Abstract
The treatment of patients with moderate to severe Crohn’s disease [CD] is still challenging. Therapeutic options include steroids, immunosuppressants, anti-TNFα agents, vedolizumab, and ustekinumab. Ustekinumab is a monoclonal antibody blocking the p40 subunit of IL-12 and IL-23. It showed to be effective and safe in randomised clinical trials and real-life studies and is currently approved for the management of CD patients who are naive to biologics and those who have already been treated with such medications. However, to date, a detailed and approved therapeutic algorithm is not available. The aim of this review is to report the most recent and updated data on the efficacy and safety of ustekinumab for the treatment of patients with moderate to severe CD and to define the optimal management of these patients.
Keywords: Inflammatory bowel diseases, Crohn’s disease, ustekinumab, algorithm
1.Introduction
Crohn’s disease [CD] is a chronic inflammatory bowel disease [IBD] that can affect the entire gastrointestinal tract from the mouth to the anus.1 CD is a progressive condition and can lead to bowel damage with the onset of abscesses, fistulas, and strictures, negatively impacting on patients’ quality of life.2 The introduction of biologic drugs has definitely revolutionised the management of these patients by significantly improving the course of the disease.2 The current therapeutic armamentarium for managing moderate-severe CD includes steroids, immunosuppressants [thiopurine and methotrexate], anti-TNFα agents [infliximab, adalimumab, and certolizumab], anti-integrins [vedolizumab and natalizumab], and an anti-interleukin 12-23 [ustekinumab].3 Ustekinumab is a fully human immunoglobulin monoclonal antibody blocking the p40 subunit of IL-12 and IL-23.4 It was approved by the U.S. Food and Drug Administration [FDA] and by the European Medicines Agency [EMA] for the treatment of CD in 2016.5,6 The purpose of this review is to summarise the available data on the efficacy and safety of ustekinumab for the treatment of patients with moderate-severe CD and to position this drug within the therapeutic algorithm.
2.Methods
We searched in PubMed, Cochrane Library, and Web of Science databases to identify all studies reporting data on efficacy and safety of ustekinumab in CD patients up to September 2021. The following Medical Subject Heading [MeSH] terms alone or matched with the Boolean operators ‘AND’ or ‘OR’ were used: ‘Crohn Disease’, ‘CD’, ‘inflammatory bowel disease’, ‘IBD’, ‘ustekinumab’, and ‘anti-IL-12/IL-23’. Three authors [FD, LPB, and SD] independently scrutinised titles and abstracts to identify eligible studies. Then, full-text articles were examined for inclusion. Abstracts from major international congresses [e.g., Digestive Disease Week, ECCO Congress, and United European Gastroenterology Week] were also checked to identify additional studies. Finally, we accurately evaluated the reference lists of the included studies for any further relevant work.
2.1. Efficacy data from randomised clinical trials and real-life studies
A phase 2a, randomised, placebo-controlled trial was conducted by Sanborn and colleagues between 2004 and 2006.7 CD patients were randomised to receive placebo, ustekinumab 90 mg subcutaneously, or ustekinumab 4.5 mg/kg intravenously. Interestingly, clinical response and clinical remission at Week 6 were achieved in a higher proportion of subjects in the intravenous arm than in the subcutaneous arm [62.0% vs 36.0% and 31.0% vs 14.0%, respectively]. A randomised, placebo-controlled, phase 2b study evaluated the efficacy and safety of ustekinumab in patients with CD.8 During the induction phase, 526 patients were randomised to receivethree different doses of intravenous ustekinumab [1 mg, 3 mg, or 6 mg per kilogram of body weight] or placebo. Responders were randomised in the maintenance study to receive ustekinumab 90 mg subcutaneously or placebo at Week 8 and Week 16. The proportion of patients who achieved the primary endpoint of clinical response (≥100-point decrease from the baseline Crohn’s Disease Activity Index [CDAI] score) at Week 6 was significantly higher in subjects treated with intravenous ustekinumab 6 mg/kg than in placebo arm [39.7% vs 23.5%, p = 0.005]. No significant difference was found with lower ustekinumab concentrations. Similarly, clinical response at Week 22 was achieved in a higher proportion of patients undergoing ustekinumab treatment compared with placebo [69.4% vs 42.5%, p < 0.001].
Ustekinumab proved to be effective for treating patients with moderate-severe CD in two phase 3, randomised, placebo-controlled, 8-week induction trials [UNITI-1 and UNITI-2] and one phase 3, randomised, placebo-controlled, 44-week maintenance trial [IM-UNITI] [Table 1].9 UNITI-1 included patients who had already been unsuccessfully treated with an anti-TNFα, and in UNITI-2, patients who were naive to anti-TNFα or who were not definable as primary or secondary non-responders to anti-TNFα were recruited. Patients completing induction studies were enrolled in the maintenance trial. In UNITI-1 and UNITI-2, patients were randomised 1:1:1 to receive a single intravenous infusion of 130 mg of ustekinumab, a weight-range–based dose of 6 mg of ustekinumab per kilogram of body weight, or placebo. Subjects who responded to induction with ustekinumab at Week 8 were randomised again 1:1:1 in the maintenance trial to receive 90 mg subcutaneous ustekinumab every 8 or 12 weeks or placebo.9 All patients who met loss of-response criteria [CDAI score ≥220 or an increase from their baseline CDAI score of ≥100 points] in the maintenance trial underwent dose adjustment. The primary endpoint of both induction trials was clinical response at Week 6, defined as a CDAI score <150 or a decrease of at least 100 points in CDAI score from baseline. On the other hand, clinical remission at Week 44 [CDAI score <150] was the primary endpoint in the maintenance trial. In total, 1369 patients were enrolled in the induction trials and 1281 continued in the maintenance phase. A significantly higher proportion of patients treated with intravenous ustekinumab at the dose of 130 mg or 6 mg/kg achieved the primary endpoint in the induction studies compared with placebo arm [34.3% and 33.7% vs 21.5% in UNITI-1, p = 0.002 and p = 0.003, respectively; 51.7% and 55.5% vs 28.7% in UNITI-2, p < 0.001 for both comparisons]. Patients receiving intravenous ustekinumab 6 mg/kg had numerically higher rates of clinical response and clinical remission and higher serum drug concentrations [6.4 μg per millilitre vs 2.1 μg per millilitre in UNITI-1 and 6.3 μg per millilitre vs 2.0 μg per millilitre in UNITI-2] compared with those treated with intravenous ustekinumab 130 mg at the end of induction phase, justifying the use of this posology.
Table 1.
Ustekinumab efficacy and safety data from randomised clinical trials
| First author | n of pts | Mean age [y] | Male n [%] | Study duration | Study arms | Primary endpoint | Main results | AEs | SAEs |
|---|---|---|---|---|---|---|---|---|---|
| Sandborn8 | 526 | 39.0 | 217 [41.3] | 36 weeks | iv UST 1 mg/kg iv UST 3 mg/kg iv UST 6 mg/kg PBO |
Clinical response at wk 6 | 36.6% 34.1% 39.7% 23.5% |
68.5% 66.2% 61.1% 71.2% |
4.6% 6.0% 6.9% 8.3% |
| Feagan9 | UNITI-1 741 UNITI-2 628 IM-UNITI 397 |
37.3 39.2 38.6 |
317 [42.8] 293 [46.7] 173 [43.6] |
8 weeks 8 weeks 44 weeks |
iv UST 130 mg; iv UST 6 mg/kg, PBO iv UST 130 mg; iv UST 6 mg/kg, PBO sc UST 90 mg e8w; sc UST 90 mg e12w, PBO |
Clinical response at wk 6 Clinical response at wk 6 Clinical remission at wk 44 |
34.3%, 33.7%, 21.5% 51.7%, 55.5%, 28.7% 53.1%, 48.8%, 35.9% |
64.6%, 65.9%, 64.9% 50.0%, 55.6%, 54.3% 81.7%, 80.3%, 83.5% |
4.9%, 7.2%, 6.1% 4.7%, 2.9%, 5.8% 9.9%, 12.1%, 15.0% |
| Sandborn10 | 237 | na | na | 252 weeks | sc UST 90 mg e8w sc UST 90 mg e12w |
Clinical remission at wk 252 | 34.4% 28.7% |
327.6 e100 py [cumulative UST] |
17.5 e100py [cumulativeUST] |
| Danese11 | 500 | na | na | 48 weeks | Treatment target arm Standard of care arm |
Endoscopic response at wk 48 | 33.6% 28.5% |
na | na |
n, number; pts, patients; y, years; AEs, adverse events; SAEs, serious adverse events; UST, ustekinumab; iv, intravenous; PBO, placebo; sc, subcutaneous; e8w, every 8 weeks; e12w,every 12 weeks; wk, week or Week; na, not available; e100py, events per 100 patient-years.
