Abstract
Introduction
One of the most important tasks in the Emergency Department (ED) is to promptly identify the patients who will benefit from hospital admission. Machine Learning (ML) techniques show promise as diagnostic aids in healthcare.
Aim of the study
Our objective was to find an algorithm using ML techniques to assist clinical decision-making in the emergency setting.
Material and methods
We assessed the following features seeking to investigate their performance in predicting hospital admission: serum levels of Urea, Creatinine, Lactate Dehydrogenase, Creatine Kinase, C-Reactive Protein, Complete Blood Count with differential, Activated Partial Thromboplastin Time, DDi-mer, International Normalized Ratio, age, gender, triage disposition to ED unit and ambulance utilization. A total of 3,204 ED visits were analyzed.
Results
The proposed algorithms generated models which demonstrated acceptable performance in predicting hospital admission of ED patients. The range of F-measure and ROC Area values of all eight evaluated algorithms were [0.679-0.708] and [0.734-0.774], respectively. The main advantages of this tool include easy access, availability, yes/no result, and low cost. The clinical implications of our approach might facilitate a shift from traditional clinical decision-making to a more sophisticated model.
Conclusions
Developing robust prognostic models with the utilization of common biomarkers is a project that might shape the future of emergency medicine. Our findings warrant confirmation with implementation in pragmatic ED trials.
Keywords: emergency department, emergency medicine, machine learning techniques, artificial intelligence, biomarkers
Introduction
The Emergency Department (ED) represents a key element of any given healthcare facility and retains a high public profile. ED staff manage patients with a huge variety of medical problems and deal with all sorts of emergencies. ED congestion resulting in delays in care remains a frequent issue that prompts the development of tools for rapid triage of high-risk patients [1]. Moreover, it is well documented that timely interventions are critical for several acute diseases [2, 3]. One of the most commonly encountered ED priorities is to quickly identify those who will need hospital admission. Traditionally, this decision relies on clinical judgment aided by the results of laboratory tests. Human factors leading to diagnostic errors occur frequently and are associated with increased morbidity and mortality [4].
Machine Learning (ML) techniques show promise as diagnostic aids in healthcare and have sparked the discussion for their wider application in the ED [5]. Developing robust prognostic models with the utilization of common biomarkers to facilitate rapid and reliable decision-making regarding hospital admission of ED patients is a project that might shape the future of emergency medicine. However, relevant data from the ED is scarce. Recent studies have focused on clinical outcome and mortality prediction [6, 7].
We assessed biochemical markers and coagulation tests that are routinely checked in patients visiting the ED, seeking to investigate their performance in predicting whether the patients will be admitted to the hospital. Our aim is to find an algorithm using ML techniques to assist clinical decision-making in the emergency setting.
Materials and methods
This research is a retrospective observational study conducted in the ED of a public tertiary care hospital in Greece that has been approved by the Institutional Review Board of Sismanogleio General Hospital (Ref. No 15177/2020, 5969/2021).
This study examines the performance of eight machine learning models based on data of the Biochemistry and Hematology Departments from ED patients. Blood samples were obtained for the measurement of biochemical and hematological parameters. The serum levels of Urea (UREA) [Normal Range (NR)=10-50 mg/dL-test principle: kinetic test with urease and glutamate dehydrogenase], Creatinine (CREA) (NR=0.5-1.5 mg/dL-kinetic colorimetric assay based on the Jaffé method), Lactate Dehydrogenase (LDH) (NR=135-225 U/L-UV assay), Creatine Kinase (CPK) (NR=25-190 U/L-UV assay), C-Reactive Protein (CRP) (NR < 6 mg/L-particle‑enhanced immunoturbidimetric assay) were measured using the Cobas 6000 c501 Analyzer (Roche Diagnostics, Mannheim, Germany). Complete blood count (CBC) samples were collected, and parameters such as White Blood Cell (WBC) (NR=4-11 K/μl-flow cytometry analysis), Neutrophil (NEUT) (NR=40-75 %-flow cytometry), Lymphocyte (LYM) (NR=20-40%-flow cytometry) and Platelet (PLT) (NR=150-400 K/μl-hydrodynamic focusing-flow cytometry) counts and Hemoglobin (HGB) (NR=12-17.5 g/dL-SLS method) were analyzed using the Sysmex XE 2100 Automated Hematology Analyzer (Sysmex Corporation, Kobe, Japan). Routine hemostasis parameters such as activated partial thromboplastin time (aPTT) (NR=24-39 sec-clotting method), DDimer (DD) (NR <500 μg/L-immunoturbidimetric assay), and International Normalized Ratio (INR) (NR=0.86-1.20-calculated) were determined in plasma using the BCS XP Automated Hemostasis Analyzer (Siemens Healthcare Diagnostics, Marburg, Germany).
