Abstract
Purpose:
Chronic lymphocytic leukemia (CLL) is an incurable illness. For some, however, CLL is indolent, requiring no treatment until disease progression. Physical symptom burden has been associated with depression, anxiety, and stress in cancer patients. Additionally, certain patient factors, i.e. individual differences, have been associated with worse psychological outcomes. There are few psychological studies of CLL, with no examination of individual differences related to physical symptom burden and psychological responses.
Patients and Methods:
A cross-sectional design was used to study the covariation of physical symptom burden with depressive and anxiety symptoms and cancer-specific stress, and then test psychosocial variables as individual difference moderators. CLL patients (N=112) receiving surveillance participated. They were Caucasian (100%), and mostly male (55%), with a mean age of 61; the majority (62.5%) had stage 0 disease. A composite measure of physical symptom burden (CLL symptoms, fatigue, pain, impaired functional status) was tested as a predictor of psychological responses (depression, anxiety, cancer-specific stress). Psychiatric history and social support were tested as moderators.
Results:
Using multiple linear regression, greater physical symptom burden covaried with greater severity on all measures of psychological response (ps<.05). Positive psychiatric history, low perceived social support, and low relationship satisfaction with one’s partner were moderators of the covariation between greater symptom burden and greater severity of psychological response (ps<.05).
Conclusion:
Findings suggest that CLL patients receiving surveillance with a psychiatric history and/or low social support are at risk for greater distress when coping with high physical symptom burden. These new data clarify the experience of CLL surveillance and identify descriptive characteristics that may aid in identifying those at heighten risk for symptom burden, stress, and anxiety or depressive symptoms.
Introduction
Chronic lymphocytic leukemia (CLL) is incurable. Most often diagnosed in the early stages, symptoms may be vague or non-specific. The disease course is variable, depending on the presence of clinical, biological, and molecular risk factors.1,2 Currently, treatment is not offered for early-stage CLL as it provides no survival advantage, making active surveillance the best option until evidence of progressive disease emerges.3
In contrast to research on psychological variables and quality of life (QoL) for solid tumor cancer patients, the literature for CLL is limited and mainly comes from clinical trials rather than studies with a QoL focus. There is little data from patients continuing in surveillance; most is from patients about to enter a treatment trial. In this literature, findings have not detected significant QoL differences between surveillance and previously treated patients.1 However, Levin et al.4 found that 25% of surveillance patients in contrast to 15% of in-treatment patients reported the use of psychotropic medications for anxiety or depression.
Despite having indolent disease, many surveillance patients remain symptomatic. In studies of solid tumor cancer patients, physical symptom burden is associated with greater severity of depressive and anxiety symptoms5–7 and cancer-specific stress.8–12 In CLL, physical symptom burden for patients undergoing treatment is linked with reduced QoL,1,13 though its relationship to stress and psychological symptoms is unknown. Common cancer-related symptoms, such as fatigue7,14–16 and pain,7,14,17 as well as poor functional status,18 also covary with psychological outcomes. Functional status captures overall physical well-being, including that associated with comorbid illnesses/conditions.
Psychological responses to cancer are not uniform. Studies of solid tumor patients have identified individual differences placing some at greater risk for worse psychological outcomes. A positive psychiatric history, for example, is predictive of later psychological distress.19–22 Inadequate social support is also relevant. Being unpartnered, or the perception that one’s social support is low, are associated with increased distress, reduced mental QoL, and increased risk of cancer mortality.23,24 Examination of individual differences in CLL patients may identify those at greater risk for worse psychological adjustment, particularly in the context of physical symptom burden.
Limited knowledge of biobehavioral responses to CLL supports the need for research, and in particular, an understanding of the CLL surveillance experience. The study had two aims. The first was to determine the association between physical symptom burden and psychological responses (symptoms of depression, generalized anxiety, and cancer-specific stress) in CLL surveillance patients. We anticipated that greater physical symptom burden would covary with greater severity of stress and psychological symptoms. The secondary aim was exploratory, examining individual difference factors (psychiatric history, social support) as moderators of the relationship of psychological responses and symptom burden. Considering the research reviewed above, we anticipated that psychiatric history and low social support would be associated with more negative psychological responses, but particularly so when symptom burden is high.
