Abstract
Background
Tenofovir disoproxil fumarate-containing pre-exposure prophylaxis (PrEP) has been associated with decreases in bone mineral density (BMD), but the bone effects of other non-tenofovir disoproxil fumarate candidate PrEP regimens are not well described.
Methods
The HPTN 069/ACTG A5305 study randomized 406 US cisgender men and transgender women, and 188 cisgender women at risk for HIV infection to one of four double-blinded regimens: (i) maraviroc; (ii) maraviroc + emtricitabine; (iii) maraviroc + tenofovir disoproxil fumarate; or (iv) tenofovir disoproxil fumarate + emtricitabine. BMD was measured in a subset of participants at the lumbar spine (LS) and hip by dual-energy X-ray absorptiometry (DXA) at baseline and 48 weeks. Percentage change in LS and hip BMD was compared between the tenofovir disoproxil fumarate- and non-tenofovir disoproxil fumarate-containing arms by Wilcoxon rank-sum tests and multiple linear regression adjusting for sex, race and baseline BMI.
Results
At baseline (n = 307), the median age was 33 years, 56% male and 43% black. At the hip, the median percentage change in BMD at 48 weeks was –1.05% in the tenofovir disoproxil fumarate arms and 0.0% in the non-tenofovir disoproxil fumarate arms (between group P = 0.001). No interaction by sex was observed. The median percentage change in LS BMD was not different between arms.
Conclusions
Tenofovir disoproxil fumarate-containing PrEP was associated with significantly greater bone loss compared with maraviroc ± emtricitabine PrEP at the hip, but not the LS. The BMD changes at the hip were similar in magnitude in men and women.
Introduction
Pre-exposure prophylaxis (PrEP) using a combination of tenofovir disoproxil fumarate and emtricitabine has been shown to reduce incident HIV infection in high-risk individuals by over 90% in those who are adherent.1 Although generally well tolerated, bone toxicity has been raised as a safety concern of tenofovir disoproxil fumarate-containing PrEP. Among persons living with HIV, tenofovir disoproxil fumarate in combination with other antiretroviral agents has been shown to decrease bone mineral density (BMD) in those who are initiating ART and in those switching from other effective regimens.2 Some studies have shown a higher risk of fracture related to tenofovir disoproxil fumarate exposure.3,4
In previous studies of individuals initiating tenofovir disoproxil fumarate/emtricitabine PrEP, BMD has been shown to decrease an average of 1%–2% at the hip and lumbar spine (LS) compared with placebo. Similar to persons living with HIV initiating ART, the BMD loss related to tenofovir disoproxil fumarate/emtricitabine occurs in the first 12 months and then levels off.5–9 In the iPrEx OLE study, discontinuation of tenofovir disoproxil fumarate/emtricitabine-containing PrEP was associated with increases in BMD to baseline values, suggesting reversibility of this effect,10 which has been confirmed by other studies.11 The majority of data regarding the effect of PrEP on bone have included cisgender men and transgender women (TGW). Studies in women have been limited to Africa; 7,11 however, thorough assessment of the bone effects of tenofovir disoproxil fumarate in these studies was limited by low (<30%) PrEP adherence.11
HPTN 069/ACTG A5305 examined the safety and tolerability over 48 weeks in US men and women of four different candidate PrEP regimens, two of which contained the cell entry inhibitor, maraviroc, but not tenofovir disoproxil fumarate. In the primary analyses, the rates of adverse events were low and did not differ by arm.12,13 Of the 406 men and 188 women randomized, 9 men and 5 women experienced bone fractures during follow-up. Given the relatively small study size, short duration and young study population, the study was not designed to be adequately powered to assess the bone safety of these regimens. For this reason, a bone substudy was conducted with the primary objective of determining whether BMD changes with tenofovir disoproxil fumarate-containing PrEP differed from non-tenofovir disoproxil fumarate-containing PrEP over 48 weeks of follow-up.