All raw data was retrieved from a standard Hospital Information System (HIS) and a Laboratory Information System (LIS). The analysis was performed using the Waikato Environment for Knowledge Analysis (WEKA) [8], a Data Mining Software in Java workbench.
The flow diagram of the study is depicted in Figure 1. A total of 3,204 ED visits were analyzed during the study period (14 March – 4 May 2019). The anonymous data set under investigation contains eighteen features presented in Table 1.
Fig. 1.
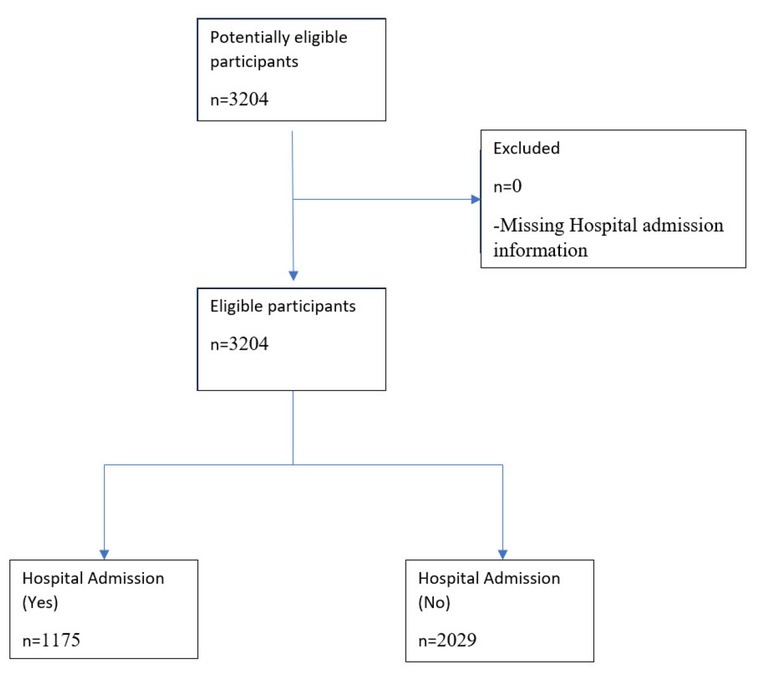
Patient flow diagram
Table 1.
Features
| Features | Type | Mean | Standard Deviation |
|---|---|---|---|
| CPK | numerical | 179.155 | 1183.877 |
| CREA | numerical | 1.06 | 0.827 |
| CRP | numerical | 39.094 | 71.48 |
| LDH | numerical | 222.327 | 156.343 |
| UREA | numerical | 45.651 | 33.616 |
| aPTT | numerical | 34.227 | 11.443 |
| DDIMER | numerical | 1422.899 | 2522.921 |
| INR | numerical | 1.131 | 0.571 |
| HGB | numerical | 12.87 | 2.13 |
| LYM | numerical | 22.085 | 11.672 |
| NEUT | numerical | 69.478 | 13.083 |
| PLT | numerical | 252.467 | 87.814 |
| WBC | numerical | 9.617 | 5.153 |
| Age | numerical; Integer* | 61.175 | 20.822 |
| Gender | categorical {Male, Female} | ||
| ED Unit | categorical {Urology, Pulmonology, Internal Medicine, Otolaryngology, Triage, Cardiology, General Surgery, Opthalmology, Vascular Surgery, Thoracic Surgery} | ||
| Ambulance | Categorical {Yes, No} | ||
| Admission | Categorical {Yes, No} |
*Patients’ age has been rounded to the nearest whole number
To assess the performance of the best-performing model (Smith and Frank 2016) for our analysis in WEKA, we have used a 10-fold cross-validation approach to avoid overfitting; Cross-validation is widely regarded as a quite reliable way to assess the quality of results from machine learning techniques. WEKA [9, 10, 11] provides detailed results for the classifiers under investigation regarding the following evaluation measures:
a. TP Rate (or Recall) is calculated as
b. FP Rate is calculated as
c. Precision is calculated as
d. F-Measure is calculated as
e. MMC is calculated as
f. The area under the Receiver Operating Characteristics (ROC) curve (AUC)
g. The PRC plot shows the relationship between precision and sensitivity.
