Uenoyama Y, Maeda K, Tsukamura H. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. The Journal of reproduction and development 2011; 57:409–415 [DOI] [PubMed] [Google Scholar]
- 20.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology 2013; 154:2750–2760 [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Millar RP, Clarke IJ, Smith JT. Evidence that Neurokinin B Controls Basal Gonadotropin-Releasing Hormone Secretion but Is Not Critical for Estrogen-Positive Feedback in Sheep. Neuroendocrinology 2015; 101:161–174 [DOI] [PubMed] [Google Scholar]
- 22.Fergani C, Mazzella L, Coolen LM, McCosh RB, Hardy SL, Newcomb N, Grachev P, Lehman MN, Goodman RL. Do Substance P and Neurokinin A Play Important Roles in the Control of LH Secretion in Ewes? Endocrinology 2016:en20161565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamura T, Wakabayashi Y, Ohkura S, Navarro VM, Okamura H. Effects of intravenous administration of neurokinin receptor subtype-selective agonists on gonadotropin-releasing hormone pulse generator activity and luteinizing hormone secretion in goats. The Journal of reproduction and development 2015; 61:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weems PW, Coolen LM, Hileman SM, Hardy S, McCosh RB, Goodman RL, Lehman MN. EVIDENCE THAT DYNORPHIN ACTS UPON KNDy AND GnRH NEURONS DURING GnRH PULSE TERMINATION IN THE EWE. Endocrinology 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, Ronnekleiv OK. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife 2016; 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grachev P, Li XF, Kinsey-Jones JS, di Domenico AL, Millar RP, Lightman SL, O’Byrne KT. Suppression of the GnRH pulse generator by neurokinin B involves a kappa-opioid receptor-dependent mechanism. Endocrinology 2012; 153:4894–4904 [DOI] [PubMed] [Google Scholar]
- 27.Mostari P, Ieda N, Deura C, Minabe S, Yamada S, Uenoyama Y, Maeda K, Tsukamura H. Dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. The Journal of reproduction and development 2013; 59:266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and kappa-opioid receptors in adult male mice. Endocrinology 2013; 154:2761–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology 2012; 153:5384–5393 [DOI] [PubMed] [Google Scholar]
- 30.Vanacker C, Moya MR, DeFazio RA, Johnson ML, Moenter SM. Long-Term Recordings of Arcuate Nucleus Kisspeptin Neurons Reveal Patterned Activity That Is Modulated by Gonadal Steroids in Male Mice. Endocrinology 2017; 158:3553–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han SY, Kane G, Cheong I, Herbison AE. Characterization of GnRH Pulse Generator Activity in Male Mice Using GCaMP Fiber Photometry. Endocrinology 2019; 160:557–567 [DOI] [PubMed] [Google Scholar]
- 32.Navarro VM, Gottsch ML, Wu M, Garcia-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 2011; 152:4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruka KA, Burger LL, Moenter SM. Both Estrogen and Androgen Modify the Response to Activation of Neurokinin-3 and kappa-Opioid Receptors in Arcuate Kisspeptin Neurons From Male Mice. Endocrinology 2016; 157:752–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith MS, Fox SR, Chatterton RT. Role of proestrous progesterone secretion in suppressing basal pulsatile LH secretion during estrus of the estrous cycle. Neuroendocrinology 1989; 50:308–314 [DOI] [PubMed] [Google Scholar]
- 35.McQuillan HJ, Han SY, Cheong I, Herbison AE. GnRH Pulse Generator Activity Across the Estrous Cycle of Female Mice. Endocrinology 2019; 160:1480–1491 [DOI] [PubMed] [Google Scholar]
- 36.Kanaya M, Iwata K, Ozawa H. Distinct dynorphin expression patterns with low- and high-dose estrogen treatment in the arcuate nucleus of female rats. Biology of reproduction 2017; 97:709–718 [DOI] [PubMed] [Google Scholar]
- 37.Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology 2012; 153:2756–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamura T, Wakabayashi Y, Sakamoto K, Matsui H, Kusaka M, Tanaka T, Ohkura S, Okamura H. The effects of chronic subcutaneous administration of an investigational kisspeptin analog, TAK-683, on gonadotropin-releasing hormone pulse generator activity in goats. Neuroendocrinology 2014; 100:250–264 [DOI] [PubMed] [Google Scholar]
