Abstract
Increasing insights into the immunopathogenesis of inflammatory bowel diseases [IBD] have led to the advent of targeted therapies that inhibit crucial mediators of the inflammatory process, thereby widening our available therapeutic armamentarium. Anti-tumour necrosis factor [anti-TNF] agents are still a mainstay of our therapeutic endeavours and the introduction of corresponding biosimilars has further widened their use. Nevertheless, only a subgroup of treated patients benefit from the initiated treatment and there is secondary non-response in the course of therapy. Initiation of subsequent therapy often poses a challenge to the treating physician, as non-response to primary anti-TNF treatment generally characterizes a patient group that is more treatment-resistant, which may be due to the immunological impregnation by prior anti-TNF exposure. At present, there is currently no guidance for the most appropriate second-line therapy after anti-TNF failure. Here, we review the efficacy of secondary biological therapy in anti-TNF-treated patients. We focus on and assess available clinical trial data of the emerging substance class of IL-23p19 inhibitors, which have demonstrated remarkable efficacy not only in anti-TNF-naïve but also refractory patients. We present molecular mechanisms that drive IL-23-mediated resistance to ongoing anti-TNF therapy and discuss the dynamic fluidity of the mucosal cytokine network in the course of therapy that perpetuates the mucosal inflammatory reaction. Translation of these findings into clinical practice might finally lead to initiation of the most appropriate therapy at the right time of the individual disease course, which would have important implications for the patient’s probability of response to treatment.
Keywords: Inflammatory bowel diseases, IL-23p19, IL-23 inhibitors, anti-TNF, molecular resistance
1. Introduction
Inflammatory bowel diseases [IBD] encompass chronic inflammatory disorders of the gastrointestinal tract whose phenotypic entities mainly comprise Crohn’s disease [CD] and ulcerative colitis [UC].1,2 The clinical course of these immune-mediated disorders is marked by unpredictable exacerbations and asymptomatic remission, causing lifelong morbidity.3 Insufficiently controlled inflammation can lead to progressive bowel damage with impaired function, heightened risk of complications and increased incidence of colitis-associated neoplasia.4–6 Optimized anti-inflammatory therapy is therefore essential for the efficient management of IBD patients.
The advent of targeted therapies has substantially improved therapeutic outcomes and made a major impact on existing therapeutic algorithms.7,8 Anti-tumour necrosis factor [TNF] inhibitors were the first targeted substance class to be approved for the treatment of patients with moderate-to-severe IBD.9 This substance class encompasses the chimeric monoclonal antibody infliximab, the monoclonal human antibody adalimumab, the fully human monoclonal antibody golimumab, the PEGylated humanized Fab′ fragment certolizumab pegol, as well as infliximab and adalimumab biosimilars.10–17 Subsequent introduction of the anti-α4β7 integrin inhibitor vedolizumab and the anti-interleukin [IL]-12/IL-23p40 antibody ustekinumab, as well as the JAK-inhibitor tofacitinib have further expanded our therapeutic options.18 Furthermore, positive phase 3 induction and maintenance trial results have been reported for representatives of novel substance classes [IL-23 inhibitors, sphingosine 1-phosphate receptor 1 modulators, selective JAK-1 inhibitors], which are all expected to further increase our therapeutic possibilities soon.19 All these agents can be applied as first-line or also subsequent treatment option. The choice of a specific therapeutic agent in individual IBD patients is often arbitrary, as there are currently no validated biomarkers available, which would predict responsiveness to available therapies in IBD and help ascertain the order in which biological treatments should be used.20–22 There are only scarce head-to-head trail data available, which can only reflect part of our considerations regarding choice of therapy. In one head-to-head trial, vedolizumab proved superior to adalimumab in inducing clinical remission in UC patients [31.3% vs 22.5%; p = 0.006], although a significant difference was reported only in biologic-naïve, but not exposed patients.23 In agreement with this, society guidelines do not express specific recommendations for therapeutic algorithms in daily clinical practice. Rather than one-size-fits-all, the choice of biologics should be tailored to the individual patient profile. Therapeutic decisions are based on patient preferences, disease phenotype, differences in the pharmacokinetic and pharmacodynamic properties of the substances, presence of extra-intestinal manifestations, and existing comorbidities.24 The strengths and limitations of each agent should be considered in conjunction with the heterogeneous disease profile in each patient.25
Here, anti-TNF inhibitors have proven to still be a mainstay in our existing therapeutic armamentarium. They have proven to be efficacious in both induction and maintenance of remission, corticosteroid-sparing effects, mucosal healing, and reduction in hospitalization and surgery, thereby redefining our current therapeutic strategies towards prevention of complications and halting the progressive course of disease, to improve the quality of life of IBD patients.5,26 In network meta-analyses, infliximab was ranked highest in biologic-naïve UC patients for clinical remission and together with vedolizumab for endoscopic remission induction.27 In CD, a very recent network meta-analysis of biologic-naive patients demonstrated that infliximab [±azathioprine], in comparison with other available treatment options, was associated with a higher odds of inducing clinical remission in luminal disease.24 Based on available randomized controlled trial data, anti-TNFs are also recommended as first-line biological therapy in specific situations, such as steroid-refractory acute severe UC,28 perianal fistulizing CD29 or prevention of postoperative disease recurrence.30 Further studies support their application in stricturing CD,31 and in patients with associated TNF-sensitive extraintestinal manifestations.32 Furthermore, after approval of respective biosimilars, anti-TNF agents are often recommended by many national healthcare institutions as first-line biological therapy in the majority of naïve patients, as they allow significant cost-savings without compromising safety or efficacy.17
Nevertheless, depending on the chosen outcome parameter and the time of treatment, approximately one-third of anti-TNF-treated patients do not demonstrate adequate response to therapy [primary non-response]. Moreover, 30–50% of initial responders are prone to lose response to therapy in the course of continued anti-TNF treatment [secondary non-response]. The annual risk for loss of infliximab response was calculated to be 13% per patient-year.33 Anti-TNF treatment can also be stopped due to occurrence of intolerable adverse events, such as increased susceptibility to serious infections or treatment-related complications [e.g. lupus-like syndromes or allergic reactions].34 This scenario poses a therapeutic challenge to the physician in daily clinical practice, as it is generally believed that non-response to primary anti-TNF treatment characterizes a patient group that is intrinsically more treatment-resistant [longer disease duration, complicated disease] or may have been immunologically embossed by prior anti-TNF exposure to inadequate response to a second agent.
