Abstract
Elevation in cardiac filing pressures is the hallmark of acute and chronic heart failure (HF). A number of pathological processes contribute to the elevation in cardiac filling pressures, including myocardial dysfunction and primary volume overload. In this review, we discuss the important role of the venous system and the concepts of stressed blood volume (SBV) and unstressed blood volume (UBV). We review how regulation of venous tone modifies the distribution of blood between these two functional compartments, the physical distribution of blood between the pulmonary and systemic circulations, and how these relate to hemodynamic abnormalities observed in HF. Finally, we review recently applied methods to estimating SBV and how they are being applied to results of clinical studies to provide new insights into resting and exercise hemodynamics and therapeutics for HF.
Keywords: Volume regulation, venous tone, exercise, heart failure, stressed blood volume, unstressed blood volume
Condensed Abstract
In this review, we discuss the important role of the venous system and the concepts of stressed blood volume (SBV) and unstressed blood volume (UBV). We review how regulation of venous tone modifies the distribution of blood between these two functional compartments, the physical distribution of blood between the pulmonary and systemic circulations, and how these relate to hemodynamic abnormalities observed in HF. Finally, we review recently applied methods to estimating SBV and how they are being applied to results of clinical studies to provide new insights into resting and exercise hemodynamics and therapeutics for HF.
Introduction
Despite decades of investigation, the mechanisms responsible for elevated cardiac filling pressures in patients with heart failure (HF) with preserved or reduced ejection fraction at rest, during exertion and during episodes of decompensation are not fully explained. Cardiac factors such as reduced inotropic reserve, blunted chronotropic reserve, impaired diastolic function and pericardial restraint have been implicated, particularly to explain abnormal exertional hemodynamics (1). Derangements in the vascular system, such as blunted vasodilatory responses, increased pulmonary arterial resistance, and decreased pulmonary arterial compliance, are also recognized. The discussion concerning such vascular effects has focused on the systemic arterial circulation because of the well-recognized relationship between cardiac afterload and cardiac performance (2), driving the belief that ventricular-vascular mismatch (or uncoupling) contributes to hemodynamic abnormalities in HF.
However, the venous system has received very little attention despite its primary role in determining cardiac filling pressures and regulating cardiac output (CO)(3). In this review, we discuss the role of the venous system and the concepts of stressed blood volume (SBV) and unstressed blood volume (UBV). We discuss how regulation of venous tone modifies the distribution of blood between these two functional compartments. Finally, we touch on recently developed methods for estimating SBV and how they are being applied to results of clinical studies to provide new insights into resting and exercise hemodynamics and HF therapeutics (Central Illustration).
Central Illustration: Venous Tone and Stressed Blood Volume.
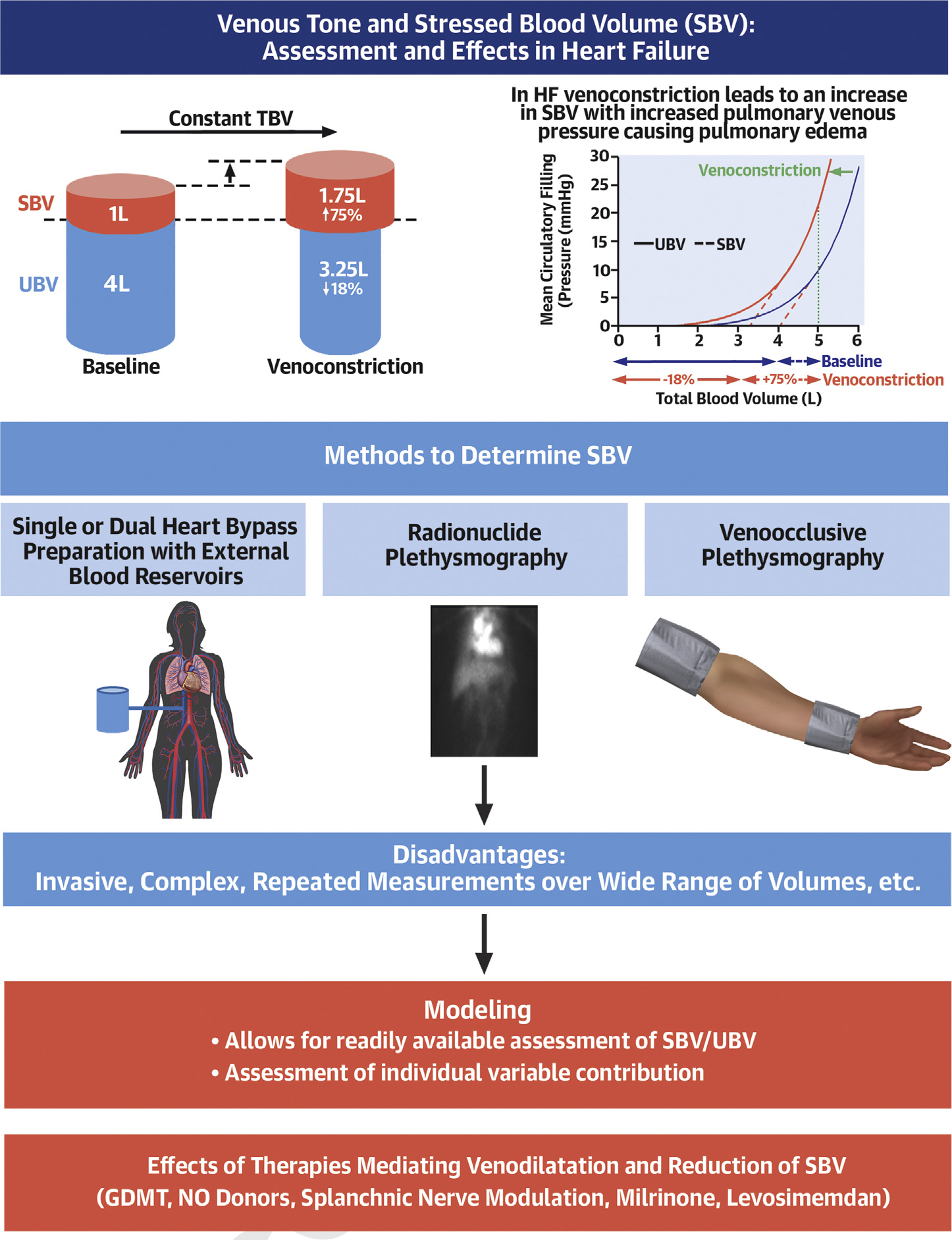
Venous tone and stressed blood volume regulation and measurement
Abbreviations: SBV–stressed blood volume, UBV–unstressed blood volume
General Considerations
Veins are not merely a conduit for the return of blood to the heart, but also serve as a functional blood reservoir. Veins contain around 70% of the total blood volume (TBV) in comparison to only ~30% contained in arteries (4). Furthermore, the highly-vascularized organs of the splanchnic compartment, such as liver, spleen, and intestines, contain approximately 20–30% of the TBV (5). Veins are more compliant than arteries (>20:1) and like arteries, have a smooth muscle layer with dense autonomic innervation. Splanchnic vessels receive more adrenergic innervation than central and peripheral vessels (6). Furthermore, the innervation is higher in the splanchnic veins than in the splanchnic arteries (7). Thus, the splanchnic venous compartment is the main site of venous capacity in animals (4) and humans (8). A basic understanding of the concepts of UBV and SBV is fundamental to understanding how regulation of venous tone exerts potent control of ventricular filling pressures and CO in health and disease. A summary of key terms and their definitions used throughout this review are outlined in Supplemental Table 1.
Basic Concepts
TBV of the body is functionally divided into two pools: UBV+SBV=TBV. Despite the central role of SBV and UBV in regulating cardiac performance, direct measurement of these parameters requires invasive methods. Specifically, quantification of SBV and UBV requires repeated measurements of mean circulatory filling pressures (MCFPs) over a range of TBVs. MCFP is the intravascular pressure when the heart is stopped, and pressures equilibrate throughout the circulation.
