Abstract
Inflammatory bowel disease (IBD) comprises a group of chronic inflammatory disorders of the gastrointestinal tract. Accumulating evidence shows that the development of IBD is always accompanied by the dysbiosis of the gut microbiota (GM), causing a decrease in prebiotic levels and an increase in harmful metabolite levels. This leads to persistent immune response and inflammation in the intestine, greatly impairing the physiological function of the gastrointestinal tract. Short-chain fatty acids (SCFAs) are produced by probiotic gut bacteria from a fiber-rich diet that cannot be digested directly. SCFAs with significant anti-inflammatory functions regulate immune function and prevent an excessive immune response, thereby delaying the clinical progression of IBD. In this review, we summarize the generation of SCFAs and their potential therapeutic effects on IBD. Furthermore, we suggest that SCFAs may modulate innate immune recognition and cytokine production to intervene in the progression of IBD. Additional randomized controlled trials and prospective cohort studies should also investigate the clinical impact of SCFA.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-022-00869-5.
Keywords: Inflammatory bowel disease, Immunomodulating activity, Therapeutic effects, Short-chain fatty acids, Gut microbiota
Background
Inflammatory bowel disease (IBD) is an autoimmune disease with two main clinical forms: Crohn's disease and ulcerative colitis (UC). IBD is currently incurable and affects the quality of life of an increasing number of people [1]. The pathogenesis of IBD is still unclear. It has been suggested that the development of IBD is related to genetics, gut microflora, and dietary habits [2, 3]. The use of agents blocking cytokines, such as tumor necrosis factor (TNF), suppresses the body's immune system and is clinically effective in patients with IBD. However, some cytokines that play a prominent role in the pathogenesis of patients with IBD, such as interleukin (IL)10 and IL17, do not have effective targeting agents [4, 5].
Short-chain fatty acids (SCFAs) produced from dietary fiber in the gut are increasingly favored by researchers for their excellent anti-inflammatory and anticancer effects [6]. Recent studies have shown that SCFAs play an active role in the treatment of inflammation-related diseases, such as hypertension, coronary artery disease, and the development of IBD [7, 8]. SCFAs may be excellent options for the prevention and mitigation of IBD. This article reviews the positive therapeutic effects of SCFAs in IBD and focuses on their modulatory effects on innate immune recognition and cytokine networks.
Levels of SCFAs in the gut are linked to the development of IBD
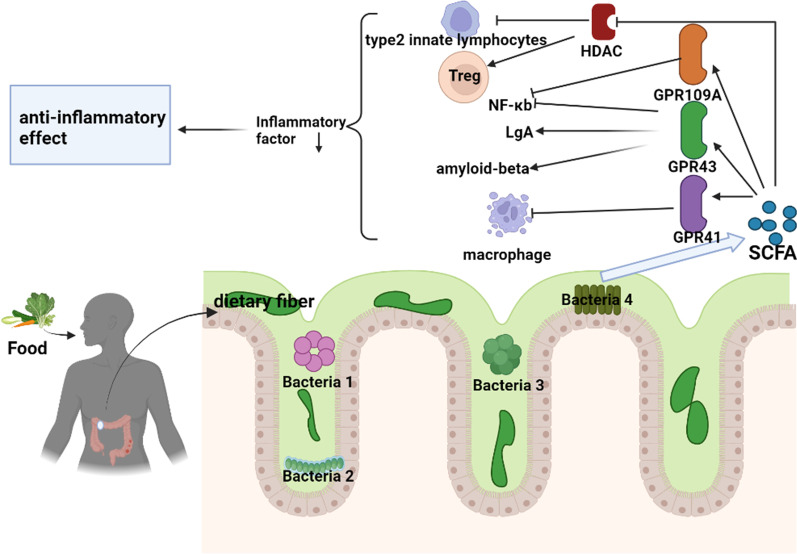
SCFAs, produced by gut microbes that metabolize dietary fiber, exhibit excellent anti-inflammatory effects (Fig. 1)—SCFAs have short chain length (not exceeding 6 carbon atoms) [9]. Prebiotic or microbially accessible carbohydrates, including plant-derived inulin, polysaccharides, and resistant starch, are substrates for SCFA synthesis [10]. However, the three main SCFAs with the highest abundance in the human gut are acetate, propionate, and butyrate. The ratio of their concentrations is 3:1:1 [11, 12]. A high fiber diet can produce approximately 400–800 mmol of SCFAs per day. The concentration and relative proportion of each SCFA in the intestine depends on the microbiota composition, substrate type, and gut transport time [12, 13]. SCFAs are mainly absorbed by colonic epithelial cells and provide energy for their vital activities [14, 15]. A small proportion of SCFAs that are not absorbed by the intestinal epithelium may exert anti-inflammatory, anticancer, and immunomodulatory functions in the gut [16]. Unused SCFAs are excreted in the feces and urine, although these are typically only about 5% of the total SCFAs [17, 18].
Fig. 1.
Dietary fiber is digested by intestinal microorganisms to form short-chain fatty acids, which exert anti-inflammatory activities through the G protein-coupled receptor pathway and histone acetylase. The cytokines refer to interleukin 23, interleukin 17 and interleukin beta, etc. They are produced by activation of innate and adaptive immunity after the microbiota is recognized by the immune system and are important contributors to the development of IBD. The Figures in this review were created with BioRender.com
The production of SCFAs is mainly regulated by the gut microbiota (GM), with Firmicutes mainly synthesizing butyrate and Bacteroides mainly synthesizing acetate and propionate [12]. Recent studies have shown that there are significant differences in gut microbial species, microbial diversity, and metabolic pathways between patients with IBD and healthy individuals [19, 20]. SCFAs are key prebiotics for maintaining intestinal health and their levels are significantly reduced in the feces of adult patients with IBD. SCFAs are involved in the development and progression of IBD [21]. Analysis of fecal microbiota composition by whole-genome birdshot sequencing previously revealed that the levels of microorganisms associated with SCFA production were substantially reduced in adult patients with IBD [22]. A human study showed that the levels of butyrate producing Faecalibacterium prausnitzii were reduced in patients with IBD [23]. Moreover, studies have shown that sodium butyrate supplementation has a positive clinical impact on patients with IBD [24]. In addition, impaired intestinal absorption of SCFAs may precede GM disorders and lead to the development of IBD [25, 26]. Before the loss of butyric acid-producing probiotics, researchers observed impaired oxidation of butyric acid at the intestinal mucosa level, which was also verified at the gene expression level [27, 28].
Anti-inflammatory mechanism of SCFAs
G protein-coupled receptors (GPCRs) are one of the major pathways that transduce signals from SCFAs, including GPR41, GPR43, and GPR109 [29]. The agonistic activity of SCFAs on GPR41 and GPR43 changes depends on concentration and is correlated with SCFA chain length. GPR43 is more active against acetate and propionate, while GPR41 is more active against SCFAs with long carbon chains [30–32]. GPR41 and GPR43 can almost be activated by all kinds of SCFAs, whereas GPR109a is mainly activated by butyrate and nicotinate [29, 33]. Recent studies have shown that, in addition to the gut, GPCR signaling can improve diseases of multiple body systems, including those of the nervous, cardiovascular, and respiratory systems [34–36]. GPCR signaling plays an anti-inflammatory role in a variety of inflammatory diseases, as shown in Table 1.