Patients with primary non-response may have altered pharmacokinetics [rapid drug clearance resulting in low trough levels] or pharmacodynamics [mechanistic failure with non-TNF-mediated inflammation], resulting in a decreased likelihood of response to a second biological agent.35 This is reflected by lower efficacy of approved therapies in anti-TNF-exposed compared with anti-TNF-naïve patients.35 Here, it is important to differentiate between the different specific reasons for discontinuing anti-TNF therapy, as this could be due to primary or secondary non-response, as well as intolerance. Available data indicate that primary non-response to initiated anti-TNF treatment should not be assessed before 8–12 weeks after commencement of therapy.36 Recent data have indicated that in patients with primary anti-TNF failure, out-of-class swap is probably preferred. Unfortunately, patients included in clinical trials are not classified as having primary non-response based on trough concentrations, but rather based on clinical history of prior response or not.35 In patients with secondary non-response, however, either an in-class switch or out-of-class swap might be options as secondary therapy.35 In patients with immunogenic failure to initial anti-TNF therapy [insufficient trough levels and detectable anti-drug antibodies], it has been shown that the addition of thiopurines to second-line anti-TNF therapy can prevent clinical failure.37
There is currently no guidance for the most appropriate second-line therapy after failure to primary anti-TNF therapy. There are no data available from direct head-to-head active comparator trials, solely in patients with previous biologic exposure. With the growing number of soon to be available therapies in the IBD field, it is important to order the sequence of treatments in patients who have previously been exposed to anti-TNF inhibitors. The currently applied clinical practice of randomly selecting second-line therapies for treatment of IBD patients may lead to futile responses, which can often only be assessed after several weeks. This is coupled with ongoing clinical repercussions and progression of bowel damage in non-responders, which may have been hindered by an initially efficacious therapy. Selection of an effective therapy, which should be based on pharmacokinetic or molecular insights regarding anti-TNF non-response, would allow individualized treatment with higher response rates and lower levels of toxicity for the patient.
In the following, the efficacy of different substance classes in IBD patients with non-response to primary anti-TNF therapy will be briefly discussed with particular reference to the apparently high effectiveness of emerging IL-23p19 inhibitors, which might be explained by a new found IL-23-mediated molecular resistance mechanism in anti-TNF non-responders.38 Improved understanding of these molecular resistance mechanisms and the changing immunological phenotype would enable us to appropriately position our different agents in the course of disease, which would be essential for optimized, personalized medicine approaches in IBD.20
2. Effectiveness of Approved Biological Therapies [Anti-TNF, Vedolizumab and Ustekinumab] After Non-Response to Previous Anti-TNF Inhibitor Treatment
2.1. Anti-TNF antagonists
The effectiveness of a second anti-TNF inhibitor treatment after previous exposure and discontinuation of the index anti-TNF agent is dependent on the reason for cessation. A systematic review and meta-analysis demonstrated that overall remission rate with the second anti-TNF agent was 30% after primary failure of the first anti-TNF agent. Most of the evaluated studies analysed the effects of switching from infliximab to adalimumab.39 When a second anti-TNF agent was applied after secondary failure to the previous one, the mean remission rate rose to 45%, which might indicate pharmacokinetic reasons [formation of anti-drug antibodies] that led to discontinuation of the index anti-TNF therapy and was overcome by the subsequent one. Overall pooled remission rates were highest [61%] when the reason for withdrawing the first anti-TNF therapy was intolerance.39 Altogether, a subgroup of patients benefited from an alternative anti-TNF agent, after failure of the first one.
2.2. Vedolizumab
The randomized GEMINI-approval trials reported efficacy of the anti-α4β7 integrin inhibitor vedolizumab in patients with previous anti-TNF therapy. Post hoc analyses of the efficacy data for 516 TNF-naïve and 960 TNF-failure CD patients from the GEMINI 2 and GEMINI 3 trials were evaluated. Clinical response rates at weeks 6 and 10 were numerically higher in the anti-TNF-naïve [40.3% and 48.4%] in comparison to the anti-TNF-exposed group [33.1% and 39.7%], respectively. The differences prevailed throughout week 52. Altogether, patients who were TNF-naïve experienced treatment benefits earlier than those who had failed prior TNF antagonist therapy, and they achieved higher overall rates of response and also remission.40 Comparable results were also found in UC patients, with post hoc analysis of efficacy data from the GEMINI 1 study of 464 TNF-naïve patients and 367 TNF-failure UC patients. Week 6 rates of response to vedolizumab and placebo were 53.1% and 26.3%, respectively, among patients naive to TNF antagonists and 39.0% and 20.6%, respectively, in patients with failure of TNF antagonists.41
Retrospective analysis of endoscopies performed at a tertiary referral centre confirmed the findings, as outcomes were significantly better in anti-TNF-naïve vs exposed patients [67.2% vs 42.0%].42 These results are also reflected in a validated scoring system to identify IBD patients with a higher likelihood of response to vedolizumab, as absence of previous treatment with a TNF antagonist was independently associated with corticosteroid-free remission upon vedolizumab therapy.43,44 Based on findings from the GEMINI trials, vedolizumab demonstrated comparable efficacy in anti-TNF-exposed patients, irrespective of primary or secondary non-response, or intolerance.40
2.3. Ustekinumab
The UNITI-1 trial included 741 moderate-to-severe CD patients who had previous exposure to two or three substances and 29.1% primary non-response as reason for failure. The primary endpoint of clinical response at week 6 was reached significantly higher in the groups that received ustekinumab at a dose of either 130 or 6 mg/kg [34.3% and 33.7%, respectively] than in the placebo group [21.5%]. In the UNITI trials, higher rates of absolute response and remission were observed in the cohort of anti-TNF-naïve patients [UNITI-2] in comparison to anti-TNF-exposed patients [UNITI-1], which might have been presumably influenced by less refractory and relatively shorter duration of disease in patients in whom only conventional therapy had been unsuccessful.45 In a recent meta-analysis, treatment response to ustekinumab was poorer in anti-TNF primary non-responders, in comparison with secondary non-responders (relative risk [RR], 0.64 [0.52–0.80]).35
In UC, a total of 961 patients were randomly assigned to receive an intravenous induction dose of ustekinumab [130 mg or weight-based dose of 6 mg/kg bodyweight] or placebo in the randomized, placebo-controlled UNIFI trial.46
In total, 51.1% of the participating patients had treatment failure to previous biological therapy, among which a total of 98.8% had treatment failure to at least one TNF antagonist. In this subpopulation, the proportions of patients who achieved clinical remission at week 8 were greater in the 130 mg/k [11.1%] and 6 mg/kg [12.8%] groups compared with patients in the placebo group [1.3%]. In maintenance, patients who were biologic failures to at least one anti-TNF comprised 99.2% of patients who had a history of biologic failure and 47.2% of patients in the primary population. The proportions of anti-TNF-exposed patients who achieved clinical remission at week 44 were numerically greater in the ustekinumab q8w group [38.9%] than in the ustekinumab q12w group [22.9%]; both ustekinumab groups were numerically greater than placebo [17.2%]. Altogether, the percentages of patients in whom each endpoint was achieved were lower across groups with previous treatment failure with biologics compared to naïve patients.