In practice, estimation of UBV and SBV starts with the measurement of TBV, often done using an indicator-dilution technique that involves injecting a known quantity of an indicator (e.g., Evans-blue dye which binds to albumin (9), or I131-radiolabeled albumin (10)) that distributes throughout the intravascular space. After injection of a known quantity of the indicator, either an equilibrium concentration (in the case of Evans-blue) or the time course of decay (in the case of I131) is measured, which is converted to total plasma volume (TPV)(9,10). TBV is then obtained from TPV/(1-hematocrit). Next, MCFP must be measured at more than one TBV, which is achieved by infusing or withdrawing a known quantity of blood (Figure 1A/B).
Figure 1. Interrelationship of stressed and unstressed blood volume.
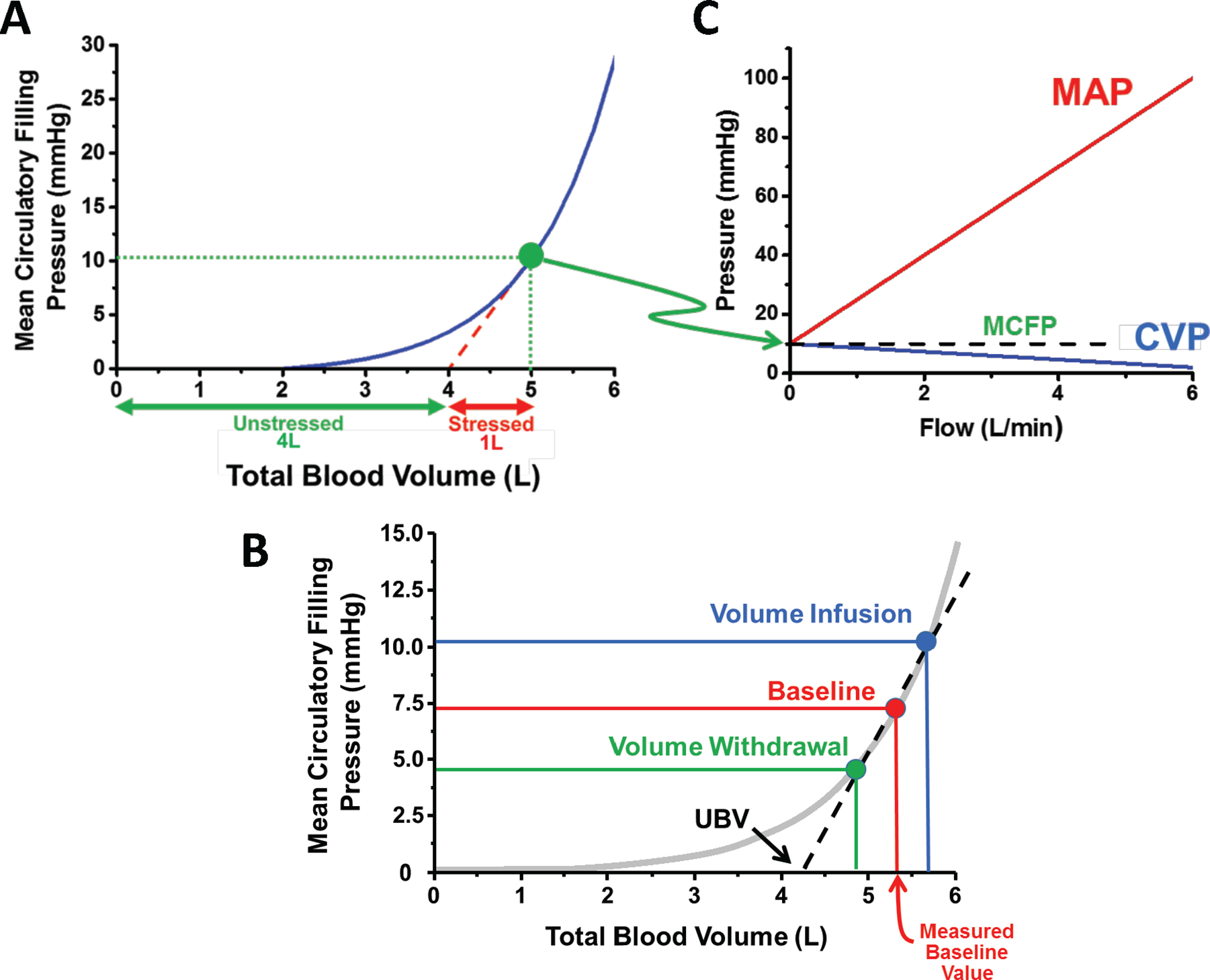
A. The sum of unstressed (UBV) and stressed blood volume is the total blood volume. The relationship between mean circulatory filling pressure (MCFP) and total blood volume is non-linear. UBV is estimated from the volume axis intercept of the linearly extrapolated MCFP-total blood volume relationship measured over a limited range of volumes (red dotted line). B. The impact of volume infusion vs withdrawal on the stressed and UBV are shown. C. MCFP is the equilibration pressure throughout the circulation when there is no blood flow. Once flow starts, for a given MCFP, central venous pressure (CVP) decreases, and mean arterial pressure (MAP) increases.
The relationship between MCFP and TBV is nonlinear, such that a significant amount of intravascular volume is required before there is any significant rise of MCFP. In shown in Figure 1A, MCFP does not rise until TBV is more than 2L and does not exceed 2.5 mmHg until TBV is nearly 4L. Furthermore, once flow in the circulatory system begins, for a given MCFP, central venous pressure (CVP) decreases, and mean arterial pressure (MAP) increases (Figure 1C).
UBV is defined as the volume required to fill the vasculature before there is any significant rise of MCFP. The portion of TBV in excess of UBV is the SBV. Under normal conditions, UBV~4/5th of TBV and only ~1/5th is SBV. Importantly, the distinction between SBV and UBV is functional, and does not suggest physically isolated volumes.
Guyton and colleagues have taught that insights into cardiovascular performance are obtained by plotting “Starling curves” on the same axes as “venous return curves” (Figure 2). A “Starling curve” is a plot of CO as a function of CVP at a given ventricular contractility, heart rate and systemic vascular resistance: CO increases with increases of CVP, a manifestation of the Frank-Starling Law of the heart. A “venous return curve” shows the rate of blood return to the right atrium as function of CVP (i.e., the plot obtained by swapping the axes of Figure 1C): increases of CVP result in decreased venous return. The venous return curve intersects the volume axis (i.e., when flow equals 0) when CVP reaches MCFP. Since CO must equal venous return in steady-state conditions, the intersection between the Starling curve and the venous return curve defines the CVP and CO for a given state (solid red dot in Figure 2). With this construct, it is easy to appreciate how MCFP is a key regulator of cardiac performance.
Figure 2. Interrelationship between Starling cardiac output curve and venous return curve.
