Summary
Background
Clinical phage therapy is often delivered alongside antibiotics. However, the phenomenon of phage-antibiotic synergy has been mostly studied in vitro. Here, we assessed the in vivo bactericidal effect of a phage-antibiotic combination on Acinetobacter baumannii AB900 using phage øFG02, which binds to capsular polysaccharides and leads to antimicrobial resensitisation in vitro.
Methods
We performed a two-stage preclinical study using a murine model of severe A. baumannii AB900 bacteraemia. In the first stage, with an endpoint of 11 h, mice (n = 4 per group) were treated with either PBS, ceftazidime, phage øFG02, or the combination of phage and ceftazidime. The second stage involved only the latter two groups (n = 5 per group), with a prolonged endpoint of 16 h. The primary outcome was the average bacterial burden from four body sites (blood, liver, kidney, and spleen). Bacterial colonies from phage-treated mice were retrieved and screened for phage-resistance.
Findings
In the first stage, the bacterial burden (CFU/g of tissue) of the combination group (median: 4.55 × 105; interquartile range [IQR]: 2.79 × 105–2.81 × 106) was significantly lower than the PBS (median: 2.42 × 109; IQR: 1.97 × 109–3.48 × 109) and ceftazidime groups (median: 3.86 × 108; IQR: 2.15 × 108–6.35 × 108), but not the phage-only group (median: 1.28 × 107; IQR: 4.71 × 106–7.13 × 107). In the second stage, the combination treatment (median: 1.72 × 106; IQR: 5.11 × 105–4.00 × 106) outperformed the phage-only treatment (median: 7.46 × 107; IQR: 1.43 × 107–1.57 × 108). Phage-resistance emerged in 96% of animals receiving phages, and all the tested isolates (n = 11) had loss-of-function mutations in genes involved in capsule biosynthesis and increased sensitivity to ceftazidime.
Interpretation
øFG02 reliably drives the in vivo evolution of A. baumannii AB900 towards a capsule-deficient, phage-resistant phenotype that is resensitised to ceftazidime. This mechanism highlights the clinical potential of using phage therapy to target A. baumannii and restore antibiotic activity.
Funding
National Health and Medical Research Council (Australia).
Keywords: Acinetobacter baumannii, Phage therapy, Antimicrobial resistance, Phage-resistance, Ceftazidime, Preclinical study, Synergism
Research in context.
Evidence before this study
Acinetobacter baumannii has been named a critical priority for the research and development of new therapeutic strategies. A search in PubMed in September 2021, with no restrictions by date or language, using the search terms “Acinetobacter baumannii” AND “phage therapy”, and excluding review articles, obtained 66 results. A manual assessment of these articles looked for either clinical case reports, preclinical models, and/or research into phage-antibiotic combinations. Five publications, all from the past 5 years, described a total of 8 clinical cases of compassionate use of phage therapy against A. baumannii (septicaemia, osteomyelitis, surgical site infection, and 5 cases of ventilator-associated pneumonia), demonstrating varying degrees of success. In all of these cases, patients received, at least for a brief period of time, phages and antibiotics simultaneously. Therefore, it was difficult to quantify the precise contribution of phage therapy to the overall outcomes, and discriminate it from that of antibiotics. A further 18 articles reported the results of preclinical experiments using phages to treat infections in either rodents or wax moth larvae. The modelled infections included pneumonia, septicaemia, and wound infections, and a great majority demonstrated the bactericidal effect of phages in vivo. However, none of these studies assessed the activity of phage-antibiotic combinations. Lastly, 5 more studies did examine the therapeutic effects of phage-antibiotic combinations, and all of them reported at least one instance of synergy, primarily when carbapenem antibiotics or colistin were added to phages. Nevertheless, these studies used either in vitro platforms (tissue culture, liquid culture or biofilms) or wax moth models. Most importantly, we could not find in the literature instances where an exact mechanism of phage-antibiotic synergy against A. baumannii has been demonstrated in action.
Added value of this study
This work presents a preclinical study, using a mammalian model, that demonstrates that a phage-antibiotic combination has a superior bactericidal effect than each of these agents individually against severe A. baumannii infection. Notably, our findings explain the mechanism through which the combination of these two agents results in a superior bactericidal effect. We confirm that, even in complex in vivo systems, treatment with a capsule-targeting phage can reliably and repeatably induce bacterial evolution towards a phenotype that is phage-resistant but resensitised to antibiotics.
Implications of all evidence available
Phage therapy is becoming increasingly promising as a therapeutic strategy against A. baumannii. To maximise their therapeutic effect, however, the biology of clinically-relevant phages needs to be better understood. A clear knowledge of the bacterial receptor used by a phage, the mechanism of bacterial phage-resistance, and its associated trade-offs, can be leveraged as a clinical intervention. The emerging evidence supports the use of appropriate phage-antibiotic combinations as a better therapeutic strategy against this critical pathogen, warranting the design of clinical trials.
Alt-text: Unlabelled box
Introduction
Antimicrobial resistance (AMR) is considered one of the biggest threats to global health. While the COVID-19 pandemic has rekindled the world's interest in the research, prevention and treatment of infectious diseases, it has also potentially contributed to worsening the problem of AMR.1 The pandemic has, for example, exacerbated the issue of inappropriate prescription of antibiotics, and the saturation of the healthcare systems has correlated to an increase in healthcare-associated, multidrug-resistant bacterial infections.1,2 In this context, Acinetobacter baumannii infections keep emerging as a leading cause of morbimortality in hospitalised patients.3, 4, 5, 6 The resilience, persistence, and AMR evolution of A. baumannii in hospital settings have been well documented,7,8 with recent estimates suggesting almost half of A. baumannii clinical isolates are multidrug-resistant.9 These precedents justify the declaration of A. baumannii as a critical priority for the research and development of new therapeutic strategies.10
In recent years, phage therapy has experienced a rebirth as a promising strategy against antimicrobial-resistant pathogens.11 Preclinical studies of phage therapy in animal models of A. baumannii infection have demonstrated encouraging results.12, 13, 14, 15, 16. Furthermore, clinical case studies have established the contribution of phage therapy in treating patients with severe A. baumannii septicaemia,17 ventilator-associated pneumonia,18,19 osteomyelitis,20 and surgical site infections.21 However, the interactions of A. baumannii with combinations of phage and traditional antimicrobial agents within an in vivo infection are currently understudied.
Therapeutic synergy occurs when the combined effect of two agents, in this case antimicrobials, is greater than the sum of their individual effects.22 Phage-antibiotic synergy (PAS) was first detected in vivo against colibacillosis in broiler chicken,23 but the term itself was coined three years later, upon observing how subinhibitory doses of antibiotics could increase the burst size of lytic phages in vitro.24 The concept has since expanded with the discovery of more mechanisms through which antibiotics and phages potentiate each other's antibacterial effects.11,25 A prime example is phage-resistance, whereby specific phages can push their hosts into evolutionary pathways that lead to phage-resistance at the cost of fitness trade-offs, including increased sensitivity to antibiotics.26, 27, 28 Compared to other clinically relevant pathogens, however, PAS research against A. baumannii is limited,29 with few studies exploring the combined effects of phages and antibiotics, and primarily doing so in vitro.30, 31, 32 More broadly, research into phage-resistance-mediated PAS should answer two key questions: (1) whether the in vitro identified mechanisms of phage-resistance repeatably occur within the substantially more complex and varied in vivo infections, and (2) if these do occur, whether in vivo combination treatments provide increased effectiveness compared to either treatment alone. Better understanding of these issues could provide important, new therapeutic insights.
Previously, we reported that phage øFG02 used the bacterial capsule of A. baumannii strain AB900 as its receptor.33 In an effort to avoid killing by this phage, A. baumannii evolved loss-of-function mutations in genes of the K locus-responsible for capsule production and export.33 The resultant capsule loss led to the sensitisation of phage-resistant mutants to beta-lactam antibiotics, the complement system, and alternative phages.33
The present work aimed to leverage our previous findings33 and move them towards clinical translation. Here, we completed a preclinical study using a murine model of severe bacteraemia and tested whether a combined phage-antibiotic regime was superior to single-agent treatments. We confirmed that, similar to in vitro, the emergence of phage-resistance in vivo occurred via capsule loss, resulting in resensitisation to and greater killing by a beta-lactam antibiotic. Finally, we used an in vitro platform to confirm that a phage-antibiotic combination achieved synergistic, rather than just additive effects against A. baumannii. In summary, we validate the repeatability and predictability of phage-resistance evolution in vivo and enable a superior phage-antibiotic combination treatment against multidrug-resistant A. baumannii in a preclinical study.
