Abstract
The interleukin-23 [IL-23] cytokine, derived predominantly from macrophages and dendritic cells in response to microbial stimulation, has emerged as a critical promoter of chronic intestinal inflammation. Genome-wide association studies linking variants in IL23R to disease protection, bolstered by experimental evidence from colitis models, and the successful application of therapies against the IL-12/IL-23 shared p40 subunit in the treatment of inflammatory bowel disease [IBD] all provide compelling evidence of a crucial role for IL-23 in disease pathogenesis. Moreover, targeting the p19 subunit specific for IL-23 has shown considerable promise in recent phase 2 studies in IBD. The relative importance of the diverse immunological pathways downstream of IL-23 in propagating mucosal inflammation in the gut, however, remains contentious. Here we review current understanding of IL-23 biology and explore its pleiotropic effects on T cells, and innate lymphoid, myeloid and intestinal epithelial cells in the context of the pathogenesis of IBD. We furthermore discuss these pathways in the light of recent evidence from clinical trials and indicate emerging targets amenable to therapeutic intervention and translation into clinical practice.
Keywords: Interleukin-23, Crohn’s disease, ulcerative colitis
1. Introduction
Crohn’s disease [CD] and ulcerative colitis [UC] represent the two main clinical forms of chronic inflammatory bowel disease [IBD].1,2 Susceptibility to both conditions is determined by a complex interplay of genetic and environmental factors, which centre on an aberrant immune response to microbiota in the gastrointestinal tract.2 Emerging evidence from a plethora of different functional and genetic studies clearly indicates both unique and shared pathogenetic mechanisms of critical importance in the genesis and propagation of intestinal inflammation in the two conditions. CD is strongly linked to perturbed recognition and handling of intestinal microbiota by the innate immune system,3–7 and a state of impaired bacterial clearance.7 Furthermore, a critical role for autophagy, a fundamental catabolic pathway enabling the targeting of cytoplasmic cargo [including organelles and invasive bacteria] to lysosomal compartments for degradation,8 is now well established in CD.9,10 Autophagy is closely connected with the endoplasmic reticulum [ER] stress response [unfolded protein response, UPR] in highly secretory cells such as Paneth cells localized to the small intestine, with impaired autophagy and non-resolved ER stress coercing to trigger the development of severe spontaneous transmural ileitis.11 Specific deletion of Atg16l1 in intestinal epithelial cells [IECs] in mice, one of the key autophagy genes associated with CD susceptibility,12 and homozygosity for the ATG16L1 risk variant T300A in humans, results in an exaggerated UPR favouring inflammation and cell death, characterized by increased expression of the ER stress sensor IRE1α in intestinal epithelial crypts as a critical promoter of ileal inflammation.13 Enhanced ER stress is itself a unifying feature of CD and UC epithelium and can be both environmentally and genetically determined, for example by deletion of the UPR transcription factor XBP1.14
Indeed, there appears to be considerable mechanistic overlap in the pathogenesis of CD and UC, consistent with the high number of concordant genetic susceptibility loci between the two phenotypes,15 and the efficacy of therapies such as tumour necrosis factor [TNF] blockade in both conditions.16,17 A crucial area of shared importance in the pathogenesis of both UC and CD relates to the role of the pro-inflammatory interleukin-23 [IL-23] cytokine. Large genome-wide association studies [GWAS] have identified variants in the IL23R gene,18 encoding the specific receptor for IL-23, and which modify susceptibility to both CD and UC, in common with a number of other autoimmune diseases such as psoriasis and ankylosing spondylitis,19,20 pointing to a pivotal role for IL-23 in the pathogenesis of these conditions. First discovered over 20 years ago,21 a wealth of evidence now implicates wide-ranging and diverse effects of IL-23 with broad-reaching implications for our understanding of disease pathogenesis and therapeutics. Indeed, clinical therapies antagonizing IL-23 and the closely related cytokine IL-12 are now approved and in mainstream use for the treatment of IBD.22 In this review, we discuss current understanding of fundamental IL-23 biology, explore its pleiotropic effects and the downstream effector pathways contributing to the development of intestinal inflammation, and discuss current and new potential avenues for therapies targeting IL-23 in the treatment of IBD.
2. IL-23: A Pleiotropic Cytokine with Distinct Pro-Inflammatory Effects
IL-23 is a heterodimeric, pro-inflammatory cytokine at the interface of innate and adaptive immunity. It was first reported by Oppmann et al. in 2000, who discovered a p19 protein that formed a covalently linked complex with the IL-12p40 subunit, giving rise to the biologically active IL-23 protein.21 This interaction distinguishes the molecule from the related cytokine IL-12, in which the shared p40 subunit is covalently linked to a distinct p35 subunit via disulphide bonds to form the prototypical heterodimeric cytokine classically associated with promotion of CD4+ Th1 and CD8+ cytotoxic responses.22 The IL-12 cytokine family also encompasses the Th1-promoting IL-27 and anti-inflammatory IL-35 cytokines, formed by the EBI3/p28 and EBI3/p35 heterodimers respectively.22,23
IL-23 exerts its biological effects through engagement with a specific receptor complex highly expressed on the surface of activated CD4+ T cells,21 group 3 innate lymphoid cells [ILCs],24 and γδ T cells,25 and also at lower levels on intestinal epithelial cells,26 monocytes and macrophages,27 neutrophils,28 natural killer [NK] cells and invariant NKT cells.29 The IL-23R chain forms a heterodimeric complex with IL-12Rβ1 to form the IL-23 receptor.30 The p19 subunit of the IL-23 ligand establishes a critical interaction with the N-terminal immunoglobulin domain of the IL-23R,31,32 triggering conformational changes in IL-23 with the p40 subunit concurrently binding to IL-12Rβ1, thus bridging the IL-23R and IL-12Rβ1 subunits.32 Upon ligand engagement with IL-23R, phosphorylation of Janus kinase 2 [JAK2, a protein constitutively associated with the IL-23R] ensues in parallel with tyrosine kinase 2 [TYK2] and the IL-23R itself, preferentially resulting in phosphorylation, homodimerization and activation of the STAT3 [signal transducer and activator of transcription 3] transcription factor [Figure 1], and to a lesser degree the STAT1, STAT4 and STAT5 transcription factors.30 By contrast, IL-12R signalling, which also requires activation of JAK2 and TYK2, predominantly activates STAT4,33 mechanistically accounting for the distinct biological effects of the two cytokines.
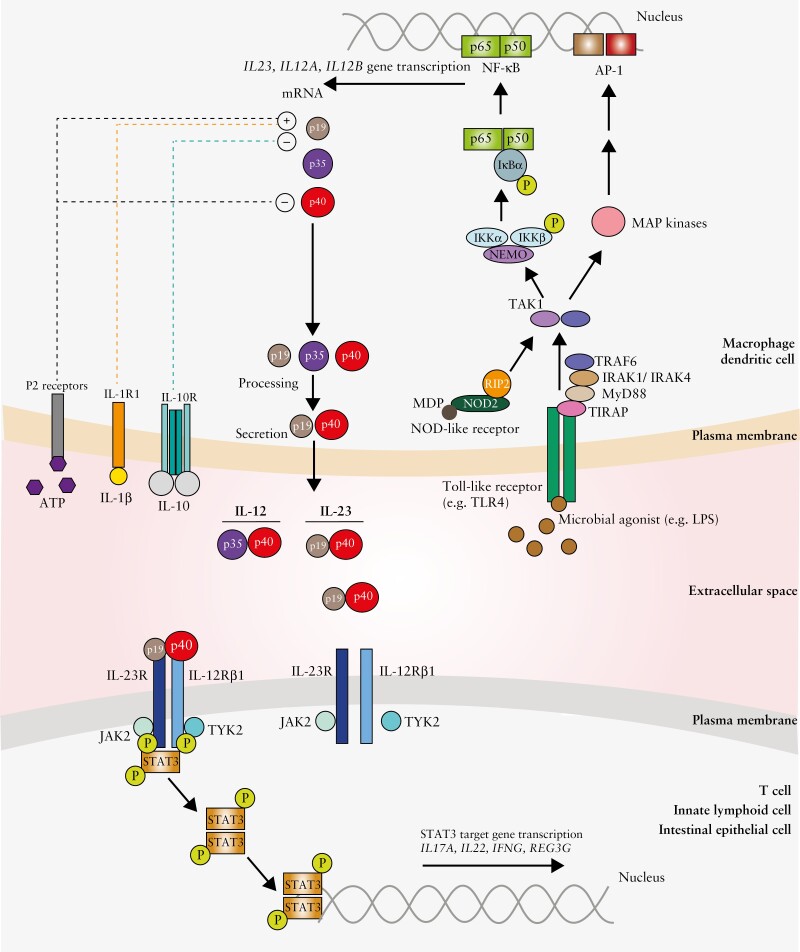
Figure 1.
Generation of the pro-inflammatory cytokine IL-23 and its downstream signalling via the IL-23 receptor.
Schematic depicts the production of IL-23 by macrophages and dendritic cells and its effects via the IL-23 receptor. In response to stimulation of Toll-like receptors and NOD-like receptors by microbial agonists, activation of intracellular signalling cascades ensues, resulting in activation of transcription factors such as NF-κB and AP-1. This enables cytokine gene transcription, including IL23A, IL12A and IL12B, which encode the p19, p35 and p40 subunits of the IL-12 and IL-23 cytokines respectively. Further critical regulatory signals modulating IL-23 release include cytokines such as IL-1β and IL-10 and adenosine nucleotides such as ATP, signalling via specific cell surface receptors. IL-23, a heterodimeric protein comprising the p19 and shared p40 subunit with IL-12, binds its cognate receptor expressed on the surface of cells such as T lymphocytes, innate lymphoid cells and intestinal epithelial cells. This engagement results in phosphorylation of JAK2 and TYK2 associated with the IL-23R and subsequently phosphorylation, homodimerization and activation of STAT3. This consequently triggers induction of STAT3 target genes such as IL17A, IL22, IFNG and REG3G.
