Abstract
This is the first report of large presacral Tarlov cysts (cerebrospinal fluid‐filled perineural cysts) diagnosed during pregnancy in which a cesarean delivery mode was selected to avoid the risk associated with vaginal delivery.
Keywords: delivery mode, pregnancy, Tarlov cyst
This is the first report of large presacral Tarlov cysts (cerebrospinal fluid‐filled perineural cysts) diagnosed during pregnancy in which a cesarean delivery mode was selected to avoid the risk associated with vaginal delivery.
1. INTRODUCTION
Tarlov cysts (TCs) were first described by Tarlov in 1938 in an autopsy report. 1 Pathologically, they are cerebrospinal fluid (CSF)‐filled perineural cysts that arise between the endoneurium and perineurium at the junction of the posterior nerve root and dorsal ganglion. 2 The cyst wall of TCs contains nerve fibers. 3 TCs are frequently found in the sacral region, mostly near the spinal canal and nerve foramen, 4 , 5 and are generally ≤2 cm in diameter. 6 TCs grow slowly, rarely extend to the endopelvic space, and seldom show natural regression. 7 , 8 , 9 Although symptomatic TCs can present with neurological symptoms such as sciatica, low back pain, lower extremity paresis, dyspareunia, and bladder/rectal disturbance, 8 the majority are asymptomatic and found by chance via magnetic resonance imaging (MRI) and computed tomography (CT) examinations performed for various lumbosacral symptoms. 4 , 10
Natural or artificial rupture of TCs has been reported to prompt symptom changes, including rapid deterioration of related symptoms. 11 , 12 , 13 Although some researchers have reported the presence of TCs in pregnancy and delivery, 2 , 14 , 15 the impact of vaginal delivery on presacral TCs has not been reported. To the best of our knowledge, this is the first report of the diagnosis of large presacral TCs in a pregnant woman in which the delivery mode was selected based on the potential effects of vaginal delivery on presacral lesions.
2. CASE REPORT
A 41‐year‐old pregnant woman (gravida 2, para 1) at 32 weeks of gestation with an otherwise normal medical history was referred from Obihiro Kosei General Hospital to JCHO Hokkaido Hospital for homecoming delivery (returning to her parents' town to give birth). Informed consent for the publication of the present case was obtained from the patient. Three years prior, she was also referred to our hospital for homecoming delivery at 32 weeks of gestation. Transvaginal echography of her pelvic cavity twice during her initial pregnancy and twice postpartum at our hospital revealed no pelvic cysts. Further, she underwent a normal vaginal delivery at 40 weeks of gestation, and no symptoms in the lower limbs, vulva, or buttock were noted.
In the second pregnancy, a right adnexal cyst was identified by transvaginal echography in a routine prenatal check‐up at Obihiro Kosei General Hospital. The patient was then diagnosed with large bilateral presacral TCs by pelvic MRI at 23 weeks of gestation. The cysts extended bilaterally from the sacral foramen to the pelvic cavity, and they were 65 mm × 23 mm and 30 mm × 32 mm on the right and left sides, respectively. The cysts were spindle‐shaped and showed a high‐intensity signal via T2‐weighted imaging, a low‐intensity signal via T1‐weighted imaging, and a high‐intensity signal via magnetic resonance myelography (Figure 1). Slight enlargement of the sacral canal was observed. An additional prepartum MRI (sagittal fat‐suppressed T2‐weighted imaging) at 34 weeks of gestation showed no change in cyst size or shape, and the minimum distance between the cyst wall and the posterior surface of the pubis was 100 mm (Figure 2A). The TCs were adjacent to the head of the fetus on the sagittal‐section, 2 cm to the right of the midline (Figure 2B).
FIGURE 1.
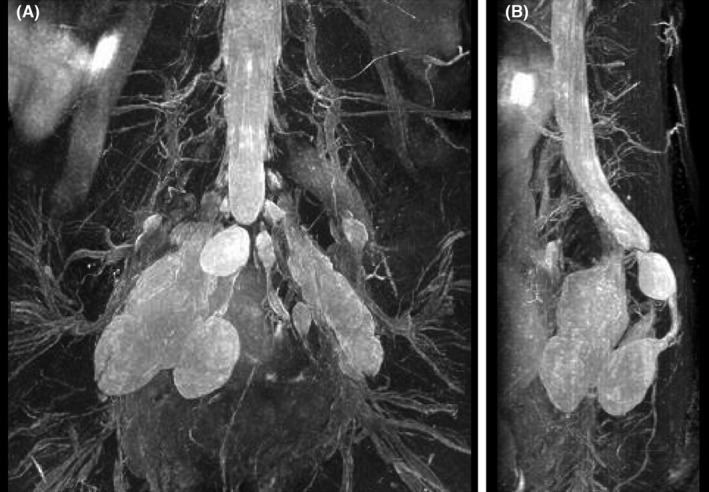
Magnetic resonance imaging findings (magnetic resonance myelography) at 23 weeks of gestation. Spindle‐shaped, bilateral cysts protruding into the pelvic cavity are shown via (A) coronal and (B) lateral views
FIGURE 2.