Methods
Study design and participants
HPTN 069/ACTG A5305 was a prospective, double-blinded, multicentre study in at-risk, HIV-uninfected men/TGW and cisgender women at 13 sites in the USA and Puerto Rico in which participants were randomized to one of three candidate PrEP regimens or a control regimen: (i) maraviroc 300 mg (Selzentry™, ViiV Healthcare, Brentford, UK); (ii) maraviroc 300 mg plus emtricitabine 200 mg (Emtriva™, Gilead Sciences, Foster City, CA, USA); (iii) maraviroc 300 mg plus tenofovir disoproxil fumarate 300 mg (Viread™, Gilead Sciences); or (iv) tenofovir disoproxil fumarate 300 mg plus emtricitabine 200 mg (control arm). As previously described, eligible participants were required to be ≥18 years and have self-reported condomless intercourse (men/TGW: anal intercourse; women: vaginal/anal intercourse) with at least one man known to be living with HIV or of unknown HIV serostatus within 90 days prior to study entry.12,13 In addition, participants were required to have calculated CLCR ≥70 mL/min (Cockcroft–Gault), a non-reactive HIV antibody test and plasma HIV RNA below the limit of detection within 14 days of study entry. Further, cisgender women were required to have a negative pregnancy test. Participants were excluded if they reported any antiretroviral drug within 90 days (e.g. for PrEP or post-exposure prophylaxis), reported active injection drug use, or had a positive hepatitis B surface antigen. The BMD substudy was offered to men/TGW until target substudy enrolment had been reached (∼200) and to all enrolled women. Participants who were >300 lbs (136 kg) were not eligible for the BMD substudy. The study (ClinicalTrials.gov identifier NCT01505114) was approved by the Institutional Review Boards of all participating institutions and all subjects provided written informed consent.
At baseline, information regarding demographics, health-related behaviours, medical conditions and prescribed medications was obtained. BMD was assessed by dual-energy X-ray absorptiometry (DXA) at the LS (L1–L4) and total hip using Hologic or Lunar scanners at baseline and at Week 48. Sites were instructed to use the same scanner and the same hip (left) at both study timepoints on the same participant. All scans were read centrally by readers blinded to treatment assignment and clinical characteristics, using a standardized protocol at the Body Composition Analysis Center, Tufts University (Boston, MA, USA). Z-scores were calculated from the site-specific BMD measurements using normative data matched for age, gender and race and, given the young age of the population, were used to summarize the baseline BMD data in accordance with International Society of Clinical Densitometry guidelines.14
Clinical and laboratory assessments
Alcohol and drug use behaviours were determined at baseline by a standardized questionnaire. Problem alcohol use was defined as an affirmative response to the question, ‘Have you ever tried and failed to control, cut down, or stop using alcohol?’. Popper use and methamphetamine use were defined as any use in the previous 3 months. 25-OH vitamin D was assessed at the local site laboratories at baseline and Week 24 and Week 48 visits. Antiretroviral drug concentration testing from plasma samples collected at Weeks 24 and 48 was performed on a random subset of men/TGW (n = 160) and all enrolled cisgender women. Maraviroc, emtricitabine and tenofovir were quantified via validated liquid chromatographic–tandem mass spectrometric (LC–MS/MS) methods, with assay limits of quantitation of 0.5 ng/mL (maraviroc) and 0.31 ng/mL (emtricitabine, tenofovir).15,16
Statistical analysis
The primary objective of this analysis was to compare the percentage change in LS and hip BMD over 48 weeks in those randomized to tenofovir disoproxil fumarate-containing versus non-tenofovir disoproxil fumarate-containing treatment arms. Two different study populations were assessed. In the modified intent-to-treat (mITT) population, participants who were randomized to treatment and had DXA results available at baseline and 48 weeks, regardless of whether the participant was receiving study medications were included. In the as-treated analysis, only those participants in the mITT population who had quantifiable drug concentrations at both 24 and 48 weeks were included. Two participants with a > 20% change over 48 weeks in the hip were excluded from analysis, as these changes were not considered to be biologically plausible and were likely related to measurement error from positioning differences.
Wilcoxon rank-sum tests were used to test the median difference of percentage change in LS and hip BMD between the two treatment groups, pooled and stratified by sex. In the as-treated analysis, we used multivariable linear models to determine the effect of tenofovir disoproxil fumarate-containing versus non-tenofovir disoproxil fumarate-containing PrEP on the 48 week percentage change in LS and hip BMD, after adjustment for sex, race and BMI. In an exploratory analysis, we examined the relationship between tenofovir concentrations (24 and 48 weeks) and 48 week percentage change in LS and hip BMD among the 100 participants (41 men/TGW and 59 women) who had tenofovir concentrations determined at both Week 24 and Week 48 and were randomized to a tenofovir disoproxil fumarate-containing arm. These multiple linear regression models were adjusted for sex, race and baseline BMI. Interactions by sex were also explored by adding tenofovir concentration × sex interaction term to the multivariable models. All statistical analyses were performed with SAS (Version 9.4, Cary, NC, USA).
