Abstract
Three experiments were conducted to compare the digestible (DE), metabolizable energy (ME), and standardized ileal digestibility (SID) of amino acids (AA) in fermented corn germ meal (FCGM) and soybean meal (SBM), and evaluate the effects of FCGM replacing SBM in growing pig diets. In Exp. 1, 18 barrows with initial body weight (BW) of 60.2 ± 3.40 kg were randomly allotted to 3 treatments with 6 replicates per treatment. The control diet used corn as the only energy ingredient, and 2 test diets were made by replacing 25.8% of corn with FCGM or SBM. The DE and ME of FCGM were less (P < 0.01) than those of SBM. In Exp. 2, 18 barrows (59.3 ± 2.52 kg BW) with ileal T-cannulas were randomly allotted to 3 treatments with 6 replicates per treatment. The SID of arginine, tryptophan and proline were greater (P < 0.05) and the tyrosine was less (P = 0.01) in SBM compared with FCGM. In Exp. 3, 144 growing pigs (56.1 ± 5.22 kg BW) were randomly allotted to 4 treatments with 6 pens per treatment (3 barrows and 3 gilts per pen). Four diets (FCGM0, FCGM10, FCGM20 and FCGM30) were formulated using FCGM to replace 0%, 10%, 20% and 30% of SBM, respectively. The ME and SID values of AA of SBM and FCGM were determined by Exp. 1 and 2. Results showed that increasing FCGM inclusion quadratically (P < 0.05) increased the average daily gain (ADG), average daily feed intake, and the levels of serum immunoglobulin G (IgG) and urea nitrogen, and linearly (P < 0.05) increased the serum IgM, the propanoic acid, butyric acid, total volatile fatty acid (VFA) and the Shannon index of microbiota in feces. Besides, the relative abundance of genus Streptococcus in FCGM0, Lactobacillus in FCGM10 and Lachnospiraceae in FCGM30 were increased (P < 0.05) compared with other treatments. In conclusion, we recommend replacing 11.80% of SBM with FCGM to obtain the optimal ADG of growing pigs. Moreover, as the ratio of FCGM replacing SBM increased in diet, the immunity, intestinal microbiota and total VFA composition of growing pigs were improved.
Keywords: Available energy, Amino acid digestibility, Fermented corn germ meal, Soybean meal, Growing pig
1. Introduction
Soybean meal (SBM) is an important protein feed ingredient in pig diet (Jezierny et al., 2010). However, the high cost of SBM and shortage of protein resources highlight an urgent need for suitable complements or substitutes for SBM (Tang et al., 2012). Corn germ meal (CGM) is a by-product of corn oil production (Estrada-Restrepo, 2017), which has been used in pet feed to partly replace corn and SBM (Li et al., 2013). Research in our lab have evaluated the nutritive value of some corn co-products, such as distillers dried grains with soluble and corn gluten meal in growing pig diet (She et al., 2015; Li et al., 2017), but there are a few studies about the CGM in growing pig diet. Corn germ meal contains 20% to 25% of crude protein (CP) and less than 3% of fat, as well as a comparable amino acid (AA) composition compared with corn, making it a potential ingredient in pig diet (NRC, 2012). The main fiber components in CGM are cellulose and arabinoxylan (Jaworski et al., 2015), which apparent total digestive tract digestibility is less than 50% (Urriola et al., 2010). The production of CGM is smaller than other corn co-products, but CGM is easily available on the market, and is cost-effective when combined with other ingredients in pig diets. However, Estrada-Restrepo (2017) reported that increasing the proportion of CGM in diet linearly decreased the carcass yield of growing-finishing pigs. Weber et al. (2010) also observed that diet containing 38.69% CGM significantly reduced feed efficiency in growing pigs. The negative impacts of CGM on pigs might due to its high fiber content, several other studies also demonstrated that high fiber content of CGM restricted its use in animal diets (Lee et al., 2012; Zhang et al., 2013).
One of the effective ways to reduce fiber content in ingredient is fermentation. Supriyati et al. (2015) found that crude fiber content of rice bran was significantly reduced after fermentation by Bacillus amyloliquefaciens. Wizna et al. (2009) also observed that crude fiber content was decreased when cassava waste was fermented. In addition, previous studies reported that the ferment bacteria (such as Bacillus and Lactobacillus strains in feed) could produce dietary enzymes (such as cellulase, amylase, lipase, phytase and protease) that degraded complex polysaccharides and other large nutrients into small and more digestible particles, which made feed ingredients more valuable for animals (Ouoba et al., 2003; Hu et al., 2016). However, there are almost no information about the effects of fermentation on CGM, and few researches about the application of fermented corn germ meal (FCGM) in growing pigs. Therefore, the aim of this study was to: 1) determine and compare the digestible (DE) and metabolizable energy (ME) as well as the standardized ileal digestibility (SID) of AA in FCGM and SBM; 2) evaluate the effects of partial replacement of SBM by FCGM on growth performance, serum parameters and the intestinal health of growing pigs, and obtain the appropriate ratio of FCGM to replace SBM in growing pig diet.
2. Materials and methods
The protocols of these 3 experiments were approved by the Institutional Animal Care and Use Committee of China Agricultural University (No. AW62701202-1-2, Beijing, China). The SBM and FCGM were sourced by China Oil and Foodstuffs Corporation (Beijing, China). The analyzed nutrient composition of FCGM and SBM are presented in Table 1.
Table 1.
Composition of soybean meal and fermented corn germ meal (as-fed basis).
| Item | SBM | FCGM |
|---|---|---|
| Common nutrient components, % | ||
| Dry matter | 89.10 | 85.80 |
| Crude protein | 46.56 | 20.40 |
| Organic matter | 93.42 | 90.50 |
| Neutral detergent fiber | 10.90 | 30.55 |
| Acid detergent fiber | 5.20 | 6.43 |
| Essential amino acids, % | ||
| Arginine | 3.23 | 0.52 |
| Histidine | 1.66 | 0.69 |
| Isoleucine | 1.76 | 0.79 |
| Leucine | 3.18 | 1.83 |
| Lysine | 2.88 | 0.82 |
| Methionine | 0.70 | 0.40 |
| Phenylalanine | 2.06 | 0.98 |
| Threonine | 2.11 | 0.79 |
| Tryptophan | 0.62 | 0.21 |
| Valine | 2.14 | 1.75 |
| Non-essential amino acids, % | ||
| Alanine | 1.93 | 1.74 |
| Asparagine | 4.77 | 1.60 |
| Cystine | 0.77 | 0.44 |
| Glutamine | 6.60 | 3.47 |
| Glycine | 1.89 | 1.02 |
| Proline | 2.37 | 1.84 |
| Serine | 2.10 | 1.03 |
| Tyrosine | 1.46 | 0.72 |
| Antigenic proteins, mg/g | ||
| Glycinin | 36.58 | – |
| β-conglycinin | 105.34 | – |
| Trypsin inhibitor, TIU/mg | 5.76 | – |
| Short-chain fatty acids, mmol/kg | ||
| Acetic acid | – | 110.24 |
| Propionic acid | – | 74.50 |
| Butyric acid | – | 4.37 |
| Total | – | 189.11 |
| Live bacteria, CFU/g | – | |
| Lactobacillus | – | 4.00 × 106 |
| Bacillus subtilis | – | 6.00 × 108 |
SBM = soybean meal; FCGM = fermented corn germ meal; TIU = trypsin inhibitor units; CFU = colony forming units.
2.1. Experimental design and sample collection
Exp. 1
Eighteen crossbred barrows (BW = 60.2 ± 3.40 kg) were randomly divided into 3 treatments equally, and the DE and ME contents of FCGM and SBM were determined by the difference method. The control diet included 97.0% of corn as the energy ingredient, and the other 2 diets were made by replacing 25.8% of corn in the control diet with FCGM or SBM (Table 2).
Table 2.
Ingredient composition of the diets in Exp. 1 (%, as-fed basis).
| Item | Corn | SBM | FCGM |
|---|---|---|---|
| Ingredients | |||
| Corn | 97.00 | 72.00 | 72.00 |
| SBM | – | 25.00 | – |
| FCGM | – | – | 25.00 |
| Dicalcium phosphate | 1.40 | 1.40 | 1.40 |
| Limestone | 0.75 | 0.75 | 0.75 |
| Salt | 0.35 | 0.35 | 0.35 |
| Vitamin and trace element premix1 | 0.50 | 0.50 | 0.50 |
| Analyzed nutrient levels | |||
| GE, MJ/kg | 15.80 | 16.05 | 15.95 |
| DM | 88.76 | 89.05 | 87.64 |
| CP | 7.72 | 16.58 | 10.75 |
| Ca | 0.63 | 0.66 | 0.62 |
SBM = soybean meal; FCGM = fermented corn germ meal; GE = gross energy; DM = dry matter; CP = crude protein.