Table 1.
GPCRs signaling inhibits inflammatory diseases
| Inflammatory diseases | GPCRs | Functions |
|---|---|---|
| Inflammatory bowel disease | GPR43 | Promoting the production of IgA, suppressing intestinal inflammation [37, 38] |
| Increasing Amphiregulin expression levels in dendritic cells to promote tissue repair [39] | ||
| Inhibiting nuclear factor kappa-B activity [40] | ||
| GPR41 | Regulating macrophage activity [41] | |
| GPR109a | Inhibiting AKT and NF-κB p65 signaling pathways [42] | |
| Inhibiting IL-23 production [43] | ||
| Improving pathological angiogenesis and inflammatory changes [44] | ||
| Diabetic nephropathy | GPR43 | Inhibiting high glucose-induced NF-κB activation and oxidative stress [45] |
| GPR43 and GPR109A | Inhibiting inflammation in renal tubular cells and podocytes under hyperglycemic conditions [46] | |
| Vascular inflammation | GPR41 and GPR43 | Inhibiting pro-inflammatory cytokine production in LPS- or TNFα-stimulated HUVECs [47] |
| GPR109A | Playing an anti-atherosclerotic role [48] | |
| Nonalcoholic fatty liver disease | GPR43 | Inhibiting hepatic steatosis [37] |
| Rheumatoid arthritis | GPR43 | Significantly inhibiting the expression of key inflammatory factors in rheumatoid arthritis [49] |
| Osteoarthritis | GPR43 | Reducing the expression levels of pro-inflammatory mediators, pro-inflammatory adipokines, and adhesion molecules in chondrocytes [50] |
| Chronic rhinosinusitis | GPR41 and GPR43 | Reducing extent of fibrin deposition and growth of nasal polyps [51] |
| Alzheimer's disease | GPR41 | Inhibiting the ERK/JNK/NF-κB pathway to exert anti-neuroinflammatory effects [52] |
| GPR43 | Promoting amyloid-beta clearance and inhibiting cellular senescence [53] | |
| GPR109A | Protecting neurons [54] |
In addition, the extremely small size of the SCFAs allows them to enter the nucleus directly and act as inhibitors of histone deacetylase (HDAC) [55]. Histone acetylation is a process that can promote the loosening of the chromatin structure of target genes to enhance gene transcription, but HDAC inhibits this process [56]. SCFAs suppress inflammatory diseases in the body through the HDAC pathway, the inactivation of which leads to the development of autoimmune diseases. SCFAs promote the differentiation of regulatory T cells (Tregs) by inhibiting HDAC activity, and Tregs secrete protective cytokines, such as IL10, to suppress inflammation [57]. Butyrate inhibits the release of inflammatory factors by inhibiting HDAC activity in type 2 innate lymphocytes, ultimately improving airway inflammation [58].
SCFAs regulate the recognition of innate immune sensors to influence the occurrence of intestinal inflammation
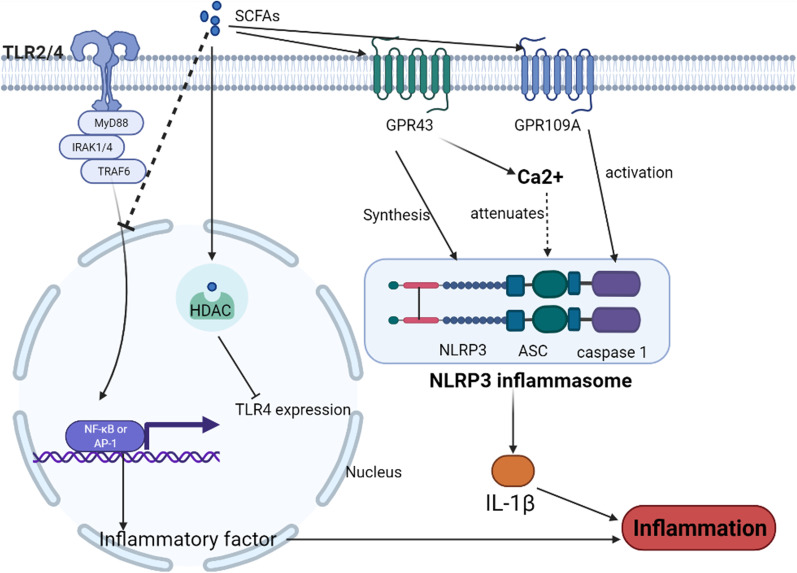
The crosstalk between innate immunity and microbes is an important cause of altered GM and persistent intestinal inflammation [59]. Intestinal epithelial cells (IECs) are the mainstay of innate immunity in the gut [60]. Recognition of microorganisms by innate immune cells, such as IECs, is the beginning of activation of the innate immune system, and the main recognition of the immune system occurs through pattern recognition receptors (PRRs) [61]. PRRs are important mediators of communication between the immune system and microorganisms, and disturbances in their signaling can lead to dysregulation of the intestinal microbiota and the development of IBD [62, 63]. However, SCFAs can regulate the recognition of the innate immune system, which plays an important role in suppressing intestinal inflammation (Fig. 2).
Fig. 2.
Short-chain fatty acids (SCFAs) inhibit the progression of inflammatory bowel disease (IBD) by regulating innate immune sensors Toll-like receptors (TLRs) and nucleotide-binding and oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflam-masomes and play a role in inhibiting the progression of IBD. SCFAs not only inhibit TLR sig-naling but also inhibit TLR4 expression by suppressing the histone acetylation pathway. SCFAs also prevent the progression of IBD by regulating the assembly and attenuation of NLRP3 in-flammasomes. The Figures in this review were created with BioRender.com
Toll-like receptors (TLRs)
TLRs are the typical PRRs associated with IBD development [60]. TLRs are type I transmembrane receptors that are widely expressed in all types of immune cells. After the recognition of microorganisms, TLRs dimerize to activate downstream adapters [64]. The adapter protein transmits the signal into the cell to eventually activate the transcription factors nuclear factor kappa-B and activator protein-1, leading to the expression of inflammatory factors [65]. In addition, TLRs facilitate antigen presentation by DCs and assist in the initiation of adaptive immunity [66]. The activation of these mechanisms leads to inflammation. However, there is a strong correlation between TLR-related inflammation and IBD, and whether TLRs have a protective or destructive effect on the intestinal tract remains controversial [67, 68]. The expression levels of some TLRs in active UC are elevated and accompanied by enhanced signaling, while those are attenuated in the quiescent phase [69]. In contrast, TLR signaling is required for the salvaging of colonic injury in mice [70]. We believe that the complex relationship between TLR signaling and IBD may be due to the following reasons: (1) TLRs are in a highly sensitive state and are extremely susceptible to activation, resulting in the development of persistent inflammation; (2) when TLR signaling is too weak, it tends to lead to an imbalance of the intestinal microbiota and damage of the intestinal mucosa, ultimately leading to inflammation. In conclusion, controlling the TLR signal intensity within a relatively stable range is beneficial to stop the occurrence of IBD.