Functionally, IL-23 plays a role in the host response to pathogens. Mice lacking expression of either IL-23p19 or the shared p40 subunit are unable to clear Citrobacter rodentium after oral challenge and succumb to lethal infection.34 Moreover, mice deficient in p40 demonstrate greater susceptibility to Mycobacterium tuberculosis infection and compromised antigen-specific interferon γ [IFNγ] responses, compared to either mice lacking p35 subunit expression or wild-type mice.35 Furthermore, absence of p19 increased mortality and diminished IL-17 responses in a Klebsiella pneumoniae infection model.36 Concordantly, single nucleotide polymorphisms [SNPs] in the locus containing the IL23R gene have been associated with susceptibility to infectious disease such as leprosy,37 and loss of function mutations in the IL12B gene [encoding the shared p40 subunit] linked with mycobacterial disease and disseminated Gram-negative bacterial infections.38
3. Genetic, Functional Studies and Interventional Trials Link IL-23 to Intestinal Inflammation
It is well established that CD and UC have a substantial genetic component, and GWAS have identified genetic variants in the IL23R region associated with CD and UC susceptibility.18,20,39 A relatively uncommon coding variant [rs11209026] resulting in substitution of arginine for glutamine at position 381 [R381Q] is markedly protective against the development of IBD in Caucasian populations.18 The R381Q variant is localized to the cytoplasmic region of the IL-23R protein, close to the binding site of JAK2, and leads to hypomorphic function.40 The presence of glutamine at position 381 decreases stability of the IL-23R protein, thereby diminishing receptor expression at the cell surface and thus STAT3/STAT4 activation in response to IL-23.41 Targeted resequencing of susceptibility loci identified two further protective rare coding variants in the IL23R gene, resulting in amino acid substitutions G149R and V362I, both of which have an allele frequency of <2% in healthy populations and similarly result in loss of function.42,43 Moreover, non-coding variants in intronic regions of the IL23R gene have been associated with IBD susceptibility independently of rs11209026 [R381Q].18
Reinforcing the importance of IL-23 in the pathogenesis of IBD, common variants in regions neighbouring the IL12B gene, which encodes the shared p40 subunit of IL-12 and IL-23, are associated with IBD susceptibility.44 Meta-analysis concordantly identified associated SNPs in regions containing the JAK2, TYK2 and STAT3 genes as shared risk factors for CD and UC.15 As discussed above, the products of these genes are important in intracellular signalling transduction downstream of IL-23. Identifying causal variants and understanding their precise impact on immune cell biology remains a considerable challenge, given that many disease-associated SNPs localize to non-coding genomic regions and exist in linkage disequilibrium with other polymorphisms. Fine mapping of the TYK2 genomic locus with SNP imputation and haplotype inference, using data across five different autoimmune diseases including CD and UC, has identified the probable causal variant in this region as rs3453644345; the protective coding variant results in an amino acid substitution [P1104A] in the TYK2 catalytic kinase domain. Whilst this protective variant indeed diminishes STAT3 phosphorylation in memory T cells upon IL-23 receptor stimulation,45 pleiotropic effects including on type I IFN receptor signalling, in which TYK2 plays a shared role,45,46 should also be noted. This may explain the broad-ranging associations of rs34536443 with other seemingly distinct autoimmune diseases.47,48
Alongside the genetic evidence, experimental data from a plethora of animal models corroborate a critical role for IL-23 in the generation of intestinal inflammation. Increased levels of IL-23p19 in inflamed mucosa are a consistent feature of experimental colitis.49 Mice deficient in p19, but not the p35 subunit of IL-12 [which is notably also shared with IL-35], are protected from the spontaneous enterocolitis that occurs upon genetic deletion of the anti-inflammatory cytokine IL-10.50 In adoptive transfer colitis models, in which naive CD4+ CD45RBhigh T cells are transferred into syngeneic immunodeficient mice,51 IL-23 unequivocally promotes inflammation. The colitis induced following adoptive T cell transfer was markedly diminished in recipient mice lacking expression of the shared p40 or IL-23p19 subunits, but not the p35 subunit.49 Genetic absence of the IL-23R in the adoptively transferred CD4+ CD45RBhigh T cells diminished their colitogenic potential, corroborating the importance of IL-23.52 Furthermore, anti-IL-23p19 treatment also prevented the development of colitis in SCID mice upon transfer of caecal bacterial antigen-specific CD4+ T cells, and furthermore ameliorated established colitis in this model.53 Contrastingly, in TNBS-induced colitis, deficiency of IL-23p19 surprisingly enhanced the severity of colitis.54 This may relate to enhanced IL-12 production by dendritic cells [DCs] due to loss of counter-regulation when IL-23p19 is absent, as neutralization of p40 improved features of intestinal inflammation in this context.54
IL-23 is essential in murine colitis models dependent on the innate immune system. Colitis triggered by the pathogenic bacterium Helicobacter hepaticus in immunodeficient mice was attenuated by treatment with an anti-IL-23p19 antibody.49 TRUC mice [T-bet−/− ×Rag2−/−], which lack expression of the T-box transcription factor T-bet as well as mature T and B lymphocytes, develop a spontaneous distal continuous colitis strikingly akin to human UC,55 which is dependent on innate lymphoid cells [discussed below] and the intestinal microbiota.55,56 Anti-IL-23p19 administration dramatically improved spontaneous colitis in the TRUC model compared to isotype controls.56 Treatment of T and B cell-deficient Rag1−/− mice with a stimulatory anti-CD40 antibody triggers a distinctive innate immune-dependent colitis and a systemic wasting disease associated with elevated serum pro-inflammatory cytokine levels.57 The mucosal inflammation observed is dependent on IL-23 production, which is upregulated in colonic DCs and monocytes upon CD40 stimulation, as co-treatment with a neutralizing anti-IL-23p19 antibody dramatically abrogated the development of colitis.57 Intriguingly, features of systemic disease observed in this model were dependent on IL-12 rather than IL-23, highlighting important mechanistic differences in the generation of localized mucosal vs systemic inflammatory responses.57
The effective translation of therapies directed against IL-23 into clinical practice moreover substantiates the detrimental role played by this cytokine in IBD pathogenesis. Biological therapies targeting the shared p40 subunit of IL-12 and IL-23 were initially evaluated in CD, stemming from the classical paradigm of CD as a Th1-associated autoimmune disease driven by IL-12 production by DCs.22 The first phase 2 study of a targeted monoclonal anti-p40 treatment [ABT-874] in active CD indicated a significantly improved rate of clinical response compared to placebo following 7 weeks of treatment, which was associated with a reduction in levels of IL-12, TNFα and IFNγ released by stimulated lamina propria mononuclear cells isolated from colonic biopsies.58 Subsequently, clear-cut evidence of the efficacy of p40 blockade in CD, in the form of the fully humanized IgG1 monoclonal ustekinumab, was established in the phase 3 UNITI studies.59 A significantly enhanced rate of clinical response, based on Crohn’s disease activity index [CDAI] score, was observed in ustekinumab-treated groups compared to placebo, regardless of preceding anti-TNF [UNITI-1 cohort] or conventional immunomodulatory therapy [UNITI-2 cohort] failure. The higher clinical response rate corresponded to a more pronounced reduction in faecal calprotectin and serum C-reactive protein levels,59 as well as improved endoscopic activity at 8 weeks in a sub-study of participants receiving ustekinumab compared to placebo.60 Furthermore, in the maintenance IM-UNTI study, in which ustekinumab responders were continued on ustekinumab or placebo treatment, 53.1 and 48.8% of patients achieved clinical remission in the 8 and 12 weekly treatment groups respectively vs 35.9% of the placebo arm after 44 weeks of treatment.59 Concordantly, inhibition of the p40 subunit is also an effective therapeutic strategy in UC. The phase 3 UNIFI study recently demonstrated the efficacy of ustekinumab in the induction and maintenance of remission in a cohort of patients with moderate-to-severely active disease.61
Given that ustekinumab targets the shared p40 unit, the unequivocal clinical efficacy observed in the UNITI and UNIFI studies could potentially be explained by either IL-12 or IL-23 inhibition, or a combination of both. Considering the colitogenic role of IL-23 demonstrated in preclinical studies, and the unambiguous genetic evidence tying the IL23R gene with IBD susceptibility, it would be logical to hypothesize that selective p19 blockade would be equally efficacious and potentially advantageous. Brazikumab [formerly named MEDI2070] is a humanized IgG2 monoclonal antibody that binds p19,62 thus offering selective inhibition of IL-23. A phase 2a study of brazikumab in a cohort of patients with active CD refractory to TNF inhibition indeed showed improved rates of clinical response at 8 weeks compared to placebo, although endoscopic outcomes were not assessed.63 Risankizumab is another humanized monoclonal IgG1 developed against the p19 subunit. A multicentre, randomized controlled phase 2 study of patients with active CD indeed revealed superiority of risankizumab over placebo in the induction of clinical remission after 12 weeks of therapy.64 Extended induction treatment further enhanced the clinical response and remission rate after 26 weeks, which was sustained at 52 weeks for the majority of patients in the open label maintenance phase of the study.65
Mirikizumab [LY3074828], a distinct humanized monoclonal IgG4 antibody that neutralizes the p19 subunit, was recently evaluated in phase 2 studies in UC and CD.66,67 In the UC study, patients were assigned to receive 50, 200 or 600 mg mirikizumab or placebo; in former two treatment arms dose escalation based on plasma drug levels was conducted. Although the difference in primary outcome, clinical remission between the high-dose mirikizumab and placebo group at 12 weeks, was not statistically significant, improved clinical response rates were seen in all treatment groups which reached nominal statistical significance compared to the placebo arm.66 In CD, treatment with either 200, 600 or 1000 mg mirikizumab was associated with a higher rate of endoscopic response following 12 weeks of treatment compared to placebo.67 Overall, selective targeting of p19 thus appears to be a promising therapeutic strategy in both CD and UC [details of recent and current phase 2 and 3 studies are summarized in Tables 1 and 2].
Table 1.
