Abstract
Competitive PCR was used to monitor the survival of a 520-bp DNA target sequence from a recombinant plasmid, pVACMC1, after admixture of the plasmid with freshly sampled human saliva. The fraction of the target remaining amplifiable ranged from 40 to 65% after 10 min of exposure to saliva samples from five subjects and from 6 to 25% after 60 min of exposure. pVACMC1 plasmid DNA that had been exposed to degradation by fresh saliva was capable of transforming naturally competent Streptococcus gordonii DL1 to erythromycin resistance, although transforming activity decreased rapidly, with a half-life of approximately 50 s. S. gordonii DL1 transformants were obtained in the presence of filter-sterilized saliva and a 1-μg/ml final concentration of pVACMC1 DNA. Addition of filter-sterilized saliva instead of heat-inactivated horse serum to S. gordonii DL1 cells induced competence, although with slightly lower efficiency. These findings indicate that DNA released from bacteria or food sources within the mouth has the potential to transform naturally competent oral bacteria. However, further investigations are needed to establish whether transformation of oral bacteria can occur at significant frequencies in vivo.
Interest in gene transfer in the gastrointestinal tract has been stimulated recently by the need to assess risks associated with the possible onward transfer of manipulated traits from genetically modified microorganisms or DNA-containing transgenes to gut bacteria (5, 23). More generally, gene transfer plays an important role in the evolution of gut bacteria and in their ability to adapt to environmental challenges. It is widely accepted that conjugal transfer involving plasmids and conjugative transposons, as well as bacteriophage transduction, plays a major role in gene transfer between bacteria in the gut (24). Conjugal transfer of recombinant plasmids has been demonstrated between strains of Escherichia coli and between strains of lactic acid bacteria introduced into the digestive tract of gnotobiotic mice (4, 9). By contrast, the possible role of transformation has received little attention, largely because free DNA has been considered unlikely to survive the action of gut nucleases (26). Despite this, fragments of bacteriophage M13 DNA were recently reported to survive passage through the mouse gastrointestinal tract and were even found in some mouse tissues (27, 28). Many bacteria, including representatives of the oral and gut microflora, are known to be naturally transformable (15). Uptake of DNA by competent cells in vitro occurs rapidly, at a rate of 100 nucleotides/s in the case of Streptococcus pneumoniae DP1601 (19); free DNA would therefore need to survive for only short periods of time to enable competent bacteria to be transformed. Furthermore, we know little about the effects of food components or gut microenvironments upon DNA survival in the gastrointestinal tract. Free DNA in soils is often bound to soil minerals (7) and plant polysaccharides (31, 32), where it is more resistant to nucleolytic attack (22) while still being available for the transformation of competent bacteria (3).
In assessing the fate of free DNA in the digestive tract, it is important to consider all regions, including the oral cavity and esophagus. The oral cavity is the site of first contact between incoming bacteria and free DNA in food and the resident microflora and is one of the most complex and heterogenous microbial habitats in the human body (8). Certain oral bacteria are important agents of periodontal disease, dental caries, and some systemic infections (18), and some of these species are also naturally competent (13, 29, 34). In this study, we investigate the survival of free DNA in human saliva and use competitive PCR to quantify DNA degradation. Plasmid DNA that has been exposed to degradation in human saliva is shown to be able to transform the naturally competent oral bacterium Streptococcus gordonii DL1 in vitro.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used are described in Table 1. Lactococcus lactis IL1403 was routinely maintained at 30°C in M17 broth (Oxoid) supplemented with 0.5% (wt/vol) glucose. S. gordonii DL1 and Streptococcus mutans strains were routinely maintained in brain heart infusion (BHI) broth (Difco), and E. coli XL1-Blue cultures were maintained in Luria broth (25), all at 37°C. All cultures were stored at −85°C in 10% (vol/vol) glycerol after being snap-frozen in liquid nitrogen.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or DNA source | Characteristics and/or source | Reference |
|---|---|---|
| L. lactis IL1403 | Plasmid free; Y. Duval-Iflah, | 6 |
| S. gordonii DL1 | T. R. Whitehead, NCAUR | 35 |
| E. coli XL1-Blue | Stratagene, Cambridge, United Kingdom | |
| E. coli DH5α | pVACMC1 | 35 |
| E. coli DH5α | pVACMCcomp | |
| pIL253 | Emr; Y. Duval-Iflah | 30 |
| pVACMC1 | CMCase, Cmr Emr | 35 |
| pVACMCcomp | Competitor plasmid to pVACMC1, CMCase, Cmr Emr; this study | |
| Bacteriophage λ | Boehringer Mannheim |
DNA purification and manipulation.