SCFAs can suppress intestinal inflammation by inhibiting the excessive signaling of TLRs. A high intake of dietary fiber increases the level of SCFAs in the gut and is effective in reducing the extent of TLR-mediated inflammation [71]. TLR4 and TLR2 may be key targets for IBD prevention by SCFAs [72]. Sodium butyrate acts as an HDAC inhibitor to suppress TLR4 expression [73]. In patients with IBD, butyrate inhibits the TLR2-mediated release of inflammatory factors [74, 75]. Butyric acid also reduces adapter protein expression level [76]. In addition, increased level of TLR4 signaling also reduces the abundance of SCFA-producing flora in the gut, which is highly relevant to the development of IBD [75].
NLRP3 inflammasomes
Nucleotide-binding and oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome, another innate immune recognition medium, consists of sensor NLRP3, adapter apoptosis-associated spot-like protein, and effector protein cysteinase [77], and it can trigger the activation of caspase-1 to mediate the release of IL1β [78]. This inflammatory response is also closely related to the inflammatory response of the gut. The role of NLRP3 in IBD has been under debate, with early studies suggesting that NLRP3 activation mediates IBD, and more recent studies suggesting that NLRP3 activation inhibits the development of IBD [78]. However, SCFAs regulate the NLRP3 inflammasome and prevent the development of IBD. In a previous study, SCFAs activated the NLRP3 inflammasome by binding to the receptors GPR43 and GPR109A, ultimately maintaining the health of the guts of mice [79]. Further studies have shown that SCFAs maintain intestinal health by regulating NLRP3 inflammasome assembly and attenuation. The activation of GPR43 by SCFAs is required for adequate inflammatory vesicle assembly and IL1β production, which lead to inflammation [80], and GPR43 promotes NLRP3 inflammasome attenuation through a Ca2+-dependent mechanism to suppress inflammation [81].
SCFAs promote intestinal barrier stability by regulating cytokine networks
The intestinal epithelial barrier is the first line of defense of the intestinal immune system and is composed of IECs and the mucus layer [82]. Damage to the intestinal epithelial barrier leads to bacterial translocation and ongoing inflammation and is one of the stages in the development of IBD and colon cancer. The mucus layer is a two-layered structure composed of mucin, with the loose outer layer providing a habitat for intestinal commensal bacteria and the dense inner layer preventing the downward invasion of bacteria [83]. IECs can differentiate into multiple subtypes, including IECs, cupped cells, panniculocytes, and intestinal endocrine cells, of which cupped cells secrete mucin to form the mucus layer [84]. IECs are interconnected by tight junctions (TJs) [85]. Any structural or functional disruption of the intestinal epithelial barrier has the potential to lead to IBD.
Defenders of the intestinal epithelial barrier: The IL10 family
Cytokines of the IL10 family have broad immunosuppressive effects and may prevent tissue damage caused by excessive inflammatory responses. IL10 family cytokines share similar structures, common receptors, and downstream signaling [86]. Studies have shown that IL10 and IL22 play an important role in maintaining the health of the epithelial barrier of the intestine, and they are often deficient in patients with IBD [87, 88]. There are significant differences in the expression patterns of IL10 and IL22 between patients with IBD and healthy individuals [89, 90]. As a key system in the pathogenesis of IBD, the IL10 family has also been verified in animal models. IL10-deficient mice exhibit spontaneous IBD [91]. IL22 gene delivery rapidly ameliorates colonic inflammation in IBD mice [92].
Both IL10 and IL22, when acting on IECs, activate STAT3 signaling in the cells, a key pathway for maintaining intestinal epithelial repair [86, 93]. Studies have shown that SCFAs induce activation of STAT3 signaling in IECs [94]. IL10 and IL22 activate STAT3 in intestinal epithelial stem cells and promote epithelial regeneration [95, 96]. In contrast, STAT3 signaling-deficient mice show a high susceptibility to IBD [97]. In addition, IL10 and IL22 play a beneficial regulatory role on other parts of the intestinal epithelial barrier. Both IL10 and IL22 can upregulate the expression of TJ proteins [98, 99]. Moreover, IL22 also enhances AMP expression and improves the ability of the gut to resist bacterial translocation [100].
However, IL10 and IL22 can both be induced by SCFAs and act to delay the development of IBD. Dietary fiber intake in IL10 knockout mice suppresses colitis [101]. Pentanoate can activate mechanistic target of rapamycin (mTOR) signaling in lymphocytes to promote IL10 production [102]. B lymphocyte-induced maturation protein 1 (Blimp-1) plays a key role in the production of IL10 in Th1 cells. SCFAs activate mTOR and STAT3 signaling in Th1 cells, which activates Blimp-1 protein, leading to IL10 transcription [103–105]. Activation of mTOR and STAT3 signaling appears to play a key role in the regulation of IL10 and IL22 production by SCFAs. SCFAs promote the expression of hypoxia-inducible factor 1α (HIF1α), which ultimately leads to the transcription of IL22, by activating mTOR and STAT3 signaling in CD4 + T cells and ILC3. However, SCFAs enhance the affinity of HIF-1α for the IL22 promoter by inhibiting HDAC [106]. In addition, activation of GPR43 leads to AKT and ERK signaling to promote ILC3 proliferation, which leads to increased expression level of IL22 [107].
Disruptor of the intestinal epithelial barrier: IL17
IL17A and IL17F, cytokines of the IL17 family, are evolutionarily highly conserved cytokines associated with autoimmune diseases [108]. They are mainly secreted by Th17. Moreover, IL17A and IL17F levels are higher in IBD tissues than in healthy tissues and are accompanied by an increase in the proportion of IL17-producing cells [109, 110]. Inhibition of IL17 expression in a mouse model significantly suppressed colitis [111]. IL17 signaling mediates tissue damage during IBD and has been identified as a highly promising target for IBD intervention [4, 112].
SCFAs engage in regulating the differentiation of CD4 + T cells toward Th17 and Tregs. Th17 expresses high levels of IL17, which promotes inflammation and is directed by the transcription factor retinoic acid-related orphan receptor γt (RORγt) for differentiation [113]. In contrast, Tregs mainly express IL10-like inflammatory factors and are directed to differentiate by the transcription factor forkhead box P3 (Foxp3) [114]. Naive CD4 + T cells express both Foxp3 and RORγt cytokines and the final direction of differentiation depends on the cytokines in the microenvironment [115]. Studies have shown that SCFAs play an important role in the regulation of intestinal T-cell homeostasis; SCFAs promote Treg production and inhibit Th17 production [116]. SCFAs enhance the activity of Foxp3 by inhibiting HDAC [57]. The exon 2 region of Foxp3 interacts directly with RORγt, inhibiting Th17 differentiation and promoting Treg differentiation [117], and this leads to a large reduction in local IL17 concentration in the gut and reduced extent of intestinal inflammation [118].
