Abstract
This review identifies the three-dimensional knee loads that have the highest risk of injuring the anterior cruciate ligament (ACL) in the athlete. It is the combination of the muscular resistance to a large knee flexion moment, an external reaction force generating knee compression, an internal tibial torque, and a knee abduction moment during a single-leg athletic manoeuvre such as landing from a jump, abruptly changing direction, or rapidly decelerating that results in the greatest ACL loads. While there is consensus that an anterior tibial shear force is the primary ACL loading mechanism, controversy exists regarding the secondary order of importance of transverse-plane and frontal-plane loading in ACL injury scenarios. Large knee compression forces combined with a posteriorly and inferiorly sloped tibial plateau, especially the lateral plateau—an important ACL injury risk factor—causes anterior tibial translation and internal tibial rotation, which increases ACL loading. Furthermore, while the ACL can fail under a single supramaximal loading cycle, recent evidence shows that it can also fail following repeated submaximal loading cycles due to microdamage accumulating in the ligament with each cycle. This challenges the existing dogma that non-contact ACL injuries are predominantly due to a single manoeuvre that catastrophically overloads the ACL.
Keywords: anterior cruciate ligament, knee, injury, mechanism
INTRODUCTION
An injury to the anterior cruciate ligament (ACL) can be debilitating for the athlete. For over 60 years, research has focused on trying to understand the risk factors (for recent review, see Pfeifer et al.115) and pathomechanics of non-contact ACL injuries,12 with the overarching goal of preventing these injuries. This is critical given that non-contact ACL injuries account for nearly 80% of all ACL injuries.121
To understand the aetiology of non-contact ACL injuries, scientists and clinicians have studied the anatomy of the ACL (for example, Duthon et al.23), its material properties,14 and its function in stabilising the knee joint. They have investigated the forces and moments about the knee during various athletic activities associated with ACL injury, as well as the ACL’s heterogeneous response to such loading.77 To do so, various experimental approaches have been utilised, ranging from quantitative video analyses of actual ACL injuries (for example, Della Villa et al.20) to dynamic in vitro simulations of various injury scenarios employing animals69 and human models (for example, Wojtys et al.155), to computer simulations (for example, Shin et al.127). The factors that can affect these forces and moments about the knee, and those applied to the ACL, include the geometry of the articulating surfaces of the tibiofemoral joint,8 the tibiofemoral muscle forces,126 and the friction between the athlete’s shoe and the playing surface.134 With the considerable amount of attention given to the ACL and ACL injuries, the body of research has grown exponentially. Hence, the purpose of this narrative review is to synthesise this literature to better understand the knowledge gained, to identify areas of consensus and those of debate, and to identify potential knowledge gaps. This will help guide future research aimed at better understanding the pathomechanics of non-contact ACL injuries towards the goal of better preventing these injuries.
ACL ANATOMY
ACL Attachment Locations
The ACL originates on the posterior-superior portion of the medial facet of the lateral femoral condyle and inserts anteriorly and slightly medially to the tibia’s intercondylar eminence (Figure 1).1 The shape of the ACL’s femoral origin (or colloquially ‘footprint’) has often been described as oval or elliptic.48,62,78,97,129,141,146,158 However, a more accurate description is that of a “segment of a circle” with a straight anterior border and a convex posterior border”25—a larger footprint that also includes the “fan-like extension fibres” or “indirect fibres” as described by Iriuchishima et al.46 and Suruga et al.,140 that extend and splay posteriorly to the articular cartilage of the femoral condyle (Figure 1A). For this reason, inconsistencies exist in the literature in terms of the surface area of the ACL’s femoral footprint. Often, it has been quantified as smaller than that of the tibial footprint28,45,47,62,66,125 if one excludes these indirect fibres. With their inclusion, however, the surface area of the femoral footprint is undoubtedly greater,143 as effectively shown by Iriuchishima et al.46 The tibial insertion of the ACL has been described as triangular,104 mostly C-shaped, but ovoid and double-C-shaped as well,125 duck-foot shaped118 and most recently as paw-shaped, but sometimes quadrangular or irregular (Figure 1B).62 The factors that lead to these variations in shape are as yet not understood.
Figure 1.
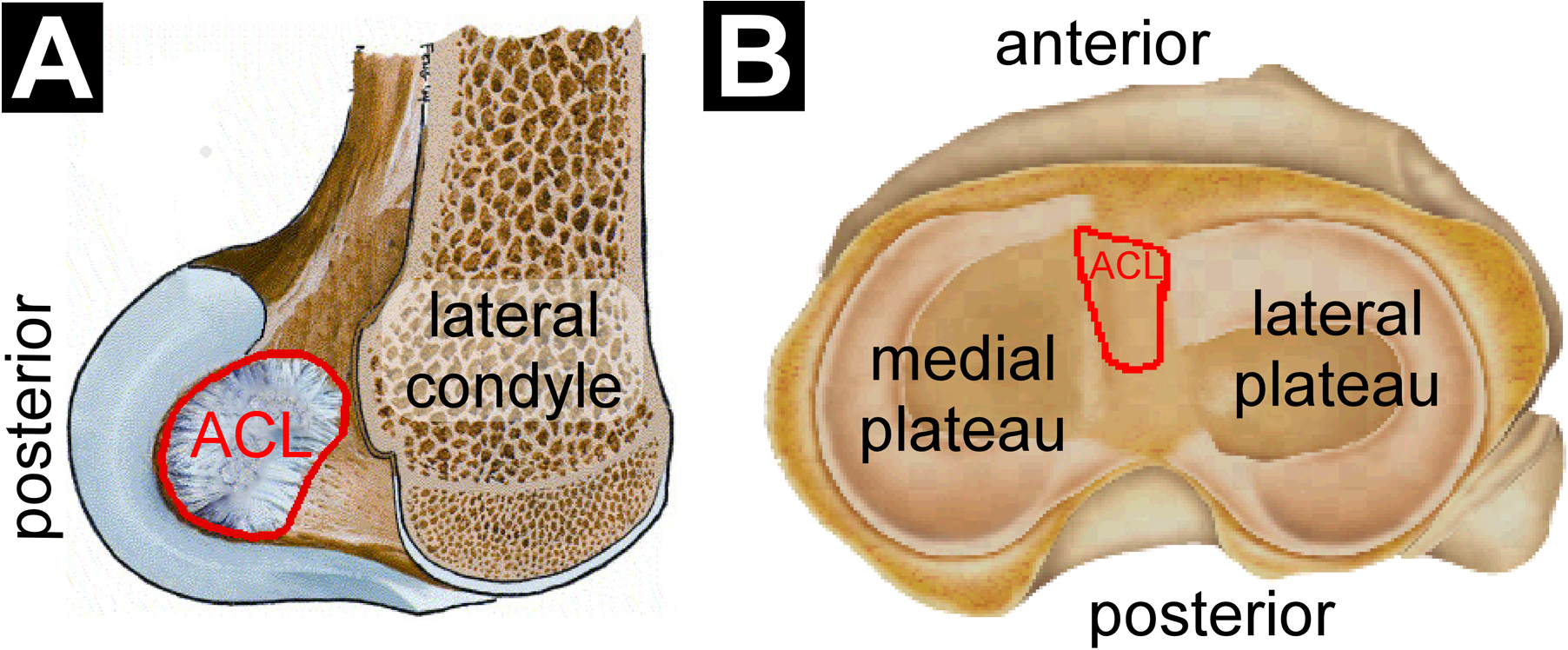
Illustration of the (A) femoral origin and (B) tibial insertion sites of the ACL (outlined in red). Modified from Chhabra et al.16 and Lord et al.75 with permission from Elsevier.
The Structure of the ACL
The ACL is most often described as a “two-bundle” structure comprising the fibres of the antero-medial (AM) and the postero-lateral (PL) bundles,112,114,132,133,137,146,161 named for the relative location of these fibres’ insertion on the tibial plateau. A third bundle, the intermediate bundle, which is situated between the AM and PL bundles as its name suggests, has also been described, but less commonly.62,79,104,145 Although controversy exists in the literature as to the number of bundles, what is of greater clinical importance is that the ACL is actually comprised of a continuum of fibres, each varying in tension during three-dimensional (3D) knee rotations.1 Although the fibre bundles function in a complementary manner,40 the AM fibres play a primary role in resisting anterior tibial translation,17,87 whereas the PL fibres play a primary role in resisting internal tibial rotation.160 The fibre contributions are also dependent on knee flexion angle, with a gradual transition from the anteriorly located fibres resisting peak loads at moderate knee flexion angles (i.e., 30°–60°) to the posteriorly located fibres resisting peak loads near full extension (i.e., 0°–15°).32,43,49,98,123 This is because the location from where the PL fibres originate on the femur rotates toward the attachment site on the tibia as the knee flexes, thereby shortening the PL fibres. Also, the bundle of PL fibres, which is narrower and shorter,53 elongates more than the AM fibres during weight bearing with a knee near full extension.43 It is not surprising, therefore, that isolated ruptures of the PL fibres, which account for 20–41% of all partial ACL ruptures,27,106,135,157 have been associated with pivoting events that include an axial rotational component; whereas isolated ruptures of the AM fibres, which account for 59–80% of all partial injuries,27,106,135,157 have been associated with predominately anterior-directed forces applied to the knee joint.130,159 It should be noted that the rate of isolated PL bundle ruptures are probably underestimated due to the difficulty in diagnosing these ruptures with conventional magnetic resonance imaging and arthroscopy.13,113,136 These isolated ruptures of the PL and AM fibres were also associated with a “less energetic” injury and a “more explosive-type” knee trauma, respectively.130,159 Accordingly, we suspect that the PL fibres, especially near the femoral enthesis,13,135,159 are at a greater risk of a non-contact injury during athletic manoeuvres which often include an axial rotation component with a knee near full extension. Also, these types of injuries frequently occur during unremarkable, routine manoeuvres—perhaps defined as “less energetic”—that the athlete has performed countless times before (refer to ‘ACL Failure Resulting from a Single Overload Event vs. Repetitive Submaximal Loading Events‘ subsection for a discussion on this topic).
BIOMECHANICAL FUNCTION OF THE ACL
Many years of research have shown that the main roles of the ACL are to resist primarily an anterior shear force on the proximal tibia relative to the distal femur, and secondarily an internal tibial torque relative to the femur and a knee abduction moment. To gain this insight, a large body of research focused on assessing ACL behaviour in response to simple, quasi-static knee loading scenarios.11,34,71,85,93 This allowed for the examination of the ACL’s response to single-plane loading (for example, Kennedy et al.55). While such loading scenarios may not be physiologically relevant to ACL injuries (i.e., no or minimal axial compression force, no significant trans-knee muscle forces, quasi-static loading, unrealistic magnitude and/or timing of applied forces), they can isolate the effect of each force/moment on ACL behaviour. This section will review this literature to summarise the ACL’s role in resisting an anterior tibial shear force, an internal tibial torque, and a knee abduction moment. This section will also focus on the function of the ACL at small knee flexion angles (0–30°) given that most non-contact ACL injuries occur with the knee close to full extension (i.e., 10–30°).20,60 Hence, unless otherwise stated, loading scenarios are reported for a knee at 0–30° of flexion.
Sagittal Plane Loading
There is widespread agreement in the literature that the ACL is the primary restraint to anterior translation of the proximal tibia relative to the distal femur, irrespective of knee flexion angle.4,11,52,55,59,85,123,142,160 It provides 70–87% of the resistance to an anterior tibial shear force applied to the knee at small flexion angles (0–30°), and somewhat less of this resistance (62–85%) at larger flexion angles (60–90°).4,11,59,142 In earlier human in vitro studies aimed at determining the role of the ACL in stabilising the knee, two main types of studies were conducted: (1) those that measured ACL strain or force in response to quasi-statically applied load(s) and (2) those that measured displacement of the tibia relative to the femur in response to a quasi-statically applied load(s) before and after transection of the ACL. The change in tibial displacement in response to the constant load (or similarly, the change in load to achieve the same tibial displacement) after ACL transection was inferred to be a measure of ACL function. In the former type of studies, an anterior tibial shear force significantly increased ACL strain,26,55 ACL length,28,42 and ACL force74,82,123,142 in ACL-intact human knee specimens. In the latter type of studies, anterior tibial translation significantly increased after ACL transection in response to the same magnitude of anterior shear force applied to the tibia.31,52,59,85,124,160 Similarly, after transection of the ACL, the anterior tibial shear force required to anteriorly translate the tibia the same distance significantly decreased.4,11,75,124 In short, there is consensus that the ACL is the primary restraint to an anterior tibial shear force and the resulting anterior tibial translation, especially at small knee flexion angles. As we shall discuss later (‘Quadriceps Contraction‘ subsection), the primary source of that anterior shear force is the patellofemoral mechanism: the resultant force produced by large quadriceps and the patellar ligament tensile forces pushes the distal femur posteriorly relative to the proximal tibia, while drawing the proximal tibia anteriorly relative to the distal femur, thereby straining the ACL.
