Highlights
-
•
The cftrtm1unc Tg(FABP-hCFTR) mouse is a commonly-used animal model of CF.
-
•
This mouse expresses human CFTR in the gut to prevent fatal intestinal obstruction.
-
•
Macrophages from this mouse fail to replicate immune dysfunction seen in patient cells.
-
•
We show ectopic expression of human CFTR transgene in macrophages from this CF mouse.
-
•
This may help to explain anomalies in the field related to use of this model.
Abstract
Macrophages represent prominent immune orchestrators of cystic fibrosis (CF) inflammation and, as such, are an ever-increasing focus of CF research with several reports of intrinsic immune dysfunction related to loss of CFTR activity in macrophages themselves. Animal models of CF have contributed, in no small part, to a deepening of our understanding of the pathophysiology of the disease and towards therapeutic development. A commonly-used animal model in CF research is the Cftrtm1Unc Tg(FABP-hCFTR) mouse, which displays gut-specific expression of a human CFTR transgene in order to rescue the high rate of early mortality in Cftr-null mice associated with severe intestinal obstruction. We find significant variation in the response to inflammatory challenge of patient macrophages and cells derived from the Cftrtm1Unc Tg(FABP-hCFTR) mouse and show that macrophages derived from this mouse exhibit aberrant expression of human CFTR. This may contribute to the absence of inflammatory changes in this model.
1. Introduction
Chronic airway inflammation underpins the progression of CF lung disease and is consequently a major focus of CF research and an important therapeutic target. There is ample evidence of dysregulated inflammatory pathways in immune cells derived from people with CF and the observation of functional CFTR expression in non-epithelial cells has uncovered a direct role for the basic defect in immune cell dysfunction in CF, particularly in monocytes and macrophages [1], [2], [3]. Rollout of disease-modifying CFTR modulators to the vast majority of people with CF may now place further emphasis on animal models to provide a means of investigating baseline differences in uncorrected, CFTR-deficient immune cells with the aim of developing novel strategies to combat chronic inflammation alongside modulator therapies and the standard treatment regimen [4]. Identification of the CFTR gene in the 1980s allowed for the generation of animal models and heralded a new wave of CF in vivo research which has helped considerably to inform a greater understanding of the pathophysiology of the disease. The mouse is the most commonly used model of CF and has many advantages, not least the ease of husbandry and low-cost relative to larger animals which are more anatomically homologous to humans (e.g. pig and ferret). However, CF mice fail to exhibit several of the most prominent hallmarks of human disease and do not develop mucus obstruction and spontaneous lung disease to anywhere near the same extent as humans with CF [5–7]. In addition, complete knockout of Cftr leads to high rates of early mortality in these models due to severe intestinal obstruction [8]. One CF mouse model designed specifically to offset this issue is the Cftrtm1Unc Tg(FABP-hCFTR) mouse which displays gut-specific expression of a human CFTR transgene, driven by the intestinal fatty acid-binding protein 2 (FABP2) promoter [9]. This allows for survival to maturity with correction of the CF phenotype restricted largely to the gut and this model is subsequently in widespread use in studying the effects of CFTR loss on other systems [10–14].
Here, we show that bone-marrow derived macrophages (BMDMs) from the Cftrtm1Unc Tg(FABP-hCFTR) mouse display significant differences to patient-derived monocyte-derived macrophages (MDMs) in response to inflammatory challenge. We then demonstrate expression of the human CFTR transgene in BMDMs from the same mouse model. Together these data suggest that this widely used mouse model has significant limitations in the study of CF associated inflammation.
2. Materials and methods
2.1. Mice
Cftrtm1Unc Tg(FABP-hCFTR)1Jaw/J were purchased from Jackson Laboratory and then backcrossed for 10 generations onto C57BL/6 J mice. Male homozygotes were bred with female heterozygotes due to poor fertility in the female homozygotes, with genotyping carried out at birth to determine deletion of murine Cftr, and the presence of the human transgene. The mice harbour the FABP-hCFTR transgene with inserted rat fatty acid-binding protein 2 (FABP2) promoter directing expression of human CFTR allowing for high expression of the transgene throughout the small intestine [9]. Mouse breeding and experimental work was carried out in accordance with the Animal (Scientific Procedures) Act 1986, PPL number 70/8884, and under the supervision of the University of Edinburgh Ethical Review Committee. All researchers were accredited by the Home Office, UK.