Among many algorithms that were evaluated for our research purposes, in this article, we present only the eight best-performing algorithms, mainly in terms of ROC area and F-Measure.
During our experiments, we retained the default settings of all classification algorithms’ original implementations provided by WEKA. Each algorithm was evaluated on two data sets; the original data set, including the missing values, and on the data set where the missing values were identified, and they were replaced with appropriate values using WEKA’s ReplaceMissing-Values filter. Furthermore, since the number of patients in our data set who met clinical criteria for hospital admission (36.7%) is less than those who did not meet (63.3%), we applied WEKA’s ClassBalancer technique [8] to prevent overfitting by reweighting the instances in the data set so that each class had the same total weight during the phase of model training.
In our investigation, we evaluated a Naive Bayes classifier [12, 13], a multinomial logistic regression model with a ridge estimator [14], two boosting techniques; AdaBoost [15] and LogitBoost [16], Classification via Regression [17], a random forest [18], a bagging method [19] and a multilayer perceptron (MLP) (a neural network trained with error backpropagation) [8, 20].
Results
The performance of each algorithm was evaluated on its ability to predict whether a patient seen in the emergency department is subsequently admitted to the hospital or not by only taking into consideration the features presented in Table 1. All algorithms were evaluated on both datasets (original with missing values, modified by using the ReplaceMissingValues filter), and the detailed results are presented in Appendix (Tables A1-A16). The classification performance’s results on the original data set, regarding the F-Measure and ROC Area of each algorithm, are summarized in Table 2 and Figure 2.
Table 2.
Weighted Average values of F-Measure and ROC Area for all methods (10-fold cross-validation)
| F-Measure | ROC Area | |
|---|---|---|
| NaiveBayes | 0.679 | 0.734 |
| Logistic Regression | 0.697 | 0.762 |
| Ada boost | 0.685 | 0.753 |
| Logit boost | 0.708 | 0.774 |
| ClassificationViaRegression | 0.691 | 0.760 |
| Random Forest | 0.689 | 0.757 |
| Bagging | 0.703 | 0.764 |
| Multilayer perceptron | 0.707 | 0.742 |
Fig. 2.
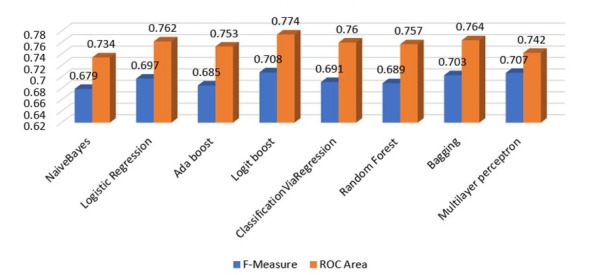
Weighted Average values of F-Measure and ROC Area for all methods (10-fold cross-validation)
According to Table 2, considering the weighted average values, it can be seen that Logit boost slightly outperformed other models with respect to both F-measure and ROC Area with values of 0.708 and 0.774, respectively. We can also observe that the range of F-measure and ROC Area values of all eight algorithms that were evaluated are [0.679-0.708] and [0.734-0.774], respectively, and they can be considered acceptable [21].