Similarly, in the maintenance trial, clinical remission was achieved in a significantly higher proportion of patients treated with 90 mg subcutaneous ustekinumab every 8 or 12 weeks than placebo [53.1% and 48.8% vs 35.9%, p = 0.005 and p = 0.04, respectively]. Interestingly, mean C-reactive protein and faecal calprotectin values remained unchanged or were reduced from baseline in a greater proportion of ustekinumab-treated patients than placebo.9 The clinical and biochemical improvements could be seen as early as the third week of starting ustekinumab treatment.9 Recently, the results of the IM-UNITI long-term extension [LTE] trial have been published reporting the 5-year efficacy data of ustekinumab in CD.10 All patients who completed the maintenance study were eligible for the LTE phase. Patients treated with placebo were discontinued and no dose adjustment was allowed. In total, 237 patients entered the LTE study and about half of them [124/237; 52.3%] completed the 5-year follow-up. At Week 252, clinical remission was achieved by 28.7% of patients treated with ustekinumab every 12 weeks and by 34.4% of those treated every 8 weeks. Furthermore, the 48-week results of the STARDUST trial are available.11 This is an ongoing, phase 3b, randomised trial, which compares the efficacy of ustekinumab in CD patients who have failed at least one biologic therapy by stratifying them according to different management strategies [standard care vs treatment target]. The primary endpoint of endoscopic improvement at Week 48 (≥50% reduction in Simple Endoscopic Score-CD [SES-CD] vs baseline) was achieved in a similar proportion of patients in the treatment target group and in the control arm [33.6%% vs 28.5%, p > 0.05]. Likewise, rates of corticosteroid-free clinical remission were not different between the study groups [56.4% and 63.3%, p > 0.05].
So far, SUSTAIN is the study with the highest number of patients [463] which has evaluated the efficacy of ustekinumab in a real-life setting of CD patients [Table 2].12 Clinical remission and clinical response were found in a high proportion of subjects at Week 16 [56.0% and 70.0%] and the probability of maintaining the drug after 1 year of therapy exceeded 80.0%. The Initiative on Crohn and Colitis [ICC] Registry is a Dutch prospective registry of IBD patients starting new therapies for IBD.13 This registry reports efficacy data from 252 patients with CD treated with ustekinumab and followed up for 2 years. About one-third of patients [34.0%] was in clinical remission without the use of steroids after 2 years of therapy. In addition, one-fifth of patients [21.5%] achieved biochemical remission at Week 104. Real-world long-term efficacy data of ustekinumab in CD have also been reported in the Spanish ENEIDA registry.14 Over 400 CD patients were treated with ustekinumab and recruited in the study. After 1 year of treatment, two-thirds of patients who were in clinical disease activity at baseline (Harvey-Bradshaw Index [HBI] >4) achieved clinical remission [290/295, 64.4%]. In about half of the patients there was a normalisation of faecal calprotectin values at Week 52 [54.0%]. A retrospective, observational, multicentre study by GETECCU evaluated the efficacy of re-induction with intravenous ustekinumab in 53 patients successfully treated with ustekinumab and who then experienced a loss of response [HBI ≥5].15 Surprisingly, about half of the subjects [23, 43.4%] achieved clinical remission [HBI ≤4] at Week 16 after re-induction and no infusion reactions or additional adverse events [AEs] were observed. Fumery et al. instead investigated the efficacy of the treatment regimen with ustekinumab every 4 weeks in patients who had lost response to therapy every 8 weeks.16 After 2 months, approximately one-third and two-thirds of patients achieved clinical response [61/100, 61.0%] or clinical remission [31, 31.0%], respectively. Instead, after a mean follow-up of 8 months, the majority of patients [61.0%] were still on ustekinumab and approximately half of the subjects [49.0%] were in steroid-free clinical remission.
Table 2.
Ustekinumab efficacy and safety data from real-life studies
| First author | n of pts | Mean age [y] | Male n [%] | Study duration | Study arms | Primary endpoint | Main results | AEs | SAEs |
|---|---|---|---|---|---|---|---|---|---|
| Chaparro12 | 463 | 45 | na | 15 months | sc UST 90 mg e8w sc UST 90 mg e12w |
Clinical remission at wk 16 | 56.0% [cumulative UST] | na | na |
| Straatmijer13 | 252 | 41 | 100 [39.7%] | 104 weeks | sc UST 90 mg e8w sc UST 90 mg e12w |
Corticosteroid-free clinical remission at wk 104 | 34.0% [cumulative UST] | 99 e100py [cumulative UST] |
9 e100py [cumulative UST] |
| Iborra14 | 407 | 45.3 | 195 [48.0%] | 52 weeks | sc UST 90 mg e4w sc UST 90 mg e8w sc UST 90 mg e12w |
Clinical remission at wk 52 | 60.0% 72.0% 89.0% |
14.7% [cumulative UST] | na |
| Bermejo15 | 53 | 45 | 26 [49.1%] | 16 weeks | sc UST 90 mg e4w sc UST 90 mg e8w sc UST 90 mg e12w |
Clinical remission at wk 16 | 43.3% [cumulative UST] | na | 1.9% |
| Fumery16 | 100 | 35 | 48 [48.0%] | 8.2 months | sc UST 90 mg e4w | Corticosteroid-free clinical remission at 6 months | 49.0% | 12.0% | 5.0% |
n, number; pts, patients; y, years; AEs, adverse events; SAEs, serious adverse events; UST, ustekinumab; iv, intravenous; PBO, placebo; sc, subcutaneous; e8w, every 8 weeks; e12w,every 12 weeks; wk, week or Week; na, not available; e100py, events per 100 patient-years.
