Abstract
Interleukin 23 [IL-23] plays a key role in the pathogenesis of both Crohn’s disease [CD] and ulcerative colitis [UC], promoting a Th17 cell-related immune response. The combined blockade of IL-23 and IL-12 with ustekinumab has been demonstrated to be safe and effective in the treatment of inflammatory bowel disease [IBD]. Studies on preclinical models and observations of other immune-mediated diseases, such as psoriasis, suggest that the selective inhibition of IL-23 could be beneficial in IBD. Four monoclonal antibodies [risankizumab, mirikizumab, brazikumab and guselkumab] are currently in advance clinical trials for either CD or UC. In this review, we provide an overview of the main results from published studies of selective anti IL-23 agents.
Keywords: Interleukin 23, interleukin 12, Crohn’s disease, ulcerative colitis, anti-IL23 monoclonal antibodies
1. Background
Inflammatory bowel diseases [IBD], such as Crohn’s disease [CD] and ulcerative colitis [UC], are chronic immune-mediated conditions affecting the gastrointestinal tract, and their treatment is based on an increasing repertoire of drugs, both biologics and oral synthetics that target the adaptive immune system. While the pathogenesis of IBD is not completely clear, robust evidence points to an underlying dysregulated immune response to gut microbiota in genetically susceptible individuals. Specific cytokines in the immune network are attractive as therapeutic targets to control inflammation and maintain disease remission. In the last two decades several advanced therapeutic agents have been developed including monoclonal antibodies and synthetic small molecule drugs. These agents inhibit specific molecular pathways involved in the pathogenesis of the disease such as tumour-necrosis-factor [TNF], integrins, Janus kinase [JAK], a family of non-receptor tyrosine kinases and interleukin 12 and 23 [IL-12/IL-23] with the ultimate goal of treating the disease while minimizing side effects.
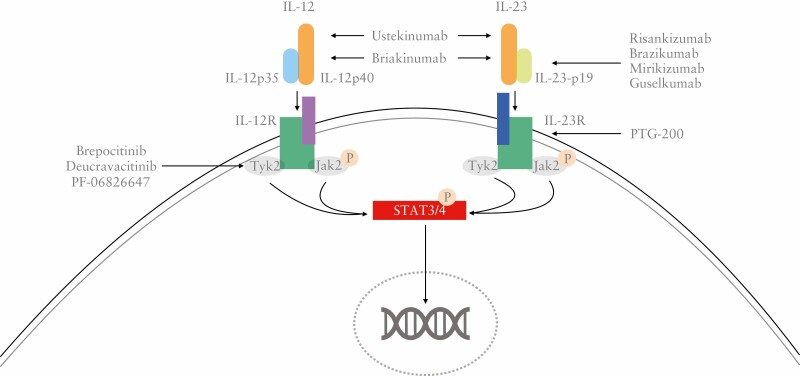
IL-12 is a heterodimeric cytokine formed by a p35 and p40 subunit. It was initially identified as a natural killer [NK] cell stimulating factor in response to mitogens1 and later shown to be derived from dendritic cells as well as macrophages and neutrophils in response to antigens.2 The IL-12 family includes heterodimeric cytokines IL-12, IL-23, IL-27 and IL-35. IL-12 acts in steering T-cell differentiation towards the Th1 interferon [IFN]-gamma-producing lineage,3 and more broadly, bridging innate and adaptive immune responses.4 Blockade of IL-12 p40 was effective in several animal models of immune-mediated conditions, including colitis.5 The observation that agents targeting only the p35 subunit did not provide the same benefit or even worsened inflammation led to the discovery of a new interleukin, IL-23, constituted by the same p40 subunit and another specific p19 subunit.6 This suggested that the therapeutic action of agents targeting p40 was due to the combined blockade of both IL-12 and IL-23 rather than IL-12 alone.6 In keeping with this hypothesis, further studies elucidated how IL-23 and its receptor [IL-23R] exert a complex immunoregulatory effect on T helper 17 [Th17] cells with IL-17A as the main effector molecule.4,7 Furthermore, IL-23 induces a strong pro-inflammatory activity activating several different cells other than Th17. Indeed, when IL-23 binds to its specific receptor, IL-23R, this activates the Janus kinases JAK-2 and Tyk-2, which in turn induce nuclear translocation of the transcription factors STAT3 and STAT4.8 Some functions of IL-23 are specific and differ from those of IL-12. The latter acts mainly on naïve T cells, whereas IL-23 preferentially activates memory T cells expressing IL-23R.8,9 In addition, genetic studies showed that polymorphisms of IL-23R are associated with CD and UC, and loss of function mutations of IL-23R are protective towards intestinal inflammation, further supporting the role IL-23 in the pathogenesis of IBD.10
Increased levels of IL-2311 and IL-17A12 were found in murine models of IBD, and confirmed in UC patients,13 in whom levels of IL-17A also reflected clinical disease severity and predicted the course of disease.14 Building on this, blockade of IL-23 and IL-17 appeared to be an effective therapeutic strategy in animal models of IBD, supporting the role of these cytokines in the pathogenesis of IBD.5,11,12,15
With regard to IL-17, however, clinical trials in CD of secukinumab, an anti-IL17 monoclonal antibody,16 and brodalumab, an anti IL-17R,17 were disappointing, with no evidence of efficacy and even worsening of the disease, probably as a result of its deleterious effect on the epithelial barrier.18 This highlighted how increased levels of a cytokine in patients’ serum and tissues do not necessarily imply an irreplaceable role for the cytokine in the pathogenesis of that disease.19
On the IL-12/IL-23 axis, the first agents targeting the two cytokines to be investigated in humans were briakinumab and ustekinumab; a third antibody, SMART, targeting exclusively IL-12 in its dimeric form was discontinued in 2003 due to a probable lack of efficacy.4,20 The development of briakinumab, an anti-p40 monoclonal antibody, began before IL-23 was discovered, and it later became clear that its biological effect was exerted through the inhibition of both IL-12 and IL-23 cytokines that share the same p40 subunit. In the proof-of-concept study of briakinumab in moderate to severe CD, patients in the treatment arm had higher response rates after 7 weeks but benefits were not sustained at week 18 and rates of remission were not statistically different.21 Development of briakinumab, which was also investigated in psoriasis, multiple sclerosis and rheumatoid arthritis, was later discontinued in all indications due to safety concerns regarding a possible increase in major adverse cardiovascular events [MACE].22
