Abstract
Objectives
To describe the prevalence of colistin heteroresistance in carbapenem-resistant Pseudomonas aeruginosa (CRPA) and evaluate the association with clinical outcomes.
Methods
Colistin heteroresistance was evaluated in CRPA isolates collected from patients without cystic fibrosis in Atlanta, Georgia, USA using two definitions: HR1, growth at 4 and 8 mg/L of colistin at a frequency ≥1 × 10−6 the main population; and HR2, growth at a colistin concentration ≥8× the MIC of the main population at a frequency ≥1 × 10−7. A modified population analysis profile (mPAP) technique was compared with reference PAP for detecting heteroresistance. For adults hospitalized at the time of or within 1 week of CRPA culture, multivariable logistic regression estimated the association between heteroresistance and 90 day mortality.
Results
Of 143 colistin-susceptible CRPA isolates, 8 (6%) met the HR1 definition and 37 (26%) met the HR2 definition. Compared with the reference PAP, mPAP had a sensitivity and specificity of 50% and 100% for HR1 and 32% and 99% for HR2. Of 82 hospitalized patients, 45 (56%) were male and the median age was 63 years (IQR 49–73). Heteroresistance was not associated with 90 day mortality using HR1 (0% in heteroresistant versus 22% in non-heteroresistant group; P = 0.6) or HR2 (12% in heteroresistant versus 24% in non-heteroresistant group; P = 0.4; adjusted OR 0.8; 95% CI 0.2–3.4).
Conclusions
Colistin heteroresistance was identified in up to 26% of patients with CRPA in our sample, although the prevalence varied depending on the definition. We did not observe an apparent association between colistin heteroresistance and 90 day mortality.
Introduction
Carbapenem-resistant Pseudomonas aeruginosa (CRPA) has been categorized by the WHO as one of the most concerning MDR organisms.1 In the USA, CRPA is the most common carbapenem-resistant Gram-negative pathogen in hospitalized patients.2 Patients infected with CRPA have prolonged hospitalizations and in-hospital mortality rates can be ≥50%.3,4 Antibiotic treatment options for CRPA are limited, and polymyxin-based regimens (colistin or polymyxin B) may be necessary for XDR isolates despite the potential for toxicity.5–9
Antibiotic heteroresistance may be important for antibiotic selection in patients with limited treatment options. Heteroresistance is broadly defined as the presence of a subpopulation of bacteria that has an increased level of resistance compared with the main population of bacteria.10 The resistant subpopulation is often not detected in clinical laboratories nor reflected in the MIC that clinicians use to guide treatment decisions.11,12 The resistant subpopulation can be selected during antibiotic exposure leading to concern that this could cause treatment failure.10 Band et al.13,14 showed that colistin heteroresistance can cause clinical treatment failure in mice infected with Enterobacterales, but the significance of heteroresistance in Gram-negative bacteria on patient outcomes remains uncertain.10,15
Understanding the clinical significance of heteroresistance is complex as there is not a uniformly agreed-upon definition of heteroresistance. In a recent review, Andersson et al.10 proposed that heteroresistance be defined as a subpopulation of resistant cells that grows at an antibiotic concentration ≥8× the MIC of the main clinically susceptible population with a frequency of ≥1 × 10−7 the main population. Band et al.,16 however, defined heteroresistance as a resistant subpopulation of bacteria that grows at an antibiotic concentration ≥2× the clinical breakpoint (defined by CLSI or EUCAST) with a frequency of ≥1 × 10−6 the main population. Unfortunately, many studies evaluating polymyxin heteroresistance in P. aeruginosa have not followed either of these definitions.10,17–20 Population analysis profile (PAP) is considered the gold standard to detect heteroresistance; however, it is expensive and time-consuming.10 Other methods to detect and classify heteroresistance are not standardized.
Using CRPA isolates collected through surveillance in the USA, this study aimed to: (1) estimate the prevalence of colistin heteroresistance using the above two different definitions; (2) compare methods of detecting colistin heteroresistance; and (3) evaluate the association between colistin heteroresistance and 90 day mortality.