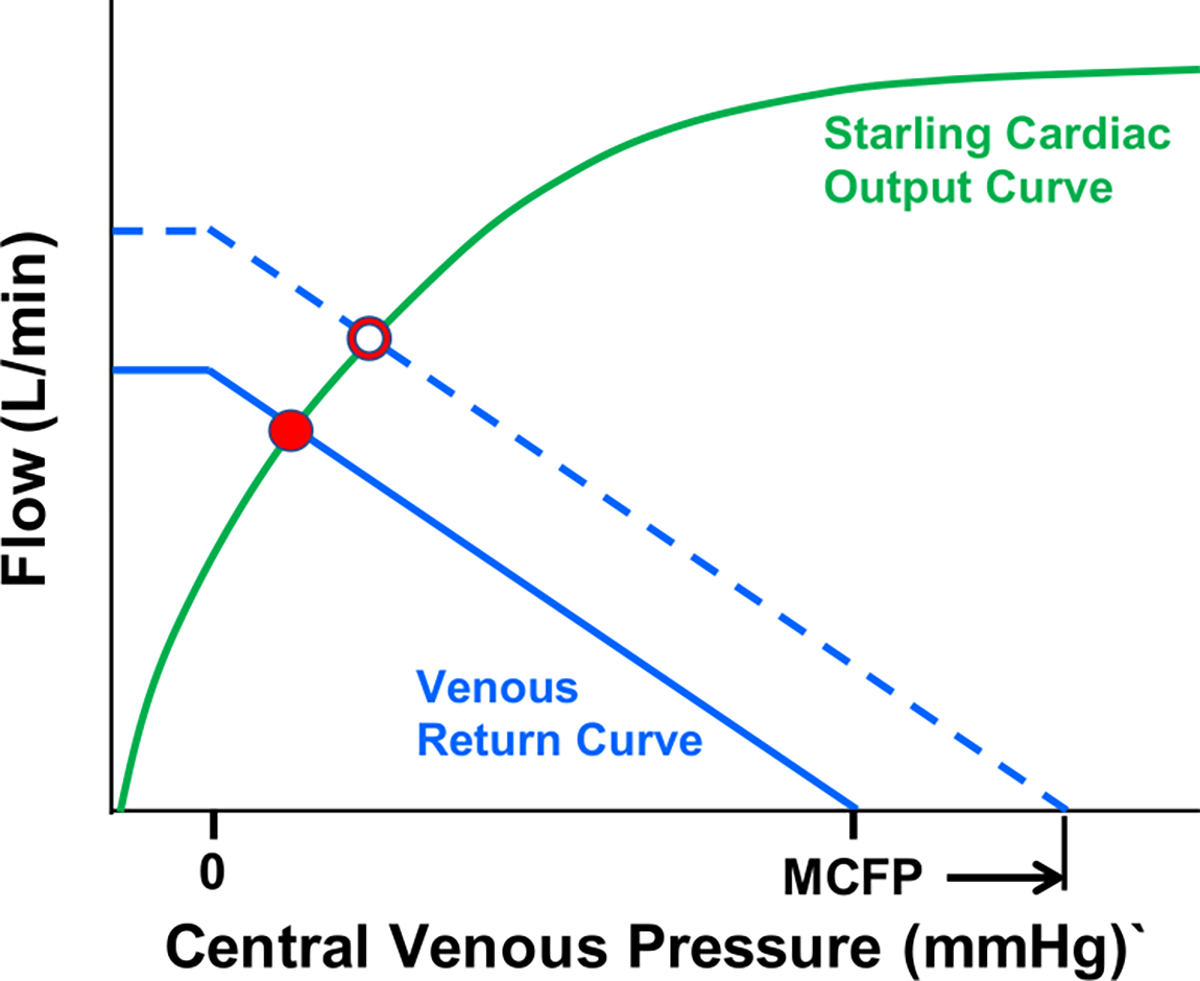
The intersection between the Starling curve and the venous return curve determines the cardiac output (solid red dot). The venous return curve intersects the central venous pressure axis at the mean circulatory filling pressure (MCFP). An increase of MCFP shifts the venous return curve rightward in parallel manner and intersects the Starling-curve at a higher value of cardiac output (open red dot).
Regulation of SBV and MCFP
With fixed vascular tone, MCFP and SBV trend in parallel (Figure 1A/B). For example, as blood is infused (increased TBV) or withdrawn (decreased TBV), SBV and MCFP increase and decrease, respectively, since UBV is constant. Conceptually, this reflects the clinical course for patients with HF: filling pressures rise as fluid accumulates in early stages of an exacerbation and fall with diuresis.
However, regulation of venous tone offers a potent means of modifying SBV in the setting of constant TBV. Experimental studies demonstrate that with sympathetic activation and vasoconstrictive pharmacologic agents, the MCFP-TBV relationship shifts leftwards towards lower volumes with the steep portion of the curve shifting in roughly parallel manner (Figure 3)(11). Thus, increased venous tone decreases UBV with complementary increase of SBV, while compliance (ΔV/ΔP in the typical working range of pressures) remains relatively constant. The example of Figure 3 depicts an ~0.75L shift of the MCFP-TBV relationship, which results in a 75% increase of SBV (from 1.00 to 1.75L) but only an 18.8% reduction of UBV (from 4.00 to 3.25L); this shift resulted in a large, 12.5mmHg increase of MCFP. Thus, relatively small changes in venous tone can achieve large changes of MCFP. In contrast to the effects of increased sympathetic tone, sympathetic blockade and venodilators (e.g., pharmacological sympatholytics or nitrates) shift the MCFP-TBV curve rightward, increasing UBV and decreasing SBV.
Figure 3. Effects of venoconstriction on stressed and unstressed blood volume.
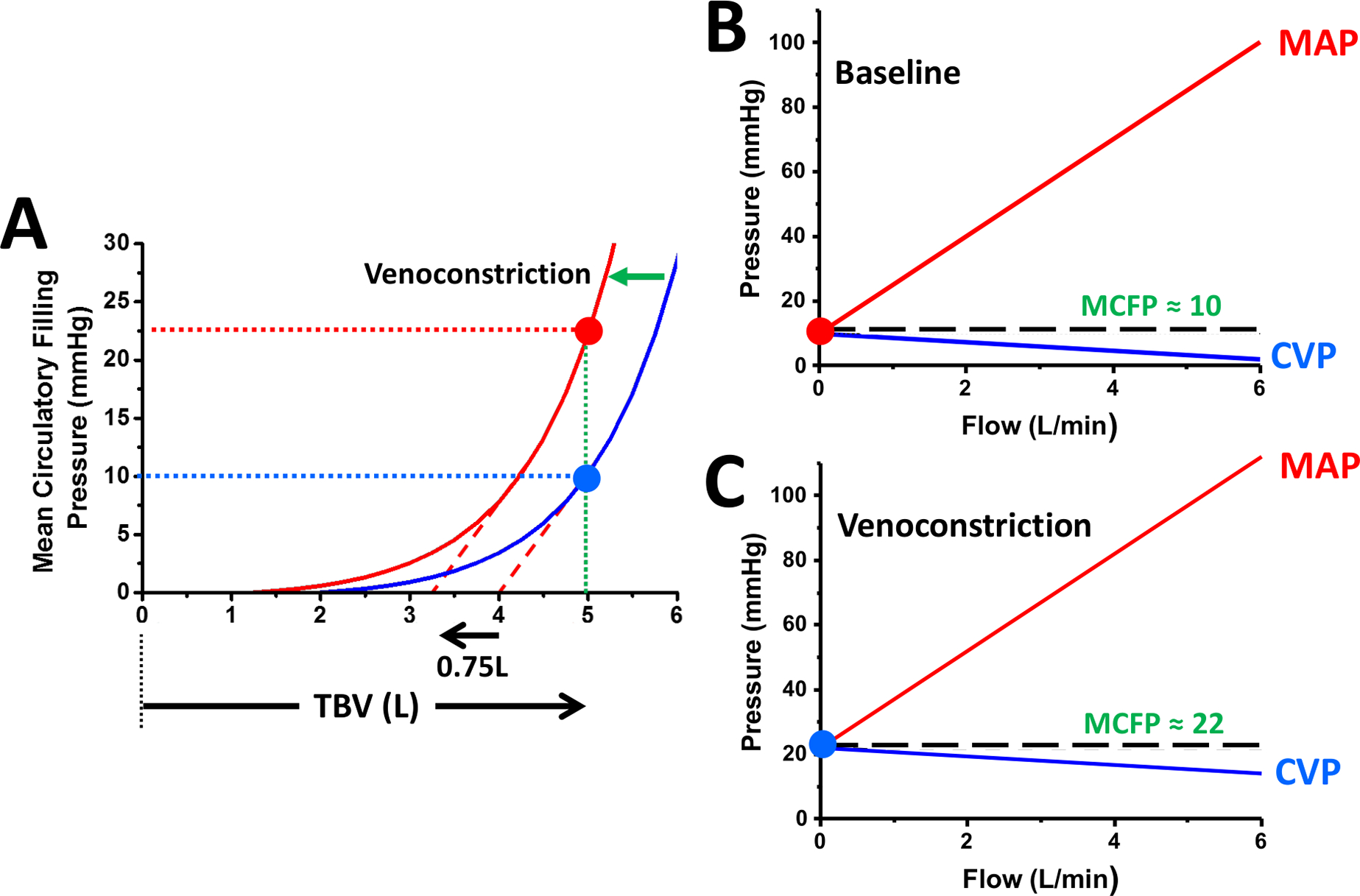
A. Effect of venoconstriction (e.g., via sympathetic hyperactivation) on the relationship between mean circulatory filling pressures (MCFP), blood volume. A 0.75L increase of stressed blood volume (SBV) resulted in an increase of MCFP from 10 to 23mmHg. B. The relationship between cardiac output (CO), mean arterial pressure (MAP) and central venous pressure (CVP). When CO is 0, MAP and CVP equilibrate at the MCFP. As CO is increased, MAP increases and CVP decreases. C. In the vasoconstricted case, despite constant total blood volume (TBV), MCFP is increased and both MAP and CVP are higher at any given CO compared to the baseline state.
