Abstract
Background and Aims
The advent of biologic drugs revolutionised the treatment of many chronic inflammatory diseases in rheumatology, dermatology, and gastroenterology. The introduction of different targeted agents closely followed the increase in knowledge of pathogenic mechanisms. The identification of IL-23 as a master regulator of ‘pathogenic’ inflammation and the consequent efficacy of IL-23 blocking agents were first proofed in psoriasis and then in other inflammatory diseases such as psoriatic arthritis and Crohn’s disease.
Methods
We reviewed all available results from anti-Il-23 clinical trials for psoriasis, focusing on data of IBDologists’ interest. Regarding guselkumab, we analysed data from phase III clinical trials VOYAGE1, VOYAGE2, and NAVIGATE. For risankizumab, we reported efficacy and safety results from UltIMMa-1, UltIMMa-2, and IMMvent clinical trials, and tildrakizumab was evaluated by analysing data from reSURFACE1 and reSURFACE2 studies.
Results
Data from all the clinical trials that we reported showed both the efficacy of all three anti-IL-23 drugs in psoriasis and the safety of this class; in particular, no gastrointestinal side effects were observed in those studies. IL-23 blockers have shown promising short- and long-term results in psoriasis, with a major safety profile and no negative interactions with gastrointestinal system.
Conclusions
Anti-IL-23 indication for psoriatic arthritis is very recent and for IBD is still to come. Therefore, dermatologists are accumulating long-term experience with these drugs, both in clinical trials and in real-world evidence, which can help gastroenterologists in the management of IBD patients.
Keywords: IBD, psoriasis, anti-IL-23
1. Introduction
Psoriasis is a chronic autoimmune disorder affecting up to 2-4% of population worldwide.1 Psoriasis is mainly known for affecting skin and adnexa [hair and nails] with a massive impact on patient’s life quality. Furthermore, recent evidences have highlighted the systemic nature of the disease that is frequently associated to other autoimmune [including psoriatic arthritis, autoimmune thyroidits, uveitis, inflammatory bowel diseases] or cardiometabolic components [such as arterial hypertension, acute ischaemic cardiovascular events, dyslipidaemia, diabetes].2,3 For these reasons, early systemic treatment of psoriasis may be helpful not only for achieving skin clearance but also for preventing comorbidities’ progression.4
Historically, psoriasis was treated with topical therapies such as coal tar, vitamin D derivatives and topical corticosteroids which still keep an important role in slight forms of the disease. Topical treatments are also useful in combination with systemic therapies for managing moderate-severe manifestations of psoriasis 5 However, topical therapies have low compliance rates due to the discomfort of daily applications and the difficulties in reaching adequate skin clearance.
Conventional systemic treatments are immunosuppressants or immunomodulators [e.g., cyclosporine, acitretine, methotrexate, or dymethilfumarate] which are indicated in moderate-severe forms of psoriasis. These drugs have shown good clinical results; however there is a lack of randomised clinical trials [RCTs]. Furthermore, long-term usage of these immunosuppressants or immunomodulators is controversial due to their specific side effects.
Availability of biologic drugs has dramatically changed the approach to immune-mediated diseases in both dermatology and gastroenterology. Anti-TNF agents were the first approved drugs to be used for managing psoriasis and also to treat inflammatory bowel diseases [IBD], such as Crohn’s disease [CD] and ulcerative colitis [UC], as in the systemic treatment of psoriasis.6–10 Unfortunately, this drug class often loses efficacy and leads to side effects such as tuberculosis reactivation or paradoxical psoriasis and, for this reason, new therapeutic options have been investigated. Ustekinumab is a monoclonal antibody targeting the shared p40 subunit of IL12/23, which has been widely used both in dermatology and IBD as in the systemic treatment of psoriasis.11,12 However, in recent years IL-17 and IL-23 inhibitors have been investigated to improve clinical outcomes in a various range of immunological conditions.
Dermatology has also been the leading field for developing IL-17 inhibitors but this therapeutic class, despite a massive impact on psoriasis clearance, has failed clinical trials on IBD in not providing significant clinical benefits.13 Furthermore, anti-IL17 drugs have been reported to be associated with paradoxical gastrointestinal effects, such as latent IBD appearance or disease exacerbation.14,15
Recently, IL23 blockers [e.g., guselkumab, risankizumab, and tildrakizumab] have been investigated and approved for treating plaque-type psoriasis, and mirikizumab was tried in psoriasis but will only be marketed for IBD treatment.16 IL-23 is a cytokine normally involved in antibacterial and antifungal immune response. Dysregulated IL-23 production is related to autoimmunity and, in particular, IL-23 acts at the very early stages of psoriasis pathogenesis, promoting and maintaining T-helper [TH]17 cell differentiation and clonal expansion pathogenesis because it is released, together with TNF-alpha, by activated dendritic cells [DCs] and regulates the differentiation and clonal expansion of CD4+ and CD8+ cells [T17 cells]. T17 cells are responsible for the production of IL-17, IL-26, IL-29, TNF-alpha and other effector cytokines. IL-17 and TNF-alpha simulate epidermal keratinocyte proliferation leading to psoriasis plaques and to a feed-forward inflammatory response. Furthermore, the release of keratinocyte-derived antimicrobial peptides, such as LL-37/cathelicidine, amplifies immune response.17
As of December 2021, IL-23 inhibitors are approved in Italy for patients with moderate-to-severe psoriasis [Psoriasis Area Severity Index or PASI ≥10 and/or Body Surface Index or BSA ≥10% or PASI <10 + BSA <10% in case of facial, genital, nails, or head involvement] who have already failed one or more than one systemic conventional treatment [cyclosporine, methotrexate, acitretine, or phototherapy].18 In some Italian regions, patients must also have failed an anti-TNF alpha drug befor accessing IL-23 inhibitors treatment.
Clinical trials in dermatology could provide useful data on these drugs currently under investigation for IBD.
2. Methods and Results
We report the most significant clinical trials that evaluated the efficacy and safety of guselkumab, risankizumab, and tildrakizumab. The main efficacy endpoints include the variation in PASI [Psoriasis Activity and Severity Index] and IGA [Investigator’s Global Assessment] scores from baseline. PASI-75, PASI-90, and PASI-100 represent a clinical improvement of 75%, 90%, and 100% from baseline PASI, respectively.19 IGA scores range from 0 [absence of disease] to 4 [very severe disease]. In clinical trials, the percentage of patients achieving an IGA score of 0 or 1 [absence of lesions or very mild disease] is usually used as an efficacy endpoint. An overview on the three anti-IL-23 monoclonal antibodies currently approved for the management of psoriasis is shown in Table 1 and 2.
Table 1.
Anti-IL-23 monoclonal antibodies currently approved for the management of psoriasis.
| Guselkumaba | Tildrakizumabb | Risankizumabc | |
|---|---|---|---|
| Antibody | Human monoclonal IgG1λ | Humanised monoclonal IgG1κ | Humanised monoclonal IgG1 |
| Mechanism of action | Binding to the p19 subunit of IL-23 receptor | Binding to the p19 subunit of IL-23 receptor | Binding to the p19 subunit of IL-23 receptor |
| Dose and frequency of administration | 100 mg s.c. at Weeks 0, 4, then every 8 weeks | 100 mg s.c. at Weeks 0, 4, then every 12 weeks | 150 mg s.c. at weeks 0,4, then every 12 weeks |
| Major side effects | Injections site reactions, upper respiratory infections, nasopharyngitis | Injections site reactions, upper respiratory infections, nasopharyngitis, headache, arthralgia | Viral upper respiratory tract infection, upper respiratory tract infection, urinary tract infection, influenza and headache |
S.c., subcutaneous.
aData from VOYAGE 1 and VOYAGE 2 trials.
bData from reSURFACE1 and reSURFACE2 trials.
cData from UltIMMa-1 and UltIMMa-2 trials.