Methods
Participants
Patients (N=112) had stage 0 disease (0: 62.5%; I: 32.1%, II: 4.5%), with a mean time since diagnosis of 4.6 years (range: 0–16 years). They were non-Hispanic Caucasians (100%) with a mean age of 61 (range: 37–76 years) and most were male (55%). Most (63.1%) participants completed a college degree or above, and were either employed full-time (46.7%) or retired (37.1%). Nearly half (48.5%) reported a household income of ≥ $100K/year. The majority of participants were married/partnered (87.7%). Based on household zip codes, 75% resided within-state of the cancer center.
Procedures
Patients returning for follow-up at a hematologic oncology clinic at a National Cancer Institute-designated comprehensive cancer center were approached for study participation. Inclusion criteria were: CLL diagnosis (0-II), no history of cancer treatment, time since diagnosis greater than 3 months, between the ages of 20 and 80 (inclusive), and ability/ willingness to provide written consent.
One hundred-thirty-nine (139) patients were eligible; 126 consented (91% accrual). Medical chart review obtained disease-specific information (date of diagnosis, stage of disease, treatment history). Three individuals were removed due to ineligibility (1: stage IV disease; 2: history of radiation treatment); 11 did not complete the assessment due to loss of interest. Remaining participants (N=112) completed a 45-minute telephone assessment of interviewer-rated and self-report measures for sociodemographic information, physical symptoms, and psychological responses.
Measures
Physical Symptom Burden.
Symptom burden was operationalized using a composite of physical and functional variables, as has been done previously.25,26 This methodology mapped on to our model of symptom burden and offered statistical simplicity.27 Four measures contributed to the composite. (1) Frequency and severity of CLL symptoms were assessed using a symptom list generated by the physician investigators, consistent with CLL symptom descriptions from national sources.28 Nine symptoms/signs were assessed: fatigue, enlarged lymph nodes, infection, night sweats, excessive bleeding/bruising, weakness, shortness of breath, unintentional weight loss, and fever. The frequency of each symptom/sign was rated from 0 (none in the past month) to 3 (more than three times a week); total scores range from 0 to 27. (2) Pain is not a typical symptom/sign of CLL, but is a common cancer-related symptom, and was therefore examined. Pain interference with daily life was assessed using the Brief Pain Inventory (BPI).29 Seven items were rated on a scale ranging from 0 (none) to 10 (completely); total scores ranged from 0 to 70. (3) Fatigue interference with daily life was assessed using the Fatigue Symptom Inventory (FSI).30,31 The 7 items of the interference scale were scored from 0 (none) to 10 (extremely); total score ranges from 0 to 70. (4) Functional status was measured using the single-item interviewer-rated Karnofsky Performance Status Scale (KPS)32 with 10-point intervals ranging from 0 (Dead) to 100 (Normal).
Pearson intercorrelations between CLL symptoms/signs and the remaining three measures supported the calculation of a physical symptom burden composite. (To eliminate redundancy, the fatigue interference item was included in the physical symptom composite and the fatigue item on the CLL symptom scale was omitted.) All correlations among the four physical symptom measures were significant (rs=.30 to .86, ps<.01), excepting pain with CLL symptoms (r=.24, p>.05). Scores for functional status were reversed for ease of interpretation. Standardized z-scores for the four measures were calculated and summed. Significant correlations (rs=.94 to .99, ps<.01) were revealed between the composite score and four modified versions of the composite that systematically omitted one individual physical symptom measure (e.g. first score omitted CLL symptoms only; second score omitted fatigue only, etc.), providing support for its use.
Psychological responses.
Depressive symptoms.
The Center for Epidemiological Studies Depression Scale (CES-D)33 is a 20-item measure of depressive symptoms. Item scores range from 0 (rarely/none of the time) to 3 (most/all of the time). Total scores range from 0 to 60. A clinical cut-off score of 16 is indicative of clinically relevant symptomatology. Cronbach’s alpha was 0.90.
Anxiety symptoms.
The Generalized Anxiety Disorder Questionnaire-IV (GAD-Q-IV)34 consists of 9 items to assess symptoms of generalized anxiety disorder. Eight of the 9 items are quantitative, scored either dichotomously (0 for absence; 1 for presence) or on a 9-point scale (0, none, to 8, very severe). The one qualitative item was not used. Total scores range from 0 to 27. A clinical cut-off of 5.7 is indicative of clinically relevant symptomatology.34,35 Cronbach’s alpha was 0.78.
Stress.
