Abstract
Pathogenic Escherichia coli O157:H7, as well as nonpathogenic strains ATCC 11775 and ATCC 23716, grew exponentially in wounds on Golden Delicious apple fruit. The exponential growth occurred over a longer time period on fruit inoculated with a lower concentration of the bacterium than on fruit inoculated with a higher concentration. The bacterium reached the maximum population supported in the wounds regardless of the initial inoculum concentrations. Populations of E. coli O157:H7 in various concentrations of sterilized apple juice and unsterilized cider declined over time and declined more quickly in diluted juice and cider. The decline was greater in the unsterilized cider than in juice, which may have resulted from the interaction of E. coli O157:H7 with natural populations of yeasts that increased with time. Experiments on the transmission of E. coli by fruit flies, collected from a compost pile of decaying apples and peaches, were conducted with strain F-11775, a fluorescent transformant of nonpathogenic E. coli ATCC 11775. Fruit flies were easily contaminated externally and internally with E. coli F-11775 after contact with the bacterium source. The flies transmitted this bacterium to uncontaminated apple wounds, resulting in a high incidence of contaminated wounds. Populations of the bacterium in apple wounds increased significantly during the first 48 h after transmission. Further studies under commercial conditions are necessary to confirm these findings.
Fruits and vegetables contain nutrients necessary for the rapid growth of food-borne pathogens, yet outbreaks of illness caused by ingestion of fruits and vegetables are less frequent than outbreaks from other foods (1–4, 10). This is due, in part, to external barriers such as the peel and rind, which prevent microorganisms from entering and subsequently growing in the interiors of fruits and vegetables (10). However, in some cases, such as on wounded fruit or fresh cut fruit slices, this external barrier is broken, thus creating an opportunity for bacterial colonization.
Escherichia coli O157:H7 was shown to cause hemorrhagic colitis and gastroenteritis in the United States for the first time in 1982 (23). Since then food-borne outbreaks have been associated with various meats and fresh produce (11, 15, 24). In the fall of 1996, there were four outbreaks of food-borne illness related to contaminated unpasteurized fresh apple cider, including the E. coli serotype O157:H7 outbreak which resulted in the death of a 16-month-old girl. This was in addition to earlier outbreaks from unpasteurized apple cider in 1991 and 1993 (6, 24). The actual source of contamination of apple cider in these outbreaks was not determined, but various potential contamination sources and events before and after harvest have been suggested (5, 24). Many enteric food-borne pathogens, including E. coli O157:H7, have reservoirs in healthy animals (22, 24, 27). This bacterium was found in the feces of birds (25), domestic animals (22, 27), and feral animals (e.g., deer) (22).
A fundamental question that must be answered is whether enterohemorrhagic E. coli must be deposited on fruit in numbers sufficient to cause human illness after consumption of the fruit or its products or if low initial levels of this bacterium deposited on fruit can reproduce, increasing the risk of human illness.
The present study was conducted to determine the population dynamics of E. coli on wounded apple tissue, in apple juice, and in apple cider after inoculation with various levels of the bacterium. Since our earlier work with wounded apples demonstrated bacterial contamination of apple wounds by fruit flies (unpublished observation), the potential of fruit flies to transmit E. coli to wounded apples was also examined.
MATERIALS AND METHODS
E. coli strains and inoculum preparation.
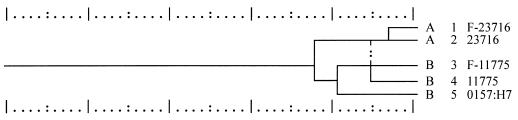
Two nonpathogenic strains of E. coli, ATCC 11775 and ATCC 23716, and one pathogenic serotype, O157:H7, were used. The two nonpathogenic strains had been modified to contain the green fluorescent protein (14). This allowed them to be easily distinguished from any background populations under UV light. BIOLOG tests (Biolog Inc., Hayward, Calif.) of these strains on GN plates resulted in a tight clustering of strains ATCC 11775, F-11775, and O157:H7 after the data were subjected to the MLCLUST program (Fig. 1).
FIG. 1.
Dendrogram of BIOLOG profiles of nonpathogenic fluorescent and nonfluorescent strains of E. coli and pathogenic O157:H7. BIOLOG GN plates were read 4 h after application of the bacteria. Results from the BIOLOG test were subjected to cluster analysis with the MLCLUST program based on the unweighted pair group method with arithmetic means. The scale is in units of taxonomic distance. To help distinguish patterns in the dendrogram, the strains are divided into groups designated by letters.