- 39.Goodman RL, Inskeep EK. Control of the ovarian cycle of the sheep. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction, Fourth Edition. Vol 2. Amsterdam: Elsevier; 2015:1259–1305. [Google Scholar]
- 40.Nestor CC, Bedenbaugh MN, Hileman SM, Coolen LM, Lehman MN, Goodman RL. Regulation of GnRH pulsatility in sheep. Reproduction 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr., Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. The New England journal of medicine 2003; 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 42.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proceedings of the National Academy of Sciences of the United States of America 2003; 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. The Journal of clinical endocrinology and metabolism 2005; 90:6609–6615 [DOI] [PubMed] [Google Scholar]
- 44.Jayasena CN, Nijher GM, Comninos AN, Abbara A, Januszewki A, Vaal ML, Sriskandarajah L, Murphy KG, Farzad Z, Ghatei MA, Bloom SR, Dhillo WS. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. The Journal of clinical endocrinology and metabolism 2011; 96:E1963–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 2007; 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 46.Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. The Journal of clinical endocrinology and metabolism 2007; 92:1137–1144 [DOI] [PubMed] [Google Scholar]
- 47.Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. The New England journal of medicine 2012; 366:629–635 [DOI] [PubMed] [Google Scholar]
- 48.Martinez de la Escalera G, Choi AL, Weiner RI. Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1–1 GnRH neuronal cell line. Proceedings of the National Academy of Sciences of the United States of America 1992; 89:1852–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young J, George JT, Tello JA, Francou B, Bouligand J, Guiochon-Mantel A, Brailly-Tabard S, Anderson RA, Millar RP. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology 2013; 97:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. Journal of neuroendocrinology 2009; 21:1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Byrne KT, Knobil E. Electrophysiological approaches to gonadotrophin releasing hormone pulse generator activity in the rhesus monkey. Human reproduction 1993; 8 Suppl 2:37–40 [DOI] [PubMed] [Google Scholar]
- 52.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 2008; 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology 2006; 147:1007–1013 [DOI] [PubMed] [Google Scholar]
- 54.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 2008; 149:4387–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore AM, Prescott M, Czieselsky K, Desroziers E, Yip SH, Campbell RE, Herbison AE. Synaptic Innervation of the GnRH Neuron Distal Dendron in Female Mice. Endocrinology 2018; 159:3200–3208 [DOI] [PubMed] [Google Scholar]
- 56.Watanabe G, Terasawa E. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology 1989; 125:92–99 [DOI] [PubMed] [Google Scholar]
- 57.Guerriero KA, Keen KL, Terasawa E. Developmental increase in kisspeptin-54 release in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta). Endocrinology 2012; 153:1887–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terasawa E, Guerriero KA, Plant TM. Kisspeptin and puberty in mammals. Advances in experimental medicine and biology 2013; 784:253–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molnar CS, Vida B, Sipos MT, Ciofi P, Borsay BA, Racz K, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Hrabovszky E. Morphological evidence for enhanced kisspeptin and neurokinin B signaling in the infundibular nucleus of the aging man. Endocrinology 2012; 153:5428–5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. The Journal of clinical endocrinology and metabolism 2007; 92:2744–2750 [DOI] [PubMed] [Google Scholar]