The Impact of Events Scale-Revised (IES-R)36 is a 22-item measure that assesses intrusive thoughts, physiological arousal, and hypervigilance. Items were worded with regard to having CLL. Item scores range from 0 (not at all) to 4 (extremely). Total scores range from 0 to 88. A cut-off score of 37 is used to identify symptomatology in the clinical range.37 Cronbach’s alpha was 0.91.
Individual differences.
Psychiatric History was assessed with three items: psychiatric diagnosis in one’s lifetime (diagnostic history); medication use to manage mood, anxiety, or sleep (pharmacotherapy history); and therapy/counseling for emotional or psychological problems (psychotherapy history). Scores for each were 0 for absence, 1 for presence. Items were summed with a total score ranging from 0 to 3.
Social support was assessed with measures of perceived support and additionally, relationship satisfaction for partnered individuals. The Interpersonal Support Evaluations List (ISEL)38 is comprised of 40 statements assessing perceived availability of social support. Items were scored as 1 (probably false) or 2 (probably true), with total scores ranging from 40 to 80. Cronbach’s alpha was .86. For partnered participants, the satisfaction item of the Dyadic Adjustment Scale (DAS),39 a common approach for assessing relationship satisfaction,40 was used and is rated on an 8-point scale ranging from 0 (extremely unhappy) to 7 (perfect).
Analytic strategy
Descriptive statistics, Pearson and Spearman correlations, and multiple linear regression (SPSS version 21.0)41 were conducted. Demographic (age, gender, education, employment, household income) and disease relevant variables (stage of disease, time since diagnosis) were considered as controls.
Multiple linear regression first tested the covariation between physical symptom burden and each psychological response variable in independent models. Second, individual difference factors were tested as moderators between physical symptom burden and psychological response. Dummy coding transformed psychiatric history to a dichotomous variable (0=absence vs. 1=presence). Perceived social support and relationship satisfaction were standardized by calculating z-scores. The regression model was as follows: (a) control variable(s); (b) physical symptom burden; (c) moderator; and, (d) interaction term (predictor X moderator).
Results
Descriptive
Mean scores for physical symptoms and psychological response variables are reported in Table 1. Eighty percent (80.4%) of the patients reported CLL symptoms. Of the CLL symptoms, fatigue was most commonly endorsed (57.9%); however patients’ reports of fatigue interference with daily activities was relatively low (M=11.4). Nearly one third of the sample (32.7%) endorsed pain unrelated to CLL.
Table 1.
Mean scores and prevalence rates for physical symptom and psychological response variables (N=112).
| Variable | Mean (Std. Dev)/Prevalence (%)1 |
|---|---|
| CLL symptoms (total scale) | 5.6 (4.8) |
| None | 19.6% |
| Fatigue | 57.9% |
| Enlarged lymph nodes | 38.3% |
| Infections | 37.4% |
| Night sweats | 27.4% |
| Excessive bleeding/bruising | 21.5% |
| Weakness | 15.9% |
| Shortness of breath | 15.9% |
| Fever | 3.7% |
| Significant weight loss | 3.7% |
| Fatigue (FSI) | 11.4 (13.1) |
| Pain (BPI) | 13.7 (13.1) |
| Functional status (KPS) | 88.69 (10.74) |
| Depressive symptoms (CES-D) | 10.2 (8.1) |
| Generalized anxiety symptoms (GAD-Q-IV) | 3.8 (6.5) |
| Cancer-specific stress (IES-R) | 13.7 (12.4) |
Percent endorsing the presence of the symptom in the last month; Coded 0=none, 1=some.
The data provided important clinical detail. Using published cutoffs for “clinically significant symptoms” on self-report measures, 6.3% reported significant stress (> 37) specific to CLL (IES-R). Twenty percent (20.5%) met the CES-D criterion (> 16) for significant depressive symptoms, 20.5% for generalized anxiety symptoms (> 5.7) on the GAD-Q-IV; 11.6% had depressive and anxiety symptom comorbidity. Two-thirds (66%) reported a positive psychiatric history in some form. Thirty-two percent (32.1%) reported “yes” to having received a psychiatric diagnosis in their lifetime. Of them, the most prevalent self-reported diagnoses were mood [27.2%, Major Depressive Disorder (MDD); 3.2%, Bipolar Disorder] and anxiety disorders [8.7%, Generalized Anxiety Disorder (GAD); 2.9%, Post Traumatic Stress Disorder]; 5.4% reported MDD/GAD comorbidity. Fifty-five percent (55.7%) responded “yes” to current or past pharmacotherapy, with commonly reported medications being antidepressants (29.5%), anxiolytics (19.6 %), and over-the-counter or prescribed sleep aids (15.7%). Forty six percent (45.6%) reported “yes” to a history of psychotherapy for emotional or psychological problems. Treatment was sought primarily with psychologists (68.8%), psychiatrists (29.5%), and family counselors (23.8%).