Methods
Bacterial strains, phages, storage and culture conditions
Wild type Acinetobacter baumannii AB900 was previously described by Adams et al.34 It is a clinical isolate originally obtained from a perineal wound of a patient at the Walter Reed National Military Medical Centre (USA), that harbours the resistance determinants ampC (cephalosporinase), blaOXA-51-like (carbapenemase), and dhfrX (dihydrofolate reductase), making it resistant to, among others, the third-generation cephalosporin ceftazidime (MIC ≥ 32 μg/ml).34 AB900 contains the type-11 K locus (for capsule synthesis) and type-8 OC locus (for production of the outer membrane's lipooligosaccharide outer core).35 The phage-resistant mutant strain of AB900 (øFG02-R AB900) obtained in vitro was characterised by Gordillo Altamirano et al.33 Bacteria were cultured using lysogeny broth (LB) (Sigma-Aldrich), at 37 °C with aeration, supplemented with agar when required.
Phages øFG02 and øLK01 were originally isolated from raw sewage collected in Victoria, Australia, using the A. baumannii clinical strains AB900 and AB5075, respectively, as hosts of isolation.33 A summary of the known characteristics of øFG02 is presented in Supplementary Table 1, including experimental confirmation of its lytic lifestyle in Supplementary Fig. 1.33,36 Phage øLK01 has not been further characterised.33 Phage stocks were amplified and purified using the Phage-on-Tap protocol37 and stored at 4 °C.
Murine model of A. baumannii bacteraemia
The model has been previously described.33 In summary, the experiments involved female, 6-to-10 weeks old, BALB/c mice, weighing at least 15 g; each animal was an experimental unit. In all cases, mice were randomly allocated to the different experimental groups. Late exponential phase bacterial inoculums of A. baumannii were prepared to the desired concentration depending on the experiment: 1 × 106 CFU for phage dosing, 2 × 106 CFU for ceftazidime dosing, and 5 × 106 CFU for both stages of the preclinical study. Inoculum size correlates with time of survival in untreated animals, with 5 × 106 CFU resulting in 100% mortality at 12 h, whereas 1 × 106 CFU enables survival to at least 24 h. In all cases, bacterial inoculums were calculated in 100 μl of PBS and mixed in a 1:1 ratio with 6% porcine stomach mucin (Sigma-Aldrich) in PBS, to enhance bacterial virulence,38 for a total volume of 200 μl. Infection and treatments were all performed via intraperitoneal injection. All treatments were calculated for a volume of 200 μl, and the amount of injections received by each mouse was the same (using PBS if no other treatment was scheduled for a specific mouse at a specific timepoint).
Treatments for the phage dosing experiments (n = 4 mice per group; 8 total) were either a single dose of phage øFG02, at a multiplicity of infection (MOI) of 1, 1 h post infection (hpi) (once-a-day or q.d. group) or two doses, at 1 h and 12 hpi (twice-a-day or b.i.d group). In the ceftazidime dosing experiments (n = 3 per group; 12 total), treatments consisted of PBS (control) or a single dose of ceftazidime, delivered at 1 hpi, at either 12.5 mg/kg (low dose group), 25 mg/kg (medium dose group), or 50 mg/kg (high dose group), doses that correlate with standard veterinary and human treatments.39,40 The first stage of the preclinical study (n = 4 per group; 16 total) involved treatments with either PBS (control), ceftazidime at 25 mg/kg every 8 h (antibiotic), phage øFG02 at a MOI of 1 as a single dose (phage) or the combination of the ceftazidime and phage treatments. The latter two treatments were used for the second, independent, stage of the preclinical study (n = 5 per group; 10 total).
The endpoints were set depending on the aim of each experiment. For phage and ceftazidime dosing experiments, the endpoints were set at the end of the duration of action, i.e. right before the next dose in a clinical context would have been scheduled (24 h for phage dosing and 12 h for ceftazidime dosing). For the preclinical study, the endpoints were shortened considering the higher inoculum used. They were set at 12 h for the first stage and 16 h for the second stage. In all cases, a researcher blinded to the treatments received by the animals would follow them at regular intervals and, when needed, the programmed endpoints would be modified based upon animal wellbeing (humane endpoint).
At the endpoints, mice were euthanised by CO2 asphyxiation. Blood and organs were collected, weighed and homogenised in PBS. Blood and organ suspensions were then serially diluted in PBS and the bacterial burden quantified by colony forming unit (CFU) counting, and normalised by organ weight, constituting our primary outcome. In mice that received phage treatment, the blood and organ suspensions were pelleted by centrifugation, and the supernatant serially diluted and plated with the soft overlay assay for plaque forming unit (PFU) quantification. Screening for phage-resistant mutants was performed as described below. Animals were housed at the Monash Animal Research Platform Facility, Monash University, with experiments starting after at least 48 h of acclimatization.
Screening for phage-resistant mutants
Bacterial colonies isolated from mice were patch-plated using sterile pipette tips onto fresh LB agar plates and individual wells of a microtiter plate filled with 200 μl of LB containing 106 PFU of phage øFG02. The agar plates were incubated overnight, while the microtiter plates were incubated for 16 h with OD600 measured at 15 min intervals. The growth curves of the putative phage-resistant mutants were compared to those of wild type AB900. Colonies with growth curves suggestive of phage-resistance underwent two rounds of single-colony purification starting from the previously prepared agar patch plates. Finally, the phenotype of each putative phage-resistant colony was confirmed by repeating the growth assay in liquid culture in the presence of phage, and the standard soft overlay assay. For the phage-resistant strains that underwent further characterisation, a final efficiency of plating assay was performed using a concentration of 109 PFU of øFG02 per plate, expecting a complete absence of plaques.
Isolation of bacterial genomic DNA, sequencing and variant calling
The GenElute™ Bacterial Genomic DNA Kit protocol (Sigma-Aldrich) was used for DNA isolation. Bacterial genomic DNA was tested for purity using a Nanodrop (NanoDrop Technologies), Qubit fluorometer (Life Technologies), and 1% agarose gel electrophoresis, then vacuum dried into a pellet for transport. Sequencing was performed using the Illumina® HiSeq 150 bp paired-end platform at the Genewiz® facilities (Suzhou, China).
The raw reads of each isolate's genome were examined using FastQC v0.11.9.41 Raw reads were quality trimmed to remove adaptor sequences and reads of quality <20 in a sliding window of 4 bp using Trimmomatic v0.39.42 Nucleotide variants were identified using “Snippy 4.3.6 - fast bacterial variant calling from NGS reads” (https://github.com/tseemann/snippy) with default settings, using the draft genome of wild type AB900 (NCBI Genbank accession number: JAAVKC010000001-JAAVKC010000043) as the reference. Snippy output files (*.csv) were then manually examined for the coverage and functional effect of variants. Synonymous, <20 read coverage and self-aligning variants (as described in Gordillo Altamirano et al.33) were removed from further analysis. Finally, frameshift variants were visualised in Integrative Genomics Viewer (IGV) v2.7.243 to examine the disruption of coding sequences.
Adsorption assay
Bacteria from overnight cultures and phages from pure lysates were mixed at a MOI of 0.01 (106 PFU/ml to 108 CFU/ml) in LB. The suspensions were incubated at 37 °C with aeration. At 0 and 20 min, samples of the suspensions were transferred into chloroform-saturated PBS, vortexed, and then centrifuged at 3500 × g for 3 min. The supernatant was diluted in PBS and plated in duplicate for quantification of free phage particles.
Scanning electron microscopy
A 20 μl droplet of 5 times-PBS washed AB900 bacterial suspension (∼107 CFU/ml) was placed onto a fresh gold-coated silicon wafer (5 × 5 mm). The cells were allowed to settle for 5 min, followed by washing with PBS. Then, a 20 μl droplet of phage lysate (∼109 PFU/ml) was added, incubated for 20 min, and washed with PBS. The wafer was then placed in 2.5% glutaraldehyde in PBS solution for 15 min, washed with deionized water and dehydrated by immersing in increasing concentrations of ethanol for 3 min each. Residual ethanol was removed with a critical point dryer EM CPD300 (Leica). The sample was mounted on a standard metal SEM stub and then coated with a ∼5 nm thick gold layer using a sputter coater SCD 005 (BAL-TEC AG). The samples were examined within the FEG-SEM ThermoFisher Elstar G4 at an accelerating voltage of 2 kV, secondary electron mode, and a working distance of 3 mm, operating in immersion mode with the through lens detector. Images were colourised using the AKVIS Coloriage Software.