- 61.Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. Influence of age and 17beta-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology 2010; 151:3783–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alcin E, Sahu A, Ramaswamy S, Hutz ED, Keen KL, Terasawa E, Bethea CL, Plant TM. Ovarian regulation of kisspeptin neurones in the arcuate nucleus of the rhesus monkey (macaca mulatta). Journal of neuroendocrinology 2013; 25:488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta). Journal of neuroendocrinology 2007; 19:432–438 [DOI] [PubMed] [Google Scholar]
- 64.Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Interactions Between Neurokinin B and Kisspeptin in Mediating Estrogen Feedback in Healthy Women. The Journal of clinical endocrinology and metabolism 2016; 101:4628–4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lippincott MF, Chan YM, Rivera Morales D, Seminara SB. Continuous Kisspeptin Administration in Postmenopausal Women: Impact of Estradiol on Luteinizing Hormone Secretion. The Journal of clinical endocrinology and metabolism 2017; 102:2091–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nature genetics 2009; 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rance NE, Young WS 3rd. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 1991; 128:2239–2247 [DOI] [PubMed] [Google Scholar]
- 68.Goodman RL, Ohkura S, Okamura H, Coolen LM, Lehman MN. The KNDy hypothesis for generation of GnRH pulses: Evidence from sheep and goats. In: Plant TM, Herbison A, eds. The GnRH Neuron and its Control. Hoboken NJ John Wiley and Sons, Ltd; 2018:289–324. [Google Scholar]
- 69.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 2010; 151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramaswamy S, Dwarki K, Ali B, Gibbs RB, Plant TM. The decline in pulsatile GnRH release, as reflected by circulating LH concentrations, during the infant-juvenile transition in the agonadal male rhesus monkey (Macaca mulatta) is associated with a reduction in kisspeptin content of KNDy neurons of the arcuate nucleus in the hypothalamus. Endocrinology 2013; 154:1845–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. The European journal of neuroscience 2010; 31:1984–1998 [DOI] [PubMed] [Google Scholar]
- 72.Hrabovszky E, Molnar CS, Sipos MT, Vida B, Ciofi P, Borsay BA, Sarkadi L, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Kallo I, Liposits Z. Sexual dimorphism of kisspeptin and neurokinin B immunoreactive neurons in the infundibular nucleus of aged men and women. Frontiers in endocrinology 2011; 2:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hrabovszky E, Sipos MT, Molnar CS, Ciofi P, Borsay BA, Gergely P, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology 2012; 153:4978–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hrabovszky E, Takacs S, Gocz B, Skrapits K. New Perspectives for Anatomical and Molecular Studies of Kisspeptin Neurons in the Aging Human Brain. Neuroendocrinology 2019; [DOI] [PubMed] [Google Scholar]
- 75.Skrapits K, Borsay BA, Herczeg L, Ciofi P, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Hrabovszky E. Colocalization of cocaine- and amphetamine-regulated transcript with kisspeptin and neurokinin B in the human infundibular region. PloS one 2014; 9:e103977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skrapits K, Borsay BA, Herczeg L, Ciofi P, Liposits Z, Hrabovszky E. Neuropeptide co-expression in hypothalamic kisspeptin neurons of laboratory animals and the human. Frontiers in neuroscience 2015; 9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonca BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. The Journal of clinical endocrinology and metabolism 2010; 95:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sidhoum VF, Chan YM, Lippincott MF, Balasubramanian R, Quinton R, Plummer L, Dwyer A, Pitteloud N, Hayes FJ, Hall JE, Martin KA, Boepple PA, Seminara SB. Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. The Journal of clinical endocrinology and metabolism 2014; 99:861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lippincott MF, Leon S, Chan YM, Fergani C, Talbi R, Farooqi IS, Jones CM, Arlt W, Stewart SE, Cole TR, Terasawa E, Hall JE, Shaw ND, Navarro VM, Seminara SB. Hypothalamic Reproductive Endocrine Pulse Generator Activity Independent of Neurokinin B and Dynorphin Signaling. The Journal of clinical endocrinology and metabolism 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. The Journal of clinical endocrinology and metabolism 2010; 95:2287–2295 [DOI] [PubMed] [Google Scholar]