Only age and stage of disease were identified as controls. Significant associations were revealed for age with depressive symptoms (r= −.20, p<.05) and CLL-specific stress (r= −.26, p<.01), and stage of disease with generalized anxiety (r= −.20, p<.05).
Analyses
As predicted, multiple linear regressions revealed that greater physical symptom burden covaried with greater depressive symptoms [β=.44, 95% Confidence Interval (C.I.) =.84, 1.96], generalized anxiety symptoms [β=.37, 95% C.I. =.52, 1.46], and cancer-specific stress [β=.20, 95% C.I. =.047, 1.85] (all ps<.05).
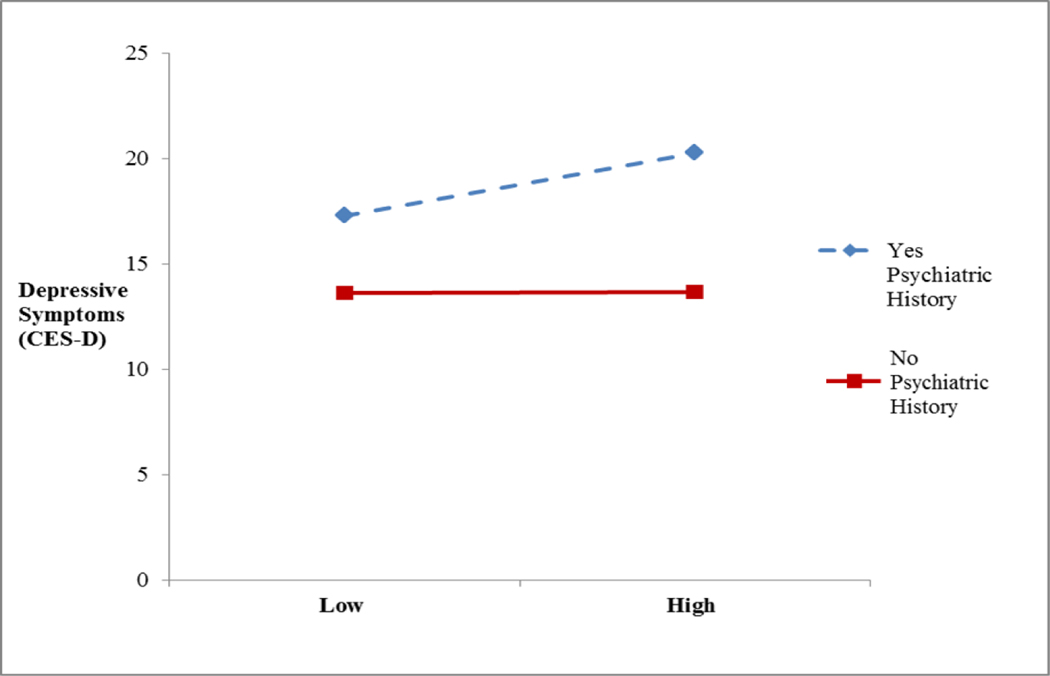
Psychiatric history, perceived social support, and relationship satisfaction were tested as moderators of the covariation between physical symptom burden and psychological responses. Psychiatric history was a significant moderator [β=.41, 95% C.I. =.10, 2.87, p<.05]. For ease of interpretation, Figure 1 displays the relationship between depressive symptoms (CES-D) and symptom burden in the context of the absence or presence of a psychiatric history. Overall, those with a psychiatric history report a significantly higher current level of depressive symptoms [β=.31, 95% C.I. =2.19, 8.10, p<.01]; however the co-occurrence of greater physical symptom burden is associated with even higher levels of depressive symptoms. In contrast, psychiatric history was not a significant moderator for physical symptom burden and generalized anxiety symptoms (GAD-Q-IV) or cancer-specific stress (IES-R).
Figure 1.
Moderation effect of psychiatric history associated with greater depressive symptoms when physical symptom burden is high. No significant main effect for physical symptom burden. Psychiatric history was a significant main effect (β=.31, 95% C.I. =2.19, 8.10, p<.01).