All plasmids used are described in Table 1. Plasmid DNA was purified from E. coli cultures by the alkaline lysis method (2) and was isolated from streptococci and lactococci by the method of O’Sullivan and Klaenhammer (21). L. lactis IL1403 chromosomal DNA was prepared by the method of Leenhouts et al. (14), and DNA was dissolved in sterile distilled water (dH2O). Bacteriophage λ DNA was purchased from Boehringer Mannheim. Restriction digestion was carried out according to the manufacturer’s instructions (Promega). The concentration of DNA in all samples was determined by a GeneQuant II apparatus (Pharmacia Biotech).
Simulation of oral and gastrointestinal conditions.
Saliva was taken prior to tooth brushing in the morning and was sampled from five different healthy volunteers. Volunteers were two males and three females aged between 25 and 35 years. To simulate stomach conditions, saliva was acidified to pH 1 to 2 or pH 3 to 4 with concentrated hydrochloric acid. The effect of small intestinal conditions was stimulated with 1.5 mg (wt/vol) of pancreas acetone powder (Sigma) per ml dissolved in 150 mM NaHCO3 (20). Colonic lumen conditions were simulated with a 20% (wt/vol) human fecal slurry dissolved in one-fourth strength Ringers solution.
DNA degradation experiments.
Except where otherwise stated, 0.15 μg of DNA, dissolved in 10 μl of sterile dH2O, was added to 30 μl of saliva, acidified saliva, pancreas acetone solution, or fecal slurry and mixed thoroughly. Samples were incubated at 37°C, and at set time intervals degradation was stopped by the addition of 80 μl of phenol-chloroform (1:1) followed by thorough mixing. The aqueous layer was removed after centrifugation (Jouan A14 microcentrifuge, 10 min, 17,746 × g) and was further purified by precipitation of DNA with 160 μl of ethanol, a wash with 70% (vol/vol) ethanol, and resuspension of DNA in 15 μl of sterile dH2O. Samples of the aqueous phase (10 μl) were run on agarose gels containing 0.15 μg of ethidium bromide per ml, and the remainder was used for PCR amplification.
PCR.
PCR amplification of a 520-bp fragment of plasmid pVACMC1 was carried out by capillary PCR with a Corbett Research FTS-4000 thermal sequencer using primers CMCP2 (5’-GACAAGACAAAGAAGACTCC-3’) and pVACrev (5’-AGCGATCCTTGAAGCTGTC-3’) (Applied Biosystems). Samples (2 μl) containing template DNA were added to a standard PCR mixture (11), except for the addition of extra MgCl2 (6.5 mM final concentration). Amplification was carried out with the following cycle times: one cycle of 94°C for 3 min, 58°C for 10 s, and 72°C for 15 s, followed by 31 cycles of 94°C for 10 s, 58°C for 10 s, and 72°C for 15 s, with a final elongation step of 72°C for 5 min and a ramp rate of 2°C/s. Direct amplification of target DNA from transformant colonies was carried out according to the protocol of Güssow and Clackson (10). After PCR amplification, samples were analyzed on a 1.4% (wt/vol) ethidium bromide-stained agarose gel.
Competitive PCR.
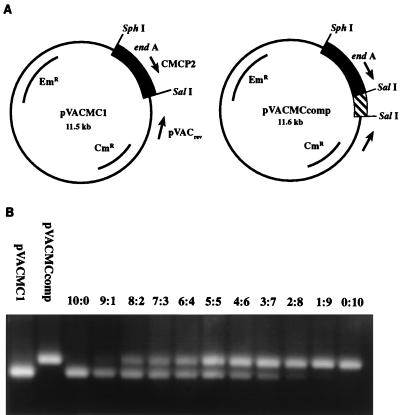
Quantitative detection of the concentration of the 520-bp PCR product of pVACMC1 was carried out by competitive PCR. A competitor plasmid was constructed by the introduction of a 100-bp fragment of DNA from the 16S rRNA gene of Prevotella albensis M384 at the end of the Ruminococcus flavefaciens endoglucanase A (endA) gene (Fig. 1A). Upon amplification with the CMCP2 and pVACrev primers described above, the competitor plasmid gave a 620-bp band easily distinguishable from the 520-bp band produced by the target on a 1.4% agarose gel (Fig. 1B).