2.2. Macrophage culture and treatment
Bone marrow-derived macrophages (BMDMs) were obtained by flushing bone marrow from femurs and tibias and plated at 3 × 106 per well in 6-well plates for 7 days in Dulbecco's Modified Eagle Medium (DMEM) + glutMAX (Thermo Fisher Scientific) supplemented with 10% heat-inactivated foetal calf serum (HI-FCS), 1% penicillin/streptomycin (P/S), 1% l-glutamine (all Thermo Fisher Scientific) and 20% M-CSF-containing L929 medium to induce macrophage differentiation. After 7 days, BMDMs were detached using ice-cold cation-free Dulbecco's phosphate buffered saline (DPBS−/−) (Thermo Fisher Scientific) + 1% BSA (Sigma) + 3 mM EDTA (Thermo Fisher Scientific), counted and plated overnight at 0.25 × 106/well on a 48-well culture plate.
Peripheral blood was collected from clinically stable patients (no intravenous antibiotics required in the 2 weeks previous to sample collection) with CF attending the Scottish National CF Service at the Edinburgh Western General Hospital. Lung transplant recipients were excluded. Written consent was given by patients and the study was approved by the East of Scotland Research Ethics Committee (15/ES/0094). All individuals recruited had at least one copy of the ΔF508 mutation with another disease-causing mutation (four ΔF508/ΔF508 homozygous, one ΔF508/1717 + 1G > A, one ΔF508/D1152H, ΔF508/G542X and one ΔF508/Q493X). Peripheral blood was also collected from age- and sex-matched healthy adult human volunteers under Ethical Review (AMREC Reference number 15-HV-013) under the project number CIRBRP009; this included written informed consent. Peripheral blood mononuclear cells (PBMCs) were isolated by Percoll (GE Healthcare) density gradient centrifugation [15]. Monocytes were isolated from the PBMCs by pan monocyte negative selection using MACS cell separation, according to manufacturer's instructions (Miltenyi Biotec). Isolated monocytes were plated at 0.8 × 106/well into 24-well tissue culture plates (Sigma-Aldrich) and cultured in Iscove's Modified Dulbecco's Medium (IMDM) supplemented with 10% HI-FCS, 1% P/S, 1% l-glutamine, and 100 ng/ml human recombinant macrophage colony-stimulating factor (M-CSF; PeproTech) for 7 days to induce differentiation.
Lipopolysaccharide (LPS) challenge of human or mouse macrophages was carried out with 100 ng/ml Pseudomonas aeruginosa 10 LPS (Sigma-Aldrich) for the relevant time points.
2.3. Inflammatory gene expression and cytokine secretion analysis
RNA extraction from cell lysates was performed using the RNeasy Mini Kit (Qiagen) with on-column DNase I digestion also carried out before final elution using the RNase-Free DNase Set (Qiagen), both as per manufacturer's instructions. cDNA was synthesised using a master mix containing reverse transcriptase and other reagents from the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) as well as RNase Inhibitor (Thermo Fisher Scientific), according to manufacturer's instructions.
Quantitative real time PCR (qRT-PCR) was carried out on StepOnePlus™ Real-Time PCR System (Applied Biosystems) using SYBR green to assess for expression of various macrophage-associated inflammatory genes. CT values were obtained for each sample and expression fold change was measured using the 2−ΔΔCt method with normalisation against 18S rRNA.
Supernatants of murine BMDM cultures were collected and assayed for levels of several inflammatory cytokines and chemokines using the LEGENDPlex™ Mouse Inflammation Panel cytokine bead array kit, as per manufacturer's instruction (BioLegend). Human TNF-α secretion in MDM supernatants was measured by ELISA (R&D Systems).
2.4. CFTR/CFTR gene and protein expression analysis
Prescence of CFTR mRNA was confirmed using gel electrophoresis and cDNA obtained from fully differentiated BMDMs, with cDNA from human bronchial epithelial cell line, 16HBE, used for human control. Sequence homology prevented design of human CFTR-specific primers and so cross-species CFTR specific primers (mouse Cftr/human CFTR: (5′−3′) Forward – GGAGAGCATACCAGCAGTGACT; Reverse - TTCCAAGGAGCCACAGCACAAC) were used in combination with mouse CFTR specific primers (mouse Cftr: (5′−3′) Forward – CCATCAGCAAGCTGAAAGCAGG; Reverse - GTAGGGTTGTAATGCCGAGACG). SYBR green qRT-PCR was carried out to quantify CFTR mRNA abundance between samples.