Ustekinumab was the second monoclonal antibody targeting IL-12/IL-23 to be developed. Initially licensed for psoriasis and psoriatic arthritis, it received regulatory approval for CD in 2016 and UC in 2019. In CD, a phase 2a trial,23 a phase 2b trial [CERTIFI]24 and two phase 3 trials [UNITI-I and UNITI-2] in both naïve and anti-TNF-experienced patients, including inadequate responders to anti-TNF patients, demonstrated the efficacy of intravenous induction treatment in reduction of Crohn’s disease activity index [CDAI] and, in a substudy, an improvement in simple endoscopic score [SES-CD].25,26 The IM-UNITI trial proved the efficacy of a subcutaneous maintenance treatment every 8 or 12 weeks to week 52 for those who responded in the two induction trials.25 Similarly positive findings came from the UNIFI trial in UC,27 with results showing efficacy in terms of clinical remission, as well as endoscopic [Mayo endoscopic subscore ≤1] and histological [according to Geboes, Nancy or Robarts histological indexes] remission.28 Additional long-term data from both trials and real world cohorts confirmed sustained benefits of maintenance therapy through 5 years in CD and 2 years in UC.29,30 In both CD and UC, the efficacy of ustekinumab can be observed even after 6–8 weeks, demonstrating a relatively fast onset of action. Accordingly, some case reports described the successful use of ustekinumab alone or in combination with cyclosporine as rescue therapy for acute severe CD31 and UC.32 The overall safety profile of ustekinumab appeared to be good with low infection rates similar to placebo,33,34 and differently from briakinumab, no signal regarding MACE has been detected.35
Growing evidence supported the investigation of selective anti-IL-23 agents in IBD. First, as already mentioned, some preclinical studies showed how IL-12 selective blockade was not beneficial in IBD.6 Second, other studies in animal models suggested that inhibition of IL-23 alone was more effective than that of combined IL-12/IL-23.11,36 Finally, positive results on IL-23 selective agents in plaque psoriasis were published in 2015.37,38 More recently, a phase III clinical trial in plaque psoriasis suggested that risankizumab, a monoclonal antibody targeting IL-23, achieved higher rates of disease improvement compared with ustekinumab, an IL-12/IL-23 inhibitor.39
2. Mechanism of action: Distinctiveness of IL-23 antagonism from IL-12/23 antagonism
IL-12 drives the Th1 pathway [Figure 1] characterized by signature cytokines IFNγ and TNF, and stimulates group 1 innate lymphoid cells [ILCs]. By contrast, IL-23 promotes the Th17 pathway and stimulates group 3 ILCs and invariant NKT cells. There is evidence that a Th17 cytokine, IL-22, plays a role in promoting colonic neoplasia, an important complication of long-standing colonic IBD, and so mechanistically IL-23 antagonism may be relevant.40 In small animal models, IL-23 antagonism may have an inhibitory effect on intestinal fibrosis.41 However, differences in efficacy between IL-12/23 [p40] inhibition and IL-23 [p19] inhibition in diseases such as psoriasis may also be due to characteristics and dosage of drugs rather than distinctive mechanism of actions. Ileal and colonic biopsies analysed by bulk RNA sequencing (RNAseq) analysis from the phase II study of risankizumab in CD highlighted a wide range of disease-relevant pathways modulated by risankizumab treatment, which included epithelial biology, second messenger-mediated signalling, immune response, lymphocyte and leukocyte activation, lymphocyte differentiation and cell–cell adhesion.42
Figure 1.
Overview of IL12 and IL23’s pathways in the pathogenesis of inflammatory bowel disease.
Several agents specifically targeting IL-23 are currently under investigation for both CD and UC, in this review we aim to provide an overview of those that are in an advanced stage of development [Figure 2].
Figure 2.
Overview of agents targeting the IL12-IL23 pathway.
2.1. Risankizumab
Risankizumab [Skyrizi] is a humanized IgG1 monoclonal antibody that selectively inhibits IL-23 by targeting its p19 subunit. In 2019 it was approved by both the FDA and EMA for the treatment of moderate-to-severe plaque psoriasis.43
2.1.1. Crohn’s disease
In the field of IBD, risankizumab has been studied in moderate-to-severe CD in a phase II randomized, placebo-controlled trial [NCT02031276].44 This study included 121 patients with moderate-to-severe disease (defined as CDAI score of 220–450 and a CDEIS [Crohn’s disease endoscopic index of severity] ≥7 or ≥4 for isolated ileitis). Nearly all participants [93%] had previous exposure to anti-TNF or vedolizumab, a marker of drug resistance and worse response. The trial consisted of three treatment periods starting with a 12-week induction in which patients were randomized 1:1:1 to receive 600 mg IV risankizumab, 200 mg IV risankizumab or placebo at weeks 0, 4 and 8. The primary endpoint, clinical remission [CDAI < 150] at week 12, was achieved in 30.5% of patients who received any dose of risankizumab compared to 15.4% in the placebo group [p = 0.0489]. Risankizumab was superior to placebo also in the secondary endpoints such as clinical response [defined as CDAI < 150 or ≥100-point decrease] [39% vs 20.5%], endoscopic remission [CDEIS ≤ 4 or ≤2 for isolated ileitis] [17% vs 3%], endoscopic response [≥50% drop in CDEIS] [32% vs 13%], and deep remission [clinical and endoscopic remission] [7% vs 0%]. All outcomes favoured the 600-mg dosage.44 In the second period, the open-label extension of the study, patients who achieved deep remission at week 12 underwent a drug washout, whereas the remaining patients received 12 additional weeks of the high-dose, 600-mg, risankizumab every 4 weeks. At week 26, 53% of patients were in clinical remission, 55% originally in the placebo arm, 59% in the 200-mg risankizumab arm and 47% in the 600-mg arm.45 Subsequently, all patients in clinical remission at week 26, regardless of their previous randomization, received 180 mg SC risankizumab maintenance for another 26 weeks. At week 52, 71% of participants were in clinical remission, 35% in endoscopic remission and 29% in deep remission. Finally, an open-label extension study enrolled 65 patients who had either achieved clinical response without clinical remission at week 26 after the extended induction phase, or who had achieved either clinical response or remission at week 52 in a maintenance study while receiving SC risankizumab 180 mg q8w.46 In the open-label extension study, participants received risankizumab for a median 33 months, and during this period efficacy outcomes were maintained [proportion of patients in clinical remission >71% and endoscopic remission >42%].46