Table 2.
Efficacy and safety end points of pivotal phase III clinical trials evaluating risankizumab, tildrakizumab, and guselkumab.
| Risankizumab [UltIMMa-1] | Risankizumab [UltIMMa-2] | Tildrakizumab 100 mg [reSURFACE 1]b | Tildrakizumab 100 mg [reSURFACE2]b | Guselkumab [VOYAGE 1] | Guselkumab [VOYAGE 2] | |
|---|---|---|---|---|---|---|
| Number of patients receiving the study drug | 304 | 294 | 309 | 307 | 334 | 496 |
| PASI-90 at Week 16 | 229 [75·3%] | 220 [74·8%] | 107 [35%]a | 119 [39%]a | 241 [73.3] | 347 [70.0] |
| sPGA 0 or 1 at Week 16 | 267 [87·8%] | 246 [83·7%] | 179 [58%]a | 168 [55%]a | 280 [85.1] | 417 [84.1] |
| PASI-100 at Week 16 | 109 [35·9%] | 149 [50·7%] | 43 [14%]a | 38 [12%]a | 123 [37.4] | 169 [34.1] |
| PASI-90 at Week 52 | 249 [81·9%] | 237 [80·6%] | N/A | N/A | 251 [76.3]c | N/Ad |
| PASI-100 at Week 52 | 171 [56·3%] | 175 [59·5%] | N/A | N/A | 156 [47.4]c | N/Ad |
| sPGA 0 at Week 52 | 175 [57·6%] | 175 [59·5%] | N/A | N/A | 166 [50.5]c | N/Ad |
| Severe adverse events | 6 [2·0%] | 7 [2·4%] | 5 [2%] | 4 [1%] | 8 [2.4] | 2 [1.0] |
| Adjudicated major adverse cardiovascular event | 0 | 0 | 1 [<1%] | 0 | 1 [0.3] | 0 |
| Serious infections | 1 [0·3%] | 3 [1·0%] | 1 [<1%] | 0 | 0 | 1 [0.5] |
N/A, not available.
aThe primary efficacy endpoint in both resurface 1 and 2 was PASI response at Week 12.
breSURFACE trials design does not allow to evaluate PASI and PGA responses after Week 24.
cVOYAGE 1 evaluated PASI and PGA responses up to Week 48.
dVOYAGE 2 design does not allow to evaluate PASI and PGA responses after Week 24.
2.1. Guselkumab
Guselkumab [Tremfya®] is the first fully human immunoglobulin G1 λ [IgG1λ] monoclonal antibody [mAb] that selectively targets and binds to IL-23; it is approved for the treatment of moderate to severe plaque psoriasis in several countries. The blocking action of guselkumab is due to its interaction with the cell surface receptor that usually is bound by the IL23 p19 subunit, subsequently inhibiting the IL-23-mediated signalling pathway.
The recommended dose is 100 mg as a single dose, followed by a further dose after 4 weeks and then 100 mg every 8 weeks.20 Guselkumab is supplied as a single-use 1-mL pre-filled syringe containing one dose, which can be also administered by patients themselves after proper training from health care professionals. Guselkumab has no absolute contraindications.
In a randomised, double-blind, placebo-controlled study which evaluated the safety, tolerability, and clinical response of guselkumab in patients with moderate-to-severe plaque psoriasis, the analyses of lesional and non-lesional skin biopsy specimens showed at Week 12 that a single dose of this anti-IL-23-specific mAb 10-300 mg] was associated with reduced [p <0.05] T cell expression and decreased inflammatory CD11c+ dendritic cell counts and epidermal thickness from baseline.21 In patients who achieved a PASI-50 response to guselkumab at Week 12 [≥50% reduction of the Psoriasis Area and Severity Index score], or at Week 1 and at Week 12, a statistically significant [p <0.05] decrease in serum IL-17A levels was detected, while no changes were seen in placebo group. Also, a dose-dependent reduction in the expression of IL-17A pathway-related genes was observed. Compared with baseline, mRNA expression for IL-17A, IL-22, and IL-17F in patients receiving guselkumab was decreased at Week 12 [with 2-,10-, and 26-fold change]. On the other hand, an increase in mRNA expression for TNF-γ was observed, underlining how guselkumab’s therapeutic effects are primarily due to its action on IL-23/Th17 pathway, almost totally sparing the IL-12/Th1 pathway.21
In phase II and III trials, guselkumab was associated with reduced serum concentrations of IL-17A, IL-17F, and IL-22 compared with placebo in patients with plaque psoriasis.21 In VOYAGE 1 [NCT02207231], a phase III, randomised, double-blind, placebo- and active comparator- controlled trial, patients were randomised to placebo, guselkumab, or adalimumab for 16 weeks, after which patients taking placebo switched to guselkumab. At baseline, a total of 837 patients were randomised into three groups: placebo [n = 174], guselkumab [n = 329], or adalimumab [n = 334]. Patients treated with guselkumab had significantly [p ≤0.001 vs baseline] decreased levels of IL-17A, IL-17F, and IL-22 from baseline at Week 4 and these effects were sustained at Week 24 and Week 48 [p ≤0.001].22 Guselkumab’s inhibition of IL-17A, IL-17F, and IL-22 was seen to be more prolonged compared with adalimumab’s, underlining a more effective action on Th17 and Th22 cells [involved in the production of these cytokines].23 At Weeks 4, 24, and 48, in patients treated with guselkumab, serum IL-17F levels were significantly [p ≤0.05] reduced and IL-17A and IL-22 levels were significantly decreased at Week 48.21,23 In the VOYAGE 1 trial, these results were consistent with the clinical responses observed with guselkumab treatment.24
In VOYAGE 2 [NCT02207244], which included a PASI-90, response-based re-randomisation at Week 28, a sustained response in the withdrawal group [patients switched to placebo from guselkumab] was associated with continued reductions of serum IL-17A, IL-17F, and IL-22 levels at Week 48. On the other hand, a loss of response [i.e. PASI <75] in this group was associated with significantly [p ≤0.05] increased levels of these cytokines at Week 48.25
Three large, randomised, double-blind, phase III trials were conducted to evaluate the clinical efficacy of guselkumab in patients with moderate to severe vulgar psoriasis [VOYAGE 1,24 VOYAGE 2,25 and NAVIGATE26]. Both VOYAGE 1 and VOYAGE 2 had a placebo-controlled period [0-16 weeks] and an adalimumab-controlled period [0-24 or 0-48 weeks, respectively].
According to the results of VOYAGE 1, at Week 16 a significantly higher percentage of patients taking guselkumab achieved PASI-90 [≥90% reduction of the Psoriasis Area and Severity Index score] compared with placebo [73.3% vs 2.9%]. Moreover at Week 16, the proportion of patients treated with guselkumab achieving IGA 0/1, compared with placebo, was significantly higher [85.1% vs 6.9%]. Also, clinical responses to guselkumab were significantly better compared with adalimumab at Week 16, as measured by the percentage of patients reaching PASI-90 [73.3% vs 49.7%], PASI 75 [91.2% vs 73.1%], and IGA 0/1 [85.1% vs 65.9%]. Better performances compared with adalimumab were maintained at Week 24 (IGA 0/1 [84.2% vs 61.7%] and PASI-90 [80.2% vs 53.0%]) and at Week 48 [respectively, 80.5% vs 55.4%, and 76.3% vs 47.9%]. In addition, after Week 16, patients switching from placebo group to guselkumab achieved similar clinical responses to those patients randomised at first into the guselkumab group.24
In VOYAGE 2, in addiction, at Week 28 patients underwent a second randomisation, based on their PASI-90 response. At Week 48, a significantly higher proportion of patients in the maintenance group [patients who continued guselkumab injections every 8 weeks] maintained PASI-90 responses, compared with the withdrawal group [patients who were re-randomised to placebo at Week 28]. In this group, the median time to loss of PASI-90 response was 15.2 weeks.25
In VOYAGE 1, significantly better improvement from baseline in DLQI [Dermatology Life Quality Index] score at Week 16 was also observed in patients taking guselkumab vs placebo group [mean change -11.2 vs -0.6]. Likewise, a greater percentage of patients treated with guselkumab achieved DLQI score 0/1, compared with placebo and adalimumab [both p <0.001]. Similar responses were maintained at Week 24 and Week 48.