In the setting of acute blood loss, the UBV can functionally serve as a reservoir for autotransfusion into the SBV pool. Controlled hemorrhage experiments in humans show that over a large range, every milliliter of blood removed from the body is “replaced” by ½ milliliter of blood by splanchnic venoconstriction (12). Furthermore, up to 10–12% of blood volume loss is tolerated without changes in blood pressure, heart rate or CVP.
Physical exercise is a major cardiovascular stressor that requires augmentation of CO to meet the increased metabolic needs of working muscles. In healthy adults, exercise steepens the slope of the Frank-Starling curve due to increased cardiac contractility, increased heart rate, and decreased systemic vascular resistance. Yet, the modulation of CO with exercise is more strongly determined by cardiac preload augmentation via increased SBV. Upright exercise in humans has been associated with a 23% reduction in splanchnic blood volume (liver 18%, kidney 24%, spleen 46%) and a 30% reduction of leg blood volume, the latter also being mediated by the effects of the muscle pump (4,13). Since leg blood volume is a third of splanchnic blood volume, the majority of recruited blood volume originates from the abdominal compartment (4,13). In comparison, with light supine exercise, the reduction of splanchnic blood volume has been estimated to be ~34% (14). Because the cardiovascular circulation is a closed system, the decrease in venous capacity during exercise increases blood volume in the heart and lungs by ~38% (13).
Catecholamines (endogenous or exogenous) act as the primary mediator of splanchnic vascular tone (15), largely via alpha adrenergic receptors. Endogenous release of norepinephrine makes up more than 40% of the total body norepinephrine spillover (16). Naturally, (patho)physiological increases in catecholamine release will lead to decreased splanchnic vascular capacitance and increases in eSBV such as can occur with acute decompensated heart failure, cardiac dysfunction (e.g., cardiogenic shock), hypoxia (sleep apnea), physical stress (exercise) or emotional stress.
In addition to pharmacologic and physiological stimuli, preclinical and human studies have shown that direct stimulation of the splanchnic nerve increases SBV and increases CVP, PA pressures and CO (17). The changes were shown to be driven by translocation of blood from the splanchnic compartment, which occurred within seconds of stimulation onset and subsided within minutes after the stimulation ended. While these regulatory mechanisms evolved to enhance the odds of survival and ability to exercise, sympathetic modulation of venous tone can contribute unfavorably in the setting of acute and chronic HF.
Studying the venous system in humans
Sympathetic and pharmacologic regulation of vascular compliance and capacity has been investigated extensively for both the systemic (18) and pulmonary circulation (19). Such experiments have involved either: 1) single or dual heart bypass preparations with external blood reservoirs; or 2) radio-labeled blood cell scintigraphy during volume infusion and withdrawal maneuvers. There are few comparable experiments in humans largely because the required methods are highly invasive and/or cumbersome to execute.
Magder and DeVarennes quantified SBV in patients undergoing hypothermic circulatory arrest during cardiac surgery (20). Similar to experiments in animals, cardiac surgery patients are connected to a cardiopulmonary bypass system with a reservoir, and MCFP can be reduced to 0 mmHg. Accordingly, the amount of blood that translocates to the external reservoir at the point when filling pressure reaches 0 mmHg is the SBV. SBV averaged 1290±296mL, or 20.2±1.0mL/kg, which amounted to ~30% of predicted TBV. Similarly, Mass et al. measured MCFP, SBV, and vascular compliance in 15 intubated postoperative patients (21). These patients were subjected to a sequence of breath-hold, volume infusions and withdrawals, and arm stop-flow maneuvers while measuring CO and arterial and venous pressures. They found that SBV was 1265±541mL, an average of 28.5% of predicted TBV and systemic vascular compliance was 0.97±0.49mL·mmHg−1·kg−1, which was similar to values reported in normal animals (4,11).
There are also other less invasive techniques. Venous occlusion plethysmography can index flow and segmental blood volume in arms and legs and assess the effects of vasoactive drugs (22). The application of a tight tourniquet to the proximal portion of a limb interrupts venous outflow but does not obstruct arterial inflow. A venous cannula is used to measure pressure. Plethysmography simultaneously estimates changes in limb volume over time. Plotting measured pressure versus estimated limb volume yields a venous pressure-volume relationship. This technique is limited to the exploration of limb physiology, which may or may not be reflective of the splanchnic bed or lungs.
Radionuclide plethysmography (radio-labeled albumin or red blood cells) images the blood pool of a region of interest. This technique can estimate acute changes of regional blood volume and vascular capacity (23). For example, radionuclide plethysmography has been used to assess splanchnic capacity humans (8,13) with and without cardiovascular disease, at rest and with exercise (13). This technique is time consuming and technically challenging, limited by the use of radioactive material, and the fact that it measures relative (not absolute) volumes in a region of interest. These factors have limited widespread adoption and resulted in the lack of recent investigations into SBV. New approaches are reviewed in greater detail in the Supplemental Figure 1–7.
Evidence for the role of stressed blood volume in heart failure
Increased RV and LV filling pressures (i.e., central and pulmonary venous pressures) at rest, and more so during exercise, are key hallmarks of HF and are believed to be important determinants of exercise intolerance (24). One paradigm explaining elevation in cardiac filling pressures in HF is abnormal LV and RV function leading to increased diastolic pressures, which transmit backwards to the pulmonary and systemic venous circulation. Others relate filling pressure elevation to blood and plasma volume expansion due to renal sodium retention. However, accumulating evidence suggests these paradigms do not fully explain the hemodynamic alterations in HF, as both cardiac dysfunction and blood volume expansion each only partially explain the increase in filling pressures (25) (Supplemental Figure 8). Accumulating evidence indicates that hemodynamic abnormalities in HF are also strongly driven by decreased splanchnic venous capacity and increased SBV which, in turn, is mediated by neurohormonal activation (26,27).