Results from two phase 3 trials, ADVANCE [NCT03105128] and MOTIVATE [NCT03104413], have recently been presented.47–49 ADVANCE is a double-blind, randomized, placebo-controlled trial that assessed the efficacy and safety of risankizumab for induction therapy of moderate to severe CD. A total of 850 patients [490 previously exposed to biologics] were randomized 2:2:1 to receive IV risankizumab 600 mg, 1200 mg or placebo at baseline, week 4 and week 8. For regulatory requirements, the clinical response endpoint varied between USA [CDAI-based] and outside USA analyses [stool frequency- and abdominal pain-based]. At week 12. risankizumab was superior to placebo in all primary endpoints including CDAI-based clinical remission [CDAI < 150] [45.2% in the 600-mg group, 41.6% in the 1000-mg group vs 25.2% in placebo], clinical remission based on patient-reported stool frequency [SF] and abdominal pain [SF ≤ 2.8 and not worse than baseline and abdominal pain score ≤1 and not worse than baseline] [43% and 41% vs 21%], and endoscopic response [at least 50% decrease in centrally-read SES-CD][40.3% and 32.2% vs 12.0%].47
The twin study, MOTIVATE, only included CD patients intolerant or who had inadequate response to biologics. A total of 569 participants were randomized 1:1:1 to receive the same doses of risankizumab [600 or 1200 mg IV every 4 weeks] or placebo. Efficacy was measured with the same clinical and endoscopic endpoints. Consistently with the more refractory population, efficacy was numerically slightly lower. At week 12, CDAI-based clinical remission was achieved by 42% and 41% in the risankizumab 600-mg and 1200-mg arms compared to 19% in placebo, clinical remission defined on normalized stool frequency and abdominal pain in 35% and 39% in the same groups vs 19% placebo, and endoscopic response in 29% and 34% vs 11%. All comparisons with placebo were statistically significant.48,49
Further analysis of the ADVANCE and MOTIVATE trials demonstrated that risankizumab at both 600 and 1200 mg induced higher clinical response and remission compared to placebo as early as week 4.50
Patients who responded to the 12-week induction treatment in either ADVANCE or MOTIVATE trials were enrolled in the phase 3 maintenance study FORTIFY trial [NCT03105102]. A total of 462 participants were re-randomized 1:1:1 to receive 180 or 360 mg of SC risankizumab or placebo, every 8 weeks. At week 52 clinical remission according to CDAI [US analysis] occurred in 55% of the 180-mg group, 52% in the 360-mg group compared to 41% in placebo; in the outside US analysis, clinical remission, defined on stool frequency and abdominal pain, occurred in 46% and 52% vs 40% in the same groups, but the difference between the 180-mg group and placebo did not reach statistical significance. The rate of endoscopic response, a co-primary endpoint, was 47% in both treatment groups compared to 22% in placebo.51
2.1.2. Safety
Numerous studies in dermatology showed a reassuring safety profile for risankizumab, although the doses used are less than half of those in CD. A meta-analysis of clinical trials evaluating risankizumab for plaque psoriasis showed no difference in serious adverse events [SAEs] compared to placebo (odds ratio [OR] 0.86, p = 0.18), but it did find an increase in risk number of infections [OR 1.44, p = 0.02].52 In the field of IBD no new safety signals were detected. The first period of the phase 2 trial, albeit short, did not find any difference in adverse events between placebo [82%] and any risankizumab arm [78% for 200 mg and 75.6% for 600 mg], and furthermore the majority of SAEs were worsening of CD.44 Results from the open-label extension and maintenance periods, which included a total of 72 patient-years, were consistent with the previous induction period. The most common AEs were arthralgia [22% of patients], headache [20%], abdominal pain [18%], nasopharyngitis [16%], nausea [16%] and pyrexia [13%], the majority of which were mild or moderate and considered unrelated to treatment.45 Severe AEs, mainly worsening of CD and intestinal obstruction, occurred in 11% of patients, while serious infections, with no apparent common pattern, were recorded in 4%. In the overall analysis of all trial periods, eight cases of fungal infection were recorded.45 Safety data from the phase 3 ADVANCE study were also encouraging, with no significant differences between risankizumab and placebo in rates of AEs [56.3% in the 600-mg group, 51.3% in the 1200-mg group vs 56.5% in placebo]. Rate of serious AEs and serious infections [7.2% and 3.8% vs 15.1%; 0.8% and 0.5% vs 3.8%] were numerically, although not significantly, less frequent in risankizumab over placebo.47
Consistently with previous findings, in the phase 3 FORTIFY maintenance period no new safety risks were observed. SAEs occurred in 12.3% and 13.4% of patients in the risankizumab 180- and 360-mg groups, respectively, compared to 12.5% in the control group, whereas no significant difference was found in infectious risk. Two major cardiovascular events were recorded, one in the 360-mg arm and one in the placebo, and both were deemed unrelated to the study drug.53 Similarly, in the open-label long-term extension study of the phase 2 trial in CD no new safety signal was detected, and the rate of serious AEs was 24.6/100 patient-years although mainly related to the gastrointestinal tract and thus possibly reflecting disease activity. No major cardiovascular events, deaths or reactivation of latent tuberculosis occurred.46
Development of trial emergent anti-drug antibodies occurred in 4% [3/76]44 of patients during the phase 2 induction trial, 8% in the maintenance phase45 and 12.5% [8/65] in the open-label long-term extension study.46 In all cases titres were low and antibodies were not neutralizing.
Overall, risankizumab results in CD are promising. Interestingly, a phase 3 head-to-head study comparing risankizumab to ustekinumab for CD is also recruiting and expected to finish in 2023 [NCT04524611].
Phase II and III trials assessing risankizumab in UC are under way [NCT03398148,54 NCT0339813551] with results expected in 2023. In addition, more safety data could emerge from ongoing trials in hidradenitis suppurativa [NCT03926169], ankylosing spondylitis [NCT02047110], asthma [NCT02443298] and atopic dermatitis [NCT03706040].