The efficacy of guselkumab in both VOYAGE trials was mantained at Week 24 and Week 100, as measured by IGA 0/1, PASI-90, and PASI-100 responses. In both trials, at Week 100 these responses were consistent in the guselkumab group, and placebo -> guselkumab, and adalimumab -> guselkumab groups [IGA 0/1 response in 73%, 81%, and 83%, respectively, in VOYAGE 1; 75%, 76%, and 81%, respectively, in VOYAGE 2].27
Guselkumab was generally well tolerated in patients with vulgar psoriasis in both the VOYAGE trials. During the first 16 weeks [placebo-controlled period] percentages of patients with at least one adverse event [AE] were similar across the groups [49% of patients receiving guselkumab, compared with 50% of adalimumab group and 47% of placebo group]. The most commonly reported AEs were nasopharyngitis and upper respiratory tract infections. Serious AEs [SAEs]were reported in similar proportions in each group.24,25
Another phase III, randomised, double-blind trial was the NAVIGATE study [NCT02203032]. This trial was designed including a 16-week open-label period, a 28-week randomised, active-treatment period, and a 16-week follow-up period. During the open-label period, all patients received ustekinumab [45 mg or 90 mg, based on patients’ weight] at Weeks 0 and 4. At Week 16, patients who had an inadequate response [IGA >1] were randomised into two groups: one switched to guselkumab 100 mg at Weeks 16, 20, and then every 8 weeks; the other group continued ustekinumab at Week 16 and then every 12 weeks. Patients achieving an IGA of 0 or 1 at Week 16 continued receiving open-label ustekinumab at Week 16 and then every 12 weeks.26 After randomisation, a significantly higher percentage of patients receiving guselkumab achieved an IGA of 0/1 response, compared with ustekinumab, from Weeks 16 to 28 [31% and 14%, respectively]. The guselkumab group had a significantly higher mean number of visits at which patients had an IGA score of 0 or 1 and at least a two-grade improvement relative to Week 16 from Week 28 through Week 40 [primary endpoint] compared with the ustekinumab group [1.5 vs 0.7; p <0.001]. The mean number of visits at which patients had a PASI-90, compared with baseline, between Week 28 and Week 40, was significantly higher in patients receiving guselkumab than in the randomised ustekinumab group [2.2 vs1.1; p <0.001].26 Moreover, patients switching to guselkumab after failing to achieve an adequate response to ustekinumab, had an improvement of overall quality of life as assessed by DLQI scores. At Week 52, 9% of guselkumab recipients were sign-free and 20% were symptom-free [compared with 3% and 10% in the ustekinumab group]. Also, a higher percentage of patients receiving guselkumab achieved a DLQI score of 0/1 compared with ustekinumab [39% vs 19%].26
The ECLIPSE study [NCT03090100] was conducted after the already-mentioned trials.28 This was the first comparator study of an IL-23p19 inhibitor, guselkumab, versus an IL-17A inhibitor, secukinumab. The aim of this study was to show clinical superiority of guselkumab versus secukinumab at Week 48: 1048 patients with plaque psoriasis were enrolled, 534 were randomised to receive guselkumab and 514 to receive secukinumab. A significantly higher percentage of patients receiving guselkumab achieved a PASI-90 response at Week 48 compared with secukinumab group [84% vs 70%; p <0·0001]. However, the proportion of patients in the guselkumab group achieving a PASI-75 response at both Week 12 and Week 48 was not significantly higher [85% vs 80% in secukinumab group; p = 0.0616]. In both treatment groups, percentages of patients with AEs were similar and, more in general, safety findings were not different from registrational trials observations.28
In conclusion, it is possible to say that guselkumab, compared with IL-17 inhibitors, can offer similarly high PASI responses while offering a few practical and safety advantages. In fact, in clinical trials which evaluated efficacy and safety of anti-IL-17 drugs, both new cases and exacerbations of IBD have been reported.29 In comparison, currently no phase III clinical trial for anti-IL-23 mAbs has reported an increased risk of IBD. Also, these drugs do not appear to be related to an higher risk of Candida infections, in contrast to IL-17 inhibitors.30 Additionally, data from VOYAGE 1 and VOYAGE 2 demonstrate that guselkumab also has excellent performances in treating psoriasis in areas such as the scalp, palms, soles, and fingernails, which are well known to be difficult to treat.27 It is relevant to mention that these clinical studies underline a marked improvement in the QoL of psoriasis patients treated with guselkumab.27
2.2. Risankizumab
Risankizumab [Skyrizi®] is a humanised IgG1 monoclonal antibody, approved for moderate-severe chronic plaque psoriasis, which selectively binds the unique p19 subunit of human IL-23.31 Skyrizi® is available in 75-mg pre-filled syringes for subcutaneous use. The recommended dose of Skyrizi® is 150 mg [two injections]. The first two doses of 150 mg are given 4 weeks apart and subsequent doses are given every 12 weeks.32
Risankinumab was compared with ustekinumab in a phase II, dose-ranging, multicentre, randomised trial which included 166 patients affected by moderate-to-severe psoriasis.33 Patients were randomised to receive subcutaneous injections of risankizumab [18-mg single dose at Week 0 or 90- or 180-mg doses at Weeks 0, 4, and 16] or ustekinumab [45 or 90 mg, according to body weight, at Weeks 0, 4, and 16].34 The primary endpoint of this trial was a 90% or greater improvement in the PASI score at Week 12. Risankizumab showed clinical superiority to ustekinumab, as 77% of the patients [90- and 180-mg groups pooled] achieved PASI-90 or greater, compared with 40% in patients receiving ustekinumab. Additionally at Week 12, 63%, 98%, and 88% of the patients receiving 18 mg, 90 mg, and 180 mg of risankizumab, respectively, achieved PASI-75 compared with 72% in ustekinumab-treated patients. PASI-100 responses were detected in 14%, 41%, and 48% of participants in the 18-, 90-, and 180-mg