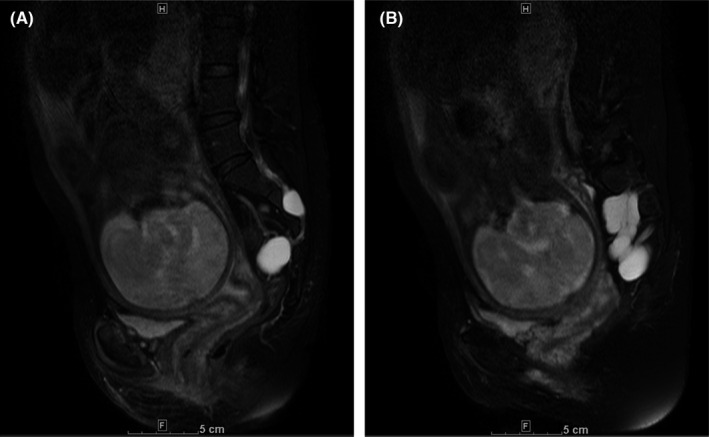
Magnetic resonance imaging findings (sagittal fat‐suppressed T2‐weighted imaging) at 34 weeks of gestation. (A) The minimum distance between the cyst wall and posterior surface of the pubis was 100 mm and (B) Tarlov cysts adjacent to the baby's head on the sagittal‐section 2 cm to the right of the midline
After an extensive literature review, we consulted with orthopedic spine specialists to discuss the optimal delivery mode for the patient. Risk of neurological symptom development due to cyst compression or rupture during vaginal delivery was a major concern. The possibility of symptom exacerbation due to vaginal delivery was determined to be low based on the following: (1) lesions were likely present when the patient had a vaginal delivery 3 years before and (2) although the TCs were large, they were presumed to be elastic and located in the retroperitoneal space. However, although non‐life‐threatening, the concern that cyst rupture could cause chronic pain in the sacral nerve area and low‐pressure headaches remained. Both vaginal delivery and cesarean section were considered as delivery mode options. After two elaborate explanations were given and counseling was performed at our prenatal clinic, the patient and her family decided that she would undergo cesarean section.
Regarding cesarean section anesthetization methods, the anesthesiology team discussed the following: (1) concerns about toxic effects of anesthesia on cyst wall nerves due to possible intracystic local anesthetic accumulation and (2) uncertainty regarding spinal effects of the anesthetic. Therefore, the team decided not to perform spinal anesthesia. An elective cesarean section was performed at 37 weeks of gestation using general anesthesia. Epidural anesthesia was not used due to difficulty inserting an epidural catheter.
A vacuum delivery device was used to deliver the baby's head during the cesarean section to avoid pressure on the anterior sacrum. Six days postoperatively, the appearance and size of presacral TCs remained unchanged when viewed via transvaginal echography, and no indications of cyst rupture were observed (Figure 3). The patient was aware of slight numbness in lateral and back sides of her right lower limb at 20 weeks of gestation, a symptom that attenuated at 30 weeks of gestation. No exacerbation of the symptom was observed after the cesarean section. No neurologic symptoms of the vulva or buttocks were observed throughout the clinical course.
FIGURE 3.