2.2. Mirikizumab
Mirikizumab [LY3074828] is another humanized IgG4-variant monoclonal antibody selectively targeting the IL-23 p19 subunit in advanced development for several indications including UC and CD.
2.3. Ulcerative colitis
The efficacy of mirikizumab in UC was assessed in the AMAC study, a randomized, double-blind, placebo-controlled phase 2 clinical trial, conducted in 14 countries between 2016 and 2017.55 Patients with moderate to severe UC, defined as total Mayo score of 6–12, were randomized 1:1:1:1 to receive a 12-week induction with 50 mg mirikizumab exposure-based [EB] dosing, 200 mg mirikizumab EB dosing, 600 mg mirikizumab fixed-dose or placebo, all administered intravenously every 4 weeks. The exposure-based dosing allowed for dose adjustments titrated on drug serum concentration. The primary endpoint of the study was clinical remission [Mayo subscores 0 for rectal bleeding, ≥1 decrease from baseline for stool frequency, and 0 or 1 for centrally read endoscopy] at week 12. This was achieved by 15.9, 22.6 and 11.5% of patients in the 50-, 200- and 600-mg groups vs 4.8% in the placebo arm. The difference with placebo was statistically significant only for the 200-mg EB group [p = 0.004], whereas the 50-mg EB and 600-mg fixed dose did not reach significance [p = 0.066 and p = 0.142, respectively]. The secondary endpoint included clinical response at week 12 that was significantly higher in all the three treatment groups [41.3%, 59.7% and 49.2%] compared to placebo [20.6%], with greater benefit for the 200-mg EB regimen, and endoscopic improvement that was significantly higher in the 50- and 200-mg EB groups [23.8% and 30.6% respectively vs 6.3% in placebo], but not in the 600-mg fixed dose [13.1%]. Endoscopic remission was observed only in 2.7% of treated patients compared with 1.6% of the placebo group.55
Although the primary endpoints of the induction trial were not entirely met, in the open-label extended induction trial, 12 additional weeks of high doses of mirikizumab provided incremental benefits.56 In this second study period, patients who completed the original induction trial were re-randomized to receive 600 or 1000 mg extension-induction. Of those who previously did not respond to mirikizumab induction, 15% and 9.4% respectively achieved clinical remission and 50% and 43.8% clinical response. Endoscopic improvement was reported in 20% and 15.6% of the two groups, while endoscopic remission only in 3% of the 1000-mg group and in none of the 600-mg group. In patients who had previously received placebo and were therefore naïve to mirikizumab, 12 weeks of mirikizumab induced clinical remission in 25% in both the 600- and 1000-mg groups, clinical response in 58.3 and 71.9%, respectively, endoscopic improvement in 25 and 37%, respectively, and endoscopic remission only in 9.3% of patients receiving 1000 mg and in none in those receiving 600 mg.56 These findings were in line with those observed in the 200-mg group in the induction phase. Of note, in the extended induction trial patients previously exposed to multiple biologics were proportionally more represented than in the original induction trial, a difference that is likely to negatively influence the efficacy.
In the maintenance phase, a total of 93 patients who responded to the induction treatment were randomized 1:1 to receive 200 mg mirikizumab every 12 [q12w] or 4 [q4w] weeks. At week 52, clinical remission was achieved in 39.7 and 53.7% of patients in the q12w and q4w arms, respectively, and endoscopic improvement [centrally read Mayo endoscopic subscore 0 or 1] in 28.3 and 14.9% in the q12w and q4w groups, respectively. Another signal of mirikizumab sustained benefit was the high percentage of patients [35%] with clinical response, but not remission, at week 12 who later achieved clinical remission at week 52.55 In addition, significant improvements in patient-reported quality of life assessed through the SF-36 v2 questionnaire were observed at both weeks 12 and 52.57 Overall, although mirikizumab did not meet the primary endpoint at week 12, evidence of its efficacy in inducing and maintaining clinical and endoscopic response is robust.
Several trials of mirikizumab in UC are ongoing. LUCENT 1 [NCT03518086] and LUCENT 2 [NCT03524092] are phase 3 trials assessing induction and maintenance treatment, and LUCENT 3 [NCT03519945] is a long-term open-label extension of treatment trial; SHINE 1 [NCT04004611] is a unique phase 2 open-label trial in children and teenagers aged 2–17 years; and LUCENT ACT [NCT04469062] is a placebo and active treatment controlled study comparing mirikizumab with vedolizumab and placebo.
2.3.1. Crohn’s disease
Mirikizumab is also being investigated in CD. Results from the phase 2 SERENITY trial [NCT02891226] have been recently presented.58–60 A total of 191 patients with moderate to severe ileal or colonic CD were randomized 2:1:1:2 to receive placebo, 200, 400, 600 or 1000 mg of IV mirikizumab at weeks 0, 4 and 8. The primary endpoint of endoscopic response [defined as at least 50% reduction from baseline SES-CD] at week 12 was achieved by all mirikizumab groups [25.8, 37.5 and 43.8% in the 200-, 600- and 1000-mg groups, respectively] compared to placebo [10.9%]. In total, 15.6 and 20.3% of patients receiving 600 and 1000 mg mirikizumab reached endoscopic remission compared to 1.6% of those on placebo, whereas for the 200-mg group the difference was not significant.58 Changes in clinical criteria such as CDAI, stool frequency [SF] and abdominal pain [AP] confirmed mirikizumab efficacy. Statistically significant improvement of CDAI and SF could be observed as early as week 4 for some dosages, and at week 12 the high dosages groups [600 and 1000 mg] demonstrated superiority compared to placebo for all three clinical outcomes.59
In the maintenance trial, patients previously treated with mirikizumab who achieved at least a 1 point reduction in SES-CD were randomized 1:1 to continue the IV treatment q4w or 300 mg mirikizumab SC q4w. Results in the two groups were encouraging, with endoscopic response in 58.5 and 58.7% in the IV and SC groups, respectively, endoscopic remission was maintained from week 12 to week 52 in 50.0 and 64.3%, and CDAI remission occurred in 39.0 and 56.5% in the same groups. Treatment-emergent AEs were similar in the two arms, occurring in 75.6 and 76.1%, with only two SAEs in the SC group.60
The phase 3 VIVID 1 trial [NCT03926130] and its open-label extension study VIVID 2 [NCT04232553] are underway.