Each kilogram of diet provided the following amounts of vitamins and minerals: Mn, 50 mg (MnO); Fe, 125 mg (FeSO4·H2O); Zn, 125 mg (ZnO); Cu, 150 mg (CuSO4·5H2O); I, 50 mg (CaI2); Se, 0.30 mg (Na2SeO3); retinyl acetic acid, 4,500 IU; cholecalciferol, 1,350 IU; DL-α-tocopheryl acetic acid, 13.5 mg; menadione sodium bisulfite complex, 2.7 mg; niacin, 18 mg; vitamin B12, 27.6 μg; thiamine, 0.6 mg; pyridoxine, 0.9 mg; riboflavin, 1.8 mg; D-calcium-pantothenate, 10.8 mg; nicotinic acid, 30.3 mg; choline chloride, 210 mg.
Pigs were housed in metabolic cages with an environment maintained at 24 ± 2 °C and had free access to drinking water. The feed supply was equal to 4% of BW and was divided equally into 2 parts and fed at 08:00 and 17:00 every day. The feed intake was recorded every feeding time, and the whole experiment period was divided into 7 d of adaptation to diet and 5 d of feces and urine collection. The feces were collected by plastic bags when they appeared and stored at −20 °C. Buckets with 10 mL of 6 mol/L HCl per 1,000 mL were used to collect urine. The urine volume was recorded daily and 10% of the urine was stored at −20 °C. Finally, the feces and urine of each experimental pig were separately mixed and then 300 g feces and 45 mL urine were taken as subsamples. Prior to analysis, the subsamples of feces were dried at 65 °C for 72 h and the urine subsamples (4 mL) were dried in a crucible with quantitative filter paper (BKMAM, Hunan, China) at 65 °C for 8 h (Pan et al., 2016).
Exp. 2
Eighteen crossbred barrows (BW = 59.3 ± 2.52 kg) fitted with T-cannulas at the distal ileum as the method of Stein et al. (1998) were randomly divided into 3 diets equally (Table 3). A nitrogen (N)-free diet was made to calculated the endogenous AA losses, and 62% of FCGM or 27.5% of SBM as the sole AA source were contained in the other 2 test diets.
Table 3.
Ingredient composition of the experimental diets in Exp. 2 (%, as-fed basis).
| Item | N-free diet | SBM | FCGM |
|---|---|---|---|
| Ingredients | |||
| Corn starch | 73.35 | 57.50 | 23.00 |
| SBM | – | 27.50 | – |
| FCGM | – | – | 62.00 |
| Sucrose | 15.00 | 10.00 | 10.00 |
| Soybean oil | 3.00 | 2.00 | 2.00 |
| Cellulose acetic acid1 | 4.00 | – | – |
| Dicalcium phosphate | 3.00 | 1.20 | 1.20 |
| Limestone | – | 0.70 | 0.70 |
| Salt | 0.45 | 0.30 | 0.30 |
| Magnesium oxide | 0.10 | – | – |
| Potassium carbonate | 0.30 | – | – |
| Chromic oxide | 0.30 | 0.30 | 0.30 |
| Vitamin and trace element premix2 | 0.50 | 0.50 | 0.50 |
| Analyzed nutrient levels | |||
| DM | 90.86 | 89.25 | 88.02 |
| CP | 0.45 | 12.46 | 13.66 |
| Essential amino acids | |||
| Arginine | 0.01 | 0.85 | 0.35 |
| Histidine | – | 0.42 | 0.41 |
| Isoleucine | 0.01 | 0.50 | 0.45 |
| Leucine | 0.04 | 0.83 | 1.10 |
| Lysine | 0.01 | 0.74 | 0.49 |
| Methionine | 0.03 | 0.16 | 0.26 |
| Phenylalanine | 0.02 | 0.56 | 0.58 |
| Threonine | 0.01 | 0.54 | 0.48 |
| Tryptophan | – | 0.15 | 0.15 |
| Valine | 0.02 | 0.60 | 1.11 |
| Non-essential amino acids | |||
| Alanine | 0.02 | 0.53 | 1.08 |
| Asparagine | 0.02 | 1.38 | 0.98 |
| Cystine | – | 0.23 | 0.26 |
| Glutamine | – | 2.06 | 2.16 |
| Glycine | 0.02 | 0.55 | 0.62 |
| Proline | – | 0.59 | 1.12 |
| Serine | 0.01 | 0.61 | 0.68 |
| Tyrosine | 0.02 | 0.36 | 0.43 |
SBM = soybean meal; FCGM = fermented corn germ meal; DM = dry matter; CP = crude protein.
Made by Chemical Reagents Company, Beijing, China.
The vitamin and trace element premix compositions were the same as those in Exp. 1.
The metabolic cages, environment and feeding method were the same as those in Exp. 1. The whole experimental period was divided into 14 d of recovery, 5 d of adaptation to diet, and 2 d of ileal digesta collection. Plastic bags were used to collect the digesta from 08:00 to 17:00 and removed to −20 °C refrigerator whenever the digesta were full or at least every 30 min. Further processing of the digesta samples was as described by Pan et al. (2017). At the end of the experiment, digesta sample per pig was thawed, mixed, subsampled, and then lyophilized in a vacuum freeze-dryer (Tofflon Freezing Drying systems, Minhang District, Shanghai, China).
Exp. 3
One hundred and forty-four crossbred growing pigs (BW = 56.1 ± 5.22 kg) were allocated to 4 groups in a randomized complete way with 6 pens per group and 6 pigs per pen (3 barrows and 3 gilts). Four diets (FCGM0, FCGM10, FCGM20 and FCGM30) were formulated using FCGM to replace 0%, 10%, 20% and 30% of SBM in diet. The diets of Exp. 3 (Table 4) were formulated according to NRC (2012) of growing pigs and the values of ME and SID of AA of FCGM and SBM were determined in Exp. 1 and 2.
Table 4.
Ingredient composition and nutrient levels of the experimental diets in Exp. 3 (%, as-fed basis).
| Item | Dietary treatments1 |
|||
|---|---|---|---|---|
| FCGM0 | FCGM10 | FCGM20 | FCGM30 | |
| Ingredients | ||||
| Corn | 64.16 | 64.71 | 64.84 | 64.01 |
| Soybean meal | 20.00 | 18.00 | 16.00 | 14.00 |
| FCGM | 0.00 | 5.00 | 10.00 | 15.00 |
| Wheat bran | 10.00 | 6.50 | 3.40 | 1.30 |
| Soybean oil | 2.20 | 2.20 | 2.20 | 2.20 |
| Dicalcium phosphate | 1.20 | 0.90 | 0.60 | 0.40 |
| Limestone | 0.90 | 1.10 | 1.30 | 1.41 |
| Sodium chloride | 0.30 | 0.30 | 0.30 | 0.30 |
| L-Lysine HCl | 0.30 | 0.34 | 0.38 | 0.40 |
| DL-Methionine | 0.05 | 0.05 | 0.05 | 0.05 |
| L-Threonine | 0.08 | 0.08 | 0.10 | 0.10 |
| L-Tryptophan | 0.06 | 0.07 | 0.08 | 0.08 |
| Vitamin and mineral premix2 | 0.50 | 0.50 | 0.50 | 0.50 |
| Chromic oxide | 0.25 | 0.25 | 0.25 | 0.25 |
| Analyzed nutrient levels | ||||
| GE, MJ/kg | 16.89 | 16.84 | 16.81 | 16.77 |
| CP | 16.10 | 15.96 | 15.93 | 15.87 |
| CF | 2.81 | 2.88 | 3.00 | 3.41 |
| NDF | 14.76 | 14.87 | 15.03 | 15.39 |
| ADF | 3.87 | 4.02 | 4.16 | 4.32 |
| Calculated nutrient levels3 | ||||
| Metabolizable energy, MJ/kg | 13.94 | 13.94 | 13.94 | 13.94 |
| SID lysine | 0.88 | 0.88 | 0.88 | 0.88 |
| SID methionine | 0.27 | 0.27 | 0.27 | 0.27 |
| SID threonine | 0.54 | 0.54 | 0.54 | 0.54 |
| SID tryptophan | 0.21 | 0.21 | 0.21 | 0.21 |
FCGM = fermented corn germ meal; GE = gross energy; CP = crude protein; CF = crude fiber; NDF = neutral detergent fiber; ADF = acid detergent fiber; SID = standard ileal digestible.
FCGM0, FCGM10, FCGM20 and FCGM30 diets were made by substituting fermented corn germ meal for 0%, 10%, 20% and 30% soybean meal.