BMDMs were derived for protein analysis from femurs of mice (Cftrtm1Unc Tg(FABP-hCFTR and WT littermate) obtained from collaborators at Imperial College London. BMDMs were culture for 7 days, as before, and lysed on ice using RIPA buffer (Thermo Fisher Scientific) supplemented with cOmplete™ Protease Inhibitor Cocktail (Roche). SDS polyacrylamide gel electrophoresis was carried out using precast 4–12% Bis-Tris gels and MOPS SDS running buffer for 90 min at 100 V. Proteins were transferred to a PVDF membrane and blocked for 1 h with TBST + 5% milk before being incubated with anti-CFTR primary antibody (1:1000; CF3; Novus Biologicals) or anti β-Actin primary antibody (1:2000; C4; Santa Cruz Biotechnology) overnight at 4 °C. HRP-linked goat anti-mouse secondary antibody (1:5000; Jackson ImmunoResearch) was then incubated with the membrane for 1 hour before exposure to chemiluminescence substrate (Thermo Fisher Scientific) was added to the membranes for 1 min before detection of chemiluminescence on x-ray film.
3. Results
3.1. Major disparities exist in the proinflammatory response of human and mouse CF macrophages to LPS challenge
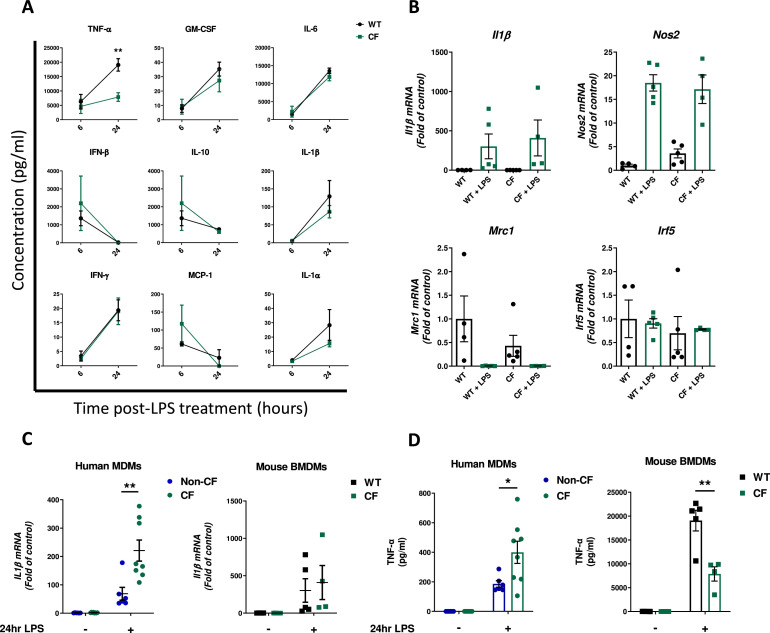
Analysis of cytokine secretion by mouse BMDMs at 6 and 24 h following treatment with P. aeruginosa LPS found no significant differences in release of several key inflammatory cytokines with the exception of TNF-α, secretion of which by CF macrophages was significantly reduced compared to wild-type (WT) (Fig. 1A). There were no significant differences in expression of several murine gene markers of macrophage polarisation between LPS-activated WT and CF BMDMs (Fig. 1B). The activated immune phenotype of the CF BMDMs was then compared to patient MDMs, subjected to the same LPS challenge. Expression of proinflammatory Il1β, as measured by qRT-PCR, remained highly upregulated after 24 stimulation with LPS compared to untreated controls but there was no significant difference in the magnitude of this upregulation between CF and WT BMDMs, however, expression of the human orthologue was significantly elevated in patient MDMs at the same time point relative to non-CF controls (Fig. 1C). Furthermore, whilst secretion of TNF-α was upregulated in LPS-treated CF MDMs at 24 h, release of the equivalent cytokine in mice was significantly blunted in activated CF BMDMs compared to WT (Fig. 1D).
Fig 1.