Investigations in animal models of acute and chronic HF confirm a reduction in venous capacity. Acute HF induced by cardiac ischemia in canines reduced splanchnic vascular volume by displacing the splanchnic venous pressure–volume relation to the left without a significant change in compliance (Supplemental Figure 9)(28). Redistribution of splanchnic volume occurred despite an increase in portal venous pressures, mostly due to active vasoconstriction rather than passive mechanisms of volume recruitment in the setting of neurohormonal hyperactivation (29). Baroreflex mediated venoconstriction accounted for about 80% of the increase in LV end-diastolic pressure, while LV dysfunction itself only accounted for 20% (28,30). SBV-increase has also been observed in chronic HF. In multiple animal models of HF, total vascular capacity was reduced, in some cases ~50% (31).
A recent study using these techniques provided new insights into the role of venous capacitance and distribution of blood volume between the SBV and UBV in humans (32). As compared to controls, patients with HFpEF displayed an 11% higher TBV, but more striking 81% higher SBV, with a greater proportion of TBV as SBV (44% vs. 30%, p<0.0001, Figure 4A), suggesting reduced venous capacitance in HFpEF at rest. During exercise, there was a greater absolute increase in SBV in patients with HFpEF compared to controls. Despite higher SBV at rest and with exercise, the ability to augment CO was depressed, indicating that in HFpEF, the heart is operating on the plateau of the Frank-Starling curve, where increases in SBV are not translated to increases in tissue perfusion (Figure 4B). This study identified a greater role for abnormalities in venous capacitance with excess body fat. SBV correlated with the body mass index (Figure 4C), but the increase in SBV could not be fully explained by adipose-associated volume expansion because the ratio of SBV/TBV also increased linearly with increasing body mass. These data identify a new mechanism that may predispose patients with obesity to develop HFpEF, and suggest that patients with the obese phenotype may be better-positioned to respond favorably to therapies targeting SBV (32).
Figure 4. Stressed and unstressed volume in HFpEF, obesity phenotype.
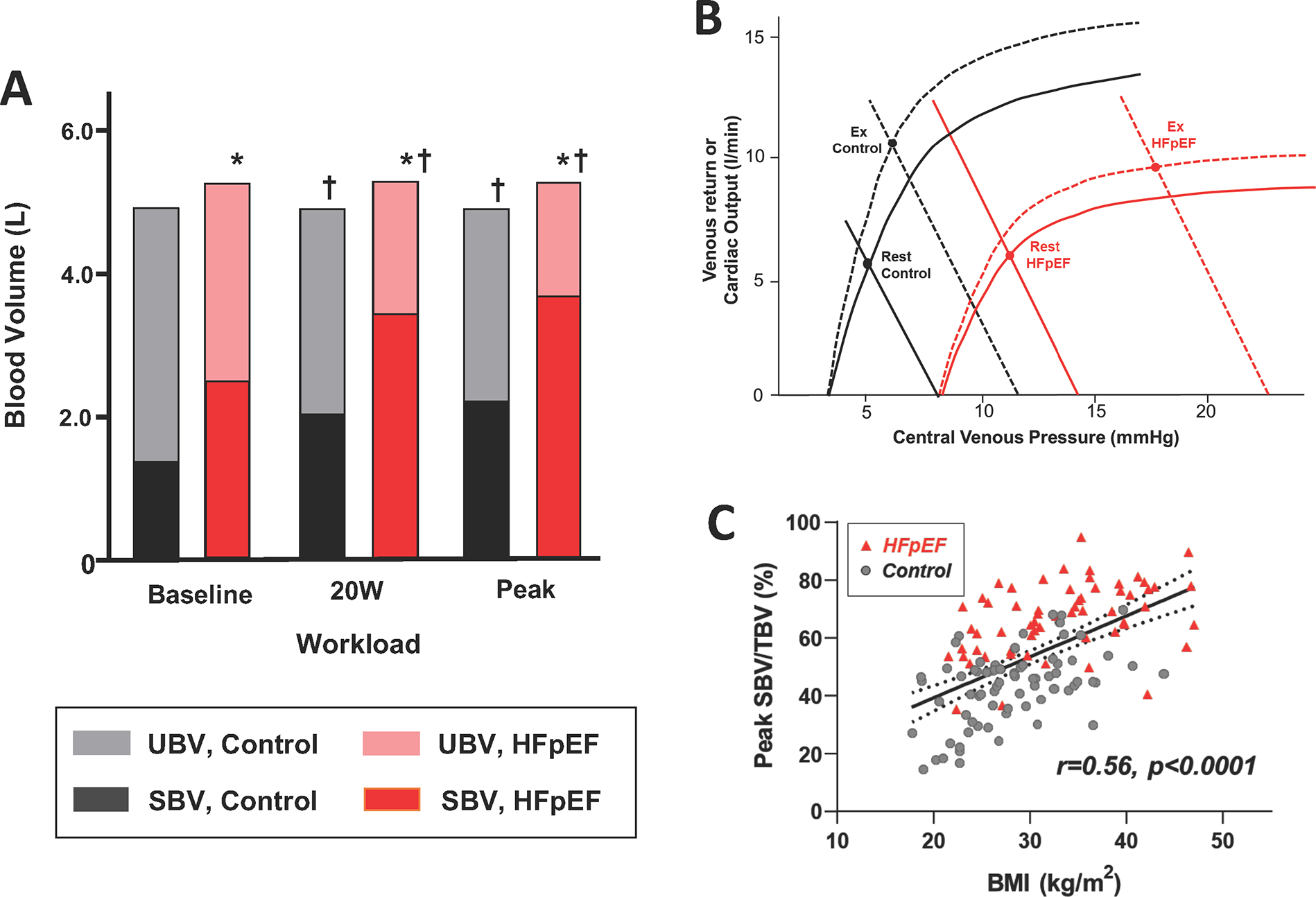
A. Comparison of total blood volume, stressed blood volume (SBV) and unstressed blood volume (UBV) at rest and during exercise in controls and patients with HFpEF. B. Explanation of why, despite a greater increase of SBV during exercise in HFpEF patients, they have a markedly blunted increase of cardiac output. C. Relationship between percent of blood ascribed to SBV as a function of body mass index (BMI). Adapted from (32).
Finally, the role of SBV in cardiogenic shock has also been investigated with focus on the difference between shock due to acute myocardial infarction (AMI-CS) or due to decompensated chronic heart failure (HF-CS)(33). While SBV was similarly elevated in these two subgroups, patients with HF-CS shock who died had a significantly higher SBV than those who survived.
To date there are no data to suggest that venous physiology in patients with HF depends on ejection fractions (EF) or other factors related to specific HF phenotypes. Compared to controls, we have seen similar increases in eSBV in patients with HFrEF, HFpEF and PH-HFpEF (Supplemental Figure 8). More specifically, eSBV is greater in HF than in controls and increases by a greater amount during exercise than in controls. Interestingly, the percent of TBV residing in the stressed component is greater in HF than in normals, and this does not seem to depend on the EF phenotype.
In contrast, while eSBV increases with TBV in obesity, eSBV expressed as a percent of TBV also increases with the body mass index (BMI), indicating fundamental difference related to excess body fat (32). However, obesity appears to have a similar impact on venous physiology and volume distribution in people with and without HF. Patients with greater abnormalities in RV-PA coupling display higher eSBV in cross sectional analysis, but the direction of causality remains unclear (32). Whether the influence of venous tone modulation on hemodynamics or clinical outcomes will depend on EF or other factors (e.g., RV dysfunction with or without pulmonary hypertension) is unknown at this time. However, as with lack of differential adverse events with pharmacologic vasodilator therapy, early experience with splanchnic nerve denervation has not uncovered any such concerns in patients with common forms of HFpEF (34). These considerations would be of more concern for patients with infiltrative cardiac diseases, such as amyloid, with severe diastolic dysfunction characterized by small RV and/or LV chambers and leftward-shifted end-diastolic pressure-volume relationships (35).