The vitamin and trace element premix compositions were the same as those in Exp. 1.
The values were calculated according to feed composition and NRC (2012). Metabolizable energy and digestible amino acids of soybean meal and FCGM were obtained in Exp. 1 and 2.
Pigs had free access to diets and water in concrete-floored pens. The pigs were weighed on d 1 and 30. The feed intake of each pen during the experiment period was recorded, and the average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (F:G) were calculated. About 1 kg fresh feces each pen was collected during d 28 to 30, mixed, and dried at 65 °C for 72 h. Diet samples (500 g) and 5 mL fresh feces (on d 28) were taken and kept at −20 °C until analysis. At 06:00 on d 30, a blood sample was collected through the jugular vein from a pig whose weight was close to the average weight per pen. The blood sample was centrifuged at 3,000 × g for 10 min at 4 °C to obtain the serum sample and was kept at −80 °C until analysis.
2.2. Chemical analysis
The dry matter (DM), CP, ether extract (EE), calcium, phosphorus or ash contents of FCGM, SBM, diets and feces samples were analyzed according to the Association of Official Analytical Chemists (AOAC, 2012). The gross energy (GE) of diets, feces and urine were measured by an automatic isoperibol oxygen bomb calorimeter (Parr 6300 calorimeter; Parr Instrument Company, Moline, IL). The detailed analysis process of AA and chromium content were described by Pan et al. (2017) and Williams et al. (1962), respectively. The neutral detergent fiber (NDF) and acid detergent fiber (ADF) content were determined by the procedures of Van Soest et al. (1991). In addition, the level of immunoglobulin A (IgA), IgG and IgM in serum were analyzed as the operation steps of the kit (Nanjing Jiancheng Institute of Bioengineering, Nanjing). And the serum urea nitrogen (UN), total cholesterol (TC) and triglycerides (TG) were analyzed by automatic biochemical analyzer (CX9, Beckman, California, USA).
The quantification of volatile fatty acid (VFA) in fecal samples were detected. Approximately 1.5 g of fresh fecal sample was placed in a centrifuge tube, mixed with 1.5 mL of sterile water, and centrifuged at 15,000 × g for 15 min at 4 °C. The supernatant was transferred with a gas chromatography vial, mixed with 200 mL metaphosphoric acid, placed in ice for 30 min, and centrifuge at 15,000 × g for 15 min at 4 °C. The VFA content in the fecal sample was measured by a Hewlett Packard 5890 gas chromatograph (HP, Pennsylvania, USA) according to the procedure mentioned by Long et al. (2018).
The microbiota community in feces was also analyzed according Ma et al. (2021). Briefly, after genomic DNA extraction, 1% agarose gel (Thermo Scientific, MA, USA) was used to detect the extracted genomic DNA. The primers, V338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and V806R (5′-GGACTACHVGGGTWTCTAAT-3′), targeting variable region V3 to V4 were put into use (Munyaka et al., 2015). Mastercycler Gradient (Eppendorf, Germany) was used to do PCR and Miseq platform (Allwegene, Beijing, China) was used to do deep sequencing, and Illumina Analysis Pipeline Version 2.6 (Illumina, San Diego, USA) was used to do image analysis, base calling as well as error estimation. Firstly, the raw data was screened, and the data, which the sequences less than 230 bp in length, with quality score less than 20, ambiguous bases, or do not match primer sequence and barcode label well, were eliminated. All qualified reads are clustered into operational taxonomic units (OTU) with a similarity threshold of 97% (Edgar, 2013). Sparse curves are generated, and richness and diversity indexes are calculated. Alpha and beta diversity were tested by QIIME (University of California, San Diego, USA).
2.3. Calculations
In Exp. 1, the method of Adeola (2001) was used to determine the DE and ME of SBM or FCGM as follows:
| DEd = (GEi − GEf) × Fi |
| DEf = [DEd − (100% − X%) × DEd/0.97]/X% |
| MEd = (GEi − GEf − GEu)/Fi |
| MEf = [MEd − (100% − X%) × MEd/0.97]/X% |
in which the GEi, GEf, GEu and Fi represent the total GE intake, the total GE content in feces and urine, and the total feed intake over the 5-d collection period, respectively. The DEd and DEf represent the DE values in diet and SBM or FCGM, MEd and MEf represent the ME values in diet and SBM or FCGM, respectively. The X% is the percentage of energy supplied by SBM or FCGM in the basal diet.
In Exp. 2, the method of Stein et al. (2007) was used to determine the SID of AA: AID (%) = [1 − (AAd/AAf) × (Crf/Crd)] × 100%, in which the subscripts “d” and “f” represent the concentrations (g/kg DM) of the substance in ileal digesta and test diet, respectively. The endogenous loss of AA was calculated as: IAAend = [AAd × (Crf/Crd)], in which the subscript “d” represented the concentrations (g/kg DM) of the substance in ileal digesta of pigs fed N-free diet, and the subscript “f” represented the concentrations (g/kg DM) of Cr in N-free diet. Finally, the SID was calculated as follows: SID (%) = AID + (IAAend/AAf) × 100%.
2.4. Statistical analysis
The data of Exp. 1 and 2 were analyzed using SAS (version 9.2, 2008) with a Student's t-test for unpaired data with each pig as an experimental unit. The data except for the microbiota in Exp. 3 were analyzed using the MIXED procedure of SAS with pen as the experimental unit. The linear and quadratic effects caused by the different ratio of FCGM replacing SBM in diets were calculated by polynomial contrasts. A nonlinear regression (NLIN) model, ADG = a × (Ratio of FCGM replacing SBM)2 + b × (Ratio of FCGM replacing SBM) + c, was used to evaluate the optimal ratio of FCGM replacing SBM in diet to the ADG of growing pigs, and the a, b, c are constants (Robbins et al., 2006). Linear discriminant analysis effect size (LEfSe) used the KruskalWallis rank sum test to analyze the difference in the abundance of the microbiota in feces. The linear discriminant analysis (LDA) scores (threshold = ≥3) were used to indicate the size of the effect. Significant differences among groups were defined as P ≤ 0.05, and the highly significant difference among groups was defined as P ≤ 0.01.
3. Results
3.1. Chemical composition of SBM and FCGM
The concentration of CP, OM and the AA in SBM are higher than that in FCGM, whereas the NDF and ADF in SBM are lower than those in FCGM (Table 1). However, SBM contained glycinin (36.58 mg/g), β-conglycinin (105.34 mg/g) and trypsin inhibitor (5.76 TIU/mg), which were not found in FCGM. The fermentation process resulted in an increase of Lactobacillus and Bacillus subtilis counts in FCGM which maintained at about 4.00 × 106 and 6.00 × 108 CFU/g. In addition, the total amount of acetic acid, propionic acid and butyric acid contained in FCGM reached 189.11 mmol/kg.
3.2. Energy concentration in Exp. 1
The contents of DE and ME in diet of SBM group were greater than those of FCGM group (P < 0.01; Table 5). Besides, the DE and ME of FCGM or SBM were 13.75 and 12.13 MJ/kg, or 15.43 and 14.76 MJ/kg, respectively. Both DE and ME of SBM were higher than those of FCGM (P < 0.01).
Table 5.
The digestible and metabolizable energy values in diets and ingredients in Exp. 1 (as-fed basis).
| Item | Corn | SBM | FCGM | SEM1 | P-value |
|---|---|---|---|---|---|
| Energy in diets, MJ/kg | |||||
| Digestible energy | 14.19b | 14.39a | 13.97c | 0.06 | <0.01 |
| Metabolizable energy | 13.78a | 13.97a | 13.26b | 0.07 | <0.01 |
| Energy in ingredients, MJ/kg | |||||
| Digestible energy | 14.63ab | 15.43a | 13.75b | 0.31 | 0.01 |
| Metabolizable energy | 14.20a | 14.96a | 12.13b | 0.36 | <0.01 |
SBM = soybean meal; FCGM = fermented corn germ meal.
Values in a row with no common superscripts differ significantly (P < 0.05).
SEM stands for the standard error of the mean (n = 6).
3.3. Amino acid digestibility in Exp. 2
The SID of arginine, tryptophan and proline were greater (P < 0.05) in SBM than in FCGM, whereas the SID of tyrosine was lower (P = 0.01) in SBM compared with FCGM (Table 6).
Table 6.