Immunonutrition therapy is a viable option for IBD
Based on the remarkable contribution of IL10 in suppressing colonic inflammation in mice, there is a consensus to use recombinant IL10 to treat IBD. However, in a double-blind trial, the rhuIL-10 treatment group showed no significant difference [119]. This may be due to the relatively low bioavailability of IL10 to the intestinal mucosa [120]. To overcome this difficulty, researchers attempted to engineer IL10-producing probiotics to deliver IL10; however, this too has been unsuccessful [88, 121]. Moreover, IL10 may promote the development of cancer to some extent. In conclusion, there is still no effective IL10 treatment available for IBD [122].
Although IL17 plays an important role in the development of IBD, the use of IL17 blockers in the clinical treatment of IBD has not yet yielded effective results and has even exacerbated IBD [4, 112, 123]. IL17A inhibits spontaneous colitis in IL10-/- mice via the inducible nitric oxide synthase pathway. Ablation of IL17A leads to severe colitis [124]. IL17 blockers have been used to treat patients with psoriasis, which can exacerbate IBD [125]. These results suggest that the presence of IL17A may also prevent further deterioration of IBD. The use of IL17A as a target for the treatment of IBD may require a more modest approach.
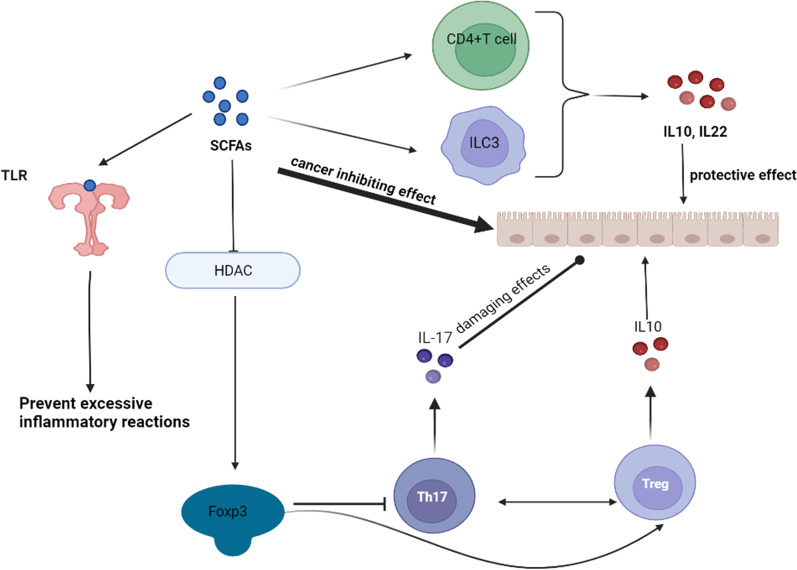
SCFAs, the microbial products that are beneficial to the host, can be supplemented through the diet. It not only regulates the recognition of innate immune sensors in the gut, but also the cytokine network in the gut, thus, achieving the goal of stopping the over-reaction of the immune system and inhibiting progression (Fig. 3). The main reasons are as follows: (1) SCFAs can regulate TLRs and NLRP3 inflammasomes to prevent the occurrence of excessive inflammatory responses; (2) SCFAs can promote the expression of IL10 and inhibit the expression of IL17. They can effectively prevent cancer due to repeated damage-repair on top of the treatment of IBD; (3) the ability of SCFAs to promote apoptosis and inhibit the activity of cancer cells has been reviewed in many studies [126, 127]; (4) in addition, no side effects have been observed with SCFAs for the treatment of IBD.
Fig. 3.
Short-chain fatty acids (SCFAs) are microbial products that can be applied as potential immunonutrition therapies for inflammatory bowel diseases. SCFAs can prevent the development of excessive immune responses by modulating the recognition function of innate immunity, and SCFAs can also play a role in protecting the intestinal barrier by promoting the production of interleukin (IL)10 and inhibiting that of IL17. In addition, SCFAs have excellent anticancer effects. The Figures in this review were created with BioRender.com
Outlook: SCFAs have a potential therapeutic effect on IBD
As an autoimmune disease, IBD has a serious impact on the quality of life of patients, but there is no cure for this disease. The pathogenesis of IBD is still unclear and it is now understood that the development of IBD is associated with genetic factors, dietary habits, and intestinal flora. Targeted inflammatory cytokine blockers, such as TNF and JAK blockers, have been clinically effective in relieving symptoms in patients with IBD. However, they also increase the probability of patients developing infectious diseases [128]. Some cytokines, such as IL10 and IL17, play a prominent role in the development of IBD, yet there are no effective IL10/17-targeted drugs available for clinical use. SCFAs, which are the microbial products that are beneficial to the host, can be supplemented through the diet. This not only regulates the recognition of innate immune sensors in the gut but also the cytokine network in the gut, thus, achieving the goal of stopping the over-reaction of the immune system and inhibiting the progression of IBD. Moreover, SCFAs also play anticancer roles and can effectively stop the development of cancer in patients with IBD. In a preliminary double-blind, placebo-controlled study, Facchin et al. demonstrated that sodium butyrate supplementation increased the growth of SCFA-producing bacteria and improved the inflammatory response in patients with IBD [24]. However, other randomized controlled trials and prospective cohort studies should also investigate the clinical impact of SCFA as one of the future directions to improve the quality of life of patients with IBD.
Acknowledgements
The Figures in this review were created with BioRender.com.
Abbreviations
- AMP
Antimicrobial peptides
- AP-1
Activator protein 1
- Blimp-1
B lymphocyte-induced maturation protein 1
- Foxp3
Forkhead box P3
- GM
Gut microbiota
- GPCRs
G protein-coupled receptors
- HDAC
Histone deacetylase
- HIF1α
Hypoxia-inducible factor 1α
- IBD
Inflammatory bowel disease
- IEC
Intestinal epithelial cells
- IL10
Interleukin 10
- iNOS
Inducible nitric oxide synthase
- mTOR
Mechanistic target of rapamycin
- NF-κB
Nuclear-factor kappa-B
- NLRP3
Nucleotide-binding and oligomerisation domain-like receptor familiy pyrin domain- containing3
- PRR
Pattern recognition receptors
- RORγt
Retinoic acid-related orphan receptor γt
- SCFA
Short chain fatty acids
- TJ
Tight junctions
- TNF
Tumor Necrosis Factor
- TLRs
Toll-like receptors
- Treg
Regulatory T cells
- UC
Ulcerative colitis
Author contributions
ZZ, HZ drafted the manuscript. TC researched the literature and drafted figures. ZZ and LS counted and plotted the tables. DT and DW critically revised the article for important intellectual content. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the Training Project of Key Talents of Youth Medicine in Jiangsu province, China [No. QNRC2016330], the Graduate Research- Innovation Project in Jiangsu province [No. SJCX21_1644], the Academic Science and Technology Innovation Fund for College Students [No. 202011117056Y], the Social Development-Health Care Project of Yangzhou, Jiangsu Province [No. YZ2018087], the Social Development-Health Care Project of Yangzhou, Jiangsu Province [No. YZ2021075], and High-level talent “six one projects” top talent scientific research project of Jiangsu Province [No. LGY2019034]. The funding bodies had no role in the design of the study; in the collection, analysis, and interpretation of the data; and in writing the manuscript.