Results
Of the 594 participants enrolled in HPTN 069/ACTG A5305 (406 men/TGW and 188 women), 397 (212 men/TGW and 185 women) were enrolled in the BMD substudy. Of these, 307 (170 men/2 TGW and 135 cisgender women) completed study follow-up and had a spine or hip DXA scan at both baseline and at 48 weeks (mITT Population) (Table 1). The median age was 30 years in men/TGW and 37 years in women. The percentage of black participants was lower in men/TGW than in women (26.7% versus 64.4%). The median BMI was 26 kg/m2 in men/TGW and 31 kg/m2 in women. Demographic characteristics of subjects who participated in the BMD substudy were balanced in those randomized to tenofovir disoproxil fumarate-containing or non-tenofovir disoproxil fumarate-containing PrEP and were similar to those who did not participate among men (data not shown). Among 188 cisgender women enrolled, 185 (98.4%) enrolled in the BMD substudy. Among men/TGW, the median (Q1, Q3) Z-score at baseline was –0.5 (–1.3, 0.4) at the LS and –0.3 (–0.9, 0.3) at the hip. Among cisgender women, the median (Q1, Q3) Z-score at baseline was 0.2 (–0.5, 1.2) at the LS and 0.3 (–0.5, 1.1) at the hip.
Table 1.
Baseline characteristics of study population with DXA
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| TDF containing | non-TDF containing | total | TDF containing | non-TDF containing | total | |
| N | 88 | 84 | 172a | 71 | 64 | 135 |
| Baseline | ||||||
| Age (years) | 32 (25, 40) | 29 (25, 38) | 30 (25, 39) | 39 (29, 48) | 36 (28, 47) | 37 (28, 48) |
| Black race | 21 (24) | 25 (30) | 46 (27) | 47 (66) | 40 (63) | 87 (64) |
| BMI (kg/m2) | 27 (23, 30) | 26 (23, 30) | 26 (23, 30) | 31 (26, 38) | 31 (25, 36) | 31 (25, 37) |
| LS Z-score | –0.6 (–1.5, 0.4) | –0.4 (–1.1, 0.6) | –0.5 (–1.3, 0.4) | 0.2 (–0.5, 1.3) | 0.3 (–0.5, 1.1) | 0.2 (–0.5, 1.2) |
| Hip Z-score | –0.3 (–0.9, 0.4) | –0.4 (–1.1, 0.2) | –0.3 (–0.9, 0.3) | 0.4 (–0.7, 1.2) | 0.2 (–0.3, 1.1) | 0.3 (–0.5, 1.1) |
TDF, tenofovir disoproxil fumarate.
Data are given as median (Q1, Q3) or n (%).
Two participants (one in the non-tenofovir disoproxil fumarate-containing arm and one in the tenofovir disoproxil fumarate-containing arm) identified as female, transsexual, or transgender.
Of the 307 participants with DXA data, 198 (83 men/TGW and 115 women) had drug concentrations measured at both Week 24 and Week 48. Of these, 72 men (87%) and 63 women (55%) had quantifiable concentrations of assigned medications at both timepoints and are included in the as-treated analysis (Table 2). Among those in the tenofovir disoproxil fumarate-containing arms, tenofovir concentrations were comparable between men and cisgender women. The population was 41.5% black, and 46.7% women. The median age was 32 years (27, 44) and the median BMI was 28 kg/m2 (24, 32). Alcohol and drug use was common. The prevalence of vitamin D deficiency (25-OH vitamin D < 20 ng/dL) was 36.3%. Characteristics were similar in those who were randomized to a tenofovir disoproxil fumarate- and non-tenofovir disoproxil fumarate-containing PrEP regimen.
Table 2.