FIG. 1.
Construction of the competitor plasmid to pVACMC1 and its use in competitive PCR. (A) Plasmid pVACMC1 and its competitor, pVACMCcomp, obtained by insertion of a 100-bp SalI fragment (see text). (B) Trial competitive PCR experiment in which different ratios of competitor plasmid and pVACMC1 were subjected to PCR amplification with the forward PCR primer CMCP2 and the reverse primer pVACrev. The R. flavefaciens cellulase gene, endA, encodes CMCase activity.
The concentration of saliva-degraded pVACMC1 was determined by adding known concentrations of pVACMCcomp to degraded pVACMC1. PCR amplification was carried out with primers CMCP2 and pVACrev, and the products were separated on 1.4% (wt/vol) agarose gels. An initial concentration range of 0.1 to 1.0 μg of competitor plasmid per ml was used; if no crossover point was observed, fivefold dilutions of competitor were used until a crossover point was obtained. At the point where the intensity of target and competitor bands is identical (crossover), the concentration of competitor plasmid is equal to that of the degraded pVACMC1 (Fig. 1B).
Transformation of oral streptococci with pVACMC1 plasmid DNA.
Transformation of S. gordonii DL1 with pVACMC1 was carried out with the protocol of Macrina et al. (16). In the experiment represented by Fig. 5, heat-inactivated horse serum was replaced with the same volume of either horse serum, filter-sterilized saliva, or sterile dH2O as an inducer of competence development. Competent cells (330 μl) were aliquoted into sterile Eppendorf tubes containing a minimal volume of transforming DNA (final concentration, 1.0 μg/ml) and incubated aerobically, with shaking, for 2 h at 37°C. Transformants were plated on BHI agar containing 10 μg of erythromycin per ml and incubated anaerobically at 37°C for 48 to 72 h. Transformation, in all cases, was confirmed by growth on selective media, analysis of plasmid DNA, PCR amplification of colony lysates, and demonstration of carboxymethylcellulase (CMCase) activity. For the experiment represented by Fig. 3, a higher initial concentration, 30 μg of pVACMC1 DNA per ml, was used in order to monitor the decrease in concentration of transforming DNA following exposure to saliva.
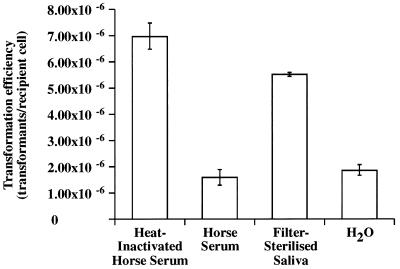
FIG. 5.
Transformation of S. gordonii DL1 with pVACMC1 after competence induction by different growth supplements. The transformation protocol described in Materials and Methods was followed except that heat-inactivated horse serum was replaced with the other growth supplements.
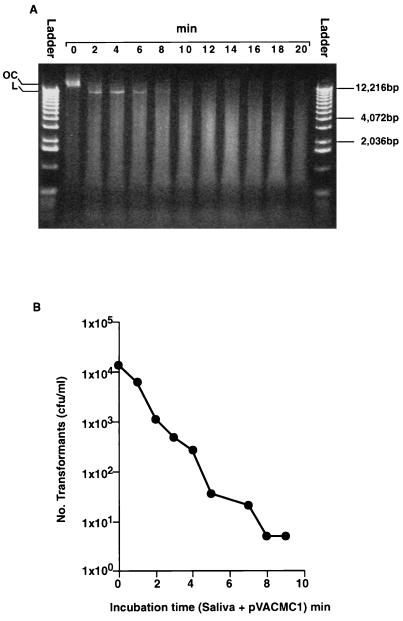
FIG. 3.
(A) Survival of pVACMC1 DNA in human saliva analyzed on a 0.8% (wt/vol) agarose gel. A 1-kb DNA ladder (Gibco BRL), 0.5 to 12.2 kbp, was used. OC, open circular; L, linear. A faint band of covalently closed circular DNA was visible in lane 0). (B) Transformation of S. gordonii DL1 with pVACMC1 DNA that had been previously exposed to fresh, whole saliva for the times indicated.
Transformation of S. gordonii DL1 with pVACMC1 in the presence of filter-sterilized saliva.