risankizumab groups, respectively, compared with 18% in the ustekinumab group.34 Higher percentages of patients achieving sPGA scores of 0 or 1 were observed in the risankizumab groups [58%, 90%, and 88% of patients in the 18-, 90-, and 180-mg groups, respectively] compared with 62% in the ustekinumab group. Moreover at Week 24, the proportions of patients who achieved a PASI-75 response were 53%, 90%, and 88% for 18-, 90-, and 180-mg dosing regimens of risankizumab, respectively, compared with 70% in the ustekinumab group. Additionally at Week 24, PASI-90 responses were also assessed, with 28%, 63%, and 81% of risankizumab-treated patients achieving this outcome [18 mg, 90 mg, and 180 mg, respectively], compared with 55% in the ustekinumab group. Interestingly, significant improvements in PASI score were first observed as early as Week 2. Clinical responses were generally maintained for up to 20 weeks after the final dose of risankizumab [Week 36], in contrast to reduction in clinical responses observed in ustekinumab-treated patients from Week 24.33
Two phase III studies [UltIMMa-1 and UltIMMa-2] were developed to assess the efficacy and safety of risankizumab compared with placebo or ustekinumab in patients with a diagnosis of moderate to severe chronic plaque psoriasis.35 In these studies, patients were randomised in a ratio of 3:1:1 [506 in UltIMMa-1 and 491 in UltIMMa-2] to receive risankizumab, ustekinumab, or placebo. Randomisation was also stratified by weight [≤100 kg vs >100 kg] and previous exposure to TNF inhibitor. For the first 16-week period [part A], patients were randomised to receive either 150 mg risankizumab, ustekinumab [based on patient’s weight: 45 mg for patients with body weight ≤100 kg or 90 mg for patients with body weight>100 kg], or placebo subcutaneously at Weeks 0 and 4. In part B [Weeks 16–52], patients originally allocated to placebo were switched to 150 mg risankizumab at Week 16; during this phase, patients received the study drug subcutaneously at Weeks 16, 28, and 40. Efficacy outcomes were assessed at Weeks 4, 8, 12, 16, 22, 28, 34, 40, 46, and 52, and safety was evaluated throughout the study. The co-primary endpoints were the proportions of patients achieving PASI-90 and sPGA 0 or 1 at Week 16.35,36 In both studies, the percentage of participants reaching PASI-90 and sPGA 0 or 1 at Week 16 was higher among patients treated with risankizumab. At Week 16, in UltIMMa-1, PASI-90 was achieved by 75.3% of patients treated with risankizumab compared with 42.0% receiving ustekinumab and 4.9% receiving placebo, and in UltIMMa-2 by 74.8% of patients receiving risankizumab compared with 47.5% receiving ustekinumab and 2.0% receiving placebo [p <0.0001 vs placebo and ustekinumab for both studies]. sPGA 0 or 1 was achieved by 87.8% of risankizumab-treated patients compared with 63.0% of ustekinumab-treated patients and 7.8% of placebo-treated patients in UltIMMa-1, and by 83.7% risankizumab-treated patients compared with 61.6% of ustekinumab-treated patients and 5.1% of placebo-treated patients in UltIMMa-2 [p <0.0001 vs placebo and ustekinumab for both studies].35
Patients receiving placebo during part A of the studies who were switched to risankizumab at Week 16, achieved similar response rates for PASI, sPGA, DLQI and PSS at Week 52 compared with those treated with risankizumab since Week 0.35 In both UltIMMa-1 and UltIMMa-2, among patients who achieved a PASI-90 response at Week 16, the percentage of patients continuing risankizumab who maintained PASI-90 response through Week 52 was significantly higher, compared with the ustekinumab group. At Week 52, 88.4% of patients on continuous risankizumab maintained PASI-90 response [vs 73.3% of patients on ustekinumab; p = 0.0009].35
Data from IMMvent, a Phase III randomised, double-blind, active-controlled study, which evaluates the efficacy and safety profiles of risankizumab compared with adalimumab in patients with moderate-to-severe vulgar psoriasis, were recently released.37 Participants were randomised 1:1 to receive 150 mg risankizumab [at baseline, Weeks 4, 16, and 28] or adalimumab [80 mg at baseline, 40 mg every other week from Week 1]. At Week 16, patients from the adalimumab group achieving PASI-50 but failing to reach PASI-90 were re-randomised 1:1 to either continue the same drug or switch to risankizumab [Weeks 16, 20, and 32]. At Week 16, the percentages of patients achieving PASI-75, PASI-90, PASI-100, and sPGA 0/1 were significantly higher among risankizumab-treated patients compared with those receiving adalimumab [90.7% vs 71.7%; 72.4% vs 47.4%; 39.9% vs 23.0%; 83.7% vs 60.2%, respectively].37 Additionally, among patients originally treated with adalimumab with a PASI-50 to <PASI-90 responses at Week 16, the proportion of patients switching to risankizumab and achieving PASI-90 and PAS- 100 responses at Week 44 were significantly higher compared with patients continuing on adalimumab [66.0% vs 21.4%; 39.6% vs 7.1%, respectively].37
In the Phase III studies UltIMMa-1 and UltIMMa-2, adverse events rates were also assessed. AE rates were similar between groups in both trials.19 During part A in both trials the most frequently reported AES were viral upper respiratory tract infections, other upper respiratory tract infections, psoriasis, and diarrhoea.36 In part A of UltIMMa-1, AEs occurred in 49.7% of the patients treated with risankizumab, 50.0% on ustekinumab, and 51.0% on placebo; during part A of UltIMMa-2, AEs were reported in 45.6% of the patients in the risankizumab group, 53.5% on ustekinumab, and 45.9% on placebo. Percentages of SAEs were also very low: they were reported in 2.3% of the patients receiving risankizumab, 8.0% of ustekinumab-treated patients, and 2.9% of patients receiving placebo in UltIMMa-1, and in 2.0% of risankizumab-treated patients, 3.0% of ustekinumab-treated patients, and 1.0% of placebo-treated patients in UltIMMa-2. The proportions of serious infections were similar across the treatment groups in both studies: in UltIMMa-1 they occurred in 0.3% in the risankizumab group and 3.0% in the