2.4. Conclusion
Overall, the available data suggest that patients who failed anti-TNF therapy represent a group with more refractory disease, as approved biological treatment options seem to perform less efficiently in these patients compared to anti-TNF-naïve patients. However, within the group of anti-TNF-exposed patients, those with prior primary non-response to anti-TNF agents may less likely respond to subsequent anti-TNF or ustekinumab therapy than those with secondary non-response or intolerance.35,39 Primary loss of response might be caused by inadequate trough concentrations due to rapid, non-immune-mediated drug clearance or due to mechanistic molecular failure, mediated by non-TNF signalling pathways.35
3. Effectiveness of IL-23p19 Inhibitors After Non-Response to Previous Anti-TNF Inhibitor Treatment
Accumulating preclinical evidence suggest that IL-23p19 is mainly involved in IBD pathogenesis.47 It is predominantly produced by macrophages and antigen presenting cells and promotes the generation of pathogenic Th17 cells, which in turn produce IL-17, IL-22, interferon [IFN]γ and TNF.48 IL-23 also inhibits regulatory T-cell responses in the intestine, thereby enhancing mucosal inflammation.49 In addition, genome-wide association studies [GWAS] have analysed polymorphism in the gene encoding IL23R and identified genetic variants in the IL23R region that were associated with CD as wells as with UC susceptibility.50,51 Correspondingly, elevated IL-23 levels were found in the inflamed mucosa of CD and UC patients, further emphasizing its key role in IBD pathogenesis.52,53 These findings led to the development of selective IL-23p19 inhibitors being tested in several phase 2 or phase 3 randomized controlled trials, which have already been completed or are underway. Available evidence suggests that selective blockade of IL-23p19 is an effective and safe treatment option for both IBD entities, qualifying them as potential first-line therapies in IBD. In the following, specific focus is placed on the effectiveness of IL-23p19 inhibitors to patients previously exposed to anti-TNF therapy.
3.1. Brazikumab
Brazikumab [formerly named MEDI2070] is a human IgG2 monoclonal antibody directed against IL-23p19. Its efficacy and safety were assessed in a phase 2, randomized, placebo-controlled trial. Participating patients had to fulfil the criteria of moderate-to-severe (Crohn’s disease activity index [CDAI] 220–450) CD and accompanying biochemical (C-reactive protein [CRP] ≥5 mg/L, faecal calprotectin ≥250 µg/g] or endoscopic [≥3 non-anastomotic ulcers, each >0.5 cm in diameter or ≥10 aphthous ulcers involving ≥10 cm of contiguous intestine] evidence of active inflammation. Patients were required to also have been exposed to at least one induction regimen of a TNF antagonist consisting of at least two doses at least 2 weeks apart. The primary outcome was clinical response at week 8 [CDAI < 150 or ≥ 100-point reduction from baseline]. Endoscopic outcomes were not assessed. A total of 119 patients were randomized to receive brazikumab 700 mg intravenously [n = 59] or placebo [n = 60] at weeks 0 and 4, with stratification by the number of prior anti-TNF agents applied [1 vs > 1]. The primary outcome was clinical response at week 8. Altogether, 37 patients had previous exposure to one, 70 patients to two and 12 patients to three or more anti-TNF agents in the study. Reasons for TNF antagonist discontinuation were primary [n = 46] or secondary [n = 67] non-response, treatment intolerance [n = 53] or other/not identified [n = 20]. A significantly greater proportion of patients receiving brazikumab reached the outcome of clinical remission or clinical response at week 8 compared to patients receiving placebo [49.2% vs 26.7%; p = 0.01]. The prespecified composite outcome of clinical response and ≥ 50% reduction in faecal calprotectin or CRP levels from baseline was achieved by 42.4% of the brazikumab-treated patients in comparison to 10% of the placebo group [p < 0.001]. At week 12, efficacy of brazikumab in comparison to placebo was 37.3% vs 28.3% for clinical response [p = 0.29] and 37.3% vs 8.3% for the composite clinical and biomarker endpoint [p < 0.001]. There was no statistical difference regarding clinical remission at week 8 or 12 between the brazikumab and placebo groups. Clinical response at week 24 occurred in 53.8% of patients who continued to receive open-label brazikumab and in 57.7% of patients who had received placebo during the double-blind period and open-label brazikumab thereafter. Patients with a baseline IL-22 concentration > 15.6 pg/mL were more likely to respond to brazikumab. The safety profile of brazikumab was similar to placebo with comparable serious adverse events and overall adverse events.54 Altogether, brazikumab demonstrated effectiveness in this study cohort of anti-TNF-exposed patients.
3.2. Mirikizumab
Mirikizumab is a humanized IgG4-variant monoclonal antibody against IL-23p19. Its efficacy and safety were assessed in a phase 2, randomized, placebo-controlled trial in moderate to severely active UC patients [Mayo Clinical Score 6–12, with Mayo Endoscopic Score ≥ 2]. The primary endpoint was clinical remission [Mayo subscores of 0 for rectal bleeding, with 1-point decrease from baseline for stool frequency, and 0 or 1 for endoscopy] at week 12. Of the 249 recruited UC patients, 63% had prior exposure to a biological agent. Patients were randomized to intravenous mirikizumab 50 mg with dose adjustment, 200 mg with dose adjustment or 600 mg fixed dose, or placebo at weeks 0, 4 and 8. Induction exposure was adjusted in the 50- and 200-mg groups according to measured drug serum concentrations. At week 12, 15.9% [p = 0.066], 22.6% [p = 0.004] and 11.5% [p = 0.142] of patients in the 50-, 200- and 600-mg groups achieved clinical remission, respectively, compared with 4.8% of patients given placebo. The result for the primary endpoint of clinical remission at week 12 for the mirikizumab 600-mg group did not reach statistical significance. Thus, all subsequent p values comparing clinical remission at week 12 are not controlled for multiplicity and were considered nominal. A similar pattern was seen for clinical remission across dose groups among the biologic-naive and biologic-experienced groups, with clinical remission rates numerically higher in all treatment groups among biologic-naive patients. In the biologic-naïve group [n = 92], 29.2% [Δ = 20.5%; p = 0.137], 36.4% [Δ = 27.7%; p = 0.035] and 17.4% [Δ = 8.7%; p = 0.665] of patients in the 50-, 200- and 600-mg groups achieved clinical remission, respectively, compared with 8.7% of patients given placebo. In the biologic-experienced group [n = 157], 7.7% [Δ = 5.2%; p = 0.359], 15% [Δ = 12.5%; p = 0.108] and 7.9% [Δ = 5.4%; p = 0.352] of patients in the 50-, 200- and 600-mg groups achieved clinical remission, respectively, compared with 2.5% of patients given placebo.
At week 52, 46.8% and 37.0% of mirikizumab responders treated with 200 mg mirikizumab every 4 and every 12 weeks, respectively, achieved clinical remission. In biologic-naïve patients, 47.6% and 36% and in biologic-experienced patients, 46.2% and 38.1% treated with 200 mg mirikizumab every 4 and every 12 weeks, respectively, achieved clinical remission. There was no safety signal recorded when comparing mirikizumab and placebo.
Altogether, trial results suggest that mirikizumab could be an effective therapy in UC patients.55 The number of previously failed biological therapies could be an indicator for the patient group that might benefit from a longer initial dosing regimen, although additional studies are needed to investigate this.56 Furthermore, a large-scale gene expression study demonstrated that mirikizumab treatment significantly affected mucosal transcripts involved in resistance to anti-TNF therapy in UC patients.57,58
3.3. Guselkumab
Guselkumab is a fully human IgG1-lambda monoclonal antibody targeting IL-23p19. Currently, a phase 2/3, randomized, double-blind, placebo- and active-controlled, parallel-group trial in moderately-to-severely active CD [GALAXI-1] is ongoing. Recently, results from the interim analysis of the phase 2, dose ranging trial [GALAXI-1] assessing the efficacy and safety of guselkumab in CD have been published. A total of 309 CD patients were randomized to 200, 600 or 1200 mg intravenous guselkumab every 4 weeks, with ustekinumab [6 mg/kg i.v. at week 0 and then 90 mg s.c. at week 8] and placebo as reference arms. The study was not powered to assess differences between guselkumab and ustekinumab. The primary outcome was the change from baseline in CDAI score at week 12. At week 12, the primary endpoint was achieved, with significantly greater LS mean reductions from baseline in CDAI score observed for the guselkumab 200 mg (−160.4), 600 mg (−138.9),and 1200 mg (−144.9) groups compared with placebo (−36.2); p < 0.05 for all comparisons.