Serum killing assay
The working volume of human serum (Sigma-Aldrich, Australia) was divided in half, storing one half at 4°C and heat-inactivating the other (56°C for 30 min). In glass test tubes, aliquots of 5 ml of 50% serum (either active or heat-inactivated) in PBS were inoculated to 105 CFU/ml of bacteria in exponential growth phase. The tubes were thoroughly mixed and incubated. At the starting timepoint and at 60 min, samples from each tube were extracted to perform serial dilutions and CFU counts. Escherichia coli DH5-alpha was used as a control of serum activity (susceptible to human serum and resistant to heat-inactivated serum).
Antimicrobial susceptibility testing
Minimum inhibitory concentrations to ceftazidime and imipenem (Sigma-Aldrich) were assessed using the microbroth dilution protocol44 and interpreted using the Clinical and Laboratory Standard Institute guidelines. E. coli strains ATCC 25922 and ATCC 35218 were used as quality controls for each batch of tests. The minimum bactericidal concentrations of ceftazidime were calculated using two methodologies (three technical replicates per strain, as detailed below), both starting from an incubated microbroth dilution test. First, 5 μl droplets from a range of wells (128 μg/ml to 2 μg/ml of ceftazidime, and the growth control) were streaked onto independent sections of a fresh (no antibiotic) Mueller Hinton agar plate, incubated overnight, and assessed for colony growth (two independent replicates). Alternatively, the microtiter plate was centrifuged (3500 × g for 10 min), the supernatant carefully aspirated and replaced with an equal volume of fresh Mueller Hinton broth, and the plate incubated for 16 h, at 37 °C, with continuous shaking, measuring the OD600 every 15 min. The resulting growth curves were analysed for bacterial growth (one replicate). Sensitivity to phage øLK01 was tested with the standard spot assay.
In vitro phage-antibiotic synergy experiments
The methodology, previously reported in,45 was slightly modified here. AB900 was grown to exponential phase in LB, then adjusted to a concentration of 5 × 105 CFU/100 μl. Each well of the microtiter plate containing the checkerboard of phage øFG02 (range: 102–108 PFU/ml) and ceftazidime (range: 1–512 μg/ml) concentrations was inoculated with 5 × 105 CFU. The plate was incubated for 16 h, at 37°C, with continuous shaking, measuring the OD600 every 15 min.
Data representation and statistical analyses
Graphing and statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, Inc.). For animal experiments with two groups, the variances of the outcome variables were compared to select the appropriate statistical tests. When variances were similar (bodyweight loss, proportion of phage-resistant mutants), a two-tailed t-test was used after applying the Shapiro-Wilk test to verify normal distribution; for unequal variances (bacterial and phage quantification), a two-tailed Mann-Whitney U-test was used instead. In experiments with multiple groups, comparison between groups were performed strictly following the pre-established hypotheses and tested with the Kruskal-Wallis test with Dunn's correction for multiple comparisons. Unless specified otherwise, all in vitro experiments were performed in triplicate, with at least two technical replicates each. For synograms, the raw OD data were first normalised with the negative control. Where necessary, the area under the bacterial growth curve (AUC) was calculated as well. To calculate the percent reduction in either OD or AUC, the following formula was used45: Reduction (%) = [(GrowthControl − Treatment)/GrowthControl] × 100. Two-way analysis of variance (ANOVA) was employed on interaction plots to analyse possible synergism between phage and antibiotics, after assessing the data for normality using the Shapiro-Wilk test. For all statistical analyses, the threshold value of two-tailed p < 0.05 was used for statistical significance.
Ethics statement
All protocols involving animals were reviewed and approved by the AMREP (Alfred Medical Research and Education Precinct) Animal Ethics Committee (Project ID: E/1781/2018/M) and complied with the National Health and Medical Research Council guidelines. The reporting of animal research in this study complied with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.46
Role of funders
The funders of the study did not have any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Results
The combination of phage øFG02 with ceftazidime is superior to single-agent treatments against Acinetobacter baumannii bacteraemia
Using an established murine model of A. baumannii bacteraemia,33 we first set out to validate the ideal dosage and administration timings for the phage and antibiotic treatments. We had previously determined a starting bacterial inoculum of mid-106 CFU per mouse consistently produced a 100% lethality rate without effective treatment at 12 h,33 mirroring the severity of A. baumannii bacteraemia in humans.47,48 First we compared the effect of once-a-day (q.d.) versus twice-a-day (b.i.d) intraperitoneal (IP) administration of phages on animal bodyweight and average bacterial and phage counts at 24 hpi. To ensure mouse survival to 24 h, the starting inoculum was reduced to low-106 CFU/mouse; and treatment with phage øFG02 at a multiplicity of infection (MOI) of 1 was administered at either 1 h-only or at 1 h and 12 hpi. We found that b.i.d. phage treatment did not provide any significant benefits compared to q.d. administration (Supplementary Fig. 2). Next, we investigated whether there was any significant difference in antibacterial effect between varying doses of the beta-lactam ceftazidime, representing low (12.5 mg/kg), medium (25 mg/kg) and high (50 mg/kg) doses in animals and adult humans.39,40 Mice were infected with low-106 CFU/mouse of A. baumannii and the antibiotic administered as a single dose 1 hpi, with an endpoint at 12 h. Consistent with AB900’s resistance to ceftazidime in vitro, we observed only a slight reduction in the bacterial burden in the animals at 12 h, with no significant differences observed between the various doses of ceftazidime (Supplementary Fig. 3). These data provided the necessary phage and antibiotic parameters for our subsequent treatment experiments.
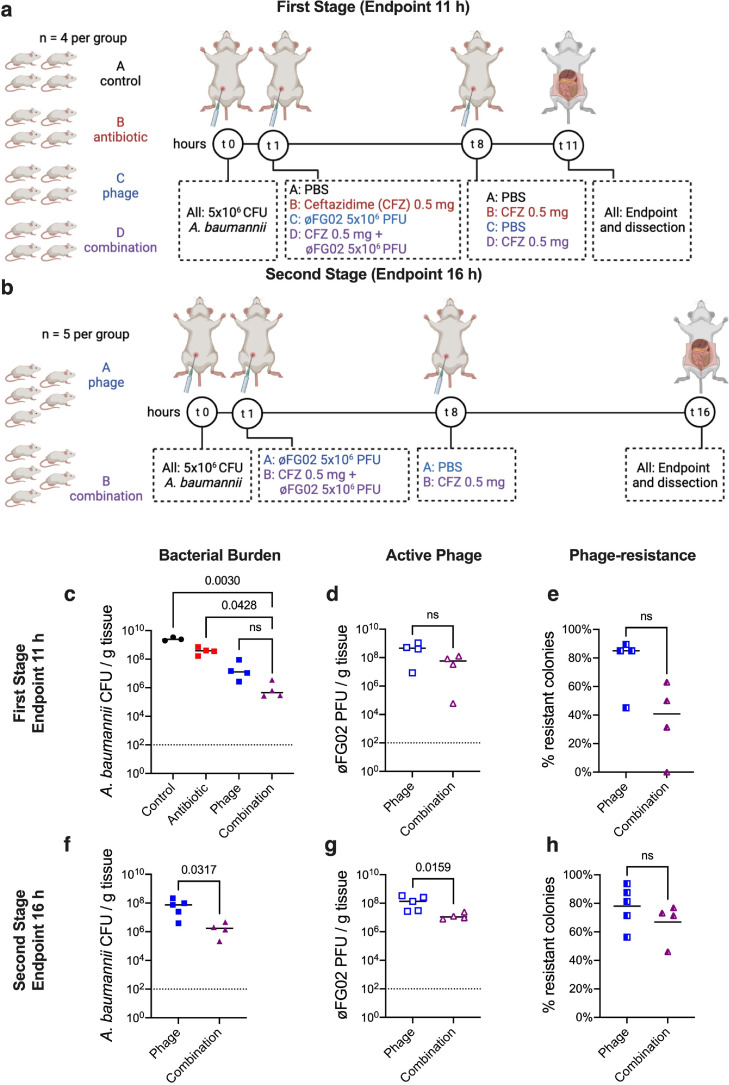
We then carried out a two-staged preclinical study comparing four treatments against A. baumannii AB900 bacteraemia (Figure 1a,b). In the first stage, groups of mice (n = 4) were treated with either (i) PBS as a negative control; (ii) 25 mg/kg ceftazidime every 8 h; (iii) phage øFG02 at a MOI of 1 as a single dose; or iv) the combination of the ceftazidime and phage treatments. The main measured outcome was the average bacterial burden from four body sites per mouse (blood, liver, kidney, and spleen). Mice were euthanised at 11 hpi when humane endpoints were reached in the negative control and antibiotic-only groups. Compared to the other groups, combination treatment reduced bacterial density by 3.7, 2.9, and 1.4 log, against the control, antibiotic-only, and phage-only groups, respectively (Figure 1c); with data analysis in individual organs showing the same trend (Supplementary Fig. 4). While the combination treatment showed the strongest bactericidal effect, it did not achieve statistical significance when compared to the phage-only treatment (Figure 1c) (Kruskal-Wallis test with Dunn's correction for multiple comparisons, p = 0.003 vs control, p = 0.0428 vs antibiotic-only, p = 0.851 vs phage-only). Notably, there was no difference in the numbers of active phage particles or phage-resistant bacterial colonies retrieved from the mice receiving either phage-only or combination treatment (Mann-Whitney U-test, p = 0.2 and p = 0.08, respectively; two-tailed) (Figure 1d,e).