ustekinumab group, and in UltIMMa-2 they were reported in 1.0% of the patients in both risankizumab and ustekinumab groups.36 In part B in both UltIMMa-1 and UltIMMa-2, the most frequent AEs were viral upper respiratory tract infection, other upper respiratory tract infection, urinary tract infection, influenza, and headache. In part B of UltIMMa-1, AEs occurred in comparable percentages across the groups [61.3% of the patients continuing on risankizumab, 66.7% on ustekinumab, and 67.0% of the patients switching from placebo to risankizumab], whereas in part B of UltIMMa-2, AEs occurred in 55.7% of the patients treated with risankizumab continuously, 74.5% of patients in the ustekinumab group, and 64.9% of the patients switching to risankizumab. In UltIMMa-1, SAEs were reported in 5.4% of risankizumab-treated patients, 4.0% of ustekinumab-treated patients, and 3.1% of the patients switching to risankizumab, and in UltIMMa-2 SAEs were observed in 4.5% of risankizumab-treated patients, 4.3% of ustekinumab-treated patients, and 3.2% of the patients switching to risankizumab.36 During part B, serious infections occurred in 0.7% of the patients on risankizumab in both UltIMMa-1 and UltIMMa-2, and in 1.0% of both the patients treated with ustekinumab and switching from placebo to risankizumab in UltIMMa-2. In part A, malignancies were observed in two patients receiving risankizumab [one squamous cell carcinoma in UltIMMa-1, one basal cell carcinoma in UltIMMa-2] and in one patient assigned to the placebo group [one squamous cell carcinoma in UltIMMa-1].36 Significantly, there were no events of tuberculosis, opportunistic infections, major adverse cardiovascular events [MACEs], or serious hypersensitivity across both studies. During part B of both studies, malignancies were reported in one patient on continuous risankizumab [a basal cell carcinoma in UltIMMa-2], in one patient on ustekinumab [a prostate cancer in UltIMMa-2], and in two patients switching from placebo to risankizumab [one patient with both basal cell and squamous cell carcinoma in UltIMMa-1, one patient with breast cancer in UltIMMa-2].36
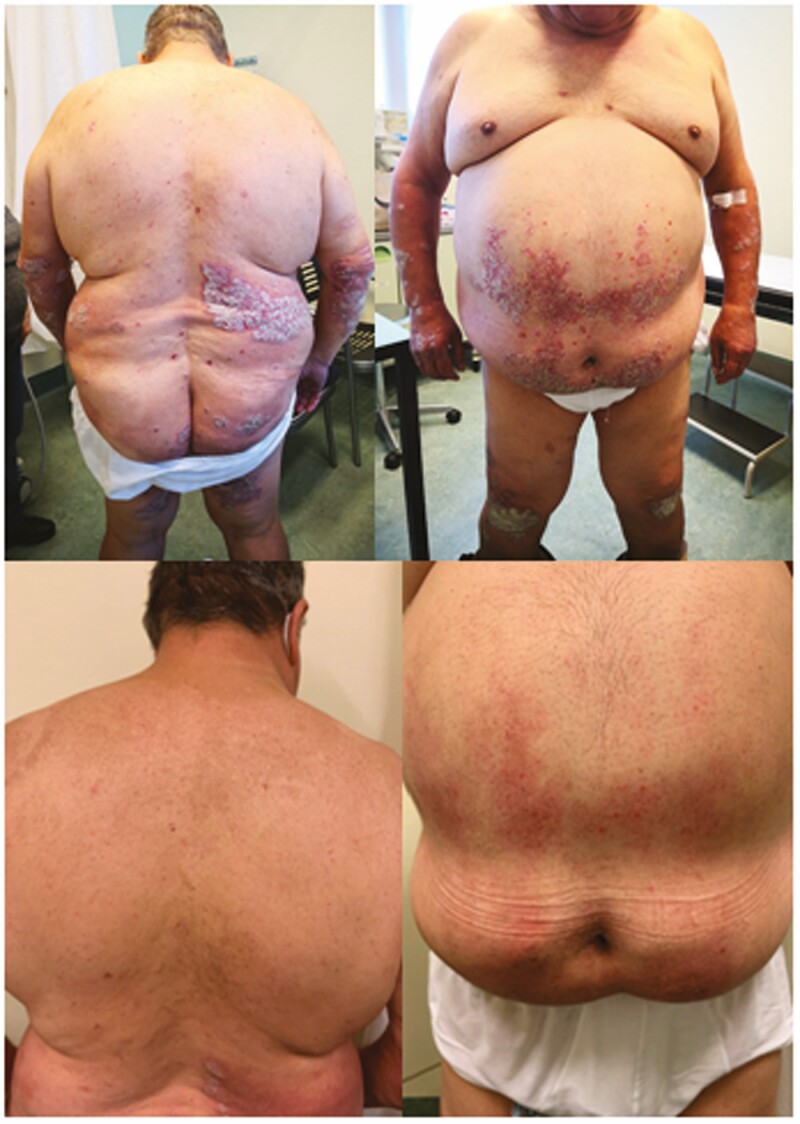
A recently published retrospective study included 66 consecutive adults with moderate-to-severe psoriasis vulgaris treated with risankizumab in monotherapy up to Week 40 in a ‘real-life’ setting. At Week 40, 98.7%, 85.7%, and 62.3% of patients achieved a Psoriasis Area and Severity Index [PASI] reduction ≥75% [PASI-75], PASI-90, and PASI-100, respectively]. Patients who had not responded to two or more previous biologic treatments were significantly less likely to achieve PASI-75/90 at Week 16 and PASI-90/100 at Week 40 compared with those who had been previously treated with only one biologic, and compared with those treated with risankizumab as a first-line biologic. Increasing body mass index decreased the chances of reaching PASI-90 at Week 40. No significant safety findings were recorded throughout the study, and none of the patients had to interrupt the treatment. These data suggest the efficacy of risankizumab for plaque psoriasis in ‘real-life’ clinical setting.38 A clinical picture of a patient affected by severe plaque psoriasis before starting the treatment with risankizumab and after 16 weeks is shown in Figure 1.
Figure 1.
Clinical appearance of a patient before receiving risankizumab and after 16 weeks of treatment.
According to the state of current knowledge, risankizumab is an anti-IL-23 monoclonal antibody for the treatment of plaque psoriasis that offers a good safety profile so far and an excellent dosing regimen. Future studies with more patients and longer follow-up periods will help establish a more complete safety profile.
2.3. Tildrakizumab
Tildrakizumab [Ilumetri®] is a humanised human immunoglobulin G1 λ [IgG1κ] monoclonal antibody [mAb] that selectively binds to the p19 subunit of IL-23, currently approved for the treatment of moderate-to-severe psoriasis.39 In the EU and Australia, Ilumetri® is available as a solution in pre-filled syringes for injection under the skin. The recommended dose is one 100-mg injection, followed by a further dose after 4 weeks and then an injection every 12 weeks. The dose may be increased to 200 mg in certain patients, for example patients badly affected by the disease or with body weight over 90 kg.40
In a randomised, placebo-controlled, parallel-group, dose-finding phase 2b trial, efficacy and safety data of tildrakizumab in 355 patients were reported.41 Patients were randomised to receive subcutaneous tildrakizumab [5 mg, 25 mg, 100 mg, or 200 mg] or placebo at Weeks 0 and 4. At Week 16, participants were re-randomised on the basis of their responding status: PASI-75 responders who received 5mg or 25 mg tildrakizumab continued their respective treatment every 12 weeks; PASI-75 responders receiving 100 mg or 200 mg tildrakizumab were re‐randomised to continue on the same or on a reduced dose [100–25 mg and 200–100 mg, respectively] every 12 weeks; patients who failed to achieve a PASI-75 response at Week 16 were randomised to receive 100 mg tildrakizumab [for placebo, and the 5‐ and 25‐mg groups] or 200 mg tildrakizumab [for the 100‐mg group]; non responders receiving 200 mg tildrakizumab remained in 200 mg group. After Week 52, patients underwent a 20-week follow-up period. The primary endpoint of this study was the percentage of participants who achieved a PASI-75 response at Week 16.41 A higher proportion of patients receiving tildrakizumab achieved PASI-75, compared with placebo [74% of patients receiving a 200-mg dose, 66% in the 100-mg group, 64% in the 25-mg group, and 33% in the 5-mg group, vs 4% in the placebo group, p <0.001 for each treatment subgroup vs placebo]. Moreover, patients randomised to 100 mg or 200 mg tildrakizumab at Week 16 mantained clinical efficacy at Week 52, whereas responders who were re-randomised to 25 mg and all the patients who continued 5 mg and 25 mg tildrakizumab had a loss in efficacy.41