Availability of data and material
Not applicable.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhilin Zhang, Huan Zhang, and Tian Chen have contributed equally to this work.
Contributor Information
Zhilin Zhang, Email: zhilinzhang2021@163.com.
Huan Zhang, Email: 2962845218@qq.com.
Tian Chen, Email: 1924499535@qq.com.
Lin Shi, Email: sltmddmv@163.com.
Daorong Wang, Email: tangdong1981yz@qq.com.
Dong Tang, Email: 83392785@qq.com.
References
- 1.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 2.Lee M, Chang EB. Inflammatory bowel diseases (IBD) and the microbiome-searching the crime scene for clues. Gastroenterology. 2021;160:524–537. doi: 10.1053/j.gastro.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marion-Letellier R, Savoye G, Ghosh S. IBD: in food we trust. J Crohns Colitis. 2016;10:1351–1361. doi: 10.1093/ecco-jcc/jjw106. [DOI] [PubMed] [Google Scholar]
- 4.Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol. 2019;16:185–196. doi: 10.1038/s41575-018-0084-8. [DOI] [PubMed] [Google Scholar]
- 5.Marafini I, Sedda S, Dinallo V, Monteleone G. Inflammatory cytokines: from discoveries to therapies in IBD. Expert Opin Biol Ther. 2019;19:1207–1217. doi: 10.1080/14712598.2019.1652267. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80:37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- 7.Verhaar BJH, Prodan A, Nieuwdorp M, Muller M. Gut microbiota in hypertension and atherosclerosis: a review. Nutrients. 2020;12:2982. doi: 10.3390/nu12102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 9.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 10.Overby HB, Ferguson JF. Gut microbiota-derived short-chain fatty acids facilitate microbiota: host cross talk and modulate obesity and hypertension. Curr Hypertens Rep. 2021;23:8. doi: 10.1007/s11906-020-01125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4:e121. doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 13.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 14.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Q, Ouyang J, Sun F, Yang J. Short-chain fatty acids: a soldier fighting against inflammation and protecting from tumorigenesis in people with diabetes. Front Immunol. 2020;11:590685. doi: 10.3389/fimmu.2020.590685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CM, et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595:541–555. doi: 10.1113/JP272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziętek M, Celewicz Z, Szczuko M. Short-chain fatty acids, maternal microbiota and metabolism in pregnancy. Nutrients. 2021;13:1244. doi: 10.3390/nu13041244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14:573–584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 21.Deleu S, Machiels K, Raes J, Verbeke K, Vermeire S. Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? EBioMedicine. 2021;66:103293. doi: 10.1016/j.ebiom.2021.103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo Sasso G, Khachatryan L, Kondylis A, Battey JND, Sierro N, Danilova NA, Grigoryeva TV, Markelova MI, Khusnutdinova DR, Laikov AV, et al. Inflammatory bowel disease-associated changes in the gut: focus on Kazan patients. Inflamm Bowel Dis. 2021;27:418–433. doi: 10.1093/ibd/izaa188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 24.Facchin S, Vitulo N, Calgaro M, Buda A, Romualdi C, Pohl D, Perini B, Lorenzon G, Marinelli C, D'Incà R, et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol Motil. 2020;32:e13914. doi: 10.1111/nmo.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takagi T, Naito Y, Higashimura Y, Ushiroda C, Mizushima K, Ohashi Y, Yasukawa Z, Ozeki M, Tokunaga M, Okubo T, et al. Partially hydrolysed guar gum ameliorates murine intestinal inflammation in association with modulating luminal microbiota and SCFA. Br J Nutr. 2016;116:1199–1205. doi: 10.1017/S0007114516003068. [DOI] [PubMed] [Google Scholar]
- 26.Koleva PT, Valcheva RS, Sun X, Gänzle MG, Dieleman LA. Inulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic rats. Br J Nutr. 2012;108:1633–1643. doi: 10.1017/S0007114511007203. [DOI] [PubMed] [Google Scholar]
- 27.De Preter V, Arijs I, Windey K, Vanhove W, Vermeire S, Schuit F, Rutgeerts P, Verbeke K. Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm Bowel Dis. 2012;18:1127–1136. doi: 10.1002/ibd.21894. [DOI] [PubMed] [Google Scholar]
- 28.Roediger WE. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet. 1980;2:712–715. doi: 10.1016/S0140-6736(80)91934-0. [DOI] [PubMed] [Google Scholar]
- 29.Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52:1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–1052. doi: 10.1016/S0006-291X(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 31.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 32.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 33.Bishehsari F, Engen PA, Preite NZ, Tuncil YE, Naqib A, Shaikh M, Rossi M, Wilber S, Green SJ, Hamaker BR, et al. Dietary fiber treatment corrects the composition of gut microbiota, promotes SCFA production, and suppresses colon carcinogenesis. Genes (Basel) 2018;9:102. doi: 10.3390/genes9020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6:133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzetto L, Fava F, Tuohy KM, Selmi C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex. J Autoimmun. 2018;92:12–34. doi: 10.1016/j.jaut.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Yip W, Hughes MR, Li Y, Cait A, Hirst M, Mohn WW, McNagny KM. Butyrate shapes immune cell fate and function in allergic asthma. Front Immunol. 2021;12:628453. doi: 10.3389/fimmu.2021.628453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, Huang X, Xiao Y, Yao S, Zhao Q, et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017;10:946–956. doi: 10.1038/mi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, Bernalier-Donadille A, Denis S, Hofman P, Bonnet R, et al. Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E. coli infection and intestinal inflammation. Sci Rep. 2016;6:19032. doi: 10.1038/srep19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiu W, Chen Q, Wang Z, Wang J, Zhou Z. Microbiota-derived short chain fatty acid promotion of Amphiregulin expression by dendritic cells is regulated by GPR43 and Blimp-1. Biochem Biophys Res Commun. 2020;533:282–288. doi: 10.1016/j.bbrc.2020.09.027. [DOI] [PubMed] [Google Scholar]