Competent cells of S. gordonii DL1 were prepared as described by Macrina et al. (16). Competent cells (165 μl) were added to sterile Eppendorf tubes and mixed with an equal volume of filter-sterilized saliva. pVACMC1 DNA (in a minimal volume of dH2O) was added to the mixture to give a final concentration in the transformation mixture of 1 μg/ml. The transformation mixture was incubated at 37°C, with shaking, for 0 to 240 min. At set time points, samples of the transformation mixture were removed and diluted appropriately, and 100 μl was plated onto selective agar (BHI agar plus 10 μg of erythromycin per ml) and incubated anaerobically at 37°C for 48 to 72 h. Transformation, in all cases, was confirmed by growth on selective media, analysis of plasmid DNA, PCR amplification of colony lysates, and demonstration of CMCase activity.
Determination of CMCase activity.
Transformant cells were plated onto BHI agar containing 0.1% (wt/vol) medium-viscosity carboxymethylcellulose (Sigma) and stained with 1.0 mg of Congo red per ml following overnight incubation at 37°C to detect the expression of the endoglucanase A (endA) gene of R. flavefaciens (6).
RESULTS
Survival of plasmid DNA sequences in human saliva monitored by competitive PCR and transformation of S. gordonii DL1.
The survival of DNA added to human saliva to a final concentration of 3.75 μg/ml was followed in preliminary experiments by agarose gel electrophoresis. This DNA concentration allowed visual assessment of degradation with L. lactis IL1403 chromosomal DNA, plasmid DNA (pVACMC1 and pIL253), and linear bacteriophage λ DNA. In all cases, DNA was visible in an incompletely degraded state for at least 3.5 min after addition to human saliva and for up to 1 min with acidified saliva (pH 3 to 4) (data not shown). By contrast, DNA added to acidified saliva (pH 2 or less), pancreas acetone solution, or human fecal slurry was undetectable by this approach after 30 s (data not shown).
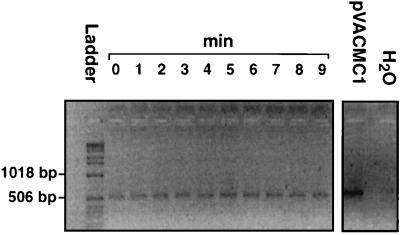
In order to achieve more sensitive detection of surviving DNA sequences present in a particular recombinant plasmid, a 520-bp portion of plasmid pVACMC1 was amplified by PCR following exposure to saliva samples. pVACMC1 comprises the E. coli/gram-positive shuttle vector pVA838 carrying a cloned cellulase-encoding fragment from the rumen anaerobic bacterium R. flavefaciens (17, 35). The two PCR primers were designed to give highly specific detection, since one recognizes vector sequences and the other a sequence in the cloned cellulase fragment (Fig. 1A). As can be seen from Fig. 2, a 520-bp fragment of pVACMC1 was still amplifiable after 9 min of incubation in saliva, and amplifiable target DNA was still detectable even after 24 h of incubation (data not shown).
FIG. 2.
Persistence of a 520-bp fragment of pVACMC1 DNA after incubation with fresh, whole saliva for 9 min. PCR amplification of degraded DNA was carried out as described in Materials and Methods, and products were analyzed on a 1.4% (wt/vol) agarose gel. A 1-kb DNA ladder (Gibco BRL), 0.5 to 12.2 kbp, was used.
Quantitative detection of saliva-degraded pVACMC1 sequences was carried out by competitive PCR. A competitor plasmid was constructed (Fig. 1A) and used as described in Materials and Methods. A typical competitive PCR gel is shown in Fig. 1B. Survival of the 520-bp fragment was estimated during incubation of pVACMC1 with saliva from five different volunteers (Table 2). After 10 min of exposure to saliva, between 35 and 61% of the target DNA had been degraded, while between 75 and 94% of the target had been degraded after 1 h.
TABLE 2.