Efficacy and safety profiles of tildrakizumab were evaluated in two phase 3 pivotal trials, reSURFACE 1 [NCT01722331] and reSURFACE 2 [NCT01729754]. These were two three-part, parallel-group, double-blind, randomised controlled studies which compared tildrakizumab 100 mg and 200 mg with placebo and etanercept in patients with moderate-to-severe plaque psoriasis.42 In reSURFACE 1, during the first part of the study, 772 participants were randomised [1:2:2] to receive subcutaneous placebo or tildrakizumab 100 mg or 200 mg at Weeks 0 and 4. In the second part, starting at Week 12, patients from the placebo group were re-randomised [1:1] to receive active treatment [tildrakizumab 100 mg or 200 mg] at Week 12 and Week 16 and then every 12 weeks to Week 28. reSURFACE 2 featured 1090 participants and in addition included a third treatment group, in which patients received etanercept 50 mg twice a week [part 1] and once a week [part 2]. In reSURFACE 2, at baseline participants were randomised [2:2:1:2] to tildrakizumab 200 mg, tildrakizumab 100 mg, placebo, or etanercept 50 mg. In part 2, the placebo group was re-randomised [1:1] to tildrakizumab 200 mg or 100 mg. In part 3 of both reSURFACE1 and reSURFACE2, patients receiving tildrakizumab who either achieved a PASI-75 response or a partial response [PASI ≥50 and PASI <75] were re-randomised at Week 28 to continue the same treatment, a different dose of tildrakizumab, or placebo, until Week 64 [reSURFACE1] or Week 52 [reSURFACE2]. Primary endpoints were the percentages of patients achieving PASI-75 and a PGA 0/1 response [with ≥2-grade score reduction from baseline] at Week 12.43 In both studies, tildrakizumab demonstrated effectiveness in the treatment of moderate-to-severe chronic psoriasis. Higher percentages of patients receiving tildrakizumab achieved the co-primary endpoints of a PASI-75 response and a PGA 0/1 response at Week 12. In reSURFACE1, at Week 12, PASI-75 was achieved by 62% of patients receiving 200 mg tildrakizumab, 64% of patients in the 100 mg tildrakizumab group, and 6% in the placebo arm [p <0.0001 for both treatment arms]. In reSURFACE2, patients treated with tildrakizumab had better responses compared with both placebo and etanercept [66%, 61%, 6%, 48% of patients achieving PASI-75, respectively, for tildrakizumab 200 mg, tildrakizumab 100 mg, placebo, and etanercept; [p <0.0001 for comparison of both tildrakizumab groups vs placebo; p <0.0001 for 200 mg vs etanercept; p <0.001 for 100 mg vs etanercept].43
The efficacy of tildrakizumab 100 mg and 200 mg was assessed over cumulative treatment periods of up to 148 weeks in the extension period of the reSURFACE studies.39 PASI-75 or better responses were maintained in 91.2% of patients who continued tildrakizumab 100 mg and in 92.4% of those continuing tildrakizumab 200 mg. Moreover, 67.6% and 69.0% of patients treated continuously with 100 mg and 200 mg of tildrakizumab, respectively, had PASI-90 responses at Week 148. In reSURFACE1, at Week 28 a subgroup of PASI-75 responders was switched to placebo. From this subgroup, 54% and 47% of patients formerly treated with tildrakizumab 100 mg and 200 mg, respectively, had a relapse. The median time to relapse was long [226 days and 258 days in the 100-mg and 200-mg groups, respectively].44 In addition, in reSURFACE 2, etanercept partial- and non-responders who were re-randomised to tildrakizumab 200 mg in part 3 of the base study, maintained better response rates 2 years into the extension study.39 The proportions of participants switched to tildrakizumab who achieved PASI-75 and -90 responses were 86.4 and 43.7%, respectively, at Week 52 and 87.0 and 56.5%, at Week 148.44
Regarding patient-reported outcomes, at Week 12 a higher percentage of participants receiving tildrakizumab had DLQI scores of 0 or 1, compared with placebo, in both phase 3 clinical trials [42% and 44% for tildrakizumab 200 mg and 100 mg, respectively, vs 5%, p <0.001 in reSURFACE1; 40% and 47%, respectively, vs 8% in reSURFACE2, p <0.001].43 In reSURFACE2, tildrakizumab 200 mg was also associated with higher rates of patients achieving these scores compared with etanercept 50 mg [47% vs 36%, p = 0.0029].42,43
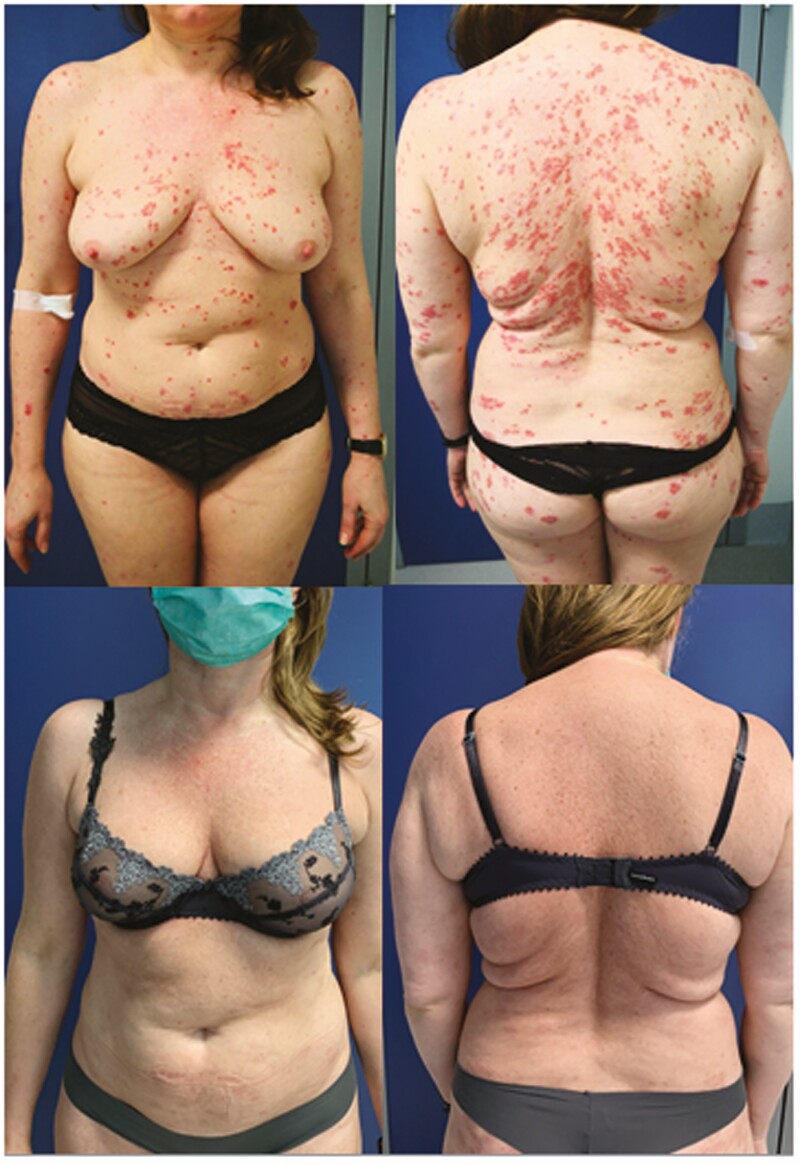
Safety assessments were also performed for up to 64 weeks in both reSURFACE1 and reSURFACE2.19 During the placebo-controlled phase, compared with placebo and etanercept, percentages of treatment-emergent adverse events [TEAEs; range 47.9–54.0%], serious TEAEs [range 1.4–2.3%], study discontinuations due to AEs [range 0.6–1.9%], major adverse cardiovascular events [MACEs; range 0.0–0.1%], and severe infections [range 0.0–0.3%] were similar in tildrakizumab 100 mg and tildrakizumab 200 mg groups. In the full trial period, serious TEAEs and discontinuations due to AEs with tildrakizumab [both 100 mg and 200 mg] were similar or even lower than with placebo and lower than with etanercept. Rates of severe infections [range 0.9–2.0%] were comparable among all groups.43 The most common adverse event was nasopharyngitis. In reSURFACE 2, injection-site erythema was also among the most common adverse events reported. Many of these adverse events were also recorded in the etanercept group.43 In both studies, no reported cases of inflammatory bowel disease or suicide were observed. During the placebo-controlled period, cutaneous Candida infections were infrequent [0.1%, 0.3%, 0.0%, and 0.0% for the tildrakizumab 100 mg, tildrakizumab 200 mg, placebo, and etanercept groups, respectively], and in the full trial period, exposure-adjusted rates were 0.2%, 0.7%, 0.0%, and 0.0%, respectively. Also, oral candidiasis was infrequent, with frequencies of 0.1%,0.3%, 0.0%, and 0.0% during the first phase [percentages from the tildrakizumab 100 mg, tildrakizumab 200 mg, placebo, and etanercept groups, respectively], and exposure-adjusted rates of 0.2%, 0.7%, 0.0%, and 0.0%, respectively, during the full trial period up to Week 64.43 Data regarding the efficacy profile of tildrakizumab in a real-world setting are currently lacking. Figure 2 shows the clinical improvement after 16 weeks of therapy in a patient affected by severe plaque psoriasis receiving tildrakizumab.