2.2. Safety data from randomised clinical trials and real-life studies
In UNITI-1, the percentages of adverse events [AEs] were similar between ustekinumab 130 mg, ustekinumab 6 mg/kg, and placebo [64.6%, 65.9%, and 64.9%, respectively]. Similarly, no significant difference in the rate of serious AEs was found [4.9%, 7.2%, and 6.1%, respectively]. In UNITI-2, patients treated with ustekinumab and those in the placebo arm had comparable rates of AEs [50.0%, 55.6%, and 54.3%] and serious AEs [4.7%, 2.9%, and 5.8%]. At the end of the maintenance study, incidence of AEs and serious AEs was similar among patients treated with ustekinumab every 8 weeks, ustekinumab every 12 weeks, and placebo [81.7%, 80.3%, and 83.5% and 9.9%, 12.1%, and 15.0%, respectively]. The most frequent AEs were arthralgia, headache, nasopharyngitis, and exacerbations of CD. Importantly, in the IM-UNITI LTE trial the number of safety events per 100 patient-years was not statistically different in placebo and combined ustekinumab groups regarding AEs [440.3 vs 327.6], serious AEs [19.3 vs 17.5], infections [99.8 vs 93.8], and serious infections [3.9 vs 3.4].10 Of note, the incidence of malignancies per 100 patient-years was 1.70 in the placebo group and 1.06 in the combined ustekinumab group, accounting for a total of 10 malignancies [excluding non-melanoma skin cancer] during the study period. In the ICC registry, 81 possibly and 18 probably related AEs were noted during the study period.13 The most common AEs were headache, skin reaction, and musculoskeletal complaints. Severe infections occurred in 13 patients [53.8% of patients were simultaneously treated with an immunosuppressant] and malignancies were diagnosed in three cases [two patients were concurrently treated with a thiopurine]. One death unrelated to the drug was also detected. In the ENEIDA registry, AEs were detected in only a small percentage of patients [14.7%] and consisted mainly of bacterial infections [34.0%].14
2.3. Pharmokinetics and pharmacodynamics
Drug concentration analysis from pivotal induction clinical trials showed that ustekinumab concentration at Week 8 in the 130 mg- or 6 mg/kg-dose groups were comparable between UNITI-1 and UNITI-2 [2.1 mg/mL and 6.4 mg/Ml vs 2.0 mg/mL and 6.3 mg/mL, respectively].17 Steady state was achieved after the second maintenance dose and mean ustekinumab concentrations were on average 3-fold higher in the every Week 8 group than in the every Week 12 group [2.0-2.2 µg/mL vs 0.6-0.8 µg/mL]. Ustekinumab serum levels were not affected by any combination therapy with thiopurine or methotrexate. Interestingly, patients with higher drug concentrations were associated with higher rates of clinical [55.3% and 70.8% in the two lower quartiles vs 77.1% and 81.3% in the two higher quartiles, p = 0.002], endoscopic [24.0%, 19.2%, and 28.0% in the three higher quartiles vs 7.7% in the lowest quartile, p = 0.054], and biochemical (median C-reactive protein [CRP] concentrations at Week 54 were 3.3 mg/L, 3.3 mg/L and 2.7 mg/L in the three higher quartiles vs 10.4 mg/L in the lowest quartile, p = 0.008) remission. Subjects with ustekinumab concentrations ranging from 0.8 to 1.4 mg/mL or greater were more likely to be in clinical remission than those with lower drug values. Antibodies to ustekinumab were found in only 27 patients [2.3%] and then were no longer identified at subsequent dosages. They were not related to AEs or injection site reactions. In a real-life study by Battat et al., patients with endoscopic response to treatment had a higher mean drug concentration value than those without endoscopic response [4.7 μg/mL vs 3.8 μg/mL, p = 0.03].18 Additionally, patients with high drug concentrations [>4.7 μg/mL] had higher endoscopic response rates at Week 26 and lower CRP concentrations than patients with lower drug levels. No anti-ustekinumab antibodies were identified among the 62 enrolled subjects. On the other hand, in a multicentere cross-sectional study investigating the impact of ustekinumab therapeutic drug monitoring [TDM] on clinical decision making, therapeutic decisions were not influenced by TDM [p = 1.0] and drug concentrations were not associated with clinical disease outcomes.19 Similarly, a retrospective study by Mechie et al. showed that ustekinumab serum concentrations did not affect disease outcomes.20 To date, the desirable threshold for maintenance levels of ustekinumab is not known and further studies are needed to define the role of TDM on disease monitoring and management.
2.4. Specific situations
2.4.1. Pregnancy
The Pregnancy in Inflammatory bowel disease And Neonatal Outcomes [PIANO] study was a multicentric prospective observational study enrolling 1490 pregnant IBD women [18 treated with ustekinumab] [Table 3].21 There were 1431 live births [96%]. Among the 18 patients treated with ustekinumab, no increased risk of congenital malformations, spontaneous abortions, preterm birth, low birthweight, or infections over the first year of life was reported. A French multicentre retrospective study evaluated the maternal and fetal outcomes of 29 pregnant women on ustekinumab therapy.22 Most of the pregnancies led to live births [26, 90.0%], whereas spontaneous abortions [2, 7.0%] or elective termination [1, 3.0%] occurred in a limited proportion of cases. The incidence of prematurity, spontaneous abortion, congenital malformations, and maternal complications was comparable to that of patients treated with anti-TNFα. The DUMBO prospective registry is also noteworthy.23 It is an ongoing Spanish multicentre initiative to evaluate the outcomes of pregnant women with IBD. To date, 433 patients have been recruited in this study, including 17 women receiving ustekinumab. SAEs occurred in four pregnancies exposed to ustekinumab but were judged non-related with the drug. In the multivariate analysis, adjusted by disease activity, patients treated with biologics were not associated with higher risk of SAEs (odds ratio [OR] = 0.8; 95% confidence interval [CI] = 0.2-0.3].
Table 3:
Key findings of ustekinumab studies on specific subpopulations of patients with Crohn’s disease.
| First author | n of pts | Mean age [y] | Male n [%] | Study duration | UST-treated patients | Study aim | Primary endpoint | Main results |
|---|---|---|---|---|---|---|---|---|
| Mahadevan21 | 1490 | 32.0 | 0 [0.0%] | 21 months | 18 | To assess pregnancy outcomes in patients exposed to thiopurines and biologics | Rates of congenital malformations, SAB, preterm birth, LBW, and infections | Drug exposure did not increase the rate of congenital malformations, SAB, preterm birth, LBW, and infections over the first year of life |
| Wils22 | 73 | na | 0 [0.0%] | na | 29 | To assess maternal and neonatal complications of VDZ or UST in pregnant IBD pts | Rates of congenital malformations, SAB, preterm birth, LBW, and infections | No negative signal on maternal or neonatal outcomes |
| Chaparro23 | 433 | 34 | 0 [0.0%] | 5 years | 17 | To evaluate the risk of SAEs during pregnancy and the predictive factors of it | Rates of SAEs | Immunomodulators and biologics do not increase the risk of SAEs during pregnancy |
| Rosh24 | 44 | 13.0 | 18 [41.0%] | 16 weeks | 44 | To evaluate pharmacokinetics, safety/tolerability, and efficacy of UST in children with CD | To compare the pharmacokinetics of UST in paediatric and adult CD pts | The pharmacokinetics/safety profiles were generally consistent with those observed in adults with CD |
| Kim25 | 38 | 12.5 | na | 62 weeks | 38 | To analyse the long-term efficacy of UST in paediatric CD pts | Response to therapy | UST has long-term efficacy with no observed safety concerns. |
| Garg26 | 117 | 69.6 | 59 [50.5%] | 1.3 years | 117 | To assess the safety and efficacy of UST in elderly CD | To compare efficacy and safety of UST in elderly and young CD pts | UST is safe and effective in elderly CD |
| Asscher27 | 410 | 45.0 | 175 [42.7%] | 103.4 weeks | 207 | To evaluate the association between age and comorbidity with safety and efficacy outcomes of VDZ and UST in IBD | Infections, hospitalisations, treatment-related AEs, clinical response, and clinical remission | Comorbidity, but not age, is associated with an increased risk of hospitalisations on either treatment |
| Tursi28 | 15 | 42.0 | 9 [60.0%] | 12 months | 15 | To evaluate the efficacy of UST in operated CD patients | Clinical remission at 6 months | This is the first report on the use of ust in post-operative CD recurrence in patients previously refractory to biologics |
| Buisson29 | 63 | 37.0 | 15 [23.8%] | 6 months | 32 | To compare the efficacy of UST vs azathioprine in preventing endoscopic POR in CD | Endoscopic POR evaluated 6 months after intestinal resection | UST seems to be more effective than azathioprine in preventing endoscopic POR in this cohort of CD pts |
| Narula30 | 576 | 33.0 | 286 [49.6%] | na | 163 | To describe the clinical and endoscopic outcomes of CD pts with non-passable strictures | The likelihood that non-passable stenosis could be converted to passable stenosis | Pts with non-passable strictures can achieve symptomatic and endoscopic remission when receiving CD therapies |
| El Ouali31 | 21 | 44.0 | 11 [52.0%] | 8 months | 15 | To evaluate the outcomes of VDZ and UST in CD pts with symptomatic strictures | Time to recurrence of obstructive symptoms, time to surgical intervention | UST and VDZ may be initial options after failure of anti-TNF agents |
| Sands32 | na | na | na | 8 weeks | na | To report efficacy of UST in the treatment of perianal CD | Fistula response and complete fistula resolution | There is a consistent signal for efficacy in fistula healing that approached statistical significance in the combined analysis of fistula resolution, despite a relatively small number of pts |
| Chapuis-Biron33 | 207 | 37.7 | 75 [36.2%] | 48 weeks | 207 | To assess the efficacy of ust in perianal CD and predictors of clinical success | Clinical success at 6 months, with no need for medical or surgical treatment | UST appears as a potential effective therapeutic option in perianal refractory CD |
| Narula34 | 1398 | 38.6 | 445 [31.9%] | 52 weeks | 527 | To evaluate the efficacy of UST in treatment of EIMs | EIM resolution at Week 6 | UST did not lead to significant resolution of EIMs for CD compared with placebo at Weeks 6 and 52 |
| Tursi35 | 24 | 49.0 | 13 [54.2%] | 6 months | 24 | To report the efficacy of UST for the treatments of EIMs in CD | Remission | EIMs associated with CD respond well to UST |
| Phillips 36 |
28 | 37.0 | 8 [28.5] | na | 19 | To report the efficacy of UST to treat refractory cutaneous lesions | Remission | UST appears to be useful for different cutaneous lesions including metastatic CD, pyoderma gangrenosum, and erythema nodosum |
n, number; pts, patients; y, years; AEs, adverse events; SAEs, serious adverse events; SAB, spontaneous abortion; LBW, low birthweight;VDZ, vedolizumab; uUST, ustekinumab;IBD, inflammatory bowel disease; na, not available; CD, Crohn’s disease; POR, post-operative recurrence; EIMs, extraintestinal manifestasions.