Transverse Plane Loading
Evidence of the ACL’s role in maintaining transverse-plane rotational stability of the knee, however, is less consistent. Some studies show that the ACL plays a secondary role in resisting internal tibial rotation at small knee flexion angles,55,59,71,75,83,96,122,127 providing 10–20% of the resistance to an internal tibial torque;4,59 meanwhile other studies show a minimal85,93,103 or negligible role.34,64,74,99,100,156 All but one of the studies that measured ACL loading in response to quasi-statically applied internal tibial torque reported a significant increase in ACL strain26,55,83,127 or ACL force.35,96 There is consensus, therefore, that an internal tibial torque significantly loads the ACL but only at small knee flexion angles (0–30°). As the flexion angle increases, the ACL’s resistance to an internal tibial torque decreases to a trivial role.59,83,96 The disagreement between studies is among those that measured changes in internal tibial rotation in response to a constant internal tibial torque (or similarly, the change in internal tibial torque to achieve the same magnitude of internal tibial rotation) before and after ACL transection. While several studies showed a significant increase in internal tibial rotation (or a decrease in internal tibial torque),4,31,33,50,59,71,75,107,122 a few studies showed a small increase85,93,103 and others showed no increase.34,64,99,100,156 Part of the disagreement in the literature may relate to how the change in tibial displacement following ACL transection is interpreted. Although most investigators interpret it as a measure of ACL function, we argue that it is mostly a measure of the tissue that is left intact in the ACL-deficient knee. Part of the disagreement may also relate to how an internal tibial torque loads the ACL. In the presence of an internal tibial torque, coupled anterior tibial translation occurs (see ‘Mechanical Coupling Between Knee Loads and Displacements’ section below for biomechanical explanation).33,34,74,156 The consensus that internal tibial torque significantly strains the ACL, therefore, may be due to this coupled anterior tibial translation and to a smaller extent, internal tibial rotation. Consequently, ACL transection would cause large increases in anterior tibial translation50 and smaller increases in internal tibial rotation in response to a constant internal tibial torque.34,64,85,93,99,100,103,156 Hence, studies that assess the role of the ACL in resisting internal tibial torque via ACL transection are probably underestimating it, partly due to the mechanical coupling of anterior tibial translation with this axial torque.
Frontal Plane Loading
Evidence of the ACL’s role in maintaining frontal-plane rotational stability of the knee is contradictory. Some studies show that the ACL plays a minor but significant role in resisting a knee abduction moment,4,26,42,83,88,127 providing 10% of the resistance at small flexion angles;4 meanwhile other studies show a minimal85,103 or negligeable role.31,34,55 Most studies that measured ACL loading in response to a quasi-statically applied knee abduction moment reported a significant increase in strain26,83,120,127 or length;42 meanwhile Kennedy et al.55 reported a mild relaxation of the ACL under knee abduction loading. The effect of a knee abduction moment on ACL strain/length, however, may be dependent on knee flexion angle, with a trend of increasing effect with increasing knee flexion angle.42,96 For example, Miyasaka et al.96 reported a significant increase in ACL strain when the knee was flexed at 30°, but no increase at smaller flexion angles (i.e., 0° and 15°). Since most non-contact ACL injuries occur with the knee flexed at 10–30°,20,60 knee abduction loading may play a role in the mechanism of only a fraction of these injuries—those for which the knee flexion angle is at the greater end of this range. Similar to transverse-plane loading, there is disagreement among those studies that measured changes in knee abduction angle in response to a constant abduction moment before and after ACL transection. While two studies showed a significant increase in knee abduction angle,4,88 two studies showed a minor increase85,103 and two others showed no increase.31,34 Mechanical coupling of knee motions also occurs in the presence of a knee abduction moment (see ‘Mechanical Coupling Between Knee Loads and Displacements’ subsection below for details). The significant increases in ACL strain reported under a knee abduction moment, therefore, seem to be largely due to a coupled anterior tibial translation and to a smaller extent, coupled internal tibial rotation. Consequently, ACL transection can cause significant increases in anterior tibial translation30,51 and internal tibial rotation30,51,88 that can parallel and perhaps cause those increases in knee abduction angle.88
Simultaneous Multiplane Loading
Given that an anterior tibial shear force, an internal tibial torque, and a knee abduction moment can all load the ACL, it is not surprising that the combination of these forces and moments induces the greatest load on the ACL (Figure 2).81 This loading has also produced the greatest anterior subluxation of the medial and lateral tibial plateau during a simulated pivot shift in human knee specimens at about 25°of knee flexion.105 Additionally, the combination of an internal tibial torque and a knee abduction moment produces significantly greater ACL forces and strain than either an internal tibial torque51 or a knee abduction moment alone.81,127 The magnitude of the effect of multiplanar loading on ACL loads, however, appears to be dependent on knee flexion angle, with internal tibial torque generating larger effects at very small knee flexion angles (−5° (hyperextension) to 10°) and knee abduction moments generating larger effects at larger angles (≥20°).82 In short, multiplanar knee loading produces the greatest load on the ACL but appears to be moderated by the knee flexion angle at which loading is applied.
Figure 2.
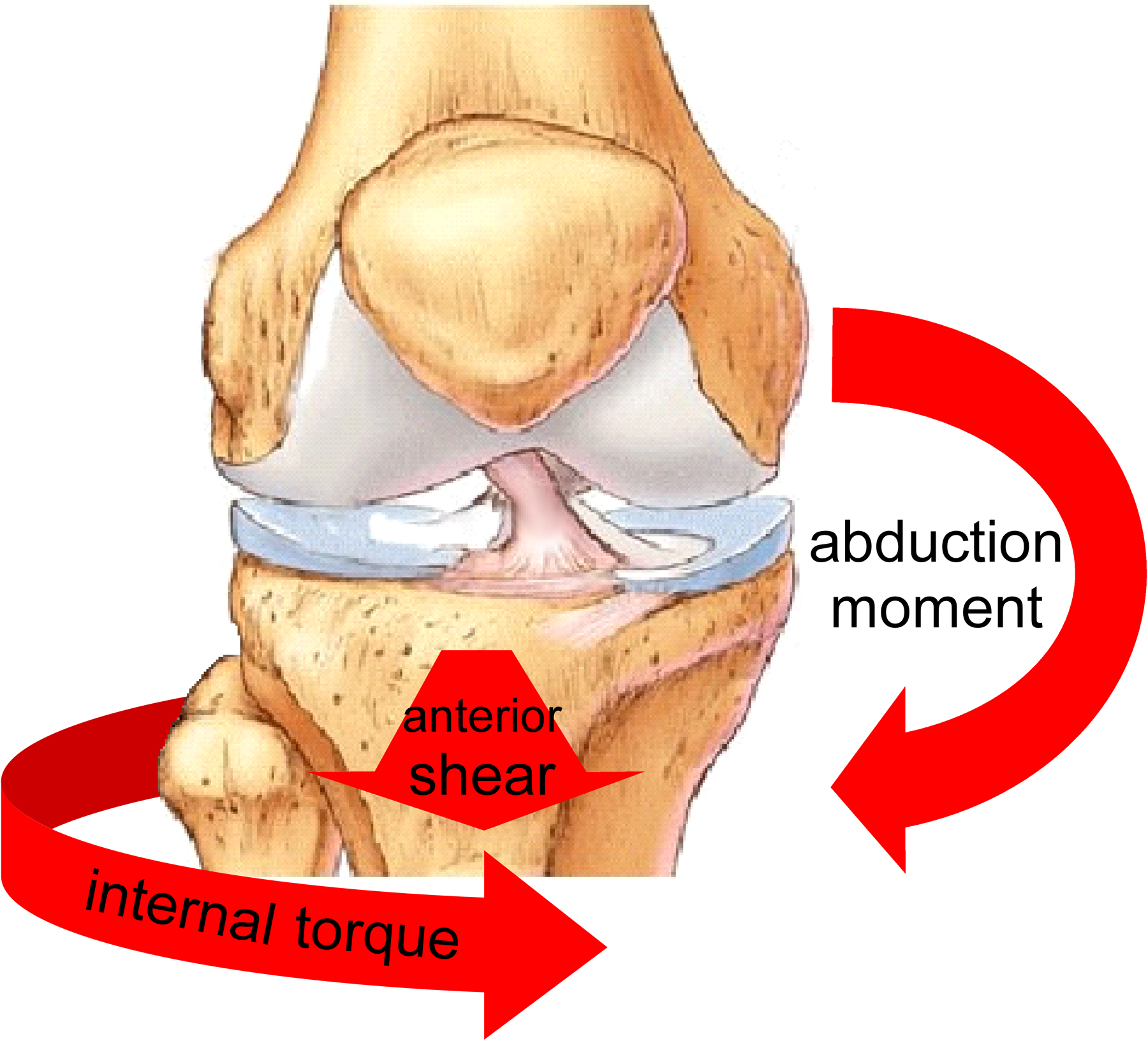
The combination of an anterior tibial shear force, an internal tibial torque, and a knee abduction moment induces the greatest load on the ACL.
Mechanical Coupling Between Knee Loads and Displacements
Although one can attempt to isolate the effect of a single force or moment on ACL behaviour with simple quasi-static loading scenarios using a single-plane approach, the resulting knee displacement will almost always be multiplanar. This is because of the complex interaction between loads applied to the knee and the knee’s kinematic response (Table 1). For instance, an anterior tibial shear force induces not only anterior tibial translation but also internal tibial rotation29,32,34,42 with minimal to no knee abduction rotation (Table 1).32,42,68 In addition, an internal tibial torque induces coupled anterior tibial translation33,34,74,156 due to the location of the centre of rotation of the tibia in combination with the geometry of the articulating surfaces of the tibiofemoral joint. With a centre of rotation located at the medial tibial plateau, internal tibial rotation includes a component of anterior translation of the centre and lateral margin of the tibial plateau (Figure 3).33 Under a knee abduction load, coupled anterior tibial translation30,32,51 and internal tibial rotation30,32,42,51,88,120 occurs (Table 1). Ren et al.120 showed that a pure knee abduction moment causes an internal tibial torque, and thus a significant increase in ACL strain. It has also been proposed that internal tibial rotation can cause knee abduction rotation by tilting the longitudinal axis of the tibia in the frontal plane, thereby inducing abduction of the tibia relative to the femur. This is because of the posterior-inferior-directed slope of the tibial plateau.88 Not surprisingly, therefore, a combination of an internal tibial torque and a knee abduction moment induces large coupled anterior tibial translation.32,52,160 Mechanical coupling of knee displacements is also induced when an axial compressive force is applied to the knee, such as during a landing. This force can induce anterior tibial translation and internal tibial rotation due to the geometry of the articulating surfaces of the tibiofemoral joint (Figure 4). How and why this occurs is explained later in this review (see both ‘Axial Knee Compressive Force’ subsections).
Table 1.
List of knee loads and the associated coupled knee displacements.
| Knee Loading | Coupled Knee Displacements | Figure Illustrating Coupling |
|---|---|---|
| anterior tibial shear force | anterior tibial translation internal tibial rotation |
N/A |
| internal tibial torque | internal tibial rotation anterior tibial translation |
Figure 3 |
| knee abduction moment | knee abduction rotation anterior tibial translation internal tibial rotation |
N/A |
| axial knee compression force | anterior tibial translation internal tibial rotation |
Figure 4A
Figure 4B |
Figure 3.