Figure 1.
Preclinical study of phage-antibiotic combination therapy against A. baumannii bacteraemia. a and b: Experimental design for the two-staged preclinical study. The experiments began with the intraperitoneal injection of 5 × 106 CFU/mouse of A. baumannii AB900. Treatments were also delivered intraperitoneally, at 1 h and 8 h post-infection. At the endpoints of 11 h or 16 h, blood, liver, kidney and spleen from each mouse were obtained for bacterial and phage quantification. c to e: Results of the first stage of the study, endpoint at 11 h, comparing control (black), antibiotic-only (red), phage-only (blue), and combined (purple) therapies. f to h: Results of the second stage of the study, endpoint at 16 h, comparing phage-only (blue), and combined (purple) therapies. Average bacterial burdens from 4 body sites (c and f) are represented by solid symbols, average active phage counts (d and g) by outline-only symbols, and proportion of phage-resistant colonies (e and h) by shaded symbols. Each data point represents one mouse, bars are medians and dotted lines represent the assays’ detection limit. Statistical analysis was performed using the Kruskal-Wallis test with Dunn's correction for multiple comparisons (c), Mann-Whitney U-test (d, e f, and g), and unpaired t-test (h). Data by organ from panels c-d and f-g available in Supplementary Fig. 4. Two data points have been excluded due to suspected incorrect administration (intraintestinal, intramuscular or subdermal) of either inoculum or phage treatment. In the missing data point from c (control group) suspected incorrect administration of the inoculum lead to no infection being established, noticed by undetectable levels of bacteria at the endpoint. In the missing data point from f-h (combination group), suspected incorrect administration of the phage treatment led to the animal reaching the endpoint at 12 h instead of 16 h, alongside undetectable levels of phage from all organs. ns: not significant.
In the second, independent, stage of the study (n = 5 per group), we excluded the negative control and antibiotic-only groups, allowing the extension of the endpoint to 16 h. Here we observed that the combination treatment achieved a sustained suppression of the bacterial burden, whereas the phage-only group experienced a resurgence in bacterial burden, for a final 1.6 log difference (median: 8.38 × 107 vs 2.08 × 106 CFU/g of tissue; Mann-Whitney U-test; p = 0.03; two-tailed) (Figure 1f). The higher bacterial load in the phage-only group was correlated to higher phage quantities (median: 1.35 × 108 vs 1.08 × 107 PFU/g of tissue; Mann-Whitney U-test; p = 0.0159; two-tailed) (Figure 1g), and both groups had a similar proportion of phage-resistant mutants (unpaired t-test; p = 0.29; two-tailed) (Figure 1h). In summary, our preclinical study demonstrated greater efficacy with the combination of ceftazidime and phage øFG02 compared to either antibiotic-only or phage-only approaches against A. baumannii bloodstream infection.
Phage-resistant A. baumannii mutants emerge in vivo via a repeatable evolutionary pathway that leads to antimicrobial resensitisation
We hypothesised that the success of the combination therapy in vivo was due to the emergence of phage-resistant mutants exhibiting antimicrobial resensitisation.33 To explore this hypothesis, we screened for phage-resistant mutants as an outcome measure from all phage or combination treated mice across all experiments (Figure 1e,h, Supplementary Fig. 2d). A minimum of 15 bacterial colonies per mouse were randomly selected, from different body sites. Following two rounds of single-colony purification, the sensitivity of these colonies to phage øFG02 was assessed with growth curves and the standard soft agar overlay assay. In total, phage-resistant colonies were isolated from 24 out of 25 mice (96%). The frequency of phage-resistance emergence was not necessarily comparable between mice across different experiments, ranging from as little as 5% of colonies per mouse up to as many as 94% (Supplementary Fig. 5). This range was likely due to variations in starting inoculums, number of phage doses, and length of follow-up. We did however observe phage-resistant isolates within all four organs. This result confirmed that A. baumannii AB900 can become resistant to phage øFG02 in vivo.
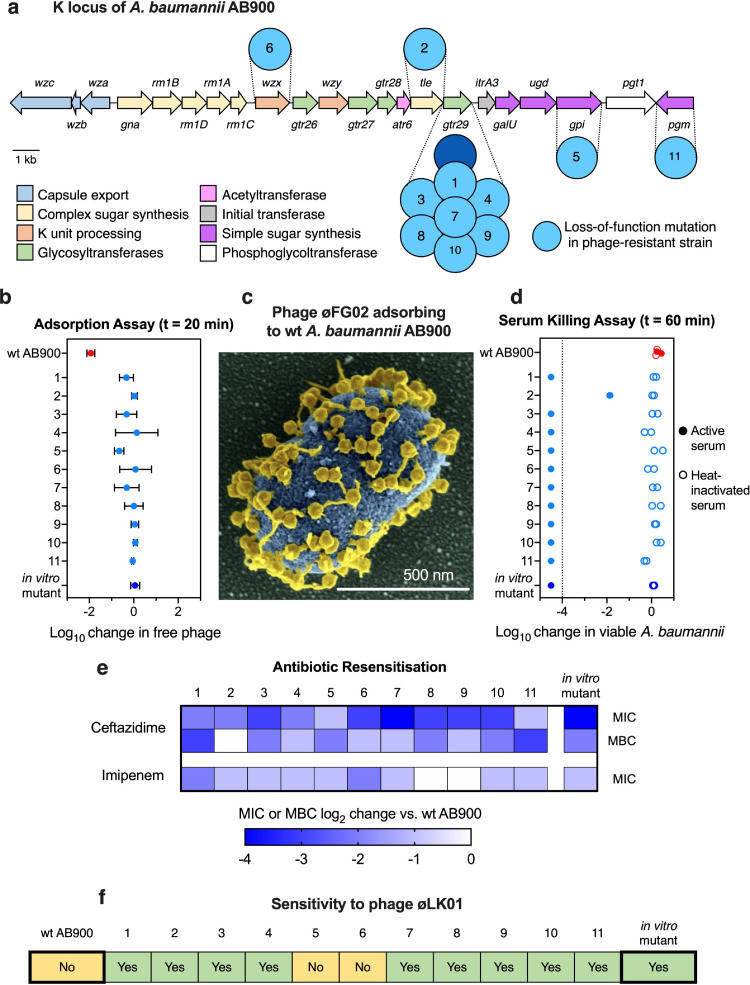
In vitro resistance to phage øFG02 in AB900 had previously been determined to occur via loss-of-function mutations in genes associated with capsule production.33 We next sought to verify if the mechanisms of phage resistance in our in vivo derived isolates were the same as those that evolved in vitro. We randomly selected one phage-resistant isolate from 11 animals (Supplementary Fig. 5), reconfirmed their phage-resistant phenotype with an efficiency of plating assay using 109 PFU of øFG02 per plate (Supplementary Fig. 6), and prepared them for whole genome sequencing. We compared the genomes of these strains to the wild type's (NCBI BioProject accession number SAMN14483301) to identify mutations. Similar to that observed in vitro,33 each in vivo phage-resistant isolate harboured mutations in genes of the K locus (Table 1).35,49 Interestingly, the affected gene in 7 of the 11 isolates was gtr29, a glycosyltransferase previously found to have lost its function in vitro.33 Furthermore, two of these strains had the same mutation, a 3-nucleotide deletion resulting in the loss of the tyrosine residue at position 204. The remaining isolates contained mutations in genes involved in the production of simple and complex sugars used for the capsule (tle: talose epimerase, gpi: glucose-6-phosphate isomerase, and pgm: phosphoglucomutase), and in the export of the capsule (K) subunits into the periplasm (wzx: oligosaccharide flippase) (Figure 2a).50 We next confirmed that these mutations inhibited the adsorption of phage øFG02 (Figure 2b). Over a period of 20 min, more than 99% of øFG02 particles were able to attach to their wild type host, AB900 (also visualised with scanning electron microscopy in Figure 2c); however, øFG02 particles did not adsorb to any of the 11 in vivo phage-resistant strains. Finally, we tested if we could recapitulate the phenomenon of antimicrobial resensitisation in these strains, using human serum, two antibiotics and one alternative phage.33 In a human serum killing assay, we observed that 10 of the phage-resistant mutants were eradicated by human serum after only 60 min of coincubation, with this bactericidal effect disappearing if heat-inactivation of the serum was performed beforehand (Figure 2d). Next, we used the microbroth dilution method, and a subsequent subculture into fresh media, to determine the minimum inhibitory (MIC) and bactericidal (MBC) concentrations, respectively, of ceftazidime and imipenem (Figure 2e, Supplementary Table 2, and Supplementary Figs. 7 and 8). Compared to wild type AB900, all 11 phage-resistant strains had reductions in the MIC of ceftazidime, 10 of them carried a corresponding reduction in the MBC of the antibiotic, and 9 had a reduced MIC to imipenem. Likewise, 9 out of 11 strains had become susceptible to øLK01, a phage to which wildtype AB900 is resistant (Figure 2f). Taken together, these results suggest that inhibition of phage adsorption through disruption of capsule synthesis is a repeatable mechanism of resistance used by A. baumannii AB900 against phage øFG02; and it consistently results in the trade-off of antimicrobial resensitisation. These findings explain the success of combination therapy in our preclinical experiments, supporting our hypothesis.