The standardized ileal digestibility (SID) of AA in soybean meal (SBM) and fermented corn germ meal (FCGM) in Exp. 2.
| Item | SBM | FCGM | SEM1 | P-value |
|---|---|---|---|---|
| Essential amino acids, % | ||||
| Arginine | 89.02a | 86.25b | 0.39 | 0.04 |
| Histidine | 86.69 | 87.35 | 1.35 | 0.74 |
| Isoleucine | 87.22 | 86.62 | 0.92 | 0.67 |
| Leucine | 87.06 | 88.62 | 1.38 | 0.46 |
| Lysine | 84.59 | 84.63 | 2.63 | 0.99 |
| Methionine | 84.09 | 85.27 | 3.76 | 0.83 |
| Phenylalanine | 84.47 | 88.60 | 1.95 | 0.19 |
| Threonine | 83.55 | 85.84 | 1.79 | 0.41 |
| Tryptophan | 86.21a | 75.27b | 2.45 | 0.03 |
| Valine | 83.82 | 88.16 | 2.56 | 0.23 |
| Non-essential amino acids, % | ||||
| Alanine | 83.10 | 85.23 | 1.54 | 0.37 |
| Asparagine | 84.92 | 86.67 | 2.30 | 0.61 |
| Cystine | 82.16 | 83.32 | 2.05 | 0.70 |
| Glutamine | 85.80 | 88.50 | 1.20 | 0.17 |
| Glycine | 85.71 | 82.78 | 2.52 | 0.45 |
| Proline | 97.39a | 93.30b | 2.73 | <0.01 |
| Serine | 83.73 | 84.22 | 1.17 | 0.78 |
| Tyrosine | 81.77b | 89.92a | 1.46 | 0.01 |
a,bValues in a row with no common superscripts differ significantly (P < 0.05).
SEM stands for the standard error of the mean (n = 6).
3.4. Growth performance of growing pigs in Exp. 3
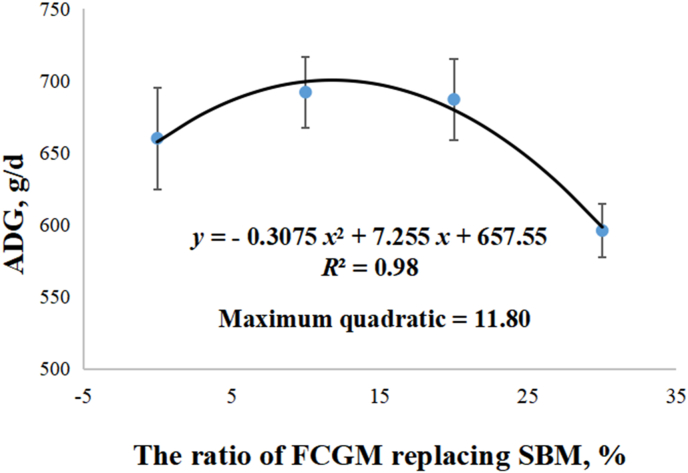
The ADG and ADFI of growing pigs showed quadratic (P < 0.05) effects in response to increasing the ratio of FCGM replacing SBM in diet (Table 7). Fig. 1 shows the quadratic model of ADG of growing pigs plotted against the ratio of FCGM replacing SBM in diet. The quadratic curve equation was: ADG = −0.3075 × (Ratio of FCGM replacing SBM)2 + 7.255 × (Ratio of FCGM replacing SBM) + 657.55, and the R2 was equal to 0.98. When the ratio of FCGM replacing SBM in diet was equal to 11.80%, the ADG reached the highest point.
Table 7.
Effect of replacing soybean meal with fermented corn germ meal (FCGM) in the diets on growth performance of growing pigs.
| Item | Dietary treatments1 |
SEM2 |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| FCGM0 | FCGM10 | FCGM20 | FCGM30 | Linear | Quadratic | |||
| Initial BW, kg | 56.0 | 56.0 | 56.0 | 56.1 | 3.93 | 0.96 | 0.93 | |
| Final BW, kg | 75.8 | 76.8 | 76.6 | 74.0 | 4.32 | 0.11 | 0.06 | |
| ADG, g/d | 660 | 693 | 687 | 597 | 21.30 | 0.06 | 0.01 | |
| ADFI, g/d | 2036 | 2090 | 2148 | 2017 | 56.50 | 0.46 | 0.03 | |
| F:G | 3.08 | 3.01 | 3.13 | 3.38 | 0.09 | 0.11 | 0.57 | |
BW = body weight; ADG = average daily gain; ADFI = average daily feed intake; F:G = feed conversion ratio.
FCGM0, FCGM10, FCGM20 and FCGM30 diets were made by substituting fermented corn germ meal for 0%, 10%, 20% and 30% soybean meal.
SEM stands for the standard error of the mean (n = 6).
Fig. 1.
Quadratic model of average daily gain (ADG) of growing pigs plotted against the ratio of fermented corn germ meal (FCGM) replacing soybean meal (SBM) in diet. The quadratic curve equation was: ADG = −0.3075 × (Ratio of FCGM replacing SBM)2 + 7.255 × (Ratio of FCGM replacing SBM) + 657.55, and the R2 was equal to 0.98. When the ratio of FCGM replacing SBM in diet was equal to 11.80%, the ADG (699.88 g/d) reached the highest point.
3.5. Apparent total tract digestibility of nutrient in growing pigs in Exp. 3
Table 8 shows the effects of increasing FCGM in diet on the apparent total tract digestibility (ATTD) of nutrient in growing pigs. The ATTD of ADF and NDF increased quadratically (P < 0.01) and linearly (P = 0.02) as the ratio of FCGM replacing SBM increased in diet, respectively.
Table 8.
Effect of replacing soybean meal with fermented corn germ meal (FCGM) in the diets on nutrient utilization of growing pigs (%).
| Item | Dietary treatments1 |
SEM2 |
P-value |
||||
|---|---|---|---|---|---|---|---|
| FCGM0 | FCGM10 | FCGM20 | FCGM30 | Linear | Quadratic | ||
| GE | 81.7 | 81.9 | 82.4 | 80.4 | 0.54 | 0.15 | 0.16 |
| CP | 77.7 | 79.2 | 79.8 | 78.8 | 0.74 | 0.27 | 0.09 |
| OM | 82.4 | 81.9 | 81.7 | 81.6 | 0.03 | 0.14 | 0.56 |
| ADF | 41.5 | 40.5 | 46.2 | 44.5 | 1.81 | 0.08 | <0.01 |
| NDF | 50.5 | 45.9 | 54.8 | 54.8 | 1.91 | 0.02 | 0.26 |
GE = gross energy; CP = crude protein; OM = organic matter; ADF = acid detergent fiber; NDF = and neutral detergent fiber.
FCGM0, FCGM10, FCGM20 and FCGM30 diets were made by substituting fermented corn germ meal for 0%, 10%, 20% and 30% soybean meal.
SEM stands for the standard error of the mean (n = 6).
3.6. Serum parameters of growing pigs in Exp. 3
Table 9 shows the effects of the increasing ratio of FCGM replacing SBM in diet on the serum parameters of growing pigs. Serum IgG and UN concentrations increased quadratically (P = 0.04), and the concentration of IgM raised linearly (P = 0.03) with the enhancement of FCGM level in diet.
Table 9.
Effect of replacing soybean meal with fermented corn germ meal (FCGM) in the diets on serum parameters of growing pigs.
| Item | Dietary treatments1 |
SEM2 |
P-value |
||||
|---|---|---|---|---|---|---|---|
| FCGM0 | FCGM10 | FCGM20 | FCGM30 | Linear | Quadratic | ||
| IgA, g/L | 0.90 | 1.11 | 1.04 | 0.91 | 0.08 | 0.92 | 0.16 |
| IgG, g/L | 8.41 | 9.26 | 9.12 | 8.28 | 0.37 | 0.74 | 0.04 |
| IgM, g/L | 0.74 | 0.80 | 0.76 | 0.94 | 0.05 | 0.03 | 0.24 |
| UN, mmol/L | 3.28 | 2.84 | 2.14 | 2.79 | 0.23 | 0.06 | 0.04 |
| TC, mmol/L | 2.34 | 2.18 | 2.46 | 2.23 | 0.09 | 0.90 | 0.70 |
| TG, mmol/L | 0.39 | 0.43 | 0.52 | 0.47 | 0.04 | 0.13 | 0.32 |
IgA = immunoglobulin A; IgG = immunoglobulin G; IgM = immunoglobulin M; UN = urea nitrogen; TC = total cholesterol; TG = total triglycerides.
FCGM0, FCGM10, FCGM20 and FCGM30 diets were made by substituting fermented corn germ meal for 0%, 10%, 20% and 30% soybean meal.
SEM stands for the standard error of the mean (n = 6).
3.7. The VFA in fecal samples of growing pigs in Exp. 3
The results of VFA in feces showed that the propanoic acid, butyric acid and total VFA increased linearly (P < 0.05) as the ratio of FCGM replacing SBM increased in diet (Table 10).