Most of the experiments were conducted with the nonpathogenic strain F-11775, and some were done with the pathogenic strain. The inocula were prepared by transfer from a slant culture of E. coli O157:H7 grown on nutrient yeast dextrose agar medium (Difco, Detroit, Mich.) or a culture of the nonpathogenic strains grown on Luria Bertani (LB) agar plates to LB broth (Difco) in Erlenmeyer flasks, incubated on a rotary shaker (150 rpm) overnight at 26°C. The cultures were harvested by centrifugation at 7,000 × g, resuspended in 0.85% NaCl solution, and adjusted to the desired concentration by measurement of the absorbance (420 nm) with a spectrophotometer. In experiments with the inoculation of apple juice and cider the original concentration of approximately 108 CFU/ml was used, and with the inoculation of apple tissue the original concentration and 100- and 10,000-fold dilutions of the bacterial suspension were used.
Inoculation and recovery of E. coli from apple juice and cider.
Apple cider and juice were made from apples that were surface sterilized with 70% ethanol and ground in a household juicer previously sanitized with antibacterial soap and boiling water. The foam portion was discarded and the remaining fresh unpasteurized cider was used for the experiments. To make the juice, cider was centrifuged at 23,500 × g, and the clear supernatant was sterilized by being passed through a 0.22-μm-pore-size Millipore filter. The full-strength apple juice and apple juice diluted to 50 and 25% had pHs of 3.7, 3.62, and 3.55, respectively. The pH of cider was the same as that of apple juice. One hundred-milliliter volumes of the cider or juice at full strength and diluted to 50 and 25% with water were added to 250-ml Erlenmeyer flasks and inoculated with 1 ml of an E. coli O157:H7 suspension at a concentration of 108 CFU/ml. The flasks were held at 26°C on a shaker at 150 rpm. Samples were collected immediately after inoculation and after incubation for 24, 48, 72, and 144 h. The samples were diluted in 0.85% saline, plated on LB agar with a spiral plater (Spiral Biotech, Bethesda, Md.) in duplicate, and incubated at 24°C for 48 h. The colonies were counted with a laser bacterial colony counter (model 500 A; Spiral Biotech), and the numbers of CFU per milliliter were determined with the BEN 4.0 software program (Spiral Biotech). Three flasks of each cider and juice concentration were inoculated with 108 CFU/ml.
Inoculation and recovery of E. coli from fruit.
Golden Delicious apples were wounded (one wound per fruit) by aseptic removal of a cylinder of tissue 3 mm in diameter and 3 mm deep midway between the calyx and stem end. The apples were placed in plastic boxes on fruit pack trays, and 25 μl of the E. coli suspension was deposited in each wound. There were three concentrations of the bacterium 104, 106, and 108 CFU/ml, for each of the three strains. The levels of E. coli in the wounds were determined within 1 h after application (0 h) and after 24, 48, 72, and 144 h of incubation at 24°C (ambient temperature). To recover E. coli, a cylinder of apple tissue (1 cm in diameter and 1 cm deep) containing the entire wound was removed with a sterile cork borer, put in a stomacher bag with 4.5 ml of 0.05 M phosphate buffer (pH 6.5), and blended in a Stomacher 80 blender (Seward Medical, London, England) for 2 min at normal speed. The resulting slurry was filtered though glass wool, plated in duplicate on LB medium by using the spiral plater, and incubated at 24°C for 48 h. Colony enumeration was conducted with the laser counter as described above. There were three replications per treatment, and treatments were arranged in a completely randomized block design.
Preparation of fruit fly cultures.