Perceived social support (ISEL) was a significant moderator of the relationships of physical symptom burden with depressive symptoms [β = −.17, 95% C.I. = −1.06, −.07, p<.05] and with cancer-specific stress [β = −.21, 95% C.I. = −1.94, −.10, p<.05]. There was a significant main effect for greater physical symptom burden associated with greater depressive symptomatology [β=.38, 95% C.I. = .72, 1.70, p<.001]. Moderation effects show that when physical symptom burden was high, low perceived social support was related to greater depressive symptoms and higher cancer-specific stress. Social support was not a significant moderator for physical symptom burden and generalized anxiety (p>.05).
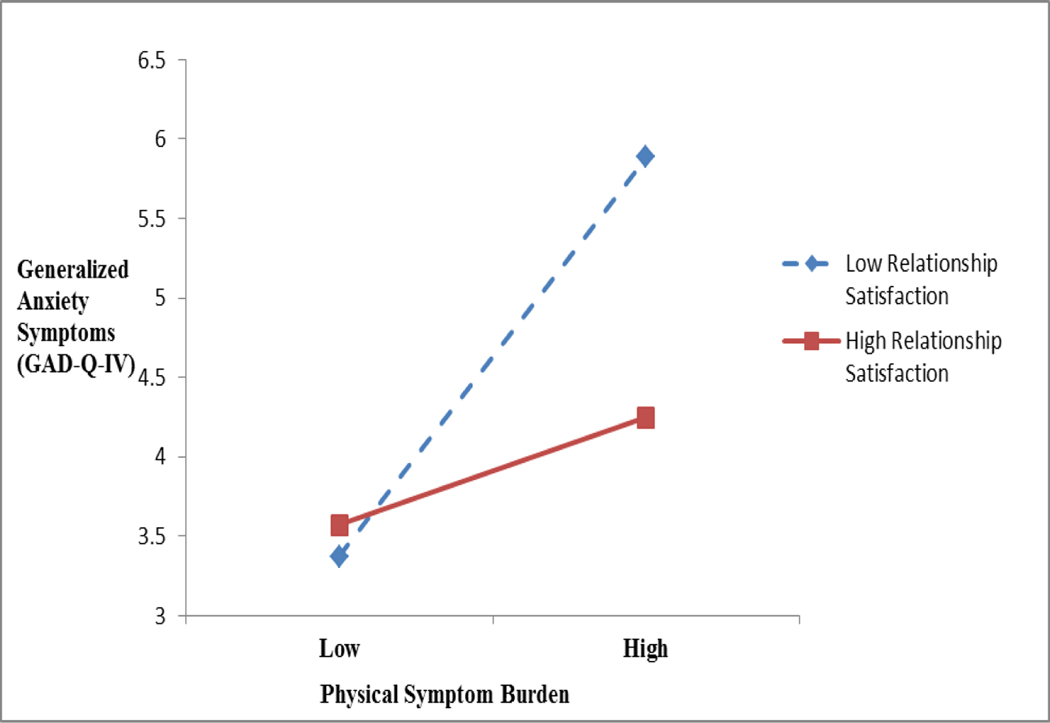
For patients with partners (n=98 of 112), relationship satisfaction (DAS) was a significant moderator of physical symptom burden with generalized anxiety symptoms [β=−.21, 95% C.I. = −.86, −.06, p<.05] and with cancer-specific stress [β=−.24, 95% C.I. = −1.82, −.18, p<.05], although not depressive symptoms (p > .05). There was a significant main effect of greater physical symptom burden associated with greater generalized anxiety [β=.33, 95% C.I. = .35, 1.24, p<.01]. Generalized anxiety was similarly endorsed for both the high and low perceived support groups when symptom burden was low. However, when symptom burden was high, low relationship satisfaction was associated with greater generalized anxiety symptoms (Figure 2).
Figure 2.
Moderation effect of low relationship satisfaction associated with greater generalized anxiety symptoms when physical symptom burden is high. Physical symptom burden was a significant main effect (β=.33, 95% C.I. = .35, 1.24, p<.01). Relationship satisfaction was not a significant main effect.
Discussion
Few studies have examined psychological and quality of life variables in CLL. The present research examined physical symptom burden, psychological response, and individual difference variables in CLL patients receiving surveillance, an understudied group. Results show that CLL patients receiving surveillance experience greater physical symptom burden associated with greater severity of depressive, anxiety, and cancer-specific stress. Additionally, this research identifies individual differences in psychiatric history and social support as moderators of this relationship.