Inflammatory Phenotype in Human CF MDMs is not replicated in Macrophages Derived from Cftrtm1Unc Tg(FABPhCFTR) Mouse. A. Secretion of various inflammatory cytokines in supernatants of WT or CF BMDMs following activation with 100 ng/ml LPS for 6 and 24 h (n = 5 WT and 4 CF). B. Expression of gene markers of macrophage polarisation (Classical/M1 – Il1β, Nos2, Irf5. Alternatively-activated/M2 – Mrc1) by CF BMDMs compared to WT at 24 h with or without LPS treatment (n = 4 WT and 4 CF). C. Comparison of human IL1β expression by CF and non-CF human MDMs at 24 h post LPS with murine Il1β expression by CF and WT BMDMs at the same time point following the same stimulation (Human n = 6 nonCF and 8 CF). D. Supernatant levels of human TNF-α produced by human MDMs and murine TNF-α by mouse BMDMs at 24 h post Pseudomonas LPS (Human n = 6 non-CF and 8 CF). * = P ≤ 0.05, ** = P ≤ 0.01. Obtained via 2-way ANOVA with Tukey's multiple comparison post-hoc test. Data are presented as mean ± SEM.
3.2. BMDMs derived from Cftrtm1unc tg(fabp-hcftr) mouse express human cftr transgene and cftr protein
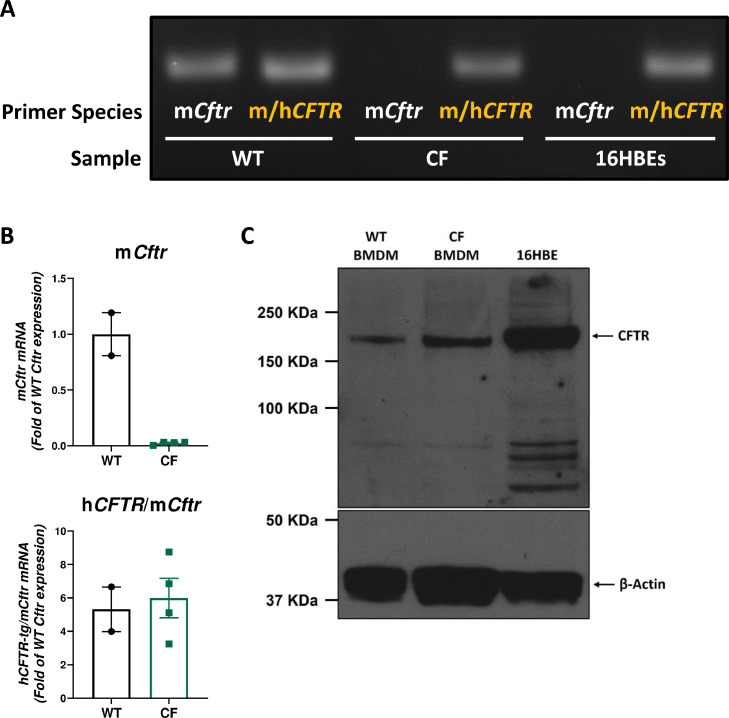
Given that mouse BMDMs failed to display a clear functional inflammatory defect, of the nature associated with CFTR deficiency in human cells, the expression of both Cftr and the human CFTR transgene were assessed. The presence of human CFTR mRNA in murine CF (Cftrtm1Unc Tg(FABP-hCFTR)) BMDMs was confirmed by gel electrophoresis following PCR using mouse/human CFTR primers in combination with mouse Cftr-specific primers. This showed that, as expected, WT macrophages express only murine Cftr and human bronchial epithelial cells (16HBEs) express only the human gene. In contrast, BMDMs derived from the Cftrtm1Unc Tg(FABP-hCFTR) mouse express no murine Cftr (as expected due to the Cftr deletion) but do display expression of the CFTR transgene (Fig. 2A). This was further underlined by quantitative analysis of gene expression by qRT-PCR, showing levels of human CFTR expression in Cftrtm1Unc Tg(FABP-hCFTR) BMDM that were comparable to Cftr expression in WT mice (Fig. 2B). Femurs were then obtained from Cftrtm1Unc Tg(FABP-hCFTR) mice from a separate institution (Imperial College London) and utilised to generate BMDMs, as before. Western blot analysis confirmed expression of CFTR protein in BMDMs from the CF mice, as well as in WT littermates and 16HBEs (Fig. 2C).