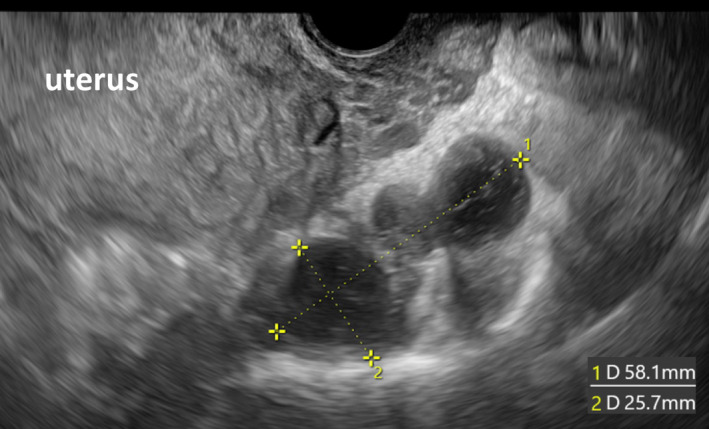
Transvaginal echography findings 6 days post‐cesarean section are shown. The appearance and size of presacral Tarlov cysts remained unchanged, and no findings were suggestive of cyst rupture
3. DISCUSSION
Tarlov cysts are cystic lesions formed between the pia mater‐derived endoneurium and the dura‐derived perineurium at the junction of the posterior nerve root and dorsal ganglion, which mostly occur at the S2–S3 levels. 5 The proposed etiology of TCs includes genetic disorders, inflammation, degeneration, and trauma. Cyst development and dilation are thought to be derived from hydrostatic pressure and pulsation of the CSF, which leads to intermittent influx via a ball‐valve mechanism. 16
Asymptomatic TCs are common 7 , 16 ; however, presacral TCs that protrude into the endopelvic space are very rare. 7 , 17 Enlarged lesions may cause abdominal pain and hydronephrosis, 18 , 19 and in the practice of gynecology, they are often first recognized as adnexal cysts by transvaginal echography and eventually diagnosed as presacral TCs by MRI or CT. 14 , 20 , 21 , 22 , 23 , 24 The posterolateral location of TCs, the presence of a normal ovary detected on the ipsilateral side, and immobility on respiration are features that identify presacral TCs vs. adnexal cysts via transvaginal echography. 14 , 20 Invasive procedures to treat presacral TCs based on their misdiagnosis as adnexal cysts have the potential to cause severe complications. Severe postoperative headache due to low intracranial pressure after laparoscopic cystectomy and fenestration 9 , 18 and severe pain from the buttocks to the thighs requiring additional neurosurgical repair surgery have been reported. 3 Furthermore, Kim et al. reported that immediately after cyst alcohol fixation was performed for presacral TCs, severe headache and vomiting occurred, and cauda equina syndrome was observed, including neurogenic bladder and anal sphincter weakness. It took 18 months for the patient to recover completely. 24 When treating adnexal cysts invasively, careful exclusion of presacral TCs is essential. MRI, which was used in the present case, is useful for obtaining an accurate diagnosis. 21 , 24
There are limited reports of TCs during pregnancy and delivery. Marino et al. reported that in a cohort of 138 female patients, six (4.35%) had worsening symptoms during pregnancy. However, detailed information, including the number of pregnant women included, TC size, the presence or absence of presacral lesions, and TC symptoms, were not clearly described. 2 H'ng et al. described a patient with TCs in which symptoms (bilateral hip and groin pain and difficulty ambulating) worsened 1 month after delivery; however, no information about the delivery itself was reported. 14 Pfund et al. reported that spinal anesthesia was effectively used during a cesarean section in a pregnant woman with symptomatic TCs who experienced intermittent low back pain and dysesthesia before pregnancy. 15 The report focused principally on the use of anesthesia in a patient with TCs and did not describe the process used to determine the delivery mode. The study also differed from the present case because TCs were not presacral.
In the present case, when delivery mode was discussed with orthopedic spine specialists, we focused on whether vaginal delivery would increase the risk of new symptom development that could impair the quality of life and activities of daily living of the patient. Cyst growth has been reported to be slow, with an average growth rate estimated at <1 mm per year. 8 Therefore, it is likely that the cysts were present at the time of the patient's previous pregnancy, which occurred 3 years prior. The TCs were presacral, large, and presumably elastic. Further, they extended into the retroperitoneal space. Therefore, we supposed that a vaginal delivery would likely be smooth, and cyst rupture would be avoided.
On the contrary, a case of TCs with rapid growth (~1 cm over a year) has been reported. 24 Further, the Valsalva maneuver reportedly enhances neurological symptoms of TCs. 25 Therefore, the possibility of rapid cyst growth and new symptom onset due to bearing down during vaginal delivery could not be completely ruled out. In addition to direct cyst compression by the head of the baby, obstetrical interventions such as vacuum delivery or uterine fundal compression may increase the risk of cyst rupture. TC rupture has the potential to trigger dramatic symptom improvement 17 ; however, chronic numbness and pain in the sacral nerve area and headache due to low intracranial pressure may occur. 3 , 9 , 12 , 13 Moreover, chronic pain and discomfort associated with TCs often lead to mood disorders. 2 Both vaginal delivery and cesarean section were proposed as delivery mode options, and the patient was provided with detailed explanations of both methods and counseling. Consequently, a cesarean delivery mode was selected.