The majority (80.4%) of participants reported CLL-related symptoms; the most common of which was fatigue (57.9%). About one-third (32.7%) also reported concurrent pain unrelated to CLL. Our use of a physical symptom burden composite score simplified analyses examining the covariation between physical symptoms and psychological response. Increases in physical symptom burden were consistently associated with increased depression (CES-D) and anxiety (GAD-Q-IV) symptoms, and cancer-specific stress (IES-R). While these cross-sectional results do not elucidate the temporal nature of the relationship, these findings demonstrate the relevance of symptom burden and its covariation with poorer psychological well-being in patients with indolent disease.
Data regarding psychiatric history and symptoms is extensive. Patients had received surveillance for an average of 5 years, although some as long as 16 years. Only six percent reported significant stress related to CLL; however mood symptoms were more common. On self-report measures, 20.5% reported current clinically significant symptom levels of depression or generalized anxiety, whereas an additional 11.6% met the cutoff for both. A population–based cohort study of a mixed sample of 1,300 cancer patients found 26% with depressive symptoms across three assessments in 6 years.42 In the present study, the self-report measure data are consistent with patients’ verbal reports of lifetime diagnoses and treatments received. Most common was Major Depressive Disorder at 27.2%. Generalized Anxiety Disorder also occurred (8.7%) and was often comorbid with MDD (5.4%) as is common among those with43 and without cancer.44 Consistent with these data, patients acknowledged receipt of antidepressants (29.5%) and/or anxiolytics (19.6 %) and/or psychotherapy (45.6%). The only comparable data is that from Levin and colleagues4 finding that 25% of surveillance patients studied (N= 57) reported current pharmacotherapy use. It is unknown whether psychiatric diagnoses or treatments preceded CLL diagnosis, but regardless, the moderating role of psychiatric history suggests that it not only heightens the likelihood of depressive symptoms but does so particularly when there is a high symptom burden. Collectively, these data suggest that psychiatric history warrants assessment in an untreated group of patients who may benefit from ongoing psychiatric treatment following CLL diagnosis.
Social support variables-perceived support and relationship satisfaction- were also significant moderators, though in somewhat different manners. Low perceived support was associated with poor psychological response (depression, stress) in the context of higher symptom burden. For partnered patients, relationship satisfaction demonstrated a buffering effect, whereby a high degree of satisfaction in one’s relationship was protective in the face of high symptom burden. Relationship satisfaction was associated with less generalized anxiety when physical symptom burden was high; however the degree of relationship satisfaction was not impactful when symptom burden was low.
Methodological aspects of the study are noted. The study sample was large and more representative of patients in collaborative trials (who tend to be younger). With CLL being a low incidence disease, patients are more often treated and followed at large centers. Thus, this sample was not a “local” one, with 75% coming from around state and another 25% from other states. Only 12.3% were unpartnered. With a predominantly male sample, higher partnered rates would be expected, however.45 With the majority being partnered, the perceived social support results are probably not generalizable to the unpartnered. Regarding other psychological data, psychiatric history came from patients’ self-reports rather than diagnostic interviews. However, the self-report measures provide some confirming evidence as current depressive and anxiety symptom rates were comparable, and it is also the case that both MDD and GAD are chronic conditions for many. Thus far, this is the only CLL study that has provided both psychiatric history and symptom assessments of anxiety and depression.
Study findings highlight the covariation between physical symptom burden and psychological responses, and identify individual differences factors in early-stage, untreated CLL, an understudied patient group. Like their treated counterparts, patients receiving surveillance may also experience psychological responses related to their physical well-being, and contend with individual difference factors that further impact adjustment. Further examination of group differences and risk factors (e.g. gender, age, or medical comorbidity) would be a clinically informative future direction of research. Greater emphasis should also be placed on the assessment of psychiatric history in CLL, including the use of brief self-report measures that can be administered in a busy clinical setting. Identifying potential risk factors and providing appropriate biopsychosocial intervention can improve the psychosocial adjustment of these patients.
Acknowledgements
This research was supported by the National Cancer Institute (CA098133), the Alumni Grants for Graduate Research and Scholarship fund (OSU), and the Critical Difference for Women research grant (OSU). We thank the Division of Hematology clinical staff for their generous assistance. We also thank the professional and research staff of the Stress and Immunity Cancer Projects for their comments on manuscript drafts.