Summary of novel therapies targeting IL-23 and the IL-23R signalling pathway in Crohn’s disease. Summary of novel therapies targeting IL-23 and its downstream signalling pathway with details of key phase 2 and phase 3 studies in Crohn’s disease [shaded area indicates phase 2 and 3 studies currently in progress or not yet reported in full]
| Target | Drug | Evidence | Number of included patients | Sex and mean age of included patients | Key primary and secondary outcomes [or anticipated outcomes] | Reference or Clinicaltrials.gov identifier |
|---|---|---|---|---|---|---|
| Shared IL-12/IL-23 p40 subunit | Briakinumab [ABT-874] |
Phase 2 study |
n = 79 Cohort 1 n = 40 Cohort 2 n = 39 |
Cohort 1: M/F n = 12/28 Mean age: 36.9 years [placebo] 43.1 years [1 mg/kg group] 42.5 years [3 mg/kg group] Cohort 2 M/F n = 14/25 Mean age: 41.0 years [placebo] 39.9 years [1 mg/kg group] 39.8 years [3 mg/kg group] |
Primary: safety evaluation of use in Crohn’s disease. Secondary: superior clinical response [fall in CDAI score of >100 at week 7] vs placebo. |
Mannon et al. N Engl J Med 2004;351[20]:2069–79 |
| Ustekinumab | Phase 3 randomized placebo-controlled trials [UNITI-1, UNITI-2 and IM-UNITI studies] |
UNITI-1: n = 741 [Failed anti-TNF] UNITI-2: n = 628 [Failed conventional therapy] IM-UNITI: n = 397 |
UNITI-1: M/F n = 317/424 Mean age: 37.3 years [placebo] 37.4 years [130 mg group] 37.3 years [6 mg/kg group] UNITI-2: M/F n = 293/335 Mean age: 40.2 years [placebo] 39.1 years [130 mg group] 38.4 years [6 mg/kg group] IM-UNITI: M/F 173/224 39.5 years [placebo] 37.9 years [12 weekly group] 38.6 [8 weekly group] |
UNITI-1: Significantly enhanced clinical response [fall in CDAI score of >100, or CDAI <150] at week 6 vs placebo. Response rate: 34.3% [130 mg group], 33.7% [6 mg/kg group] vs 21.5% [placebo]. UNITI-2: Significantly improved clinical response rate at week 6 vs placebo. Response rate: 51.7% [130 mg group], 55.5% [6 mg/kg group] vs 28.7% [placebo]. IM-UNITI: Significantly improved rate of clinical remission [CDAI < 150] at week 44 of treatment vs placebo. Remission rate: 53.1% [8 weekly group], 48.8% [12 weekly group] vs 35.9% [placebo group]. |
Feagan et al. N Engl J Med 2016;375[20]:1946–60 | |
| IL-23 p19 subunit | Brazikumab [MEDI2070] | Phase 2a randomized placebo-controlled trial | n = 119 | M/F n = 45/74 Mean age: 38.1 years [placebo] 34.9 years [MEDI2070] |
Significantly improved clinical response rate [reduction in CDAI score of at least 100, or CDAI <150] after 8 weeks of treatment [49.2%] compared to placebo [26.7%]. | Sands et al. Gastroenterology 2017;153[1]:77–86.e6. |
| Risankizumab | Phase 2 randomized placebo-controlled trial | n = 121 | M/F n = 47/74 Mean age: 36 years [placebo] 39 years [pooled risankizumab groups] |
Significantly higher rate of clinical remission [CDAI < 150] in 200 mg and 600 mg pooled treatment groups [30.5%] after 12 weeks of treatment vs placebo [15.4%]. | Feagan et al. Lancet 2017;389:1699–709. | |
| Mirikizumab | Phase 2 randomized placebo-controlled trial [SERENITY] | n = 191 | M/F n = 93/98 Mean age: 39.0 years [placebo] 38.1 years [200 mg group] 40.4 years [600 mg group] 37.7 years [1000 mg group] |
Higher percentage of participants achieving at least a 50% decrease in simple endoscopic activity score-Crohn’s disease [SES-CD] in all treatment groups compared to placebo [reaching pre-specified significance threshold of 0.1]. | Sands et al. Gastroenterology 2022;162:495–508. | |
| IL-23 receptor | JNJ-67864238 | Phase 2 randomized placebo-controlled trial [PRISM] | n = 48 | Difference in CDAI after 12 weeks of treatment compared to baseline. Study terminated early as futility criteria met. |
NCT04102111 | |
| IL-23 p19 subunit | Risankizumab | Phase 3 randomized placebo-controlled trials [MOTIVATE and ADVANCE] |
n = 931 [NCT03105128] n = 618 [NCT03104413] |
Percentage with moderate–severely active disease achieving clinical remission at week 12 [CDAI]; and proportion achieving endoscopic response. |
NCT03105128 NCT03104413 |
|
| Guselkumab | Phase 2/3 randomized placebo and active controlled trial [GALAXI] | Estimated n ~2000 | Difference in CDAI after 12 weeks of treatment compared to baseline [phase 2 study]. Clinical remission [CDAI < 150] and endoscopic response after 12 weeks of treatment [phase 3 study]. |
NCT03466411 | ||
| JAK1/TYK2 | Brepocitinib | Phase 2 randomized placebo-controlled trial [NCT03395184] | Estimated n ~250 | Proportion of patients achieving improvement in endoscopic activity [50% reduction in SES-CD]. | NCT03395184 | |
| TYK2 | Deucravacitinib | Phase 2a randomized placebo-controlled trial [LATTICE-CD] | Estimated n ~240 | Percentage of patients in clinical remission and percentage demonstrating endoscopic response after 12 weeks of treatment. | NCT03599622 |
Table 2.
Summary of novel therapies targeting IL-23 and the IL-23R signalling pathway in ulcerative colitis. Summary of novel therapies targeting IL-23 and its downstream signalling pathway with details of key phase 2 and phase 3 studies in ulcerative colitis [shaded area indicates phase 2 and 3 studies currently in progress or not yet reported in full]
| Target | Drug | Evidence | Number of included patients | Sex and age range of included patients | Summary of primary outcomes, or anticipated primary outcomes if not yet reported | Reference or Clinicaltrials.gov identifier |
|---|---|---|---|---|---|---|
| Shared IL-12/IL-23 p40 subunit | Ustekinumab | Phase 3 randomized placebo-controlled trials [UNIFI study]. |
n = 961 [induction study] n = 523 [maintenance study] |
Induction trial: M/F n = 582/379 Mean age: 41.2 years [placebo] 42.2 years [130 mg group] 41.7 years [6 mg/kg group] Maintenance trial: M/F n = 297/226 Mean age: 42.0 years [placebo] 40.7 years [12 weekly group] 39.5 years [8 weekly group] |
Induction trial: clinical remission significantly higher after 8 weeks of treatment [15.6%, 130 mg group and 15.5%, 6 mg/kg group] compared to placebo [5.3%]. Maintenance trial: significantly enhanced rate of clinical remission after 44 weeks [in 8 and 12-weekly treatment arms] compared to placebo. |
Sands et al. N Engl J Med 2019;381:1201–14 |
| IL-23 p19 subunit | Mirikizumab | Phase 2 randomized placebo-controlled trial | n = 249 | M/F n = 149/100 Mean age: 42.6 years [placebo] 41.8 years [50mg] 43.4 years [200 mg] 42.4 years [600 mg] |
Clinical remission after 12 weeks of treatment compared to placebo—difference not statistically significant. | Sandborn et al. Gastroenterology 2020;158:537–49 e10 |
| IL-23 p19 subunit | Mirikizumab | Phase 3 randomized placebo-controlled trial [LUCENT 1, LUCENT 2] | Estimated ~ 1160 participants [NCT03518086] Estimated ~1044 participants [NCT03524092] |
Clinical remission [determined using modified Mayo score] after 12 weeks of treatment [LUCENT 1]. Clinical remission after 40 weeks of treatment [LUCENT 2]. |
NCT03518086 NCT03524092 |
|
| Risankizumab | Phase 3 randomized placebo-controlled study | Estimated ~942 participants | Percentage of participants achieving clinical remission at week 52 [determined using adapted Mayo score]. | NCT03398135 | ||
| Guselkumab | Phase 2b/3 randomized placebo-controlled trial [QUASAR] | Estimated ~1000 participants | Clinical response [induction study 1] after 12 weeks of treatment Clinical remission [induction study 2]. Clinical remission after 44 weeks of treatment [maintenance study]. |
NCT04033445 | ||
| Brazikumab | Phase 2 study | Estimated ~256 participants | Clinical remission [defined by modified Mayo score] after 10 weeks of treatment. | NCT03616821 | ||
| JAK1/TYK2 | Brepocitinib | Phase 2b randomized, placebo-controlled study | Estimated ~319 participants | Total Mayo score of participants after 8 weeks of treatment. | NCT02958865 | |
| TYK2 | Deucravacitinib | Phase 2 randomized, placebo-controlled trial | Estimated ~131 participants | Clinical remission according to modified Mayo score following 12 weeks of treatment. | NCT03934216 |
It could also be envisaged that targeting IL-23R directly, or its downstream signalling molecules such as JAK2 and TYK2, might be correspondingly efficacious. Tofacitinib, an orally active pan-JAK inhibitor with weaker inhibitory effects on TYK2, has demonstrated efficacy in the induction and maintenance of remission in UC in phase 3 studies compared to placebo68 and is now approved for use in UC. Originally developed as a JAK3 inhibitor, tofacitinib has been noted to exert suppressive effects on Th17 cell differentiation in vitro, and to diminish IL-23R expression, alongside other broad-ranging immunomodulatory effects.69 Further more selective small molecules targeting TYK2 are presently under evaluation in both CD and UC, several of which have concurrent inhibitory effects on Janus kinases.70 Brepocitinib [PF-06700841], an orally administered dual inhibitor of JAK1 and TYK2, potently inhibits STAT3 activation in response to IFNα, IL-23 and IL-27 stimulation of whole blood, and furthermore diminishes STAT4 activation in response to IL-12.71 This compound is currently under investigation in phase 2 studies of moderate to severe CD [NCT03395184] and UC [NCT02958865], having been generally well tolerated and showing clinical benefit in plaque psoriasis.72 Deucravacitinib [BMS-986165] is a novel allosteric inhibitor selective for TYK2, which binds the pseudokinase protein domain and thereby stabilizes autoinhibitory interactions with the neighbouring functional catalytic site.73,74 Biologically, deucravacitinib dampens STAT3 phosphorylation in CD161+ CD3+ Th17 cells in response to IL-23 stimulation, together with functional effects on B cells and monocyte differentiation attributed to concurrent blockade of type I IFN signalling.75In vivo, deucravacitinib protected SCID mice from excessive weight loss in both anti-CD40-induced colitis and following adoptive transfer of CD4+CD45RBhigh T cells; histological analysis of colonic tissue demonstrated a favourable effect of deucravacitinib equivalent to that of anti-p40 treatment in both colitis models.75 Deucravacitinib has now entered phase 2 studies in CD [LATTICE-CD, NCT03599622] and UC [NCT04613518] [see also Tables 1 and 2]. Pharmacologically targeting the IL-23R itself has not yet successfully materialized in IBD; a phase 2 study of JNJ-67864238, a locally acting oral peptide antagonizing IL-23R, was recently terminated after meeting criteria for futility [NCT04102111]. It will be interesting to determine whether the efficacy of inhibitors targeting IL-23R signalling is influenced by the presence of protective or disease-associated variants in IL23R and related genes.