Plasmid DNA survival in human saliva
| Incubation time | % DNA degradationa
|
||||||
|---|---|---|---|---|---|---|---|
| Volunteer 1 | Volunteer 2 | Volunteer 3 | Volunteer 4 | Volunteer 5 | Mean | SE | |
| 0 min | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 min | 57 | 61 | 60 | 51 | 35 | 52 | 7 |
| 60 min | 85 | 94 | 78 | 75 | 80 | 82 | 9 |
| 360 min | 99 | 99 | ND | 93 | 99 | 97 | 10 |
| 24 h | 99 | 99 | 99 | 97 | 99 | 99 | 10 |
DNA degradation was estimated by competitive PCR following incubation of plasmid pVACMC1 with fresh whole saliva from five different volunteers. Percent degradation was calculated after estimation of the DNA concentration (in micrograms per milliliter) at time zero and at the subsequent times shown. Saliva sampling was conducted as described in Materials and Methods. ND, not determined.
In the experiment represented by Fig. 3, pVACMC1 was exposed to human saliva for varying time intervals and plasmid DNA was immediately extracted to inhibit further degradation. Half of the partially degraded DNA was run on a 0.8% (wt/vol) agarose gel (Fig. 3A), and the other half was used to transform competent cells of S. gordonii DL1 (Fig. 3B). The object of this experiment was to determine for how long DNA fragments of a size sufficient to effect transformation remained, and very high initial concentrations of DNA (30 μg/ml) were used to allow sensitive detection. Degradation of pVACMC1 is evident from the disappearance of the band corresponding to the open circular form of the plasmid, which is visible at time zero, and from the initial increase and subsequent decrease in intensity of the band representing the linear form of the plasmid between 4 and 10 min (Fig. 3A). Saliva-degraded pVACMC1 DNA showed a rapidly decreasing capacity to transform S. gordonii DL1 (half-life, approximately 50 s) to erythromycin resistance, and the transformant yield after 2 min of degradation was some 10-fold lower than for unexposed DNA (Fig. 3B). The authenticity of transformants was confirmed by demonstrating the presence of a plasmid of the expected size and restriction profile, by PCR amplification with the specific CMCP2 and pVACrev primer pair and by the production of CMCase activity. Transformation efficiencies up to 9 min were all >1 × 10−7, which is higher than the spontaneous mutation rate to erythromycin resistance (<1 × 10−8) of this strain (data not shown). Successful transformation in gram-positive bacteria involves reconstitution of a functional plasmid from different partially degraded molecules (15), and this would be expected to require fragments significantly larger than the 520-bp PCR target studied, since replication functions, the endoglucanase gene, and the selectable marker must also be present.
Transformation of S. gordonii DL1 by plasmid DNA in the presence of human saliva.
Streptococci are normal components of the flora of the oropharyngeal mucosa and also occur in supragingival plaque and dental caries (12, 29, 33); a number of these species, including strains of S. gordonii and S. mutans, are reported to be naturally competent. S. gordonii DL1 (Streptococcus sanguis Challis), a naturally transformable strain originally isolated from human serum (13), was already known to be transformable by pVACMC1 in vitro (35) and was chosen here as a test recipient.
Transformation was demonstrated when competent S. gordonii DL1 cells and plasmid DNA, at a final concentration of 1.0 μg/ml, were simultaneously added to filter-sterilized saliva, as shown in Fig. 4. Similar results were obtained for saliva from a second volunteer (results not shown). The apparent delay in the appearance of transformants is assumed to be due to the time required to build up a sufficient plasmid copy number and level of gene expression to achieve growth upon plating on media containing erythromycin. A lag phase was also observed in the absence of saliva (Fig. 4). Whole saliva gave a background of erythromycin-resistant colonies (approximately 106 CFU/ml from a total population of 108 CFU/ml), and native bacteria present in whole saliva would be expected to compete with the test strain for the added DNA. Nevertheless, approximately 0.1% of erythromycin-resistant colonies showed CMCase activity in agar plate tests in similar transformation experiments with whole saliva. These contained amplifiable pVACMC1 plasmid DNA and must have arisen by transformation. Degradation of pVACMC1 in filter-sterile saliva, estimated by competitive PCR, occurred at a rate of approximately 60% of that in whole saliva.
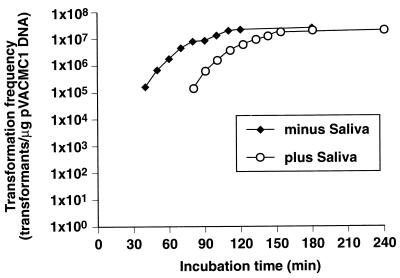
FIG. 4.