2.3.2. Safety
Mirikizumab showed a favourable safety profile in the phase 2 trials in both UC and CD. During the maintenance phase in UC, although treatment-emergent AEs were observed in around 70% of patients the great majority were mild, such as nasopharyngitis, headache and arthralgia. Only 2.2% of patients in the q12w had to discontinue treatment due to AEs and none in the q4w arm. All the SAEs recorded were considered to be unrelated to the drug. One patient with severe UC suffered a colonic perforation after a sigmoidoscopy; the remaining SAEs were worsening of UC, gastroenteritis, appendicitis, respiratory tract infection and non-melanoma skin cancer. No hypersensitivity reactions were observed.
2.4. Brazikumab
Brazikumab [MEDI2070, formely AMG139] is an IgG2 human monoclonal antibody targeting the IL-23 p19 subunit. A phase 2a trial [NCT01714726] assessed the safety and efficacy of brazikumab for induction treatment in 119 CD patients previously non-responders to anti-TNF.61 Participants were randomized 1:1 to receive 700 mg IV brazikumab [at the time known as MEDI2070] at baseline and week 4 or placebo. After week 12 patients entered the open label extension study [OLE] and continued to receive 210 mg brazikumab SC every 4 weeks through week 100 [week 112 from baseline], although efficacy analysis are not available for the OLE.
At week 8 the treatment arm had a significantly higher proportion of clinical response [CDAI decrease >100 points] and clinical remission [CDAI < 150] than the placebo group [49.2% vs 26.7% and 27.1% vs 15.0%, respectively]. Moreover, when considering a composite outcome of CDAI plus reduction of 50% in either faecal calprotectin or C-reactive protein, the difference was 42.4% vs 10% [p < 0.001] in the same groups. the benefits of brazikumab were sustained also through week 24 in patients who received continuous treatment compared with placebo. Unfortunately, the study did not provide endoscopic nor imaging assessment of the disease.61
Brazikumab’s safety profile appears reassuring. In the induction phase, placebo and treatment arms recorded similar proportions of treatment-emergent AEs [65 and 67%, respectively] and SAEs [8% in both]. The OLE study did not detect any new safety signal; overall 11.5% of patients suffered a treatment-emergent AE that required discontinuation, and 19.2% experienced an SAE, half of which were CD-related. Clinically relevant infectious complications were numerically greater in the placebo group than in the active treatment [seven vs four] and no opportunistic infections were observed. Only three of 199 patients developed antidrug antibodies. Of note, levels of IL-22, a cytokine regulated by IL-23, were found to be predictive of response to brazikumab treatment. If confirmed this could help in selecting patients for more accurate treatment, but this observation has not yet been replicated.61
A phase 2b/3 INTREPID programme is underway to assess brazikumab compared to placebo or adalimumab in CD.
Brazikumab is also been investigated in UC in the EXPEDITION trial [NCT03616821], a head to head trial comparing brazikumab against vedolizumab. After the 10-week period, patients previously enrolled in the EXPEDITION [NCT04277546] study were included in an additional open-label extended induction study.
2.5. Guselkumab
Guselkumab [Tremfya] is a fully human IgG1-lambda monoclonal antibody targeting IL-23 approved for treatment of plaque psoriasis and psoriatic arthritis and currently under investigation in IBD.
Results from the interim analysis of the phase 2 trial GALAXY 1 assessing the efficacy and safety of guselkumab in CD have recently been presented.62 The study was designed to compare three different dosages of guselkumab [200, 600 and 1200 mg], administered IV every 4 weeks, with placebo and with ustekinumab [6 mg/kg IV and then 90 mg SC at week 8] as reference. A total of 250 patients, half of whom were previously exposed to biologics, were randomized 1:1:1:1:1 into the five arms. At week 12 rates of clinical remission [CDAI < 150] were significantly higher in all guselkumab groups [54.0, 58.0 and 50.0% for 200, 600 and 1200 mg, respectively] and ustekinumab [44.9%] compared with placebo [15.7%]. Similarly, more patients had a clinical response [CDAI decrease of at least 100 points] with guselkumab [66, 68 and 64%] and ustekinumab [67.3%] than with placebo [23.5%]. Finally, evaluation of the combined clinical and biochemical markers also supported guselkumab’s efficacy. Although the study was not powered to assess differences between guselkumab and ustekinumab, the two drugs appeared to achieve similar rates of all outcomes. Importantly, in all guselkumab groups, the CDAI decrease was clearly appreciable as early as week 4.62
Guselkumab has also been investigated in combination therapy with golimumab, an anti-TNF, for patients with active moderate to severe UC in the phase 2a randomized, double-blind, active controlled VEGA study [NCT03662542]. In parallel, the placebo-controlled phase 2b/3 QUASAR trial [NCT04033445] is actively recruiting and results are expected by 2026.
Safety data of guselkumab are not yet available in the setting of IBD. However, the general safety profile can be inferred from pivotal trials and real world experiences in psoriasis and psoriatic arthritis. In these indications, guselkumab had rates of AE similar to placebo, with no significant increase of infectious risk and no particular safety signal.63,64 A note of caution is worthwhile, as doses in trials for IBD are roughly double those approved for psoriasis and psoriatic arthritis [Table 1].
Table 1.