Two glass boxes (22.5 by 22.5 by 26.0 cm), open on top and containing 10 fresh apples cut in half, were put outside near a compost pile of decaying apples and peaches in October 1997. In each glass box were two plastic vials (3.2 cm wide and 9.5 cm high) with about 20 ml of Drosophila diet (Formula 4-24 instant Drosophila medium; Carolina Biological Supply Company) made with 1 part dry medium, 1.5 parts apple cider, and 8 to 10 grains of yeast. After 1 day in the field, the vials were collected and a foam stopper was placed in the top of each vial, trapping 10 to 30 flies per vial. The vials were placed in a growth chamber at 21°C with a 12-h/12-h light/dark cycle. After 1 week the adult flies were removed from the vials and F1 flies began to emerge after 14 days. After one generation, the laboratory culture contained a single species and all parasites had been removed. The fruit flies were identified as Drosophila melanogaster Meigen by Tam Nguyen, Department of Entomology, American Museum of Natural History, New York, N.Y. Three times each week, a new culture vial was initiated with 15 to 30 laboratory-reared flies per vial. Adult flies were removed after 1 week so that any new flies in the vials would be newly emerged flies. Newly emerged flies were collected by anesthetizing the flies with CO2, after which they were then kept on an ice pack for introduction into the chamber for E. coli studies.
Contamination of fruit flies with E. coli.
Experiments on the contamination of fruit flies with E. coli and the transmission of E. coli to apple wounds were conducted with glass chambers (22.5 by 22.5 by 26.0 cm). One side of the chamber was covered with a removable screen with a mesh size small enough to contain the fruit flies. Ten fruit flies were put into each chamber in an open small petri dish (5-cm diameter), and filter paper soaked in a suspension of E. coli ATCCF-11775 at approximately 8 × 108 CFU/ml in 20% apple juice was placed in another small petri plate. Two vials (1 cm wide and 3.5 cm high) filled with water were inverted on the filter paper to prevent rapid desiccation of the filter (the filter paper remained moist for approximately 18 h). The chambers were closed with the screen, and fruit flies, after waking up, were allowed to feed on the juice. There were four chambers, each designated for a different feeding period. The fruit flies were sampled after 2, 6, 24, and 48 h. To collect fruit flies, a chamber was flushed with CO2 until all flies were anesthetized. Then the chamber was opened and the flies were placed in 10-cm-diameter petri plates with LB agar medium (one fly per plate); after waking up, the flies were allowed to walk for 10 min on the medium. The plates on which the flies walked were rated for growth of the bacterium after incubation for 48 h at 24°C according to the following scale: 0, no growth; 1, 1 to 10 colonies per plate, 2, >10 colonies per plate; and 3, trails. The identity of E. coli ATCCF-11775 was confirmed by fluorescence under UV light.
To determine the level of internal contamination of fruit flies with E. coli, at each sampling time, after the flies had been allowed to walk for 10 min on the agar plate, the plates containing the flies were placed in a freezer for approximately 10 min to anesthetize the flies. Each fly was then placed in a test tube with 5 ml of 70% ethanol for 1 min for surface sterilization. After sterilization the flies were blotted on paper, transferred to test tubes with 5 ml of sterile distilled water, blotted on paper again, and ground with a mortar and pestle in 5 ml of saline. The suspension was plated on LB agar with the spiral plater and incubated for 48 h at 30°C. Enumeration of the colonies was conducted with a laser counter as described above.
Transmission of E. coli to apple wounds by fruit flies.
The experiments for testing transmission to apple wounds were conducted in the glass chambers described above for contamination of fruit flies with E. coli. Golden Delicious apples were wounded as described above, except that the wounds were made close to the stem end, and six apples were placed in each chamber. Two 5-cm-diameter petri dishes, one with 10 anesthetized fruit flies and the other with a filter paper disk saturated with E. coli F-11775, were placed in the center of each chamber as described above. The chambers were closed with the screen and fruit was exposed to fruit fly contamination for 7, 24, and 48 h. At each time, CO2 gas from a cylinder was introduced into the boxes until all flies were anesthetized. The fruit was removed from the chambers, and the individual flies were placed in mortars containing 4.5 ml of 0.85% saline. The flies were ground with a pestle, and the resulting slurry was filtered though glass wool-packed syringes, diluted, and plated on LB agar with the spiral plater. The plates were incubated at 30°C and colonies were counted as described above.
To determine the efficacy of transmission, E. coli was recovered from wounds of two (of the six) fruits immediately after removal from the chamber, from two other fruits after 24 h of incubation, and from the last two pieces of fruit after 48 h of incubation. Thus, there were three times of exposure to fruit fly inoculation (each in a separate chamber), and for each exposure time there were three incubation periods (0, 24, and 48 h) after which E. coli was recovered from the wounds. The experiment was repeated twice.