Table 1.
Mutations harboured by the in vivo-obtained phage-resistant AB900 isolates. Whole genomic DNA short-read sequenced, assembled and compared to wild type AB900. All affected genes are in the K locus.
| Isolate | Tissue of isolation | Treatment received | Mutation Type | Affected Gene | nt Position | Effect | Product and function |
|---|---|---|---|---|---|---|---|
| 1 | Kidney | Phage only | Insertion (1 nt) | gtr29 | 877/1041 | Frameshift | Glycosyltransferase 29: elongates the K unit adding sugars |
| 2 | Blood | Phage only | Substitution (1 nt) | tle | 401/1134 | Gly134Asp | Talose epimerase: synthesis of complex sugars for the K unit |
| 3 | Spleen | Phage only | Deletion (20 nt) | gtr29 | 865/1041 | Frameshift | Glycosyltransferase 29: elongates the K unit adding sugars |
| 4 | Liver | Phage only | Deletion (3 nt) | gtr29 | 610/1041 | Tyr204del | Glycosyltransferase 29: elongates the K unit adding sugars |
| 5 | Kidney | Phage only | Insertion (1 nt) | gpi | 163/1671 | Frameshift | Glucose-6-phosphate isomerase: synthesis of simple sugars for the K unit |
| 6 | Spleen | Phage only | Insertion (1 nt) | wzx | 254/1269 | Frameshift | Oligosaccharide flippase: translocates the K unit into the periplasm |
| 7 | Blood | Phage only | Deletion (1 nt) | gtr29 | 819/1041 | Frameshift | Glycosyltransferase 29: elongates the K unit adding sugars |
| 8 | Spleen | Phage only | Deletion (1 nt) | gtr29 | 193/1041 | Frameshift | Glycosyltransferase 29: elongates the K unit adding sugars |
| 9 | Liver | Phage and antibiotic | Deletion (3 nt) | gtr29 | 212/1041 | Frameshift | Glycosyltransferase 29: elongates the K unit adding sugars |
| 10 | Liver | Phage and antibiotic | Deletion (3 nt) | gtr29 | 610/1041 | Tyr204del | Glycosyltransferase 29: elongates the K unit adding sugars |
| 11 | Liver | Phage and antibiotic | Insertion (1 nt) | pgm | 979/1371 | Frameshift | Phosphoglucomutase: synthesis of simple sugar for the K unit |
nt: nucleotide.
Figure 2.
The mechanism and trade-offs of phage-resistance in A. baumannii AB900 are repeatable in vivo. a: Schematic representation of the genes of the K locus of AB900, based on.49,50 The high-level functions of their products are colour-coded; the dark blue circle represents the mutated gene in the previously described in vitro-obtained phage-resistant mutant,33 while the light blue circles represent the mutated genes in the in vivo-obtained phage-resistant isolates from this study (designated with the numbers on Table 1); scale bar = 1 kb. b: Adsorption assay. Log10 reduction in free phage titres 20 min after mixing phages and hosts. øFG02 was mixed with either wild type (red), in vitro phage-resistant (blue), or each of the 11 in vivo-obtained phage-resistant isolates (light blue). Phages only adsorb to wild type AB900. One-way ANOVA comparison between wild type AB900 and each of the phage-resistant isolates; p < 0.05 for all cases; two-tailed; all other comparisons were non-significant. Data are mean ± s.e.m. (n = 3). c: Colourised scanning electron microscopy image of øFG02 particles (yellow) adsorbing to the capsule of wild type AB900 (blue). Scale bar = 500 nm. d: Human serum killing assay. Log10 reduction in viable A. baumannii after 60 min of incubation in 50% active (solid symbols) or heat-inactivated (outline-only symbols) human serum in PBS. Dotted line represents the assay's limit of detection. (n = 2 for each of the conditions). e: Antibiotic resensitisation in phage-resistant isolates. The minimum inhibitory concentrations (MIC) of ceftazidime and imipenem were measured using the microbroth dilution method. For ceftazidime, the minimum bactericidal concentration (MBC) was measured by subculturing wells of the microbroth dilution tests into fresh media without antibiotic. The median (n = 3) of the log2 reduction in MIC and MBC of phage-resistant strains, compared to the wild type, is represented by the intensity of the blue shading of the cells. Raw data values and methods are available in Supplementary Table 2, and Supplementary Figs. 7 and 8. f: Changes in the phage sensitivity pattern. The infectivity of phage øLK01 was tested in each of the 11 strains, with nine being susceptible (green, as in the in vitro mutant) and two being resistant (yellow, as in wild type AB900)
The combined effect of ceftazidime and phage øFG02 against A. baumannii AB900 is synergistic
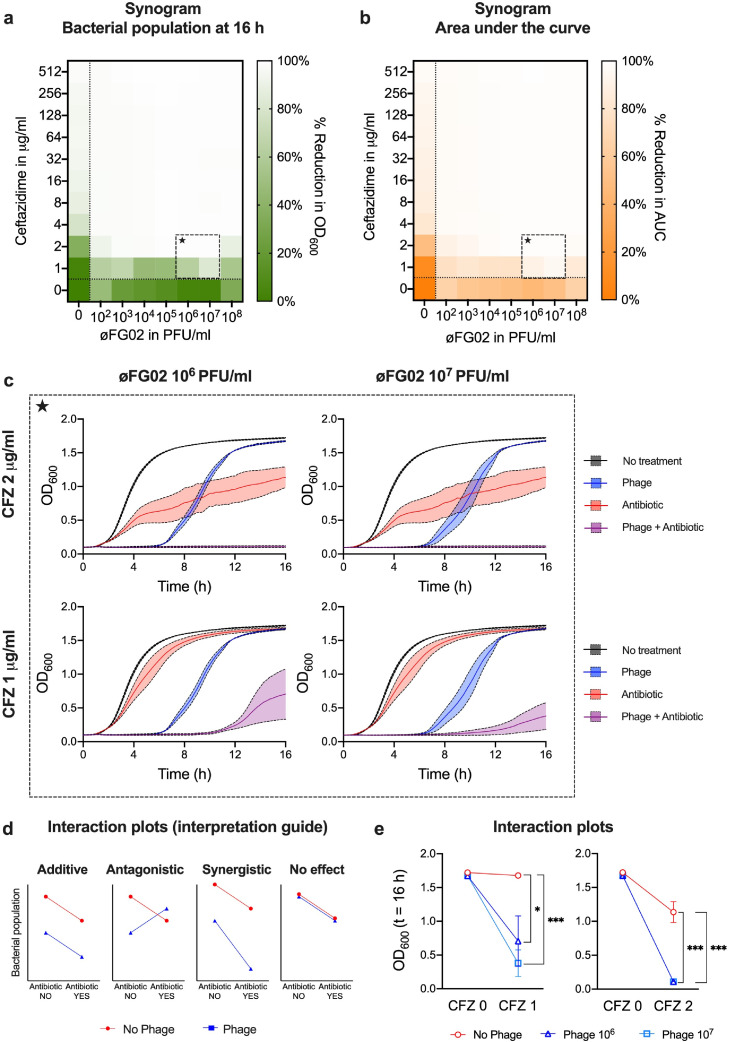
The results of our preclinical study suggest that ceftazidime and øFG02 achieve at least an additive effect when combined against A. baumannii AB900. A hypothesis of phage-antibiotic synergy (PAS) could be made, after corroborating that resensitisation to ceftazidime is a repeatable effect of resistance to øFG02. However, in vivo confirmation of antimicrobial synergy is notoriously fickle,51 and most in vitro and computational platforms that test for synergy may not cater for the unique biology of phages.22,52 Here, we tested our hypothesis using the synogram, an in vitro model based on the gold standard time-kill curve53 and the traditional checkerboard assay, and validated for phage-antibiotic combinations.45 With the synogram, we assessed the antimicrobial effect of ceftazidime at concentrations of 1 to 512 μg/ml and phage øFG02 at concentrations of 102 to 108 PFU/ml. Varying combinations of both agents were assessed and a no-treatment control was used. The measured outcomes were the size of the bacterial population at 16 h (Figure 3a) and the area under the bacterial growth curve (Figure 3b). By analysing individual wells of the synogram, we observed that all phage concentrations eventually lead to the emergence of phage-resistance, and most importantly, that even subinhibitory doses of phage and ceftazidime achieved sustained bacterial suppression when used in combination (Figure 3c). Finally, we used these data to construct interaction plots (Figure 3d,e) comparing the endpoint effect of ceftazidime when it was used with and without phage øFG02. The plots demonstrate that the addition of øFG02 increased the bactericidal effect of ceftazidime by more than the phage's bactericidal effect alone (divergent lines in the plots, Figure 3e) (two-way ANOVA; “phage” factor; p = 0.0264 for CFZ 1 μg/ml with øFG02 106 PFU/ml; and p = 0.0001 for CFZ 1 μg/ml with øFG02 107 PFU/ml; CFZ 2 μg/ml with øFG02 106 PFU/ml; and CFZ 2 μg/ml with øFG02 107 PFU/ml). As such, we could confirm the existence of synergy between ceftazidime and øFG02 against A. baumannii AB900, at least in vitro.