2.4.2.Paediatric patients
The pharmacokinetics, safety/tolerability, and efficacy of ustekinumab in paediatric CD population were assessed in the UniStar phase 1, multicentre study.24 A total of 44 patients aged 2 to 17 years [body weight ≥10 kg] were randomised 1: 1 to receive intravenous ustekinumab at the dose of 130 mg vs 390 mg in patients ≥40 kg or 3 mg/kg vs 9 mg/kg in patients <40 kg. After the induction dose, all patients received at Week 8 a single subcutaneous ustekinumab dose based on body weight [90 mg in patients ≥40 kg or 2 mg/ kg in patients <40 kg]. Serum ustekinumab concentrations were generally similar to those in adult patients except for children with a body weight <40 kg who had lower concentrations. Overall, 22.0% and 16.0% of patients in the low-dose group and 29.0% and 11.1% in the high-dose arm achieved clinical [PCDAI <10] and endoscopic [SES-CD ≤2] remission at Week 16. Approximately three-quarters of patients [73.0%] experienced at least one AEs, and serious AEs were reported more in the low-dose group than in the high-dose group [26.0% vs 5.0%] and consisted mainly of CD exacerbations. Furthermore, a retrospective cohort study by Kim and colleagues reported the long-term efficacy data of ustekinumab in 38 paediatric CD patients previously exposed to anti-TNFα.25 Median duration on ustekinumab therapy was 62 weeks and most patients achieved clinical remission [60.5%] at the last available follow-up.
2.4.3.Elderly
A retrospective cohort study by Garg et al. enrolled 117 CD patients treated with ustekinumab, by stratifying them into elderly [age ≥65 years] and non-elderly [<65 years].26 No significant difference was found in the rate of steroid-free remission and mucosal healing between elderly and non-elderly groups [30.0% vs 54.1%, p = 0.22 and 25.9% vs 29.5%, p = 0.74, respectively]. Moreover, infusion reactions [2.6% vs 6.4%, p = 0.77], infections [5.2% vs 7.7%, p = 0.7], and postsurgical complications [0.0% vs 6.7%, p = 0.99] occurred in a similar proportion between the study arms. Instead, a prospective multicentre cohort study investigated the impact of patient age and comorbidities on safety and efficacy outcomes in 207 ustekinumab-treated patients.27 Age at baseline [≥60 years or <60 years] was not associated with efficacy (hazard ratio[HR] 0.977, 95% CI 0.955-1.000, p = 0.054) and safety [HR 0.987, 95% CI 0.956-1.018 p = 0.397] outcomes, whereas comorbidity (assessed using the Charlson Comorbidity Index [CCI]) was an independent predictor of hospitalisation [OR 1.621, 95% CI 1.034-2.541, p = 0.035].
2.4.4.Operated patients
Tursi et al. reported promising data on the efficacy of ustekinumab in post- operative CD recurrence.28 Fifteen patients with active CD [defined as HBI ≥5 or Rutgeert’s score ≥2] already treated with anti- TNFα agents or vedolizumab were included. After a median follow-up time of 6 months, 12 patients [80.0%] achieved clinical remission [HBI ≤4] and 11 patients [73.3%] reached mucosal healing [Rutgeert’s score ≤1]. Another multicentre retrospective cohort study compared the rate of endoscopic post-operative recurrence [POC] in 63 CD patients treated with ustekinumab or azathioprine.29 A propensity score analysis was applied to compare the two groups. After 6 months, endoscopic POR occurred in a lower rate in the ustekinumab arm than in the azathioprine group [28.0% vs 54.5%, p = 0.029].
2.4.5.Strictures
A post-hoc analysis of three large CD clinical trial programmes described the clinical and endoscopic outcomes of 150 CD patients with strictures after treatment with infliximab, ustekinumab, or azathioprine.30 Strictures were defined according to the Simple Endoscopic Score for Crohn’s Disease [SES-CD]. After 1 year of treatment, more than half of the patients with non-passable stenosis [62.5%] achieved resolution of the stricture or an improvement in the stricture that was passable. Clinical remission and endoscopic remission were detected in 52.4% and 37.5% of patients at the end of the study, respectively. However, a significant lower rate of clinical improvement was found in patients with non-passable strictures at baseline compared with those with passable or no strictures [adjusted odds ratio 0.17, 95% CI 0.03–0.99, p = 0.048]. A recent pilot study by El Oauli et al. reported data about 15 CD patients with stricturing disease treated with ustekinumab.31 All patients continued ustekinumab after 6 months of therapy, but 18.0% and 40.0% of them required dose escalation or a corticosteroid course, respectively.
2.4.6. Perianal disease
The efficacy of ustekinumab for the management of perianal disease in CD patients was evaluated in the phase 2 CERTIFY study and in the UNITI induction trials.32,37 Up to 15.5% of patients included in these studies had active perianal disease at baseline [defined by physical examination]. Fistula response [≥50% reduction in draining fistulas] and complete fistula resolution [100% reduction] were assessed after 8 weeks of ustekinumab therapy. The overall rate of fistula response to ustekinumab was numerically higher in patients treated with ustekinumab compared with the placebo group [26.0% vs 16.9%, p = 0.14]. Similarly, a higher proportion of ustekinumab-treated patients experienced fistula resolution than those receiving placebo at Week 8 [24.7% vs 14.1%, p = 0.07]. In the IM-UNITI LTE study, 61/567 ustekinumab-treated patients had active perianal disease at baseline.10 After 5 years of follow-up, most patients with data available [24/31, 77.4%] experienced fistula response [defined as a ≥50% reduction in the number of draining fistulas]. A French multicentre retrospective cohort study was specifically designed to assess the efficacy of ustekinumab in perianal CD.33 The primary endpoint of this study was clinical success at 6 months defined as resolution of perianal disease with no need for specific medical or surgical therapy. Among the 148 patients with active perianal disease at baseline, the primary endpoint was achieved in 57 cases [38.5%] after treatment with ustekinumab at the standard dose. Finally, a systematic review and meta-analysis by Attauabia et al. reported the efficacy of ustekinumab in perianal CD from nine observational cohort studies.38 The cumulative response and remission rates after 8 weeks of treatment were 41.0% and 17.1%, respectively. Interestingly, the response rates were higher after 54 weeks of treatment whereas the remission rates remained stable [55.9% and 16.7%, respectively].