Transformation of S. gordonii DL1 with pVACMC1 in the presence and absence of filter-sterilized saliva. Competent cells were exposed to DNA and saliva or sterile dH2O for the times shown before plating on selective medium to identify transformants. Maximum transformation frequencies for the saliva of a second volunteer were 3.7 × 105. Experiments were carried out with different samples of S. gordonii DL1 competent cells.
In all of the experiments reported so far, S. gordonii DL1 was made competent according to standard procedures (16) by addition of 10% (vol/vol) heat-inactivated horse serum to the growth medium. In the experiment represented by Fig. 5, the effect of substituting alternative growth medium supplements for heat-inactivated horse serum upon competence development and subsequent transformation efficiency was studied. Replacement of heat-inactivated horse serum by untreated horse serum decreased transformant yield, and similar results were obtained with both heat-inactivated and untreated human serum (data not shown). However, the addition of filter-sterilized human saliva contributed to competence development and gave transformation efficiencies only 20% lower than those obtained with heat-inactivated horse serum (Fig. 5). In the absence of any apparent inducer of competence development, in the case of water supplementation, transformation efficiency was threefold lower than with heat-inactivated horse serum.
DISCUSSION
This work demonstrates that free DNA can survive for a significant time in samples of human saliva in an incompletely degraded state, since between 40 and 65% of a 520-bp target region within plasmid pVACMC1 was found to be amplifiable after 10 min of incubation with saliva. Since experiments were done in vitro and involved a 4:1 admixture of saliva and added DNA solution, the precise kinetics of degradation do not exactly reproduce those occurring in vivo. However, over short time intervals the admixture of freshly sampled saliva with the DNA-containing sample must be considered a reasonable simulation of the admixture of ingested food with saliva in the mouth. High initial concentrations of DNA were studied here in order to test the ability of a plasmid that had been exposed to salivary nucleases to transform naturally competent cells of S. gordonii DL1, a species that forms part of the oral microflora. The rapid decrease observed in transforming activity with time of exposure to saliva, which decayed with a half-life of approximately 50 s, reflects the decreasing population of transforming DNA molecules. Natural transformation of gram-positive bacteria is thought to involve reconstitution of an active plasmid from more than one partially degraded molecule of plasmid DNA within the cell exhibiting two-hit kinetics (1, 15). Therefore, the transformation frequency is expected to change in proportion to the square of the DNA concentration.
We also show here that naturally competent S. gordonii DL1 can be transformed by plasmid DNA in the presence of human saliva. It is not known what proportion of oral streptococci develop competence under in vivo conditions in the oral cavity, and we have not attempted to address this question directly here. However, our findings do show that heat-inactivated horse serum, which is routinely added to S. gordonii DL1 cells to enhance development of “natural” competence, is not an absolute requirement for competence development. Furthermore, our data suggest that human saliva may itself contain factors that promote competence development. Thus, there is no reason to assume that S. gordonii and probably other oral streptococci, such as S. mutans, that show natural transformability under laboratory conditions (29, 34) are not capable of transformation at low frequencies in the oral cavity in vivo. The transformation experiments reported here were deliberately performed at high cell densities and with high initial DNA concentrations (1.0 μg/ml) in order to maximize detection of transformant numbers, and we make no attempt here to estimate the frequency of such events in vivo. A plasmid concentration of 1 μg/ml would correspond approximately to the complete plasmid content of 1 ml of a bacterial culture of 109 cells/ml, assuming a copy number of 50 per cell for a 10-kb plasmid, and is very unlikely to occur naturally. Assuming two-hit kinetics (1), it is predicted that transformation frequencies would be lower by a factor of 106 for a plasmid concentration of 1 ng/ml than those reported here for a 1-μg/ml plasmid concentration, but it is very difficult to extrapolate from homogeneous pure cultures to conditions in vivo, particularly for colonies or biofilms where cells and DNA may be highly localized. The question of whether quantitatively significant rates of natural genetic transformation occur in vivo in the oral cavity therefore remains open and requires further investigation, although it should be clear that even very infrequent transformation events can be highly significant if the transforming DNA bestows a selective advantage on the recipient. DNA is constantly released from ingested plant, animal, or microbial cells in different regions of the gut. Although naked DNA is predicted to have less chance of survival under conditions prevailing lower down the digestive tract than in the oral cavity due to the effects of stomach acid and pancreatic nucleases, survival of bacteriophage M13 DNA has been demonstrated in the rat gut (27). In conclusion, the possibility of microbial transformation events occurring in a variety of gut microenvironments cannot be ruled out and deserves attention as a potential factor in the adaptation and evolution of gut microorganisms.