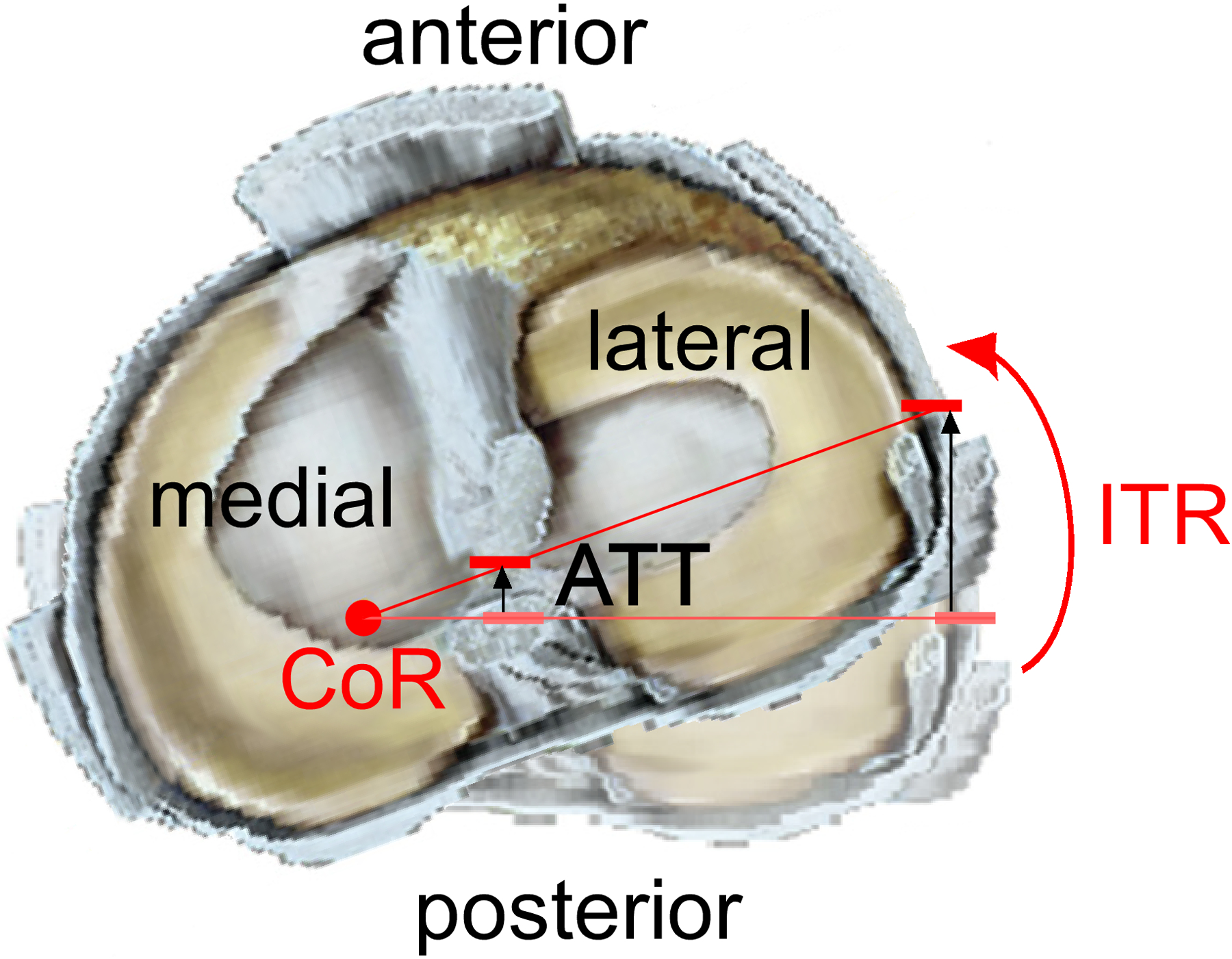
Superior view of a right knee illustrating how an internal tibial torque can produce internal tibial rotation (ITR) and coupled anterior tibial translation (ATT). Because the centre of rotation (CoR) of the tibia is located at the medial tibial plateau, the centre and lateral margin of the tibial plateau translate anteriorly as the tibia rotates internally relative to the femur. Modified from Gardner et al.33 with permission from Elsevier.
Figure 4.
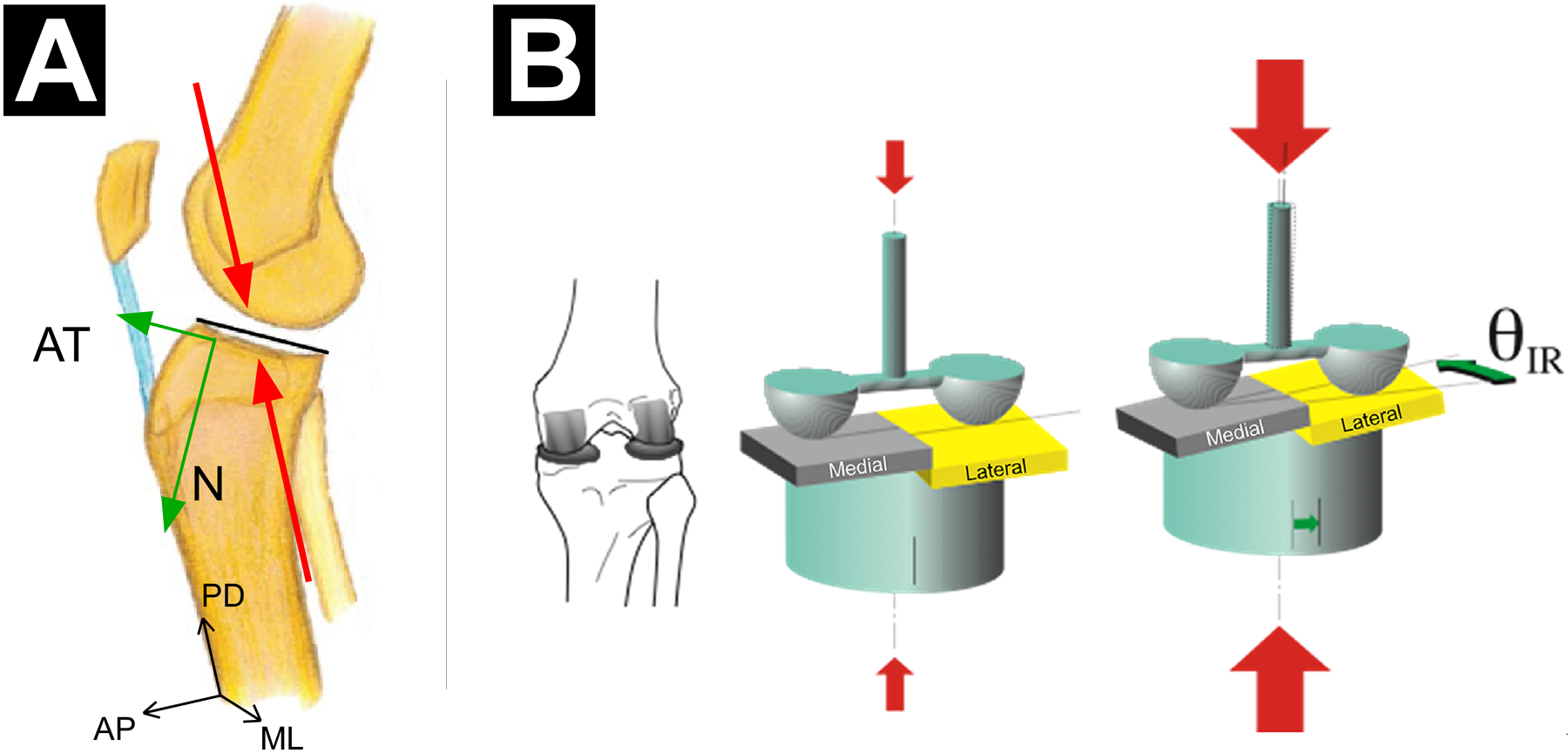
(A) Sagittal-plane view of a right knee illustrating how an axial knee compression force can induce coupled anterior tibial translation because of the geometry of the articulating surfaces of the tibiofemoral joint. As a result of the posterior-inferior-directed slope of the lateral and medial tibial plateau, the knee compression force (red arrows, acting along the proximal-distal (PD) axis of the tibia) has a component that induces an anterior tibial shear force (green arrow, AT), in addition to the normal force (green arrow, N, force component perpendicular to the surface of the tibial plateau). (B) Postero-superior three-quarter view of a right knee illustrating how a large axial knee compression force (large red arrows) can induce coupled internal tibial rotation. As a result of the steeper posterior-inferior-directed slope of the lateral tibial plateau in comparison with that of the medial tibial plateau, the lateral femoral condyle essentially pushes the lateral tibial plateau anteriorly more than the medial femoral condyle pushes the medial tibial plateau thereby causing internal tibial rotation (IR). Reproduced from Wojtys et al.155
A REVIEW OF ACL LOADING MECHANISMS
Most non-contact ACL injuries occur during athletic manoeuvres that include sudden dynamic loading of the knee joint, such as landing from a jump, cutting, pivoting, and decelerating.20,101,138,149 To study these manoeuvres and how they load the ACL, in vitro models have been used that allow for precise measurement of the external forces and moments at the knee joint, as well as the resulting ACL strain and knee displacements. Such models aim to replicate knee loading that occurs during in vivo athletic manoeuvres. They provide a more accurate understanding of non-contact ACL injury mechanisms than the quasi-static models presented in the previous section (‘Biomechanical Function of the ACL‘) that ignore physiologically relevant loading. Hence, this section will be utilising data obtained from these dynamic impulsive in vitro loading models to gain insight into how the three main forces/moments identified in the previous section—anterior tibial shear force, internal tibial torque, and knee abduction moment—can be produced during athletic manoeuvres.
Anterior Tibial Shear Force
Axial Knee Compressive Force.
During athletic manoeuvres such as jump landings, large axial compressive forces act on the tibiofemoral joint. These forces are produced by trans-knee muscle contractions, especially of the quadriceps to oppose the large flexion moment that occurs during these manoeuvres, and by the large ground reaction force acting across the knee, which also contributes to the flexion moment. The vertical ground reaction force reaches 3–4 times bodyweight during landings in a controlled laboratory environment,36,110,144 and can exceed this magnitude in the field. Evidence indicates that axial knee compressive forces can significantly load the ACL.80,84,94,150 This is because of the geometry of the articulating surfaces of the tibiofemoral joint: these joint compressive forces induce coupled anterior tibial translation,80,84,94,151 as well as internal tibial rotation84,94 (Table 1) and some knee abduction84—all knee displacements that are known to load the ACL (see ‘Biomechanical Function of the ACL‘ section for details). Although these compression forces induce the greatest knee displacements at larger knee flexion angles (30–50°), significant displacements also occur at smaller flexion angles (10–30°)80,84 when most non-contact ACL injuries occur.20,60 The knee joint compression force acting along the longitudinal axis of the tibia has an anterior tibial shear force component which produces anterior tibial translation as a result of the posterior-inferior-directed slope of the lateral and medial tibial plateau (Figure 4A). In fact, Wang et al.151 found that the magnitude of anterior tibial translation produced under an axial knee compression force was positively correlated with both the medial and lateral slopes of the tibial plateau: as the slopes increased, so did tibial translation. It is not surprising, therefore, that extensive evidence exists that the magnitude of the posterior-inferior-directed slope of the tibial plateau is a risk factor for ACL injury, especially that of the lateral tibial plateau.10,24,37,38,119,131,139,148,162 ACL-injured knees have a larger lateral tibial slope than uninjured control knees.10,24,37,38,119,131,139,147,148 Also, a greater slope of the lateral tibial plateau has been associated with greater anterior tibial acceleration,92 and therefore greater ACL strain in vitro.72,92
Quadriceps Contraction.