Table 10.
Effect of replacing soybean meal with fermented corn germ meal (FCGM) in the diets on fecal volatile fatty acids of growing pigs (mmol/kg).
| Item | Dietary treatments1 |
SEM2 |
P-value |
||||
|---|---|---|---|---|---|---|---|
| FCGM0 | FCGM10 | FCGM20 | FCGM30 | Linear | Quadratic | ||
| Acetic acid | 68.41 | 76.26 | 79.52 | 84.68 | 5.95 | 0.08 | 0.83 |
| Propionic acid | 24.15 | 29.45 | 30.00 | 32.27 | 2.35 | 0.04 | 0.53 |
| Isobutyric acid | 1.56 | 1.62 | 1.92 | 1.60 | 0.18 | 0.65 | 0.33 |
| Butyric acid | 9.36 | 13.01 | 16.30 | 17.74 | 1.85 | <0.01 | 0.57 |
| Isovaleric acid | 4.55 | 5.03 | 5.78 | 5.03 | 0.49 | 0.34 | 0.24 |
| Valeric acid | 1.44 | 2.66 | 2.54 | 2.63 | 0.54 | 0.19 | 0.32 |
| Total volatile fatty acid | 109 | 128 | 136 | 144 | 8.23 | 0.01 | 0.53 |
FCGM0, FCGM10, FCGM20 and FCGM30 diets were made by substituting fermented corn germ meal for 0%, 10%, 20% and 30% soybean meal.
SEM stands for the standard error of the mean (n = 6).
3.8. The 16S rRNA gene sequence in feces of growing pigs in Exp. 3
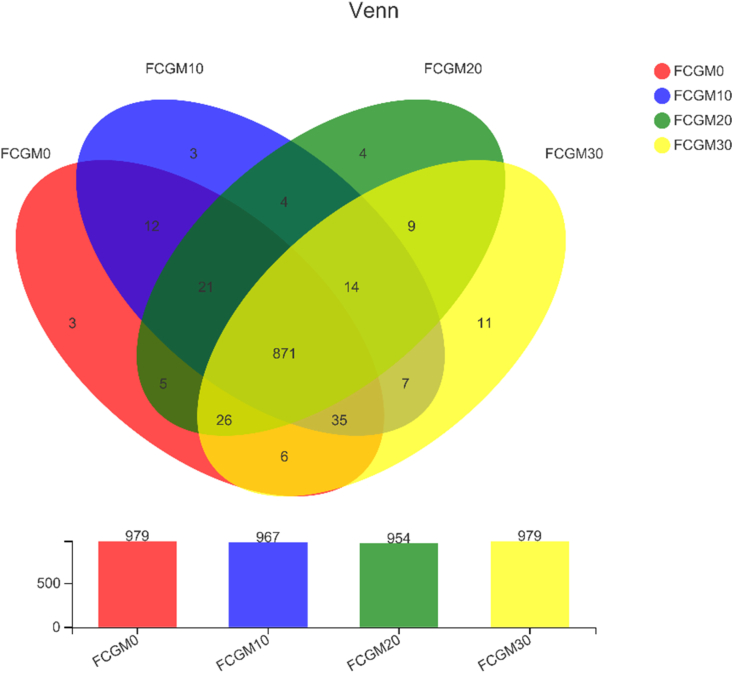
There were 871 common OTU and 21 unique OTU among 4 treatments in Exp. 3 (Fig. 2). Table 11 shows the results of alpha diversity analysis of which the Shannon index increased linearly with the ratio of FCGM replacing SBM in diet raised (P < 0.01).
Fig. 2.
Microbial OTU Venn diagram in fecal samples of growing pigs as affected by different ratio of fermented corn germ meal (FCGM) replacing soybean meal in diet. FCGM0, FCGM10, FCGM20 and FCGM30 diets were made by substituting FCGM for 0%, 10%, 20% and 30% soybean meal (n = 6). OUT = operational taxonomic units.
Table 11.
Effect of replacing soybean meal with fermented corn germ meal (FCGM) in the diets on the diversity of bacterial community in feces of growing pigs.
| Item | Dietary treatments1 |
SEM2 |
P-value |
||||
|---|---|---|---|---|---|---|---|
| FCGM0 | FCGM10 | FCGM20 | FCGM30 | Linear | Quadratic | ||
| Sobs | 676 | 702 | 709 | 700 | 29.8 | 0.56 | 0.57 |
| Shannon | 4.41 | 4.57 | 4.80 | 4.93 | 0.12 | <0.01 | 0.94 |
| Simpson | 0.04 | 0.04 | 0.05 | 0.06 | 0.01 | 0.16 | 0.98 |
| Ace | 811 | 733 | 793 | 807 | 31.2 | 0.74 | 0.17 |
| Chao | 820 | 735 | 803 | 820 | 32.1 | 0.64 | 0.14 |
FCGM0, FCGM10, FCGM20 and FCGM30 diets were made by substituting fermented corn germ meal for 0%, 10%, 20% and 30% soybean meal.
SEM stands for the standard error of the mean (n = 6).
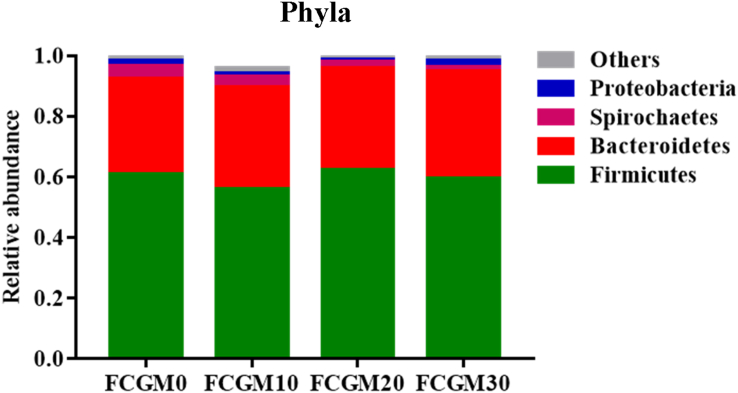
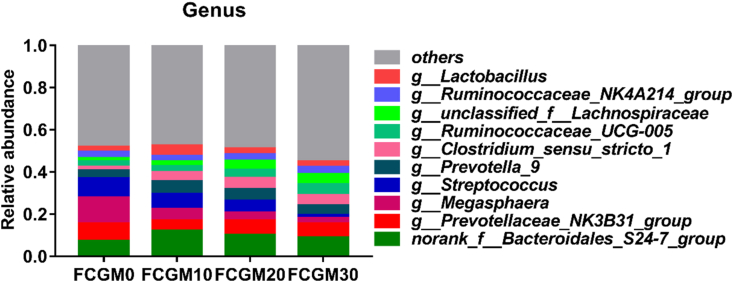
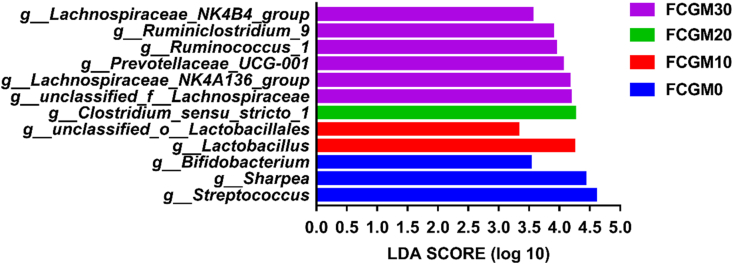
The relative abundances of the 4 most common taxa in different treatments at the phylum level are shown in Fig. 3. Firmicutes and Bacteroidetes were the most abundant, followed by Spirochaetes and Proteobacteria, and no significant difference was found at the phylum level among treatments. Fig. 4, Fig. 5 show the top 10 genus-level bacterial communities in relative abundance and the dominant microorganisms with taxa LDA value higher than 3.0 analyzed by LEfSe, respectively. The FCGM0 group had abundant Streptococcus (P < 0.05) genus, the FCGM10 group was abundant in Lactobacillus (P < 0.05) genus, group FCGM20 had high Clostridium_ sensu_ stricto (P < 0.05) and group FCGM30 had high Lachnospiraceae, Ruminiclostridium and Ruminococcus genus (P < 0.05).
Fig. 3.
Fecal microbial community at the phylum level of growing pigs as affected by different ratio of fermented corn germ meal (FCGM) replacing soybean meal in diet (relative abundances below 0.05% were grouped as “others”). FCGM0, FCGM10, FCGM20 and FCGM30 diets were made by substituting FCGM for 0%, 10%, 20% and 30% soybean meal (n = 6).