ACKNOWLEDGMENTS
This work was supported by a grant from the United Kingdom Ministry of Agriculture, Fisheries and Food, as part of the Novel Foods Programme.
We thank Terry Whitehead for the donation of S. gordonii DL1 and for help with streptococcal transformations. We also thank Colin Stewart for valuable contributions during discussions.
REFERENCES
- 1.Behnke D. Plasmid transformation of Streptococcus sanguis (Challis) occurs by circular and linear molecules. Mol Gen Genet. 1981;182:490–497. doi: 10.1007/BF00293940. [DOI] [PubMed] [Google Scholar]
- 2.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamier B, Lorenz M G, Wackernagel W. Natural transformation of Acinetobacter calcoaceticus by plasmid DNA adsorbed on sand and groundwater aquifer material. Appl Environ Microbiol. 1993;59:1662–1667. doi: 10.1128/aem.59.5.1662-1667.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duval-Iflah Y, Gainche I, Ouriet M-F, Lett M-C, Hubert J-C. Recombinant DNA transfer to Escherichia coli of human faecal origin in vitro and in digestive tract of gnotobiotic mice. FEMS Microbiol Ecol. 1994;15:79–88. [Google Scholar]
- 5.Ehrlich S D. Biotechnology action programme (Biotechnology R & D in the EC, part II). Commission of the European Communities catalogue of BAP achievements on risk assessment for the period 1985–1990. Brussels, Belgium: Cordis-RTD Publications; 1990. Gene transfer from and survival of genetically modified lactic acid bacteria; pp. 1–91. [Google Scholar]
- 6.Flint H J, McPherson C A, Bisset J. Molecular cloning of genes from Ruminococcus flavefaciens encoding xylanase and β(1-3,1-4)glucanase activities. Appl Environ Microbiol. 1989;55:1230–1233. doi: 10.1128/aem.55.5.1230-1233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallori E, Bazzicalupo M, Dalcanto L, Fani R, Nannipieri P, Vettori C, Stotzky G. Transformation of Bacillus subtilis by DNA bound on clay in non-sterile soil. FEMS Microbiol Ecol. 1994;15:119–126. [Google Scholar]
- 8.Gibbons R J, van Houte J. On the formation of dental plaques. J Periodontol. 1973;44:347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- 9.Gruzza M, Fons M, Ouriet M F, Duval-Iflah Y, Ducluzeau R. Study of gene transfer in vitro and in the digestive tract of gnotobiotic mice from Lactococcus lactis strains to various strains belonging to human intestinal flora. Microb Releases. 1994;2:183–189. [PubMed] [Google Scholar]
- 10.Güssow D, Clackson T. Direct clone characterisation from plaques and colonies by the polymerase chain reaction. Nucleic Acids Res. 1989;17:4000. doi: 10.1093/nar/17.10.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Innis M A, Gelfand D H. Optimisation of PCR’s. In: Innis M A, et al., editors. PCR protocols: a guide to methods and applications. London, United Kingdom: Academic Press Inc.; 1990. [Google Scholar]
- 12.Jenkinson H F, Terry S D, McNab R, Tannock G W. Inactivation of the gene encoding surface protein SspA in Streptococcus gordonii DL1 affects cell interactions with human salivary agglutinin and oral actinomyces. Infect Immun. 1993;61:3199–3208. doi: 10.1128/iai.61.8.3199-3208.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuramitsu H K, Trapa V. Genetic exchange between oral streptococci during mixed growth. J Gen Microbiol. 1984;130:2497–2500. doi: 10.1099/00221287-130-10-2497. [DOI] [PubMed] [Google Scholar]
- 14.Leenhouts K J, Kok J, Venema G. Stability of integrated plasmids in the chromosome of Lactococcus lactis. Appl Environ Microbiol. 1990;56:2726–2735. doi: 10.1128/aem.56.9.2726-2735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenz M G, Wackernagel W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev. 1994;58:563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macrina F L, Keeler C L, Jr, Jones K R, Wood P H. Molecular characterization of unique deletion mutants of the streptococcal plasmid pAMβ1. Plasmid. 1980;4:8–16. doi: 10.1016/0147-619x(80)90079-7. [DOI] [PubMed] [Google Scholar]