This work is original research; all authors have contributed and agree to submission to JCO and have no conflicts of interest. This research was supported by the National Cancer Institute (CA098133), the Alumni Grants for Graduate Research and Scholarship fund (OSU), and the Critical Difference for Women research grant (OSU).
Contributor Information
Eleshia Morrison, Department of Psychiatry and Psychology Mayo Clinic 200 First Street SW Rochester, MN 55905
Joseph M. Flynn, Department of Internal Medicine Division of Hematology The Ohio State University 410 West 12th Ave. Columbus, OH 43210.
Jeffrey Jones, Department of Internal Medicine Division of Hematology The Ohio State University 410 West 12th Ave. Columbus, OH 43210.
John C. Byrd, Department of Internal Medicine Division of Hematology The Ohio State University 410 West 12th Ave. Columbus, OH 43210
Barbara L. Andersen, Department of Psychology 1835 Neil Avenue The Ohio State University Columbus, OH 43210
References
- 1.Holzner B, Kemmler G, Kopp M, et al. : Quality of life of patients with chronic lymphocytic leukemia: results of a longitudinal investigation over 1 yr. Eur J Haematol 72: 381–389, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Singer C, Goldstone A: Chronic lymphocytic leukaemia and other chronic lymphoid leukaemias, in Souhami R, Tannock L, Hohenberger P, et al. (eds): Oxford Textbook of Oncology. New York, NY, Oxford University Press Inc, 2002, pp 2254–2282 [Google Scholar]
- 3.Hallek M, Cheson BD, Catovsky D, et al. : Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute–Working Group 1996 guidelines. Blood 111: 5446–5456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin TT, Riskind JH, Li Y, et al. : Depression, anxiety and quality of life in a chronic lymphocytic leukemia cohort. Gen Hosp Psychiatry 29: 251–256, 2007b [DOI] [PubMed] [Google Scholar]
- 5.Brown LF, Kroenke K: Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics 50:440–447, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrow GR, Roscoe JA, Hickok JT, et al. : Nausea and emesis: evidence for a biobehavioral perspective. Support Care Cancer 10: 96–105, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Harrington CB, Hansen JA, Moskowitz M, et al. : It’s not over when it’s over: long-term symptoms in cancer survivors-a systematic review. Int J Psychiatry Med 40:163–181, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Carpenter KM, Fowler JM, Maxwell GL, et al. : Direct and buffering effects of social support among gynecologic cancer survivors. Ann Behav Med 39:79–90, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jim HS, Andrykowski MA, Munster PN, et al. : Physical symptoms/side effects during breast cancer treatment predict posttreatment distress. Ann Behav Med 34: 200–208, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Mykletun A, Dahl AA, Haaland CF, et al. : Side effects and cancer-related stress determine quality of life in long-term survivors of testicular cancer. J Clin Oncol 23: 3061–3068, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Yang H-C, Brothers B, Andersen BL: Stress and quality of life in breast cancer recurrence: moderation or mediation of coping? Ann Behav Med 35, 188–197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wettergren L, Langius A, Bjorkholm M, et al. : Post-traumatic stress symptoms in patients undergoing autologous stem cell transplantation. Acta Oncol 38: 475–480, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Shanafelt TD, Bowen D, Venkat C, et al. : Quality of life in chronic lymphocytic leukemia: an international survey of 1482 patients. Br J Haematol 139: 255–264, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Cheng KKF, Lee DTF: Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol 78:127–137, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Minton O, Stone P: How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Res Treat 112: 5–13, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Mosher CE, Redd WH, Rini CM, et al. : Physical, psychological, and social sequelae following hematopoietic stem cell transplantation: a review of the literature. Psychooncology 18: 113–127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen L, Koch L, Brenner H, et al. : Quality of life among long-term (>= 5 years) colorectal cancer survivors - Systematic review. Eur J Cancer 46: 2879–2888, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Dodd MJ, Cho MH, Cooper BA, et al. : The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs 14: 101–110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Leeuw JRJ, de Graeff A, Ros WJG, et al. : Prediction of depressive symptomatology after treatment of head and neck cancer: The influence of pre-treatment physical and depressive symptoms, coping, and social support. Head Neck 22: 799–807, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Harrison J, Maguire P: Predictors of psychiatric morbidity in cancer patients. Br