- 40.Park BO, Kang JS, Paudel S, Park SG, Park BC, Han SB, Kwak YS, Kim JH, Kim S. Novel GPR43 agonists exert an anti-inflammatory effect in a colitis model. Biomol Ther (Seoul) 2021;30(1):48. doi: 10.4062/biomolther.2021.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hertati A, Hayashi S, Ogata H, Miyata K, Kato R, Yamamoto T, Kadowaki M. Morphological elucidation of short-chain fatty acid receptor GPR41-positive enteric sensory neurons in the colon of mice with dextran sulfate sodium-induced colitis. Heliyon. 2020;6:e05647. doi: 10.1016/j.heliyon.2020.e05647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen G, Ran X, Li B, Li Y, He D, Huang B, Fu S, Liu J, Wang W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine. 2018;30:317–325. doi: 10.1016/j.ebiom.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhatt B, Zeng P, Zhu H, Sivaprakasam S, Li S, Xiao H, Dong L, Shiao P, Kolhe R, Patel N, et al. Gpr109a limits microbiota-induced IL-23 production to constrain ILC3-mediated colonic inflammation. J Immunol. 2018;200:2905–2914. doi: 10.4049/jimmunol.1701625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salem HA, Wadie W. Effect of niacin on inflammation and angiogenesis in a murine model of ulcerative colitis. Sci Rep. 2017;7:7139. doi: 10.1038/s41598-017-07280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang W, Man Y, Gao C, Zhou L, Gu J, Xu H, Wan Q, Long Y, Chai L, Xu Y, Xu Y. Short-chain fatty acids ameliorate diabetic nephropathy via GPR43-mediated inhibition of oxidative stress and NF-κB signaling. Oxid Med Cell Longev. 2020;2020:4074832. doi: 10.1155/2020/4074832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li YJ, Chen X, Kwan TK, Loh YW, Singer J, Liu Y, Ma J, Tan J, Macia L, Mackay CR, et al. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid-mediated activation of g protein-coupled receptors GPR43 and GPR109A. J Am Soc Nephrol. 2020;31:1267–1281. doi: 10.1681/ASN.2019101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li M, van Esch B, Henricks PAJ, Folkerts G, Garssen J. The Anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front Pharmacol. 2018;9:533. doi: 10.3389/fphar.2018.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Digby JE, Martinez F, Jefferson A, Ruparelia N, Chai J, Wamil M, Greaves DR, Choudhury RP. Anti-inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms. Arterioscler Thromb Vasc Biol. 2012;32:669–676. doi: 10.1161/ATVBAHA.111.241836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C, Chang J, Wu W, Deng Y, Zhou P, Jiang W, Wang C, Huang F. Activation of GPR43 suppresses TNF-α-induced inflammatory response in human fibroblast-like synoviocytes. Arch Biochem Biophys. 2020;684:108297. doi: 10.1016/j.abb.2020.108297. [DOI] [PubMed] [Google Scholar]
- 50.Pirozzi C, Francisco V, Guida FD, Gómez R, Lago F, Pino J, Meli R, Gualillo O. Butyrate modulates inflammation in chondrocytes via GPR43 receptor. Cell Physiol Biochem. 2018;51:228–243. doi: 10.1159/000495203. [DOI] [PubMed] [Google Scholar]
- 51.Imoto Y, Kato A, Takabayashi T, Sakashita M, Norton JE, Suh LA, Carter RG, Weibman AR, Hulse KE, Stevens W, et al. Short-chain fatty acids induce tissue plasminogen activator in airway epithelial cells via GPR41&43. Clin Exp Allergy. 2018;48:544–554. doi: 10.1111/cea.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Li H, Gong T, Chen W, Mao S, Kong Y, Yu J, Sun J. Anti-neuroinflammatory effect of short-chain fatty acid acetate against Alzheimer's disease via upregulating GPR41 and inhibiting ERK/JNK/NF-κB. J Agric Food Chem. 2020;68:7152–7161. doi: 10.1021/acs.jafc.0c02807. [DOI] [PubMed] [Google Scholar]
- 53.Razazan A, Karunakar P, Mishra SP, Sharma S, Miller B, Jain S, Yadav H. Activation of microbiota sensing—free fatty acid receptor 2 signaling ameliorates amyloid-β induced neurotoxicity by modulating proteolysis-senescence axis. Front Aging Neurosci. 2021;13:735933. doi: 10.3389/fnagi.2021.735933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun J, Yuan B, Wu Y, Gong Y, Guo W, Fu S, Luan Y, Wang W. Sodium butyrate protects N2a cells against Aβ toxicity in vitro. Mediat Inflamm. 2020;2020:7605160. doi: 10.1155/2020/7605160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Visekruna A, Luu M. The role of short-chain fatty acids and bile acids in intestinal and liver function, inflammation, and carcinogenesis. Front Cell Dev Biol. 2021;9:703218. doi: 10.3389/fcell.2021.703218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56–63. doi: 10.1016/j.neulet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thio CL, Chi PY, Lai AC, Chang YJ. Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J Allergy Clin Immunol. 2018;142:1867–1883.e1812. doi: 10.1016/j.jaci.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 59.Dai Z, Zhang J, Wu Q, Fang H, Shi C, Li Z, Lin C, Tang D, Wang D. Intestinal microbiota: a new force in cancer immunotherapy. Cell Commun Signal. 2020;18:90. doi: 10.1186/s12964-020-00599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Burdette BE, Esparza AN, Zhu H, Wang S. Gasdermin D in pyroptosis. Acta Pharm Sin B. 2021;11:2768–2782. doi: 10.1016/j.apsb.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plaza-Díaz J, Ruiz-Ojeda FJ, Vilchez-Padial LM, Gil A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients. 2017;9:555. doi: 10.3390/nu9060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burgueño JF, Abreu MT. Epithelial toll-like receptors and their role in gut homeostasis and disease. Nat Rev Gastroenterol Hepatol. 2020;17:263–278. doi: 10.1038/s41575-019-0261-4. [DOI] [PubMed] [Google Scholar]
- 64.Lin C, Zhao S, Zhu Y, Fan Z, Wang J, Zhang B, Chen Y. Microbiota-gut-brain axis and toll-like receptors in Alzheimer's disease. Comput Struct Biotechnol J. 2019;17:1309–1317. doi: 10.1016/j.csbj.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell. 2020;180:1044–1066. doi: 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uto T, Fukaya T, Takagi H, Arimura K, Nakamura T, Kojima N, Malissen B, Sato K. Clec4A4 is a regulatory receptor for dendritic cells that impairs inflammation and T-cell immunity. Nat Commun. 2016;7:11273. doi: 10.1038/ncomms11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu Y, Li X, Liu S, Zhang Y, Zhang D. Toll-like receptors and inflammatory bowel disease. Front Immunol. 2018;9:72. doi: 10.3389/fimmu.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kordjazy N, Haj-Mirzaian A, Haj-Mirzaian A, Rohani MM, Gelfand EW, Rezaei N, Abdolghaffari AH. Role of toll-like receptors in inflammatory bowel disease. Pharmacol Res. 2018;129:204–215. doi: 10.1016/j.phrs.