Another sagittal-plane loading mechanism of the ACL is via the activation and stretch of the quadriceps during athletic manoeuvres.2,102 For example, when the tensile force on an active muscle exceeds the force that the muscle can generate, the muscle is forcibly lengthened in a so-called ‘eccentric’ contraction. This lengthening can increase the muscle tension by 70% above its maximum isometric value,54 a phenomenon that athletes exploit to create high muscle forces, such as during a stretch-shortening cycle. When the quadriceps is forcibly stretched as the knee flexes slightly during an athletic manoeuvre, therefore, a large quadriceps force is produced. There is consensus that the quadriceps muscle force acts via the patellofemoral mechanism to significantly increase anterior tibial translation21,41 and internal tibial rotation,41 thereby significantly loading the ACL.9,22,70,86,150,153 This occurs because of the angle at which the quadriceps inserts onto the tibia via the patellar tendon; the anterior-superior-directed tensile force developed by the quadriceps at its tibial insertion results in an anterior tibial shear component and an axial knee compression component, both of which can strain the ACL (Table 1, Figure 4A). Near full knee extension (0–20°), the anterior tibial shear force component is greatest because of the relatively large insertion angle at the tibia. At larger knee flexion angles (>20°), this component is smaller, and the axial compressive force component is larger.126 Hamstrings co-contraction can reduce ACL loading22,70,111,154 by developing a posterior tibial shear force to oppose the anterior tibial shear force. Near full knee extension (~0°), however, the hamstrings are much less efficient because of the small angle at which they insert at the tibia, and therefore they develop relatively small posterior tibial shear forces.111 On that account, can a large quadriceps force produce ACL loading high enough to injure it? Some disagreement exist in the literature,21,89,90,116 but the general consensus is that this cannot occur under physiological knee loading scenarios.90,116 In landing and deceleration tasks, in the sagittal-plane, peak ACL forces result from the complex interaction between the anterior tibial shear force produced by quadriceps contraction and knee compression, and the posterior tibial shear force induced by the ground reaction force applied to the lower leg.90,116
Internal Tibial Torque
Similar to the quasi-static knee loading models presented earlier (‘Biomechanical Function of the ACL‘ section), in vitro dynamic knee loading models have consistently revealed that the application of an internal tibial torque significantly loads the ACL.108 These models simulated landing manoeuvres by applying realistic knee loads consisting of an impulsive knee compression force and knee flexion moment, as well as trans-knee muscle forces.56,57,72,108 They showed that adding an internal tibial torque, thus simulating a pivot landing, significantly increased ACL strain,56,57,72,108 as well as coupled anterior tibial translation,72,108 in comparison to landing without pivot. Such an internal tibial torque can be produced upon initial ground contact when the resultant ground reaction force is directed posteriorly and medially with respect to the shank (Figure 5). This can occur in a number of scenarios with varying combinations of joint mechanics at initial ground contact, such as suddenly decelerating and changing direction or axially rotating the trunk towards the ipsilateral knee prior to and upon ground contact.18 The magnitude of this torque is moderated by two main factors: (1) axial knee compressive force interacting with the geometry of the articulating surfaces of the tibiofemoral joint and (2) coefficient of friction between an athlete’s shoe and the playing surface.
Figure 5.
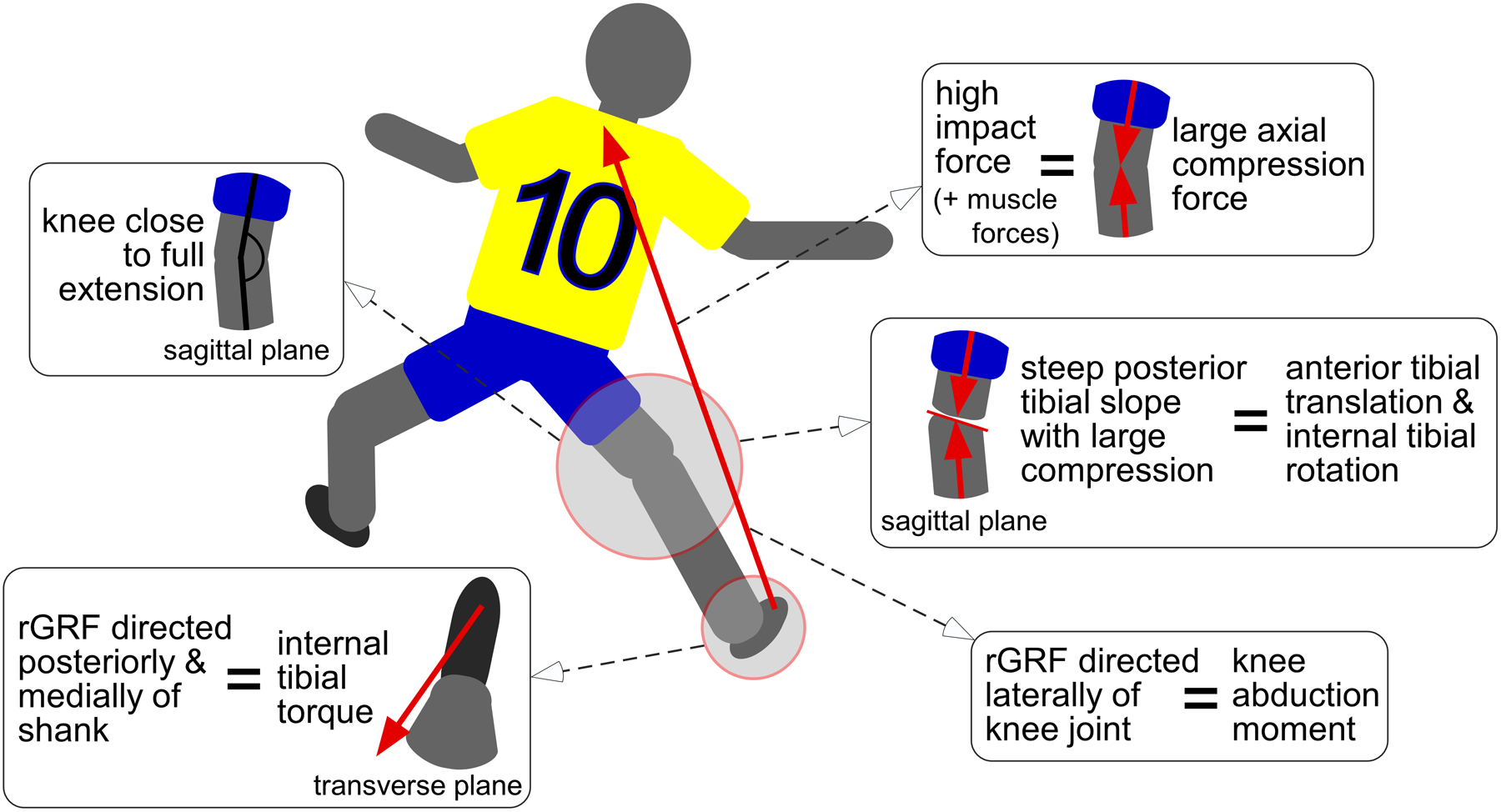
A rear-view illustration of an athlete landing and cutting to the left. The combination of the muscular resistance to a large knee flexion moment and a large upward external reaction force (rGRF) generating knee compression, an internal tibial torque, and a knee abduction moment during a single-leg athletic manoeuvre such as landing from a jump, abruptly changing direction, or rapidly decelerating with a knee close to full extension has the highest risk of injuring the ACL in the athlete. The large knee compression force resulting from the rGRF and the trans-knee muscle forces can induce coupled anterior tibial translation and internal tibial rotation due to the posterior-inferior-directed slope of the tibial plateau, especially that of the lateral compartment. An internal tibial torque can be produced when the resultant ground reaction force (rGRF) applied under the ball of the foot is directed posteriorly and medially with respect to the shank as the athlete decelerates and pushes laterally. In the frontal plane, a knee abduction moment is produced when the rGRF is directed laterally with respect to the knee joint centre.
Axial Knee Compressive Force.
Large knee compressive forces can significantly increase ACL loads80,84,94,150 not only because of their anterior shear force component but also because of the coupled internal tibial rotation41,84,94 due to the geometry of the articulating surfaces of the tibiofemoral joint (Table 1). This is especially true at larger knee flexion angles (30–50°), but also at smaller flexion angles (10–30°)80,84 when most non-contact ACL injuries occur.20,60 The coupled internal tibial rotation results from the steeper posterior-inferior-directed slope of the lateral tibial plateau in comparison with that of the medial tibial plateau. The lateral femoral condyle essentially pushes the steeper sloped lateral tibial plateau anteriorly more than the medial femoral condyle pushes the medial tibial plateau thereby causing internal tibial rotation (Figure 4B).155 This concept is supported by McLean et al.,91 who found that during a dynamic in vivo single-leg land-and-pivot manoeuvre, peak internal tibial rotation was significantly associated with the medial-to-lateral tibial slope ratio, which was governed by the lateral tibial slope. In addition, Wang et al.151 found that the magnitude of internal tibial rotation produced under an axial compression force was positively correlated with the lateral tibial slope as well as with the lateral-to-medial tibial slope difference: the steeper the slope and the greater the difference between slopes, the greater the rotation.151 This explains why a steep slope of the tibial plateau, especially of its lateral compartment, has been frequently identified as a significant risk factor for ACL injury.
Effect of Friction Between Shoe and Ground.
Upon initial ground contact during an athletic manoeuvre with an axial rotation component (e.g., pivot landing; plant-and-pivot task), the magnitude of internal tibial torque that can be applied to the knee joint is also governed by the coefficient of friction between the athlete’s shoe and the ground. A higher coefficient can induce higher knee torques. For example, artificial grass with sand/rubber infill and soccer shoes with blade-type cleats produced greater axial torques of the lower leg than natural grass and stud-type cleats, respectively.134 Therefore, one might expect athletes to generate greater internal tibial torque, thereby leading to greater risk of ACL injuries, under those high-friction shoe-playing surface conditions.39,63,76 For instance, ACL injury rates in the NFL were found to be 67% higher on artificial surfaces (FieldTurf) than on natural grass surface.39 Also, high school American football players using a cleat design shoe with higher rotational traction were more than three times more likely to injure their ACL than in a cleat with lower rotational traction.63 It must be noted, however, that not all evidence supports a higher risk of ACL injury on artificial turf.3,44 Nevertheless, biomechanical principles tell us that the coefficient of friction between the athlete’s shoe is an important factor in the production of internal tibial torque. The controversy in the literature simply reveals the complex interaction between the factors that determine the magnitude and 3D direction of the knee and ACL loads.
Knee Abduction Moment
Amongst studies utilising in vitro dynamic knee loading models with impulsive loading previously described, there appears to be agreement that the application of a knee abduction moment significantly loads the ACL.56,57,127,152 For instance, Withrow et al.152 demonstrated that adding a knee abduction moment to a neutral landing (impulsive knee compression force, knee flexion moment, trans-knee muscle forces) increased peak ACL strain by 30%. The presence of a knee abduction moment not only increases the range of abduction rotation upon landing,57 but it also increases anterior tibial translation and internal tibial rotation due to mechanical coupling.30,32,51,109,120,155 It begs the question, which knee displacement dominates in terms of ACL loading? Although this coupled internal tibial rotation with a knee abduction moment has been demonstrated frequently, evidence against it exists.57 This disagreement in the literature is discussed in greater detail below (‘Worst-Case Knee Loading Direction for the ACL’ subsection). Generally, a knee abduction moment is produced upon initial ground contact when the resultant ground reaction force is directed laterally with respect to the knee joint centre117 to help the athlete accelerate in a contralateral direction (Figure 5).
Effect of Knee Flexion Angle
The angle of knee flexion can affect the magnitude of ACL loading, with this relation being modulated by the knee loading scenario. For instance, without any loading applied to a knee joint specimen being moved quasi-statically from full extension (0°) to 90°, ACL forces are highest in full extension and sharply decrease as the flexion angle increases with minimal forces measured at 30° and beyond.96 Given that these associations between knee flexion angle and ACL loading are presented throughout this review within their respective sections in terms of loading scenario, a mere summary is presented here in tabular format (Table 2). In short, ACL loading tends to increase as the knee flexion angle decreases, which may explain why most non-contact ACL injuries occur with the knee close to full extension.20,60
Table 2.