The classification performance’s results of F-Measure and ROC Area on the data set where the missing values have been replaced by using WEKA’s ReplaceMissing-Values filter are summarized in Table 3 and Figure 3.
Table 3.
Weighted Average values of F-Measure and ROC Area for all methods -ReplaceMissingValues filters (10-fold cross-validation)
| F-Measure | ROC Area | |
|---|---|---|
| NaiveBayes | 0.663 | 0.741 |
| Logistic Regression | 0.696 | 0.765 |
| Ada boost | 0.674 | 0.731 |
| Logit boost | 0.704 | 0.757 |
| ClassificationViaRegression | 0.691 | 0.758 |
| Random Forest | 0.723 | 0.789 |
| Bagging | 0.712 | 0.775 |
| Multilayer perceptron | 0.697 | 0.740 |
Fig. 3.
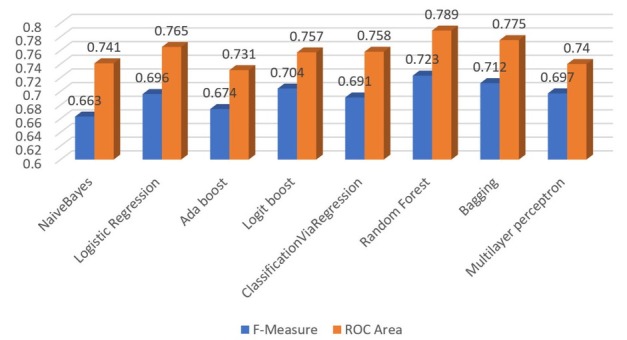
Weighted Average values of F-Measure and ROC Area for all methods - ReplaceMissingValues filters (10-fold cross-validation)
According to Table 3, considering the weighted average values, it can be seen that the Random Forest slightly outperformed other models with respect to both F-measure and ROC Area with values of 0.723 and 0.789, respectively. Additionally, we can observe that the range of F-measure and ROC Area values of all eight algorithms are [0.663-0.723] and [0.731-0.789], respectively, and as previously noted, they can also be considered acceptable. Furthermore, we were positively surprised to see that the impact of missing values on the classifiers’ performance was less pronounced than we initially thought.
Furthermore, since the admitted patients were 1175 versus 2029 that were not admitted, we applied the WEKA’s ClassBalancer technique on both datasets and re-evaluate the performance of the two classifiers (Logit boost and Random Forest). After the application of the ClassBalancer filter in the original data set, we observe that the performance of Logit boost (F-measure:0.693; ROC area:0.773) (Table A17) is quite similar to this of the imbalanced data set (F-measure:0.708; ROC area:0.774). Similar behavior, we also observe in the performance of Random Forest before (F-measure:0.723; ROC area:0.789) and after (F-measure:0.704; ROC area:0.784) (Table A18) the application of Class-Balancer filter in the data set where the missing values have been replaced by using the ReplaceMissingValues filter.
Discussion
Based on the data from 3,204 adult ED visits, using common laboratory tests and basic demographics, we evaluated eight ML algorithms that generated models that can reliably predict the hospital admission of patients seen in the ED. Our study utilized pre-existing patient data from a standard HIS and LIS. Therefore, the methods proposed here can serve as a valuable tool for the clinician to decide whether to admit or not an ED patient. The main advantages of this tool include easy access, availability, yes/no result, and low cost. The clinical implications of our approach might be significant and might facilitate a shift from traditional clinical decision-making to a more sophisticated model.
The application of machine learning techniques in the ED is not entirely new. Yet, it is not considered the standard of care. Current efforts are aiming to develop and integrate clinical decision support systems able to provide objective criteria to healthcare professionals. Our study is consistent with previous research showing that logistic regression is the most frequently used technique for model design. The area under the receiver operating curve (AUC) is the most frequently used performance measure [22]. Moreover, the major goal of such predictive tools is to identify high-risk patients accurately and differentiate them from stable, low-risk patients that can be safely discharged from the ED [23] and communicate this identification to the medical expert who can take this information into account while making a decision on admission or discharge.