Stressed blood volume as a target in heart failure
Despite a strong evidence base and proposed importance of SBV several decades ago (36), the role of venous tone and SBV as a potential target for therapeutics has remained speculative due to lack of evidence until very recently. We now review different therapies whose action appear, in part, to be mediated by venodilation and reductions of SBV.
Guideline directed medical therapy (GDMT)
Several pharmacologic agents which are components of GDMT for HFrEF such as sympatholytics (alpha/beta-blockers), renin-angiotensin-aldosterone system blockers (i.e., angiotensin-converting enzyme inhibitors, aldosterone inhibitors, etc.) and sodium-glucose linked transporter 2 inhibitors (SGLT2i) have proven benefits on neurohormonal balance. Further, there is evidence these drugs reduce (e)SBV in HF (28,37). To what degree SBV changes contribute to the observed clinical benefits with these drugs is unclear.
NO donors
As noted above, nitroglycerine and NO donors with preferential effects on venous capacity are well known to reduced cardiac filling pressures. As shown in the preclinical study depicted in Supplemental Figure 9A, this is at least partially mediated by venodilation. Indeed, drugs like nitroglycerin (primary venodilator) and nitroprusside (mixed arterial-venodilator) are commonly used to treat acute decompensated HF. Among Black patients with NYHA-class III/IV HFrEF, treatment with fixed dose isosorbide dinitrate plus hydralazine was associated with reductions in all-cause death and time-to-first HF hospitalization, with improvement in quality-of-life scores (38). A different nitrate, isosorbide mononitrate, failed to improve activity levels or 6-minute walk distance in HFpEF (39). The reasons for the neutral response are not clear but may relate to excessive and tonic venodilation in a patient population characterized by labile blood pressure swings due to ventricular-vascular stiffening (2). Inorganic nitrite provides more targeted NO provision with less effect at rest because conversion from nitrite is facilitated during conditions of exercise including venous hypoxia and acidosis. In this light, a number of placebo-controlled studies have revealed marked reductions in right and left heart filling pressures with nitrite during exercise, consistent with reductions in SBV (40). While one trial did not demonstrate an improvement in exercise capacity with nitrite (41), this may relate to the inhaled drug delivery route, and multiple trials are ongoing.
Splanchnic Nerve Modulation
The splanchnic sympathetic nerves innervate the highly vascularized organs of the abdomen. The concept of splanchnic nerve blockade emerged following the observation that significant hemodynamic effects of splanchnic nerve stimulation in healthy animals and humans (17) and the observation in HF of acute or chronic SBV increase resulting in acute rises in cardiac filling pressures (42). Temporary splanchnic nerve modulation in patients hospitalized for decompensated HF or chronic ambulatory HF resulted in a reduction in right and left sided filling pressures (43–46). A reduction in resting and exercise induced pressure elevations was primarily explained by a reduction in SBV following splanchnic nerve blockade (Figure 5A)(44). Similar effects on filling pressures have recently been reported with surgical ablation of the splanchnic nerve (47); percutaneous approaches to achieving the same effects are currently under investigation (48).
Figure 5. Impact of therapeutic interventions on estimated stressed blood volume.
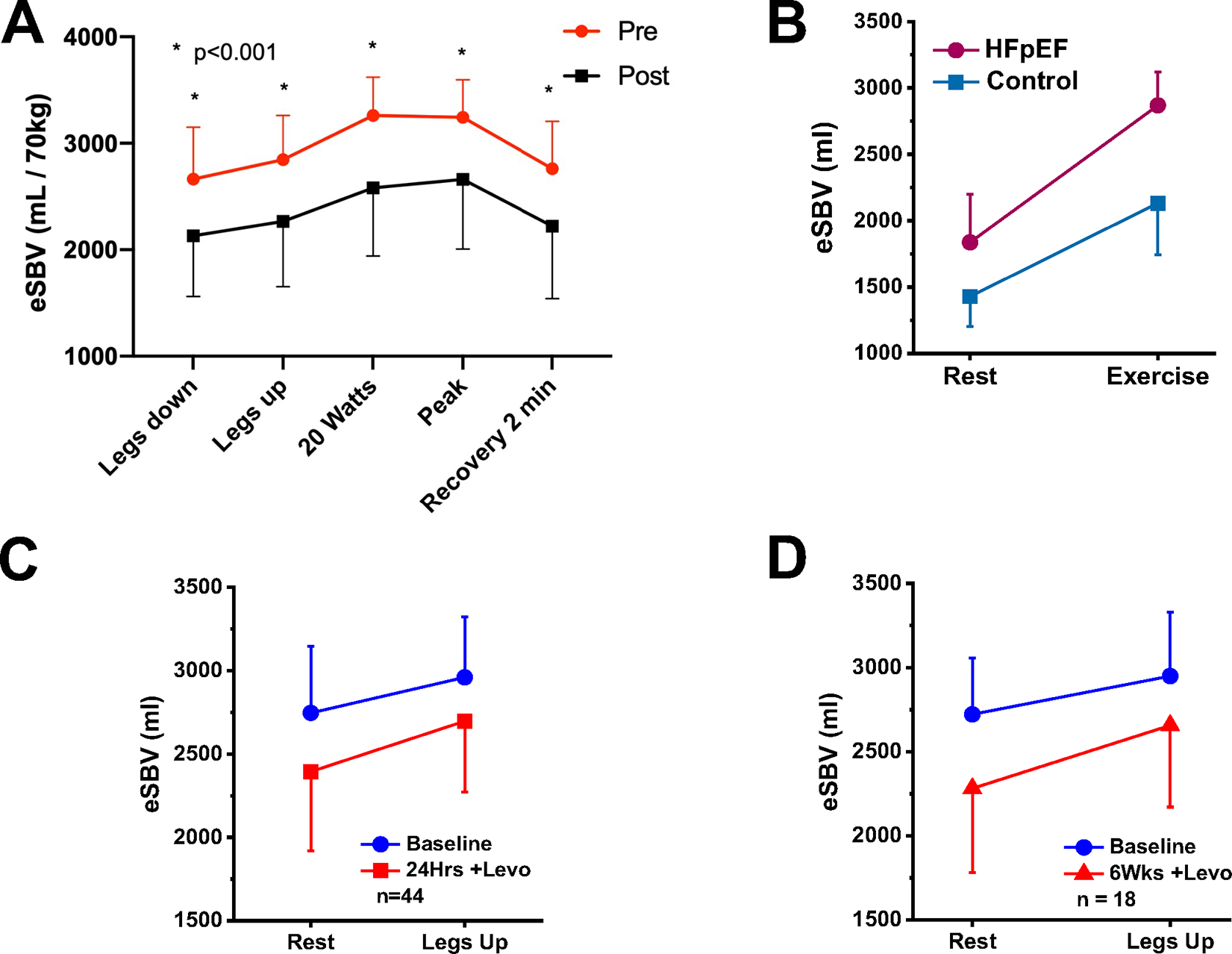
A: Estimated stressed blood volume (eSBV) and effects of splanchnic nerve blockade in ambulatory heart failure. *indicates an adjusted p<0.001 for a pairwise comparison with the pre-SNB value. Abbreviations: HF-heart failure, eSBV-estimated stressed blood volume, SNB-splanchnic nerve block. B: Impact of acute intravenous milrinone on estimated exercise hemodynamics in patients with heart failure and preserved ejection fraction (HFpEF). C. Effect of a 24 hours levosimendan infusion on eSBV at rest and with leg elevation. D. Effect of weekly levosimendan infusion on eSBV at rest and with leg elevation at 6 weeks.