Materials and methods
Isolate collection and analysis
In this observational study, we analysed data from the CDC-funded Georgia Emerging Infections Program’s (EIP) Multi-site Gram-Negative Surveillance Initiative (MuGSI). Georgia EIP performed active population and laboratory-based surveillance for CRPA (MIC ≥8 mg/L for doripenem, imipenem or meropenem) isolated from sterile sites, urine, respiratory tracts and wounds in metropolitan Atlanta, Georgia, USA (population ∼4.1 million) from 1 August 2016 to 31 July 2018. Cases were identified by routine queries of laboratory automated testing instruments in the catchment area and, where possible, a convenience sample of isolates was collected from local laboratories. We analysed all available incident CRPA isolates linked to adults without cystic fibrosis (CF). An incident case was the first CRPA isolate during a 30 day period. Colistin susceptibility was determined by reference broth microdilution according to CLSI 2020 guidelines.21 All additional antibiotic susceptibility was determined by local clinical laboratory testing. All colistin-susceptible isolates (MIC ≤ 2 mg/L) underwent testing for heteroresistance.
Heteroresistance assays
Cells from frozen stocks were streaked onto Mueller–Hinton agar (MHA) II (Becton Dickinson and Co., Sparks, MD, USA) plates and grown overnight at 37°C. We used a starting inoculum of ∼1 × 109 cells (OD600 = 1.0). For reference PAP testing, 100 μL of serial dilutions were spiral plated on MHA II plates containing increasing concentrations of colistin (0, 4, 8 and 16 mg/L). For the modified PAP (mPAP), 10 μL drops of serial dilutions were used on MHA II plates containing the same colistin concentrations. Colonies were counted after 48 h at 37°C, and the frequency of any resistant subpopulation was determined by dividing the number of colonies growing on the highest concentration of colistin by the number of colonies growing on antibiotic-free plates. Two biological replicates were tested by each method, and both replicates had to meet the heteroresistance definition to be considered heteroresistant.
Heteroresistance definitions
We compared two definitions of heteroresistance. The first definition (HR1) was proposed by Band et al.16 and defined as growth of a resistant subpopulation of cells at 4 and 8 mg/L of colistin (1× and 2× the EUCAST susceptibility breakpoint) at a frequency of ≥1 × 10−6 (0.0001%) the main population grown on antibiotic-free media. The second definition (HR2) was proposed by Andersson et al.10 and defined as growth of a resistant subpopulation of cells at a colistin concentration ≥8× the MIC of the main population, at a frequency of ≥1 × 10−7 (0.00001%).
Data sources for clinical characteristics and outcomes
Information on patient demographics (age, sex and race) and clinical characteristics including Charlson comorbidity index (CCI), residence 3 days prior to culture, admission to the ICU in the week prior, length of stay, culture source and colistin use were obtained through medical record review. Data on all-cause mortality were obtained through the Georgia Vital Statistics program.
Statistical analysis
We used proportions to describe the prevalence of heteroresistance and to calculate the sensitivity and specificity of mPAP compared with the reference PAP technique.
For the clinical outcome analysis, we only included incident CRPA cases from adults who had complete medical record review and were hospitalized at the time of, or within 1 week of, CRPA culture. Only the first case meeting inclusion criteria for each patient were analysed. We compared clinical characteristics and outcomes of patients stratified by the presence of a heteroresistant isolate using chi-squared and Fisher’s exact tests for categorical variables and Mann–Whitney U-tests for continuous variables. Univariable and multivariable logistic regression estimated the association between heteroresistance (exposure) and 90 day mortality (outcome). In the multivariable model, we initially included all covariates that a priori were considered clinically relevant confounders: residence prior to culture; CCI; admission to the ICU in the last week; and lower respiratory culture source. We removed residence prior to culture from the final model as there were zero deaths in one category, which prevented model convergence. All analyses were performed independently, once using the HR1 definition and then again using the HR2 definition as the main exposure. Multivariable modelling could not be performed using the HR1 definition as no participants died by 90 days in this group. A complete case analysis was performed without imputation. We also performed two sensitivity analyses: one where we changed the outcome to 30 day mortality; and a second where we analysed all unique patients, not just those hospitalized. All analyses were performed in SAS version 9.4.
Ethics
The Emory University Institutional Review Board approved this study, granting a waiver of informed consent.