Figure 2.
Plaque psoriasis in a patient treated with tildrakizumab.
According to the state of current knowledge, head-to-head comparative clinical trials of tildrakizumab with other anti-IL-23, anti-IL-12/IL-23, and anti-IL-17 mAbs [long-term evaluations included] are still lacking.45 Further evidences are still needed to assess the efficacy of tildrakizumab compared with guselkumab or anti-IL-17 mAbs, such as ixekizumab and secukinumab.
3. Discussion
Dermatological clinical trials on anti-IL23 agents highlighted important clues for IBD specialists and other specialists approaching the use of this therapeutic class at least for two reasons:
First, considering the safety side, an important characteristic of IL-23 inhibitors not shared by TNF inhibitors is the low rate of antidrug antibodies [ADA] developed during treatment [<3% in a randomised controlled trial on tildrakizumab and even less with risankizumab and guselkumab] with low effects on efficacy and no safety interactions.46 The low rate of ADA development throughout treatment period supports the long-term potential of these drugs, which have shown also in real-life practice a frequent, long-term, clinical remission of psoriasis, even on difficult-to-treat areas such as the scalp and the palm/soles.47
In general, IL-23 blockers could be considered safer as compared with IL-17 inhibitors and TNF inhibitors, with very low incidence of upper respiratory tract infections [as frequent as in placebo arms] but especially on the gastrointestinal profile, considering the fairly null risk of developing or reactivating IBD in anti-IL-23 treated patients. A few complicated COVID-19 infections have been reported in patients receiving anti-IL-17 drugs,34 but no negative impact of the anti-IL-23 class was found on the outcome of COVID-19 disease.48,49
Second, the efficacy outcomes have pointed out the outstanding results on short-term efficacy and, in particular, on long-term maintenance of skin clearance that characterises IL-23 blockers compared with IL-17 or TNF-alpha inhibitors. In this context, one key feature of IL-23 inhibitors shown in psoriasis clinical trials is the possibility of drug withdrawal with long-term remission of the disease and without significant implications on the future effectiveness of the anti-IL23 antibody. This observation, mainly coming from risankizumab and guselkumab studies, is thought to be linked to the ability of IL-23 inhibitors to reduce the number of tissue resident memory T cells [TRM cells] in the skin of treated patients,50 whose number is proportional to disease severity and to the time to relapse after standard treatments. TRM cells with skin tropism are identified by the expression of the cutaneous lymphocyte-associated antigen [CLA] and by CD103 and CD69 tissue residency markers.51
TRM cells have the ability to recirculate in skin and blood and they usually persist at sites of healed lesions or in treated resistant plaques, and this may be the reason for clinical re-occurrence in the same body regions.
De Jesus Gil et al.52 demonstrated that IL-23, together with IL-15, acts as stimulator on IL-17F/A production by CLA+ memory T cells and autologous epidermal cells. In this immunological landscape, IL-23 inhibitors seem to decrease IL-17 signalling and TRM amounts in psoriatic lesions more than do IL-17A selective blockers.53 Consequently, IL-23 inhibitors might be considered as modifiers of the natural course of the disease.
In conclusion, IL-23 blockers have shown promising short- and long-term results in psoriasi,s with a major safety profile and no negative interactions with gastrointestinal system. The flexibility of these drugs is an important advantage to be counted in daily life practice. Should the promise of ‘disease modification’ be maintained also in IBD, we have no doubt that this new class of drugs will be the first choice also for our IBDologists colleagues.
More specific clinical trials on IBD together with our preliminary clinical experience could confirm the findings coming from investigative clinical trials on psoriasis.
Contributor Information
Mario Valenti, Dermatology Unit, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele (MI), Italy; Dermatology Unit, IRCCS Humanitas Research Hospital, Rozzano (MI), Italy.
Alessandra Narcisi, Dermatology Unit, IRCCS Humanitas Research Hospital, Rozzano (MI), Italy.
Giulia Pavia, Dermatology Unit, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele (MI), Italy; Dermatology Unit, IRCCS Humanitas Research Hospital, Rozzano (MI), Italy.
Luigi Gargiulo, Dermatology Unit, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele (MI), Italy; Dermatology Unit, IRCCS Humanitas Research Hospital, Rozzano (MI), Italy.
Antonio Costanzo, Dermatology Unit, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele (MI), Italy; Dermatology Unit, IRCCS Humanitas Research Hospital, Rozzano (MI), Italy.
Funding
AC is supported by grants from Fondazione Cariplo [COVISKIN 1833073], Italian Ministry of Health [CO-2018-12365521] and Horizon 2020 projects IMMUNIVERSE, WINTHER, and INNODERM. This paper was published as part of a supplement financially supported by AbbVie.
Conflict of Interest
AC has acted as speaker or consultant for Abbvie, Galderma, Almirall, Janssen, UCB, Sanofi, Novartis, Lilly, Sandoz, Biogen, Pfizer.
Author Contributions
MV wrote the manuscript; AN revised the manuscript and collected data; GP revised the manuscript and collected data; LG revised the manuscript and collected data; AC wrote the manuscript and supervised data collection.
References
- 1. Reid C, Griffiths CEM. Psoriasis and treatment: past, present and future aspects. Acta Derm Venereol 2020;100:adv00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Campanati A, Marani A, Martina E, Diotallevi F, Radi G, Offidani A. Psoriasis as an immune-mediated and inflammatory systemic disease: from pathophysiology to novel therapeutic approaches. Biomedicines 2021;9:1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cai J, Cui L, Wang Y, Li Y, Zhang X, Shi Y. Cardiometabolic comorbidities in patients with psoriasis: focusing on risk, biological therapy, and pathogenesis. Front Pharmacol 2021;12:774808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Narcisi A, Valenti M, De Simone C, et al. Effects of TNF-α inhibition on pre-clinical enthesitis: observational study on 49 psoriatic patients. J Dermatolog Treat 2021:1–4. [DOI] [PubMed] [Google Scholar]
- 5. Okwundu N, Cardwell LA, Cline AE, Richardson IM, Feldman SR. Adherence to topical treatment can improve treatment-resistant moderate psoriasis. Cutis 2020;105:89–91;E2;E3. [PubMed] [Google Scholar]
- 6. Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol 2020;182:840–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaudhari U, Romano P, Mulcahy LD, et al. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet 2001;357:1842–7. [DOI] [PubMed] [Google Scholar]
- 8. Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomised, controlled phase III trial. J Am Acad Dermatol 2008;58:106–15. [DOI] [PubMed] [Google Scholar]
- 9. Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn’s disease: a prospective, randomised trial. J Crohns Colitis 2016;10:1259–66. [DOI] [PubMed] [Google Scholar]
- 10. Seagrove AC, Alam MF, Alrubaiy L, et al. Randomised controlled trial. Comparison Of iNfliximab and ciclosporin in STeroid Resistant Ulcerative Colitis: trial design and protocol [CONSTRUCT]. BMJ Open 2014;4:e005091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 12. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. [DOI] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomised trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 2008;135:1130–41. [DOI] [PubMed] [Google Scholar]
- 14. Fauny M, Moulin D, D’Amico F, et al. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann Rheum Dis 2020;79:1132–8. [DOI] [PubMed] [Google Scholar]
- 15. Verstockt B, Ferrante M, Vermeire S, Van Assche G. New treatment options for inflammatory bowel diseases. J Gastroenterol 2018;53:585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol 2018;53:49–63. [DOI] [PubMed] [Google Scholar]
- 17. Lande R, Botti E, Jandus C, et al. The antimicrobial peptide LL37 is a T cell autoantigen in psoriasis. Nat Commun 2014;5:5621. Erratum in: Nat Commun2015;6: 6595. [DOI] [PubMed] [Google Scholar]
- 18. Burlando M, Baldari M, Brunasso Vernetti AM, et al. Consensus on the place in therapy of biologics in the treatment of patients with chronic plaque psoriasis. Italy J Dermatol Venerol 2021, May 3. Doi: 10.23736/S2784-8671.21.06984-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19. Ashcroft DM, Wan Po AL, Williams HC, Griffiths CE. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol 1999;141:185–91. [DOI] [PubMed] [Google Scholar]
- 20. European Medicines Agency. Summary of Product Characteristics: Tremfya 100 mg Solution for Injection. 2017. http://www.ema.europa.eu/ Accessed November 29, 2021.