Summary of key efficacy findings
| Drug | IBD type | Phase | Patients | Induction efficacy* | Maintenance efficacy*‡ |
|---|---|---|---|---|---|
| Risankizumab | CD | 2 | 121 | Figures for combined dosages - clinical remission 30.5% vs 15.4% - clinical response 39% vs 20.5% - endoscopic remission 17% vs 3% - endoscopic response 32% vs 13% |
- clinical remission 71% - endoscopic remission 35% - endoscopic + histological remission 29% |
| CD | 3 ADVANCE | 850 | Figures for 600 and 1200 mg - Clinical remission [CDAI] 45%, 42% vs 25% in placebo - endoscopic response 40%, 32% and 12% |
Figures for 180 and 360 mg - clinical remission [CDAI] 55%, 52% vs 41% - endoscopic response 47%, 47% vs 22% |
|
| CD | 3 MOTIVATE | 569 | Figures for 600 and 1200 mg - Clinical remission [CDAI] 42%, 41% vs 19% in placebo - endoscopic response 29%, 34% and 11% |
||
| Mirikizumab | CD | 2 | 191 | Figures for 200, 600 and 1000 mg - endoscopic response 25.8%, 37.5%, 43.8%, vs 10.9% in placebo - CDAI change −82, −142, −104 vs −31 placebo |
Figures for IV and SC groups - endoscopic response 58% and 58% - endoscopic remission 50% and 64% - clinical remission 39% and 56.5% |
| UC | 2 | 249 | Figures for combined dosages - clinical remission 17.4% vs 4.8% - clinical response 50.0% vs 20.6% - endoscopic remission 2.7% vs 1.6% - endoscopic improvement 22.6% vs 6.3% |
Figures for q4w and q8w - clinical remission 46.8%, 37.0% vs 7.7% - clinical response 80.9%, 76.1% vs 53.8% - endoscopic remission 14.9%, 28.3% vs 7.7% - endoscopic improvement 57.4%, 47.8% vs 15.4% |
|
| Brazikumab | CD | 2 | 119 | Figures for combined dosages - clinical remission 27.1% vs 15.0% - clinical response 49.2% vs 26.7% |
|
| Guselkumab | CD | 2 | 250 | Figures for combined dosages - clinical remission 54.0 % vs 15.7% - clinical response 67.3% vs 23.5% |
CD, Crohn’s disease; UC, ulcerative colitis; IBD, inflammatory bowel disease; q4w, every 4 weeks; q8w, every 8 weeks; SC, subcutaneous; CDAI, Crohn’s disease activity index score.
*Induction data refer to 12 weeks of treatment except for brazikumab [week 8], and maintenance data refer to 52 weeks of treatment.
†All comparisons with placebo were statistically significant.
‡Participants of maintenance studies are a subgroup of total participants of the induction phases.
3. Conclusion
The well-established efficacy of IL-12/IL-23 blockade with ustekinumab provides an encouraging stepping stone for IL-23-selective agents. In CD, phase 2 trials of risankizumab, mirikizumab, guselkumab and brazikumab showed promising results, with clear benefits over placebo in terms of clinical response, and, in the case of mirikizumab and risankizumab, also endoscopic response. Efficacy signals were detected as early as after 4 weeks, supporting a rapid action, a desirable characteristic especially in the setting of more severe disease. Moreover, a comparison between guselkumab and ustekinumab, although underpowered, suggest a similar efficacy profile.
In line with data from other indications, the safety profile of all anti-IL-23 agents appears particularly favourable, with no clear increase in infection risk, and no relevant signals related to neoplastic risks or cardiovascular complications detected across studies in IBD. All IL-23-selective monoclonal antibodies allow for subcutaneous maintenance administration with no major concerns regarding injection site reaction. Related to this, data on immunogenicity are also reassuring with relatively modest rates of anti-drug antibodies detected.
From a trial perspective, the ongoing registration studies of anti IL23 agents will provide several exciting novelties. Different studies with active treatment as reference are underway comparing brazikumab and mirikizumab with vedolizumab in UC, and brazikumab with adalimumab, and risankizumab and guselkumab with ustekinumab in CD. Evidence from these studies will be extremely important to position anti-IL-23 agents in the therapeutic algorithm. Secondly, a trial assessing the combination therapy of guselkumab and golimumab in UC, on top of providing useful data on guselkumab’s efficacy and safety, will expand our knowledge on combination biologics, an exciting new field in which blockade of IL-23 could play a major role. An ongoing trial of mirikizumab is also addressing the general lack of data on biologics in children with IBD. Finally, methodological improvements to trial designs such as endoscopic central reading, combined histo-endoscopic endpoints and active dose adjustments are gaining ground.
Monoclonal antibodies are not the only class of drugs targeting the IL-23 pathway under investigation. PTG-200, an orally administered peptide targeting IL-23R has shown encouraging results in animal models and healthy volunteers,65,66 and a phase 2 clinical trial [NCT04102111] in CD is currently ongoing. If results were positive this could lead to an additional expansion of the therapeutic armamentarium with convenient orally administered agents. IL-23 is also central to another promising therapeutic strategy for IBD, the inhibition of tyrosine kinase 2 [Tyk2].67 In fact, Tyk2, a type of JAK kinase, mediates and regulates the signal transduction downstream of IL-23, as well as IL-12 and type 1 IFN receptors. In in vitro and animal models, selective allosteric inhibitors of Tyk2, such as brepocitinib and deucravacitinib, provide benefits similar to those of JAK inhibitors with limited toxicities. Ongoing phase 2 trials will clarify the potential of these compounds.67
In conclusion, increasing evidence suggests that the blockade of IL-23 is an effective and safe therapeutic target for both CD and UC. Several clinical trials have been completed or underway and will provide more robust data in the coming years, including regarding long-term efficacy and safety.
Contributor Information
Tommaso Lorenzo Parigi, Institute of Immunology and Immunotherapy NIHR Birmingham Biomedical Research Centre, University Hospitals NHS Foundation Trust and University of Birmingham, Birmingham, UK; Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy.
Marietta Iacucci, Institute of Immunology and Immunotherapy NIHR Birmingham Biomedical Research Centre, University Hospitals NHS Foundation Trust and University of Birmingham, Birmingham, UK.
Subrata Ghosh, APC Microbiome Ireland Centre, College of Medicine and Health, University College Cork, Ireland.
Funding
This paper was published as part of a supplement financially supported by AbbVie.
Conflicts of Interest
S.G. has participated in steering committees by Janssen, Boehringer-Ingelheim, Abbvie, Galapagos, Bristol Myers Squibb and Receptos, Advisory committees by Takeda, Pfizer, Janssen, Ferring, Abbvie, BMS, Gilead, Galapagos and Celltrion, and speaker commitments by Abbvie, Janssen, Celltrion, Pfizer, Takeda, Galapagos and Gilead. M.I. and T.L.P. have no relevant conflicts of interest.
Author Contributions
T.P.: writing manuscript, literature search. M.I. and S.G.: concept of the review, editing and finalization.
Data: publicly available sources.