Results
Prevalence of heteroresistance using reference PAP
Of the 1508 incident CRPA cases from patients without CF, 147 isolates were available for analysis. Four (3%) were resistant to colistin and not further analysed. Of the 143 colistin-susceptible isolates, the majority (n = 95; 66%) had a colistin MIC of 1 mg/L. Fourteen (10%) were isolated from a normally sterile site, 55 (39%) from the lower respiratory tract, 50 (35%) from urine and 24 (17%) from wounds (Table S1, available as Supplementary data at JAC Online). One hundred and nine (76%) were non-susceptible to ≥3 antibiotic classes (75% of the isolates meeting the HR1 definition of heteroresistance and 84% of isolates meeting the HR2 definition were non-susceptible to ≥3 antibiotic classes) (Table S2 and S3). Using the PAP technique, 8 (6%) of the 143 isolates met the HR1 definition and an additional 29 isolates (n = 37; 26%) met the HR2 definition of heteroresistance. Of the 29 isolates that met the HR2 but not the HR1 definition, 24 would have met HR1 if growth was required at 1× and 2× the clinical breakpoint (component of HR1 definition) but the frequency requirement was ≥1 × 10−7 (component of HR2 definition). Only 1 of the 29 isolates would have met HR1 if growth had to occur at 8× the main population MIC (component of HR2 definition), but the frequency requirement was ≥1 × 10−6 (component of HR1 definition).
Comparison of methods to detect heteroresistance
With the mPAP technique using 10 μL drops, 4 (2.8%) isolates met HR1 and 13 (9%) met HR2. Compared with the reference PAP, mPAP had a specificity and sensitivity of 100% and 50%, respectively, for HR1, and 99% and 32%, respectively, for HR2 (Table 1). Additionally, we often observed formation of ‘rings’ above the susceptible breakpoint when using the mPAP technique with a high concentration of cells (∼1 × 109) (Figure 1). These ring formations may be explained by a phenomenon known as the ‘coffee ring’ effect, where particles in a liquid droplet move to the perimeter in the evaporation process, leaving a ring.22
Table 1.
Comparing mPAP with reference PAP testing
| HR1a |
HR2b |
|||||
|---|---|---|---|---|---|---|
| Positive reference PAP | Negative reference PAP | Total | Positive reference PAP | Negative reference PAP | Total | |
| Positive mPAP | 4 | 0 | 4 | 12 | 1 | 13 |
| Negative mPAP | 4 | 135 | 139 | 25 | 105 | 130 |
| Total | 8 | 135 | 143 | 37 | 106 | 143 |
HR1 definition of heteroresistance: growth of a resistant subpopulation of cells at 4 and 8 mg/L of colistin at a frequency of at least 1 × 10−6 the main population grown on antibiotic-free media.
HR2 definition of heteroresistance: growth of a resistant subpopulation of cells at a colistin concentration ≥8× higher than the main population, at a frequency of at least 1 × 10−7.
Figure 1.
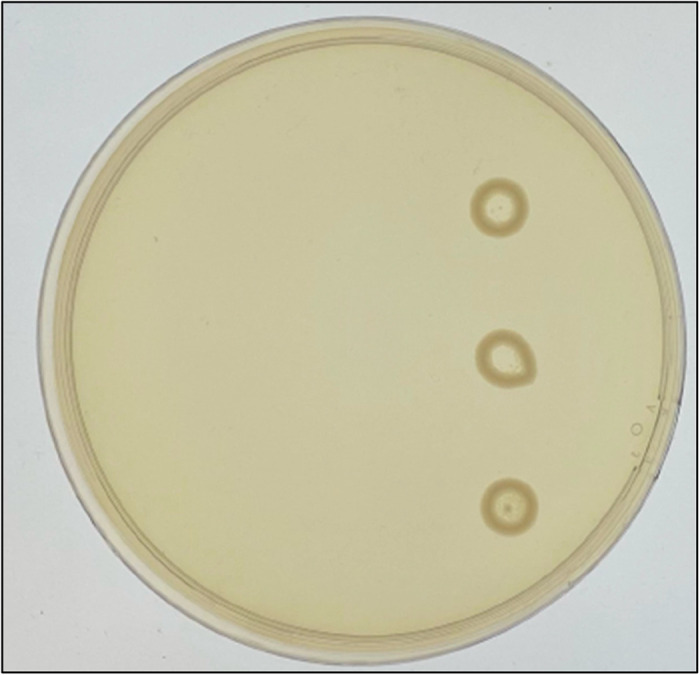
A representative example of the ‘coffee ring’ formations observed in the mPAP technique on agar plates with at least 2 mg/L colistin. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Clinical characteristics and outcomes
Of the 143 isolates, 131 had complete medical record review, and 82 were from unique adults hospitalized at the time of or within 1 week of CRPA culture. The median age was 63 years (IQR 49–73). Most (n = 45; 56%) were male and 35 (43%) had CCI > 2. The lower respiratory tract was the most frequent culture site (n = 28; 34%) and 12 (15%) were from a normally sterile site. Using either the HR1 or HR2 definition, there were few differences observed in demographics or clinical characteristics between patients with and without a heteroresistant isolate. However, patients with HR2 isolates tended to remain in the hospital longer after the culture was obtained than patients without an HR2 isolate (median 16 versus 9 days, P = 0.09) (Table 2). Colistin use was able to be assessed in 65 of the 82 patients, and only 2 (3%) patients received colistin within 2 weeks of culture.