Figure 3.
Synergy between ceftazidime (CFZ) and phage øFG02 in vitro. a and b: Synograms of ceftazidime (CFZ) (1 to 512 μg/ml, rows) and phage øFG02 (102 to 108 PFU/ml, columns); wells below or to the left of the dotted lines contained single treatment of phage or antibiotic, respectively; the bottom left well served as a no-treatment control; the mean reduction in bacterial density at the endpoint (t = 16 h; n = 3) is colour coded by the shades of green (a), while the reduction in the area under the curve (AUC) is in orange (b). Wells of combination treatment that are analysed in panel c are delineated with the dashed squares containing a star. c: Growth curves of A. baumannii AB900 with varying doses of phage øFG02 (blue), ceftazidime (red), both (purple), or neither (black). Data are mean ± s.e.m (n = 3). Curves show how the combination of subinhibitory doses of phage and antibiotic achieves effective bacterial suppression. d and e: Interaction plots of the antimicrobial effects of ceftazidime and phage øFG02 suggest the existence of synergy (interpretation key presented in d, as per45: additive effect would result in parallel lines, antagonism in convergent lines, synergy in divergent lines, no effect in superimposed lines). Data are mean ± s.e.m (n = 3). Two-way ANOVA for statistical analysis; “phage” factor; * p < 0.05; ***p = 0.0001. OD600=optical density at 600 nm.
Discussion
Clinical translation of new therapeutic strategies against A. baumannii, although extremely necessary, can be challenging. Here, we have advanced the translation of a phage-antibiotic combination strategy taking advantage of the knowledge of phage receptor54 and the mechanism for the emergence of phage-resistance33 in A. baumannii. In this study, we have successfully demonstrated that the combination of ceftazidime with phage øFG02 is better than each agent individually in a preclinical model of bacteraemia. The superiority of this approach can be explained by understanding the interactions between phages, their bacterial and mammalian hosts, and antibiotics.
Previous in vitro findings illustrated the emergence of AB900 resistance against phage øFG02 through capsule loss, resulting in decreased fitness and antimicrobial resensitisation.33 Here, we also tackled a logical subsequent question: whether the evolution of similar mutations would occur in a complex in vivo environment.55 This work provides evidence that for this phage-host pair, this mechanism of bacterial phage-resistance in vivo is repeatable and predictable. Phage-resistant mutants emerged in 24 out of 25 animals receiving phage øFG02, either alone or in combination, and all of the 11 randomly selected resistant isolates contained mutations in genes of the K locus, resulting in abolition of phage adsorption. Most importantly, all phage-resistant mutants showed increased sensitivity to ceftazidime, with 9 of the 11 becoming either vulnerable to an alternative phage, øLK01, or more sensitive to imipenem. Of note, populations of 10 of the 11 strains could be eradicated by 60 min of incubation in human serum, although this finding might not have contributed to the results in the murine models given the poor complement activity of mouse sera.56 In any case, our findings support a model in which the combination treatment acts as a staggered “one-two punch”, with initial phage dosing partially killing the bacterial population and driving the emergence of phage-resistant variants, which are then resensitised to and killed by the antibiotic.
Phages that induce repeatable and predictable phage-resistant phenotypes in their hosts have been previously described.57,58 These phages can certainly be clinically useful, but it is worth remembering that not all phage-host pairs will behave in the same way.59, 60, 61 Interestingly, while the locus of loss-of-function mutations in our phage-resistant strains was repeatable, and mutations primarily clustered in genes involved in the early steps of capsule production,62 the specific genes, types of mutations and nucleotide positions were not all the same, suggesting a degree of flexibility and diversity in the variants. It is entirely possible for some of these variants to differ in other fitness trade-offs. Our findings do not completely exclude the chance of alternative, unrelated, mechanisms of resistance against øFG02. However, they do suggest that, if possible, those mechanisms might require a more complicated evolutionary route to emerge, or are present in lower proportions, arguably making them irrelevant in the clinical setting. Finally, and although unseen here, another possibility is the occurrence of subsequent compensatory mutations or reversion to the wild type phenotype.
During optimisation experiments, we observed that once-a-day (q.d.) and twice-a-day (b.i.d) phage administration regimes appeared to be equivalent. Namely, we observed no differences in neither their antibacterial effect, phage levels, or emergence rate of phage-resistance. While these findings successfully guided the design of our preclinical study, deeper research into the pharmacology of phage therapy is certainly warranted. It is becoming increasingly apparent that the interactions between phages and eukaryotic cells also need to be accounted for when planning phage therapy regimes, as factors such as mammalian cell type and phage size can influence cellular uptake of phage and thus, phage bioavailability.63
While the resulting in vivo model reflects the severity of A. baumannii bacteraemia in humans, our analyses stemming from it still present some limitations. Firstly, by sampling only four body sites (blood, liver, kidney, and spleen), we could possibly be missing body sites that act as bacterial reservoirs, or where phages do not reach in sufficient quantity to trigger the emergence of phage-resistant variants. Second, the arguably short endpoint of 16 h makes it hard to establish conclusions on the long-term sustainability of the combined therapy, and the model was not amenable to a prolonged survival experiment. Third, the use of the intraperitoneal administration route, which may not have a perfect correlation with the intravenous route used in humans, could influence phage bioavailability and effectiveness at specific body sites.64
A further limitation is that our results are restricted to the antibiotic ceftazidime and phage øFG02. However, we hypothesise that similar results could be obtained with other phages that target A. baumannii’s capsule, thus inducing capsule loss upon emergence of phage-resistance. It could even be possible for similar phenomena to emerge with other capsulated pathogens, as suggested by preliminary observations in Klebsiella pneumoniae65 and Pseudomonas aeruginosa.66 A final caveat regarding phage øFG02 is that, despite multiple attempts,33 its genome remains unsequenced. Here we have used a phenotypic assay36 to rule out lysogenic behaviour in øFG02 (Supplementary Fig. 1) but it would be advisable to obtain sequencing data before using it in a clinical setting.