The hectic pace of work and the stressful setting of the ED have negative consequences on patient safety [24, 25]. It is well established that human factors play an important role in the efficiency of healthcare systems. Different error types have different underlying mechanisms and require specific methods of risk management [26]. A fearful shortcoming for the emergency physician is to fail to admit a seriously ill patient. Our methods might be useful to reduce these errors while explicitly acknowledging that they are meant to aid and not substitute clinical judgment.
In summary, we present an inexpensive clinical decision support tool derived from readily available patient data. This tool is intended to aid the emergency physician regarding hospital admission decisions, as the development of machine learning models represents a rapidly evolving field in healthcare.
Limitations
This study is not without limitations. In our analysis, we did not include clinical parameters such as the vital signs and the Emergency Severity Index (ESI) [27]. We aimed to investigate whether our model can identify hospital admissions without taking into account clinical data. Thus, we included limited input variables in order to present a low-cost decision support tool with the minimum available data from our HIS. Τhere were also missing values in the data we collected and analyzed; for example, not all of the analyzed ED visits had all the laboratory investigations available. Furthermore, our preliminary findings have not yet been followed up by an implementation phase, and the proposed algorithms have not been validated in a pragmatic ED trial. Therefore, future research is warranted in order to demonstrate whether they can actually improve care.
Conclusions
In this study, we evaluated a collection of very popular ML classifiers on data from an ED. The proposed algorithms generated models which demonstrated acceptable performance in predicting hospital admission of ED patients based on common biochemical markers, coagulation tests, basic demographics, ambulance utilization, and triage disposition to the ED unit. Our research confirms the prevalent current notion that the utilization of artificial intelligence may have a favorable impact on the future of emergency medicine.
Appendix
Table A1.
Performance results by class of NaiveBayes (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.360 | 0.091 | 0.696 | 0.360 | 0.474 | 0.330 | 0.734 | 0.602 | Yes | |
| 0.909 | 0.640 | 0.710 | 0.909 | 0.797 | 0.330 | 0.734 | 0.811 | No | |
| Weighted Avg. | 0.708 | 0.439 | 0.705 | 0.708 | 0.679 | 0.330 | 0. 734 | 0.734 |
Table A2.
Performance results by class of NaiveBayes –ReplaceMissingValues filters (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.329 | 0.091 | 0.677 | 0.329 | 0.442 | 0.300 | 0.741 | 0.603 | Yes | |
| 0.909 | 0.671 | 0.700 | 0.909 | 0.791 | 0.300 | 0.741 | 0.822 | No | |
| Weighted Avg. | 0.696 | 0.458 | 0.692 | 0.696 | 0.663 | 0.300 | 0.741 | 0.742 |
Table A3.
Performance results by class of Logistic Regression (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.456 | 0.142 | 0.650 | 0.456 | 0.536 | 0.346 | 0.762 | 0.641 | Yes | |
| 0.858 | 0.544 | 0.732 | 0.858 | 0.790 | 0.346 | 0.762 | 0.838 | No | |
| Weighted Avg. | 0.711 | 0.396 | 0.702 | 0.711 | 0.697 | 0.346 | 0.762 | 0.766 |
Table A4.
Performance results by class of Logistic Regression–ReplaceMissingValues filters (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.456 | 0.143 | 0.649 | 0.456 | 0.536 | 0.345 | 0.765 | 0.643 | Yes | |
| 0.857 | 0.544 | 0.731 | 0.857 | 0.789 | 0.345 | 0.765 | 0.841 | No | |
| Weighted Avg. | 0.710 | 0.397 | 0.701 | 0.710 | 0.696 | 0.345 | 0.765 | 0.768 |
Table A5.
Performance results by class of AdaBoost (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admision | |
|---|---|---|---|---|---|---|---|---|---|
| 0.423 | 0.137 | 0.642 | 0.423 | 0.510 | 0.323 | 0.753 | 0.620 | Yes | |
| 0.863 | 0.577 | 0.721 | 0.863 | 0.786 | 0.323 | 0.753 | 0.836 | No | |
| Weighted Avg. | 0.702 | 0.415 | 0.692 | 0.702 | 0.685 | 0.323 | 0.753 | 0.757 |
Table A6.