2.4.7. Extraintestinal manifestations
A post-hoc analysis of the UNITI trials evaluated the efficacy of ustekinumab for the treatment of extraintestinal manifestations [EIMs] in CD.34 The primary outcome was the overall EIM resolution between ustekinumab and placebo-treated patients at Week 6, and the secondary outcome was the overall EIM resolution in both arms at Week 52. In total, 504 patients experienced EIMs and were included in the analysis. Most patients had one EIM [36.0%] and the most frequent EIMs at baseline were arthritis or arthralgia [50.1%], erythema nodosum [3.0%], iritis or uveitis [2.4%], and pyoderma gangrenosum [0.5%]. No significant improvement in EIMs was seen in ustekinumab-treated patients compared with placebo at Week 6 [36.9% vs 39.1%, p = 0.564]. Similarly, there was no statistically significant difference at Week 52 [76.4% vs 80.0%, p = 0.542]. In addition, a similar proportion of de novo EIMs was found at Week 52 between ustekinumab and placebo arms [1.1% vs 0.0%, p > 0.05]. Of note, a recent systematic review by Guillo and colleagues including nine studies [eight retrospective and one prospective] investigated the efficacy of ustekinumab for the management of EIMs in IBD.39 Ustekinumab showed to be effective for treating arthralgia and psoriatic arthritis. A high rate of response to ustekinumab was found also in patients with dermatological manifestations [e.g., psoriasis, pyoderma gangrenosum, and erythema nodosum], but no efficacy was detected in subjects with axial spondyloarthritis. Moreover, Tursi and colleagues reported their experience with 24 patients with IBD and EIMs [17 rheumatological manifestations, five dermatological manifestations, one uveitis, and one sclerosing cholangitis] treated with ustekinumab.35 After a mean follow-up of 6 months, almost all CD patients treated with ustekinumab reached a favourable outcome of EIMs. A multicentre case series evaluated the efficacy of ustekinumab for the management of different types of anti-TNF refractory cutaneous lesions in 19 IBD patients.36 Ustekinumab showed to be effective in inducing remission of metastatic CD [in five cases], erythema nodosum [in four cases], and pyoderma gangrenosum [in three cases].
2.5. Comparison between ustekinumab and other biologic drugs
A post-hoc analysis of randomised clinical trials, conducted by Narula et al., compared the efficacy and rapidity of action of infliximab and ustekinumab in 420 biologic-naïve CD patients [Table 4].40 Disease outcomes were rates of clinical response, clinical remission, and decreased faecal calprotectin after 6 weeks of therapy. Interestingly, no significant differences were found in the rate of clinical response [58.4% vs 54.9%], clinical remission [44.9% vs 37.9%], or improvement in faecal calprotectin [42.3% vs 34.7%] between infliximab and ustekinumab groups. Comparative data between ustekinumab and vedolizumab as second-line therapy after failure of an anti-TNF in CD come from the Dutch ICC registry.41 Corticosteroid-free clinical remission [HBI ≤4] and biochemical remission [defined as a CRP ≤5 mg/L and a faecal calprotectin ≤250 µg/g] were evaluated in 128 vedolizumab- and 85 ustekinumab-treated CD patients after at least 1 year of therapy. Data analysis, adjusted for confounding factors, revealed that patients treated with ustekinumab had a higher rate of steroid-free clinical remission [OR: 2.58, 95% CI: 1.36-4.90, p = 0.004] and biochemical remission [OR: 2.34, 95% CI: 1.10-4.96, p = 0.027] compared with patients receiving vedolizumab. On the other hand, no significant difference between the two drugs was found in infection rate [OR: 1.26, 95% CI: 0.63-2.54, p = 0.517], AEs [OR: 1.33, 95% CI: 0.62-2.81, p = 0.464], or hospitalisations [OR: 0.67, 95% CI: 0.32-1.39, p = 0.282].
Table 4:
Comparative studies between ustekinumab and other biological drugs
| First author | n of pts | Mean age [y] | Male n [%] | Study duration | Study arms | Primary endpoint | Main results | AEs |
|---|---|---|---|---|---|---|---|---|
| Narula40 | 420 | 37.0 | 211 [50.2] | 6 wks | 206 iv UST 6 mg/kg at wk 0 214 iv IFX 5 mg/kg at wk 0-2 |
Clinical remission at wk 6 | 44.9% 37.9% |
na |
| Biemans41 | 213 | 37.6 | 78 [36.6] | 104 wks | 128 iv VDZ 300 mg at wk 0-2-6 and then e8w 85 iv UST 6 mg/kg at wk 0 and then sc UST 90 mg e8w or e12w |
Corticosteroid-free clinical remission at wk 52 | 26.8% 45.9% |
140.9 e100 py 85.0 e100 py |
| Townsend42 | 130 | 42.9 | 51 [39.2] | 52 wks | 85 iv VDZ 300 mg at wk 0-2-6 and then e8w 45 iv UST 6 mg/kg at wk 0 and then sc UST 90 mg e8w |
Steroid-free clinical remission at end of induction therapy and during maintenance therapy | 11.8% and 24.7% 28.9% and 42.2% |
5.9% 2.2% |
| Manlay43 | 312 | 39.1 | 118 [37.8%] | 16.5 months | 88 iv VDZ 300 mg at wk 0-2-6 and then e8w or e4w 224 iv UST 6 mg/kg at wk 0 and then sc UST 90 mg e8w or e4w |
Corticosteroid-free clinical remission at wk 54 | 40.5% 51.5% |
6.8% 4.9% |
| Albshesh44 | 204 | 41.5 | 96 [47.0] | 52 wks | 156 patients treated with VDZ as second-line therapy 48 patients treated with UST as second-line therapy |
Clinical response at week 16–22 | 55.5% 56.2% |
8.3% 12.5% |
| Irving45 | 386 | na | na | 52 wks | 191 pts UST 6 mg/kg at wk 0 and then sc UST 90 mg e8w 195 pts ADA 160–80 mg at wk 0-2 and then 40 mg e2w |
Clinical remission at wk 52 | 64.9% 61.0% |
80.1% 77.9% |
| Ko 46 |
2499 | 47.7 | 1184 [47.3] | 8219 py | 1146 IFX 1359 ADA 162 VDZ 251 UST |
Persistence rate at 12 months | 68.1% 64.2% 73.5% 80.0% |
na |
n, number; pts, patients; y, years; AEs, adverse events; SAEs, serious adverse events; UST, ustekinumab; iv, intravenous; PBO, placebo; sc, subcutaneous; e8w, every 8 weeks; e12w,every 12 weeks; wk, week or Week; na, not available; e100py, events per 100 patient-years; ADA, adalimumab; IFX, infliximab.
Another study by Townsend et al. compared the 12-month efficacy of ustekinumab and vedolizumab in anti-TNF-refractory CD.42 A total of 130 patients [85 treated with vedolizumab and 45 with ustekinumab] were recruited. After adjusting for confounding factors, ustekinumab was showed a higher proportion of steroid-free remission than vedolizumab at both 2 months [OR: 2.79, 95% CI: 1.06-7.39, p = 0.038] and 12 months [OR: 2.01, 95% CI: 0.89-4.56, p = 0.095]. Furthermore, ustekinumab ensured greater persistence towards therapy than vedolizumab after 12 months of follow-up [84.4% vs 61.5%, p = 0.007]. These data were also confirmed by Manlay and colleagues in a propensity study of 312 CD patients treated with ustekinumab or vedolizumab refractory to anti-TNF therapy [ustekinumab = 224 and vedolizumab = 88].43 Interestingly, a multicentre retrospective cohort study by Albshesh et al. compared vedolizumab and ustekinumab as third-line treatment in CD patients.44 In total, 204 non-responders to anti-TNFα agents were included. Three-quarters of the patients [76.0%] were treated with vedolizumab as second-line and ustekinumab as third-line therapy [group A], and the remaining patients [24.0%] were treated first with ustekinumab and then with anti-integrin [group B]. The primary outcome of clinical response at Weeks 16–22 [defined by a reduction of HBI ≥3] occurred in a similar proportion between the study groups [55.5% vs 56.2%, p = 0.9]. SEAVUE was the first head-to-head, multicentre, controlled, randomised trial comparing the efficacy and safety of ustekinumab and adalimumab in 386 biologic-naïve adult patients with moderate-severe CD.45 Subjects were randomised 1:1 to ustekinumab [6 mg/kg intravenously for the induction phase and then ustekinumab 90 mg subcutaneously every 8 weeks for maintenance] or adalimumab [160/80 mg subcutaneously at Weeks 0 and 2 and then 40 mg subcutaneously every 2 weeks]. The primary endpoint was clinical remission at Week 52 [defined as CDAI <150]. No difference between ustekinumab and adalimumab was identified in the rate of clinical remission at Week 52 [65% vs 61%; 95% CI: -5.5%-13.5%, p = 0.417]. In addition, ustekinumab and adalimumab had a similar proportion of corticosteroid-free clinical remission [28.5% vs 30.7%, p = 0.485] and endoscopic remission [60.7% vs 57.4%, p = 0.631] at Week 52. Regarding safety, no significant differences in the number of AEs [80.1% vs 77.9%] or serious AEs [13.1% vs 16.4%] were reported. However, ustekinumab-treated patients had a numerically lower rate of injection site reactions [1.0% vs 10.3%] and infections [34.0% vs 40.5%]. Despite these promising data, it is important to point out that patients enrolled in the adalimumab arm were not allowed weekly drug optimisation, thus preventing firm conclusions from being drawn. A retrospective Australian population-based study evaluated persistence of biologic agents in IBD.46 Nearly 3000 patients treated with anti-TNFα, ustekinumab, or vedolizumab were included and followed for 8219 person-years. Ustekinumab showed a higher persistence rate in CD at 12 months compared with vedolizumab, infliximab, and adalimumab [80.0% vs 73.5%, 68.1%, and 64.2%, respectively, p = 0.01].