Transmission of E. coli from fruit to fruit by fruit flies.
The experiment for testing transmission from fruit to fruit was similar to the previous one on the transmission of E. coli F-11775 to apple wounds by fruit flies, except that wounds on three apples were inoculated with E. coli F-11775 (25 μl of aqueous suspension; 108 CFU/ml) 1 day (incubated at 24°C) before the fruit was placed in a chamber, and the other three apples were wounded just before being placed in a chamber. Only a 5-cm-diameter petri dish with 10 anesthetized fruit flies was placed in the center of the chamber. Also, E. coli was recovered from three fruits immediately after inoculation. In one set of three boxes, the fruit flies were allowed to serve as a vector between the contaminated and uncontaminated apples for 24 h; for another set 48 h was the amount of time used. After exposure, the chambers were placed on dry ice in plastic boxes and the tops were covered with identical boxes. The fruit flies were anesthetized by the CO2 evolving from the dry ice and the low temperature and then were removed. From each of the three boxes from each exposure time, one originally uninoculated fruit was removed immediately after opening, one was removed after 24 h of incubation, and one was removed after 48 h of incubation, and E. coli was recovered from the wounds of these fruits as described above. An additional chamber was a control with no fruit flies, where uninoculated fruit was assayed for E. coli after 48 h of incubation. The experiment was conducted twice.
RESULTS
Population dynamics of E. coli strains on apple tissue.
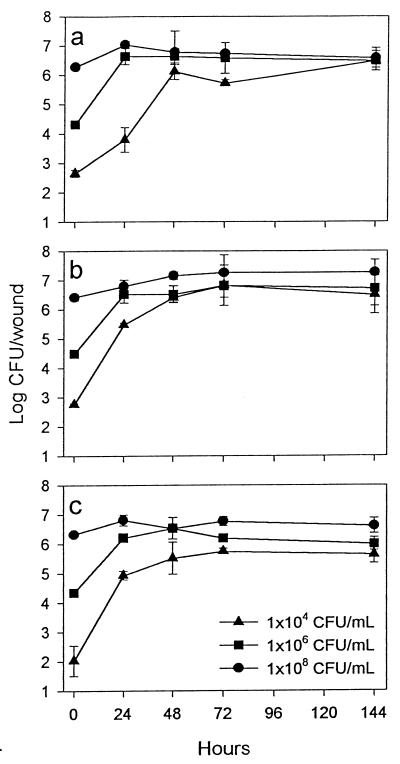
The populations of all three strains of E. coli increased exponentially after inoculation of apple tissue (Fig. 2). The greatest extent of increase was observed with the smallest inoculum (2.5 × 102 CFU/wound); populations increased approximately 3 log units during the first 48 h of incubation. Inoculation with 2.5 × 104 and 2.5 × 106 CFU/wound resulted in increases of approximately 2 log units and 1 log unit, respectively. From 48 to 144 h, populations changed only slightly.
FIG. 2.
Recovery of E. coli ATCC F-23716 (a), F-11775 (b), and O157:H7 (c) from exposed apple tissue. Golden Delicious apples were wounded and then inoculated with one of the three concentrations of each strain of the bacterium, and populations were recovered after incubation at 24°C for various periods of time. Bars represent standard errors of the means.
Survival of E. coli O157:H7 in apple juice and cider.
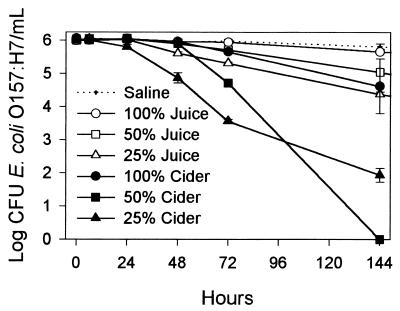
Recovery of E. coli O157:H7 from sterilized apple juice and recovery from unsterilized cider differed (Fig. 3). In both cases populations of E. coli O157:H7 declined; however, a greater decline occurred in apple cider. Generally, the more diluted the juice or cider, the greater the decline in bacterial populations. After 144 h, the population declines in juice were about 1.5, 1, and 0.5 log unit in 25, 50, and 100% juice, respectively. Population declines in cider were significantly greater than in juice from 48 to 144 h of incubation and after 144 h ranged from 1.5 log units for 100% cider to 4 log units in 25% cider and 6 log units in 50% cider. During the first 72 h of incubation, population declines in cider and juice at various concentrations followed the same pattern. A significant growth of cider yeasts was observed from the recovery at 48 h to the end of the experiment.