Milrinone
A recent study explored the impact of acute intravenous milrinone on exercise hemodynamics in patients with HFpEF (49). The original hypothesis for choosing milrinone was that its pulmonary and systemic arterial vasodilator effects and inotropic effects (on the right ventricles) would act to reduce CVP and PCWP while permitting greater exercise-induced increases of cardiac output. Indeed, milrinone was shown to increase heart rate, LV contractility and to reduce SVR. Unexpectedly, however, analysis showed that estimated SBV was also decreased by milrinone (Figure 5B). Furthermore, while the changes of heart rate, contractility and SVR were the main contributors to increased exercise-induced CO, the reduction of SBV appeared most responsible for reductions of CVP and PCWP (50). Venodilatory effects of milrinone may be mediated by activating ATP-dependent potassium channels of venous smooth muscles causing muscle relaxation (51). Venodilatory effects of increased cAMP and subsequent PKA activation by milrinone should also be considered.
Levosimendan
Similar to the study of milrinone described above, levosimendan was studied in view of its pulmonary and systemic arterial vasodilator effects and inotropic effects (on left and right ventricles) in patients with pulmonary hypertension associated with HFpEF(52). In contrast to milrinone which showed effects on multiple parameters, a 24-hour levosimendan infusion decreased resting and exercise CVP and PCWP in the absence of evidence of pulmonary or arterial dilatatory effects and without increase in cardiac output (Figure 5C). Similar results were obtained following 6, weekly levosimendan infusion which, by echocardiography, also showed no detectible improvement of LV or RV contractility. Rather, analysis showed that, in comparison to placebo, the hemodynamic effects at rest and in response to passive leg raise were due to a reduction in estimated SBV (Figure 5D)(53). Though not widely appreciated, levosimendan has venodilatory effects that are mediated through activation of ATP-dependent potassium channels (54).
Summary and Conclusions
The role of venous system tone and its regulation of SBV in HF has been largely ignored and, until recently, has not been exploited as a therapeutic target. While direct and repeated measurements of SBV and venous tone are difficult in the clinical setting, especially during exercise or in response to therapies, analytical approaches have recently been developed and indirectly validated for this purpose. These methods have provided new insights into the pathophysiology of HF. Additionally, several recent studies have suggested a primary role of venodilation and reductions of SBV in explaining reductions of CVP and PCWP. All told, the evidence suggests that venodilation may be a viable target for HF for reducing resting and exercise cardiac filling pressures. It will be important to determine whether hemodynamic effects will translate into improvements of symptoms, exercise tolerance and mortality. Use of existing techniques and development of novel ways to investigate the venous system are needed. Future studies should consider the impact of the impact of drug- and device-based therapies on the venous system and the potential impact on SBV and UBV.
Supplementary Material
Highlights:
Venous tone regulates the distribution of blood volume between stressed and unstressed compartments.
This distribution in turn influences pressures in the pulmonary and systemic circulations, contributing to hemodynamic abnormalities in heart failure
Venodilation and reduction of stressed blood volume underlie the therapeutic effects of many of the drugs and devices used for management of heart failure.
Disclosures:
Fudim was supported by NHLBI K23HL151744 from the National Heart, Lung, and Blood Institute (NHLBI), the American Heart Association grant No 20IPA35310955, Mario Family Award, Duke Chair’s Award, Translating Duke Health Award, Bayer and BTG Specialty Pharmaceuticals. He receives consulting fees from AxonTherapies, Bodyport, CVRx, Daxor, Edwards LifeSciences, NXT Biomedical, Zoll, Viscardia. Borlaug: Research funding from NIH/NHLBI (R01 HL128526, U01 HL125205); AstraZeneca; Corvia; Medtronic; Mesoblast; GlaxoSmithKline; TENAX; Advisory board/consulting for: Merck, Novartis, Lilly, Novo Nordisk.
Shah: consults for AxonTherapies. Burkhoff: Institutional grants from Abiomed, Ancora and Fire 1; consulting fees from PVLoops LLC and Axon Therapeutics. All other authors report no relevant disclosures.
Abbreviations:
- HFrEF
heart failure with reduced ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- CVP
Central venous pressure
- MCFP
Mean circulatory filling pressure
- MPFP
Mean pulmonary filling pressure
- MSFP
Mean systemic filling pressure
- SBV
Stressed blood volume
- PCWP
pulmonary capillary wedge pressure
- TBV
Total blood volume
- UBV
Unstressed blood volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2014;11:507–15. [DOI] [PubMed] [Google Scholar]
- 2.Reddy YNV, Andersen MJ, Obokata M et al. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fudim M, Sobotka PA, Dunlap ME. Extracardiac Abnormalities of Preload Reserve: Mechanisms Underlying Exercise Limitation in Heart Failure with Preserved Ejection Fraction, Autonomic Dysfunction, and Liver Disease. Circ Heart Fail 2021;14:e007308. [DOI] [PubMed] [Google Scholar]
- 4.Rothe CF. Reflex control of veins and vascular capacitance. Physiol Rev 1983;63:1281–342. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd JT, Vanhoutte PM. Role of the venous system in circulatory control. Mayo Clin Proc 1978;53:247–55. [PubMed] [Google Scholar]
- 6.Smiseth OA, Mjos OD. A reproducible and stable model of acute ischaemic left ventricular failure in dogs. Clin Physiol 1982;2:225–239. [DOI] [PubMed] [Google Scholar]
- 7.Birch DJ, Turmaine M, Boulos PB, Burnstock G. Sympathetic innervation of human mesenteric artery and vein. J Vasc Res 2008;45:323–32. [DOI] [PubMed] [Google Scholar]
- 8.Manyari DE, Wang Z, Cohen J, Tyberg JV. Assessment of the human splanchnic venous volume-pressure relation using radionuclide plethysmography. Effect of nitroglycerin. Circulation 1993;87:1142–51. [DOI] [PubMed] [Google Scholar]
- 9.Haneda K, Horiuchi T. A method for measurement of total circulating blood volume using indocyanine green. Tohoku J Exp Med 1986;148:49–56. [DOI] [PubMed] [Google Scholar]
- 10.Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation 1977;56:605–612. [DOI] [PubMed] [Google Scholar]
- 11.Rothe CF. Physiology of venous return. An unappreciated boost to the heart. Arch Intern Med 1986;146:977–82. [PubMed] [Google Scholar]
- 12.Price HL, Deutsch S, Marshall BE, Stephen GW, Behar MG, Neufeld GR. Hemodynamic and metabolic effects of hemorrhage in man, with particular reference to the splanchnic circulation. Circ Res 1966;18:469–74. [DOI] [PubMed] [Google Scholar]
- 13.Flamm SD, Taki J, Moore R et al. Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation 1990;81:1550–9. [DOI] [PubMed] [Google Scholar]
- 14.Bradley SE, Childs AW, Combes B, Cournand A, Wade OL, Wheeler HO. The effect of exercise on the splanchnic blood flow and splanchnic blood volume in normal man. Clin Sci 1956;15:457–63. [PubMed] [Google Scholar]
- 15.Gelman S, Mushlin Phillip S, Weiskopf Richard B. Catecholamine-induced Changes in the Splanchnic Circulation Affecting Systemic Hemodynamics. Anesthesiology 2004;100:434–439. [DOI] [PubMed] [Google Scholar]
- 16.Aneman A, Eisenhofer G, Olbe L et al. Sympathetic discharge to mesenteric organs and the liver. Evidence for substantial mesenteric organ norepinephrine spillover. J Clin Invest 1996;97:1640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fudim M, Yalamuri S, Herbert JT, Liu PR, Patel MR, Sandler A. Raising the pressure: Hemodynamic effects of splanchnic nerve stimulation. Journal of applied physiology (Bethesda, Md : 1985) 2017;123:126–127. [DOI] [PubMed] [Google Scholar]