In conclusion, this report is the first to emphasize that prudent consideration is required and that adequate information for informed choice should be provided to the patient and her family when selecting a delivery mode in pregnant patients with large presacral TCs. The future accumulation of evidence will contribute to establishing improved management strategies for TCs in pregnant women.
AUTHOR CONTRIBUTION
AK collected the data and drafted the manuscript. HM, MS, TK, MY, MM, YO, and YO managed the patient. MS performed the diagnostic imaging and advice. TY supervised manuscript preparation.
CONFLICTS OF INTEREST
None declared.
ETHICAL APPROVAL
An ethical review is not required for this type of article. Written informed consent for the publication of this article was obtained from the patient.
CONSENT
Published with written consent from the patient.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Kanagawa A, Matsumiya H, Sasaki M, et al. Large presacral Tarlov cysts in pregnancy. Clin Case Rep. 2022;10:e05837. doi: 10.1002/ccr3.5837
Funding information
None declared
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are included in the article.
REFERENCES
- 1. Tarlov IM. Perineural cysts of the spinal nerve roots. Arch NeurPsych. 1938;40(6):1067‐1074. doi: 10.1001/archneurpsyc.1938.02270120017001 [DOI] [Google Scholar]
- 2. Marino D, Carluccio MA, Di Donato I, et al. Tarlov cysts: clinical evaluation of an Italian cohort of patients. Neurol Sci. 2013;34(9):1679‐1682. doi: 10.1007/s10072-013-1321-0 [DOI] [PubMed] [Google Scholar]
- 3. Hirst JE, Torode H, Sears W, Cousins MJ. Beware the Tarlov cyst. J Minim Invasive Gynecol. 2009;16(1):78‐80. doi: 10.1016/j.jmig.2008.09.580 [DOI] [PubMed] [Google Scholar]
- 4. Langdown AJ, Grundy JR, Birch NC. The clinical relevance of Tarlov cysts. J Spinal Disord Tech. 2005;18(1):29‐33. doi: 10.1097/01.bsd.0000133495.78245.71 [DOI] [PubMed] [Google Scholar]
- 5. Murphy K, Oaklander AL, Elias G, Kathuria S, Long DM. Treatment of 213 patients with symptomatic Tarlov cysts by CT‐guided percutaneous injection of fibrin sealant. AJNR Am J Neuroradiol. 2016;37(2):373‐379. doi: 10.3174/ajnr.A4517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim VM, Khanna R, Kalinkin O, Castellanos ME, Hibner M. Evaluating the discordant relationship between Tarlov cysts and symptoms of pudendal neuralgia. Am J Obstet Gynecol. 2020;222(1):70.e1‐70.e6. doi: 10.1016/j.ajog.2019.07.021 [DOI] [PubMed] [Google Scholar]
- 7. Kuhn FP, Hammoud S, Lefèvre‐Colau MM, Poiraudeau S, Feydy A. Prevalence of simple and complex sacral perineural Tarlov cysts in a French cohort of adults and children. J Neuroradiol. 2017;44(1):38‐43. doi: 10.1016/j.neurad.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 8. Yang AI, Rinehart CD, McShane BJ, Hitti FL, Welch WC. Growth of lumbosacral perineural (Tarlov) cysts: a natural history analysis. Neurosurgery. 2020;86(1):88‐92. doi: 10.1093/neuros/nyy586 [DOI] [PubMed] [Google Scholar]
- 9. Zhu H, Shen L, Chen Z, Yang M, Zheng X. Giant Tarlov cysts with rare pelvic extension: report of 3 cases and literature review. World Neurosurg. 2020;139:505‐511. doi: 10.1016/j.wneu.2020.04.112 [DOI] [PubMed] [Google Scholar]