Table 2.
Characteristics and clinical outcomes of hospitalized patients with CRPA, stratified by two definitions of heteroresistance (HR1 and HR2) to colistin
| Characteristic or outcome | HR1a |
HR2c |
|||||
|---|---|---|---|---|---|---|---|
| Total (N = 82) | Not heteroresistant (N = 77) | Heteroresistant (N = 5) | P valueb | Not heteroresistant (N = 58) | Heteroresistant (N = 24) | P valued | |
| Age category (years), n (%) | 0.7 | 0.2 | |||||
| 19–49 | 21 (26) | 19 (25) | 2 (40) | 18 (31) | 3 (13) | ||
| 50–64 | 24 (29) | 22 (29) | 2 (40) | 14 (24) | 10 (42) | ||
| 65–79 | 28 (34) | 27 (35) | 1 (20) | 20 (34) | 8 (33) | ||
| >79 | 9 (11) | 9 (12) | 0 (0) | 6 (10) | 3 (13) | ||
| Male (N = 81), n (%) | 45 (56) | 43 (57) | 2 (40) | 0.7 | 32 (56) | 13 (54) | 0.9 |
| Race (N = 79), n (%) | 0.4 | 0.7 | |||||
| Black | 47 (59) | 45 (61) | 2 (40) | 34 (62) | 13 (54) | ||
| White | 31 (39) | 28 (38) | 3 (60) | 20 (36) | 11 (46) | ||
| Other or multiracial | 1 (1) | 1 (1) | 0 (0) | 1 (2) | 0 (0) | ||
| CCI > 2, n (%) | 35 (43) | 34 (44) | 1 (20) | 0.4 | 28 (48) | 7 (29) | 0.11 |
| Residence 3 days prior to culture, n (%) | 0.7 | 0.8 | |||||
| Inpatient | 41 (50) | 38 (49) | 3 (60) | 29 (50) | 12 (50) | ||
| LTCF or LTACH | 17 (21) | 17 (22) | 0 (0) | 11 (19) | 6 (25) | ||
| Private residence | 24 (29) | 22 (29) | 2 (40) | 18 (31) | 6 (25) | ||
| Admitted to ICU in prior week, n (%) | 24 (29) | 23 (30) | 1 (20) | 1.0 | 20 (34) | 4 (17) | 0.11 |
| Culture source, n (%) | 1.0 | 0.16 | |||||
| Sterile site | 12 (15) | 11 (14) | 1 (20) | 11 (19) | 1 (4) | ||
| Lower respiratory tract | 28 (34) | 26 (34) | 2 (40) | 20 (34) | 8 (33) | ||
| Urine | 25 (30) | 24 (31) | 1 (20) | 14 (24) | 11 (46) | ||
| Wound | 17 (21) | 16 (21) | 1 (6) | 13 (22) | 4 (17) | ||
| Days from admission to discharge, median (IQR) | 16 (7–49) | 15 (6–44) | 21 (13–60) | 0.4 | 15 (6–44) | 19 (9–64) | 0.5 |
| Days from culture to discharge, median (IQR) | 10 (6–24) | 10 (6–24) | 17 (11–47) | 0.3 | 9 (5–20) | 16 (9–28) | 0.09 |
| 90 day mortality, n (%) | 17 (21) | 17 (22) | 0 (0) | 0.6 | 14 (24) | 3 (12) | 0.4 |
LTCF, long-term care facility; LTACH, long-term acute care hospital.
Patients with an isolate that met criteria for the HR1 definition: growth of a resistant subpopulation of cells at 4 and 8 mg/L of colistin at a frequency of least 1 × 10−6 the main population on antibiotic-free media.