Previous studies have shown evidence of synergy between A. baumannii-specific phages and the beta-lactams imipenem32 and meropenem.30,32 as well as the outer-membrane disrupting agent colistin30,31,67 either in vitro or using the Galleria mellonella larvae system. However, our study demonstrates the superiority of a phage-antibiotic combination using a mammalian model, furthering its translational potential. Furthermore, we confirmed that, at least in vitro, ceftazidime and phage øFG02 achieve synergistic, instead of just additive, effects.
Further research in the field will surely lead to the discovery of innovative uses of combination therapies using phages, rather than proposing phages as a substitute to antibiotics.11 As an example, in the reviewed clinical cases of phage therapy use against A. baumannii, the patients received-at least for a brief period-phages and antibiotics simultaneously17, 18, 19, 20, 21; and in at least one of those cases, therapeutic synergy may have contributed to the patient's favourable outcome.17 In fact, it is reasonable to believe that the translation of phage therapy into widespread clinical use could be spearheaded by the use of phage-antibiotic combinations. In this study, we have provided insights that further advance the development of combination-based therapeutic approaches using phages against the top priority pathogen A. baumannii.
Contributors
F.G.A., A.Y.P. and J.J.B. conceived the study. F.G.A., and X.K. performed in vivo experiments. F.G.A. performed characterisation of phage-resistant mutants and in vitro synergy testing. D.S. led the bioinformatic analyses, with F.G.A. providing further input. D.K. performed scanning electron microscopy. A.Y.P. and J.J.B. provided resources for experimental work. A.Y.P. and J.J.B. supervised and funded the project. F.G.A. wrote the original draft, with J.J.B. providing further feedback and editing. Underlying data has been verified by F.G.A., X.K., and J.J.B. (in vivo experiments), F.G.A., D.S., and J.J.B. (bioinformatics), and F.G.A., and J.J.B. (in vitro experiments). All authors were involved in reviewing and editing the final manuscript.
Data sharing statement
Sequencing data are available with the NCBI BioProject accession number: PRJNA608808. This work did not involve the creation of novel code for bioinformatics analyses. All the used software is freely accessible and appropriately referenced. Raw data from all experiments are available upon reasonable request.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
Fernando L. Gordillo Altamirano acknowledges the support from Monash University through the Monash Postgraduate Research Scholarships that funded his doctoral studies, and the Monash Postgraduate Publication Award (Round 1, 2021). Anton Y. Peleg is supported by a National Health and Medical Research Council Practitioner Fellowship (APP1117940). This work, including the efforts of Jeremy J. Barr, was funded by the National Health and Medical Research Council (NHMRC: 1156588), Perpetual Trustees Australia award (2018HIG00007), and Frontier Health Medical Research (RFRHPI000017). We thank the Monash Ramaciotti Cryo-EM platform for the use of the facility and Alex de Marco for granting access to the SEM; as well as the MASSIVE high performance computing facility (www.massive.org.au) for providing us cluster time for data analysis. We also thank Dr María José Fierro for her comments on the structure and readability of the manuscript. Figure 1a,b was made using BioRender (biorender.com).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104045.
Contributor Information
Anton Y. Peleg, Email: anton.peleg@monash.edu.
Jeremy J. Barr, Email: jeremy.barr@monash.edu.
Appendix. Supplementary materials
References
- 1.Getahun H., Smith I., Trivedi K., Paulin S., Balkhy HH. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull World Health Organ. 2020;98:442. doi: 10.2471/BLT.20.268573. 442A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruiz J. Enhanced antibiotic resistance as a collateral COVID-19 pandemic effect? J Hosp Infect. 2021;107:114–115. doi: 10.1016/j.jhin.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lescure F.-.X., Bouadma L., Nguyen D., et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottesman T., Fedorowsky R., Yerushalmi R., Lellouche J., Nutman A. An outbreak of carbapenem-resistant Acinetobacter baumannii in a COVID-19 dedicated hospital. Infect Prev Pract. 2021;3 doi: 10.1016/j.infpip.2021.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharifipour E., Shams S., Esmkhani M., et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis. 2020;20:646. doi: 10.1186/s12879-020-05374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuehn BM. Drug-resistant bacteria outbreak linked to COVID-19 patient surge. JAMA. 2021;325:335. doi: 10.1001/jama.2020.26113. [DOI] [PubMed] [Google Scholar]
- 7.Hawkey J., Ascher D.B., Judd L.M., et al. Evolution of carbapenem resistance in Acinetobacter baumannii during a prolonged infection. Microb Genom. 2018;4 doi: 10.1099/mgen.0.000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peleg A.Y., Seifert H., Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner-Lastinger L.M., Abner S., Edwards J.R., et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the national healthcare safety network, 2015–2017. Infect Control Hosp Epidemiol. 2020;41:1–18. doi: 10.1017/ice.2019.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. [Internet]. Geneva; Switzerland: World Health Organization. [2017; cited January 2021]. Available from: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1
- 11.Gordillo Altamirano F.L., Barr JJ. Phage therapy in the postantibiotic era. Clin Microbiol Rev. 2019;32 doi: 10.1128/CMR.00066-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua Y., Luo T., Yang Y., et al. Phage therapy as a promising new treatment for lung infection caused by carbapenem-resistant Acinetobacter baumannii in mice. Front Microbiol. 2017;8:2659. doi: 10.3389/fmicb.2017.02659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon J., Park J.H., Yong D. Efficacy of bacteriophage treatment against carbapenem-resistant Acinetobacter baumannii in Galleria mellonella larvae and a mouse model of acute pneumonia. BMC Microbiol. 2019;19:70. doi: 10.1186/s12866-019-1443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leshkasheli L., Kutateladze M., Balarjishvili N., et al. Efficacy of newly isolated and highly potent bacteriophages in a mouse model of extensively drug-resistant Acinetobacter baumannii bacteraemia. J Glob Antimicrob Resist. 2019;19:255–261. doi: 10.1016/j.jgar.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Wintachai P., Naknaen A., Pomwised R., Voravuthikunchai S.P., Smith DR. Isolation and characterization of Siphoviridae phage infecting extensively drug-resistant Acinetobacter baumannii and evaluation of therapeutic efficacy in vitro and in vivo. J Med Microbiol. 2019;68:1096–1108. doi: 10.1099/jmm.0.001002. [DOI] [PubMed] [Google Scholar]
- 16.Regeimbal J.M., Jacobs A.C., Corey B.W., et al. Personalized therapeutic cocktail of wild environmental phages rescues mice from Acinetobacter baumannii wound infections. Antimicrob Agents Chemother. 2016;60:5806–5816. doi: 10.1128/AAC.02877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schooley R.T., Biswas B., Gill J.J., et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017;61:e00954-17. doi: 10.1128/AAC.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan X., Chen H., Zhang M., et al. Clinical experience of personalized phage therapy against carbapenem-resistant Acinetobacter baumannii lung infection in a patient with chronic obstructive pulmonary disease. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.631585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu N., Dai J., Guo M., et al. Pre-optimized phage therapy on secondary Acinetobacter baumannii infection in four critical COVID-19 patients. Emerg Microbes Infect. 2021;10:612–618. doi: 10.1080/22221751.2021.1902754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nir-Paz R., Gelman D., Khouri A., et al. Successful treatment of antibiotic-resistant, poly-microbial bone infection with bacteriophages and antibiotics combination. Clin Infect Dis. 2019;69:2015–2018. doi: 10.1093/cid/ciz222. [DOI] [PubMed] [Google Scholar]
- 21.LaVergne S., Hamilton T., Biswas B., Kumaraswamy M., Schooley R.T., Wooten D. Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. Open Forum Infect Dis. 2018;5:ofy064. doi: 10.1093/ofid/ofy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doern CD. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol. 2014;52:4124. doi: 10.1128/JCM.01121-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huff W.E., Huff G.R., Rath N.C., Balog J.M., Donoghue AM. Therapeutic efficacy of bacteriophage and Baytril (enrofloxacin) individually and in combination to treat colibacillosis in broilers. Poult Sci. 2004;83:1944–1947. doi: 10.1093/ps/83.12.1944. [DOI] [PubMed] [Google Scholar]
- 24.Comeau A.M., Tetart F., Trojet S.N., Prere M.F., Krisch HM. Phage-antibiotic synergy (PAS): beta-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One. 2007;2:e799. doi: 10.1371/journal.pone.0000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Anany A.M., Fatima R., Hynes AP. Temperate phage-antibiotic synergy eradicates bacteria through depletion of lysogens. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109172. [DOI] [PubMed] [Google Scholar]