Performance results by class of AdaBoost–ReplaceMissingValues filters (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admision | |
|---|---|---|---|---|---|---|---|---|---|
| 0.396 | 0.133 | 0.634 | 0.396 | 0.487 | 0.302 | 0.731 | 0.604 | Yes | |
| 0.867 | 0.604 | 0.713 | 0.867 | 0.782 | 0.302 | 0.731 | 0.815 | No | |
| Weighted Avg. | 0.694 | 0.431 | 0.684 | 0.694 | 0.674 | 0.302 | 0.731 | 0.738 |
Table A7.
Performance results by class of LogitBoost (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.489 | 0.147 | 0.658 | 0.489 | 0.561 | 0.370 | 0.774 | 0.657 | Yes | |
| 0.853 | 0.511 | 0.742 | 0.853 | 0.794 | 0.370 | 0.774 | 0.854 | No | |
| Weighted Avg. | 0.719 | 0.377 | 0.711 | 0.719 | 0.708 | 0.370 | 0.774 | 0.782 |
Table A8.
Performance results by class of LogitBoost –ReplaceMissingValues filters (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.464 | 0.135 | 0.666 | 0.464 | 0.547 | 0.364 | 0.757 | 0.641 | Yes | |
| 0.865 | 0.536 | 0.736 | 0.865 | 0.795 | 0.364 | 0.757 | 0.837 | No | |
| Weighted Avg. | 0.718 | 0.389 | 0.710 | 0.718 | 0.704 | 0.364 | 0.757 | 0.765 |
Table A9.
Performance results by class of ClassificationViaRegression (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.447 | 0.145 | 0.641 | 0.447 | 0.527 | 0.334 | 0.760 | 0.639 | Yes | |
| 0.855 | 0.553 | 0.727 | 0.855 | 0.786 | 0.334 | 0.760 | 0.839 | No | |
| Weighted Avg. | 0.705 | 0.403 | 0.696 | 0.705 | 0.691 | 0.334 | 0.760 | 0.766 |
Table A10.
Performance results by class of ClassificationViaRegression–ReplaceMissingValues filters (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.447 | 0.145 | 0.641 | 0.447 | 0.527 | 0.334 | 0.758 | 0.638 | Yes | |
| 0.855 | 0.553 | 0.727 | 0.855 | 0.786 | 0.334 | 0.758 | 0.837 | No | |
| Weighted Avg. | 0.705 | 0.403 | 0.696 | 0.705 | 0.691 | 0.334 | 0.758 | 0.764 |
Table A11.
Performance results by class of Random Forest (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.394 | 0.103 | 0.689 | 0.394 | 0.501 | 0.345 | 0.757 | 0.650 | Yes | |
| 0.897 | 0.606 | 0.719 | 0.897 | 0.798 | 0.345 | 0.757 | 0.832 | No | |
| Weighted Avg. | 0.713 | 0.422 | 0.708 | 0.713 | 0.689 | 0.345 | 0.757 | 0.765 |
Table A12.
Performance results by class of Random Forest–ReplaceMissingValues filters (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.540 | 0.161 | 0.660 | 0.540 | 0.594 | 0.399 | 0.789 | 0.676 | Yes | |
| 0.839 | 0.460 | 0.759 | 0.839 | 0.797 | 0.399 | 0.789 | 0.858 | No | |
| Weighted Avg. | 0.729 | 0.350 | 0.723 | 0.729 | 0.723 | 0.399 | 0.789 | 0.791 |
Table A13.
Performance results by class of Bagging (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.471 | 0.142 | 0.657 | 0.471 | 0.549 | 0.360 | 0.764 | 0.654 | Yes | |
| 0.858 | 0.529 | 0.737 | 0.858 | 0.793 | 0.360 | 0.764 | 0.840 | No | |
| Weighted Avg. | 0.716 | 0.387 | 0.708 | 0.716 | 0.703 | 0.360 | 0.764 | 0.772 |
Table A14.