Fig 2.
Aberrant Expression of Human CFTR Transgene in BMDMs Derived from Cftrtm1Unc Tg(FABP-hCFTR) Mouse. A. Qualitative analysis by PCR gel electrophoresis of CFTR gene expression in WT and CF BMDMs and human bronchial epithelial cell line, 16HBEs. mCftr denotes mouse Cftr-specific transcript amplification whereas m/hCFTR primers bind both human and mouse CFTR. B. Quantitative measurement of CFTR expression in CF BMDMs by qRT-PCR. Data are presented as mean ± SEM. C. The presence of CFTR protein in lysates from BMDMs derived from Cftrtm1Unc Tg(FABP-hCFTR) mice or WT littermates and from 16HBEs was detected by western blot. β-Actin protein detection is shown as loading control.
4. Discussion
This study underlines key differences in the inflammatory response between macrophages from people with CF and macrophages from a commonly used murine model of CF. Inflammation is a cornerstone of CF lung disease and investigation of immune cell dysfunction in CF is now a central aspect of CF research with the aim of identifying novel ways to dampen chronic inflammation therapeutically and slow lung function decline. Macrophages represent one major target of such investigations and have been shown to display intrinsic, CFTR-dependent immune dysregulation in CF that likely contributes to exacerbation of the chronic inflammatory landscape [16]. This phenotype has been shown in various models of CF including in the CFTR−/- pig and in mice using myeloid-specific deletion of Cftr, whether through use of bone marrow chimeras or LysMCre conditional models, demonstrating a role for CFTR-deficient macrophages in the aggravation of airway inflammation and thwarting of effective resolution in such mice following inflammatory or infectious challenge [17], [18], [19]. Animal models of CF that are easy to breed and analogous, in key aspects of disease manifestation, to people with CF are highly important to the field CF research. The mouse is the most commonly used model, with the Cftrtm1Unc Tg(FABP-hCFTR) gut-correction model in widespread use. Here, we show that BMDMs derived from the Cftrtm1Unc Tg(FABP-hCFTR) mouse do not display a hyperinflammatory response to activation and, as such, fail to replicate a key aspect of human CF macrophage dysfunction. Heightened proinflammatory cytokine secretion in CF BMDMs in response to LPS has been reported in other CF mice including CFTR-nulls and models generated to mimic known clinical mutations (e.g. ΔF508, G551D) [18,20]. Following on from this, we find that macrophages derived from the Cftrtm1Unc Tg(FABP-hCFTR) mouse display aberrant expression of the human CFTR transgene, which may underpin the absence of this CF-associated inflammatory phenotype. This is consistent with a previous report of human CFTR expression in alveolar macrophages derived from the same mouse, even in those that had been newly purchased prior to generations of inbreeding and the potential for genetic drift that accompanies that [21]. We also obtained bones from mice of the same model from a different institution and generated BMDMs as before, to find CFTR protein expression in macrophages derived from the CF mouse as well as WT littermates, therefore allowing us to conclude that this ectopic expression is likely present in all animals generated using this model, and was not simply a spontaneous, isolated occurrence. Furthermore, the observation that expression of human CFTR transgene in these BMDMs actually reduces the TNF response to LPS suggests that the presence of human WT CFTR may actually be anti-inflammatory in these cells. Highlighting this phenotype in BMDMs is particularly relevant to CF research, given that lineage-tracing studies have shown significant replenishment, or even complete replacement, of tissue-resident alveolar macrophages with blood-derived macrophages in the lung following infection or chronic inflammation [22], [23], [24], [25]. Consequently, it stands to reason that, in the context of severe CF lung disease, blood-derived macrophages likely become the predominant macrophage population responsible for mediating proper pulmonary resolution and repair. This has important implications for use of the Cftrtm1Unc Tg(FABP-hCFTR) model. A better understanding of aberrant transgene expression in this mouse may also go some way to explaining certain anomalies in our understanding of macrophage function in CF more generally. Notably, the use of the gut-corrected Tg(FABP-hCFTR) CF mouse to propose Cftr-dependent regulation of phagosomal acidification as a key mechanism behind defective intracellular bacterial killing in CF macrophages was a major finding in the field, but spawned much controversy and has ultimately not proven reproducible despite the use of more accurate ratiometric methods with greater pH sensitivity than the fluorescein-based techniques employed in the original study [26], [27], [28]. One explanation for this ectopic transgene expression may be that murine macrophages express Fabp2. Although further investigation of this would be necessary for mechanistic confirmation, it is worth noting that expression of other FABPs has been shown previously in macrophages [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]. These findings suggest that, whilst the Cftrtm1Unc Tg(FABP-hCFTR) mouse serves as a useful model of human disease and allows for drastically improved ease of husbandry relative the complete knockouts; other strains of CF mouse, such as ones harbouring human disease-specific mutations, should be considered for the study of myeloid cell dysfunction in CF.