Relation between knee flexion angle (0–90°) and the magnitude of ACL loading for various knee loading scenarios. The section of the review where this information is discussed is also presented.
| Knee Loading | Relation Between Flexion Angle and ACL Loading | Review Section |
|---|---|---|
| anterior tibial shear force | greatest ACL loading at 15–30°; moderate at 0° and 60°; lowest at 90° | Biomechanical Function of the ACL / Sagittal Plane Loading |
| internal tibial torque | ACL loading increases as knee flexion decreases; significant loading at 0–30° | Biomechanical Function of the ACL / Transverse Plane Loading |
| knee abduction moment | ACL loading decreases as knee flexion decreases | Biomechanical Function of the ACL / Frontal Plane Loading |
| quadriceps contraction | ACL loading increases as knee flexion decreases; significant loading at 0–20° | A Review of ACL Loading Mechanisms / Anterior Tibial Shear Force / Quadriceps Contraction |
| axial knee compressive force | ACL loading, ATT, ITR and ABD decrease as knee flexion decreases; | A Review of ACL Loading Mechanisms / Anterior Tibial Shear Force & Internal Tibial Torque / Axial Knee Compressive Force |
ATT: anterior tibial translation; ITR: internal tibial rotation: ABD: knee abduction angle
Worst-Case Knee Loading Direction for the ACL
There is consensus that the 3D combination of knee joint compression and a knee flexion moment with an anterior tibial shear force, internal tibial torque, and a knee abduction moment (Figure 5) results in the greatest loads in the ACL during single-leg athletic manoeuvres, such as jump landings, abrupt turns, and sudden decelerations, performed with a knee close to full extension (i.e., 0–30°) than any of the forces or moments alone.5,7,56,58,67,109,127
Controversy exists, however, whether a knee abduction moment is secondary to an internal tibial torque with regard to its effect on ACL loading or vice versa5,7,58,109 (both are secondary to an anterior tibial shear force which is the primary ACL loading mechanism, as presented in the ‘Biomechanical Function of the ACL‘ section). Oh et al.109 argued that it is the internal tibial torque that primarily causes large peak ACL strain. First, their in vitro dynamic knee loading model of a single-leg pivot landing (i.e., impulsive knee compression force, knee flexion moment, trans-knee muscle forces, as well as an axial tibial torque and a frontal-plane moment) was very sensitive to the direction of axial tibial torque108,109 but not to the direction of the frontal-plane moment.109 Peak ACL strain was found to be significantly greater when an internal tibial torque was applied in comparison with an external tibial torque, regardless of whether the frontal-plane moment was abduction or adduction. On the other hand, peak ACL strain remained the same whether a knee abduction or adduction moment was applied in the presence of an axial tibial rotation, regardless of its direction.109 Second, the application of a knee abduction moment increased peak ACL strain by generating a coupled internal tibial rotation before a medial knee joint space opening could occur (see ‘Mechanical Coupling Between Knee Loads and Displacements’ subsection for biomechanical explanation and evidence). If internal tibial rotation was not coupled with a knee abduction moment, a larger medial knee joint opening would have to occur to strain the ACL. However, a concomitant injury to the medial collateral ligament injury with ACL injury only occurs 4–27% of the time.65,95 Therefore, evidence supports the notion that it is the internal tibial rotation induced mainly by the knee compression forces and an internal tibial torque, and also by a knee abduction moment, that plays a greater role in loading the ACL (secondary to an anterior tibial shear force, the primary ACL loading mechanism).
Conversely, a group working with a different in vitro dynamic knee loading model argued the opposite—that internal tibial rotation is secondary to knee abduction motion and anterior tibial translation in increasing ACL loading.7,56–58,67 First, while investigating the timing sequence of multiplanar knee kinematics during an in vitro simulated landing, Kiapour et al.58 found that internal tibial rotation increased and peaked significantly later than knee flexion, anterior tibial translation, knee abduction, and ACL strain. However, such time lag in internal tibial rotation has not been reported in other in vitro-simulated dynamic landing models107 or in in vivo single-leg landings.61 Second, minimal coupled internal tibial rotation was found with a knee abduction moment, but significant coupled knee abduction motion was present with an internal tibial torque.57 This result contradicts much of the literature, which shows a strong mechanical coupling between a knee abduction moment and internal tibial rotation,30,32,42,51,109 as discussed earlier (see ‘Mechanical Coupling Between Knee Loads and Displacements’ subsection). Additionally, there is concern with how the external moment were applied to the knee in this in vitro dynamic landing model. Prior to the simulated ground impact, the knee is preloaded with an external anterior tibial shear force, knee abduction moment, and internal tibial torque, in addition to the trans-knee muscle forces.6 It is unusual for the knee to be placed under static external pre-loads prior to ground contact during a landing in vivo. In contrast, the in vitro dynamic knee loading model previously discussed applied impulsive axial compression, knee flexion moment, internal tibial torque, and knee abduction moment.107–109 Hence, evidence appears to favour an internal tibial torque as the second most critical to ACL loading, with a knee abduction moment having a tertiary order effect.
ACL Failure Resulting from a Single Overload Event vs. Repetitive Submaximal Loading Events
Current dogma considers implicitly that an ACL tear happens under a single loading cycle that exceeds the ultimate tensile strength (UTS) of the healthy ligament (one cycle on x-axis above UTS* on y-axis in Figure 6).20,101,128 While such a single overload injury event can and does happen, newer research suggests that partial or complete ACL tears can also occur after repetitive near-maximal loading events (below the healthy ligament’s UTS), each causing enough microdamage to accumulate and coalesce to weaken the structure. Unable to be repaired in time, the ligament fails under a submaximal load in a seemingly normal manoeuvre that has been performed numerous times before without injury (Figure 6).15,19,73,155 While little can be done to modulate the intensity of an athlete during competition, it is possible to adjust their training to limit the number and intensity of the unnecessarily strenuous (for the ACL) repetitions in training, especially of the likely very small subset of manoeuvres known to significantly load the ACL to near maximum levels. If these could be recognised and counted, they could be limited to allow sufficient time for any microdamage to repair and for the ultimate strength of the ACL to increase back to that of a healthy ligament. Based on this review, the loading mechanism most likely to induce ACL failure, whether in a single or under repetitive loading, would be a manoeuvre that gives rise to the combination of knee joint compression, anterior tibial shear, internal tibial torque and knee abduction moment during a jump landing, abrupt change of direction, or sudden stop.
Figure 6.
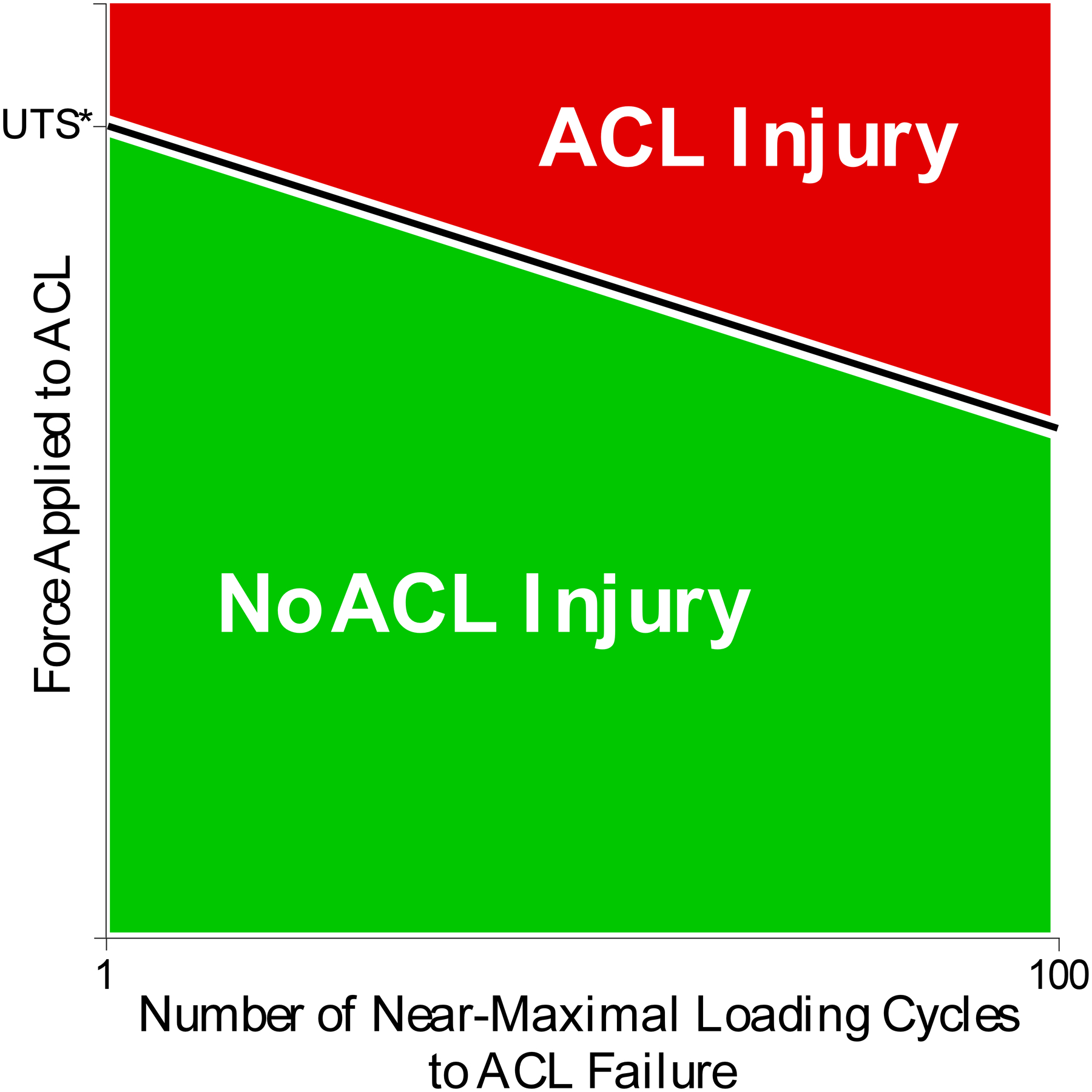
Diagram illustrating the inverse relationship between force applied to the ACL during athletic manoeuvres and the number of near-maximal loading cycles it can withstand before failing. Evidence suggests that as the force applied to the ACL decreases per loading cycle, the number of loading cycles leading to ACL failure increases, and vice versa. The precise nature of this relationship is unknown, however, and most likely depends on many factors such as age, the time interval between cycles and the ACL’s structural properties. It should be noted that these near-maximal ACL loading cycles represent a very small fraction of the total ACL loading cycles that occur in a typical practice session. UTS* refers to the ultimate tensile strength of a healthy ligament.
CONCLUSIONS
In summary, there is consensus that the 3D combination of knee joint compression and a knee flexion moment combined with an anterior tibial shear force, internal tibial torque, and a knee abduction moment results in the greatest loads in the ACL during single-leg athletic manoeuvres including jump landings, abrupt turns, and sudden decelerations performed with a knee close to full extension (i.e., 0–30°). While there is consensus that an anterior tibial shear force is the primary loading mechanism of the ACL, especially at small knee flexion angles, controversy still exists regarding the secondary order of importance of transverse-plane and frontal-plane loading in ACL injury scenarios. In addition, mechanical coupling between 3D knee loads and displacements adds to the complexity of characterising ACL loading mechanisms. Due to the geometry of the articulating surfaces of the tibiofemoral joint, single-plane loading usually causes multiplane knee displacements. For instance, large knee compression forces combined with a posteriorly and inferiorly sloped tibial plateau, especially with a steeper lateral plateau, causes anterior tibial translation and internal tibial rotation and increases ACL loading. Accordingly, a steep lateral tibial slope has been identified repeatedly as an important risk factor for ACL injury. Lastly, recent evidence has emerged suggesting that while the ACL can fail under a single supramaximal loading cycle, it can also fail under repetitive submaximal loading due to microdamage accumulating with each successive loading cycle, thereby weakening the ligament. This challenges the widely accepted view that an ACL injury only occurs during a single loading event and has implications for better ACL injury prevention in the future.