Clinical remission [CDAI < 150] at week 12 was reached by 57.4, 55.6 and 45.9% in the 200-, 600- and 1200-mg guselkumab groups, respectively, while 46.0% in the ustekinumab and 16.4% in the placebo group fulfilled that criterion. All results of the guselkumab treatment groups were statistically significant in comparison to placebo. In total, 54.4% of patients had an inadequate response to, or intolerance to biologic therapy: 53.1% failed at least one anti-TNF inhibitor, 8.7% failed vedolizumab, and 7.4% failed at least one anti-TNF drug and vedolizumab. In the subgroup of patients with inadequate response or intolerance to prior biologic therapy, 47.5% in the combined guselkumab group and 10.0% in the placebo group achieved clinical remission at week 12. Consistent with the overall population, no apparent dose response was observed across analysed endpoints.
Overall endoscopic response (at least 50% improvement from baseline in Simple Endoscopic Score for Crohn Disease [SES-CD] score or SES-CD score ≤2) at week 12 was observed in 37.7, 36.5 and 32.8% in the 200-, 600- and 1200-mg guselkumab groups, respectively, as well as 28.6% in the ustekinumab and 11.5% in the placebo group. In biologic-naïve patients, 37.9, 42.9 and 44.4% in the 200-, 600- and 1200-mg guselkumab groups, respectively, as well as 46.2% in the ustekinumab and 9.7% in the placebo group reached endoscopic response in comparison to 37.5, 31.4 and 23.5 in the 200-, 600- and 1200-mg guselkumab groups, respectively, as well as 16.2% in the ustekinumab and 13.3% in the placebo group of biologic-exposed patients. Guselkumab was well tolerated in the study. Altogether, a remarkably high efficacy of guselkumab treatment was observed in the group of biologic-exposed patients, especially regarding the outcome measure of endoscopic improvement. Further trial data are awaited.59
3.4. Risankizumab
Risankizumab is a humanized IgG1 monoclonal antibody directed against IL-23p19. Its efficacy and safety were initially studied in a phase II randomized, placebo-controlled trial in CD patients. Study outcomes were reported for a 12-week, double-blinded intravenous period, a 14-week, open-label, intravenous therapy or wash-out period, and a 26-week subcutaneous treatment period. In total, 121 patients with moderate-to-severe disease (CDAI 220–450 and a Crohn's Disease Endoscopic Index of Severity [CDEIS] score ≥ 7, or ≥ 4 for isolated ileitis) were randomized to receive 200 or 600 mg risankizumab, or placebo by intravenous infusion at weeks 0, 4 and 8. Nearly all patients [93.4%] had previous exposure to anti-TNF agents. In the pooled risankizumab treatment group, 22, 57 and 13% experienced one, two or three or more previous anti-TNF therapies. Significantly more patients in the pooled risankizumab dose groups achieved the primary endpoint of clinical remission at week 12 compared to placebo [30.5% vs 15.4%; p = 0.0489]. Clinical response [CDAI < 150 or ≥ 100-point decrease] was also significantly more fulfilled by risankizumab- compared to placebo-treated patients [39% vs 20.5%; p = 0.0273], which was also evident for endoscopic remission [CDEIS ≤ 4 or ≤ 2 for isolated ileitis; 17% vs 3%; p = 0.0015]. At week 12, a significantly higher proportion of patients given 600 mg risankizumab achieved clinical remission compared to placebo [36.6% vs 15%]. No significant difference was observed for patients treated with 200 mg risankizumab [p = 0.308]. Furthermore, significantly greater proportions of patients receiving 600 mg risankizumab achieved endoscopic remission [20% vs 3%] or had an endoscopic response [37% vs 13%] compared to placebo. Treatment with risankizumab decreased IL-22 expression in ileum biopsies and in circulation, consistent with the expected effects of blockade of the IL-23 axis.60
In a subsequent trial, patients not achieving deep remission [CDAI < 150 and CEDEI ≤ 4 or ≤ 2 for patients with isolated ileitis] at week 12 received extended open-label induction therapy with intravenous risankizumab [600 mg] every 4 weeks for 12 weeks [weeks 14–26]. Patients in deep remission [n = 6] entered a 12-week washout phase. At week 26, 53% of patients were in clinical remission, 55% originally in the placebo arm, 59% in the 200-mg risankizumab arm and 47% in the 600-mg risankizumab arm. Enrolled patients had a mean disease duration of 14 years and nearly all had been previously exposed to one or more TNF inhibitors, indicating that long disease duration and previous treatment exposure can delay successful treatment responses.61
In an open-label extension study, enrolled patients had achieved clinical response without clinical remission at week 26, or clinical response and/or remission at week 52 in the parent phase 2 study, and received open-label subcutaneous risankizumab 180 mg every 8 weeks. Efficacy outcomes were maintained during the study [clinical remission >71%] and endoscopic remission >42%].62
Recently, results from two phase 3 trials with risankizumab in moderate-to-severe CD were presented. In the double-blind, randomized, ADVANCE study, 490 of the recruited 850 patients were previously exposed to biological therapy. In total, 239 had one previous and 251 more than one biological therapy beforehand, with 110 exposed to ustekinumab. Patients were given intravenous risankizumab 600 mg, risankizumab 1200 mg, or placebo at baseline, and weeks 4 and 8. The co-primary endpoint at week 12 was clinical remission (outside USA: average daily stool frequency [SF] ≤ 2.8 and not worse than baseline AND average daily abdominal pain score [APS] ≤ 1 and not worse than baseline; USA: CDAI < 150) and endoscopic response [decrease in SES-CD > 50% from baseline or for subjects with isolated ileal disease and a baseline SES-CD of 4, at least a 2-point reduction from baseline]. Risankizumab reached the primary endpoint on a statistically significant level in comparison to placebo [CDAI < 150: 45.2% in the 600-mg risankizumab group, 41.6% in the 1200-mg risankizumab group vs 25.2% in the placebo group; SF/APS: 43.5% and 41.0% vs 21.7%], and endoscopic response [40.3% and 32.2% vs 12.0%]. When only looking at the subgroup of biologic-experienced patients, all risankizumab doses showed superior efficacy compared to placebo for clinical remission [CDAI < 150: 42.5% in the 600-mg risankizumab group, 37.4% in the 1200-mg risankizumab group vs 25.8% in the placebo group; SF/APS: 40.5% and 38.9% vs 22.7%] and endoscopic response [32.9% and 23.5% vs 11.4%]. Altogether, risankizumab efficacy was numerically lower than in biologic-naïve patients. In the MOTIVATE study [n = 569], 47.1% of patients were previously exposed to one and 52.9% to two or more biological therapies. Among them, 19.1% were exposed to ustekinumab. Risankizumab reached the co-primary endpoint on a statistically significant level in comparison to placebo [CDAI < 150: 42.5% in the 600-mg risankizumab group, 40.3% in the 1200-mg risankizumab group vs 19.8% in the placebo group; SF/APS: 34.6% and 39.3% vs 19.3%], and endoscopic response [28.8% and 34.2% vs 11.2%].63
Finally, risankizumab responders to induction therapy at week 12 of the ADVANCE and MOTIVATE studies were enrolled into the phase 3 maintenance study FORTIFY [n = 542], and received 180 or 360 mg of subcutaneous risankizumab or placebo, every 8 weeks. In total, 73.1% of patients were previously exposed to at least one biological therapy. The primary endpoint of clinical remission within the USA [CDAI <150] was reached with statistical significance for both risankizumab groups in comparison with placebo [55.4% in the 180-mg risankizumab group, 52.2% in the 360-mg risankizumab group vs 40.9% in the placebo group]. Clinical remission based on the outside of the USA defined endpoint [SF ≤ 2.8 and APS ≤ 1] was reached by risankizumab 360 mg on a statistically significant level [51.8% vs placebo 39.6%], but not by risankizumab 180 mg [46.5%]. The co-primary endpoint of endoscopic response was reached on a statistically significant level by both risankizumab dose groups [47.1% in the 180-mg group, 46.5% in the 360-mg group vs 22% in the placebo group]. Risankizumab therapy was generally safe and well tolerated.64
3.5. Overall assessment of IL-23p19 inhibitor efficacy in anti-TNF refractory patients
There is general consensus, backed up by clinical trial data, that patients who failed previous treatment with anti-TNF agents represent a group of more refractory disease. This is especially evident in patients with primary non-response to anti-TNF agents, who are intrinsically more difficult to treat with second-line biologics. Overall, the generally lessened efficacy of second-line biological therapy in anti-TNF-exposed patients has direct clinical implications as these patients are at a higher risk for poor outcomes.65 Choice of the secondary therapy therefore has critical clinical implications for the patient to prevent deleterious outcomes.