- 81.Francou B, Bouligand J, Voican A, Amazit L, Trabado S, Fagart J, Meduri G, Brailly-Tabard S, Chanson P, Lecomte P, Guiochon-Mantel A, Young J. Normosmic congenital hypogonadotropic hypogonadism due to TAC3/TACR3 mutations: characterization of neuroendocrine phenotypes and novel mutations. PloS one 2011; 6:e25614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coiro V, Volpi R, Capretti L, Caiazza A, Marcato A, Bocchi R, Colla R, Rossi G, Chiodera P. Luteinizing hormone response to an intravenous infusion of substance P in normal men. Metabolism: clinical and experimental 1992; 41:689–691 [DOI] [PubMed] [Google Scholar]
- 83.Hrabovszky E, Borsay BA, Racz K, Herczeg L, Ciofi P, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Substance P immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular region. PloS one 2013; 8:e72369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Skorupskaite K, George JT, Veldhuis JD, Anderson RA. Neurokinin B Regulates Gonadotropin Secretion, Ovarian Follicle Growth, and the Timing of Ovulation in Healthy Women. The Journal of clinical endocrinology and metabolism 2018; 103:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Neurokinin 3 receptor antagonism decreases gonadotropin and testosterone secretion in healthy men. Clinical endocrinology 2017; 87:748–756 [DOI] [PubMed] [Google Scholar]
- 86.Fraser GL, Ramael S, Hoveyda HR, Gheyle L, Combalbert J. The NK3 Receptor Antagonist ESN364 Suppresses Sex Hormones in Men and Women. The Journal of clinical endocrinology and metabolism 2016; 101:417–426 [DOI] [PubMed] [Google Scholar]
- 87.Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Neurokinin 3 Receptor Antagonism Reveals Roles for Neurokinin B in the Regulation of Gonadotropin Secretion and Hot Flashes in Postmenopausal Women. Neuroendocrinology 2018; 106:148–157 [DOI] [PubMed] [Google Scholar]
- 88.George JT, Kakkar R, Marshall J, Scott ML, Finkelman RD, Ho TW, Veldhuis J, Skorupskaite K, Anderson RA, McIntosh S, Webber L. Neurokinin B Receptor Antagonism in Women With Polycystic Ovary Syndrome: A Randomized, Placebo-Controlled Trial. The Journal of clinical endocrinology and metabolism 2016; 101:4313–4321 [DOI] [PubMed] [Google Scholar]
- 89.Kalil B, Ramaswamy S, Plant TM. The Distribution of Substance P and Kisspeptin in the Mediobasal Hypothalamus of the Male Rhesus Monkey and a Comparison of Intravenous Administration of These Peptides to Release GnRH as Reflected by LH Secretion. Neuroendocrinology 2016; 103:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fraser GL, Hoveyda HR, Clarke IJ, Ramaswamy S, Plant TM, Rose C, Millar RP. The NK3 Receptor Antagonist ESN364 Interrupts Pulsatile LH Secretion and Moderates Levels of Ovarian Hormones Throughout the Menstrual Cycle. Endocrinology 2015; 156:4214–4225 [DOI] [PubMed] [Google Scholar]
- 91.Narayanaswamy S, Prague JK, Jayasena CN, Papadopoulou DA, Mizamtsidi M, Shah AJ, Bassett P, Comninos AN, Abbara A, Bloom SR, Veldhuis JD, Dhillo WS. Investigating the KNDy Hypothesis in Humans by Coadministration of Kisspeptin, Neurokinin B, and Naltrexone in Men. The Journal of clinical endocrinology and metabolism 2016; 101:3429–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology 2011; 94:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garcia JP, Guerriero KA, Keen KL, Kenealy BP, Seminara SB, Terasawa E. Kisspeptin and Neurokinin B Signaling Network Underlies the Pubertal Increase in GnRH Release in Female Rhesus Monkeys. Endocrinology 2017; 158:3269–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia JP, Keen KL, Kenealy BP, Seminara SB, Terasawa E. Role of Kisspeptin and Neurokinin B Signaling in Male Rhesus Monkey Puberty. Endocrinology 2018; 159:3048–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yen SS, Quigley ME, Reid RL, Ropert JF, Cetel NS. Neuroendocrinology of opioid peptides and their role in the control of gonadotropin and prolactin secretion. American journal of obstetrics and gynecology 1985; 152:485–493 [DOI] [PubMed] [Google Scholar]