References
- 1. Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med 1989;170:827–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol 1995;154:5071–9. [PubMed] [Google Scholar]
- 3. Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000;100:655–69. [DOI] [PubMed] [Google Scholar]
- 4. Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol 2019;16:185–96. [DOI] [PubMed] [Google Scholar]
- 5. Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med 1995;182:1281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003;421:744–8. [DOI] [PubMed] [Google Scholar]
- 7. Patel DD, Kuchroo VK. Th17 cell pathway in human immunity: lessons from genetics and therapeutic interventions. Immunity 2015;43:1040–51. [DOI] [PubMed] [Google Scholar]
- 8. Neurath MF. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev 2019;45:1–8. [DOI] [PubMed] [Google Scholar]
- 9. Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000;13:715–25. [DOI] [PubMed] [Google Scholar]
- 10. Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006;314:1461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 2006;116:1310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leppkes M, Becker C, Ivanov II, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology 2009;136:257–67. [DOI] [PubMed] [Google Scholar]
- 13. Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003;52:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohman L, Dahlén R, Isaksson S, et al. Serum IL-17A in newly diagnosed treatment-naive patients with ulcerative colitis reflects clinical disease severity and predicts the course of disease. Inflamm Bowel Dis 2013;19:2433–9. [DOI] [PubMed] [Google Scholar]
- 15. Kullberg MC, Jankovic D, Feng CG, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med 2006;203:2485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hueber W, Sands BE, Lewitzky S, et al. ; Secukinumab in Crohn’s Disease Study Group. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012;61:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am J Gastroenterol 2016;111:1599–607. [DOI] [PubMed] [Google Scholar]
- 18. Maxwell JR, Zhang Y, Brown WA, et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity 2015;43:739–50. [DOI] [PubMed] [Google Scholar]
- 19. Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis 2018;77:175–87. [DOI] [PubMed] [Google Scholar]
- 20. Teng MW, Bowman EP, McElwee JJ, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med 2015;21:719–29. [DOI] [PubMed] [Google Scholar]
- 21. Mannon PJ, Fuss IJ, Mayer L, et al. ; Anti-IL-12 Crohn’s Disease Study Group. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med 2004;351:2069–79. [DOI] [PubMed] [Google Scholar]
- 22. Langley RG, Papp K, Gottlieb AB, et al. Safety results from a pooled analysis of randomized, controlled phase II and III clinical trials and interim data from an open-label extension trial of the interleukin-12/23 monoclonal antibody, briakinumab, in moderate to severe psoriasis. J Eur Acad Dermatol Venereol 2013;27:1252–61. [DOI] [PubMed] [Google Scholar]
- 23. Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 2008;135:1130–41. [DOI] [PubMed] [Google Scholar]
- 24. Sandborn WJ, Gasink C, Gao LL, et al. ; CERTIFI Study Group. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 2012;367:1519–28. [DOI] [PubMed] [Google Scholar]
- 25. Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 26. Rutgeerts P, Gasink C, Chan D, et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology 2018;155:1045–58. [DOI] [PubMed] [Google Scholar]
- 27. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. [DOI] [PubMed] [Google Scholar]
- 28. Li K, Marano C, Zhang H, et al. Relationship between combined histologic and endoscopic endpoints and efficacy of ustekinumab treatment in patients with ulcerative colitis. Gastroenterology 2020;159:2052–64. [DOI] [PubMed] [Google Scholar]
- 29. Adedokun O, Panaccione R, Hisamatsu T, et al. S0845 pharmacokinetics and immunogenicity of maintenance therapy with Ustekinumab: 2-year results from the UNIFI long-term extension study. Am J Gastroenterol 2020;115:S437. [Google Scholar]
- 30. Hong SJ, Krugliak Cleveland N, Akiyama S, et al. Real -world effectiveness and safety of Ustekinumab for ulcerative colitis from 2 Tertiary IBD Centers in the United States. Crohns Colitis 360 2021;3. Doi: 10.1093/crocol/otab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hooper A, Matro R, Coyle W, Rosenblatt A, Konijeti GG. S2269 efficacy of intravenous Ustekinumab as induction therapy for patients hospitalized with severe inflammatory bowel disease: a case series. Am J Gastroenterol 2020;115:S1200. [Google Scholar]
- 32. Shaffer SR, Traboulsi C, Cleveland NK, Rubin DT. S2261 combining cyclosporine with Ustekinumab in acute severe ulcerative colitis: report of 2 cases. Am J Gastroenterol 2020;115:S1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hanauer SB, Sandborn WJ, Feagan BG, et al. IM-UNITI: three-year efficacy, safety, and immunogenicity of Ustekinumab treatment of Crohn’s disease. J Crohns Colitis 2020;14:23–32. [DOI] [PubMed] [Google Scholar]
- 34. Panaccione R, Danese S, Sandborn WJ, et al. Ustekinumab is effective and safe for ulcerative colitis through 2 years of maintenance therapy. Aliment Pharmacol Ther 2020;52:1658–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghosh S, Ott E, Gasink C, Miao Y, Colombel JF. Safety of Ustekinumab in IBD: a comprehensive analysis of major cardiovascular events (MACE) through 5 years in CD and 2 years in UC. Gastroenterology 2021;160:S-37. [Google Scholar]
- 36. Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest 2006;116:1218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Papp K, Thaçi D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol 2015;173:930–9. [DOI] [PubMed] [Google Scholar]
- 38. Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of Guselkumab versus Adalimumab for plaque psoriasis. N Engl J Med 2015;373:136–44. [DOI] [PubMed] [Google Scholar]
- 39. Strober B, Menter A, Leonardi C, et al. Efficacy of Risankizumab in patients with moderate-to-severe plaque psoriasis by baseline demographics, disease characteristics and prior biologic therapy: an integrated analysis of the phase III UltIMMa-1 and UltIMMa-2 studies. J Eur Acad Dermatol Venereol 2020;34:2830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature 2006;442:461–5. [DOI] [PubMed] [Google Scholar]
- 41. Mathur R, Alam MM, Zhao XF, et al. Induction of autophagy in Cx3cr1+ mononuclear cells limits IL-23/IL-22 axis-mediated intestinal fibrosis. Mucosal Immunol 2019;12:612–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Visvanathan S, Baum P, Salas A, et al. Selective IL-23 inhibition by Risankizumab modulates the molecular profile in the colon and ileum of patients with active Crohn’s disease: results from a randomised phase II biopsy sub-study. J Crohns Colitis 2018;12:1170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus Ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med 2017;376:1551–60. [DOI] [PubMed] [Google Scholar]
- 44. Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor Risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 2017;389:1699–709. [DOI] [PubMed] [Google Scholar]
- 45. Feagan BG, Panés J, Ferrante M, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol 2018;3:671–80. [DOI] [PubMed] [Google Scholar]
- 46. Ferrante M, Feagan BG, Panés J, et al. Long-term safety and efficacy of Risankizumab treatment in patients with Crohn’s disease: results from the phase 2 open-label extension study. J Crohns Colitis. Doi: 10.1093/ecco-jcc/jjab093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. D’Haens GR, Colombel JF, Bossuyt PJJ, et al. Risankizumab induction therapy in patients with moderate-to-severe Crohn’s disease with intolerance or inadequate response to conventional and/or biologic therapy: results from the phase 3 advance study. Gastroenterology 2021;161:E28. [Google Scholar]
- 48. Press releases | AbbVie News Center. https://news.abbvie.com/news/press-releases/?start_row=51. Accessed July 24, 2021.