In the presented trial data of the specific IL-23p19 inhibitors, there was remarkable efficacy demonstrated by all substances, especially also in the subgroup of patients previously exposed to biological therapy. In all of the presented trials the group of biologic-experienced patients mainly comprised anti-TNF-exposed patients, which could be indicative of a non-TNF-mediated inflammatory signalling pathway that perpetuates mucosal inflammation in this IBD subgroup.
In a recently updated network meta-analysis in UC, seven randomized controlled trials including 1580 patients with moderate-to-severe UC with prior exposure to TNF antagonists were analysed. These included subgroup analysis of trials of adalimumab, vedolizumab, tofacitinib and ustekinumab. Ustekinumab (surface under the cumulative ranking curve [SUCRA], 0.87) and tofacitinib [SUCRA, 0.87] were ranked highest for induction of clinical remission with ustekinumab superior to vedolizumab (ustekinumab vs vedolizumab: odds ratio [OR], 5.99; 95% confidence interval [CI], 1.13–31.76) and adalimumab (ustekinumab vs adalimumab: [OR, 10.71; 95% CI, 2.01–57.20]) in anti-TNF-exposed patients.27
Recently, an updated systematic review and network meta-analysis that assessed the comparative efficacy and safety of biologics in patients with CD was published. It also comprised ten randomized controlled trials including 2479 patients with previous exposure to biological therapy, where effectiveness of the second-line biological was evaluated comparatively. In the study, phase 3 results of risankizumab and, in a sensitivity analysis, interim results at week 12 from the phase 2 induction data of the phase 2/3 GALAXI study were included. In the direct meta-analysis, ustekinumab (OR 2.55 [95% CI 1.39–4.69]) and risankizumab (2.64 [1.89–3.68]) were associated with a significantly higher odds of inducing clinical remission than placebo. There was moderate confidence in estimates supporting risankizumab (OR 2.10 [95% CI 1.12–3.92]) over vedolizumab for inducing remission in patients previously exposed to a biologic.24
No substance was clearly superior to others for induction of a clinical response, but the overall ranking was highest for risankizumab [SUCRA 0.87] and ustekinumab [SUCRA 0.67]. These data indicate that IL-23 blockade might be the preferred mechanism of action in patients who have been previously exposed to TNF antagonists, after either primary or secondary loss of response. Real-world observations have recently indicated that ustekinumab is more effective than vedolizumab in anti-TNF-exposed CD patients.66,67 Adalimumab (OR 3.55 [95% CI 1.82–6.93]) was also associated with a higher odds of inducing clinical remission, but only after loss of response or intolerance to infliximab, but is likely to be of limited use in primary TNF non-responders.
Altogether, these data underline that in moderate-to-severe IBD patients who have not previously responded to TNF antagonist therapy, blockade of IL-23 by risankizumab and ustekinumab are most likely to induce remission and are therefore a preferable second-line therapy.
3.6. Molecular mechanisms that drive IL-23-mediated resistance against anti-TNF therapy
Multiple different molecular signalling pathways are likely to be activated in IBD and are destined to change during the course of disease due to highly complex underlying molecular events. Fittingly, it could be shown that different pathogenetic pathways are only activated contemporarily in the inflamed intestinal mucosa of IBD patients.68,69 In early mucosal inflammation in the neo-terminal ileum before endoscopic recurrence, heightened expression of Th1-related cytokines, TNF and IL17A [slightly] was found. Transition from this stage to endoscopic recurrence was marked by high levels of Th1 cytokines, a marked increase in IL17A, and induction of IL-6 and IL-23, while established lesions were characterized by a mixed Th1–Th17 profile with absent TNF induction.70 These findings support those in experimental colitis models, where the initial phase of the inflammation was driven by Th1 cytokines, while the later phases were associated with mixed Th1/Th17 cell responses.71,72 In supernatants of T cell clones derived from intestinal biopsies of paediatric CD patients, that were stimulated with IL-12, heightened IFN-γ levels in early but not in late CD were demonstrated.73 These data indicate that the mucosal cytokine profile in CD is not stable and may vary in the course of the disease and that mucosal T cell immunoregulation similarly succumbs to changes during progression of disease, with potential implications for therapeutic approaches. As IBD is driven by molecular processes, it is conceivable that drug interactions should have an effect on its target and disease pathways. Interference with one particular signalling pathway might have an impact on other relevant inflammatory cascades that perpetuate the inflammatory reaction. In cancer therapy, various molecular mechanisms that drive therapeutic resistance to targeted therapies, thereby driving disease progression within a year of initiating treatment, have been identified.74 As such, an improved understanding of molecular resistance mechanisms is essential to optimize personalized therapy in cancer, and the same approach is likely to be beneficial in IBD as well.20 The concept of molecular resistance derived from modifications in the composition of mucosal immune cells in response to therapeutic pressure has recently been introduced to the IBD field.38
Here, a significant upregulation of mucosal IL23p19, IL23R and IL17A but not IL-12p40 expression could be found in anti-TNF non-responders during ongoing anti-TNF treatment. These effects were absent in anti-TNF responders. Non-responsive patients also exhibited an IL-23-mediated expansion of apoptosis-resistant mucosal T cells expressing TNFR2 and the IL-23 receptor, with decreased susceptibility to anti-TNF-induced apoptosis. These findings indicate that IL-23 could be a key driver of molecular resistance to TNF antagonists during ongoing therapy.38 These data indicate that T cell plasticity and therapy-induced changes in immune profiles may explain the need for different biological treatments at different stages of disease.