- 10. Klepinowski T, Orbik W, Sagan L. Global incidence of spinal perineural Tarlov’s cysts and their morphological characteristics: a meta‐analysis of 13,266 subjects. Surg Radiol Anat. 2021;43(6):855‐863. doi: 10.1007/s00276-020-02644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duja CM, Berna C, Kremer S, Géronimus C, Kopferschmitt J, Bilbault P. Confusion after spine injury: cerebral fat embolism after traumatic rupture of a Tarlov cyst: case report. BMC Emerg Med. 2010;15(10):18. doi: 10.1186/1471-227X-10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sivakumar W, Ravindra VM, Cutler A, Couldwell WT. Intracranial hypotension in the setting of concurrent perineural cyst rupture and subarachnoid hemorrhage. J Clin Neurosci. 2014;21(6):1063‐1065. doi: 10.1016/j.jocn.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 13. Qasem F, McCallum C, Armstrong P. Epidural blood patch treatment for headache caused by a ruptured Tarlov cyst. Can J Anaesth. 2017;64(9):983‐984. doi: 10.1007/s12630-017-0916-y [DOI] [PubMed] [Google Scholar]
- 14. H'ng MWC, Wanigasiri UIDK, Ong CL. Perineural (Tarlov) cysts mimicking adnexal masses: a report of three cases. Ultrasound Obstet Gynecol. 2009;34(2):230‐233. doi: 10.1002/uog.6448 [DOI] [PubMed] [Google Scholar]
- 15. Pfund N, Oh A, Cyna A. Successful spinal anaesthesia in a patient with a Tarlov cyst. Int J Obstet Anesth. 2018;34:96‐98. doi: 10.1016/j.ijoa.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 16. Paulsen RD, Call GA, Murtagh FR. Prevalence and percutaneous drainage of cysts of the sacral nerve root sheath (Tarlov cysts). AJNR Am J Neuroradiol. 1994;15(2):293‐297; discussion 298‐299. [PMC free article] [PubMed] [Google Scholar]
- 17. Akahori S, Nishimura Y, Eguchi K, et al. Spontaneous rupture of a huge presacral Tarlov cyst leading to dramatic neurologic recovery. World Neurosurg. 2021;145:306‐310. doi: 10.1016/j.wneu.2020.09.098 [DOI] [PubMed] [Google Scholar]
- 18. Wang B, Pu F, Wu Q, Zhang Z, Shao Z. Presacral Tarlov cyst as an unusual cause of abdominal pain: new case and literature review. World Neurosurg. 2018;110:79‐84. doi: 10.1016/j.wneu.2017.10.135 [DOI] [PubMed] [Google Scholar]
- 19. Paterakis K, Brotis A, Rountas BM, et al. A giant Tarlov cyst presenting with hydronephrosis in a patient with Marfan syndrome: a case report and review of the literature. World Neurosurg. 2019;126:581‐587. doi: 10.1016/j.wneu.2019.02.222 [DOI] [PubMed] [Google Scholar]
- 20. McClure MJ, Atri M, Haider MA, Murphy J. Perineural cysts presenting as complex adnexal cystic masses on transvaginal sonography. AJR Am J Roentgenol. 2001;177(6):1313‐1318. doi: 10.2214/ajr.177.6.1771313 [DOI] [PubMed] [Google Scholar]
- 21. Saboo SS, Di Salvo D. Perineural cysts resembling complex cystic adnexal masses on transvaginal sonography. J Clin Ultrasound. 2013;41(1):55‐58. doi: 10.1002/jcu.20899 [DOI] [PubMed] [Google Scholar]
- 22. Ahmadi F, Akhbari F. Adnexal masses or perineural (tarlov) cysts? Differentiation by imaging techniques: a case report. Int J Reprod Biomed. 2017;15(9):589‐592. doi: 10.29252/ijrm.15.9.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boukobza M, Roussel A, Fernandez‐Rodriguez P, Laissy JP. Giant multiple and bilateral presacral Tarlov cysts mimicking adnexal mass—imaging features. Int Med Case Rep J. 2018;22(11):181‐184. doi: 10.2147/IMCRJ.S147791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim S, Lee HJ, Park JH, Kim T, Nam K. Tarlov cysts misdiagnosed as adnexal masses in pelvic sonography: a literature review. Front Med (Lausanne). 2020;30(7):577301. doi: 10.3389/fmed.2020.577301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mummaneni PV, Pitts LH, McCormack BM, Corroo JM, Weinstein PR. Microsurgical treatment of symptomatic sacral Tarlov cysts. Neurosurgery. 2000;47(1):74‐79; discussion 78‐79. doi: 10.1097/00006123-200007000-00016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included in the article.