Characteristics of subset with quantifiable plasma drug concentrations at Week 24 and Week 48a (N = 135)
| TDF containing | Non-TDF containing | Total | P value | |
|---|---|---|---|---|
| N | 71 | 64 | 135 | |
| Age (years) | 32 (27, 45) | 33 (27, 44) | 32 (27, 44) | 0.86 |
| Sex (male) | 35 (49) | 37 (58) | 72a (53) | 0.32 |
| Race (Black) | 33 (47) | 23 (36) | 56 (42) | 0.22 |
| BMI (kg/m2) | 28 (24, 33) | 27 (24, 31) | 28 (24, 32) | 0.75 |
| Problem alcohol use | 5 (7) | 6 (10) | 11 (8) | 0.64 |
| Popper use | 15 (21) | 12 (19) | 27 (20) | 0.77 |
| Methamphetamine use | 2 (3) | 8 (13) | 10 (8) | 0.05 |
| 25 OH vitamin D < 20 ng/dL | 27 (38) | 22 (34) | 49 (36) | 0.66 |
| On study | ||||
| Receiving MVC | 32 (45) | 64 (100) | 96 (71) | <0.001 |
| 24 week TFV concentrations (ng/mL) | – | |||
| Men | 72 (49, 122) | |||
| Women | 70 (48, 119) | |||
| 48 week TFV concentrations (ng/mL) | – | |||
| Men | 67 (44, 114) | |||
| Women | 64 (36, 129) |
MVC, maraviroc; TFV, tenofovir; TDF, tenofovir disoproxil fumarate.
Data are given as median (Q1, Q3) or n (%).
All were cisgender men.
Changes in BMD
Overall in the mITT population, the median (Q1, Q3) percentage change in LS BMD was –0.41% (–2.5%, 1.6%) in the tenofovir disoproxil fumarate arms and 0.07% (–2.1%, 1.67%) in the non-tenofovir disoproxil fumarate arms (between group P = 0.36). The median percentage change in hip BMD was –1.05% (–2.9%, 0.59%) in the tenofovir disoproxil fumarate arms and 0.0% (–1.7%, 1.3%) in the non-tenofovir disoproxil fumarate arms (between group P = 0.001).
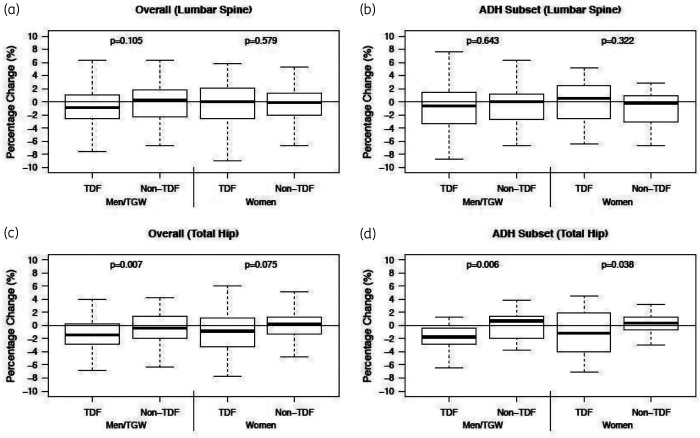
In analyses stratified by sex (Figure 1), the median LS BMD change was –0.86% in the tenofovir disoproxil fumarate arms and 0.31% in the non-tenofovir disoproxil fumarate arms (between group P = 0.105) among men/TGW. At the total hip, the median of change was –1.4% in the tenofovir disoproxil fumarate arms and –0.42% in the non-tenofovir disoproxil fumarate arms (between group P = 0.007). Among the women in the mITT population, the median LS BMD change was 0.04% in the tenofovir disoproxil fumarate arms and –0.09% in the non-tenofovir disoproxil fumarate arms (between group P = 0.58). At the hip, the median of change was –0.83% in the tenofovir disoproxil fumarate arms and 0.18% in the non-tenofovir disoproxil fumarate arms (between group P = 0.075).
Figure 1.
Percentage change in BMD 48 weeks after initiating tenofovir disoproxil fumarate-containing or non-tenofovir disoproxil fumarate-containing PrEP in HIV-negative men and women at the spine (a) and hip (c) in the overall study and in adherent subset (i.e as-treated population) with quantifiable plasma drug concentrations at Weeks 24 and 48 (b, d). TDF, tenofovir disoproxil fumarate; ADH, adherent.