Performance results by class of Bagging–ReplaceMissingValues filters (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.515 | 0.161 | 0.649 | 0.515 | 0.574 | 0.375 | 0.775 | 0.654 | Yes | |
| 0.839 | 0.485 | 0.749 | 0.839 | 0.791 | 0.375 | 0.775 | 0.852 | No | |
| Weighted Avg. | 0.720 | 0.366 | 0.712 | 0.720 | 0.712 | 0.375 | 0.775 | 0.779 |
Table A15.
Performance results by class of Multilayer perceptron (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.542 | 0.190 | 0.623 | 0.542 | 0.580 | 0.364 | 0.742 | 0.622 | Yes | |
| 0.810 | 0.458 | 0.753 | 0.810 | 0.781 | 0.364 | 0.742 | 0.815 | No | |
| Weighted Avg. | 0.712 | 0.360 | 0.705 | 0.712 | 0.707 | 0.364 | 0.742 | 0.744 |
Table A16.
Performance results by class of Multilayer perceptron–ReplaceMissingValues filters (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.480 | 0.161 | 0.633 | 0.480 | 0.546 | 0.343 | 0.740 | 0.617 | Yes | |
| 0.839 | 0.520 | 0.736 | 0.839 | 0.784 | 0.343 | 0.740 | 0.808 | No | |
| Weighted Avg. | 0.707 | 0.388 | 0.698 | 0.707 | 0.697 | 0.343 | 0.740 | 0.738 |
Table A17.
Performance results by class of Logit Boost– ClassBalancer filter (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.685 | 0.300 | 0.696 | 0.685 | 0.690 | 0.385 | 0.773 | 0.758 | Yes | |
| 0.700 | 0.315 | 0.690 | 0.700 | 0.695 | 0.385 | 0.773 | 0.779 | No | |
| Weighted Avg. | 0.693 | 0.307 | 0.693 | 0.693 | 0.693 | 0.385 | 0.773 | 0.769 |
Table A18.
Performance results by class of Random Forest– ReplaceMissingValues and ClassBalancer filters (10-fold cross-validation)
| TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area | Admission | |
|---|---|---|---|---|---|---|---|---|---|
| 0.653 | 0.243 | 0.729 | 0.653 | 0.689 | 0.412 | 0.784 | 0.767 | Yes | |
| 0.757 | 0.347 | 0.686 | 0.757 | 0.720 | 0.412 | 0.784 | 0.783 | No | |
| Weighted Avg. | 0.705 | 0.295 | 0.707 | 0.705 | 0.704 | 0.412 | 0.784 | 0.775 |
Footnotes
Conflict of interest
The authors report no conflict of interest.
References
- 1.Sun BC, Hsia RY, Weiss RE. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med. 2013;61(6):605–611. doi: 10.1016/j.annemergmed.2012.10.026. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaieski DF, Agarwal AK, Mikkelsen ME. The impact of ED crowding on early interventions and mortality in patients with severe sepsis. Am J Emerg Med. 2017;35(7):953–960. doi: 10.1016/j.ajem.2017.01.061. et al. [DOI] [PubMed] [Google Scholar]
- 3.Rathore SS, Curtis JP, Chen J. National Cardiovascular Data Registry. Association of door-to-balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: national cohort study. BMJ. 2009;338:b1807. doi: 10.1136/bmj.b1807. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hautz WE, Kämmer JE, Hautz SC. Diagnostic error increases mortality and length of hospital stay in patients presenting through the emergency room. Scand J Trauma Resusc Emerg Med. 2019;27(1):54. doi: 10.1186/s13049-019-0629-z. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feretzakis G, Loupelis E, Sakagianni A. Using Machine Learning Techniques to Aid Empirical Antibiotic Therapy Decisions in the Intensive Care Unit of a General Hospital in Greece. Antibiotics (Basel) 2020;9(2):50. doi: 10.3390/antibiotics9020050. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raita Y, Goto T, Faridi MK, Brown DFM, Camargo CA Jr, Hasegawa K. Emergency department triage prediction of clinical outcomes using machine learning models. Crit Care. 2019;23(1):64. doi: 10.1186/s13054-019-2351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan L, Zhang HT, Goncalves J. An interpretable mortality prediction model for COVID-19 patients. Nat Mach Intell. 2020;2:283–288. et al. [Google Scholar]
- 8.Hall M, Frank E, Holmes G, Pfahringer B, Reutemann P, Witten I. The WEKA data mining software. ACM SIGKDD Explorations Newsletter. 2009;11(1):10–18. [Google Scholar]
- 9.Kasperczuk A, Dardzinska A. Comparative Evaluation of the Different Data Mining Techniques Used for the Medical Database. Acta Mechanica Et Automatica. 2016;10(3):233. –. [Google Scholar]
- 10.Bouckaert R. Naive Bayes Classifiers That Perform Well with Continuous Variables. Lecture Notes in Computer Science. 2004;3339:1089–1094. [Google Scholar]
- 11.Han J, Pei J, Yin Y. Mining frequent patterns without candidate generation. ACM SIGMOD International Conference on Management of Data. 2000. pp. 1–12. –.