- 96.Rossmanith WG, Wirth U, Sterzik K, Yen SS. The effects of prolonged opioidergic blockade on LH pulsatile secretion during the menstrual cycle. Journal of endocrinological investigation 1989; 12:245–252 [DOI] [PubMed] [Google Scholar]
- 97.Brownstein MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proceedings of the National Academy of Sciences of the United States of America 1993; 90:5391–5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends in neurosciences 1995; 18:22–29 [DOI] [PubMed] [Google Scholar]
- 99.Abe J, Okamura H, Kitamura T, Ibata Y, Minamino N, Matsuo H, Paull WK. Immunocytochemical demonstration of dynorphin(PH-8P)-like immunoreactive elements in the human hypothalamus. The Journal of comparative neurology 1988; 276:508–513 [DOI] [PubMed] [Google Scholar]
- 100.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. The Journal of comparative neurology 2006; 498:712–726 [DOI] [PubMed] [Google Scholar]
- 101.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 2007; 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 102.Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. Journal of neuroendocrinology 2008; 20:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.True C, Takahashi D, Kirigiti M, Lindsley SR, Moctezuma C, Arik A, Smith MS, Kievit P, Grove KL. Arcuate nucleus neuropeptide coexpression and connections to gonadotrophin-releasing hormone neurones in the female rhesus macaque. Journal of neuroendocrinology 2017; 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic Acid levels in a subset of dynorphin neurons in the sheep. Endocrinology 2005; 146:1835–1842 [DOI] [PubMed] [Google Scholar]
- 105.Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides 2009; 30:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quigley ME, Yen SS. The role of endogenous opiates in LH secretion during the menstrual cycle. The Journal of clinical endocrinology and metabolism 1980; 51:179–181 [DOI] [PubMed] [Google Scholar]
- 107.Reid RL, Quigley ME, Yen SS. The disappearance of opioidergic regulation of gonadotropin secretion in postmenopausal women. The Journal of clinical endocrinology and metabolism 1983; 57:1107–1110 [DOI] [PubMed] [Google Scholar]
- 108.DeFazio J, Verheugen C, Chetkowski R, Nass T, Judd HL, Meldrum DR. The effects of naloxone on hot flashes and gonadotropin secretion in postmenopausal women. The Journal of clinical endocrinology and metabolism 1984; 58:578–581 [DOI] [PubMed] [Google Scholar]
- 109.Melis GB, Paoletti AM, Gambacciani M, Mais V, Fioretti P. Evidence that estrogens inhibit LH secretion through opioids in postmenopausal women using naloxone. Neuroendocrinology 1984; 39:60–63 [DOI] [PubMed] [Google Scholar]
- 110.Casper RF, Alapin-Rubillovitz S. Progestins increase endogenous opioid peptide activity in postmenopausal women. The Journal of clinical endocrinology and metabolism 1985; 60:34–36 [DOI] [PubMed] [Google Scholar]
- 111.Shoupe D, Montz FJ, Lobo RA. The effects of estrogen and progestin on endogenous opioid activity in oophorectomized women. The Journal of clinical endocrinology and metabolism 1985; 60:178–183 [DOI] [PubMed] [Google Scholar]
- 112.Veldhuis JD, Rogol AD, Samojlik E, Ertel NH. Role of endogenous opiates in the expression of negative feedback actions of androgen and estrogen on pulsatile properties of luteinizing hormone secretion in man. The Journal of clinical investigation 1984; 74:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goodman RL, Gibson M, Skinner DC, Lehman MN. Neuroendocrine control of pulsatile GnRH secretion during the ovarian cycle: evidence from the ewe. Reproduction 2002; 59:41–56 [PubMed] [Google Scholar]