Compared patients with an HR1 isolate with those that did not, using χ2 or Fisher’s exact test for categorical variables and Mann–Whitney U-test for continuous variables.
Patients with an isolate that met criteria for the HR2 definition: growth of a resistant subpopulation of cells at a colistin concentration at least 8× higher than the main population MIC, at a frequency of at least 1 × 10−7.
Compared patients with an HR2 isolate with those that did not, using χ2 or Fisher’s exact test for categorical variables and Mann–Whitney U-test for continuous variables.
Seventeen (21%) of the 82 patients died within 90 days. Heteroresistance was not associated with 90 day mortality; using HR1, 0 (0%) patients with a heteroresistant isolate versus 17 (22%) without a heteroresistant isolate died (P = 0.6), and using HR2, 3 (12%) patients with a heteroresistant isolate versus 14 (24%) without a heteroresistant isolate died (P = 0.4; Table 2). In a multivariable analysis, having an HR2 isolate was not a risk factor for 90 day mortality (adjusted OR 0.8; 95% CI 0.2–3.4) after controlling for elevated CCI, being admitted to the ICU in the prior week, and CRPA culture being obtained from a lower respiratory source (Table 3). In sensitivity analyses, we did not find an association between heteroresistance (HR1 or HR2) and 30 day mortality. Similarly, there was no association between heteroresistance and 90 day mortality when all evaluable patients were included (data not shown).
Table 3.
Results of the logistic regression model assessing the association between heteroresistance (HR2 definitiona) and 90 day mortality
| Variable | Univariable OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|
| Heteroresistant CRPA isolate | 0.4 (0.1–1.7) | 0.8 (0.2–3.4) |
| CCI > 2 | 4.4 (1.4–14.0) | 5.0 (1.4–18.0) |
| Admitted to the ICU in prior week | 5.2 (1.7–16.2) | 4.0 (1.02–15.6) |
| Lower respiratory tract culture | 3.7 (1.2–11.3) | 2.2 (0.6–8.3) |
Growth of a resistant subpopulation of cells at a colistin concentration at least 8× higher than the main population MIC, at a frequency of at least 1 × 10−7.
Discussion
In our sample of CRPA isolates from Atlanta, GA, USA, the frequency of heteroresistance ranged from 6% to 26% depending on the definition of heteroresistance applied. The prevalence estimates were determined through PAP testing, the gold standard for detecting heteroresistance. The mPAP technique was more efficient, but had poor sensitivity (≤50%), especially when using the more inclusive HR2 definition. Heteroresistance was not associated with increased 90 day mortality, regardless of the definition of heteroresistance, although this was primarily in patients who did not receive colistin.
The HR1 definition, adapted from Band et al.,16 was the most stringent and only identified eight CRPA isolates as heteroresistant. The HR2 definition proposed by Andersson et al.10 allowed for a decreased frequency of the resistant subpopulation by an order of magnitude and only required growth at 8× the MIC of the main population, but not necessarily reaching 1× or 2× the breakpoint. Therefore, the HR2 definition classified an additional 29 isolates as being heteroresistant, almost all of which were due to the change in the frequency requirement. Prior literature has also reported wide ranges of prevalence estimates for colistin heteroresistance in P. aeruginosa, and this may differ based on location, culture source and antibiotic susceptibility pattern. One study of 152 P. aeruginosa isolates from blood cultures in a tertiary care centre in Hungary identified colistin heteroresistance in 41 (27%).20 In contrast, a study from a hospital in China found 9 (4%) heteroresistant isolates among 231 carbapenem-non-susceptible P. aeruginosa isolates.19 Neither of these studies used the HR1 or HR2 definitions, and instead defined heteroresistance as a subpopulation with growth at a colistin concentration >2 mg/L with no minimum frequency requirement.19,20
The mPAP technique is quicker and uses fewer resources than the reference PAP but performed poorly as a screening method for colistin heteroresistance. We attribute the low sensitivity to potential inaccuracy in dropping 10 μL onto a plate (as opposed to spreading 100 μL) with such a low frequency of resistant cells. Lastly, in the mPAP method with colistin we often noted the ‘coffee ring’ phenomenon where the drop of bacterial culture left a ring of cells along the droplet perimeter.22 These ring formations could have led to inaccuracies in counting resistant cells.