REFERENCES
- 1.Arnoczky SP. Anatomy of the anterior cruciate ligament. Clin Orthop Relat Res. 1983(172):19–25. [PubMed] [Google Scholar]
- 2.Bai D, Okada Y, Fukumoto T, Ogawa M, Tanaka Y. The muscle pre-activity timing of the hamstrings and quadriceps during 180 degrees and 360 degrees rotational jump landings in healthy female subjects. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2019;17:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balazs GC, Pavey GJ, Brelin AM, Pickett A, Keblish DJ, Rue JP. Risk of anterior cruciate ligament injury in athletes on synthetic playing surfaces: A systematic review. Am J Sports Med. 2015;43(7):1798–1804. [DOI] [PubMed] [Google Scholar]
- 4.Ball S, Stephen JM, El-Daou H, Williams A, Amis AA. The medial ligaments and the ACL restrain anteromedial laxity of the knee. Knee Surg Sports Traumatol Arthrosc. 2020;28(12):3700–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates NA, Nesbitt RJ, Shearn JT, Myer GD, Hewett TE. Knee abduction affects greater magnitude of change in ACL and MCL strains than matched internal tibial rotation in vitro. Clin Orthop Relat Res. 2017;475(10):2385–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates NA, Schilaty ND, Nagelli CV, Krych AJ, Hewett TE. Novel mechanical impact simulator designed to generate clinically relevant anterior cruciate ligament ruptures. Clin Biomech (Bristol, Avon). 2017;44:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates NA, Schilaty ND, Nagelli CV, Krych AJ, Hewett TE. Multiplanar loading of the knee and its influence on anterior cruciate ligament and medial collateral ligament strain during simulated landings and noncontact tears. Am J Sports Med. 2019;47(8):1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayer S, Meredith SJ, Wilson K, et al. Knee morphological risk factors for anterior cruciate ligament injury: a systematic review. J Bone Joint Surg Am. 2020;102(8):703–718. [DOI] [PubMed] [Google Scholar]
- 9.Beynnon B, Howe JG, Pope MH, Johnson RJ, Fleming BC. The measurement of anterior cruciate ligament strain in vivo. Int Orthop. 1992;16(1):1–12. [DOI] [PubMed] [Google Scholar]
- 10.Bojicic KM, Beaulieu ML, Imaizumi Krieger DY, Ashton-Miller JA, Wojtys EM. Association between lateral posterior tibial slope, body mass index, and ACL injury risk. Orthop J Sports Med. 2017;5(2):2325967116688664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62(2):259–270. [PubMed] [Google Scholar]
- 12.Carlson VR, Sheehan FT, Boden BP. Video analysis of anterior cruciate ligament (ACL) injuries: a systematic review. JBJS Rev. 2016;4(11):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carulli C, Innocenti M, Roselli G, Sirleo L, Matassi F, Innocenti M. Partial rupture of anterior cruciate ligament: preliminary experience of selective reconstruction. J Orthop Traumatol. 2020;21(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandrashekar N, Mansouri H, Slauterbeck J, Hashemi J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. J Biomech. 2006;39(16):2943–2950. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Kim J, Shao W, et al. An anterior cruciate ligament failure mechanism. Am J Sports Med. 2019;47(9):2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chhabra A, Zelle BA, Feng MT, Fu FH. The arthroscopic appearance of a normal anterior cruciate ligament in a posterior cruciate ligament-deficient knee: the posterolateral bundle (PLB) sign. Arthroscopy. 2005;21(10):1267. [DOI] [PubMed] [Google Scholar]
- 17.Christel PS, Akgun U, Yasar T, Karahan M, Demirel B. The contribution of each anterior cruciate ligament bundle to the Lachman test: a cadaver investigation. J Bone Joint Surg Br. 2012;94(1):68–74. [DOI] [PubMed] [Google Scholar]
- 18.Critchley ML, Davis DJ, Keener MM, et al. The effects of mid-flight whole-body and trunk rotation on landing mechanics: implications for anterior cruciate ligament injuries. Sports Biomech. 2020;19(4):421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csapo R, Juras V, Heinzle B, Trattnig S, Fink C. Compositional MRI of the anterior cruciate ligament of professional alpine ski racers: preliminary report on seasonal changes and load sensitivity. Eur Radiol Exp. 2020;4(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Della Villa F, Buckthorpe M, Grassi A, et al. Systematic video analysis of ACL injuries in professional male football (soccer): injury mechanisms, situational patterns and biomechanics study on 134 consecutive cases. Br J Sports Med. 2020;54(23):1423–1432. [DOI] [PubMed] [Google Scholar]
- 21.DeMorat G, Weinhold P, Blackburn T, Chudik S, Garrett W. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. Am J Sports Med. 2004;32(2):477–483. [DOI] [PubMed] [Google Scholar]
- 22.Draganich LF, Vahey JW. An in vitro study of anterior cruciate ligament strain induced by quadriceps and hamstrings forces. J Orthop Res. 1990;8(1):57–63. [DOI] [PubMed] [Google Scholar]
- 23.Duthon VB, Barea C, Abrassart S, Fasel JH, Fritschy D, Ménétrey J. Anatomy of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):204–213. [DOI] [PubMed] [Google Scholar]
- 24.Elmansori A, Lording T, Dumas R, Elmajri K, Neyret P, Lustig S. Proximal tibial bony and meniscal slopes are higher in ACL injured subjects than controls: a comparative MRI study. Knee Surg Sports Traumatol Arthrosc. 2017;25(5):1598–1605. [DOI] [PubMed] [Google Scholar]
- 25.Ferretti M, Ekdahl M, Shen W, Fu FH. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy. 2007;23(11):1218–1225. [DOI] [PubMed] [Google Scholar]
- 26.Fleming BC, Renstrom PA, Beynnon BD, et al. The effect of weightbearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34(2):163–170. [DOI] [PubMed] [Google Scholar]
- 27.Fok AW, Yau WP. Associations between isolated bundle tear of anterior cruciate ligament, time from injury to surgery, and clinical tests. J Orthop Surg (Hong Kong). 2014;22(2):209–213. [DOI] [PubMed] [Google Scholar]
- 28.Fujimaki Y, Thorhauer E, Sasaki Y, Smolinski P, Tashman S, Fu FH. Quantitative in situ analysis of the anterior cruciate ligament: length, midsubstance cross-sectional area, and insertion site areas. Am J Sports Med. 2016;44(1):118–125. [DOI] [PubMed] [Google Scholar]
- 29.Fukubayashi T, Torzilli PA, Sherman MF, Warren RF. An in vitro biomechanical evaluation of anterior-posterior motion of the knee. Tibial displacement, rotation, and torque. J Bone Joint Surg Am. 1982;64(2):258–264. [PubMed] [Google Scholar]
- 30.Fukuda Y, Woo SL, Loh JC, et al. A quantitative analysis of valgus torque on the ACL: a human cadaveric study. J Orthop Res. 2003;21(6):1107–1112. [DOI] [PubMed] [Google Scholar]
- 31.Furman W, Marshall JL, Girgis FG. The anterior cruciate ligament. A functional analysis based on postmortem studies. J Bone Joint Surg Am. 1976;58(2):179–185. [PubMed] [Google Scholar]
- 32.Gabriel MT, Wong EK, Woo SL, Yagi M, Debski RE. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res. 2004;22(1):85–89. [DOI] [PubMed] [Google Scholar]
- 33.Gardner EJ, Noyes FR, Jetter AW, Grood ES, Harms SP, Levy MS. Effect of anteromedial and posterolateral anterior cruciate ligament bundles on resisting medial and lateral tibiofemoral compartment subluxations. Arthroscopy. 2015;31(5):901–910. [DOI] [PubMed] [Google Scholar]
- 34.Gollehon DL, Torzilli PA, Warren RF. The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J Bone Joint Surg Am. 1987;69(2):233–242. [PubMed] [Google Scholar]
- 35.Hame SL, Oakes DA, Markolf KL. Injury to the anterior cruciate ligament during alpine skiing: a biomechanical analysis of tibial torque and knee flexion angle. Am J Sports Med. 2002;30(4):537–540. [DOI] [PubMed] [Google Scholar]
- 36.Hargrave MD, Carcia CR, Gansneder BM, Shultz SJ. Subtalar pronation does not influence impact forces or rate of loading during a single-leg landing. J Athl Train. 2003;38(1):18–23. [PMC free article] [PubMed] [Google Scholar]
- 37.Hashemi J, Chandrashekar N, Mansouri H, et al. Shallow medial tibial plateau and steep medial and lateral tibial slopes: new risk factors for anterior cruciate ligament injuries. Am J Sports Med. 2010;38(1):54–62. [DOI] [PubMed] [Google Scholar]
- 38.Hendrix ST, Barrett AM, Chrea B, Replogle WH, Hydrick JM, Barrett GR. Relationship between posterior-inferior tibial slope and bilateral noncontact ACL injury. Orthopedics. 2017;40(1):e136–e140. [DOI] [PubMed] [Google Scholar]
- 39.Hershman EB, Anderson R, Bergfeld JA, et al. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League games: 2000–2009 seasons. Am J Sports Med. 2012;40(10):2200–2205. [DOI] [PubMed] [Google Scholar]
- 40.Hino K, Shiraishi Y, Nishimatsu K, et al. In vivo anterior cruciate ligament length pattern assessment secondary to differences in the femoral attachment under loading condition using image-matching techniques. J Orthop Sci. 2019;24(2):294–300. [DOI] [PubMed] [Google Scholar]
- 41.Hirokawa S, Solomonow M, Lu Y, Lou ZP, D’Ambrosia R. Anterior-posterior and rotational displacement of the tibia elicited by quadriceps contraction. Am J Sports Med. 1992;20(3):299–306. [DOI] [PubMed] [Google Scholar]
- 42.Hollis JM, Takai S, Adams DJ, Horibe S, Woo SL. The effects of knee motion and external loading on the length of the anterior cruciate ligament (ACL): a kinematic study. J Biomech Eng. 1991;113(2):208–214. [DOI] [PubMed] [Google Scholar]
- 43.Hosseini A, Gill TJ, Li G. In vivo anterior cruciate ligament elongation in response to axial tibial loads. J Orthop Sci. 2009;14(3):298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howard M, Solaru S, Kang HP, et al. Epidemiology of anterior cruciate ligament injury on natural grass versus artificial turf in soccer: 10-Year data from the National Collegiate Athletic Association injury surveillance system. Orthop J Sports Med. 2020;8(7):2325967120934434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iriuchishima T, Ryu K, Aizawa S, Fu FH. Proportional evaluation of anterior cruciate ligament footprint size and knee bony morphology. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3157–3162. [DOI] [PubMed] [Google Scholar]
- 46.Iriuchishima T, Ryu K, Aizawa S, Fu FH. The difference in centre position in the ACL femoral footprint inclusive and exclusive of the fan-like extension fibres. Knee Surg Sports Traumatol Arthrosc. 2016;24(1):254–259. [DOI] [PubMed] [Google Scholar]
- 47.Iriuchishima T, Ryu K, Yorifuji H, Aizawa S, Fu FH. Commonly used ACL autograft areas do not correlate with the size of the ACL footprint or the femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2014;22(7):1573–1579. [DOI] [PubMed] [Google Scholar]
- 48.Iwahashi T, Shino K, Nakata K, et al. Direct anterior cruciate ligament insertion to the femur assessed by histology and 3-dimensional volume-rendered computed tomography. Arthroscopy. 2010;26(9 Suppl):S13–20. [DOI] [PubMed] [Google Scholar]
- 49.Jordan SS, DeFrate LE, Nha KW, Papannagari R, Gill TJ, Li G. The in vivo kinematics of the anteromedial and posterolateral bundles of the anterior cruciate ligament during weightbearing knee flexion. Am J Sports Med. 2007;35(4):547–554. [DOI] [PubMed] [Google Scholar]
- 50.Kanamori A, Woo SL, Ma CB, et al. The forces in the anterior cruciate ligament and knee kinematics during a simulated pivot shift test: A human cadaveric study using robotic technology. Arthroscopy. 2000;16(6):633–639. [DOI] [PubMed] [Google Scholar]
- 51.Kanamori A, Zeminski J, Rudy TW, Li G, Fu FH, Woo SL. The effect of axial tibial torque on the function of the anterior cruciate ligament: a biomechanical study of a simulated pivot shift test. Arthroscopy. 2002;18(4):394–398. [DOI] [PubMed] [Google Scholar]
- 52.Kato Y, Ingham SJ, Maeyama A, et al. Biomechanics of the human triple-bundle anterior cruciate ligament. Arthroscopy. 2012;28(2):247–254. [DOI] [PubMed] [Google Scholar]
- 53.Katouda M, Soejima T, Kanazawa T, Tabuchi K, Yamaki K, Nagata K. Relationship between thickness of the anteromedial bundle and thickness of the posterolateral bundle in the normal ACL. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1293–1298. [DOI] [PubMed] [Google Scholar]
- 54.Katz B The relation between force and speed in muscular contraction. J Physiol. 1939;96(1):45–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kennedy JC, Hawkins RJ, Willis RB. Strain gauge analysis of knee ligaments. Clin Orthop Relat Res. 1977(129):225–229. [DOI] [PubMed] [Google Scholar]