CRediT authorship contribution statement
Jonathan L Gillan: Conceptualization, Data curation, Methodology, Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. Gareth R Hardisty: Conceptualization, Data curation, Methodology, Investigation, Visualization, Writing – review & editing. Donald J Davidson: Conceptualization, Resources, Writing – review & editing. Robert D Gray: Conceptualization, Funding acquisition, Resources, Writing – review & editing.
Declaration of Competing Interest
None.
Acknowledgments
We thank Professor Uta Griesenbach and Professor Eric Alton for Supplying Bone Marrow from their colony of Cftrtm1UncTg(FABP-hCFTR) mice.
RDG was funded by Scottish Senior Fellowship (SCAF/16/02).
DJD was funded by an MRC Senior non-clinical Fellowship (G1002046).
We thank Lauren Melrose for genotyping the mice used in this study.
References
- 1.del Porto P., Cifani N., Guarnieri S., Di Domenico E.G., Mariggiò M.A., Spadaro F. Dysfunctional cftr alters the bactericidal activity of human macrophages against pseudomonas aeruginosa. PLoS ONE. 2011;6:e19970. doi: 10.1371/journal.pone.0019970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ettorre M., Verzè G., Caldrer S., Johansson J., Calcaterra E., Assael B.M. Electrophysiological evaluation of cystic fibrosis conductance transmembrane regulator (CFTR) expression in human monocytes. Biochim Biophys Acta Gen Subj. 2014;1840:3088–3095. doi: 10.1016/j.bbagen.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Bruscia E.M., Bonfield T.L. Cystic fibrosis lung immunity: the role of the macrophage. J Innate Immun. 2016;8:550–563. doi: 10.1159/000446825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillan J.L., Davidson D.J., Gray R.D. Targeting cystic fibrosis inflammation in the age of CFTR modulators: focus on macrophages. Eur Respir J. 2020 doi: 10.1183/13993003.03502-2020. [DOI] [PubMed] [Google Scholar]
- 5.Davidson D.J., Dorin J.R. The CF mouse: an important tool for studying cystic fibrosis. Expert Rev Mol Med. 2001;3:1–27. doi: 10.1017/s1462399401002551. [DOI] [PubMed] [Google Scholar]
- 6.Fisher J.T., Zhang Y., Engelhardt J.F. Comparative biology of cystic fibrosis animal models. Methods Mol Biol. 2011;742:311–334. doi: 10.1007/978-1-61779-120-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavelle G.M., White M.M., Browne N., McElvaney N.G., Reeves E.P. Animal models of cystic fibrosis pathology: phenotypic parallels and divergences. Biomed Res Int. 2016;2016 doi: 10.1155/2016/5258727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colledge W.H., Ratcliff R., Foster D., Williamson R., Evans M.J. Cystic fibrosis mouse with intestinal obstruction. Lancet. 1992;340:680. doi: 10.1016/0140-6736(92)92223-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhou L., Dey C.R., Wert S.E., DuVall M.D., Frizzell R.A., Whitsett J.A. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994;266:1705–1708. doi: 10.1126/science.7527588. (80-) [DOI] [PubMed] [Google Scholar]
- 10.Grubb B.R., Boucher R.C. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. 79(1 Suppl) [DOI] [PubMed] [Google Scholar]
- 11.Vij N., Mazur S., Zeitlin P.L. CFTR is a negative regulator of NFκB mediated innate immune response. PLoS ONE. 2009;4:e4664. doi: 10.1371/journal.pone.0004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorio C., Montresor A., Bolomini-Vittori M., Caldrer S., Rossi B., Dusi S. Mutations of cystic fibrosis transmembrane conductance regulator gene cause a monocyte-selective adhesion deficiency. Am J Respir Crit Care Med. 2016;193:1123–1133. doi: 10.1164/rccm.201510-1922OC. [DOI] [PubMed] [Google Scholar]
- 13.Riquelme S.A., Lozano C., Moustafa A.M., Liimatta K., Tomlinson K.L., Britto C., CFTR-PTEN-dependent mitochondrial metabolic dysfunction promotes pseudomonas aeruginosa airway infection. vol. 11. 2019. 10.1126/scitranslmed.aav4634. [DOI] [PMC free article] [PubMed]