4. Macrophages and DCs Release IL-23 Upon Microbial Stimulation
Macrophages and antigen presenting cells are the principal cellular sources of IL-23.21 In healthy non-inflamed mucosa, expression of IL-23 is greatest in the terminal ileum; lamina propria CD11c+ DCs in this region demonstrate constitutive expression of the shared p40 subunit in the presence of intestinal microbiota.76 IL-23p19 expression is increased in inflamed mucosa from patients with both UC and CD, derived predominantly from CD68+ macrophages and DCs.77 In the inflamed colon, tissue-infiltrating neutrophils also appear to contribute to IL-23 production.78 In CD, IL-23p19 mRNA levels are positively correlated with the severity of macroscopic lesions identified at endoscopy.79 Lamina propria CD14+ macrophages expressing IL-23p19 were also noted to be more frequent in CD patients with disease refractory to TNF antagonism, which was associated with accumulation of TNFR2+ IL-23R+ CD4+ T cells resistant to apoptosis in the intestine.80
DCs and macrophages upregulate IL-23 production upon encountering microbial stimuli. In the intestine, lamina propria CD14+ CD33+ macrophages have a particularly high propensity for IL-23 secretion in response to commensal bacteria such as Escherichia coli and Enterococcus faecalis, the abundance of which is increased in CD patients compared to controls.81,82 Stimulation of macrophages and DCs with antigens such as Toll-like receptor [TLR] agonists initiates intracellular signalling cascades culminating in increased IL23A gene transcription and protein secretion. Interestingly, myeloid DCs isolated from mesenteric lymph nodes of patients with CD were noted to secrete quantitatively higher levels of the shared p40 subunit and IL-23p19 in response to Enterococcus faecalis extract compared to patients with UC.83
The potency of IL-23 induction by macrophages and DCs in response to antigenic stimulation is determined by a complex interplay of factors, some intrinsic to the cell and others related to the surrounding environmental milieu. First, the nature of the microbial stimulus and specific pattern recognition receptors engaged critically determines IL-23 production relative to other cytokines such as IL-12,84 bacterial peptidoglycan stimulation for example preferentially inducing IL-23p19 over IL-12p35 in vitro,85,86 whereas the TLR4 agonist lipopolysaccharide [LPS] demonstrates capacity for both IL-12 and IL-23 upregulation, especially in the presence of IFNγ.87,88 Stimulation of the cytosolic nucleotide-binding oligomerization domain-containing protein 2 [NOD2] receptor, the protein encoded by the earliest identified CD susceptibility gene,3,4 augments IL-23 production by DCs and IL-17 production in DC and CD4+ T cell co-cultures. DCs expressing hypomorphic NOD2 variants were initially shown to have diminished capacity for IL-23 production, with loss of NOD2 in DCs leading to reduced IL-17 induction by CD4+ memory T cells.86 Somewhat paradoxically, subsequent work using activated DCs showed that NOD2 variants linked to CD also associate with decreased expression of miR-29, a microRNA capable of indirectly downregulating IL-23p19 via effects on ATF2.89 miR-29 expression in DCs itself reportedly diminished IL-23p19 production and Th17 responses in co-culture experiments. Thus, the presence of NOD2 disease-associated variants is predicted to enhance IL-23 responses, and indeed these variants were noted to augment p40 secretion from DCs upon adherent invasive Escherichia coli stimulation.89 Nonetheless, these studies clearly highlight the importance of microbe and innate immune interactions, influenced by host genetic factors, in the initial generation and propagation of the IL-23 response.
The IL-23 response is further shaped by a complex network of pro- and anti-inflammatory cytokines and other biologically active molecules. In particular, recent work identified release of IL-10 by a specific population of peripheral blood mononuclear cells [PBMCs] as an important paracrine regulator of IL-23p19 production by distinct CD14+ mononuclear cells.90 The inhibitory effect of IL-10 on pro-inflammatory cytokine production was abrogated in patient cells harbouring IL10R mutations linked to IBD, whereas IL-1R1 blockade was effective in dampening IL-23p19 expression.90 Thus, IL-10 and IL-1β are key negative and positive regulators of IL-23 responses, respectively. This raises the possibility that IL-1 receptor antagonism, currently under investigation in acute severe UC,91 might be effective in part by inhibiting the IL-23 response in individuals with IL10R mutations, or in those with a state of induced ‘functional resistance’ in IL-10R signalling occurring upon bacterial exposure proposed by the authors.90
Beyond cytokines, small molecules such as extracellular adenosine nucleotides, known to accumulate at sites of inflammation and be released under conditions of cell stress,92 enhance IL-23p19 induction by DCs in response to Escherichia coli via P2 purinergic receptor signalling whilst reciprocally suppressing IL-12 responses.93 Intracellular metabolism within DCs and macrophages appears to provide a further level of regulation in the IL-23 response. Incubation of murine bone-marrow-derived DCs with palmitic acid, a saturated fatty acid, enhanced expression of IL-23p19 following TLR stimulation.94 Mechanistically, palmitic acid was reported to inhibit the glycolytic enzyme hexokinase, leading to diminished glycolytic flux, elevated mitochondrial reactive oxygen species production and activation of the UPR to trigger IL-23 transcription.94 Although experimental colitis was not investigated in that study, mice fed a high-fat diet did demonstrate exacerbated psoriasis-like inflammation in response to topical imiquimod application, with an enhanced number of IL-23+ cDC1s present in regional lymph nodes in a manner dependent on DC expression of XBP1.94 Exogenous dietary components may therefore directly shape metabolic rewiring of immune cells to control IL-23 generation and autoimmunity. Of further note, several of the IBD-associated genes encode proteins crucially involved in metabolism.95,96 This includes FAMIN, an evolutionary conserved multifunctional enzyme that through its core catalytic activities enables a purine nucleotide cycle in macrophages.97 It would therefore be interesting to explore whether additional levels of immunometabolic regulation on the IL-23 response might exist, and how these are influenced by host genetic factors. In all, modulation of the IL-23 response by myeloid cells might therefore be accomplished through therapeutic targeting of regulatory cytokines and small molecules produced by the host, components of diet, the intestinal microbiome and its associated rich array of metabolites, and the downstream intracellular signalling pathways that they regulate.
5. Effectors of the IL-23 Response
5.1. The IL-23–Th17 axis
A major focus of the pro-inflammatory effects of IL-23 has centred on its impact on Th17 cells. Th17 cells are a subset of CD4+ T helper cells first identified in 2005,98 characterized by expression of the retinoic acid-related orphan receptor γt [RORγt] transcription factor and their signature cytokine IL-17 [also known as IL-17A], alongside others including IL-17F, IL-22 and TNFα. These cells are important in the host response to extracellular bacterial and fungal infections, although conversely play a pathogenic role in various autoimmune diseases.99 Th17 cells are present in the intestinal lamina propria under conditions of homeostasis and constitutively produce IL-17A.100 A significant increase in Th17 cells is observed in inflamed CD and UC mucosa compared to controls.101 Animal studies have revealed the importance of intestinal microbiota composition in Th17 cell induction within the intestine, and interestingly the colonization of mice with specific Gram-positive commensals known as segmental filamentous bacteria appears sufficient for Th17 cell induction.102
IL-23 does not appear to induce Th17 differentiation or IL-17A production from naïve CD4+ T cells per se, given that these cells lack significant expression of the IL-23R.21 The differentiation of Th17 cells from naïve CD4+ T cells requires distinct cytokines such as IL-6 and transforming growth factor β [TGFβ], although IL-23 does seem to have a role in their continued survival and expansion.103 Treatment of naïve CD4+ T cells with IL-6 and IL-21 in the presence of low concentrations of TGFβ in vitro suppresses expression of the transcription factor Forkhead box P3 [FoxP3], reciprocally enhancing RORγt,104 and furthermore synergistically upregulates expression of the IL-23R.104,105 This allows enhanced IL-23R signalling, which may further promote IL-17 production [Figures 2 and 3].105 Naïve CD4+ T cells from healthy volunteers heterozygous for the IBD-protective R381Q variant, isolated and differentiated towards a Th17-like phenotype, released significantly less IL-17A in response to IL-23 stimulation compared to those homozygous for the common risk variant.106 Concordantly, memory CD4+ CD45RO+ cells [in which IL-23R expression is upregulated21] from healthy individuals harbouring the R381Q variant also produced significantly lower amounts of IL-17 and IL-22 upon activation and stimulation with IL-23.107 Furthermore, isolated memory CD4+ T cells from individuals homozygous for the protective variant also demonstrated reduced IL-17A and IL-17F production in response to IL-23 in another study, compared to those homozygous for the risk allele.108
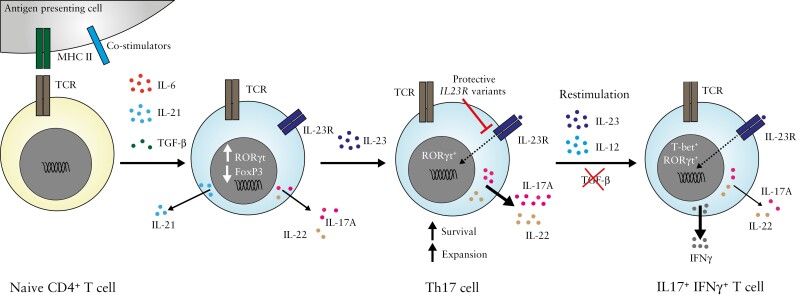
Figure 2.
The IL-23/Th17 axis.
Naïve CD4+ T cells, which lack expression of the IL-23 receptor, polarize towards a Th17 phenotype upon encountering specific signals from antigen presenting cells and cytokines such as IL-6, IL-21 and TGF-β. This results in the reciprocal up- and down-regulation of the signature RORγt and FoxP3 transcription factors respectively, alongside increased IL-23 receptor expression. IL-23 signalling via its cognate receptor promotes the survival and expansion of IL-17 and IL-22-producing Th17 cells, diminished by the presence of variants in IL23R conferring protection against autoimmune disease. Upon restimulation when IL-23/IL-12 levels are high, Th17 cells can morph into a Th1-like phenotype characterized by expression of the transcription factor T-bet and production of the colitogenic cytokine IFN-γ.
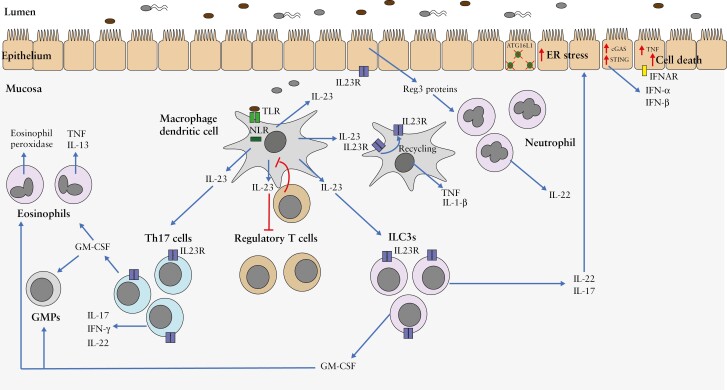
Figure 3.