Prior literature has demonstrated that linking heteroresistance to clinical outcomes is challenging. There have been many conflicting studies looking at heteroresistance in vancomycin-intermediate Staphylococcus aureus; however, there have been few studies analysing heteroresistance in Gram-negative organisms.10 To our knowledge, we are the first to analyse patient outcomes with respect to colistin heteroresistance in P. aeruginosa. While we did not observe an association between heteroresistance and 90 day mortality, few patients in this population received colistin, which may be necessary for clinical failure and could explain why we did not observe this. Colistin resistance can also lead to a fitness cost in P. aeruginosa and this could help explain why there appeared to be a non-significant trend towards improved mortality in heteroresistant isolates.23 Notably, patients with HR2 isolates had longer lengths of stay after CRPA culture, which could indicate a more complicated hospital course. Further research is needed to determine the relationship between heteroresistance and clinical outcomes in Gram-negative bacteria. More proximal measures, such as clinical failure or recurrence, may be more relevant outcomes to assess in future studies.
Strengths of this study include that we analysed many CRPA isolates linked to clinical data, allowing us to explore the association between heteroresistance and mortality in P. aeruginosa. Our study is unique because we compared two commonly used definitions of heteroresistance.
This study also has limitations. While isolates were collected through active, laboratory- and population-based surveillance, ultimately our sample was a convenience sample dependent on laboratories sharing their isolates. This may bias our sample toward larger, academic laboratories who could more easily share data and limit the generalizability. Additionally, we were unable to differentiate infected versus colonized patients in sites where colonization can occur. We restricted the outcome assessment to patients hospitalized within 1 week of culture to limit the number of patients with primarily colonization. Lastly, given the relatively modest sample size, we were only able to perform a multivariable analysis using the HR2 definition and may not have had adequate power to detect small differences in mortality.
As evidenced by this study and prior work, the microbiological definition of heteroresistance is in flux, and has previously been defined primarily based on laboratory operational considerations. While the HR1 definition could be thought of as a more restrictive definition and the HR2 definition a more inclusive definition for heteroresistance, until we are able to measure and understand the clinical impact of heteroresistance we will not be able to generate a clinically meaningful definition. This is analogous to defining a resistant isolate based on deviation from a normal distribution (biological definition) as opposed to evaluating treatment efficacy and relating this to clinical breakpoints (clinical definition). Thus, while it would be ideal to propose a standardized definition of heteroresistance from this work, without additional data linking heteroresistance to clinical outcomes, the optimal definition of heteroresistance remains undefined.
In summary, colistin heteroresistance may occur in up to approximately one-quarter of CRPA isolates in Atlanta, GA, USA; however, additional research is needed to understand how this should be tested for in clinical laboratories and what implications this has on management. The wide range of heteroresistance frequencies observed underscores the need to standardize the definition of heteroresistance and method of detection. Ideally the definition would be one that correlates with clinical outcomes; however, more data are needed to determine this. Future studies on the impact of heteroresistance should gather additional data on antibiotics administered and assess outcomes such as clinical failure and recurrence in addition to mortality.
Supplementary Material
Acknowledgements
We are grateful to all the employees at the Georgia Emerging Infections Program, who work tirelessly to collect and maintain these data, and Davina Campbell from the Division of Healthcare Quality Promotion (DHQP) at the Centers for Disease Control and Prevention for performing the colistin susceptibility testing.
Funding
This work was supported by the Centers for Disease Control and Prevention Emerging Infection Program (U50CK000485) and the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (UM1AI10468, Antibacterial Resistance Leadership Group fellowship AI141883 to J.H.A. and AI148661 to D.S.W.). Laboratory testing for heteroresistance was funded in part by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (1U19AI158080-01) and performed by Emory University’s Investigational Clinical Microbiology Core. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention or National Institutes of Health.
Transparency declarations
All authors report no conflicts of interest relevant to this work.
Author contributions
J.H.A., J.T.J., D.S.W. and S.W.S. contributed to the conception and design of the study. J.H.A., M.D., A.M.P., C.W.B., G.S. and S.W.S. contributed to the acquisition of the data. J.H.A., M.D., A.M.P., J.T.J., D.I.A., D.S.W. and S.W.S. contributed to data analysis and interpretation. J.H.A. and S.W.S. drafted the article and all authors provided critical revisions and important intellectual content. All authors gave approval of the final manuscript to be submitted.
Supplementary data
Tables S1 to S3 are available as Supplementary data at JAC Online.