J Psychiatry 165: 593–598, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Morasso G, Costantini M, Viterbori P, et al. : Predicting mood disorders in breast cancer patients. Eur J Cancer 37: 216–223, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Prieto JM, Blanch J, Atala J, et al. : Stem cell transplantation: Risk factors for psychiatric morbidity. Eur J Cancer 42: 514–520, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Pinquart M, Duberstein PR: Associations of social networks with cancer mortality: A meta-analysis. Crit Rev Oncol Hematol 75:122–137, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherman KA, Kasparian NA, Mireskandari S: Psychological adjustment among male partners in response to women’s breast/ovarian cancer risk: a theoretical review of the literature. Psychooncology 19:1–11, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Mendoza TR, Wang XS, Lu C, et al. : Measuring the Symptom Burden of Lung Cancer: The validity and utility of the lung cancer module of the M. D. Anderson Symptom Inventory. Oncologist: 16: 217–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang XS, Williams LA, Eng C, et al. : Validation and application of a module of the M. D. Anderson Symptom Inventory for measuring multiple symptoms in patients with gastrointestinal cancer (the MDASI-GI). Cancer 116: 2053–2063, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Cleeland CS, Sloan JA, Cella D, et al. : Recommendations for including multiple symptoms as endpoints in cancer clinical trials. Cancer 119: 411–420, 2013 [DOI] [PubMed] [Google Scholar]
- 28.American Cancer Society (ed): Leukemia-Chronic Lymphocytic. Atlanta, GA, American Cancer Society, 2015 [Google Scholar]
- 29.Cleeland C, Ryan K: Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 23: 129–138, 1994 [PubMed] [Google Scholar]
- 30.Hann DM, Jacobsen PB, Azzarello LM, et al. : Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res 7: 301–310, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Hann DM, Denniston MM, Baker F: Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Qual Life Res 9: 847–854, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Karnofsky DA, Burchenal JH: The clinical evaluation of chemotherapeutic agents in cancer, in MacLeod CM (ed): Evaluation of chemotherapeutic agents. New York, NY, Columbia University Press, 1949, pp.199–205 [Google Scholar]
- 33.Radloff L: The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1: 385–401, 1977 [Google Scholar]
- 34.Newman MG, Zuellig AR, Kachin KE, et al. : Preliminary reliability and validity of the generalized anxiety disorder questionnaire-IV: A revised self-report diagnostic measure of generalized anxiety disorder. Behav Ther 33: 215–233, 2002 [Google Scholar]
- 35.Luterek J, Turk C, Heimburg R, et al. : Psychometric properties of the GAD-Q-IV among individuals with clinician-assessed generalized anxiety disorder: an update. Presented at the Association for Advancement of Behavior Therapy, 2002 [Google Scholar]
- 36.Horowitz MJ, Wilner N, Alvarez W : Impact of Event Scale: a measure of subjective stress. Psychosom Med 41: 209–218, 1979 [DOI] [PubMed] [Google Scholar]
- 37.Shapinsky AC, Rapport LJ, Henderson MJ, et al. : Civilian PTSD scales: relationships with trait characteristics and everyday distress. Assessment 12: 220–230, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Cohen S, Mermelstein R, Kamarck T, et al. : Measuring the Functional Components of Social Support, in Sarason IG, Sarason BR (eds): Social Support: Theory, Research and Applications. Dordrecht, Martinus Nijhoff Publishers, 1985, pp 73–94 [Google Scholar]
- 39.Spanier G: Measuring dyadic adjustment: new scales assessing the quality of marriage and similar dyads. J Marriage Fam 38: 15–28, 1976 [Google Scholar]
- 40.Goodwin R: Overall, just how happy are you? The magical question 31 of the Spanier Dyadic Adjustment Scale. Fam Ther 19: 273–275, 1992 [Google Scholar]
- 41.Corp IBM. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp. [Google Scholar]
- 42.Burton CL, Galatzer-Levy IR, Bonanno GA: Treatment type and demographic characteristics as predictors for cancer adjustment: prospective trajectories of depressive symptoms in a population sample. Health Psychol 34: 602–609, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Brintzenhofe-Szoc KM, Levin TT, Li Y, et al. : Mixed Anxiety/Depression Symptoms in a Large Cancer Cohort: Prevalence by Cancer Type. Psychosomatics 50: 383–391, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Kessler RC, DuPont RL, Berglund P, et al. : Impairment in pure and comorbid generalized anxiety disorder and major depression at 12 months in two national surveys. Am J Psychiatry 156:1915–1923, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Aizer AA, Chen M-H, McCarthy EP, et al. : Marital status and survival in patients with cancer. J Clin Oncol 31: 3869–3876, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]