IL-23 promotes intestinal inflammation through pleiotropic effects on innate and adaptive immune cells.
Figure depicts the diverse downstream immunological effects of IL-23 produced by macrophages and dendritic cells in response to microbial stimulation. IL-23 enhances the induction and survival of Th17 cells, releasing the IL-17, IFNγ, IL-22 and GM-CSF cytokines, the last of these promoting accumulation of granulocyte-monocyte progenitor cells [GMPs] and activated eosinophils in the intestine. IL-23 inhibits the induction of regulatory T cells, and orchestrates the production of cytokines such as IL-17, IL-22 and GM-CSF from group 3 innate lymphoid cells [ILC3s]. Autocrine effects of IL-23 on macrophages including pro-inflammatory cytokine production and IL-23R recycling have also been reported. IL-23 additionally induces the production of neutrophil chemoattractant Reg proteins by intestinal epithelial cells, in turn serving as an additional source of IL-22. IL-22 exerts dichotomous effects in intestinal inflammation, including induction of ER stress in the intestinal epithelium and promotion of excessive activation of the cGAS-STING pathway in the absence of ATG16L1, leading to type I interferon production and cell death.
Based on the above observations, it would be tempting to extrapolate that IL-17, derived from Th17 cells, might be a major pathogenic factor in IBD amenable to therapeutic targeting. Despite clear experimental evidence that IL-17 plays a critical function in the host pathogen response, as well as in the development of autoimmune diseases such as arthritis and psoriasis,109 its role in the development of IBD is more controversial. First, data from animal models of colitis are rather conflicting. Neither inhibition of the IL-17 receptor nor neutralization of IL-17A and IL-17F had any appreciable effect in innate anti-CD40-induced colitis.24 In T cell transfer models of colitis, genetic absence of IL-17A in naïve CD4+ CD45RBhigh T cells did not impair their ability to induce colitis in immunodeficient hosts,110,111 and was even associated with more severe inflammatory disease in one study.112 Genetic absence of IL-17A was also reported to increase the severity of pathology in chemically induced colitis,113 but made no difference or improved disease activity parameters and survival in other studies.114–116 Administration of a neutralizing anti-IL-17 antibody significantly worsened acute dextran sodium sulphate [DSS]-induced colitis in mice.117 The exacerbated disease activity may in part reflect the loss of protective effects of IL-17A in the maintenance of gut epithelial integrity and reducing intestinal permeability.113,114
Following from these studies, clinical targeting of IL-17 has not proven effective in the treatment of CD, despite showing striking benefit in other diseases such as psoriasis.118 Secukinumab, a neutralizing human anti-IL-17A, was ineffective in a proof-of-concept study of moderate to severe CD.119 A further human anti-IL-17 receptor A monoclonal antibody, brodalumab, caused significant deterioration of CD in patients with active disease.120
Could alternative mechanisms thus explain how Th17 cells contribute to disease? Th17 cells co-express cytokines such as GM-CSF and IL-22 [discussed below], which are induced in the presence of IL-23.121,122 Furthermore, Th17 cells themselves demonstrate considerable plasticity and phenotypic heterogeneity.123,124 In the appropriate milieu, they can morph into a phenotype with a closer resemblance to Th1 cells, characterized by IFNγ production.125,126 Restimulation of Th17-polarized CD4+ T cells in the presence of either IL-12 or IL-23 in vitro actually appears to diminish IL-17A and IL-17F and enhance IFNγ expression over time, when levels of TGFβ are low or absent.127In vivo, adoptive transfer of naïve CD4+ CD45RBhigh cells deficient in IL23R expression into Rag1−/− mice reduced the proportion of IL-17A+ IFNγ+ cells in the colon, compared to recipients that received wild-type naive cells, as a consequence of diminished proliferation in the intestine.52 Thus, IFNγ-producing Th17 cells, potentiated by IL-23, could represent a critical pathogenic cell type in IBD. In support of this, transfer of IL-17+ Th17-differentiated precursor cells into Rag1−/− mice results in an accelerated and severe colitis, which is contingent on IFNγ expression by the precursor cells.128 Indeed enrichment of IFNγ+ IL-17A+ CD4+ T cells has been identified in inflamed mucosa of both CD and UC patients compared to non-inflamed controls.80,129 Additional important sources of IFNγ in the inflamed mucosa may include ILCs and NK cells, which are also responsive to IL-23.130 IFNγ is itself well established to promote colitis and its neutralization is protective in experimental models.131 Interestingly, fontolizumab, a humanized monoclonal anti-IFNγ antibody, did indicate possible benefit in moderate to severe CD compared to placebo in a multicentre phase 2 study, albeit not demonstrating any statistically significant difference in the study primary endpoint of clinical remission after 28 days of treatment.132 Inhibition of IFNγ has not to date been explored in UC and this could merit consideration.
5.2. Regulatory T cells
Regulatory CD4+ T cells [Tregs] have an important role in the promotion of tolerance and restraint of immune responses in the intestine.133 These cells develop in the thymus, but they can also be induced from peripheral naïve T cells in the appropriate context.133 Tregs are characterized by expression of the transcription factor Forkhead box P3 [FoxP3], high cell surface CD25, and production of TGFβ and the anti-inflammatory cytokines IL-10 and IL-35.133,134 Experimental models support a suppressive effect of these cells in the development of colitis. In the T cell transfer model, co-transfer of CD4+ CD45RBlow cells, which are enriched for Tregs, prevented colitis.51 Furthermore, adoptive transfer of CD4+ CD25+ Tregs could resolve established colitis in this model.135 IL-23 has been proposed to inhibit the induction of FoxP3+ CD4+ Tregs in the intestine,110 via cell-intrinsic mechanisms [Figure 3].52 Tregs can themselves act to suppress IL-23 release from intestinal macrophages via a mechanism that appears unrelated to IL-10 production, instead being dependent on cell contact and expression of latent activation gene-3 [LAG3], and consequentially inhibiting IL-22 production by ILCs as a downstream effect.136 This highlights the interdependence and connectedness of different pathways involved in the development of intestinal inflammation.
Genetic mutations affecting Treg function, for example in the FOXP3 gene, can cause the immune dysregulation, polyendocrinopathy, enteropathy, X linked syndrome [IPEX], which is associated with the development of colitis.137 However, the overwhelming majority of patients with IBD do not harbour such mutations, nor have genetic studies strongly implicated genes linked specifically to Treg differentiation or function with polygenic IBD susceptibility.1 Furthermore, isolated CD4+ CD25high Treg cells from IBD patients do not display an intrinsic functional defect in their suppressive capacity ex vivo, and FoxP3+ Treg cells accumulate in the inflamed IBD mucosa compared to non-inflamed controls.138 Nonetheless these Tregs are clearly not able to suppress inflammation in the IBD milieu, potentially due to resistance of effector CD4+ T cells to suppression by Tregs,139 which might have therapeutic implications. Cellular therapy using antigen [ovalbumin]-specific Tregs has been attempted in an open label study of 20 patients with CD, although many unanswered questions remain, including optimal dosing, timing and antigen specificity of the cells transferred.140,141 Low-dose IL-2 therapy, which preferentially expands Tregs over effector T cells, has shown promise in a humanized mouse model of colitis,142 and early studies to primarily assess safety and tolerability in UC have recently been completed [NCT02200445].
5.3. Innate lymphoid cells and the IL-23–IL-22 axis
IL-23 has emerged as a critical orchestrator of ILC function. ILCs are a family of innate immune cells derived from a common lymphoid progenitor, with absent RAG-dependent rearranged antigen receptors, and subdivided phenotypically on the basis of surface marker expression and functional characteristics.143,144 ILC1s secrete high levels of IFNγ and thereby enable the host response to intracellular pathogens, and ILC2s produce IL-4, IL-5 and IL-13 upon activation to facilitate alternative macrophage activation and tissue repair and remodelling.145 Contrastingly, ILC3s release high levels of IL-17 and IL-22 in response to IL-23 stimulation,146–148 the latter reportedly dependent on both STAT3149 and STAT5 activation.150 ILCs responsive to IL-23 are increased in inflamed ileum and colon of CD, but not UC, patients, compared to non-inflamed mucosa from control individuals.151 In experimental colitis induced by Helicobacter hepaticus in Rag−/− mice, in which typhlocolitis occurs in an IL-23-dependent fashion,49 depletion of Thy1high SCA-1+ ILCs prevented the development of colitis.152 Furthermore, depletion of the same ILCs attenuated colitis after agonistic anti-CD40 antibody administration, which corresponded to a significant reduction in IL-22, TNFα and IFNγ secretion from isolated colonic lamina propria cells.152
IL-22, a member of the IL-10 family of cytokines, has dichotomous roles in intestinal inflammation. Alongside group 3 RORγt+ ILCs, importantly IL-22 is also produced by Th17 cells, γδ T cells and neutrophilic granulocytes in response to IL-23.25,26,28,153 On the one hand, IL-22 has apparent protective roles in intestinal inflammation. Loss of IL-22 exacerbates DSS-induced colitis, and T cell-mediated colitis in adoptive transfer models.154 Furthermore IL-22 is required for optimal host defence against pathogens; mice lacking IL-22 demonstrate increased bacterial burden, impaired epithelial integrity and decreased survival following Citrobacter rodentium inoculation.155 These effects were attributed to induction of antimicrobial protein expression such as Reg3b and Reg3g, members of the secreted C-type lectin protein family.155 Reg3b is itself a STAT3 target gene and is produced by epithelial cells in response to IL-23, and can serve as a chemoattractant for neutrophils which contribute to IL-22 production in the mucosa.26 IL-22 derived from ILC3s and γδ T cells has also been reported to protect colonic LGR5+ stem cells from malignant transformation, enabling an effective DNA damage response following exposure to carcinogens.156
In spite of these beneficial effects, an emerging body of evidence suggests that pro-inflammatory effects of IL-22 can exacerbate intestinal inflammation. Neutralization of IL-22 was noted to inhibit the development of anti-CD40 antibody-induced colitis in immunodeficient hosts.24 Furthermore, the spontaneous colitis observed in macrophage-restricted IL-10Rα-deficient mice was reported to be contingent on both IL-23 expression by macrophages and IL-22 production, proposed to derive primarily from Th17 cells in this model.157 IL-22 enhances ER stress in epithelial cells,158,159 a process critically intertwined with the development of intestinal inflammation.14 Genetic deficiency and neutralization of IL-22 in the murine TRUC model of IBD dramatically inhibited the development of colitis, which correlated with diminished levels of ER stress in the mucosa.158 Notably, autophagy, and specifically the product of the ATG16L1 gene linked to CD susceptibility, appears to be a crucial regulator of IL-22 signalling and downstream biological responses in the intestinal epithelium.159 Using small intestinal organoids generated from mice lacking ATG16L1 expression in IECs, IL-22 was shown to induce ER stress and type I IFN responses, which were pathologically enhanced in the absence of ATG16L1 as a result of excessive activation of the cGAS-STING pathway.159 Accordingly, exogenous IL-22 administration in vivo exacerbated the spontaneous small intestinal inflammation observed in mice dually deficient in ATG16L1 and XBP1 in IECs [Atg16l1ΔIEC/Xbp1ΔIEC], and furthermore worsened ileal inflammation in Atg16l1ΔIEC mice acutely exposed to low-dose DSS. Inhibition of the IFNα/β receptor ameliorated the ileitis potentiated by IL-22 in the latter, highlighting an important pathogenic role for type I IFN responses in this context.159
Clearly the observations described above have important therapeutic implications. Although early translation has focused on stimulation of IL-22 signalling using recombinant IL-22 fusion proteins160 [NCT02749630], IL-22 antagonism could in fact be beneficial in certain contexts or selected patients. Indeed, serum and mucosal levels of IL-22 appear overall increased in IBD,158,161 and recent transcriptional profiling furthermore identified an IL-22 responsive gene expression signature as significantly upregulated in the inflamed IBD mucosa.158 Enrichment for this IL-22 transcriptional signature positively correlated with markers of intestinal inflammation such as faecal calprotectin levels, albeit not differentiating clinical response to p40 blockade.158 However, it is noteworthy that treatment with p19 neutralizing antibodies reduces serum IL-22 levels.63,64 In addition, patients stratified by higher baseline IL-22 level appeared to demonstrate greater benefit from selective p19 inhibition,63 further hinting at the importance of the IL-23–IL-22 axis in shaping the clinical effectiveness of p40 and selective p19 blockade. Furthermore, targeting downstream effects of IL-22, including pharmacological inhibition of ER stress, shown to efficacious in the TRUC experimental model of IBD,158 as well as antagonism of augmented type I IFN signalling, could equally represent very promising therapeutic targets in patients with underlying dysfunction of the IL-23–IL-22 axis.