Overall in the as-treated population (i.e. restricting to those with quantifiable plasma drug concentrations at Weeks 24 and 48), the median (Q1, Q3) percentage change in LS BMD was –0.11% (–2.9%, 2.0%) in the tenofovir disoproxil fumarate arms and –0.15% (–2.7%, 1.2%) in the non-tenofovir disoproxil fumarate arms (between group P = 0.73). The median percentage change in hip BMD was –1.46% (–3.3%, 0.5%) in the tenofovir disoproxil fumarate arms and 0.52% (–1.4%, 1.4%) in the non-tenofovir disoproxil fumarate arms (between group P < 0.001).
In the men, the median percentage change in LS BMD was –0.63% in the tenofovir disoproxil fumarate arms and 0.07% in the non-tenofovir disoproxil fumarate arms (between group P = 0.64). At the hip, the median of BMD change was –1.76% in the tenofovir disoproxil fumarate arms and 0.69% in the non-tenofovir disoproxil fumarate arms (between group P = 0.006). Among the women in the as-treated analysis, the median change of LS BMD was 0.52% in the tenofovir disoproxil fumarate arms and –0.23% in the non-tenofovir disoproxil fumarate arms (between group P = 0.32). At the hip, the median of BMD change was –1.18% in the tenofovir disoproxil fumarate arms and was 0.39% in the non-tenofovir disoproxil fumarate arms (between group P = 0.04) (Figure 1).
In multivariable models of the as-treated analysis set pooled by sex, the adjusted between-group difference (tenofovir disoproxil fumarate arms versus non-tenofovir disoproxil fumarate arms) was –0.22% [95% CI: (–1.36%, 0.92%), P = 0.70] for LS BMD and –1.94% [95% CI: (–3.23%, –0.65%), P = 0.003] for hip BMD. Addition of maraviroc exposure to the models did not affect estimates of the between-group differences [LS: –0.69% (95% CI –2.23%, 0.84%); hip: –2.07%% (95% CI –3.67%, –0.47%)] and the maraviroc effect was not statistically significant in either the LS (P = 0.41) or hip (P = 0.78). No tenofovir disoproxil fumarate–sex interactions were observed.
In an exploratory analysis, we examined the relationship between plasma tenofovir concentrations at 24 and 48 weeks and BMD percentage change at the LS and hip in participants who were randomized to a tenofovir disoproxil fumarate-containing regimen (Table S1, available as Supplementary data at JAC Online). In an analysis adjusted for sex, race and BMI, higher tenofovir concentrations at 24 weeks were associated with a greater BMD decrease at the hip [–0.93% for each log10 ng/mL (95% CI: –1.83, –0.03), P = 0.043], but not at the LS. Week 48 tenofovir concentrations were not associated with BMD change at hip or the LS. No tenofovir concentration–sex interactions were observed.
Discussion
In this randomized clinical trial comparing tenofovir disoproxil fumarate-containing PrEP with active non-tenofovir disoproxil fumarate-containing candidate PrEP regimens in a US population of men and women, we found that BMD changes in the LS over 48 weeks did not differ in the tenofovir disoproxil fumarate versus non-tenofovir disoproxil fumarate groups. However, hip BMD decreased to a greater extent in the tenofovir disoproxil fumarate-containing arms as compared with the non-tenofovir disoproxil fumarate, maraviroc-containing arms. As expected, the magnitude of the between-group difference in hip BMD changes was greater in those with quantifiable on-treatment drug concentrations, but no differences in the magnitude of BMD effects at the hip were observed by sex. Our findings suggest that small, but statistically significant, decreases in hip BMD are associated with tenofovir disoproxil fumarate-containing PrEP in both men and women in whom quantifiable drug concentrations were found. Although the clinical significance of these bone changes with tenofovir disoproxil fumarate-containing PrEP is not clear, our findings are reassuring for US women receiving tenofovir disoproxil fumarate-containing PrEP that the modest bone effects are similar to those seen in men.