- 56.Kiapour AM, Demetropoulos CK, Kiapour A, et al. Strain response of the anterior cruciate ligament to uniplanar and multiplanar loads during simulated landings: implications for injury mechanism. Am J Sports Med. 2016;44(8):2087–2096. [DOI] [PubMed] [Google Scholar]
- 57.Kiapour AM, Kiapour A, Goel VK, et al. Uni-directional coupling between tibiofemoral frontal and axial plane rotation supports valgus collapse mechanism of ACL injury. J Biomech. 2015;48(10):1745–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiapour AM, Quatman CE, Goel VK, Wordeman SC, Hewett TE, Demetropoulos CK. Timing sequence of multi-planar knee kinematics revealed by physiologic cadaveric simulation of landing: implications for ACL injury mechanism. Clin Biomech (Bristol, Avon). 2014;29(1):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kittl C, El-Daou H, Athwal KK, et al. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee. Am J Sports Med. 2016;44(2):345–354. [DOI] [PubMed] [Google Scholar]
- 60.Koga H, Nakamae A, Shima Y, et al. Mechanisms for noncontact anterior cruciate ligament injuries: knee joint kinematics in 10 injury situations from female team handball and basketball. Am J Sports Med. 2010;38(11):2218–2225. [DOI] [PubMed] [Google Scholar]
- 61.Koshino Y, Yamanaka M, Ezawa Y, et al. Coupling motion between rearfoot and hip and knee joints during walking and single-leg landing. J Electromyogr Kinesiol. 2017;37:75–83. [DOI] [PubMed] [Google Scholar]
- 62.Lalwani R, Srivastava R, Kotgirwar S, Athavale SA. New insights in anterior cruciate ligament morphology: implications for anterior cruciate ligament reconstruction surgeries. Anat Cell Biol. 2020;53(4):398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lambson RB, Barnhill BS, Higgins RW. Football cleat design and its effect on anterior cruciate ligament injuries. A three-year prospective study. Am J Sports Med. 1996;24(2):155–159. [DOI] [PubMed] [Google Scholar]
- 64.Lane JG, Irby SE, Kaufman K, Rangger C, Daniel DM. The anterior cruciate ligament in controlling axial rotation. An evaluation of its effect. Am J Sports Med. 1994;22(2):289–293. [DOI] [PubMed] [Google Scholar]
- 65.LaPrade RF, Wentorf FA, Fritts H, Gundry C, Hightower CD. A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy. 2007;23(12):1341–1347. [DOI] [PubMed] [Google Scholar]
- 66.Lee JK, Lee S, Seong SC, Lee MC. Anatomy of the anterior cruciate ligament insertion sites: comparison of plain radiography and three-dimensional computed tomographic imaging to anatomic dissection. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2297–2305. [DOI] [PubMed] [Google Scholar]
- 67.Levine JW, Kiapour AM, Quatman CE, et al. Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am J Sports Med. 2013;41(2):385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li G, Papannagari R, DeFrate LE, Yoo JD, Park SE, Gill TJ. The effects of ACL deficiency on mediolateral translation and varus-valgus rotation. Acta Orthop. 2007;78(3):355–360. [DOI] [PubMed] [Google Scholar]
- 69.Li G, Rudy TW, Allen C, Sakane M, Woo SL. Effect of combined axial compressive and anterior tibial loads on in situ forces in the anterior cruciate ligament: a porcine study. J Orthop Res. 1998;16(1):122–127. [DOI] [PubMed] [Google Scholar]
- 70.Li G, Rudy TW, Sakane M, Kanamori A, Ma CB, Woo SL. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J Biomech. 1999;32(4):395–400. [DOI] [PubMed] [Google Scholar]
- 71.Lipke JM, Janecki CJ, Nelson CL, et al. The role of incompetence of the anterior cruciate and lateral ligaments in anterolateral and anteromedial instability. A biomechanical study of cadaver knees. J Bone Joint Surg Am. 1981;63(6):954–960. [PubMed] [Google Scholar]
- 72.Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Morphologic characteristics help explain the gender difference in peak anterior cruciate ligament strain during a simulated pivot landing. Am J Sports Med. 2012;40(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lipps DB, Wojtys EM, Ashton-Miller JA. Anterior cruciate ligament fatigue failures in knees subjected to repeated simulated pivot landings. Am J Sports Med. 2013;41(5):1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lo J, Müller O, Wünschel M, Bauer S, Wülker N. Forces in anterior cruciate ligament during simulated weight-bearing flexion with anterior and internal rotational tibial load. J Biomech. 2008;41(9):1855–1861. [DOI] [PubMed] [Google Scholar]
- 75.Lord BR, El-Daou H, Zdanowicz U, Śmigielski R, Amis AA. The role of fibers within the tibial attachment of the anterior cruciate ligament in restraining tibial displacement. Arthroscopy. 2019;35(7):2101–2111. [DOI] [PubMed] [Google Scholar]
- 76.Loughran GJ, Vulpis CT, Murphy JP, et al. Incidence of knee injuries on artificial turf versus natural grass in National Collegiate Athletic Association american football: 2004–2005 through 2013–2014 seasons. Am J Sports Med. 2019;47(6):1294–1301. [DOI] [PubMed] [Google Scholar]
- 77.Luetkemeyer CM, Cai LY, Neu CP, Arruda EM. Full-volume displacement mapping of anterior cruciate ligament bundles with dualMRI. Extreme Mech Lett. 2018;19:7–14. [Google Scholar]
- 78.Luites JW, Wymenga AB, Blankevoort L, Kooloos JG. Description of the attachment geometry of the anteromedial and posterolateral bundles of the ACL from arthroscopic perspective for anatomical tunnel placement. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1422–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacKay JW, Whitehead H, Toms AP. Radiological evidence for the triple bundle anterior cruciate ligament. Clin Anat. 2014;27(7):1097–1102. [DOI] [PubMed] [Google Scholar]
- 80.Markolf K, Boguszewski D, Yamaguchi K, Lama C, McAllister D. Prediction of ACL force produced by tibiofemoral compression during controlled knee flexion: a new robotic testing methodology. J Biomech Eng. 2018;140(12). [DOI] [PubMed] [Google Scholar]
- 81.Markolf K, Yamaguchi K, Matthew J, McAllister D. Effects of tibiofemoral compression on ACL forces and knee kinematics under combined knee loads. J Orthop Res. 2019;37(3):631–639. [DOI] [PubMed] [Google Scholar]
- 82.Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13(6):930–935. [DOI] [PubMed] [Google Scholar]
- 83.Markolf KL, Gorek JF, Kabo JM, Shapiro MS. Direct measurement of resultant forces in the anterior cruciate ligament. An in vitro study performed with a new experimental technique. J Bone Joint Surg Am. 1990;72(4):557–567. [PubMed] [Google Scholar]
- 84.Markolf KL, Jackson SR, Foster B, McAllister DR. ACL forces and knee kinematics produced by axial tibial compression during a passive flexion-extension cycle. J Orthop Res. 2014;32(1):89–95. [DOI] [PubMed] [Google Scholar]
- 85.Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee--the contributions of the supporting structures. A quantitative in vitro study. J Bone Joint Surg Am. 1976;58(5):583–594. [PubMed] [Google Scholar]
- 86.Markolf KL, O’Neill G, Jackson SR, McAllister DR. Effects of applied quadriceps and hamstrings muscle loads on forces in the anterior and posterior cruciate ligaments. Am J Sports Med. 2004;32(5):1144–1149. [DOI] [PubMed] [Google Scholar]
- 87.Markolf KL, Park S, Jackson SR, McAllister DR. Contributions of the posterolateral bundle of the anterior cruciate ligament to anterior-posterior knee laxity and ligament forces. Arthroscopy. 2008;24(7):805–809. [DOI] [PubMed] [Google Scholar]
- 88.Matsumoto H, Suda Y, Otani T, Niki Y, Seedhom BB, Fujikawa K. Roles of the anterior cruciate ligament and the medial collateral ligament in preventing valgus instability. J Orthop Sci. 2001;6(1):28–32. [DOI] [PubMed] [Google Scholar]
- 89.McLean SG, Andrish JT, van den Bogert AJ. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. Am J Sports Med. 2005;33(7):1106; author reply 1106–1107. [DOI] [PubMed] [Google Scholar]
- 90.McLean SG, Huang X, Su A, Van Den Bogert AJ. Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clin Biomech (Bristol, Avon). 2004;19(8):828–838. [DOI] [PubMed] [Google Scholar]
- 91.McLean SG, Lucey SM, Rohrer S, Brandon C. Knee joint anatomy predicts high-risk in vivo dynamic landing knee biomechanics. Clin Biomech (Bristol, Avon). 2010;25(8):781–788. [DOI] [PubMed] [Google Scholar]
- 92.McLean SG, Oh YK, Palmer ML, et al. The relationship between anterior tibial acceleration, tibial slope, and ACL strain during a simulated jump landing task. J Bone Joint Surg Am. 2011;93(14):1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McQuade KJ, Crutcher JP, Sidles JA, Larson RV. Tibial rotation in anterior cruciate deficient knees: an in vitro study. J Orthop Sports Phys Ther. 1989;11(4):146–149. [DOI] [PubMed] [Google Scholar]
- 94.Meyer EG, Haut RC. Anterior cruciate ligament injury induced by internal tibial torsion or tibiofemoral compression. J Biomech. 2008;41(16):3377–3383. [DOI] [PubMed] [Google Scholar]
- 95.Miyasaka KC, Daniel DM, Stone ML, Hirschman P. The incience of knee ligament injuries in the general population. Am J Knee Surg. 1991;4:43–48. [Google Scholar]
- 96.Miyasaka T, Matsumoto H, Suda Y, Otani T, Toyama Y. Coordination of the anterior and posterior cruciate ligaments in constraining the varus-valgus and internal-external rotatory instability of the knee. J Orthop Sci. 2002;7(3):348–353. [DOI] [PubMed] [Google Scholar]
- 97.Mochizuki T, Muneta T, Nagase T, Shirasawa S, Akita KI, Sekiya I. Cadaveric knee observation study for describing anatomic femoral tunnel placement for two-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2006;22(4):356–361. [DOI] [PubMed] [Google Scholar]
- 98.Mommersteeg TJ, Huiskes R, Blankevoort L, Kooloos JG, Kauer JM. An inverse dynamics modeling approach to determine the restraining function of human knee ligament bundles. J Biomech. 1997;30(2):139–146. [DOI] [PubMed] [Google Scholar]
- 99.Monaco E, Ferretti A, Labianca L, et al. Navigated knee kinematics after cutting of the ACL and its secondary restraint. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):870–877. [DOI] [PubMed] [Google Scholar]
- 100.Monaco E, Maestri B, Labianca L, et al. Navigated knee kinematics after tear of the ACL and its secondary restraints: preliminary results. Orthopedics. 2010;33(10 Suppl):87–93. [DOI] [PubMed] [Google Scholar]
- 101.Montgomery C, Blackburn J, Withers D, Tierney G, Moran C, Simms C. Mechanisms of ACL injury in professional rugby union: a systematic video analysis of 36 cases. Br J Sports Med. 2018;52(15):994–1001. [DOI] [PubMed] [Google Scholar]
- 102.Nagano Y, Ida H, Akai M, Fukubayashi T. Gender differences in knee kinematics and muscle activity during single limb drop landing. Knee. 2007;14(3):218–223. [DOI] [PubMed] [Google Scholar]
- 103.Nielsen S, Ovesen J, Rasmussen O. The anterior cruciate ligament of the knee: an experimental study of its importance in rotatory knee instability. Arch Orthop Trauma Surg. 1984;103(3):170–174. [DOI] [PubMed] [Google Scholar]
- 104.Norwood LA, Cross MJ. Anterior cruciate ligament: functional anatomy of its bundles in rotatory instabilities. Am J Sports Med. 1979;7(1):23–26. [DOI] [PubMed] [Google Scholar]
- 105.Noyes FR, Jetter AW, Grood ES, Harms SP, Gardner EJ, Levy MS. Anterior cruciate ligament function in providing rotational stability assessed by medial and lateral tibiofemoral compartment translations and subluxations. Am J Sports Med. 2015;43(3):683–692. [DOI] [PubMed] [Google Scholar]
- 106.Ochi M, Adachi N, Deie M, Kanaya A. Anterior cruciate ligament augmentation procedure with a 1-incision technique: anteromedial bundle or posterolateral bundle reconstruction. Arthroscopy. 2006;22(4):463 e461–465. [DOI] [PubMed] [Google Scholar]
- 107.Oh YK, Kreinbrink JL, Ashton-Miller JA, Wojtys EM. Effect of ACL transection on internal tibial rotation in an in vitro simulated pivot landing. J Bone Joint Surg Am. 2011;93(4):372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oh YK, Kreinbrink JL, Wojtys EM, Ashton-Miller JA. Effect of axial tibial torque direction on ACL relative strain and strain rate in an in vitro simulated pivot landing. J Orthop Res. 2012;30(4):528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oh YK, Lipps DB, Ashton-Miller JA, Wojtys EM. What strains the anterior cruciate ligament during a pivot landing? Am J Sports Med. 2012;40(3):574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Orishimo KF, Kremenic IJ, Pappas E, Hagins M, Liederbach M. Comparison of landing biomechanics between male and female professional dancers. Am J Sports Med. 2009;37(11):2187–2193. [DOI] [PubMed] [Google Scholar]