- 21. Sofen H, Smith S, Matheson RT, et al. Guselkumab [an IL-23-specific mAb] demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol 2014;133:1032–40. [DOI] [PubMed] [Google Scholar]
- 22. Hamza T, Barnett JB, Li B. Interleukin 12 a key immunoregulatory cytokine in infection applications. Int J Mol Sci 2010;11:789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al-Salama ZT, Scott LJ. Guselkumab: a review in moderate to severe plaque psoriasis. Am J Clin Dermatol 2018;19:907–18. [DOI] [PubMed] [Google Scholar]
- 24. Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. J Am Acad Dermatol 2017;76:405–17. [DOI] [PubMed] [Google Scholar]
- 25. Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomised withdrawal and re-treatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol 2017;76:418–31. [DOI] [PubMed] [Google Scholar]
- 26. Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomised, double-blind, phase III NAVIGATE trial. Br J Dermatol 2018;178:114–23. [DOI] [PubMed] [Google Scholar]
- 27. Gordon K, Menter A, Ferris L, et al. Consistency of response [PASI-90 or 100 and IGA 0 or 0/1] in patients with moderate to severe psoriasis treated with guselkumab: results from the VOYAGE 1 and 2 trials [poster 6894]. In: Proceedings of the 76th Annual Meeting of the AAD; Feb 16–18, 2018; San Diego, CA.
- 28. Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis [ECLIPSE]: results from a phase 3, randomised controlled trial. Lancet 2019;394:831–9. [DOI] [PubMed] [Google Scholar]
- 29. Gordon KB, Blauvelt A, Papp KA, et al. UNCOVER-1 Study Group; UNCOVER-2 Study Group. UNCOVER-3 Study Group Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med 2016;375:345–56. [DOI] [PubMed] [Google Scholar]
- 30. Conti HR, Gaffen SL. IL-17-mediated immunity to the opportunistic fungal pathogen candida albicans. J Immunol 2015;195:780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomised, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2015;136:116–24. [DOI] [PubMed] [Google Scholar]
- 32. European Medicines Agency. Summary of Product Characteristics: Skyrizi 75 mg Solution for Injection.2019. http://www.ema.europa.eu/ Accessed December 8, 2021.
- 33. Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med 2017;376:1551–60. [DOI] [PubMed] [Google Scholar]
- 34. Facheris P, Valenti M, Pavia G, et al. Complicated coronavirus disease 2019 [COVID-19] in a psoriatic patient treated with ixekizumab. Int J Dermatol 2020;59:e267–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis [UltIMMa-1 and UltIMMa-2]: results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018;392:650–61. [DOI] [PubMed] [Google Scholar]
- 36. Machado Á, Torres T. Spotlight on risankizumab and its potential in the treatment of plaque psoriasis: evidence to date. Psoriasis 2018;8:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis [IMMvent]: a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet 2019;394:576–86. [DOI] [PubMed] [Google Scholar]
- 38. Borroni RG, Malagoli P, Gargiulo L, et al. Real-life effectiveness and safety of risankizumab in moderate-to-severe plaque psoriasis: a 40-week multicentric retrospective study. Acta Derm Venereol 2021;101:adv00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frampton JE. Tildrakizumab: a review in moderate-to-severe plaque psoriasis. Am J Clin Dermatol 2019;20:295–306. [DOI] [PubMed] [Google Scholar]
- 40. European Medicines Agency. Summary of Product Characteristics: Ilumetri 100 mg Solution for Injection.2019. http://www.ema.europa.eu/ Accessed December 8, 2021.
- 41. Papp K, Thaci D, Reich K, et al. Tildrakizumab [MK-3222], an antiinterleukin- 23p19 monoclonal antibody, improves psoriasis in a phase IIb randomised placebo-controlled trial. Br J Dermatol 2015;173:930–9. [DOI] [PubMed] [Google Scholar]
- 42. Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis [reSURFACE 1 and reSURFACE 2]: results from two randomised controlled, phase 3 trials. Lancet 2017;390:276–88. [DOI] [PubMed] [Google Scholar]
- 43. Blauvelt A, Reich K, Papp KA, et al. Safety of tildrakizumab for moderate-to-severe plaque psoriasis: pooled analysis of three randomised controlled trials. Br J Dermatol 2018;179:615–22. [DOI] [PubMed] [Google Scholar]
- 44. Reich K, Warren RB, Iversen L, et al. Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: pooled analyses of two randomised phase III clinical trials [reSURFACE 1 and reSURFACE 2] through 148 weeks. Br J Dermatol 2020;182:605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beck KM, Yang EJ, Sekhon S, et al. IL-23 inhibitors for psoriasis. Curr Dermatol Rep 2018;7:119–24. [Google Scholar]
- 46. Kimball AB, Kerbusch T, van Aarle F, et al. Assessment of the effects of immunogenicity on the pharmacokinetics, efficacy and safety of tildrakizumab. Br J Dermatol 2020;182:180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Narcisi A, Valenti M, Cortese A, et al. Anti-IL17 and anti-IL23 biologic drugs for scalp psoriasis: a single-center retrospective comparative study. Dermatol Ther 2021:e15228. [DOI] [PubMed] [Google Scholar]
- 48. Gisondi P, Facheris P, Dapavo P, et al. The impact of the COVID-19 pandemic on patients with chronic plaque psoriasis being treated with biological therapy: the Northern Italy experience. Br J Dermatol 2020;183:373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Valenti M, Pavia G, Gargiulo L, et al. Biologic therapies for plaque type psoriasis in patients with previous malignant cancer: long-term safety in a single- center real-life population. J Dermatolog Treat 2021:1–5. [DOI] [PubMed] [Google Scholar]
- 50. Mehta H, Mashiko S, Angsana J, et al. Differential changes in inflammatory mononuclear phagocyte and T cell profiles within psoriatic skin during treatment with guselkumab vs secukinumab. J Invest Dermatol 2021;141:1707–18.e9. [DOI] [PubMed] [Google Scholar]
- 51. Cheuk S, Wikén M, Blomqvist L, et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol 2014;192:3111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Jesús-Gil C, Ruiz-Romeu E, Ferran M, et al. IL-15 and IL-23 synergize to trigger Th17 response by CLA+ T cells in psoriasis. Exp Dermatol 2020;29:630–8. [DOI] [PubMed] [Google Scholar]
- 53. Vu TT, Koguchi-Yoshioka H, Watanabe R. Skin-resident memory T cells: pathogenesis and implication for the treatment of psoriasis. J Clin Med 2021;10:3822. [DOI] [PMC free article] [PubMed] [Google Scholar]