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 69.Sánchez-Muñoz F, Fonseca-Camarillo G, Villeda-Ramírez MA, Miranda-Pérez E, Mendivil EJ, Barreto-Zúñiga R, Uribe M, Bojalil R, Domínguez-López A, Yamamoto-Furusho JK. Transcript levels of toll-like receptors 5, 8 and 9 correlate with inflammatory activity in ulcerative colitis. BMC Gastroenterol. 2011;11:138. doi: 10.1186/1471-230X-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malvin NP, Seno H, Stappenbeck TS. Colonic epithelial response to injury requires Myd88 signaling in myeloid cells. Mucosal Immunol. 2012;5:194–206. doi: 10.1038/mi.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen H, Lu Z, Chen Z, Wu Y, Shen Z. Rapid fermentable substance modulates interactions between ruminal commensals and toll-like receptors in promotion of immune tolerance of goat rumen. Front Microbiol. 1812;2016:7. doi: 10.3389/fmicb.2016.01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rösch C, Taverne N, Venema K, Gruppen H, Wells JM, Schols HA. Effects of in vitro fermentation of barley β-glucan and sugar beet pectin using human fecal inocula on cytokine expression by dendritic cells. Mol Nutr Food Res. 2017;61:1600243. doi: 10.1002/mnfr.201600243. [DOI] [PubMed] [Google Scholar]
- 73.Kazemi Sefat NA, Mohammadi MM, Hadjati J, Talebi S, Ajami M, Daneshvar H. Sodium butyrate as a histone deacetylase inhibitor affects toll-like receptor 4 expression in colorectal cancer cell lines. Immunol Investig. 2019;48:759–769. doi: 10.1080/08820139.2019.1595643. [DOI] [PubMed] [Google Scholar]
- 74.Kovarik JJ, Tillinger W, Hofer J, Hölzl MA, Heinzl H, Saemann MD, Zlabinger GJ. Impaired anti-inflammatory efficacy of n-butyrate in patients with IBD. Eur J Clin Investig. 2011;41:291–298. doi: 10.1111/j.1365-2362.2010.02407.x. [DOI] [PubMed] [Google Scholar]
- 75.Li AL, Ni WW, Zhang QM, Li Y, Zhang X, Wu HY, Du P, Hou JC, Zhang Y. Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol Immunol. 2020;64:23–32. doi: 10.1111/1348-0421.12749. [DOI] [PubMed] [Google Scholar]
- 76.Sam QH, Ling H, Yew WS, Tan Z, Ravikumar S, Chang MW, Chai LYA. The divergent immunomodulatory effects of short chain fatty acids and medium chain fatty acids. Int J Mol Sci. 2021;22:6453. doi: 10.3390/ijms22126453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/S1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 78.Zhen Y, Zhang H. NLRP3 inflammasome and inflammatory bowel disease. Front Immunol. 2019;10:276. doi: 10.3389/fimmu.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 80.Vieira AT, Macia L, Galvão I, Martins FS, Canesso MC, Amaral FA, Garcia CC, Maslowski KM, De Leon E, Shim D, et al. A role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of gout. Arthritis Rheumatol. 2015;67:1646–1656. doi: 10.1002/art.39107. [DOI] [PubMed] [Google Scholar]
- 81.Xu M, Jiang Z, Wang C, Li N, Bo L, Zha Y, Bian J, Zhang Y, Deng X. Acetate attenuates inflammasome activation through GPR43-mediated Ca(2+)-dependent NLRP3 ubiquitination. Exp Mol Med. 2019;51:1–13. doi: 10.1038/s12276-019-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516–1526. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fakhoury HMA, Kvietys PR, AlKattan W, Anouti FA, Elahi MA, Karras SN, Grant WB. Vitamin D and intestinal homeostasis: barrier, microbiota, and immune modulation. J Steroid Biochem Mol Biol. 2020;200:105663. doi: 10.1016/j.jsbmb.2020.105663. [DOI] [PubMed] [Google Scholar]
- 84.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 85.Garcia-Carbonell R, Yao SJ, Das S, Guma M. Dysregulation of intestinal epithelial cell RIPK pathways promotes chronic inflammation in the IBD gut. Front Immunol. 2019;10:1094. doi: 10.3389/fimmu.2019.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ouyang W, O'Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50:871–891. doi: 10.1016/j.immuni.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 87.Keir M, Yi Y, Lu T, Ghilardi N. The role of IL-22 in intestinal health and disease. J Exp Med. 2020;217:e20192195. doi: 10.1084/jem.20192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen HD, Aljamaei HM, Stadnyk AW. The production and function of endogenous interleukin-10 in intestinal epithelial cells and gut homeostasis. Cell Mol Gastroenterol Hepatol. 2021;12:1343–1352. doi: 10.1016/j.jcmgh.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Su Y, Zhao H. Predisposition of inflammatory bowel disease is influenced by IL-8, IL-10, and IL-18 polymorphisms: a meta-analysis. Int Arch Allergy Immunol. 2020;181:799–806. doi: 10.1159/000509110. [DOI] [PubMed] [Google Scholar]
- 90.Sakemi R, Mitsuyama K, Morita M, Yoshioka S, Kuwaki K, Tokuyasu H, Fukunaga S, Mori A, Araki T, Yoshimura T, et al. Altered serum profile of the interleukin-22 system in inflammatory bowel disease. Cytokine. 2020;136:155264. doi: 10.1016/j.cyto.2020.155264. [DOI] [PubMed] [Google Scholar]
- 91.Jofra T, Galvani G, Cosorich I, De Giorgi L, Annoni A, Vecchione A, Sorini C, Falcone M, Fousteri G. Experimental colitis in IL-10-deficient mice ameliorates in the absence of PTPN22. Clin Exp Immunol. 2019;197:263–275. doi: 10.1111/cei.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ernst M, Thiem S, Nguyen PM, Eissmann M, Putoczki TL. Epithelial gp130/Stat3 functions: an intestinal signaling node in health and disease. Semin Immunol. 2014;26:29–37. doi: 10.1016/j.smim.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S, Xiao Y, Huang X, Eaves-Pyles TD, Golovko G, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11:752–762. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fay NC, Muthusamy BP, Nyugen LP, Desai RC, Taverner A, MacKay J, Seung M, Hunter T, Liu K, Chandalia A, et al. A novel fusion of IL-10 engineered to traffic across intestinal epithelium to treat colitis. J Immunol. 2020;205:3191–3204. doi: 10.4049/jimmunol.2000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lindemans CA, Calafiore M, Mertelsmann AM, O'Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stuhlmann-Laeisz C, Lang S, Chalaris A, Krzysztof P, Enge S, Eichler J, Klingmüller U, Samuel M, Ernst M, Rose-John S, Scheller J. Forced dimerization of gp130 leads to constitutive STAT3 activation, cytokine-independent growth, and blockade of differentiation of embryonic stem cells. Mol Biol Cell. 2006;17:2986–2995. doi: 10.1091/mbc.e05-12-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun X, Yang H, Nose K, Nose S, Haxhija EQ, Koga H, Feng Y, Teitelbaum DH. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;294:G139–147. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- 99.Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, Mujib S, Benko E, Kovacs C, Shin LY, et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012;5:670–680. doi: 10.1038/mi.2012.72. [DOI] [PubMed] [Google Scholar]