Our findings for men/TGW were similar to previous studies investigating similar populations. The iPrEx and CDC studies found 0.9% and 0.8% decreases in the total hip BMD at 48 weeks, respectively, in those receiving tenofovir disoproxil fumarate-containing PrEP, compared with placebo.5,6 We did not, however, observe any differences in changes in LS BMD attributable to tenofovir disoproxil fumarate use. In studies of persons living with HIV initiating ART and in the iPrEx study, tenofovir disoproxil fumarate has been associated with ∼1% net loss of BMD at the LS.17,18 The reasons for this difference by anatomical site are unclear. Since we had DXA data only at baseline and 48 weeks, it is possible that we did not observe potential rapid decreases in BMD, which subsequently returned to baseline prior to the 48 week DXA measurement. However, the lack of an effect on BMD at the LS with tenofovir disoproxil fumarate-containing PrEP was also observed in the CDC PrEP study, in which the spine BMD in both arms had a neutral or positive trajectory.6 Similar to the CDC study, our study was US based with a high percentage of white male participants. It should be noted that we also did not observe an effect of tenofovir disoproxil fumarate-containing PrEP on LS BMD in women, of whom 64% were black. It is not clear whether these or other population characteristics contributed to the observed lack of change in spine BMD related to tenofovir disoproxil fumarate-containing PrEP.
Our study had two important findings with respect to bone health in US cisgender women at risk for HIV infection initiating a tenofovir disoproxil fumarate-containing PrEP regimen. First, the BMD in our cisgender women population was normal compared with the age-, sex-, race-based reference population used to calculate Z-scores. This is in contrast to the men/TGW, in whom Z-scores were approximately 0.5 SDs lower than expected. This lower than expected BMD has been previously observed in the CDC study and iPrEx study and suggests that behavioural or environmental factors may be influencing BMD.6,19 This also suggests that these factors are not present or are not influencing BMD in at risk cisgender women. It should be noted that higher BMD is associated with increasing BMI20 and the high median BMI in the female participants could partially explain the relatively high BMD at baseline. Second, similar to the VOICE study, which examined tenofovir disoproxil fumarate-containing PrEP in young women in Uganda and Zimbabwe,11 we found no effect of tenofovir disoproxil fumarate-containing PrEP on changes in BMD over 48 weeks when examining the whole female population. However, when we restricted to the 54% of women in whom drug concentrations were measured and were quantifiable, we found that the magnitude of the changes in hip BMD related to tenofovir disoproxil fumarate was similar in the men and the women. This is in contrast to a randomized controlled trial of people living with HIV initiating tenofovir disoproxil fumarate-containing ART, in which greater bone loss was observed among women compared with men.21 Our findings suggest that hip BMD is similarly affected with tenofovir disoproxil fumarate-containing PrEP in men and women, despite differences in baseline BMD.
In an exploratory analysis, we examined the association between on-treatment plasma tenofovir concentrations and 48 weeks BMD loss and whether this relationship differed by sex. In the iPrEX-OLE demonstration project in men and TGW, a monotonic relationship was observed between concentrations of tenofovir diphosphate in dried blood spots and BMD loss at the spine and hip over a median of 24 weeks.22 In our study, we examined BMD changes over 48 weeks and found that plasma tenofovir concentrations at 24 weeks, but not at 48 weeks, were linearly associated with BMD loss at the hip. Importantly, our results suggest that the relationship between 24 week plasma tenofovir concentrations and 48 week BMD loss at the hip did not differ by sex, providing some further reassurance of the bone safety of tenofovir disoproxil fumarate-containing PrEP in women.
Three treatment arms in our study contained maraviroc, a CCR5 receptor antagonist. In addition to its expression on T cells, CCR5 is expressed on both osteoblasts and osteoclasts and one of its primary ligands on osteoblasts is MIP-1α, a protein secreted in abundance by osteoclasts,23,24 suggesting that communication between osteoblasts and osteoclasts may in part be mediated by CCR5. However, the functional consequences of CCR5 inhibition or deficiency on bone have yielded conflicting results in preclinical models.25–28 In a study of individuals living with HIV initiating ART with darunavir/ritonavir/emtricitabine, maraviroc was associated with less bone loss than tenofovir disoproxil fumarate over 48 weeks and the effect of maraviroc on bone appeared to be neutral.29 Similarly, in the present study, the presence of maraviroc did not influence the BMD trajectory, providing further evidence for its lack of effect on BMD. Despite its tolerability and apparent bone safety, maraviroc has not been further developed for PrEP use due to the simultaneous development of longer-acting PrEP regimens.