FIG. 3.
Survival of E. coli O157:H7 in apple juice and apple cider at various concentrations and in saline. Bars represent standard errors of the means.
Contamination of fruit flies with E. coli.
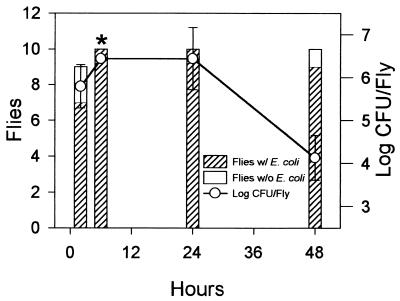
Since the population dynamics of the three strains of E. coli on apple tissue were similar, for experimental-safety reasons, we selected nonpathogenic strain F-11775 to study transmission of E. coli by fruit flies. Seven of 9 flies carried E. coli externally after 2 h of exposure to contaminated apple juice, and all 10 flies carried E. coli after exposure for 6 and 24 h. Six of 10 flies were dead after 48 h of exposure, most likely from lack of moisture, since the filters dried out after 18 h. After 48 h, three of the four live flies were contaminated externally with E. coli. The average levels of external contamination on flies exposed to contaminated juice for 2, 6, 24, and 48 h were 1.2, 2.2, 1.9, and 0.8, respectively, according to our rating scale. Internal contamination of fruit flies with E. coli followed a similar pattern, and the greatest contamination occurred during the first 24 h, after which there was a decline of 2 log units between 24 and 48 h of exposure (Fig. 4).
FIG. 4.
Internal contamination of fruit flies after exposure to E. coli F-11775-contaminated apple juice. The same flies which were rated for external contamination were surface sterilized, ground, and plated on LB agar medium, and internal populations were determined after incubation of plates at 24°C for 48 h. Bars represent standard errors of the means for contaminated flies. The asterisk indicates that the actual populations of the bacterium at 6 h were higher than recorded since the colonies on all plates were too numerous to count and they were plated at the same dilutions as for 48 h.
Transmission of E. coli to apple wounds by fruit flies.
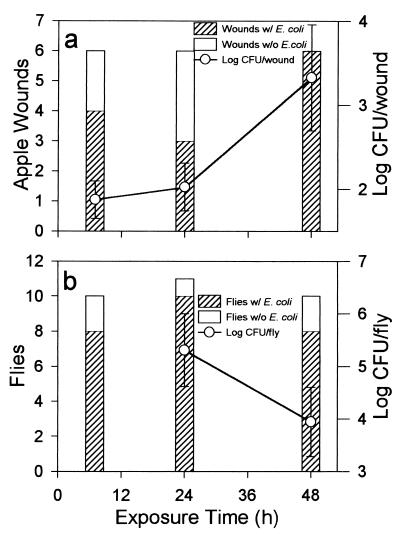
Six apples were evaluated for contamination with E. coli F-11775 by flies carrying this bacterium at each exposure period. Four, three, and all six apples (wounds) were contaminated with the bacterium after 6, 24, and 48 h of exposure, respectively (Fig. 5a). Populations of the bacterium in these wounds after 48 h of incubation following removal from the chambers increased as the exposure to fruit fly contamination increased. Most of the flies were contaminated after 6 h of exposure (Fig. 5b). There was no detectable contamination in control treatments.
FIG. 5.
Transmission of E. coli F-11775 to apple wounds by fruit flies (a) and contamination of fruit flies by E. coli F-11775 (b) after exposure of wounded apples to fruit flies and contaminated apple juice in chambers. The populations of the bacterium in apple wounds (a) and in or on fruit flies (b) were averaged for infected wounds and infected flies. Bars represent standard errors of the means.
Fruit-to-fruit transmission of E. coli by fruit flies.