3.Discussion
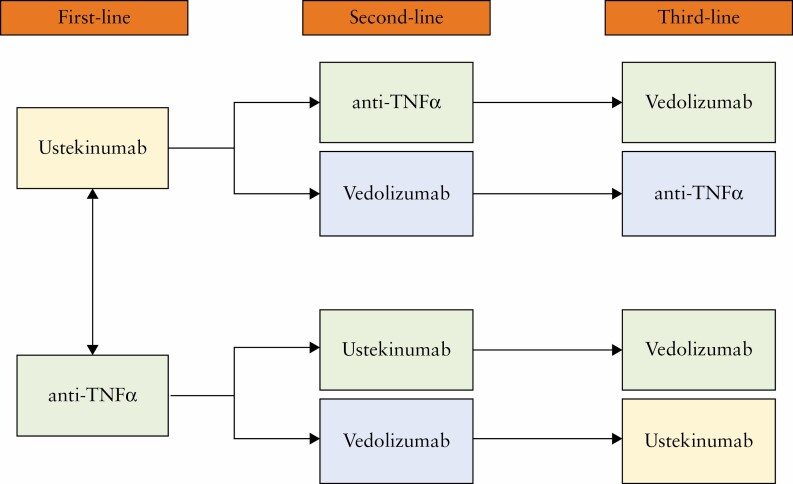
Accumulating evidence has shown the efficacy and safety of ustekinumab for the treatment of CD in both randomised clinical trials and real-life experiences. Ustekinumab is currently approved for use in patients with moderate-severe CD as first- or second-line therapy.3 However, a commonly validated algorithm for its use is not yet available and patient management is becoming increasingly personalised and tailored.47 Direct comparative studies among the biologic drugs are lacking, making the therapeutic decision challenging. The only available head-to-head trial among CD-approved molecules revealed no significant differences between ustekinumab and adalimumab, supporting use in both naive and anti-TNF-treated patients.45 Ustekinumab is a drug with a rapid mechanism of action, poor immunogenicity, and high safety profile, so it may be adopted in case of clinical disease activity in order to allow a rapid benefit for the patient, or in high-risk populations such as the elderly with multiple comorbidities.40 A recent systematic review and network meta-analysis, including 15 phase 2 and phase 3 randomised, controlled trials, showed that ustekinumab was ranked highest for induction of clinical remission compared with vedolizumab [SUCRA 0.58 vs SUCRA 0.45].48 Ustekinumab and vedolizumab have equally good safety profiles, but ustekinumab has a faster mechanism of action and appears to be more effective than anti-integrin, based on indirect studies. Although the current evidence does not preclude the use of vedolizumab as first- or second-line, we suppose that ustekinumab should be preferred and used before vedolizumab in patients with CD [Figure 1]. Moreover, costs are still a limiting factor in drug choice and ustekinumab is more expensive than anti-TNF agents [$33,798 in the first year vs ~ €6,000 per year].49–51 Instead, no difference in total costs [indirect and direct] was found between vedolizumab and ustekinumab [€97,561.08 vs €98,554.66].52 The ustekinumab patent is expected to expire by the end of 2023 in the USA and early 2024 in Europe.53 Ustekinumab biosimilars are already being tested, significantly impacting on the cost-effectiveness of the drug [Figure 2].54
Figure 1:
Proposed algorithm for use of ustekinumab in patients with moderate-severe active Crohn’s disease.
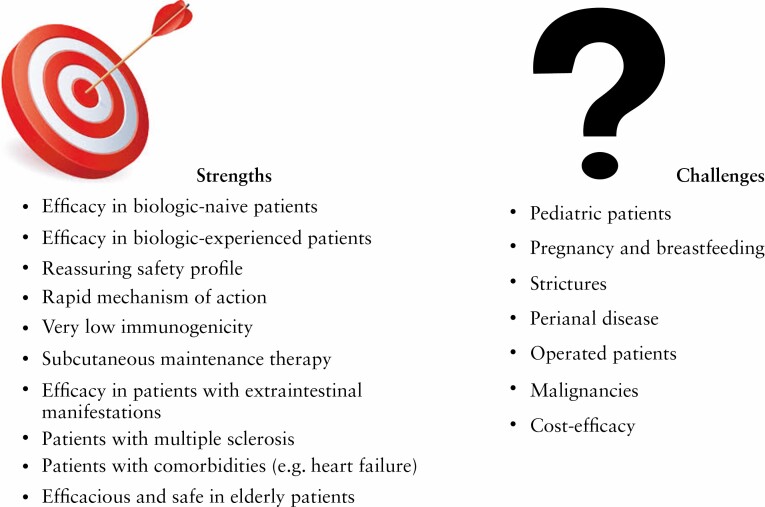
Figure 2:
Strengths and challenges of ustekinumab use in the treatment of Crohn’s disease.
It is worth underlining that therapeutic decisions are increasingly personalised, based on various factors such as extraintestinal manifestations, age of the patient, comorbidities, and oncological history. To date, there is still little evidence in paediatric patients or pregnant women. In these specific populations, anti-TNFs represent the therapeutic standard, but efficacy data of ustekinumab are promising and it can be considered a valid option in patients unsuccessfully treated with anti-TNF drugs.55–57 Ustekinumab has also proved to be effective for the management of EIMs. It is already approved and used by dermatologists and rheumatologists for the treatment of psoriasis and psoriatic arthritis, thus representing a valid alternative to traditional anti-TNF agents, unlike vedolizumab which has been associated with poor results in this setting.6,58 Regarding the treatment of perianal disease, the main medical therapy is constituted by anti-TNFs, but several studies have reported the efficacy of ustekinumab indicating the need for further studies to confirm the use of the drug in this condition.59 Similarly, further studies are needed to define the role of ustekinumab in the treatment of patients with strictures or of operated individuals in order to prevent the risk of recurrence. Finally, it is important to mention that there have been reports of biologic combination therapies, including ustekinumab + vedolizumab and ustekinumab + anti-TNF agents.60–63 This approach could be adopted in patients with EIMs. Sometimes biologic therapy is able to control intestinal disease but has less effect on EIMs. Adding a second drug could lead to improved symptom control. In this context, a balanced risk-benefit ratio is essential and the choice of a very safe drug such as ustekinumab could be a winning option.
In conclusion, randomised clinical trials and real-life studies confirm the safety and efficacy of ustekinumab for treatment of moderate-severe Crohn’s disease. Ustekinumab can be used as first- or second-line therapy and is a reliable option in specific subpopulations such as the elderly and patients with extraintestinal manifestations. Further head-to-head trials between available biologic drugs are necessary to standardise the management of patients with Crohn’s disease.
Contributor Information
Ferdinando D’Amico, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and Vita-Salute San Raffaele University, Milan, Italy.
Laurent Peyrin-Biroulet, Department of Gastroenterology and Inserm NGERE U1256, University Hospital of Nancy, University of Lorraine, Vandoeuvre-lès-Nancy, France.
Silvio Danese, Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and Vita-Salute San Raffaele University, Milan, Italy.
Funding
This paper was published as part of a supplement financially supported by AbbVie.