5.4. Myeloid cells and autocrine regulation
Infiltration of the intestinal mucosa by neutrophils is a key feature of experimental colitis and inflamed human IBD lesions, particularly UC.162 Although neutrophils exert beneficial functions in the clearance of pathogens and resolution of acute inflammation, and indeed monogenic disorders causing defective neutrophil function are associated with the development of CD-like enteritis,137,163 neutrophils may perpetuate established intestinal inflammation through the production of pro-inflammatory cytokines, chemokines and matrix metalloproteases.162,164,165 In acute infection and inflammation, IL-23 promotes neutrophil recruitment via both IL-17A-dependent and IL-17A-independent mechanisms dependent on context.166,167 IL-23 appears to indirectly promote granulopoiesis,168 and in homeostasis, efferocytosis of apoptotic neutrophils by DCs may serve to regulate neutrophil production by dampening of IL-23 production as part of a negative feedback loop.169 In experimental T cell transfer colitis, IL-23 potentiates GM-CSF production by Th17 cells, which in turn enhances extramedullary haematopoiesis and the accumulation of proliferative granulocyte–monocyte progenitor cells in the colon and spleen.165 Furthermore, GM-CSF enhances the production and activation of eosinophils, which have also been proposed to contribute to tissue damage in colitis through activity of eosinophil peroxidase. As chemical inhibition of eosinophil peroxidase improved murine experimental colitis induced by Helicobacter hepaticus and IL-10R blockade, this might represent an interesting target for future development.170
Alongside their critical role in secreting IL-23, myeloid cells such as macrophages concurrently express the IL-23R on their cell surface at low levels, implying potential autocrine effects. In vivo administration of IL-23, but not IL-12, was recognized to stimulate TNF and IL-1β expression in murine peritoneal macrophages.171 Concordantly, IL-23 stimulates the release of pro-inflammatory cytokines by human monocyte-derived macrophages, coinciding with induction of IL-23R endocytic recycling and recruitment of the IL-12Rβ1, JAK2 and other signalling intermediates to the IL-23R complex.27 Macrophages cultured from donors expressing the protective R381Q IL-23R variant appeared diminished in their capacity for cytokine induction27 and reactive oxygen and nitrogen species generation,172 and demonstrated reduced IL-23R recycling.27 The extent to which IL-23R recycling in myeloid cells contributes to the development of intestinal inflammation in vivo remains to be fully elucidated, as does whether similar mechanisms might operate in cells with correspondingly high IL-23R expression such ILCs and T cells, in which IL-23 has also been reported to enhance expression of its cognate receptor.80
6. Concluding Remarks
The prominence of IL-23 in the pathogenesis of both CD and UC, alongside other autoimmune diseases, is now unassailable. However, the downstream effector mechanisms by which IL-23 drives the manifestation of chronic IBD are a continued matter of debate and investigation, in particular the relative importance of Th1/Th17 cells, ILC3s and myeloid cells, and the colitogenic cytokines IFNγ, IL-22 and IL-17A. Despite the closely intertwined role of IL-23 in distinct autoimmune diseases, it is likely that there are important tissue-specific differences in determining how IL-23 contributes to disease; IL-17A induction potentially has a more deleterious role in the development of ankylosing spondylitis and psoriasis rather than colitis, highlighted by the clinical effectiveness of IL-17A blockade in these conditions but not in IBD. The contribution of IL-23 to inflammatory disease is modified and shaped by distinct environmental factors such as diet, the host microbiome and additional genetic determinants, evident in controlled experimental murine colitis models.173,174 These will of course be highly heterogeneous between patients.
Therapies targeting the IL-12 and IL-23 shared p40 subunit are established in current clinical practice for IBD, and recent phase 2 studies support the use of specific p19 blockade in CD and UC. Further careful evaluation of these agents continues to be needed in specific patient groups, such as in pregnancy and children with refractory IBD, although recent small-scale studies do indicate potential safety and efficacy of p40 blockade in the latter.175,176 More broadly, whilst evidence from a recent cohort study suggests superiority of p40 inhibition over vedolizumab in refractory CD,177 randomized controlled trials directly comparing the efficacy of p19 with p40 blockade, and with distinct classes of biologics, will be vital to undertake in the future. Indeed, in psoriasis, specific targeting of p19 with the monoclonal antibody guselkumab was reported to show improved efficacy compared to adalimumab.178 Identification of patients who may benefit from early use of p19 inhibition and related therapies, integrating key biomarkers that may predict responsiveness such as serum IL-22 with applicable genetic data, may also be a powerful therapeutic strategy.
Contributor Information
Gavin W Sewell, Cambridge Institute of Therapeutic Immunology and Infectious Disease, Jeffrey Cheah Biomedical Centre, University of Cambridge, Cambridge, UK; Division of Gastroenterology and Hepatology, Department of Medicine, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Arthur Kaser, Cambridge Institute of Therapeutic Immunology and Infectious Disease, Jeffrey Cheah Biomedical Centre, University of Cambridge, Cambridge, UK; Division of Gastroenterology and Hepatology, Department of Medicine, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Funding
Research in Professor Kaser’s laboratory has been supported by Wellcome Trust senior investigator awards [106260/Z/14/Z and 222497/Z/21/Z to A.K.]. G.W.S is supported by an Academy of Medical Sciences starter grant for clinical lecturers [SGL022\1008]. This paper was published as part of a supplement financially supported by AbbVie.
Conflict of Interest
A.K. has provided scientific consultancy for Applied Molecular Transport, AstraZeneca, Boehringer Ingelheim, Galapagos, Genentech, GlaxoSmithKline, Glenmark, Gilead Sciences, Hospira, Janssen, Kintai Therapeutics, Novartis, Pfizer, Roche and VHSquared. G.W.S. does not have any conflicts of interest to declare.
Author Contributions
G.W.S. and A.K. both drafted and approved the final manuscript.
Data Availability
No new data were analysed or generated in support of this review article.