- 100.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang H, Shi P, Zuo L, Dong J, Zhao J, Liu Q, Zhu W. Dietary non-digestible polysaccharides ameliorate intestinal epithelial barrier dysfunction in IL-10 knockout mice. J Crohns Colitis. 2016;10:1076–1086. doi: 10.1093/ecco-jcc/jjw065. [DOI] [PubMed] [Google Scholar]
- 102.Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, Hofmann J, Raifer H, Vachharajani N, Carrascosa LC, et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun. 2019;10:760. doi: 10.1038/s41467-019-08711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, Chen F, Xiao Y, Zhao Y, Ma C, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. doi: 10.1038/s41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rutz S, Ouyang W. Regulation of interleukin-10 expression. Adv Exp Med Biol. 2016;941:89–116. doi: 10.1007/978-94-024-0921-5_5. [DOI] [PubMed] [Google Scholar]
- 105.Chen L, Sun M, Wu W, Yang W, Huang X, Xiao Y, Ma C, Xu L, Yao S, Liu Z, Cong Y. Microbiota metabolite butyrate differentially regulates Th1 and Th17 Cells' differentiation and function in induction of colitis. Inflamm Bowel Dis. 2019;25:1450–1461. doi: 10.1093/ibd/izz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang W, Yu T, Huang X, Bilotta AJ, Xu L, Lu Y, Sun J, Pan F, Zhou J, Zhang W, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11:4457. doi: 10.1038/s41467-020-18262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chun E, Lavoie S, Fonseca-Pereira D, Bae S, Michaud M, Hoveyda HR, Fraser GL, Gallini Comeau CA, Glickman JN, Fuller MH, et al. Metabolite-sensing receptor Ffar2 regulates colonic group 3 innate lymphoid cells and gut immunity. Immunity. 2019;51:871–884.e876. doi: 10.1016/j.immuni.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50:892–906. doi: 10.1016/j.immuni.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim SW, Kim ES, Moon CM, Park JJ, Kim TI, Kim WH, Cheon JH. Genetic polymorphisms of IL-23R and IL-17A and novel insights into their associations with inflammatory bowel disease. Gut. 2011;60:1527–1536. doi: 10.1136/gut.2011.238477. [DOI] [PubMed] [Google Scholar]
- 111.Kim DJ, Kim KS, Song MY, Seo SH, Kim SJ, Yang BG, Jang MH, Sung YC. Delivery of IL-12p40 ameliorates DSS-induced colitis by suppressing IL-17A expression and inflammation in the intestinal mucosa. Clin Immunol. 2012;144:190–199. doi: 10.1016/j.clim.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 112.Schmitt H, Neurath MF, Atreya R. Role of the IL23/IL17 pathway in Crohn's disease. Front Immunol. 2021;12:622934. doi: 10.3389/fimmu.2021.622934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yan JB, Luo MM, Chen ZY, He BH. The function and role of the Th17/Treg cell balance in inflammatory bowel disease. J Immunol Res. 2020;2020:8813558. doi: 10.1155/2020/8813558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.He X, Liang B, Gu N. Th17/Treg imbalance and atherosclerosis. Dis Markers. 2020;2020:8821029. doi: 10.1155/2020/8821029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fasching P, Stradner M, Graninger W, Dejaco C, Fessler J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules. 2017;22:134. doi: 10.3390/molecules22010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hartog A, Belle FN, Bastiaans J, de Graaff P, Garssen J, Harthoorn LF, Vos AP. A potential role for regulatory T-cells in the amelioration of DSS induced colitis by dietary non-digestible polysaccharides. J Nutr Biochem. 2015;26:227–233. doi: 10.1016/j.jnutbio.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 117.Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto B, Suzuki Y, Yonezu T, Mizushima N, Watanabe N, Sato T, Inoue S, Inokuchi S. Cha-Koji, comprising green tea leaves fermented with Aspergillus luchuensisvar kawachii kitahara, increases regulatory T cell production in mice and humans. Biosci Biotechnol Biochem. 2018;82:885–892. doi: 10.1080/09168451.2018.1443789. [DOI] [PubMed] [Google Scholar]
- 119.Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, Jacyna M, Lashner BA, Gangl A, Rutgeerts P, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn's disease Crohn's Disease IL-10 Cooperative Study Group. Gastroenterology. 2000;119:1461–1472. doi: 10.1053/gast.2000.20196. [DOI] [PubMed] [Google Scholar]
- 120.Marlow GJ, van Gent D, Ferguson LR. Why interleukin-10 supplementation does not work in Crohn's disease patients. World J Gastroenterol. 2013;19:3931–3941. doi: 10.3748/wjg.v19.i25.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 122.Wei HX, Wang B, Li B. IL-10 and IL-22 in mucosal immunity: driving protection and pathology. Front Immunol. 2020;11:1315. doi: 10.3389/fimmu.2020.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Omidian Z, Ahmed R, Giwa A, Donner T, Hamad ARA. IL-17 and limits of success. Cell Immunol. 2019;339:33–40. doi: 10.1016/j.cellimm.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tachibana M, Watanabe N, Koda Y, Oya Y, Kaminuma O, Katayama K, Fan Z, Sakurai F, Kawabata K, Hiroi T, Mizuguchi H. Ablation of IL-17A leads to severe colitis in IL-10-deficient mice: implications of myeloid-derived suppressor cells and NO production. Int Immunol. 2020;32:187–201. doi: 10.1093/intimm/dxz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ju J, Dai Y, Yang J, Liu C, Fan L, Feng L, Zhao B, Zeng M, Liu Z, Sun X. Crohn's disease exacerbated by IL-17 inhibitors in patients with psoriasis: a case report. BMC Gastroenterol. 2020;20:340. doi: 10.1186/s12876-020-01474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang G, Yu Y, Wang YZ, Wang JJ, Guan R, Sun Y, Shi F, Gao J, Fu XL. Role of SCFAs in gut microbiome and glycolysis for colorectal cancer therapy. J Cell Physiol. 2019;234:17023–17049. doi: 10.1002/jcp.28436. [DOI] [PubMed] [Google Scholar]
- 127.Gomes SD, Oliveira CS, Azevedo-Silva J, Casanova MR, Barreto J, Pereira H, Chaves SR, Rodrigues LR, Casal M, Côrte-Real M, et al. The role of diet related short-chain fatty acids in colorectal cancer metabolism and survival: prevention and therapeutic implications. Curr Med Chem. 2020;27:4087–4108. doi: 10.2174/0929867325666180530102050. [DOI] [PubMed] [Google Scholar]
- 128.Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019;20:970–979. doi: 10.1038/s41590-019-0415-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.