- 14.Isopi E., Mattoscio D., Codagnone M., Mari V.C., Lamolinara A., Patruno S. Resolvin D1 reduces lung infection and inflammation activating resolution in cystic fibrosis. Front Immunol. 2020;11:581. doi: 10.3389/fimmu.2020.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray R.D., Hardisty G., Regan K.H., Smith M., Robb C.T., Duffin R. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax. 2018;73:134–144. doi: 10.1136/thoraxjnl-2017-210134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turton K.B., Ingram R.J., Valvano M.A. Macrophage dysfunction in cystic fibrosis: nature or nurture? J Leukoc Biol. 2020 doi: 10.1002/JLB.4RU0620-245R. [DOI] [PubMed] [Google Scholar]
- 17.Paemka L., McCullagh B.N., Abou Alaiwa M.H., Stoltz D.A., Dong Q., Randak C.O. Monocyte derived macrophages from CF pigs exhibit increased inflammatory responses at birth. J Cyst Fibros. 2017;16:471–474. doi: 10.1016/j.jcf.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruscia E.M., Zhang P.X., Ferreira E., Caputo C., Emerson J.W., Tuck D. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator-/-mice. Am J Respir Cell Mol Biol. 2009;40:295–304. doi: 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonfield T.L., Hodges C.A., Cotton C.U., Drumm M.L. Absence of the cystic fibrosis transmembrane regulator (Cftr) from myeloid-derived cells slows resolution of inflammation and infection. J Leukoc Biol. 2012;92:1111–1122. doi: 10.1189/jlb.0412188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas G.R., Costelloe E.A., Lunn D.P., Stacey K.J., Delaney S.J., Passey R. G551D cystic fibrosis mice exhibit abnormal regulation of inflammation in lungs and macrophages. J Immunol. 2000;164:3870–3877. doi: 10.4049/jimmunol.164.7.3870. [DOI] [PubMed] [Google Scholar]
- 21.Deriy L.V., Gomez E.A., Zhang G., Beacham D.W., Hopson J.A., Gallan A.J. Disease-causing mutations in the cystic fibrosis transmembrane conductance regulator determine the functional responses of alveolar macrophages. J Biol Chem. 2009;284:35926–35938. doi: 10.1074/jbc.M109.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misharin A.V., Morales-Nebreda L., Reyfman P.A., Cuda C.M., Walter J.M., McQuattie-Pimentel A.C. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machiels B., Dourcy M., Xiao X., Javaux J., Mesnil C., Sabatel C. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat Immunol. 2017;18:1310–1320. doi: 10.1038/ni.3857. [DOI] [PubMed] [Google Scholar]
- 24.Guilliams M., Mildner A., Yona S. Developmental and functional heterogeneity of monocytes. Immunity. 2018;49:595–613. doi: 10.1016/j.immuni.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Guilliams M., Thierry G.R., Bonnardel J., Bajenoff M. Establishment and maintenance of the macrophage Niche. Immunity. 2020;52:434–451. doi: 10.1016/j.immuni.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Di A., Brown M.E., Deriy L.V., Li C., Szeto F.L., Chen Y. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 27.Haggie P.M., Verkman A.S. Defective organellar acidification as a cause of cystic fibrosis lung disease: reexamination of a recurring hypothesis. Am J Physiol Cell Mol Physiol. 2009;296 doi: 10.1152/ajplung.00018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Law S.M., Stanfield S.J., Hardisty G.R., Dransfield I., Campbell C.J., Gray R.D. Human cystic fibrosis monocyte derived macrophages display no defect in acidification of phagolysosomes when measured by optical nanosensors. J Cyst Fibros. 2020;19:203–210. doi: 10.1016/j.jcf.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Furuhashi M., Hotamisligil G.S. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]