The clinical significance of the approximately net 2% loss of hip BMD over 48 weeks attributable to tenofovir disoproxil fumarate-containing PrEP in those who with quantifiable drug concentrations is unclear. Given that PrEP is generally taken for a relatively short period30 and the effects on BMD appear to be reversible,10,11 tenofovir disoproxil fumarate-containing PrEP is unlikely to lead to clinically significant bone effects for most individuals. However, in patients who are at higher risk of fracture, based on age or other concomitant risk factors, especially for those who expect to require PrEP for long periods, tenofovir disoproxil fumarate-limiting ‘on-demand’ PrEP or non-tenofovir disoproxil fumarate-containing PrEP may be safer alternatives, such as tenofovir alafenamide/emtricitabine.9
Our study had several limitations. We only had one post-baseline DXA scan so we were unable to define the trajectory of the BMD changes over a longer period and may have missed early changes in BMD before 48 weeks. We also did not have a DXA scanning after discontinuation and therefore could not comment on the reversibility of the bone effects. We did not assess drug concentrations in all men/TGW participants, so many participants were excluded from the as-treated analysis whose adherence to study medications was unknown. Finally, we studied a US population, so the findings may not be generalizable to other populations around the world.
In conclusion, we found loss of BMD in the hip, but not the LS, in US men and women taking tenofovir disoproxil fumarate-containing PrEP compared with non-tenofovir disoproxil fumarate-containing PrEP, among those who had quantifiable drug concentrations, with no appreciable difference in the magnitude of the effect in men and women. The magnitude of BMD effect at the hip was relatively small, so these findings should be reassuring regarding the bone safety of tenofovir disoproxil fumarate-containing PrEP in US populations.
Funding
This work was supported by the Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) through the HIV Prevention Trials Network (HPTN) [UM1-AI068619, UM1-AI068613, UM1-AI068617] and the AIDS Clinical Trials Group (ACTG) [UM1-AI-068636]. Gilead Sciences and ViiV Healthcare provided study drugs.
Transparency declarations
T.T.B. has served as a consultant to Gilead Sciences, Janssen, Merck, ViiV Healthcare, EMD-Serono and Theratechnologies. T.J.W. has received research grants (to Weill Cornell) from Gilead Sciences and GlaxoSmithKline/ViiV Healthcare and served as an ad hoc consultant to GlaxoSmithKline/ViiV Healthcare. R.J.L. has served as a consultant to Gilead Sciences, Merck and Roche. K.H.M. has received unrestricted research grants (to Fenway Health) from Gilead Sciences and GlaxoSmithKline/ViiV Healthcare and served on scientific advisory boards of Gilead Sciences and Merck, Inc. C.W.H. receives grants from ViiV Healthcare/GlaxoSmithKline, Merck and Gilead and serves on scientific advisory boards for ViiV Healthcare/GlaxoSmithKline and Merck. R.M.G., K.Y., Y.Q.C., M.A.M., K.L.K., W.C. and M.B.M. have no conflicts of interest.
Supplementary data
Table S1 is available as Supplementary data at JAC Online.
Supplementary Material
References
- 1.Riddell JT, Amico KR, Mayer KH.. HIV preexposure prophylaxis: a review. JAMA 2018; 319: 1261–8. [DOI] [PubMed] [Google Scholar]
- 2.Grant PM, Cotter AG.. Tenofovir and bone health. Curr Opin HIV AIDS 2016; 11: 326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedimo R, Maalouf NM, Zhang Set al. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS 2012; 26: 825–31. [DOI] [PubMed] [Google Scholar]
- 4.Borges AH, Hoy J, Florence Eet al. Antiretrovirals, fractures, and osteonecrosis in a large international HIV cohort. Clin Infect Dis 2017; 64: 1413–21. [DOI] [PubMed] [Google Scholar]
- 5.Mulligan K, Glidden D, Gonzales Pet al. Effects of FTC/TDF on bone mineral density in seronegative men from 4 continents: DEXA results of the global iPrEx study. Eighteenth Conference on Retroviruses and Oppourtunistic Infections, Boston, MA, USA, 2011. Abstract 94LB.