References
- 1.WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery and development of new antibiotics. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1.
- 2.Cai B, Echols R, Magee G. et al. Prevalence of carbapenem-resistant Gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis 2017; 4: ofx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aviv T, Lazarovitch T, Katz D. et al. The epidemiological impact and significance of carbapenem resistance in Pseudomonas aeruginosa bloodstream infections: a matched case–case-control analysis. Infect Control Hosp Epidemiol 2018; 39: 1262–5. [DOI] [PubMed] [Google Scholar]
- 4.Nathwani D, Raman G, Sulham K. et al. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control 2014; 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pogue JM, Kaye KS, Veve MP. et al. Ceftolozane/tazobactam vs polymyxin or aminoglycoside-based regimens for the treatment of drug-resistant Pseudomonas aeruginosa. Clin Infect Dis 2020; 71: 304–10. [DOI] [PubMed] [Google Scholar]
- 6.Horcajada JP, Montero M, Oliver A. et al. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev 2019; 32: e00031-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vicari G, Bauer SR, Neuner EA. et al. Association between colistin dose and microbiologic outcomes in patients with multidrug-resistant Gram-negative bacteremia. Clin Infect Dis 2013; 56: 398–404. [DOI] [PubMed] [Google Scholar]
- 8.Benattar YD, Omar M, Zusman O. et al. The effectiveness and safety of high-dose colistin: prospective cohort study. Clin Infect Dis 2016; 63: 1605–12. [DOI] [PubMed] [Google Scholar]
- 9.Horcajada JP, Sorlí L, Luque S. et al. Validation of a colistin plasma concentration breakpoint as a predictor of nephrotoxicity in patients treated with colistin methanesulfonate. Int J Antimicrob Agents 2016; 48: 725–7. [DOI] [PubMed] [Google Scholar]
- 10.Andersson DI, Nicoloff H, Hjort K.. Mechanisms and clinical relevance of bacterial heteroresistance. Nat Rev Microbiol 2019; 17: 479–96. [DOI] [PubMed] [Google Scholar]
- 11.Nicoloff H, Hjort K, Levin BR. et al. The high prevalence of antibiotic heteroresistance in pathogenic bacteria is mainly caused by gene amplification. Nat Microbiol 2019; 4: 504–14. [DOI] [PubMed] [Google Scholar]
- 12.Band VI, Satola SW, Smith RD. et al. Colistin heteroresistance is largely undetected among carbapenem-resistant Enterobacterales in the United States. mBio 2021; 12: e02881-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Band VI, Satola SW, Burd EM. et al. Carbapenem-resistant Klebsiella pneumoniae exhibiting clinically undetected colistin heteroresistance leads to treatment failure in a murine model of infection. mBio 2018; 9: e02448-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Band VI, Crispell EK, Napier BA. et al. Antibiotic failure mediated by a resistant subpopulation in Enterobacter cloacae. Nat Microbiol 2016; 1: 16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Halfawy OM, Valvano MA.. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin Microbiol Rev 2015; 28: 191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Band VI, Hufnagel DA, Jaggavarapu S. et al. Antibiotic combinations that exploit heteroresistance to multiple drugs effectively control infection. Nat Microbiol 2019; 4: 1627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergen PJ, Forrest A, Bulitta JB. et al. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrob Agents Chemother 2011; 55: 5134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermes DM, Pormann Pitt C, Lutz L. et al. Evaluation of heteroresistance to polymyxin B among carbapenem-susceptible and -resistant Pseudomonas aeruginosa. J Med Microbiol 2013; 62: 1184–9. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Xu C, Fang R. et al. Resistance and heteroresistance to colistin in Pseudomonas aeruginosa isolates from Wenzhou, China. Antimicrob Agents Chemother 2019; 63: e00556-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juhász E, Iván M, Pintér E. et al. Colistin resistance among blood culture isolates at a tertiary care centre in Hungary. J Glob Antimicrob Resist 2017; 11: 167–70. [DOI] [PubMed] [Google Scholar]
- 21.CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Thirtieth Edition: M100. 2020.
- 22.Shen X, Ho C-M, Wong T-S.. Minimal size of coffee ring structure. J Phys Chem B 2010; 114: 5269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J-Y, Park YK, Chung ES. et al. Evolved resistance to colistin and its loss due to genetic reversion in Pseudomonas aeruginosa. Sci Rep 2016; 6: 25543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