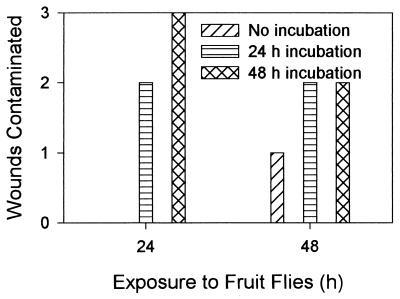
E. coli F-11775 was detected in wounds of noncontaminated apples exposed to contaminated apples and fruit flies for 24 h and incubated after removal from the chamber for 24 and 48 h, as well as on apples exposed for 48 h without incubation and after incubation for 24 and 48 h (Fig. 6). The average populations of the bacterium in the contaminated wounds were similar (approximately 105 CFU/wound) regardless of incubation time. However, there were large variations in population sizes on individual apples after 48 h of incubation, as indicated by the standard errors of the means (Table 1).
FIG. 6.
Transmission of E. coli F-11775 from apple wounds contaminated with the bacterium to noncontaminated wounds by fruit flies. Wounded contaminated and noncontaminated apples and fruit flies were placed in chambers for 24 and 48 h. Populations of the bacterium in originally noncontaminated wounds were evaluated immediately after removal from chambers and after 24 and 48 h of incubation at 24°C.
TABLE 1.
Populations of E. coli F-11775 in wounds of apples originally not contaminated with the bacterium
| Length (h) of fruit incubation after removal from chamber | Bacterium populationa (CFU/wound) after exposure to fruit flies for:
|
|
|---|---|---|
| 24 h | 48 h | |
| 0 | NDb | 5.2 × 104 |
| 24 | 2.0 × 105 ±2.4 × 105 | 1.6 × 105 ± 2.1 × 105 |
| 48 | 8.3 × 104 ± 14.0 × 104 | 1.1 × 105 ± 0.1 × 105 |
Values are means ± standard errors of the means.
ND, not detectable.
DISCUSSION
Wounded apple tissue is an excellent substrate for the growth of E. coli. The maximum populations were approximately 106 to 107 CFU/wound, regardless of the initial inoculum. The growth patterns for the nonpathogenic fluorescent strain F-11775 and pathogenic O157:H7 in apple wounds were almost identical at all three inoculation levels. Clustering together of these bacteria in the BIOLOG/MLCLUST system further indicates their physiological similarity (catabolic potential). Knowledge of microbial contamination of fresh-cut produce is limited (7, 12, 13, 20), and there is an urgent need for proactive research to reduce outbreaks of food-borne diseases (8). The ability of E. coli to grow exponentially on apple tissue is important and should be taken into consideration by the apple processing industry in the Hazard Analysis Critical Control Point system. Unsanitary handling of fruits by workers might result in contamination and a rapid population increase of the bacterium.
The growth of E. coli in highly acidic apple wounds (pH ∼3.5) is interesting because bacteria in general colonize produce whose tissue has a higher pH than normally found in apple flesh, and populations of E. coli O157:H7 had been shown in our work and by others to decline in unpasteurized apple cider (26). Filamentous molds were considered a factor in this decline (26). However, the possible use of these molds for the reduction of populations of E. coli O157:H7 in apple cider was rejected because they caused spoilage of the cider. In our work, the decreased survival of E. coli O157:H7 in unpasteurized cider compared to that in sterilized apple juice may have resulted from an interaction with natural populations not of filamentous molds but of yeasts which survived surface sterilization of apples before the cider was made. These yeasts were much more noticeable on culture plates at the later sampling times. It may be worthwhile to explore the possibility of using some of these yeasts, which do not cause spoilage and have acceptable organoleptic qualities (some of which may be used in fermented cider), for treating apple slices to prevent potential colonization by E. coli.
The growth of E. coli in apple wounds was not totally unexpected because various bacterial biological control agents, including Pseudomonas syringae, which is used commercially (BioSave 110; EcoScience Corp., Orlando, Fla.) to control Penicillium expansum (causing blue mold) and Botrytis cinerea (causing gray mold) on apples and pears after harvest, also grow exponentially in apple wounds (16, 17). The growth of E. coli and some other bacteria on apple tissue may have resulted from the ability of these bacteria to modify the adjacent microenvironment. Such modifications would be much more difficult in liquid apple juice or cider than on the solid surface of apple tissue. Similar results were observed with growth of Salmonella montevideo on tomatoes, where this bacterium grew at pH levels that would not be expected to support growth of the bacterium (28).