- 111.Pandy MG, Shelburne KB. Dependence of cruciate-ligament loading on muscle forces and external load. J Biomech. 1997;30(10):1015–1024. [DOI] [PubMed] [Google Scholar]
- 112.Peeler J, Anderson J, Piotrowski S, Stranges G. Motion of the anterior cruciate ligament during internal and external rotation at the knee: A cadaveric study. Clin Anat. 2017;30(7):861–867. [DOI] [PubMed] [Google Scholar]
- 113.Petersen W, Zantop T. Partial rupture of the anterior cruciate ligament. Arthroscopy. 2006;22(11):1143–1145. [DOI] [PubMed] [Google Scholar]
- 114.Petersen W, Zantop T. Anatomy of the anterior cruciate ligament with regard to its two bundles. Clin Orthop Relat Res. 2007;454:35–47. [DOI] [PubMed] [Google Scholar]
- 115.Pfeifer CE, Beattie PF, Sacko RS, Hand A. Risk factors associated with non-contact anterior cruciate ligament injury: a systematic review. Int J Sports Phys Ther. 2018;13(4):575–587. [PMC free article] [PubMed] [Google Scholar]
- 116.Pflum MA, Shelburne KB, Torry MR, Decker MJ, Pandy MG. Model prediction of anterior cruciate ligament force during drop-landings. Med Sci Sports Exerc. 2004;36(11):1949–1958. [DOI] [PubMed] [Google Scholar]
- 117.Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42–51. [DOI] [PubMed] [Google Scholar]
- 118.Quiles C, Constantino JA, Gañán Y, Macías D, Quiles M. Stereophotogrammetric surface anatomy of the anterior cruciate ligament’s tibial footprint: Precise osseous structure and distances to arthroscopically-relevant landmarks. Knee. 2018;25(4):531–544. [DOI] [PubMed] [Google Scholar]
- 119.Rahnemai-Azar AA, Yaseen Z, van Eck CF, Irrgang JJ, Fu FH, Musahl V. Increased lateral tibial plateau slope predisposes male college football players to anterior cruciate ligament injury. J Bone Joint Surg Am. 2016;98(12):1001–1006. [DOI] [PubMed] [Google Scholar]
- 120.Ren Y, Jacobs BJ, Nuber GW, Koh JL, Zhang LQ. Developing a 6-DOF robot to investigate multi-axis ACL injuries under valgus loading coupled with tibia internal rotation. Annu Int Conf IEEE Eng Med Biol Soc. 2010;2010:3942–3945. [DOI] [PubMed] [Google Scholar]
- 121.Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ruiz N, Filippi GJ, Gagnière B, Bowen M, Robert HE. The comparative role of the anterior cruciate ligament and anterolateral structures in controlling passive internal rotation of the knee: a biomechanical study. Arthroscopy. 2016;32(6):1053–1062. [DOI] [PubMed] [Google Scholar]
- 123.Sakane M, Fox RJ, Woo SL, Livesay GA, Li G, Fu FH. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J Orthop Res. 1997;15(2):285–293. [DOI] [PubMed] [Google Scholar]
- 124.Sakane M, Livesay GA, Fox RJ, Rudy TW, Runco TJ, Woo SL. Relative contribution of the ACL, MCL, and bony contact to the anterior stability of the knee. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):93–97. [DOI] [PubMed] [Google Scholar]
- 125.Scheffler SU, Maschewski K, Becker R, Asbach P. In-vivo three-dimensional MR imaging of the intact anterior cruciate ligament shows a variable insertion pattern of the femoral and tibial footprints. Knee Surg Sports Traumatol Arthrosc. 2018;26(12):3667–3672. [DOI] [PubMed] [Google Scholar]
- 126.Shelburne KB, Pandy MG. A musculoskeletal model of the knee for evaluating ligament forces during isometric contractions. J Biomech. 1997;30(2):163–176. [DOI] [PubMed] [Google Scholar]
- 127.Shin CS, Chaudhari AM, Andriacchi TP. Valgus plus internal rotation moments increase anterior cruciate ligament strain more than either alone. Med Sci Sports Exerc. 2011;43(8):1484–1491. [DOI] [PubMed] [Google Scholar]
- 128.Shultz SJ, Schmitz RJ, Cameron KL, et al. Anterior Cruciate Ligament Research Retreat VIII Summary Statement: An Update on Injury Risk Identification and Prevention Across the Anterior Cruciate Ligament Injury Continuum, March 14–16, 2019, Greensboro, NC. J Athl Train. 2019;54(9):970–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siebold R, Ellert T, Metz S, Metz J. Femoral insertions of the anteromedial and posterolateral bundles of the anterior cruciate ligament: morphometry and arthroscopic orientation models for double-bundle bone tunnel placement--a cadaver study. Arthroscopy. 2008;24(5):585–592. [DOI] [PubMed] [Google Scholar]
- 130.Siebold R, Fu FH. Assessment and augmentation of symptomatic anteromedial or posterolateral bundle tears of the anterior cruciate ligament. Arthroscopy. 2008;24(11):1289–1298. [DOI] [PubMed] [Google Scholar]
- 131.Simon RA, Everhart JS, Nagaraja HN, Chaudhari AM. A case-control study of anterior cruciate ligament volume, tibial plateau slopes and intercondylar notch dimensions in ACL-injured knees. J Biomech. 2010;43(9):1702–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Skelley NW, Castile RM, Cannon PC, Weber CI, Brophy RH, Lake SP. Regional variation in the mechanical and microstructural properties of the human anterior cruciate ligament. Am J Sports Med. 2016;44(11):2892–2899. [DOI] [PubMed] [Google Scholar]
- 133.Skelley NW, Castile RM, York TE, Gruev V, Lake SP, Brophy RH. Differences in the microstructural properties of the anteromedial and posterolateral bundles of the anterior cruciate ligament. Am J Sports Med. 2015;43(4):928–936. [DOI] [PubMed] [Google Scholar]
- 134.Smeets K, Jacobs P, Hertogs R, et al. Torsional injuries of the lower limb: an analysis of the frictional torque between different types of football turf and the shoe outsole. Br J Sports Med. 2012;46(15):1078–1083. [DOI] [PubMed] [Google Scholar]
- 135.Sonnery-Cottet B, Barth J, Graveleau N, Fournier Y, Hager JP, Chambat P. Arthroscopic identification of isolated tear of the posterolateral bundle of the anterior cruciate ligament. Arthroscopy. 2009;25(7):728–732. [DOI] [PubMed] [Google Scholar]
- 136.Sonnery-Cottet B, Colombet P. Partial tears of the anterior cruciate ligament. Orthop Traumatol Surg Res. 2016;102(1 Suppl):S59–67. [DOI] [PubMed] [Google Scholar]
- 137.Steckel H, Starman JS, Baums MH, Klinger HM, Schultz W, Fu FH. Anatomy of the anterior cruciate ligament double bundle structure: a macroscopic evaluation. Scand J Med Sci Sports. 2007;17(4):387–392. [DOI] [PubMed] [Google Scholar]
- 138.Stuelcken MC, Mellifont DB, Gorman AD, Sayers MG. Mechanisms of anterior cruciate ligament injuries in elite women’s netball: a systematic video analysis. J Sports Sci. 2016;34(16):1516–1522. [DOI] [PubMed] [Google Scholar]
- 139.Sturnick DR, Vacek PM, DeSarno MJ, et al. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am J Sports Med. 2015;43(4):839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Suruga M, Horaguchi T, Iriuchishima T, et al. Morphological size evaluation of the midsubstance insertion areas and the fan-like extension fibers in the femoral ACL footprint. Arch Orthop Trauma Surg. 2017;137(8):1107–1113. [DOI] [PubMed] [Google Scholar]
- 141.Takahashi M, Doi M, Abe M, Suzuki D, Nagano A. Anatomical study of the femoral and tibial insertions of the anteromedial and posterolateral bundles of human anterior cruciate ligament. Am J Sports Med. 2006;34(5):787–792. [DOI] [PubMed] [Google Scholar]
- 142.Takai S, Woo SL, Livesay GA, Adams DJ, Fu FH. Determination of the in situ loads on the human anterior cruciate ligament. J Orthop Res. 1993;11(5):686–695. [DOI] [PubMed] [Google Scholar]
- 143.Tampere T, Van Hoof T, Cromheecke M, et al. The anterior cruciate ligament: a study on its bony and soft tissue anatomy using novel 3D CT technology. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):236–244. [DOI] [PubMed] [Google Scholar]
- 144.Tamura A, Akasaka K, Otsudo T, Shiozawa J, Toda Y, Yamada K. Dynamic knee valgus alignment influences impact attenuation in the lower extremity during the deceleration phase of a single-leg landing. PLoS One. 2017;12(6):e0179810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tantisricharoenkul G, Linde-Rosen M, Araujo P, Zhou J, Smolinski P, Fu FH. Anterior cruciate ligament: an anatomical exploration in humans and in a selection of animal species. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):961–971. [DOI] [PubMed] [Google Scholar]
- 146.Tran TD, Tran QL. A cadaveric study on the anatomy of anterior cruciate ligament in Vietnamese adults. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2018;14:22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vasta S, Andrade R, Pereira R, et al. Bone morphology and morphometry of the lateral femoral condyle is a risk factor for ACL injury. Knee Surg Sports Traumatol Arthrosc. 2018;26(9):2817–2825. [DOI] [PubMed] [Google Scholar]
- 148.Waiwaiole A, Gurbani A, Motamedi K, et al. Relationship of ACL injury and posterior tibial slope with patient age, sex, and race. Orthop J Sports Med. 2016;4(11):2325967116672852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Walden M, Krosshaug T, Bjorneboe J, Andersen TE, Faul O, Hagglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wall SJ, Rose DM, Sutter EG, Belkoff SM, Boden BP. The role of axial compressive and quadriceps forces in noncontact anterior cruciate ligament injury: a cadaveric study. Am J Sports Med. 2012;40(3):568–573. [DOI] [PubMed] [Google Scholar]
- 151.Wang D, Kent RN 3rd, Amirtharaj MJ, et al. Tibiofemoral kinematics during compressive loading of the ACL-intact and ACL-sectioned knee: roles of tibial slope, medial eminence volume, and anterior laxity. J Bone Joint Surg Am. 2019;101(12):1085–1092. [DOI] [PubMed] [Google Scholar]
- 152.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech (Bristol, Avon). 2006;21(9):977–983. [DOI] [PubMed] [Google Scholar]
- 153.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The relationship between quadriceps muscle force, knee flexion, and anterior cruciate ligament strain in an in vitro simulated jump landing. Am J Sports Med. 2006;34(2):269–274. [DOI] [PubMed] [Google Scholar]
- 154.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. Effect of varying hamstring tension on anterior cruciate ligament strain during in vitro impulsive knee flexion and compression loading. J Bone Joint Surg Am. 2008;90(4):815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wojtys EM, Beaulieu ML, Ashton-Miller JA. New perspectives on ACL injury: On the role of repetitive sub-maximal knee loading in causing ACL fatigue failure. J Orthop Res. 2016;34(12):2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wünschel M, Müller O, Lo J, Obloh C, Wülker N. The anterior cruciate ligament provides resistance to externally applied anterior tibial force but not to internal rotational torque during simulated weight-bearing flexion. Arthroscopy. 2010;26(11):1520–1527. [DOI] [PubMed] [Google Scholar]
- 157.Yadav S, Singh S. Analysis of partial bundle anterior cruciate ligament tears- diagnosis and management with ACL augmentation. J Clin Orthop Trauma. 2020;11(Suppl 3):S337–S341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yasuda K, Kondo E, Ichiyama H, et al. Anatomic reconstruction of the anteromedial and posterolateral bundles of the anterior cruciate ligament using hamstring tendon grafts. Arthroscopy. 2004;20(10):1015–1025. [DOI] [PubMed] [Google Scholar]
- 159.Zantop T, Brucker PU, Vidal A, Zelle BA, Fu FH. Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res. 2007;454:48–53. [DOI] [PubMed] [Google Scholar]
- 160.Zantop T, Herbort M, Raschke MJ, Fu FH, Petersen W. The role of the anteromedial and posterolateral bundles of the anterior cruciate ligament in anterior tibial translation and internal rotation. Am J Sports Med. 2007;35(2):223–227. [DOI] [PubMed] [Google Scholar]
- 161.Zantop T, Wellmann M, Fu FH, Petersen W. Tunnel positioning of anteromedial and posterolateral bundles in anatomic anterior cruciate ligament reconstruction: anatomic and radiographic findings. Am J Sports Med. 2008;36(1):65–72. [DOI] [PubMed] [Google Scholar]
- 162.Zeng C, Yang T, Wu S, et al. Is posterior tibial slope associated with noncontact anterior cruciate ligament injury? Knee Surg Sports Traumatol Arthrosc. 2016;24(3):830–837. [DOI] [PubMed] [Google Scholar]