- 26.Torres-Barcelo C., Hochberg ME. Evolutionary rationale for phages as complements of antibiotics. Trends Microbiol. 2016;24:249–256. doi: 10.1016/j.tim.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Burmeister A.R., Turner PE. Trading-off and trading-up in the world of bacteria-phage evolution. Curr Biol. 2020;30:R1120–R11R4. doi: 10.1016/j.cub.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangalea M.R., Duerkop BA. Fitness trade-offs resulting from bacteriophage resistance potentiate synergistic antibacterial strategies. Infect Immun. 2020;88:e00926-19. doi: 10.1128/IAI.00926-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tagliaferri T.L., Jansen M., Horz H-P. Fighting pathogenic bacteria on two fronts: phages and antibiotics as combined strategy. Front Cell Infect Microbiol. 2019;9:22. doi: 10.3389/fcimb.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen M., Wahida A., Latz S., et al. Enhanced antibacterial effect of the novel T4-like bacteriophage KARL-1 in combination with antibiotics against multi-drug resistant Acinetobacter baumannii. Sci Rep. 2018;8:14140. doi: 10.1038/s41598-018-32344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Styles K.M., Thummeepak R., Leungtongkam U., et al. Investigating bacteriophages targeting the opportunistic pathogen Acinetobacter baumannii. Antibiotics. 2020;9:200. doi: 10.3390/antibiotics9040200. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blasco L., Ambroa A., Lopez M., et al. Combined use of the Ab105-2φΔCI lytic mutant phage and different antibiotics in clinical isolates of multi-resistant Acinetobacter baumannii. Microorganisms. 2019;7:556. doi: 10.3390/microorganisms7110556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordillo Altamirano F., Forsyth J.H., Patwa R., et al. Bacteriophage-resistant Acinetobacter baumannii are resensitized to antimicrobials. Nat Microbiol. 2021;6:157–161. doi: 10.1038/s41564-020-00830-7. [DOI] [PubMed] [Google Scholar]
- 34.Adams M.D., Goglin K., Molyneaux N., et al. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J Bacteriol. 2008;190:8053–8064. doi: 10.1128/JB.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenyon J.J., Hall RM. Variation in the complex carbohydrate biosynthesis loci of Acinetobacter baumannii genomes. PLoS One. 2013;8:e62160. doi: 10.1371/journal.pone.0062160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordillo Altamirano F.L., Barr JJ. Screening for lysogen activity in therapeutically relevant bacteriophages. Bio-protoc. 2021;11:e3997. doi: 10.21769/BioProtoc.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonilla N., Rojas M.I., Netto Flores Cruz G., Hung S.-.H., Rohwer F., Barr J.J. Phage on tap–a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ. 2016;4:e2261. doi: 10.7717/peerj.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris G., Holbein B.E., Zhou H., Xu H.H., Chen W. Potential mechanisms of mucin-enhanced Acinetobacter baumannii virulence in the mouse model of intraperitoneal infection. Infect Immun. 2019;87:e00591-19. doi: 10.1128/IAI.00591-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acred P. Therapeutic and kinetic properties of ceftazidime in animals. Infection. 1983;11:S44–S48. doi: 10.1007/BF01641106. [DOI] [PubMed] [Google Scholar]
- 40.Richards D.M., Ceftazidime BRN. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1985;29:105–161. doi: 10.2165/00003495-198529020-00002. [DOI] [PubMed] [Google Scholar]
- 41.Andrews S. FastQC: A Quality Control Tool For High Throughput Sequence data. [Internet] Cambridge, UK: Babraham Bioinformatics; [2010; cited November 2020]. Available from: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- 42.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorvaldsdóttir H., Robinson J.T., Mesirov JP. Integrative Genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiegand I., Hilpert K., Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 45.Gu Liu C., Green S.I., Min L., et al. Phage-antibiotic synergy is driven by a unique combination of antibacterial mechanism of action and stoichiometry. mBio. 2020;11:e01462-20. doi: 10.1128/mBio.01462-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Percie du Sert N., Hurst V., Ahluwalia A., et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ballouz T., Aridi J., Afif C., et al. Risk factors, clinical presentation, and outcome of Acinetobacter baumannii bacteremia. Front Cell Infect Microbiol. 2017;7:156. doi: 10.3389/fcimb.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papathanakos G., Andrianopoulos I., Papathanasiou A., Priavali E., Koulenti D., Koulouras V. Colistin-resistant Acinetobacter baumannii bacteremia: a serious threat for critically ill patients. Microorganisms. 2020;8:287. doi: 10.3390/microorganisms8020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh J.K., Adams F.G., Brown MH. Diversity and function of capsular polysaccharide in Acinetobacter baumannii. Front Microbiol. 2019;9:3301. doi: 10.3389/fmicb.2018.03301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kenyon J.J., Shashkov A.S., Senchenkova S.N., et al. Acinetobacter baumannii K11 and K83 capsular polysaccharides have the same 6-deoxy-l-talose-containing pentasaccharide K units but different linkages between the K units. Int J Biol Macromol. 2017;103:648–655. doi: 10.1016/j.ijbiomac.2017.05.082. [DOI] [PubMed] [Google Scholar]
- 51.Fantin B., Carbon C. In vivo antibiotic synergism: contribution of animal models. Antimicrob Agents Chemother. 1992;36:907–912. doi: 10.1128/aac.36.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torella J.P., Chait R., Kishony R. Optimal drug synergy in antimicrobial treatments. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knezevic P., Curcin S., Aleksic V., Petrusic M., Vlaski L. Phage-antibiotic synergism: a possible approach to combatting Pseudomonas aeruginosa. Res Microbiol. 2013;164:55–60. doi: 10.1016/j.resmic.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Gordillo Altamirano F.L., Barr JJ. Unlocking the next generation of phage therapy: the key is in the receptors. Curr Op Biotechnol. 2021;68:115–123. doi: 10.1016/j.copbio.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Pirnay J.-.P., Ferry T., Resch G. Recent progress toward the implementation of phage therapy in western medicine. FEMS Microbiol Rev. 2022;46:fuab040. doi: 10.1093/femsre/fuab040. [DOI] [PubMed] [Google Scholar]
- 56.Ratelade J., Verkman AS. Inhibitor(s) of the classical complement pathway in mouse serum limit the utility of mice as experimental models of neuromyelitis optica. Mol Immunol. 2014;62:104–113. doi: 10.1016/j.molimm.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan B.K., Sistrom M., Wertz J.E., Kortright K.E., Narayan D., Turner PE. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci Rep. 2016;6:26717. doi: 10.1038/srep26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chatterjee A., Johnson C.N., Luong P., et al. Bacteriophage resistance alters antibiotic-mediated intestinal expansion of enterococci. Infect Immun. 2019;87:e00085-19. doi: 10.1128/IAI.00085-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oechslin F. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses. 2018;10:351. doi: 10.3390/v10070351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burmeister A.R., Fortier A., Roush C., et al. Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc Natl Acad Sci U S A. 2020;117:11207. doi: 10.1073/pnas.1919888117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright R.C.T., Friman V.-.P., Smith M.C.M., Brockhurst MA. Resistance evolution against phage combinations depends on the timing and order of exposure. mBio. 2019;10 doi: 10.1128/mBio.01652-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai J., Dai Y., Farinha A., et al. Essential gene analysis in Acinetobacter baumannii by high-density transposon mutagenesis and CRISPR interference. J Bacteriol. 2021;203:e00565-20. doi: 10.1128/JB.00565-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bichet M.C., Chin W.H., Richards W., et al. Bacteriophage uptake by mammalian cell layers represents a potential sink that may impact phage therapy. iScience. 2021;24 doi: 10.1016/j.isci.2021.102287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dąbrowska K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med Res Rev. 2019;39:2000–2025. doi: 10.1002/med.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song L., Yang X., Huang J., et al. Phage selective pressure reduces virulence of hypervirulent Klebsiella pneumoniae through mutation of the wzc gene. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.739319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engeman E., Freyberger H.R., Corey B.W., et al. Synergistic killing and re-sensitization of Pseudomonas aeruginosa to antibiotics by phage-antibiotic combination treatment. Pharmaceuticals. 2021;14:184. doi: 10.3390/ph14030184. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X., Loh B., Gordillo Altamirano F., Yu Y., Hua X., Leptihn S. Colistin-phage combinations decrease antibiotic resistance in Acinetobacter baumannii via changes in envelope architecture. Emerg Microbes Infect. 2021;10:2205–2219. doi: 10.1080/22221751.2021.2002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.