The high frequency of external and internal contamination of fruit flies during relatively short periods of exposure to an E. coli source, and the high incidence of contamination of apple wounds with E. coli by these flies, indicates the potential for fruit flies to transmit E. coli to apples. Fruit flies have previously been shown to be important in the dissemination of fruit pathogens such as Rhizopus stolonifer, Mucor pyriformis, Geotrichum candidum, and B. cinerea (9, 18, 19). They may also transmit E. coli in an orchard, especially during harvest, when fruit flies are abundant, or during fruit handling operations after harvest. Some newly introduced apple cultivars, e.g., Gala, have a tendency to crack during some years as harvest approaches (21). This creates natural uncolonized wounds which could be colonized by E. coli, which could then be disseminated by fruit flies. However, for this to happen, fruit flies, a source of E. coli, and conditions conducive for the bacterium to grow must occur. The potential sources of E. coli for fruit contamination are numerous (5). One possible source may be bird droppings, which are plentiful on apples during harvest, as many birds feed on the maturing fruit (25). Birds are also involved in disseminating various food-borne pathogens such as Campylobacter, Salmonella, Vibrio cholerae, and Listeria species (5). Dropped apples may also be exposed to feces of domestic or feral animals (22, 24, 27). The contamination of damaged apples with E. coli O157:H7 might then be spread by fruit flies to other fruits with wounds caused by natural cracking, birds, insects, hail, or other means. Regardless of the source of the original fruit contamination, our results indicate that fruit-to-fruit transmission by fruit flies is possible and that the transmitted cells can grow rapidly in wounds. Further tests in an orchard are necessary to determine the significance of fruit flies in frequency of transmission and population dynamics of E. coli in apple wounds. Also, additional tests should be conducted in packinghouses, because the possibility for fruit-to-fruit transmission exists, especially in the vicinity of sorting lines, where fruit flies and damaged apples are plentiful and environmental conditions are less stressful for the flies and the bacterium. The conditions for contamination of apples in the orchard or packinghouse are probably low-frequency events, because there are no reports that fresh apples are the source of food-borne illness. Furthermore, the few cider outbreaks indicate that conditions for natural fruit contamination are indeed rare circumstances.
Our results indicate that we need to be vigilant and proactive in investigating microbiological safety, especially in the newly emerging and rapidly expanding fresh-cut industry, where little is known about the microbiology of the produce and thus unexpected problems may occur.
ACKNOWLEDGMENTS
We thank C. Sharer for assistance with the experiments, A. Mattrezzo for preparing cultures, and O. Planket for preparing figures.
REFERENCES
- 1.Abdul-Raouf U M, Beuchat L R, Ammar M S. Survival and growth of Escherichia coli O157:H7 on salad vegetables. Appl Environ Microbiol. 1993;59:1999–2006. doi: 10.1128/aem.59.7.1999-2006.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. Foodborne pathogens: risk and consequences. Task force report. Council for Agricultural Science and Technology no. 122. Ames, Iowa: Council for Agricultural Science and Technology; 1994. [Google Scholar]
- 3.Bean N H, Griffin P M. Foodborne diseases outbreaks in the United States, 1973–1987: pathogens, vehicles, and trends. J Food Prot. 1990;53:804–817. doi: 10.4315/0362-028X-53.9.804. [DOI] [PubMed] [Google Scholar]
- 4.Beuchat L R. Pathogenic microorganisms associated with fresh produce. J Food Prot. 1996;59:204–216. doi: 10.4315/0362-028X-59.2.204. [DOI] [PubMed] [Google Scholar]
- 5.Beuchat L R, Ryu J H. Produce handling and processing practices. Emerg Infect Dis. 1997;3:459–465. doi: 10.3201/eid0304.970407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besser R E, Lett S M, Weber J T, Doyle M P, Barrett T J, Wells J G, Griffin P M. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA. 1993;269:2217–2220. [PubMed] [Google Scholar]