- 17.Macrina F L, Tobian J A, Jones K R, Evans R P, Clewell D B. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene. 1982;19:345–353. doi: 10.1016/0378-1119(82)90025-7. [DOI] [PubMed] [Google Scholar]
- 18.Madigan M T, Martinko J M, Parker J. Biology of microorganisms. London, United Kingdom: Prentice-Hall International; 1997. [Google Scholar]
- 19.Méjean V, Claverys J-P. DNA processing during entry in transformation of Streptococcus pneumoniae. J Biol Chem. 1993;268:5594–5599. [PubMed] [Google Scholar]
- 20.Molly K, Van de Woestyne M, Verstraete W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl Microbiol Biotechnol. 1993;39:254–258. doi: 10.1007/BF00228615. [DOI] [PubMed] [Google Scholar]
- 21.O’Sullivan D J, Klaenhammer T R. High-copy-number and low-copy-number Lactococcus shuttle cloning vectors with features for clone screening. Gene. 1993;137:227–231. doi: 10.1016/0378-1119(93)90011-q. [DOI] [PubMed] [Google Scholar]
- 22.Romanowski G, Lorenz M G, Wackernagel W. Plasmid DNA in a groundwater aquifer microcosm: adsorption, DNA-ase resistance and natural genetic-transformation of Bacillus subtilis. Mol Ecol. 1993;2:171–181. doi: 10.1111/j.1365-294x.1993.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 23.Ryder M. Key issues in the deliberate release of genetically-manipulated bacteria. FEMS Microbiol Ecol. 1994;15:139–145. [Google Scholar]
- 24.Salyers A A. Gene transfer in the mammalian intestinal tract. Curr Opin Biotechnol. 1993;4:294–298. doi: 10.1016/0958-1669(93)90098-h. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Schauf C L, Moffett D F, Moffett S B. Human physiology: foundations and frontiers. St. Louis, Mo: Times Mirror/Mosby College Publishing; 1990. [Google Scholar]
- 27.Schubbert R, Lettmann C, Doerfler W. Ingested foreign (phage M13) DNA survives transiently in the gastrointestinal tract and enters the bloodstream of mice. Mol Gen Genet. 1994;242:495–504. doi: 10.1007/BF00285273. [DOI] [PubMed] [Google Scholar]
- 28.Schubbert R, Renz D, Schmitz B, Doerfler W. Foreign (M13) DNA ingested by mice reaches peripheral leukocytes, spleen, and liver via the intestinal wall mucosa and can be covalently linked to mouse DNA. Proc Natl Acad Sci USA. 1997;94:961–966. doi: 10.1073/pnas.94.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah G R, Caufield P W. Enhanced transformation of Streptococcus mutans by modifications in culture conditions. Anal Biochem. 1993;214:343–346. doi: 10.1006/abio.1993.1503. [DOI] [PubMed] [Google Scholar]
- 30.Simon D, Chopin A. Construction of a vector plasmid and its use for molecular cloning in Streptococcus lactis. Biochimie. 1988;70:559–566. doi: 10.1016/0300-9084(88)90093-4. [DOI] [PubMed] [Google Scholar]
- 31.Skujins J. Extracellular enzymes in soil. Crit Rev Microbiol. 1976;4:383–421. doi: 10.3109/10408417609102304. [DOI] [PubMed] [Google Scholar]
- 32.Stewart G J, Carlson C A. The biology of natural transformation. Annu Rev Microbiol. 1986;40:211–235. doi: 10.1146/annurev.mi.40.100186.001235. [DOI] [PubMed] [Google Scholar]
- 33.Tao L, MacAllister T J, Tanzer J M. Transformation efficiency of EMS-induced mutants of Streptococcus mutans of altered cell shape. J Dent Res. 1993;72:1032–1039. doi: 10.1177/00220345930720060701. [DOI] [PubMed] [Google Scholar]
- 34.Westergren G, Emilson C-G. Prevalence of transformable Streptococcus mutans in human dental plaque. Infect Immun. 1983;41:1386–1388. doi: 10.1128/iai.41.3.1386-1388.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitehead T R, Flint H J. Heterologous expression of an endoglucanase gene (endA) from a ruminal anaerobe Ruminococcus flavefaciens 17 in Streptococcus bovis and Streptococcus sanguis. FEMS Microbiol Lett. 1995;126:165–170. doi: 10.1111/j.1574-6968.1995.tb07411.x. [DOI] [PubMed] [Google Scholar]