Conflict of interest
FD’A declares no conflict of interest. LP-B has served as a speaker, consultant, and advisory board member for Merck, Abbvie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Hospira/Pfizer, Celltrion, Takeda, Biogaran, Boerhinger-Ingelheim, Lilly, HAC-Pharma, Index Pharmaceuticals, Amgen, Sandoz, For-ward Pharma GmbH, Celgene, Biogen, Lycera, Samsung Bioepis, Theravance. SD has served as a speaker, consultant, and advisory board member for Schering-Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma, and Vifor.
Authors’ Contributions
FD wrote the article and created tables and figures. SD and LPB critically revised the manuscript. The manuscript was approved by all authors.
References
- 1. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 2. Roda G, Chien Ng S, Kotze PG, et al. Crohn’s disease. Nat Rev Dis Primers 2020;6:22. [DOI] [PubMed] [Google Scholar]
- 3. Torres J, Bonovas S, Doherty G, et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J Crohns Colitis 2020;14:4–22. [DOI] [PubMed] [Google Scholar]
- 4. Ryan C, Thrash B, Warren RB, Menter A. The use of ustekinumab in autoimmune disease. Expert Opin Biol Ther 2010;10:587–604. [DOI] [PubMed] [Google Scholar]
- 5. Johnson & Johnson. Content Lab U.S. FDA Approves STELARA® [Ustekinumab] for Treatment of Adults With Moderately to Severely Active Crohn’s Disease. | https://www.jnj.com/media-center/press-releases/fda-approves-stelara-ustekinumab-for-treatment-of-adults-with-moderately-to-severely-active-crohns-disease Accessed September 4, 2021.
- 6. European Medicines Agency. Stelara®. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/stelara Accessed September 4, 2021.
- 7. Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomised trial of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 2008;135:1130–41. [DOI] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 2012;367:1519–28. [DOI] [PubMed] [Google Scholar]
- 9. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 10. Sandborn WJ, Rebuck R, Wang Y, et al. Five-year efficacy and safety of ustekinumab treatment in Crohn’s disease: The IM-UNITI Trial. Clin Gastroenterol Hepatol; 2021, February 19. S1542–3565[21]00203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. OP35 Effect of maintenance ustekinumab on corticosteroid-free endoscopic and clinical outcomes in patients with Crohn’s disease: Week 48 analysis of the STARDUST trial. https://www.ecco-ibd.eu/publications/congress-abstracts/item/op35-effect-of-maintenance-ustekinumab-on-corticosteroid-free-endoscopic-and-clinical-outcomes-in-patients-with-crohn-s-disease-week-48-analysis-of-the-stardust-trial.html Accessed September 12, 2021.
- 12. Abstract Book UEG Week 2020: 406. https://www.nxtbook.com/ueg/UEG/Abstracts/index.php?startid=406#/p/406 Accessed September 12, 2021.
- 13. Straatmijer T, Biemans VBC, Hoentjen F, et al. Ustekinumab for Crohn’s disease: 2-year results of the initiative on Crohn and Colitis [ICC] Registry, a nationwide prospective observational cohort study. J Crohns Colitis 2021, April 28. doi: 10.1093/ecco-jcc/jjab081. [DOI] [PubMed] [Google Scholar]
- 14. Iborra M, Beltrán B, Fernández-Clotet A, et al. Real-world long-term effectiveness of ustekinumab in Crohn’s disease: results from the ENEIDA registry. Aliment Pharmacol Ther 2020;52:1017–30. [DOI] [PubMed] [Google Scholar]
- 15. Bermejo F, Jiménez L, Algaba A, et al. Re-induction with intravenous ustekinumab in patients with crohn’s disease and a loss of response to this therapy. Inflamm Bowel Dis 2022;28:41–7, [DOI] [PubMed] [Google Scholar]
- 16. Fumery M, Peyrin-Biroulet L, Nancey S, et al. Effectiveness and safety of ustekinumab intensification at 90 mg every four weeks in Crohn’s disease: a multicenter study. J Crohns Colitis 2020, September 8;doi: 10.1093/ecco-jcc/jjaa177. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 17. Adedokun OJ, Xu Z, Gasink C, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with crohn’s disease. Gastroenterology 2018;154:1660–71. [DOI] [PubMed] [Google Scholar]
- 18. Battat R, Kopylov U, Bessissow T, et al. Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with crohn’s disease. Clin Gastroenterol Hepatol 2017;15:1427–34.e2. [DOI] [PubMed] [Google Scholar]
- 19. Afif W, Sattin B, Dajnowiec D, et al. Ustekinumab therapeutic drug monitoring-impact on clinical practice: a multicenter cross-sectional observational trial. Dig Dis Sci 2021, August 17;. doi: 10.1007/s10620-021-07173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mechie NC, Burmester M, Mavropoulou E, et al. Evaluation of ustekinumab trough levels during induction and maintenance therapy with regard to disease activity status in difficult to treat Crohn disease patients. Medicine [Baltimore] 2021;100:e25111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahadevan U, Long MD, Kane SV, et al. Pregnancy and neonatal outcomes after fetal exposure to biologics and thiopurines among women with inflammatory bowel disease. Gastroenterology 2021;160:1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wils P, Seksik P, Stefanescu C, et al. Safety of ustekinumab or vedolizumab in pregnant inflammatory bowel disease patients: a multicentre cohort study. Aliment Pharmacol Ther 2021;53:460–70. [DOI] [PubMed] [Google Scholar]
- 23. DOP52 Safety of Inflammatory Bowel Disease Drugs During Pregnancy and Breastfeeding: Mothers’ and Babies’ Outcomes [DUMBO registry]. https://www.ecco-ibd.eu/publications/congress-abstracts/item/dop52-safety-of-inflammatory-bowel-disease-drugs-during-pregnancy-and-breastfeeding-mothers-and-babies-outcomes-dumbo-registry-2.html Accessed September 10, 2021.
- 24. Rosh JR, Turner D, Griffiths A, et al. Ustekinumab in paediatric patients with moderately to severely active Crohn’s disease: pharmacokinetics, safety, and efficacy results from UNISTAR, a phase 1 study. J Crohns Colitis 2021, May 26; doi: 10.1093/ecco-jcc/jjab089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim FS, Patel PV, Stekol E, et al. Experience using ustekinumab in pediatric patients with medically refractory crohn’s disease. J Pediatr Gastroenterol Nutr 2021, July 9; doi: 10.1097/MPG.0000000000003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garg R, Aggarwal M, Butler R, et al. Real-world effectiveness and safety of ustekinumab in elderly crohn’s disease patients. Dig Dis Sci 2021, June 23; doi: 10.1007/s10620-021-07117-9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asscher VER, Biemans VBC, Pierik MJ, et al. Comorbidity, not patient age, is associated with impaired safety outcomes in vedolizumab- and ustekinumab-treated patients with inflammatory bowel disease: a prospective multicentre cohort study. Aliment Pharmacol Ther 2020;52:1366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tursi A, Mocci G, Picchio M, Elisei W, Maconi G. Letter: ustekinumab for the treatment of post-surgical and refractory Crohn’s disease. Aliment Pharmacol Ther 2021;53:859–60. [DOI] [PubMed] [Google Scholar]
- 29. Buisson A, Nancey S, Manlay L, et al. Ustekinumab is more effective than azathioprine to prevent endoscopic postoperative recurrence in Crohn’s disease. United Eur Gastroenterol J 2021;9:552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Narula N, Wong ECL, Dulai PS, Marshall JK, Colombel JF, Reinisch W. Outcomes of passable and non-passable strictures in clinical trials of Crohn’s disease: a post-hoc analysis. J Crohns Colitis 2021, March 6; doi: 10.1093/ecco-jcc/jjab045. [DOI] [PubMed] [Google Scholar]
- 31..El Ouali. S. Vedolizumab and Ustekinumab for the Treatment of Symptomatic Small Bowel Stricturing Crohn’s Disease - Results from an Observational Cohort Study. https://eposters.ddw.org/ddw/2021/ddw-2021-virtual/320964/sara.el.ouali.vedolizumab.and.ustekinumab.for.the.treatment.of.symptomatic.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3DVEDOLIZUMAB+AND+USTEKINUMAB+FOR+THE+TREATMENT+OF+SYMPTOMATIC+SMALL+BOWEL+STRICTURING+CROHN%27S+DISEASE+-+RESULTS+FROM+AN+OBSERVATIONAL+COHORT+STUDY Accessed September 12, 2021.