- 7.Brackett R E. Microbiological spoilage and pathogens in minimally processed refrigerated fruits and vegetables. In: Wiley R, editor. Minimally processed refrigerated fruits and vegetables. New York, N.Y: Chapman & Hall; 1993. pp. 269–312. [Google Scholar]
- 8.Buchanan R L. Identifying and controlling emerging foodborne pathogens: research needs. Emerg Infect Dis. 1997;3:517–521. doi: 10.3201/eid0304.970416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler E E, Braker C E., Jr The role of Drosophila melanogaster in the epidemiology of Geotrichum, Rhizopus, and other fruit rots of tomato. Phytopathology. 1963;53:1016–1029. [Google Scholar]
- 10.Centers for Disease Control. Foodborne diseases outbreaks, 5–year summary, 1983–1987. Morbid Mortal Weekly Rep. 1990;39(SS-1):15–57. [PubMed] [Google Scholar]
- 11.del Rosario B A, Beuchat L R. Survival and growth of enterohemorrhagic Escherichia coli O157:H7 in cantaloupe and watermelon. J Food Prot. 1995;58:105–107. doi: 10.4315/0362-028X-58.1.105. [DOI] [PubMed] [Google Scholar]
- 12.Diaz C, Hotchkiss J H. Comparative growth of Escherichia coli O157:H7, spoilage organisms and shelf-life of shedded iceberg lettuce stored under modified atmospheres. J Sci Food Agric. 1996;70:433–438. [Google Scholar]
- 13.Doyle M P. Fruit and vegetable safety—microbiological considerations. HortScience. 1990;25:1478–1482. [Google Scholar]
- 14.Fratamico P M, Deng M Y, Strobaugh T P, Palumbo S A. Construction and characterization of Escherichia coli O157:H7 strains expressing firefly luciferase and green fluorescent protein and their use in survival studies. J Food Prot. 1997;60:1167–1173. doi: 10.4315/0362-028X-60.10.1167. [DOI] [PubMed] [Google Scholar]
- 15.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 16.Janisiewicz W J, Jeffers S N. Efficacy of commercial formulation of two biofungicides for control of blue mold and gray mold of apples in storage. Crop Prot. 1997;16:629–633. [Google Scholar]
- 17.Janisiewicz W J, Marchi A. Control of storage rots of various pear cultivars with a saprophytic strain of Pseudomonas syringae. Plant Dis. 1992;76:555–560. [Google Scholar]
- 18.Louise C, Girard M, Kuhl G, Lopez-Ferber M. Persistence of Botrytis cinerea in its vector Drosophila melanogaster. Phytopathology. 1996;86:934–939. [Google Scholar]
- 19.Michailides T J, Spotts R A. Transmission of Mucor pyriformis to fruit of Prunus persica by Carpophilus spp. and Drosophila melanogaster. Plant Dis. 1990;74:287–291. [Google Scholar]
- 20.Nguyen T-C, Carlin F. The microbiology of minimally processed fresh fruit and vegetables. Crit Rev Food Sci Nutr. 1994;34:371–401. doi: 10.1080/10408399409527668. [DOI] [PubMed] [Google Scholar]
- 21.Opara L U, Studman C J, Banks N H. Fruit skin splitting and cracking. Hortic Rev. 1997;19:217–262. [Google Scholar]
- 22.Rice D H, Hancock D D, Besser T E. Verotoxigenic E. coli O157 colonization of wild deer and range cattle. Vet Rec. 1995;137:524. doi: 10.1136/vr.137.20.524. [DOI] [PubMed] [Google Scholar]
- 23.Riley L W, Remis R S, Helgerson S D, McGee H B, Wells J G, Davis B R, Hebert R J, Olcott E S, Johnson L M, Hargett N T, Blake P A, Cohen M L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 24.Tauxe R V. Emerging foodborne diseases: an evolving public health challenge. Emerg Infect Dis. 1997;3:425–434. doi: 10.3201/eid0304.970403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace J S, Cheasty T, Jones K. Isolation of Vero cytotoxin-producing Escherichia coli O157:H7 from wild birds. J Appl Microbiol. 1997;82:399–404. doi: 10.1046/j.1365-2672.1997.00378.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhao T, Doyle M P, Besser R E. Fate of enterohemorrhagic Escherichia coli O157:H7 in apple cider with and without preservatives. Appl Environ Microbiol. 1993;59:2526–2530. doi: 10.1128/aem.59.8.2526-2530.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao T, Doyle M P, Shere J, Garber L. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl Environ Microbiol. 1995;61:1290–1293. doi: 10.1128/aem.61.4.1290-1293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuang R-Y, Beuchat L R, Angulo F J. Fate of Salmonella montevideo on and in raw tomatoes as affected by temperature and treatment with chlorine. Appl Environ Microbiol. 1995;61:2127–2131. doi: 10.1128/aem.61.6.2127-2131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]