Fig. 4.
Fecal microbial community at the genus level of growing pigs as affected by different ratio of fermented corn germ meal (FCGM) replacing soybean meal in diet (relative abundances below 0.05% were grouped as “others”). FCGM0, FCGM10, FCGM20 and FCGM30 diets were made by substituting FCGM for 0%, 10%, 20% and 30% soybean meal (n = 6).
Fig. 5.
Fecal microbial community at the genus level of growing pigs as affected by different ratio of fermented corn germ meal (FCGM) replacing soybean meal in diet using LEfSe analyses (linear discriminant analysis score above 3, P < 0.05). FCGM0, FCGM10, FCGM20 and FCGM30 diets were made by substituting FCGM for 0%, 10%, 20% and 30% soybean meal (n = 6).
4. Discussion
As expected, Exp. 1 showed that the DE and ME were significantly lower in FCGM than in SBM, which might be related to the difference in chemical composition of SBM and FCGM. From Table 1, we could find that the CP content of SBM higher than that of FCGM, and the content of NDF and ADF of SBM were lower than that of FCGM, respectively. Noblet and Goff (2001) summarized the factors affecting the energy value of pig diets, they found that the DE and ME in ingredient were negatively correlated with the content of ADF and NDF, but positively correlated with the CP content of ingredient. Besides, previous research also reported the content of ADF in CGM was negatively correlated with its DE and ME (Shi et al., 2019). Therefore, the higher NDF and ADF as well as lower CP content in FCGM might be the main reason that caused the energy value of FCGM significantly lower than that of SBM. Besides, the available energy content of FCGM in this study can be used as a reference for pig diet formulations in production because these values were absent in NRC (2012). Although the content of AA in FCGM was lower than that in SBM, the SID values of most AA (except arginine, tryptophan, and proline) of FCGM were not significantly different from those of SBM in Exp. 2, which indicated that FCGM had a certain similarity with SBM in terms of the digestibility of AA. Besides, compared with SBM, FCGM did not contain antigen proteins (such as glycinin and β-conglycinin) or trypsin inhibitors which were harmful to the nutrient utilization and intestinal health of pigs (Dunsoford et al., 1989; Li et al., 1991). Furthermore, FCGM contains probiotics (Lactobacillus and B. subtilis) and total VFA which were beneficial to animals (Scholten et al., 1999). Therefore, although the available energy value of FCGM was lower than that of SBM, FCGM has the potential to partially replace SBM in a traditional corn–soybean meal diets and may have better feeding effects for pigs.
The effects of fermented feed in pigs have been studied for decades, however, almost no research on the effects of FCGM in diet of growing pigs. The results of Exp. 3 showed that the ADG and ADFI were increased quadratically with the ratio of FCGM replacing SBM raised in diet, which indicated that a suitable ratio of FCGM replacing SBM provided a better growth performance of growing pigs. The increased ADFI and ADG were observed when the ratio of FCGM replacing SBM was less than 20%, which might be associated with the increased levels of fiber content in diets. From the analyzed diet nutrient levels of Exp. 3, we found that the CF, NDF and ADF of diet all increased as the ratio of FCGM replacing SBM increased. Supriyati et al. (2015) found that the feed intake of animals would improve as the crude fiber raised in diet, and another research also reported that appropriate amount of fiber (below 5%) addition was beneficial for the growth performance of animals (Mateos et al., 2002). Interestingly, the ADG and ADFI of growing pigs dropped sharply when the ratio of FCGM replacing SBM in diet reached 30%, which might be related to the poor palatability of the FCGM30 diet. The palatability of fermented feed ingredients was affected by the pH value and the concentration of organic acids produced during the fermentation process (Le et al., 2016). The high concentrations of acetic acid, butyric acid and biogenic amines produced by fermentation would reduce the palatability of the diet (Missotten et al., 2015). Thus, the FCGM used in the current study contained acetic acid (110.24 mmol/kg) and butyric acid (4.37 mmol/kg), which might reduce the palatability of the diet of FCGM30 as the FCGM replacing SBM reached a large proportion (30%), and further caused a decrease in the ADFI and ADG of growing pigs.
The ATTD of ADF and NDF were increased quadratically and linearly, respectively, and the ATTD of CP tended to increase quadratically with the increase of the ratio of FCGM replacing SBM. In partial consistent with our results, Xu et al. (2019) found that the digestibility of DM, CP and GE of growing pigs fed fermented feed was increased by the method of meta-analysis. Similarly, Lee et al. (2014) also reported that increasing the content of fermented biomass of B. subtilis LS 1–2 in diet could linearly increase the digestibility of CP and GE in weaned piglets. Fermented feed could improve the digestibility of nutrients in animals, possibly because fermented feed contains the probiotics (such as Bacillus and Lactobacillus plantarum), which could produce dietary enzymes, such as cellulase and amylase, which degraded CP and carbohydrates into small peptides, free AA and other small molecular nutrients (Ouoba et al., 2003; Hu et al., 2016). Besides, fermentation also produced growth factors which could improve the activity of protease, amylase, and probiotic (Ouoba et al., 2003; Feng et al., 2007). In the current study, the FCGM analyzed to contain 4.00 × 106 CFU/g Lactobacillus and 6.00 × 108 CFU/g B. subtilis on as-fed basis, which might be the main reason that the digestibility of ADF and NDF increased with the levels of FCGM raised in diets. On the other hand, we also found the abundant Lachnospiraceae genus in feces of FCGM15 group, which could degrade non-starch polysaccharides and improve the utilization of dietary fiber (Reichardt et al., 2014). Therefore, the higher digestibility of ADF and NDF in pigs fed FCGM diets may be due to the live Lactobacillus and B. subtilis strains contained in FCGM.
It was reported that the composition of microorganisms in the intestines of animals has a strong correlation with their immune systems (Vrese and Marteau, 2007). In this research, the levels of serum IgG and IgM increased quadratically or linearly with the ratio of FCGM replacing SBM raised in diet, which might be caused by the improvement of the pig intestinal microflora. Previous studies showed that probiotics or organic acids in diet could inhibit Escherichia coli and Salmonella in the large intestine, and contribute to improve the concentrations of serum IgM and IgG of pigs (Zhu et al., 2017; Long et al., 2018). The FCGM used in the present study contained probiotics and VFA, which could favor the beneficial microorganisms and improve the intestinal balance of pigs (Peng et al., 2021). Besides, we also found that increasing the level of FCGM in diet could lead to a significant linear increase in butyric acid and total VFA in feces, which could reduce the disease sensitivity by lowering intestinal pH and modifying the immune system of pigs (Dibner and Buttin, 2002). In line with our results, Mizumachi et al. (2009) found that a fermented liquid diet prepared with L. plantarum LQ80 could improve the IgM and IgG levels in the serum of piglets. Our results indicated that the probiotics contained in FCGM as well as the high levels of the VFA in the large intestine of pigs fed FCGM diets might be the main reasons for the enhanced immunity of growing pigs.
The VFA are the main products of dietary fiber fermented by microorganisms in the large intestine, which can be rapidly absorbed by intestinal cells and have beneficial effects on animals (Bergman, 1990; Long et al., 2021). We found that increasing the ratio of FCGM replacing SBM in diet resulted in a significant linear increase in propionic acid, butyric acid and total VFA contents of feces, which was consistent with the increase in ATTD of ADF and NDF, indicating that fermented feed promoted microbial utilization of carbohydrates to produce VFA. Interestingly, the relative abundance of Lachnospiraceae was significantly higher in treatment FCGM30 than in other treatments, which could degrade non-starch polysaccharides to produce propionic acid and butyric acid (Reichardt et al., 2014; Louis et al., 2004). The VFA, especially butyric acid, was beneficial on the epithelial absorption function and cell growth of pigs (Bergman, 1990), which was also consistent with the highest digestibility of NDF and ADF as well as ADG of growing pigs appeared in diets with FCGM.