References
- 1. Uhlig HH, Powrie F. Translating immunology into therapeutic concepts for inflammatory bowel disease. Annu Rev Immunol 2018;36:755–81. [DOI] [PubMed] [Google Scholar]
- 2. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol 2010;28:573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001;411:599–603. [DOI] [PubMed] [Google Scholar]
- 4. Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001;411:603–6. [DOI] [PubMed] [Google Scholar]
- 5. Hisamatsu T, Suzuki M, Reinecker HC, et al. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 2003;124:993–1000. [DOI] [PubMed] [Google Scholar]
- 6. Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial sensor NOD2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity 2014;41:311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith AM, Rahman FZ, Hayee B, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med 2009;206:1883–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008;132:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cadwell K, Liu JY, Brown SL, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008;456:259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saitoh T, Fujita N, Jang MH, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008;456:264–8. [DOI] [PubMed] [Google Scholar]
- 11. Adolph TE, Tomczak MF, Niederreiter L, et al. Paneth cells as a site of origin for intestinal inflammation. Nature 2013;503:272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hampe J, Franke A, Rosenstiel P, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in Atg16L1. Nat Genet 2007;39:207–11. [DOI] [PubMed] [Google Scholar]
- 13. Tschurtschenthaler M, Adolph TE, Ashcroft JW, et al. Defective Atg16L1-mediated removal of IRE1alpha drives Crohn’s disease-like ileitis. J Exp Med 2017;214:401–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaser A, Lee AH, Franke A, et al. Xbp1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008;134:743–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jostins L, Ripke S, Weersma RK, et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 17. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65 e1-3. [DOI] [PubMed] [Google Scholar]
- 18. Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006;314:1461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Capon F, Di Meglio P, Szaub J, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet 2007;122:201–6. [DOI] [PubMed] [Google Scholar]
- 20. Wellcome Trust Case Control C. Association scan of 14,500 nsSNPs in four common diseases identifies variants involved in autoimmunity. Nat Genet 2007;39:1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000;13:715–25. [DOI] [PubMed] [Google Scholar]
- 22. Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol 2019;16:185–96. [DOI] [PubMed] [Google Scholar]
- 23. Neurath MF. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev 2019;45:1–8. [DOI] [PubMed] [Google Scholar]
- 24. Eken A, Singh AK, Treuting PM, Oukka M. IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol 2014;7:143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sutton CE, Lalor SJ, Sweeney CM, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009;31:331–41. [DOI] [PubMed] [Google Scholar]
- 26. Aden K, Rehman A, Falk-Paulsen M, et al. Epithelial IL-23r signaling licenses protective IL-22 responses in intestinal inflammation. Cell Rep 2016;16:2208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun R, Hedl M, Abraham C. IL23 induces IL23R recycling and amplifies innate receptor-induced signalling and cytokines in human macrophages, and the IBD-protective IL23R R381Q variant modulates these outcomes. Gut 2020;69:264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zindl CL, Lai JF, Lee YK, et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci U S A 2013;110:12768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rachitskaya AV, Hansen AM, Horai R, et al. Cutting edge: Nkt cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol 2008;180:5167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parham C, Chirica M, Timans J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol 2002;168:5699–708. [DOI] [PubMed] [Google Scholar]
- 31. Bloch Y, Bouchareychas L, Merceron R, et al. Structural activation of pro-inflammatory human cytokine IL-23 by cognate IL-23 receptor enables recruitment of the shared receptor IL-12Rbeta1. Immunity 2018;48:45–58 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glassman CR, Mathiharan YK, Jude KM, et al. Structural basis for IL-12 and IL-23 receptor sharing reveals a gateway for shaping actions on T versus NK cells. Cell 2021;184:983–99 e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bacon CM, McVicar DW, Ortaldo JR, et al. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of janus family tyrosine kinases by IL-2 and IL-12. J Exp Med 1995;181:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(h)17 lineage. Nature 2006;441:231–4. [DOI] [PubMed] [Google Scholar]
- 35. Cooper AM, Kipnis A, Turner J, et al. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol 2002;168:1322–7. [DOI] [PubMed] [Google Scholar]
- 36. Happel KI, Dubin PJ, Zheng M, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med 2005;202:761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang F, Liu H, Chen S, et al. Identification of two new loci at IL23R and RAB32 that influence susceptibility to leprosy. Nat Genet 2011;43:1247–51. [DOI] [PubMed] [Google Scholar]
- 38. Altare F, Lammas D, Revy P, et al. Inherited interleukin 12 deficiency in a child with Bacille Calmette-Guerin and Salmonella enteritidis disseminated infection. J Clin Invest 1998;102:2035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fisher SA, Tremelling M, Anderson CA, et al. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn’s disease. Nat Genet 2008;40:710–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pidasheva S, Trifari S, Phillips A, et al. Functional studies on the IBD susceptibility gene IL23R implicate reduced receptor function in the protective genetic variant R381q. PLoS One 2011;6:e25038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sivanesan D, Beauchamp C, Quinou C, et al. Il23R (interleukin 23 receptor) variants protective against inflammatory bowel diseases (IBD) display loss of function due to impaired protein stability and intracellular trafficking. J Biol Chem 2016;291:8673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rivas MA, Beaudoin M, Gardet A, et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet 2011;43:1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Momozawa Y, Mni M, Nakamura K, et al. Resequencing of positional candidates identifies low frequency IL23R coding variants protecting against inflammatory bowel disease. Nat Genet 2011;43:43–7. [DOI] [PubMed] [Google Scholar]
- 44. Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet 2010;42:1118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dendrou CA, Cortes A, Shipman L, et al. Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci Transl Med 2016;8:363ra–149.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell 1992;70:313–22. [DOI] [PubMed] [Google Scholar]
- 47. Cunninghame Graham DS, Morris DL, Bhangale TR, et al. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet 2011;7:e1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mero IL, Lorentzen AR, Ban M, et al. A rare variant of the TYK2 gene is confirmed to be associated with multiple sclerosis. Eur J Hum Genet 2010;18:502–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hue S, Ahern P, Buonocore S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med 2006;203:2473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 2006;116:1310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in c. B-17 SCID mice. Int Immunol 1993;5:1461–71. [DOI] [PubMed] [Google Scholar]
- 52. Ahern PP, Schiering C, Buonocore S, et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 2010;33:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Elson CO, Cong Y, Weaver CT, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology 2007;132:2359–70. [DOI] [PubMed] [Google Scholar]
- 54. Becker C, Dornhoff H, Neufert C, et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol 2006;177:2760–4. [DOI] [PubMed] [Google Scholar]
- 55. Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 2007;131:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Powell N, Walker AW, Stolarczyk E, et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity 2012;37:674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Uhlig HH, McKenzie BS, Hue S, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 2006;25:309–18. [DOI] [PubMed] [Google Scholar]
- 58. Mannon PJ, Fuss IJ, Mayer L, et al. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med 2004;351:2069–79. [DOI] [PubMed] [Google Scholar]
- 59. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 60. Rutgeerts P, Gasink C, Chan D, et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology 2018;155:1045–58. [DOI] [PubMed] [Google Scholar]
- 61. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. [DOI] [PubMed] [Google Scholar]
- 62. Kock K, Pan WJ, Gow JM, et al. Preclinical development of AMG 139, a human antibody specifically targeting IL-23. Br J Pharmacol 2015;172:159–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sands BE, Chen J, Feagan BG, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology 2017;153:77–86 e6. [DOI] [PubMed] [Google Scholar]
- 64. Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 2017;389:1699–709. [DOI] [PubMed] [Google Scholar]
- 65. Feagan BG, Panes J, Ferrante M, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol 2018;3:671–80. [DOI] [PubMed] [Google Scholar]
- 66. Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology 2020;158:537–49 e10. [DOI] [PubMed] [Google Scholar]
- 67. Sands BE, Peyrin-Biroulet L, Kierkus J, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology 2022;162:495–508. [DOI] [PubMed] [Google Scholar]
- 68. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 69. Ghoreschi K, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol 2011;186:4234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Danese S, Peyrin-Biroulet L. Selective tyrosine kinase 2 inhibition for treatment of inflammatory bowel disease: new hope on the rise. Inflamm Bowel Dis 2021;27:2023–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of tyk2 and jak1 for the treatment of autoimmune diseases: discovery of ((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 h-pyrazol-4-yl)amino)pyrimidin-4-yl)-3,8-diazabicyclo[3.2.1]octan-8-yl)methanone (PF-06700841). J Med Chem 2018;61:8597–612. [DOI] [PubMed] [Google Scholar]
- 72. Forman SB, Pariser DM, Poulin Y, et al. TYK2/JAK1 inhibitor PF-06700841 in patients with plaque psoriasis: phase IIa, randomized, double-blind, placebo-controlled trial. J Invest Dermatol 2020;140:2359–70 e5. [DOI] [PubMed] [Google Scholar]
- 73. Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem 2019;62:8973–95. [DOI] [PubMed] [Google Scholar]
- 74. Tokarski JS, Zupa-Fernandez A, Tredup JA, et al. Tyrosine kinase 2-mediated signal transduction in T lymphocytes is blocked by pharmacological stabilization of its pseudokinase domain. J Biol Chem 2015;290:11061–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med 2019;11. [DOI] [PubMed] [Google Scholar]
- 76. Becker C, Wirtz S, Blessing M, et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest 2003;112:693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu Z, Yadav PK, Xu X, et al. The increased expression of IL-23 in inflammatory bowel disease promotes intraepithelial and lamina propria lymphocyte inflammatory responses and cytotoxicity. J Leukoc Biol 2011;89:597–606. [DOI] [PubMed] [Google Scholar]
- 78. Kvedaraite E, Lourda M, Idestrom M, et al. Tissue-infiltrating neutrophils represent the main source of IL-23 in the colon of patients with IBD. Gut 2016;65:1632–41. [DOI] [PubMed] [Google Scholar]
- 79. Schmidt C, Giese T, Ludwig B, et al. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn’s disease but not in ulcerative colitis. Inflamm Bowel Dis 2005;11:16–23. [DOI] [PubMed] [Google Scholar]
- 80. Schmitt H, Billmeier U, Dieterich W, et al. Expansion of IL-23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti-TNF therapy in Crohn’s disease. Gut 2019;68:814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest 2008;118:2269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pascal V, Pozuelo M, Borruel N, et al. A microbial signature for Crohn’s disease. Gut 2017;66:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sakuraba A, Sato T, Kamada N, et al. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn’s disease. Gastroenterology 2009;137:1736–45. [DOI] [PubMed] [Google Scholar]
- 84. Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nat Rev Immunol 2008;8:81–6. [DOI] [PubMed] [Google Scholar]
- 85. Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem 2001;276:37692–9. [DOI] [PubMed] [Google Scholar]
- 86. van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory t cells. Immunity 2007;27:660–9. [DOI] [PubMed] [Google Scholar]
- 87. Verreck FA, de Boer T, Langenberg DM, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A 2004;101:4560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol 1998;10:1593–8. [DOI] [PubMed] [Google Scholar]
- 89. Brain O, Owens BM, Pichulik T, et al. The intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 release. Immunity 2013;39:521–36. [DOI] [PubMed] [Google Scholar]