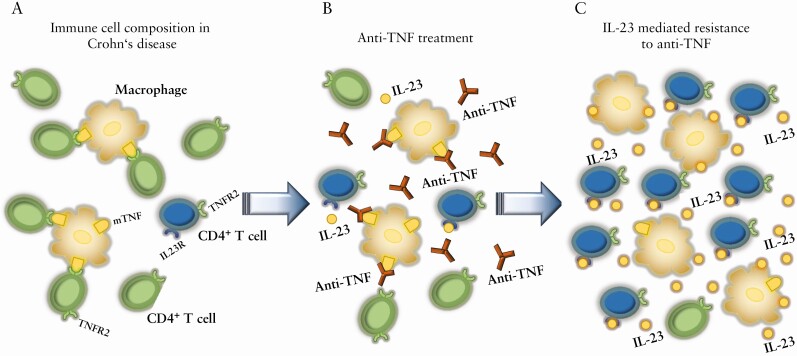
In this concept, effective anti-TNF antibody treatment is based on induction of T cell apoptosis by blocking the transmembrane TNF [mTNF]/TNF receptor 2 [TNFR2] co-stimulation pathway between CD14+ macrophages and CD4+ T cells in the mucosa.75 In non-responders, there is an accumulation of CD14+ macrophages that produced heightened amounts of IL-23 during ongoing anti-TNF therapy. This leads to the expansion of anti-TNF apoptosis-resistant T cells that express TNFR2 and IL-23R, which modifies the initial composition of cells and changes the molecular phenotype of disease. Subsequently, there is an IL-23-induced molecular resistance to anti-TNF therapy in CD patients, suggesting that targeting IL-23 might be particularly effective in these patients, which has been reflected by the previously presented trial data [Fig. 1].76
Figure 1.
Molecular mechanism of IL-23-driven resistance to anti-TNF therapy in Crohn’s disease. [A] Immune cell composition in a Crohn’s disease patient prior to the initiation of anti-TNF therapy. Perpetuation of mucosal inflammation due to an indication of apoptosis resistance in TNF receptor 2 [TNFR2]-expressing CD4+ T cells by transmembrane TNF [mTNF]-expressing CD14+ macrophages. [B] After commencement of efficacious anti-TNF therapy, there is induction of CD4+ T-cell apoptosis by binding of the anti-TNF antibody to mTNF-expressing CD14+ macrophages, thereby inhibiting the mTNF/TNFR2 co-stimulation pathway between CD14+ macrophages and CD4+ T cells in the mucosa. [C] In anti-TNF non-responders, there is heightened production of IL-23 by CD14+ macrophages, which leads to the expansion of apoptosis-resistant IL-23R+/TNFR2+ CD4+ T cells, which drive resistance to ongoing anti-TNF therapy. The expansion of IL-23R+ lymphocytes creates a novel immune phenotype that drives mucosal inflammation, non-responsive to anti-TNF therapy but potentially responsive to IL-23 inhibition.
4. Concluding Remarks
One of the main challenges in the optimized management of IBD patients with moderate-to-severe disease activity is the appropriate positioning of different targeted therapies in the course of disease. The growing therapeutic armamentarium has enabled us to choose from a variety of different substances that specifically target an inflammatory pathway involved in the immunopathogenesis of IBD. Ongoing trials with specific IL-23p19 inhibitors will offer further novel findings and the ongoing comparative trial of risankizumab with ustekinumab in CD will further help to position the substance class of IL-23p19 inhibitors in our therapeutic algorithm. Preclinical models suggest that both IL-12 and IL-23 seem to contribute to intestinal inflammation through sequential and temporarily distinct functions. Whereas IL-12 initiates the initial, primarily innate immune-cell-driven inflammatory reaction triggered by heightened exposure to bacteria in response to intestinal barrier disruption, IL-23 becomes functionally important during the chronic phase of the inflammatory response that is characterized by an increased contribution of adaptive immunity.77 These studies indicate that anti-p40 antibodies, such as ustekinumab, would probably be more effective in newly diagnosed IBD, while anti-IL-23p19 antibodies might demonstrate higher efficacy in later stages of the disease. However, these findings need to be analysed in clinical studies.
Identification of patients who have a heightened probability of responding to either of these substance classes will be important for their future clinical use. Currently existing efficacy and safety profiles qualify them as potential first-line treatment. However, resistance to previously initiated anti-TNF therapy, which is probably the most widely used first-line targeted therapeutic agent in IBD, represents an important problem in clinical practice, as these patients are prone to have poorer outcomes. Non-responsiveness to TNF inhibition is potentially a surrogate marker for a more refractory phenotype, or reflects changes in the immune response that are induced by previous anti-TNF exposure. The dynamic fluidity of the mucosal cytokine network is reflected by successional development of alternative pro-inflammatory cytokine pathways upon blockade of a single cytokine, thereby circumventing this therapeutic approach and perpetuating the inflammatory reaction.3 This mechanism has been exemplified in CD patients who are non-responsive to initiated anti-TNF therapy, where neutralization of TNF has been associated with an IL-23-driven induction for Th-17 cell responses.38 Blockade of IL-23p19 has conversely shown great efficacy in anti-TNF-resistant patients, which qualifies them for efficacious second-line targeted therapy, which has also been demonstrated in a recent comparative network meta-analysis.24 Moreover, it is theoretically also possible that IL23 inhibition might restore sensitivity to the mechanism of action of TNF antagonists in non-responders. This would suggest that therapies combining TNF- and IL-23-neutralizing antibodies could potentially achieve improvements in efficacy or duration of response than either monotherapy alone. Trial results of a proof-of-concept, phase IIa, randomized, double-blind study are awaited in this regard, as the efficacy and safety of the anti-IL-23p19 antibody guselkumab in combination with the anti-TNF agent golimumab are studied in the VEGA trial in moderate-to-severe UC patients. Moreover, Vorabody V56B2, the first anti-TNF/anti-IL-23 orally delivered intestinal protease-resistant bispecific antibody, has been presented, which might offer a novel therapeutic approach for disease-modifying activity in IBD.78
Altogether, choosing the most appropriate biological therapy at the right time has important implications for the patient’s probable response. One of the most major challenges remains to identify predictors of response to targeted therapies in IBD.20,79 They would optimally need to integrate the mode of action of the therapeutic substance, the temporarily distinct functions of specific signalling pathways in intestinal inflammation and the induction of molecular resistance mechanisms by previous biological therapies to understand mechanisms of cell–cell signalling and drug susceptibility. Altogether, the available data indicate that specific IL-23p19 inhibitors would certainly qualify themselves not only as potential first-line biological therapy, but also as a promising therapeutic approach in anti-TNF-exposed IBD patients.
Contributor Information
Raja Atreya, Medical Clinic 1, Department of Medicine, University Hospital Erlangen, University of Erlangen-Nürnberg, Erlangen, Germany; Deutsches Zentrum Immuntherapie (DZI), Erlangen, Germany.