- 18.Shoukas AA, Brunner MC. Epinephrine and the carotid sinus baroreceptor reflex. Influence on capacitive and resistive properties of the total systemic vascular bed of the dog. Circ Res 1980;47:249–57. [DOI] [PubMed] [Google Scholar]
- 19.Shoukas AA. Pressure-flow and pressure-volume relations in the entire pulmonary vascular bed of the dog determined by two-port analysis. Circ Res 1975;37:809–18. [DOI] [PubMed] [Google Scholar]
- 20.Magder S, De Varennes B. Clinical death and the measurement of stressed vascular volume. Crit Care Med 1998;26:1061–4. [DOI] [PubMed] [Google Scholar]
- 21.Maas JJ, Pinsky MR, Aarts LP, Jansen JR. Bedside assessment of total systemic vascular compliance, stressed volume, and cardiac function curves in intensive care unit patients. Anesth Analg 2012;115:880–7. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol 2001;52:631–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt M, Blackman DJ, Middleton GW, Cockcroft JR, Frenneaux MP. Assessment of venous capacitance. Radionuclide plethysmography: methodology and research applications. Br J Clin Pharmacol 2002;54:565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy YNV, Obokata M, Wiley B et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller WL, Sorimachi H, Grill DE, Fischer K, Borlaug BA. Contributions of cardiac dysfunction and volume status to central haemodynamics in chronic heart failure. Eur J Heart Fail 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fudim M, Hernandez AF, Felker GM. Role of Volume Redistribution in the Congestion of Heart Failure. Journal of the American Heart Association 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaye D, Sunagawa K, Burkhoff D. Comprehensive physiological modelling provides novel insights into HFpEF physiology. JAHA 2021;In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang SY, Manyari DE, Scott-Douglas N, Smiseth OA, Smith ER, Tyberg JV. Splanchnic venous pressure-volume relation during experimental acute ischemic heart failure. Differential effects of hydralazine, enalaprilat, and nitroglycerin. Circulation 1995;91:1205–12. [DOI] [PubMed] [Google Scholar]
- 29.Rutlen DL, Welt FG, Ilebekk A. Passive effect of reduced cardiac function on splanchnic intravascular volume. Am J Physiol 1992;262:H1361–4. [DOI] [PubMed] [Google Scholar]
- 30.Wang SY, Manyari DE, Tyberg JV. Cardiac vagal reflex modulates intestinal vascular capacitance and ventricular preload in anesthetized dogs with acute myocardial infarction. Circulation 1996;94:529–33. [DOI] [PubMed] [Google Scholar]
- 31.Ogilvie RI, Zborowska-Sluis D. Effect of chronic rapid ventricular pacing on total vascular capacitance. Circulation 1992;85:1524–30. [DOI] [PubMed] [Google Scholar]
- 32.Sorimachi H, Burkhoff D, Verbrugge FH et al. Obesity, Venous Capacitance, and Venous Compliance in Heart Failure with Preserved Ejection Fraction. Eur J Heart Fail 2021. [DOI] [PubMed] [Google Scholar]
- 33.Whitehead EH, Thayer KL, Sunagawa K et al. Estimation of stressed blood volume in patients with cardiogenic shock from acute myocardial infarction and decompensated heart failure. J Card Fail 2021;in press. [DOI] [PubMed] [Google Scholar]
- 34.Shah S ZT, Shaburishvili N, Shaishmelashvili G, Sievert H, Sievert K, Engelman Z, Burkhoff D, Shaburishvili T. Durability Of Right Greater Splanchnic Nerve Ablation For The Treatment Of Heart Failure with Preserved Ejection Fraction in an Open-label First-in-human Clinical Trial. HFSA 2021 2021. [Google Scholar]
- 35.Rosenblum H, Burkhoff D, Maurer MS. Untangling the physiology of transthyretin cardiac amyloidosis by leveraging echocardiographically derived pressure-volume indices. Eur Heart J 2020;41:1448–1450. [DOI] [PubMed] [Google Scholar]
- 36.Burkhoff D, Tyberg JV. Why does pulmonary venous pressure rise after onset of LV dysfunction: a theoretical analysis. Am J Physiol 1993;265:H1819–28. [DOI] [PubMed] [Google Scholar]
- 37.Omar M, Jensen J, Burkhoff D et al. Effect of Empagliflozin on Blood Volume Redistribution in Patients With Chronic Heart Failure and Reduced Ejection Fraction: An Analysis from the Empire HF Randomized Clinical Trial. Circ Heart Fail 2021. [DOI] [PubMed] [Google Scholar]
- 38.Taylor AL, Ziesche S, Yancy C et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004;351:2049–57. [DOI] [PubMed] [Google Scholar]
- 39.Redfield MM, Anstrom KJ, Levine JA et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med 2015;373:2314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borlaug BA, Koepp KE, Melenovsky V. Sodium Nitrite Improves Exercise Hemodynamics and Ventricular Performance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2015;66:1672–82. [DOI] [PubMed] [Google Scholar]
- 41.Borlaug BA, Anstrom KJ, Lewis GD et al. Effect of Inorganic Nitrite vs Placebo on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: The INDIE-HFpEF Randomized Clinical Trial. JAMA 2018;320:1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fudim M, Neuzil P, Malek F, Engelman ZJ, Reddy VY. Greater Splanchnic Nerve Stimulation in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2021;77:1952–1953. [DOI] [PubMed] [Google Scholar]
- 43.Fudim M, Boortz-Marx RL, Ganesh A et al. Splanchnic Nerve Block for Chronic Heart Failure. JACC Heart failure 2020;8:742–752. [DOI] [PubMed] [Google Scholar]
- 44.Fudim M, Patel MR, Boortz-Marx R et al. Splanchnic Nerve Block Mediated Changes in Stressed Blood Volume in Heart Failure. JACC Heart Fail 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fudim M, Ganesh A, Green C et al. Splanchnic nerve block for decompensated chronic heart failure: splanchnic-HF. Eur Heart J 2018;39:4255–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fudim M, Jones WS, Boortz-Marx RL et al. Splanchnic Nerve Block for Acute Heart Failure. Circulation 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malek F, Gajewski P, Zymlinski R et al. Surgical ablation of the right greater splanchnic nerve for the treatment of heart failure with preserved ejection fraction: First-in-human clinical trial. European journal of heart failure 2021. [DOI] [PubMed] [Google Scholar]
- 48.Fudim M, Ponikowski P, Burkhoff D et al. Splanchnic Nerve Modulation in Heart Failure: Mechanistic Overview, Initial Clinical Experience, and Safety Considerations. Eur J Heart Fail 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaye DM, Nanayakkara S, Vizi D, Byrne M, Mariani JA. Effects of Milrinone on Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2016;67:2554–6. [DOI] [PubMed] [Google Scholar]
- 50.Kaye DM, Byrne M, Mariani J, Nanayakkara S, Burkhoff D. Identification of physiologic treatment targets with favourable haemodynamic consequences in heart failure with preserved ejection fraction. ESC Heart Fail 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rieg AD, Suleiman S, Perez-Bouza A et al. Milrinone relaxes pulmonary veins in guinea pigs and humans. PLoS One 2014;9:e87685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burkhoff D, Borlaug BA, Shah SJ et al. Levosimendan Improves Hemodynamics and Exercise Tolerance in PH-HFpEF: Results of the Randomized Placebo-Controlled HELP Trial. JACC Heart Fail 2021;9:360–370. [DOI] [PubMed] [Google Scholar]
- 53.Brener MI, Hamid NB, Sunagawa K et al. Changes in Stressed Blood Volume with Levosimendan in Pulmonary Hypertension from Heart Failure with Preserved Ejection Fraction: Insights Regarding Mechanism of Action From the HELP Trial. J Card Fail 2021;27:1023–1026. [DOI] [PubMed] [Google Scholar]
- 54.Hohn J, Pataricza J, Petri A et al. Levosimendan interacts with potassium channel blockers in human saphenous veins. Basic Clin Pharmacol Toxicol 2004;94:271–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