Alpha diversity is the diversity of species richness in a specific ecosystem (Xu et al., 2020). In Exp. 3, the Shannon index was increased linearly with the ratio of FCGM replacing SBM in diet raised, which could be explained by the suitable growth environment for the microorganisms due to the probiotic and organic acid of FCGM in diet. And our results also showed that FCGM in diet did not affect the relative abundance of microbiota at the phylum level, but at the genus level, the relative abundance of Lachnospiraceae in diet FCGM30 and Lactobacillus in diet FCGM10 were significantly increased compared with diet FCGM0, and the relative abundance of Streptococcus in diet FCGM0 was significantly higher than other groups. The increase of Lachnospiraceae in feces might be related to the increment in dietary fiber content (Jackson and Jewell, 2019). Biddle et al. (2013) found that Lachnospiraceae specializes in degrading fiber in the diet, which was also corresponding with the improvement of VFA content in feces. Additionally, it exhibited a significant increment in the relative abundance of Lactobacillus in diet FCGM10. The increasement in Lactobacillus can produce lactic acid, which could delay the proliferation of potential pathogens including E. coli by reducing the pH of gastrointestinal and effectively improving the growth performance of pigs (Suiryanrayna and Ramana, 2015). Streptococcus, one of the major pathogens in swine, has a strong virulence and drug resistance due to the ability to form biofilms (Wang et al., 2018). Researches showed that Lactobacillus and Bacillus can inhibit the growth and activity of intestinal pathogens, such as Streptococcus (Lan et al., 2016; Sirichokchatchawan et al., 2018). Therefore, the microbiological results showed that appropriate addition of FCGM in diets could inhibit the intestinal pathogens (such as Lachnospiraceae) and enrich beneficial bacteria (such as Lactobacillus) and further promote the immunity and health status of growing pigs.
5. Conclusion
It was concluded that the DE and ME as well as the SID of arginine, tryptophan and proline were lower in FCGM than in SBM. A low ratio of FCGM replacing SBM in diet could improve the growth performance of growing pigs, and we recommend replacing 11.80% of SBM with FCGM to obtain the optimal ADG of growing pigs. Moreover, as the ratio of FCGM replacing SBM increased in diet, the immunity, intestinal microbiota and VFA composition of growing pigs were improved.
Author contributions
Tengfei He: Conceptualization, Methodology, Software; Data curation, Writing – Original draft preparation; Yuhui Zheng: Visualization, Investigation; Shenfei Long: Software, Validation; Xiangshu Piao: Supervision; Writing – Reviewing and Editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study is supported by the National Natural Science Foundation of China (31772612), the Beijing Natural Science Foundation (6202019) and the Key Research & Developmental Program of Shandong Province (2019JZZY020308).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Adeola O. In: Swine nutrition. Lewis J., Southern L.L., editors. CRC Press; Washington, DC: 2001. Digestion and balance techniques in pigs; pp. 903–916. [DOI] [Google Scholar]
- AOAC . 19th ed. Off. Assoc. Anal. Chem.; Arlington, VA: 2012. Official methods of analysis. [Google Scholar]
- Bergman E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- Biddle A., Stewart L., Blanchard J., Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5:627–640. doi: 10.3390/d5030627. [DOI] [Google Scholar]
- Dibner J.J., Buttin P. Use of organic acid as a model to study the impact of gut microflora on nutrition and metabolism. J Appl Poult Res. 2002;11:453–463. doi: 10.3109/00016347909154051. [DOI] [Google Scholar]
- Dunsoford B.R., Knabe D.A., Hacnsly W.E. Effect of dietary soybean meal on the microscopic anatomy of the small intestine in the early-weaned pig. J Anim Sci. 1989;72:1855–1863. doi: 10.2527/jas1989.6771855x. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Estrada-Restrepo J.E. University of Illinois at Urbana-Champaign; 2017. Effects of body weight and research conditions on the productive energy content of corn germ meal fed to growing-finishing pigs. [Doctoral degree thesis Dissertation] [Google Scholar]
- Feng J., Liu X., Xu Z.R., Wang Y.Z., Liu J.X. Effects of fermented soybean meal on digestive enzyme activities and intestinal morphology in broilers. Poult Sci. 2007;86(6):1149–1154. doi: 10.1093/ps/86.6.1149. [DOI] [PubMed] [Google Scholar]
- Hu Y.N., Wang Y.W., Li A.K., Wang Z.S., Zhang X.L., Yun T.T., et al. Effects of fermented rapeseed meal on antioxidant functions, serum biochemical parameters and intestinal morphology in broilers. Food Agric Immunol. 2016;27:182–193. doi: 10.1080/09540105.2015.1079592. [DOI] [Google Scholar]
- Jackson M.I., Jewell D.E. Balance of saccharolysis and proteolysis underpins improvements in stool quality induced by adding a fiber bundle containing bound polyphenols to either hydrolyzed meat or grain-rich foods. Gut Microbes. 2019;10:298–320. doi: 10.1080/19490976.2018.1526580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski N.W., Lærke H.N., Bach Knudsen K.E., Stein H.H. Carbohydrate composition and in vitro digestibility of dry matter and nonstarch polysaccharides in corn, sorghum, and wheat and coproducts from these grains. J Anim Sci. 2015;93:1103–1113. doi: 10.2527/jas.2014-8147. [DOI] [PubMed] [Google Scholar]
- Jezierny D., Mosenthin R., Bauer E. The use of grain legumes as a protein source in pig nutrition: a review. Anim Feed Sci Technol. 2010;157:111–128. doi: 10.1016/j.anifeedsci.2010.03.001. [DOI] [Google Scholar]
- Lan R.X., Lee S.I., Kim I.H. Effects of multistrain probiotics on growth performance, nutrient digestibility, blood profiles, faecal microbial shedding, faecal score and noxious gas emission in weaning pigs. J Anim Physiol Anim Nutr. 2016;100:1130–1138. doi: 10.1111/jpn.12501. [DOI] [PubMed] [Google Scholar]
- Le M.H., Galle S., Yang Y., Landero J.L., Beltranena E., Gänzle M.G., et al. Effects of feeding fermented wheat with Lactobacillus reuteri on gut morphology, intestinal fermentation, nutrient digestibility, and growth performance in weaned pigs. J Anim Sci. 2016;94:4677–4687. doi: 10.2527/jas.2016-0693. [DOI] [PubMed] [Google Scholar]
- Lee J.W., Mckeith F.K., Stein H.H. Up to 30% corn germ may be included in diets fed to growing-finishing pigs without affecting pig growth performance, carcass composition, or pork fat quality. J Anim Sci. 2012;90:4933–4942. doi: 10.2527/jas.2012-5129. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Ingale S.L., Kim J.S., Kim K.H., Lokhande Anushka, Kim E.K., et al. Effects of dietary supplementation with Bacillus subtilis LS 1–2 fermentation biomass on growth performance, nutrient digestibility, cecal microbiota and intestinal morphology of weanling pig. Anim Feed Sci Technol. 2014;188:102–110. doi: 10.1016/j.anifeedsci.2013.12.001. [DOI] [Google Scholar]
- Li D.F., Nelssen J.L., Reddy P.G., Blecha F., Klemm R.D., Giesting D.W., et al. Measuring suitability of soybean products for early-weaned pigs with immunological criteria. J Anim Sci. 1991;69:3299–3307. doi: 10.2527/1991.6983299x. [DOI] [PubMed] [Google Scholar]
- Li M.H., Robinson E.H., Oberle D.F., Lucas P.M., Bosworth B.G. Use of corn meal in diet for pond-raised channel catfish, Ictalurus punctatus. J World Aquacult Soc. 2013;44:282–287. doi: 10.1111/jwas.12019. [DOI] [Google Scholar]
- Li Z.C., Li P., Liu D.W., Li D.F., Wang F.L., Su Y.B., et al. Determination of the energy value of corn distillers dried grains with solubles containing different oil levels when fed to growing pigs. J Anim Physiol Anim Nutr. 2017;101:339–348. doi: 10.1111/jpn.12445. [DOI] [PubMed] [Google Scholar]
- Long S.F., Xu Y.T., Pan L., Wang Q.Q., Wang C.L., Wu J.Y., et al. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. Anim Feed Sci Technol. 2018;235:23–32. doi: 10.1016/j.anifeedsci.2017.08.018. [DOI] [Google Scholar]