Markus F Neurath, Medical Clinic 1, Department of Medicine, University Hospital Erlangen, University of Erlangen-Nürnberg, Erlangen, Germany; Deutsches Zentrum Immuntherapie (DZI), Erlangen, Germany.
Funding
Funding by the German Research Council (DFG) SFB1181 [project no. C02] and SFB/TRR241 [project no. C02, IBDome]. The German Research Council [DFG] funds the Heisenberg Professorship of R.A. This paper was published as part of a supplement financially supported by AbbVie.
Conflict of Interest
R.A. has served as a speaker, or consultant, or received research grants from AbbVie, Amgen, Arena Pharmaceuticals, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Cellgene, Celltrion Healthcare, DrFalk Pharma, Ferring, Fresenius Kabi, Galapagos, Gilead, GlaxoSmithKline plc, InDex Pharmaceuticals, Janssen-Cilag, Kliniksa Pharmaceuticals, Lilly, MSD Sharp & Dohme, Novartis, Pandion Therapeutics, Pfizer, Roche Pharma, Samsung Bioepsis, Stelic Institute, Sterna Biologicals, Takeda Pharma, Tillotts Pharma AG, Viatris. M.F.N. reports research grants and/or personal fees from Abbvie, MSD, Takeda, Boehringer, Roche, Pfizer, Janssen, Pentax and PPD.
Author Contributions
R.A. and M.F.N. drafted and reviewed the manuscript. Both authors approved submission of the final draft of the manuscript.
References
- 1. Gomollón F, Dignass A, Annese V, et al. ; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 2. Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers 2020;6:74. [DOI] [PubMed] [Google Scholar]
- 3. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14:329–42. [DOI] [PubMed] [Google Scholar]
- 4. Pariente B, Torres J, Burisch J, et al. Validation and update of the Lémann index to measure cumulative structural bowel damage in Crohn’s disease. Gastroenterology 2021;161:853–64.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colombel JF, Mahadevan U. Inflammatory bowel disease 2017: innovations and changing paradigms. Gastroenterology 2017;152:309–12. [DOI] [PubMed] [Google Scholar]
- 6. Torres J, Caprioli F, Katsanos KH, et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J Crohns Colitis 2016;10:1385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.BD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castaño-Milla C, Chaparro M, Gisbert JP. Systematic review with meta-analysis: the declining risk of colorectal cancer in ulcerative colitis. Aliment Pharmacol Ther 2014;39:645–59. [DOI] [PubMed] [Google Scholar]
- 9. Billmeier U, Dieterich W, Neurath MF, Atreya R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J Gastroenterol 2016;22:9300–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanauer SB, Feagan BG, Lichtenstein GR, et al. ; ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 11. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 12. Sandborn WJ, Feagan BG, Stoinov S, et al. ; PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 2007;357:228–38. [DOI] [PubMed] [Google Scholar]
- 13. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- 14. Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. ; PRECISE 2 Study Investigators. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med 2007;357:239–50. [DOI] [PubMed] [Google Scholar]
- 15. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65.e1–3. [DOI] [PubMed] [Google Scholar]
- 16. Sandborn WJ, Feagan BG, Marano C, et al. ; PURSUIT-Maintenance Study Group. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:96–109.e1. [DOI] [PubMed] [Google Scholar]
- 17. Fischer S, Cohnen S, Klenske E, et al. Long-term effectiveness, safety and immunogenicity of the biosimilar SB2 in inflammatory bowel disease patients after switching from originator infliximab. Therap Adv Gastroenterol 2021;14:1756284820982802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol 2017;14:269–78. [DOI] [PubMed] [Google Scholar]
- 19. Verstockt B, Ferrante M, Vermeire S, Van Assche G. New treatment options for inflammatory bowel diseases. J Gastroenterol 2018;53:585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Atreya R, Neurath MF. Mechanisms of molecular resistance and predictors of response to biological therapy in inflammatory bowel disease. Lancet Gastroenterol Hepatol 2018;3:790–802. [DOI] [PubMed] [Google Scholar]
- 21. Digby-Bell JL, Atreya R, Monteleone G, Powell N. Interrogating host immunity to predict treatment response in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020;17:9–20. [DOI] [PubMed] [Google Scholar]
- 22. Atreya R, Neurath MF, Siegmund B. Personalizing treatment in IBD: hype or reality in 2020? Can we predict response to anti-TNF? Front Med 2020;7:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sands BE, Peyrin-Biroulet L, Loftus EV Jr, et al. ; VARSITY Study Group. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019;381:1215–26. [DOI] [PubMed] [Google Scholar]
- 24. Singh S, Murad MH, Fumery M, et al. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn’s disease: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2021;6:1002–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shim HH, Chan PW, Chuah SW, Schwender BJ, Kong SC, Ling KL. A review of vedolizumab and ustekinumab for the treatment of inflammatory bowel diseases. JGH Open 2018;2:223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ungaro RC, Yzet C, Bossuyt P, et al. Deep remission at 1 year prevents progression of early Crohn’s disease. Gastroenterology 2020;159:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol 2020;18:2179–91.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laharie D, Bourreille A, Branche J, et al. ; Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 2012;380:1909–15. [DOI] [PubMed] [Google Scholar]
- 29. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999;340:1398–405. [DOI] [PubMed] [Google Scholar]
- 30. De Cruz P, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet 2015;385:1406–17. [DOI] [PubMed] [Google Scholar]
- 31. Bouhnik Y, Carbonnel F, Laharie D, et al. ; GETAID CREOLE Study Group. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut 2018;67:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vavricka SR, Gubler M, Gantenbein C, et al. ; Swiss IBD Cohort Study Group. Anti-TNF treatment for extraintestinal manifestations of inflammatory bowel disease in the Swiss IBD cohort study. Inflamm Bowel Dis 2017;23:1174–81. [DOI] [PubMed] [Google Scholar]
- 33. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol 2009;104:760–7. [DOI] [PubMed] [Google Scholar]
- 34. Beaugerie L, Rahier JF, Kirchgesner J. Predicting, preventing, and managing treatment-related complications in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2020;18:1324–35.e2. [DOI] [PubMed] [Google Scholar]
- 35. Singh S, George J, Boland BS, Vande Casteele N, Sandborn WJ. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: a systematic review and meta-analysis. J Crohns Colitis 2018;12:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. D’Haens GR, Panaccione R, Higgins PD, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol 2011;106:199–212; quiz 213. [DOI] [PubMed] [Google Scholar]
- 37. Roblin X, Williet N, Boschetti G, et al. Addition of azathioprine to the switch of anti-TNF in patients with IBD in clinical relapse with undetectable anti-TNF trough levels and antidrug antibodies: a prospective randomised trial. Gut 2020;69:1206–12. [DOI] [PubMed] [Google Scholar]
- 38. Schmitt H, Billmeier U, Dieterich W, et al. Expansion of IL-23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti-TNF therapy in Crohn’s disease. Gut 2019;68:814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015;41:613–23. [DOI] [PubMed] [Google Scholar]