- 49. Bossuyt PJJ, Ferrante M, Baert F, et al. Risankizumab therapy induces improvements in endoscopic endpoints in patients with moderate-to-severe Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE studies. J Crohns Colitis 2021;15:S033–S034. [Google Scholar]
- 50. Schreiber S, Ferrante M, Panaccione R, et al. Risankizumab induces early clinical remission and response in patients with moderate-to-severe Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE studies. J Crohns Colitis 2021;15:S026–S027. [Google Scholar]
- 51. AbbVie. A multicenter, randomized, double-blind, placebo controlled 52-week maintenance and an open-label extension study of the efficacy and safety of Risankizumab in subjects with ulcerative colitis. 2021. https://clinicaltrials.gov/ct2/show/NCT03398135. Accessed July 28, 2021.
- 52. Singh S, Singh S, Thangaswamy A, Thangaraju P, Varthya SB. Efficacy and safety of Risankizumab in moderate to severe psoriasis: a systematic review and meta-analysis. Dermatol Ther 2021;34:e14487. [DOI] [PubMed] [Google Scholar]
- 53. Phase 3 Maintenance Results Show Patients with Crohn’s Disease Receiving Risankizumab (SKYRIZI®) Achieved Endoscopic Response and Clinical Remission at One Year. Press Release. https://news.abbvie.com/news/press-releases/phase-3-maintenance-results-show-patients-with-crohns-disease-receiving-risankizumab-skyrizi-achieved-endoscopic-response-and-clinical-remission-at-one-year.htm. Accessed July 28, 2021. [Google Scholar]
- 54. AbbVie. A multicenter, randomized, double-blind, placebo controlled induction study to evaluate the efficacy and safety of Risankizumab in subjects with moderately to severely active ulcerative colitis. 2021. https://clinicaltrils.gov/ct2/show/NCT03398148. Accessed July 28, 2021.
- 55. Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of Mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology 2020;158:537–49.e10. [DOI] [PubMed] [Google Scholar]
- 56. Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of continued treatment with Mirikizumab in a phase 2 trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020. Doi: 10.1016/j.cgh.2020.09.028 [DOI] [PubMed] [Google Scholar]
- 57. Lichtenstein GR, Feagan BG, Tuttle JL, et al. Impact of Mirikizumab treatment on health-related quality of life in patients with ulcerative colitis: a phase 2 study analysis using the SF-36 V2 Standard. Gastroenterology 2020;158:S-S-1205. [Google Scholar]
- 58. Sands B, Sandborn WJ, Peyrin-Biroulet L, et al. Efficacy and safety of Mirikizumab (LY3074828) in a phase 2 study of patients with Crohn’s disease. United Eur Gastroent 2019;7:10–188. [Google Scholar]
- 59. Sandborn WJ, Sands BE, Hindryckx P, et al. Evaluation of symptom improvement during induction in patients with Crohn’s disease treated with Mirikizumab. Am J Gastroenterol 2020;115:S354. [Google Scholar]
- 60. Sands BE, Sandborn WJ, Peyrin-Biroulet L, et al. Efficacy and safety of Mirikizumab after 52-weeks maintenance treatment in patients with moderate-to-severe Crohn’s disease. Gastroenterology 2021;160:S-37. Doi: 10.1016/S0016-5085(21)00835-0. [DOI] [Google Scholar]
- 61. Sands BE, Chen J, Feagan BG, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology 2017;153:77–86.e6. [DOI] [PubMed] [Google Scholar]
- 62. Danese S, Sandborn WJ, Feagan BG, et al. The effect of Guselkumab induction therapy on early clinical outcome measures in patients with moderately to severely active Crohn’s disease: results from the phase 2 GALAXI 1 study. J Crohns Colitis2021;15:S027–S028. Doi: 10.1093/ecco-jcc/jjab075.027. [DOI] [Google Scholar]
- 63. Bai F, Li GG, Liu Q, Niu X, Li R, Ma H. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res 2019;2019:2546161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Galluzzo M, D’Adamio S, Campione E, Bianchi L, Talamonti M. A safety evaluation of Guselkumab for the treatment of psoriasis. Expert Opin Drug Saf 2018;17:741–51. [DOI] [PubMed] [Google Scholar]
- 65. Cheng X, Lee TY, Ledet G, et al. Safety, tolerability, and pharmacokinetics of PTG-200, an oral GI-restricted peptide antagonist of IL-23 receptor, in normal healthy volunteers. Am J Gastroenterol 2019;114:S439. [Google Scholar]
- 66. Cheng X, Taranath R, Mattheakis L, Bhandari A, Liu D. The biomarker profile of PTG-200, an oral peptide antagonist of IL-23 receptor, tracks with efficacy in preclinical model of IBD.
- 67. Danese S, Peyrin-Biroulet L. Selective tyrosine kinase 2 inhibition for treatment of inflammatory bowel disease: new hope on the rise. Inflamm Bowel Dis 2021;27:2023–2030. Doi: 10.1093/ibd/izab135 [DOI] [PMC free article] [PubMed] [Google Scholar]