- Long S.F., Liu S.J., Wang J., Mahfuz S., Piao X.S. Natural capsicum extract replacing chlortetracycline enhances performance via improving digestive enzyme activities, antioxidant capacity, anti-inflammatory function, and gut health in weaned pigs. Anim Nutr. 2021;7:305–314. doi: 10.1016/j.aninu.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P., Duncan S.H., McCrae S.I., Millar J., Jackson M.S., Flint H.J. Restricted distribution of the butyric acid kinase pathway among butyric acid-producing bacteria from the human colon. J Bacteriol. 2004;186:2099–2106. doi: 10.1128/JB.186.7.2099-0106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.Y., Piao X.S., Shang Q.H., Long S.F., Liu S.J., Mahfuz S. Mixed organic acids as an alternative to antibiotics improve serum biochemical parameters and intestinal health of weaned piglets. Anim Nutr. 2021;7:737–749. doi: 10.1016/j.aninu.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos G.G., Lazaro R., Gracia M.I. The feasibility of using nutritional modifications to replace drugs in poultry feeds. J Appl Poult Res. 2002;11:437–452. doi: 10.1093/japr/11.4.437. [DOI] [Google Scholar]
- Missotten J.A., Michiels J., Degroote J., De Smet S. Fermented liquid feed for pigs: an ancient technique for the future. J Anim Sci Biotechnol. 2015;6:4. doi: 10.1186/2049-1891-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumachi K., Aoki R., Ohmori H., Saeki M. Effect of fermented liquid diet prepared with Lactobacillus plantarum LQ80 on the immune response in weaning pigs. Animal. 2009;3:670–676. doi: 10.1017/S1751731109003978. [DOI] [PubMed] [Google Scholar]
- Munyaka P.M., Eissa N., Bernstein C.N., Khafipour E., Ghia J.E. Antepartum antibiotic treatment increases offspring susceptibility to experimental colitis: a role of the gut microbiota. PLoS One. 2015;10:536–558. doi: 10.1371/journal.pone.0142536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noblet J., Goff G.L. Effect of dietary fibre on the energy value of feeds for pigs. Anim Feed Sci Technol. 2001;90:35–52. doi: 10.1016/s0377-8401(01)00195-x. [DOI] [Google Scholar]
- NRC . 11th revised ed. National Academy Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- Ouoba L.I., Rechinger K.B., Barkholt V., Diawara B., Traore A.S., Jakobsen M. Degradation of proteins during the fermentation of African locust bean (Parkia biglobosa) by strains of Bacillus subtilis and Bacillus pumilus for production of Soumbala. J Appl Microbiol. 2003;94(3):396–402. doi: 10.1046/j.1365-2672.2003.01845.x. [DOI] [PubMed] [Google Scholar]
- Pan L., Li P., Ma X.K., Xu Y.T., Tian Q.Y., Liu L., et al. Tannin is a key factor in the determination and prediction of energy content in sorghum grains fed to growing pigs. J Anim Sci. 2016;94:2879–2889. doi: 10.2527/jas.2016-0457. [DOI] [PubMed] [Google Scholar]
- Pan L., Shang Q.H., Wu Y., Ma X.K., Long S.F., Liu L., et al. Concentration of digestible and metabolizable energy, standardized ileal digestibility, and growth performance of pigs fed diets containing sorghum produced in the United States or corn produced in China. J Anim Sci. 2017;95:4880–4892. doi: 10.2527/jas2017.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Tang Y.M., Huang Y.H. Gut health: the results of microbial and mucosal immune interactions in pigs. Anim Nutr. 2021;7(2):282–294. doi: 10.1016/j.aninu.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt N., Duncan S.H., Young P., Belenguer A., McWilliam Leitch C., Scott K.P., Flint H.J., Louis P. Phylogenetic distribution of three pathways for propionic acid production within the human gut microbiota. ISME J. 2014;8:1323–1335. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins K.R., Saxton A.M., Southern L.L. Estimation of nutrient requirements using broken-line regression analysis. J Anim Sci. 2006;84:155–165. doi: 10.2527/2006.8413_supplE155x. [DOI] [PubMed] [Google Scholar]
- Scholten R.H.J., van der Peet-Schwering C.M.C., Verstegen M.W.A., den Hartog L.A., Schrama J.W., Vesseur P.C. Fermented co-products and fermented compound diets for pigs: a review. Anim Feed Sci Technol. 1999;82:1–19. doi: 10.1016/S0377-8401(99)00096-6. [DOI] [Google Scholar]
- She Y., Su Y.B., Liu L., Huang C.F., Li J.T., Li P., et al. Effects of microbial phytase on coefficient of standardized total tract digestibility of phosphorus in growing pigs fed corn and corn co-products, wheat and wheat co-products and oilseed meals. Anim Feed Sci Technol. 2015;208:132–144. doi: 10.1016/j.anifeedsci.2015.07.011. [DOI] [Google Scholar]
- Shi M., Liu Z.Y., Wang H.L., Shi C.X., Liu L., Wang J.J., et al. Determination and prediction of the digestible and metabolizable energy contents of corn germ meal in growing pigs. Asian Australas J Anim Sci. 2019;32:405–412. doi: 10.5713/ajas.17.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichokchatchawan W., Pupa P., Praechansri P., Am-In N., Tanasupawat S., Sonthayanon P., Prapasarakul N. Autochthonous lactic acid bacteria isolated from pig faeces in Thailand show probiotic properties and antibacterial activity against enteric pathogenic bacteria. Microb Pathog. 2018;119:208–215. doi: 10.1016/j.micpath.2018.04.031. [DOI] [PubMed] [Google Scholar]
- Stein H.H., Shipley C.F., Easter R.A. Technical note: a technique for inserting a T-cannula into the distal ileum of pregnant sows. J Anim Sci. 1998;76:1433–1436. doi: 10.2527/1998.7651433x. [DOI] [PubMed] [Google Scholar]
- Stein H.H., Sève B., Fuller M.F., Moughan P.J., de Lange C.F. Committee on terminology to report AA bioavailability and digestibility. invited review: amino acid bioavailability and digestibility in pig feed ingredients: terminology and application. J Anim Sci. 2007;85:172–180. doi: 10.2527/jas.2005-742. [DOI] [PubMed] [Google Scholar]
- Suiryanrayna M.V., Ramana J.V. A review of the effects of dietary organic acids fed to swine. J Anim Sci Biotechnol. 2015;6:45. doi: 10.1186/s40104-015-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supriyati T.H., Susanti T., Susana I.W.R. Nutritional value of rice bran fermented by Bacillus amyloliquefaciens and humic substances and its utilization as a feed ingredient for broiler chickens. Asian Australas J Anim Sci. 2015;28:231–238. doi: 10.5713/ajas.14.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W., Sun H., Yao X.H., Wu Y.F., Wang X., Feng J. Effects of replacement of soybean meal by fermented cottonseed meal on growth performance, serum biochemical parameters and immune function of yellow-feathered broilers. Asian Australas J Anim Sci. 2012;25:393–400. doi: 10.5713/ajas.2011.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urriola P.E., Shurson G.C., Stein H.H. Digestibility of dietary fiber in distillers coproducts fed to growing pigs. J Anim Sci. 2010;88:2373–2381. doi: 10.2527/jas.2009-2227. [DOI] [PubMed] [Google Scholar]
- Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Vrese M.D., Marteau P.R. Probiotics and prebiotics: effects on diarrhea. J Nutr. 2007;137:803–811. doi: 10.1089/jmf.2005.067. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang Y.X., Sun L.Y., Grenier D., Yi L. Streptococcus suis biofilm: regulation, drug-resistance mechanisms, and disinfection strategies. Appl Microbiol Biotechnol. 2018;102:9121–9129. doi: 10.1007/s00253-018-9356-z. [DOI] [PubMed] [Google Scholar]
- Weber T.E., Trabue S.L., Ziemer C.J., Kerr B.J. Evaluation of elevated dietary corn fiber from corn germ meal in growing female pigs. J Anim Sci. 2010;88:192–201. doi: 10.2527/jas.2009-1896. [DOI] [PubMed] [Google Scholar]
- Williams C.H., David D.J., Iismaa O. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J Agric Sci. 1962;59:381–385. doi: 10.1017/S002185960001546X. [DOI] [Google Scholar]
- Wizna Abbas H., Rizal Y., Dharma A., Kompiang I.P. Improving the quality of tapioca by-products (onggok) as poultry feed through fermentation by Bacillus amyloliquefaciens. Paki J Nutr. 2009;8:1636–1640. doi: 10.3923/pjn.2009.1636.1640. [DOI] [Google Scholar]
- Xu B.C., Zhu L.Y., Fu J., Li Z., Wang Y.Z., Jin M.L. Overall assessment of fermented feed for pigs: a series of meta-analyses. J Anim Sci. 2019;97:4810–4821. doi: 10.1093/jas/skz350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.T., Lahaye L., He Z.X., Zhang J.X., Yang C.B., Piao X.S. Micro-encapsulated essential oils and organic acids combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88+) Anim Nutr. 2020;6:269–277. doi: 10.1016/j.aninu.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.Y., Yi J.Q., Piao X.S., Li P.F., Zeng Z.K., Wang D., et al. The metabolizable energy value, standardized ileal digestibility of amino acids in soybean meal, soy protein concentrate and fermented soybean meal, and the application of these products in early-weaned piglets. Asian Australas J Anim Sci. 2013;26:691–699. doi: 10.5713/ajas.2012.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.J., Gao M.X., Zhang R.L., Sun Z.J., Wang C.M., Yang F.F., et al. Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb Cell Fact. 2017;16:191–201. doi: 10.1186/s12934-017-0809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]