- 114.Portoghese PS, Lipkowski AW, Takemori AE. Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists. Life sciences 1987; 40:1287–1292 [DOI] [PubMed] [Google Scholar]
- 115.Ferin M, Van Vugt D, Wardlaw S. The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides. Recent progress in hormone research 1984; 40:441–485 [DOI] [PubMed] [Google Scholar]
- 116.Steele PA, Judd SJ. Role of endogenous opioids in reducing the frequency of pulsatile luteinizing hormone secretion induced by progesterone in normal women. Clinical endocrinology 1986; 25:669–674 [DOI] [PubMed] [Google Scholar]
- 117.Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology 2002; 143:4366–4374 [DOI] [PubMed] [Google Scholar]
- 118.Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 2004; 145:2959–2967 [DOI] [PubMed] [Google Scholar]
- 119.Ropert JF, Quigley ME, Yen SS. Endogenous opiates modulate pulsatile luteinizing hormone release in humans. The Journal of clinical endocrinology and metabolism 1981; 52:583–585 [DOI] [PubMed] [Google Scholar]
- 120.Van Vugt DA, Bakst G, Dyrenfurth I, Ferin M. Naloxone stimulation of luteinizing hormone secretion in the female monkey: influence of endocrine and experimental conditions. Endocrinology 1983; 113:1858–1864 [DOI] [PubMed] [Google Scholar]
- 121.Van Vugt DA, Lam NY, Ferin M. Reduced frequency of pulsatile luteinizing hormone secretion in the luteal phase of the rhesus monkey. Involvement of endogenous opiates. Endocrinology 1984; 115:1095–1101 [DOI] [PubMed] [Google Scholar]
- 122.Orstead KM, Hess DL, Spies HG. Opiatergic inhibition of pulsatile luteinizing hormone release during the menstrual cycle of rhesus macaques. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine 1987; 184:312–319 [DOI] [PubMed] [Google Scholar]
- 123.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Molecular pharmacology 1994; 45:330–334 [PubMed] [Google Scholar]
- 124.Abel TW, Rance NE. Proopiomelanocortin gene expression is decreased in the infundibular nucleus of postmenopausal women. Brain research Molecular brain research 1999; 69:202–208 [DOI] [PubMed] [Google Scholar]
- 125.Wardlaw SL, Wehrenberg WB, Ferin M, Antunes JL, Frantz AG. Effect of sex steroids on beta-endorphin in hypophyseal portal blood. The Journal of clinical endocrinology and metabolism 1982; 55:877–881 [DOI] [PubMed] [Google Scholar]
- 126.Petersen SL, LaFlamme KD. Progesterone increases levels of mu-opioid receptor mRNA in the preoptic area and arcuate nucleus of ovariectomized, estradiol-treated female rats. Brain research Molecular brain research 1997; 52:32–37 [DOI] [PubMed] [Google Scholar]
- 127.Smith YR, Zubieta JK, del Carmen MG, Dannals RF, Ravert HT, Zacur HA, Frost JJ. Brain opioid receptor measurements by positron emission tomography in normal cycling women: relationship to luteinizing hormone pulsatility and gonadal steroid hormones. The Journal of clinical endocrinology and metabolism 1998; 83:4498–4505 [DOI] [PubMed] [Google Scholar]
- 128.Gallo RV. Kappa-opioid receptor involvement in the regulation of pulsatile luteinizing hormone release during early pregnancy in the rat. Journal of neuroendocrinology 1990; 2:685–691 [DOI] [PubMed] [Google Scholar]
- 129.Goodman RL, Parfitt DB, Evans NP, Dahl GE, Karsch FJ. Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology 1995; 136:2412–2420 [DOI] [PubMed] [Google Scholar]
- 130.Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology 2010; 151:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 2000; 141:4218–4225 [DOI] [PubMed] [Google Scholar]
- 132.Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology 2012; 153:5105–5118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Iijima N, Takumi K, Sawai N, Ozawa H. An immunohistochemical study on the expressional dynamics of kisspeptin neurons relevant to GnRH neurons using a newly developed anti-kisspeptin antibody. Journal of molecular neuroscience : MN 2011; 43:146–154 [DOI] [PubMed] [Google Scholar]