- 40. Sands BE, Sandborn WJ, Van Assche G, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis 2017;23:97–106. [DOI] [PubMed] [Google Scholar]
- 41. Feagan BG, Rubin DT, Danese S, et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol 2017;15:229–39.e5. [DOI] [PubMed] [Google Scholar]
- 42. Verstockt B, Mertens E, Dreesen E, et al. Influence of drug exposure on vedolizumab-induced endoscopic remission in anti-tumour necrosis factor [TNF] naïve and anti-TNF exposed IBD patients. J Crohns Colitis 2020;14:332–41. [DOI] [PubMed] [Google Scholar]
- 43. Dulai PS, Boland BS, Singh S, et al. Development and validation of a scoring system to predict outcomes of vedolizumab treatment in patients with crohn’s disease. Gastroenterology 2018;155:687–95.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dulai PS, Singh S, Vande Casteele N, et al. Development and validation of clinical scoring tool to predict outcomes of treatment with vedolizumab in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020;18:2952–61.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 46. Sands BE, Sandborn WJ, Panaccione R, et al. ; UNIFI Study Group. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. [DOI] [PubMed] [Google Scholar]
- 47. Schmitt H, Neurath MF, Atreya R. Role of the IL23/IL17 pathway in Crohn’s disease. Front Immunol 2021;12:622934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol 2019;16:185–96. [DOI] [PubMed] [Google Scholar]
- 49. Izcue A, Hue S, Buonocore S, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity 2008;28:559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006;314:1461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fisher SA, Tremelling M, Anderson CA, et al. ; Wellcome Trust Case Control Consortium. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn’s disease. Nat Genet 2008;40:710–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kobayashi T, Okamoto S, Hisamatsu T, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut 2008;57:1682–9. [DOI] [PubMed] [Google Scholar]
- 53. Liu Z, Yadav PK, Xu X, et al. The increased expression of IL-23 in inflammatory bowel disease promotes intraepithelial and lamina propria lymphocyte inflammatory responses and cytotoxicity. J Leukoc Biol 2011;89:597–606. [DOI] [PubMed] [Google Scholar]
- 54. Sands BE, Chen J, Feagan BG, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology 2017;153:77–86.e6. [DOI] [PubMed] [Google Scholar]
- 55. Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology 2020;158:537–49.e10. [DOI] [PubMed] [Google Scholar]
- 56. Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of continued treatment with mirikizumab in a phase 2 trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol 2022;20:105–15.e14 [DOI] [PubMed] [Google Scholar]
- 57. Smillie CS, Biton M, Ordovas-Montanes J, et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 2019;178:714–30.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Steere B, Schmitz J, Powell N, et al. Dop65 mirikizumab regulates genes involved in anti-tnf resistance and ulcerative colitis disease activity. J Crohns Colitis 2020;14:S103–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sandborn WJ, D’Haens GR, Reinisch W, et al. GALAXI-1 Investigators. Guselkumab for the treatment of Crohn’s disease: induction results from the Phase 2 GALAXI-1 study. Gastroenterology 2022:S0016-5085(22)00102-0. [DOI] [PubMed] [Google Scholar]
- 60. Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 2017;389:1699–709. [DOI] [PubMed] [Google Scholar]
- 61. Feagan BG, Panés J, Ferrante M, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol 2018;3:671–80. [DOI] [PubMed] [Google Scholar]
- 62. Ferrante M, Feagan BG, Panés J, et al. Long-term safety and efficacy of risankizumab treatment in patients with crohn’s disease: results from the phase 2 open-label extension study. J Crohns Colitis 2021;15:2001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schreiber SW, Ferrante M, Panaccione R, et al. Risankizumab induces early clinical remission and response in patients with moderate-to-severe crohn’s disease: results from the phase 3 advance and motivate studies. J Crohns Colitis 2021. [Google Scholar]
- 64. Ferrante M, Liao Y, Pang Y, Pivorunas V, Hisamatsu T. Efficacy and safety of risankizumab as maintenance therapy in patients with Crohn’s disease: 52 week results from the phase 3 FORTIFY study. United European Gastroenterol J 2021. [Google Scholar]
- 65. Papamichael K, Rivals-Lerebours O, Billiet T, et al. Long-term outcome of patients with ulcerative colitis and primary non-response to infliximab. J Crohns Colitis 2016;10:1015–23. [DOI] [PubMed] [Google Scholar]
- 66. Biemans VBC, van der Woude CJ, Dijkstra G, et al. ; Dutch Initiative on Crohn and Colitis (ICC). Ustekinumab is associated with superior effectiveness outcomes compared to vedolizumab in Crohn’s disease patients with prior failure to anti-TNF treatment. Aliment Pharmacol Ther 2020;52:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Alric H, Amiot A, Kirchgesner J, et al. The effectiveness of either ustekinumab or vedolizumab in 239 patients with Crohn’s disease refractory to anti-tumour necrosis factor. Aliment Pharmacol Ther 2020;51:948–57. [DOI] [PubMed] [Google Scholar]
- 68. Verdier J, Begue B, Cerf-Bensussan N, Ruemmele FM. Compartmentalized expression of Th1 and Th17 cytokines in pediatric inflammatory bowel diseases. Inflamm Bowel Dis 2012;18:1260–6. [DOI] [PubMed] [Google Scholar]
- 69. Atreya R, Siegmund B. Location is important: differentiation between ileal and colonic Crohn’s disease. Nat Rev Gastroenterol Hepatol 2021;18:544–58. [DOI] [PubMed] [Google Scholar]
- 70. Zorzi F, Monteleone I, Sarra M, et al. Distinct profiles of effector cytokines mark the different phases of Crohn’s disease. PLoS One 2013;8:e54562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Spencer DM, Veldman GM, Banerjee S, Willis J, Levine AD. Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology 2002;122:94–105. [DOI] [PubMed] [Google Scholar]
- 72. Fichtner-Feigl S, Fuss IJ, Young CA, et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol 2007;178:5859–70. [DOI] [PubMed] [Google Scholar]
- 73. Kugathasan S, Saubermann LJ, Smith L, et al. Mucosal T-cell immunoregulation varies in early and late inflammatory bowel disease. Gut 2007;56:1696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pitt JM, Vétizou M, Daillère R, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 2016;44:1255–69. [DOI] [PubMed] [Google Scholar]
- 75. Atreya R, Zimmer M, Bartsch B, et al. Antibodies against tumor necrosis factor (TNF) induce T-cell apoptosis in patients with inflammatory bowel diseases via TNF receptor 2 and intestinal CD14+ macrophages. Gastroenterology 2011;141:2026–38. [DOI] [PubMed] [Google Scholar]
- 76. Schmitt H, Neufert C, Neurath MF, Atreya R. Resolution of Crohn’s disease. Semin Immunopathol 2019;41:737–46. [DOI] [PubMed] [Google Scholar]
- 77. Eftychi C, Schwarzer R, Vlantis K, et al. Temporally distinct functions of the cytokines IL-12 and IL-23 drive chronic colon inflammation in response to intestinal barrier impairment. Immunity 2019;51:367–80.e4. [DOI] [PubMed] [Google Scholar]
- 78. Roberts KJ, Cubitt MF, Carlton TM, et al. Preclinical development of a bispecific TNFα/IL-23 neutralising domain antibody as a novel oral treatment for inflammatory bowel disease. Sci Rep 2021;11:19422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Atreya R, Neumann H, Neufert C, et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med 2014;20:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]