- 12.Rish I. An empirical study of the naive bayes clas-sifier. InIJCAI workshop on Empirical Methods in AI. 2005.
- 13.John GH, Langley P. Eleventh Conference on Uncertainty in Artificial Intelligence. San Mateo: 1995. Estimating Continuous Distributions in Bayesian Classifiers; pp. 338–345. [Google Scholar]
- 14.le Cessie S, van Houwelingen JC. Ridge Estimators in Logistic Regression. Applied Statistics. 1992;41(1):191–201. [Google Scholar]
- 15.Freund Y, Schapire RE. Thirteenth International Conference on Machine Learning. San Francisco: 1996. Experiments with a new boosting algorithm; pp. 148–156. [Google Scholar]
- 16.Friedman J, Hastie T, Tibshirani R. Additive Logistic Regression: a Statistical View of Boosting. The Annals of Statistics. 2000;28(2):337–407. [Google Scholar]
- 17.Frank E, Wang Y, Inglis S, Holmes G, Witten IH. Using model trees for classification. Machine Learning. 1998;32:63–76. [Google Scholar]
- 18.Breiman L. Random Forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 19.Breiman L. Bagging predictors. Machine Learning. 1996;24:123–140. [Google Scholar]
- 20.Smith TC, Frank E. Introducing Machine Learning Concepts with WEKA. Methods Mol Biol. 2016;1418:353–378. doi: 10.1007/978-1-4939-3578-9_17. [DOI] [PubMed] [Google Scholar]
- 21.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–6. doi: 10.1097/JTO.0b013e3181ec173d. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes M, Vieira SM, Leite F, Palos C, Finkelstein S, Sousa JMC. Clinical Decision Support Systems for Triage in the Emergency Department using Intelligent Systems: a Review. Artif Intell Med. 2020;102:101762. doi: 10.1016/j.artmed.2019.101762. [DOI] [PubMed] [Google Scholar]
- 23.Levin S, Toerper M, Hamrock E. Machine-Learning-Based Electronic Triage More Accurately Differentiates Patients With Respect to Clinical Outcomes Compared With the Emergency Severity Index. Ann Emerg Med. 2018;71(5):565–574. doi: 10.1016/j.annemergmed.2017.08.005. et al. [DOI] [PubMed] [Google Scholar]
- 24.Weant KA, Bailey AM, Baker SN. Strategies for reducing medication errors in the emergency department. Open Access Emerg Med. 2014;6:45–55. doi: 10.2147/OAEM.S64174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morley C, Unwin M, Peterson GM, Stankovich J, Kinsman L. Emergency department crowding: A systematic review of causes, consequences and solutions. PLoS One. 2018;13(8):e0203316. doi: 10.1371/journal.pone.0203316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reason J. Understanding adverse events: human factors. Qual Health Care. 1995;4(2):80–9. doi: 10.1136/qshc.4.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González J, Soltero R. Emergency Severity Index (ESI) triage algorithm: trends after implementation in the emergency department. Bol Asoc Med P R. 2009;101(3):7–10. [PubMed] [Google Scholar]