- 134.Xu Z, Kaga S, Mochiduki A, Tsubomizu J, Adachi S, Sakai T, Inoue K, Adachi AA. Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocrine journal 2012; 59:161–171 [DOI] [PubMed] [Google Scholar]
- 135.Desroziers E, Mikkelsen JD, Duittoz A, Franceschini I. Kisspeptin-immunoreactivity changes in a sex- and hypothalamic-region-specific manner across rat postnatal development. Journal of neuroendocrinology 2012; 24:1154–1165 [DOI] [PubMed] [Google Scholar]
- 136.Overgaard A, Ruiz-Pino F, Castellano JM, Tena-Sempere M, Mikkelsen JD. Disparate changes in kisspeptin and neurokinin B expression in the arcuate nucleus after sex steroid manipulation reveal differential regulation of the two KNDy peptides in rats. Endocrinology 2014; 155:3945–3955 [DOI] [PubMed] [Google Scholar]
- 137.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. The Journal of reproduction and development 2007; 53:367–378 [DOI] [PubMed] [Google Scholar]
- 138.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 2007; 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- 139.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biology of reproduction 2009; 81:1216–1225 [DOI] [PubMed] [Google Scholar]
- 140.Takumi K, Iijima N, Ozawa H. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. Journal of molecular neuroscience : MN 2011; 43:138–145 [DOI] [PubMed] [Google Scholar]
- 141.Ruiz-Pino F, Navarro VM, Bentsen AH, Garcia-Galiano D, Sanchez-Garrido MA, Ciofi P, Steiner RA, Mikkelsen JD, Pinilla L, Tena-Sempere M. Neurokinin B and the control of the gonadotropic axis in the rat: developmental changes, sexual dimorphism, and regulation by gonadal steroids. Endocrinology 2012; 153:4818–4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chan YM, Butler JP, Pinnell NE, Pralong FP, Crowley WF Jr., Ren C, Chan KK, Seminara SB. Kisspeptin resets the hypothalamic GnRH clock in men. The Journal of clinical endocrinology and metabolism 2011; 96:E908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chan YM, Butler JP, Sidhoum VF, Pinnell NE, Seminara SB. Kisspeptin administration to women: a window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. The Journal of clinical endocrinology and metabolism 2012; 97:E1458–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proceedings of the National Academy of Sciences of the United States of America 2005; 102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jayasena CN, Abbara A, Veldhuis JD, Comninos AN, Ratnasabapathy R, De Silva A, Nijher GM, Ganiyu-Dada Z, Mehta A, Todd C, Ghatei MA, Bloom SR, Dhillo WS. Increasing LH pulsatility in women with hypothalamic amenorrhoea using intravenous infusion of Kisspeptin-54. The Journal of clinical endocrinology and metabolism 2014; 99:E953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, Doyle C, Papadopoulou DA, Bloom SR, Mohideen P, Panay N, Hunter MS, Veldhuis JD, Webber LC, Huson L, Dhillo WS. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2017; 389:1809–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. Journal of neuroendocrinology 2011; 23:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weems P, Smith J, Clarke IJ, Coolen LM, Goodman RL, Lehman MN. Effects of Season and Estradiol on KNDy Neuron Peptides, Colocalization With D2 Dopamine Receptors, and Dopaminergic Inputs in the Ewe. Endocrinology 2017; 158:831–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ahn T, Fergani C, Coolen LM, Padmanabhan V, Lehman MN. Prenatal testosterone excess decreases neurokinin 3 receptor immunoreactivity within the arcuate nucleus KNDy cell population. Journal of neuroendocrinology 2015; 27:100–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. kappa-Opioid Receptor Is Colocalized in GnRH and KNDy Cells in the Female Ovine and Rat Brain. Endocrinology 2016; 157:2367–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]