- 32. Sands BE, Gasink C, Jacobstein D, et al. Fistula healing in pivotal studies of ustekinumab in crohn’s disease. Gastroenterology 2017;152:S185. [Google Scholar]
- 33. Chapuis-Biron C, Kirchgesner J, Pariente B, et al. Ustekinumab for perianal Crohn’s disease: the biolap multicenter study from the GETAID. Am J Gastroenterol 2020;115:1812–20. [DOI] [PubMed] [Google Scholar]
- 34. Narula N, Aruljothy A, Wong ECL, et al. The impact of ustekinumab on extraintestinal manifestations of Crohn’s disease: a post hoc analysis of the UNITI studies. United Eur Gastroenterol J 2021;9:581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tursi A, Mocci G, Maconi G. Effect of ustekinumab on extraintestinal diseases in refractory Crohn’s disease. J Crohns Colitis 2021;15:1399–400. [DOI] [PubMed] [Google Scholar]
- 36. Phillips FM, Verstockt B, Sebastian S, et al. Inflammatory cutaneous lesions in inflammatory bowel disease treated with vedolizumab or ustekinumab: an ECCO CONFER multicentre case series. J Crohns Colitis 2020;14:1488–1493. [DOI] [PubMed] [Google Scholar]
- 37. Singh S, Proctor D, Scott FI, Falck-Ytter Y, Feuerstein JD. AGA technical review on the medical management of moderate to severe luminal and perianal fistulizing crohn’s disease. Gastroenterology 2021;160:2512–2556.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Attauabi M, Burisch J, Seidelin JB. Efficacy of ustekinumab for active perianal fistulizing Crohn’s disease: a systematic review and meta-analysis of the current literature. Scand J Gastroenterol 2021;56:53–8. [DOI] [PubMed] [Google Scholar]
- 39. Guillo L, D’Amico F, Danese S, Peyrin-Biroulet L. Ustekinumab for extra-intestinal manifestations of inflammatory bowel disease: a systematic literature review. J Crohns Colitis. 2021;15:1236–43. [DOI] [PubMed] [Google Scholar]
- 40. Narula N, Wong ECL, Dulai PS, et al. Comparative efficacy and rapidity of action for infliximab vs ustekinumab in biologic naïve crohn’s disease. Clin Gastroenterol Hepatol 2021, April 7; S1542–3565[21]00394-3. [DOI] [PubMed] [Google Scholar]
- 41. Biemans VBC, van der Woude CJ, Dijkstra G, et al. Ustekinumab is associated with superior effectiveness outcomes compared with vedolizumab in Crohn’s disease patients with prior failure to anti-TNF treatment. Aliment Pharmacol Ther 2020;52:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Townsend T, Razanskaite V, Dodd S, et al. Comparative effectiveness of ustekinumab or vedolizumab after one year in 130 patients with anti-TNF-refractory Crohn’s disease. Aliment Pharmacol Ther 2020;52:1341–52. [DOI] [PubMed] [Google Scholar]
- 43. Manlay L, Boschetti G, Pereira B, et al. Comparison of short- and long-term effectiveness between ustekinumab and vedolizumab in patients with Crohn’s disease refractory to anti-tumour necrosis factor therapy. Aliment Pharmacol Ther 2021;53:1289–99. [DOI] [PubMed] [Google Scholar]
- 44. Albshesh A, Taylor J, Savarino EV, et al. Effectiveness of third-class biologic treatment in Crohn’s disease: a multi-center retrospective cohort study. J Clin Med 2021;10:2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Irving PM, Sands BE, Hoops T, et al. OP02 Ustekinumab versus adalimumab for induction and maintenance therapy in moderate-to-severe Crohn’s disease: The SEAVUE study. J Crohns Colitis 2021;15[Suppl_1]:S001–2. [Google Scholar]
- 46. Ko Y, Paramsothy S, Yau Y, Leong RW. Superior treatment persistence with ustekinumab in Crohn’s disease and vedolizumab in ulcerative colitis compared with anti-TNF biological agents: real-world registry data from the Persistence Australian National IBD Cohort [PANIC] study. Aliment Pharmacol Ther 2021;54:292–301. [DOI] [PubMed] [Google Scholar]
- 47. D’Amico F, Fiorino G, Furfaro F, et al. Patient’s profiling for therapeutic management of inflammatory bowel disease: a tailored approach. Expert Rev Gastroenterol Hepatol 2020;14:765–73. [DOI] [PubMed] [Google Scholar]
- 48. Singh S, Murad MH, Fumery M, et al. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn’s disease: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2021, October 21; S2468–1253[21]00312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aliyev ER, Hay JW, Hwang C. Cost-effectiveness comparison of ustekinumab, infliximab, or adalimumab for the treatment of moderate-severe Crohn’s disease in biologic-naïve patients. Pharmacotherapy 2019;39:118–28. [DOI] [PubMed] [Google Scholar]
- 50. Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic Review Report: Ustekinumab [Stelara/Stelara I.V.]: Janssen Inc. Indication: treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a biologic or have medical contraindications to such therapies. 2020. http://www.ncbi.nlm.nih.gov/books/NBK566686/ Accessed September 18, 2021. [PubMed]
- 51. Severs M, Oldenburg B, van Bodegraven AA, Siersema PD, Mangen MJJ; initiative of Crohn’s and Colitis . The economic impact of the introduction of biosimilars in inflammatory bowel disease. J Crohns Colitis 2017;11:289–96. [DOI] [PubMed] [Google Scholar]
- 52. Holko P, Kawalec P, Pilc A. Cost-effectiveness analysis of crohn’s disease treatment with vedolizumab and ustekinumab after failure of tumor necrosis factor-α antagonist. PharmacoEconomics 2018;36:853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mehr S. The Next-Generation Biosimilar Race: A Tale of Two AutoimmuneBiologics. 2020. https://biosimilarsrr.com/2020/08/14/the-next-generation-biosimilar-race-a-tale-of-two-autoimmune-biologics/ Accessed September 18, 2021.
- 54. Gao L, Li Q, Zhang H, et al. A biosimilarity study between QX001S and ustekinumab in healthy chinese male subjects. Front Pharmacol 2021;12:675358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van der Woude C J, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis 2015;9:107–24. [DOI] [PubMed] [Google Scholar]
- 56. Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016;150:734–57.e1. [DOI] [PubMed] [Google Scholar]
- 57. van Rheenen PF, Aloi M, Assa A, et al. The medical management of paediatric Crohn’s disease: an ECCO-ESPGHAN Guideline Update. J Crohns Colitis 2020, October 7; doi: 10.1093/ecco-jcc/jjaa161. [DOI] [PubMed] [Google Scholar]
- 58. Chateau T, Bonovas S, Le Berre C, Mathieu N, Danese S, Peyrin-Biroulet L. Vedolizumab treatment in extra-intestinal manifestations in inflammatory bowel disease: a systematic review. J Crohns Colitis 2019;13:1569–77. [DOI] [PubMed] [Google Scholar]
- 59. Panés J, Rimola J. Perianal fistulizing Crohn’s disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol 2017;14:652–64. [DOI] [PubMed] [Google Scholar]
- 60. Privitera G, Onali S, Pugliese D, et al. Dual targeted therapy: a possible option for the management of refractory inflammatory bowel disease. J Crohns Colitis 2020, July 17; doi: 10.1093/ecco-jcc/jjaa149. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 61. Yang E, Panaccione N, Whitmire N, et al. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn’s disease. Aliment Pharmacol Ther 2020;51:1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ahmed W, Galati J, Kumar A, et al. Dual biologic or small molecule therapy for treatment of inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021, March 31; S1542–3565[21]00344-X. [DOI] [PubMed] [Google Scholar]
- 63. Dolinger MT, Spencer EA, Lai J, Dunkin D, Dubinsky MC. Dual biologic and small molecule therapy for the treatment of refractory pediatric inflammatory bowel disease. Inflamm Bowel Dis 2021;27:1210–4. [DOI] [PubMed] [Google Scholar]