- 90. Aschenbrenner D, Quaranta M, Banerjee S, et al. Deconvolution of monocyte responses in inflammatory bowel disease reveals an IL-1 cytokine network that regulates IL-23 in genetic and acquired IL-10 resistance. Gut 2021;70:1023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thomas MG, Bayliss C, Bond S, et al. Trial summary and protocol for a phase II randomised placebo-controlled double-blinded trial of interleukin 1 blockade in acute severe colitis: the IASO trial. BMJ Open 2019;9:e023765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol 2016;16:177–92. [DOI] [PubMed] [Google Scholar]
- 93. Schnurr M, Toy T, Shin A, et al. Extracellular nucleotide signaling by p2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood 2005;105:1582–9. [DOI] [PubMed] [Google Scholar]
- 94. Mogilenko DA, Haas JT, L’Homme L, et al. Metabolic and innate immune cues merge into a specific inflammatory response via the UPR. Cell 2019;177:1201–16 e19. [DOI] [PubMed] [Google Scholar]
- 95. Cader MZ, Boroviak K, Zhang Q, et al. C13orf31 (FAMIN) is a central regulator of immunometabolic function. Nat Immunol 2016;17:1046–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Goodman RP, Markhard AL, Shah H, et al. Hepatic NADH reductive stress underlies common variation in metabolic traits. Nature 2020;583:122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cader MZ, de Almeida Rodrigues RP, West JA, et al. FAMIN is a multifunctional purine enzyme enabling the purine nucleotide cycle. Cell 2020;180:278–95 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005;6:1123–32. [DOI] [PubMed] [Google Scholar]
- 99. Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev 2008;223:87–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006;126:1121–33. [DOI] [PubMed] [Google Scholar]
- 101. Jiang W, Su J, Zhang X, et al. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm Res 2014;63:943–50. [DOI] [PubMed] [Google Scholar]
- 102. Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009;139:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006;24:179–89. [DOI] [PubMed] [Google Scholar]
- 104. Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced FOXP3 inhibits T(h)17 cell differentiation by antagonizing RORgammat function. Nature 2008;453:236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(h)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007;8:967–74. [DOI] [PubMed] [Google Scholar]
- 106. Di Meglio P, Di Cesare A, Laggner U, et al. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PLoS One 2011;6:e17160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sarin R, Wu X, Abraham C. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc Natl Acad Sci U S A 2011;108:9560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Di Meglio P, Villanova F, Napolitano L, et al. The IL23R A/Gln381 allele promotes IL-23 unresponsiveness in human memory T-helper 17 cells and impairs Th17 responses in psoriasis patients. J Invest Dermatol 2013;133:2381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol 2003;171:6173–7. [DOI] [PubMed] [Google Scholar]
- 110. Izcue A, Hue S, Buonocore S, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity 2008;28:559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Noguchi D, Wakita D, Tajima M, et al. Blocking of IL-6 signaling pathway prevents CD4+ T cell-mediated colitis in a T(h)17-independent manner. Int Immunol 2007;19:1431–40. [DOI] [PubMed] [Google Scholar]
- 112. O’Connor W, Jr., Kamanaka M, Booth CJ, et al. A protective function for interleukin 17a in T cell-mediated intestinal inflammation. Nat Immunol 2009;10:603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 2015;43:727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Maxwell JR, Zhang Y, Brown WA, et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity 2015;43:739–50. [DOI] [PubMed] [Google Scholar]
- 115. Ito R, Kita M, Shin-Ya M, et al. Involvement of IL-17a in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun 2008;377:12–6. [DOI] [PubMed] [Google Scholar]
- 116. Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis 2006;12:382–8. [DOI] [PubMed] [Google Scholar]
- 117. Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol 2004;110:55–62. [DOI] [PubMed] [Google Scholar]
- 118. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med 2014;371:326–38. [DOI] [PubMed] [Google Scholar]
- 119. Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17a monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012;61:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am J Gastroenterol 2016;111:1599–607. [DOI] [PubMed] [Google Scholar]
- 121. Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006;203:2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. El-Behi M, Ciric B, Dai H, et al. The encephalitogenicity of T(h)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol 2011;12:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr Opin Immunol 2011;23:702–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schmitt H, Neurath MF, Atreya R. Role of the IL23/IL17 pathway in Crohn’s disease. Front Immunol 2021;12:622934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bending D, De la Pena H, Veldhoen M, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest 2009;119:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hirota K, Duarte JH, Veldhoen M, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 2011;12:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity 2009;30:92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci U S A 2015;112:7061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Globig AM, Hennecke N, Martin B, et al. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-gamma+IL-17+ coproducing CD4+ T cells in active inflammatory bowel disease. Inflamm Bowel Dis 2014;20:2321–9. [DOI] [PubMed] [Google Scholar]
- 130. Takayama T, Kamada N, Chinen H, et al. Imbalance of NKp44(+)NKp46(-) and NKp44(-)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn’s disease. Gastroenterology 2010;139:882–92, 92 e1-3. [DOI] [PubMed] [Google Scholar]
- 131. Powrie F, Leach MW, Mauze S, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in SCID mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1994;1:553–62. [DOI] [PubMed] [Google Scholar]
- 132. Hommes DW, Mikhajlova TL, Stoinov S, et al. Fontolizumab, a humanized anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn’s disease. Gut 2006;55:1131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol 2009;27:313–38. [DOI] [PubMed] [Google Scholar]
- 134. Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007;450:566–9. [DOI] [PubMed] [Google Scholar]
- 135. Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 2003;170:3939–43. [DOI] [PubMed] [Google Scholar]
- 136. Bauche D, Joyce-Shaikh B, Jain R, et al. LAG3(+) regulatory T cells restrain interleukin-23-producing CX3CR1(+) gut-resident macrophages during group 3 innate lymphoid cell-driven colitis. Immunity 2018;49:342–52 e5. [DOI] [PubMed] [Google Scholar]
- 137. Uhlig HH. Monogenic diseases associated with intestinal inflammation: implications for the understanding of inflammatory bowel disease. Gut 2013;62:1795–805. [DOI] [PubMed] [Google Scholar]
- 138. Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology 2005;128:1868–78. [DOI] [PubMed] [Google Scholar]
- 139. Fantini MC, Rizzo A, Fina D, et al. SMAD7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology 2009;136:1308–16, e1-3. [DOI] [PubMed] [Google Scholar]
- 140. Desreumaux P, Foussat A, Allez M, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology 2012;143:1207–17 e2. [DOI] [PubMed] [Google Scholar]
- 141. Clough JN, Omer OS, Tasker S, Lord GM, Irving PM. Regulatory T-cell therapy in Crohn’s disease: challenges and advances. Gut 2020;69:942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Goettel JA, Kotlarz D, Emani R, et al. Low-dose interleukin-2 ameliorates colitis in a preclinical humanized mouse model. Cell Mol Gastroenterol Hepatol 2019;8:193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Spits H, Artis D, Colonna M, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol 2013;13:145–9. [DOI] [PubMed] [Google Scholar]
- 144. Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut 2013;62:1653–64. [DOI] [PubMed] [Google Scholar]
- 145. Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 2015;348:aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Takatori H, Kanno Y, Watford WT, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 2009;206:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009;457:722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Longman RS, Diehl GE, Victorio DA, et al. CX(3)CR1(+) mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J Exp Med 2014;211:1571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Guo X, Qiu J, Tu T, et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity 2014;40:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bauche D, Joyce-Shaikh B, Fong J, et al. IL-23 and IL-2 activation of STAT5 is required for optimal IL-22 production in ILC3s during colitis. Sci Immunol 2020;5. [DOI] [PubMed] [Google Scholar]
- 151. Geremia A, Arancibia-Carcamo CV, Fleming MP, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med 2011;208:1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010;464:1371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Keir M, Yi Y, Lu T, Ghilardi N. The role of IL-22 in intestinal health and disease. J Exp Med 2020;217:e20192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zenewicz LA, Yancopoulos GD, Valenzuela DM, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 2008;29:947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zheng Y, Valdez PA, Danilenko DM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 2008;14:282–9. [DOI] [PubMed] [Google Scholar]
- 156. Gronke K, Hernandez PP, Zimmermann J, et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 2019;566:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bernshtein B, Curato C, Ioannou M, et al. IL-23-producing IL-10Ralpha-deficient gut macrophages elicit an IL-22-driven proinflammatory epithelial cell response. Sci Immunol 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Powell N, Pantazi E, Pavlidis P, et al. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut 2020;69:578–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Aden K, Tran F, Ito G, et al. ATG16L1 orchestrates interleukin-22 signaling in the intestinal epithelium via cGAS-STING. J Exp Med 2018;215:2868–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Stefanich EG, Rae J, Sukumaran S, et al. Pre-clinical and translational pharmacology of a human interleukin-22 IGG fusion protein for potential treatment of infectious or inflammatory diseases. Biochem Pharmacol 2018;152:224–35. [DOI] [PubMed] [Google Scholar]
- 161. Brand S, Beigel F, Olszak T, et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol 2006;290:G827–38. [DOI] [PubMed] [Google Scholar]
- 162. Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol 2012;5:354–66. [DOI] [PubMed] [Google Scholar]
- 163. Rahman FZ, Marks DJ, Hayee BH, et al. Phagocyte dysfunction and inflammatory bowel disease. Inflamm Bowel Dis 2008;14:1443–52. [DOI] [PubMed] [Google Scholar]
- 164. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006;6:173–82. [DOI] [PubMed] [Google Scholar]
- 165. Griseri T, McKenzie BS, Schiering C, Powrie F. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity 2012;37:1116–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. McDermott AJ, Falkowski NR, McDonald RA, et al. Interleukin-23 (IL-23), independent of IL-17 and IL-22, drives neutrophil recruitment and innate inflammation during Clostridium difficile colitis in mice. Immunology 2016;147:114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wu Q, Martin RJ, Rino JG, et al. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect 2007;9:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Smith E, Zarbock A, Stark MA, et al. IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice. J Immunol 2007;179:8274–9. [DOI] [PubMed] [Google Scholar]
- 169. Stark MA, Huo Y, Burcin TL, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 2005;22:285–94. [DOI] [PubMed] [Google Scholar]
- 170. Griseri T, Arnold IC, Pearson C, et al. Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity 2015;43:187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003;421:744–8. [DOI] [PubMed] [Google Scholar]
- 172. Sun R, Abraham C. IL23 promotes antimicrobial pathways in human macrophages, which are reduced with the IBD-protective IL23R R381Q variant. Cell Mol Gastroenterol Hepatol 2020;10:673–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Chen L, He Z, Iuga AC, et al. Diet modifies colonic microbiota and CD4[+] T-cell repertoire to induce flares of colitis in mice with myeloid-cell expression of interleukin 23. Gastroenterology 2018;155:1177–91 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. He Z, Chen L, Catalan-Dibene J, et al. Food colorants metabolized by commensal bacteria promote colitis in mice with dysregulated expression of interleukin-23. Cell Metab 2021;33:1358–71 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Takeuchi I, Arai K, Kyodo R, et al. Ustekinumab for children and adolescents with inflammatory bowel disease at a tertiary children’s hospital in Japan. J Gastroenterol Hepatol 2021;36:125–30. [DOI] [PubMed] [Google Scholar]
- 176. Rosh JR, Turner D, Griffiths A, et al. Ustekinumab in paediatric patients with moderately to severely active Crohn’s disease: pharmacokinetics, safety, and efficacy results from UNISTAR, a phase 1 study. J Crohns Colitis 2021;15:1931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Biemans VBC, van der Woude CJ, Dijkstra G, et al. Ustekinumab is associated with superior effectiveness outcomes compared to vedolizumab in Crohn’s disease patients with prior failure to anti-TNF treatment. Aliment Pharmacol Ther 2020;52:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol 2017;76:405–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were analysed or generated in support of this review article.