- 6.Liu AY, Vittinghoff E, Sellmeyer DEet al. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One 2011; 6: e23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasonde M, Niska RW, Rose Cet al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One 2014; 9: e90111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havens PL, Stephensen CB, Van Loan MDet al. Decline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxis. Clin Infect Dis 2017; 64: 317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer KH, Molina JM, Thompson MAet al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 2020; 396: 239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glidden DV, Mulligan K, McMahan Vet al. Recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2017; 76: 177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirembe BG, Kelly CW, Mgodi Net al. Bone mineral density changes among young, healthy African women receiving oral tenofovir for HIV preexposure prophylaxis. J Acquir Immune Defic Syndr 2016; 71: 287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulick RM, Wilkin TJ, Chen YQet al. Phase 2 study of the safety and tolerability of maraviroc-containing regimens to prevent HIV infection in men who have sex with men (HPTN 069/ACTG A5305). J Infect Dis 2017; 215: 238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulick RM, Wilkin TJ, Chen YQet al. Safety and tolerability of maraviroc-containing regimens to prevent HIV infection in women: a phase 2 randomized trial. Ann Intern Med 2017; 167: 384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schousboe JT, Shepherd JA, Bilezikian JPet al. Executive summary of the 2013 international society for clinical densitometry position development conference on bone densitometry. J Clin Densitom 2013; 16: 455–66. [DOI] [PubMed] [Google Scholar]
- 15.Hendrix CW, Chen BA, Guddera Vet al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PloS One 2013; 8: e55013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emory JF, Seserko LA, Marzinke MA.. Development and bioanalytical validation of a liquid chromatographic-tandem mass spectrometric (LC-MS/MS) method for the quantification of the CCR5 antagonist maraviroc in human plasma. Clin Chim Acta 2014; 431: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McComsey GA, Kitch D, Daar ESet al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011; 203: 1791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stellbrink HJ, Orkin C, Arribas JRet al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 2010; 51: 963–72. [DOI] [PubMed] [Google Scholar]
- 19.Mulligan K, Glidden DV, Anderson PLet al. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2015; 61: 572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Laet C, Kanis JA, Oden Aet al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 2005; 16: 1330–8. [DOI] [PubMed] [Google Scholar]
- 21.Kalayjian RC, Albert JM, Cremers Set al. Women have enhanced bone loss associated with phosphaturia and CD4+ cell restoration during initial antiretroviral therapy. AIDS 2018; 32: 2517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinelli MA, Glidden DV, Anderson PLet al. Impact of estimated pre-exposure prophylaxis (PrEP) adherence patterns on bone mineral density in a large PrEP demonstration project. AIDS Res Hum Retroviruses 2019; 35: 788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yano S, Mentaverri R, Kanuparthi Det al. Functional expression of β-chemokine receptors in osteoblasts: role of regulated upon activation, normal T cell expressed and secreted (RANTES) in osteoblasts and regulation of its secretion by osteoblasts and osteoclasts. Endocrinology 2005; 146: 2324–35. [DOI] [PubMed] [Google Scholar]
- 24.Han JH, Choi SJ, Kurihara Net al. Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood 2001; 97: 3349–53. [DOI] [PubMed] [Google Scholar]
- 25.Andrade I Jr., Taddei SR, Garlet GPet al. CCR5 down-regulates osteoclast function in orthodontic tooth movement. J Dent Res 2009; 88: 1037–41. [DOI] [PubMed] [Google Scholar]
- 26.Yang YF, Mukai T, Gao Pet al. A non-peptide CCR5 antagonist inhibits collagen-induced arthritis by modulating T cell migration without affecting anti-collagen T cell responses. Eur J Immunol 2002; 32: 2124–32. [DOI] [PubMed] [Google Scholar]
- 27.Vierboom MP, Zavodny PJ, Chou CCet al. Inhibition of the development of collagen-induced arthritis in rhesus monkeys by a small molecular weight antagonist of CCR5. Arthritis Rheum 2005; 52: 627–36. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto H, Kamatani N.. A CCR-5 antagonist inhibits the development of adjuvant arthritis in rats. Rheumatology (Oxford) 2006; 45: 230–2. [DOI] [PubMed] [Google Scholar]
- 29.Taiwo BO, Chan ES, Fichtenbaum CJet al. Less bone loss with maraviroc- versus tenofovir-containing antiretroviral therapy in the AIDS clinical trials group A5303 study. Clin Infect Dis 2015; 61: 1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coy KC, Hazen RJ, Kirkham HSet al. Persistence on HIV preexposure prophylaxis medication over a 2-year period among a national sample of 7148 PrEP users, United States, 2015 to 2017. J Int AIDS Soc 2019; 22: e25252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.