Abstract
BACKGROUND:
Nonsteroidal antiinflammatory drug use has been shown to increase blood pressure in nonpregnant adults. Because of this, the American College of Obstetricians and Gynecologists suggests avoiding their use in women with postpartum hypertension; however, evidence to support this recommendation is lacking.
OBJECTIVE:
Our goal was to test the hypothesis that nonsteroidal antiinflammatory drugs, such as ibuprofen, adversely affect postpartum blood pressure control in women with preeclampsia with severe features.
STUDY DESIGN:
At delivery, we randomized women with preeclampsia with severe features to receive around-the-clock oral dosing with either 600 mg of ibuprofen or 650 mg of acetaminophen every 6 hours. Dosing began within 6 hours after delivery and continued until discharge, with opioid analgesics available as needed for breakthrough pain. Study drugs were encapsulated in identical capsules such that patients, nurses, and physicians were masked to study allocation. Exclusion criteria were serum aspartate aminotransferase or alanine aminotransferase >200 mg/dL, serum creatinine >1.0 mg/dL, infectious hepatitis, gastroesophageal reflux disease, age <18 years, or current incarceration. Our primary outcome was the duration of severe-range hypertension, defined as the time (in hours) from delivery to the last blood pressure ≥ 160/110 mm Hg. Secondary outcomes were time from delivery to last blood pressure ≥150/100 mm Hg, mean arterial pressure, need for antihypertensive medication at discharge, prolongation of hospital stay for blood pressure control, postpartum use of short-acting antihypertensives for acute blood pressure control, and opioid use for breakthrough pain. We analyzed all outcome data according to intention-to-treat principles.
RESULTS:
We assessed 154 women for eligibility, of whom 100 met entry criteria, agreed to participate, and were randomized to receive postpartum ibuprofen or acetaminophen for first-line pain control. Seven patients crossed over or did not receive their allocated study drug, and 93 completed the study protocol in their assigned groups. We found no differences in baseline characteristics between groups, including mode of delivery, body mass index, parity, race, chronic hypertension, and maximum blood pressure prior to delivery. We did not find a difference in the duration of severe-range hypertension in the ibuprofen vs acetaminophen groups (35.3 vs 38.0 hours, P = .30). There were no differences between groups in the secondary outcome measures of time from delivery to last blood pressure ≥150/100 mm Hg, postpartum mean arterial pressure, maximum postpartum systolic or diastolic blood pressures, any postpartum blood pressure ≥160/110 mm Hg, short-acting antihypertensive use for acute blood pressure control, length of postpartum stay, need to extend postpartum stay for blood pressure control, antihypertensive use at discharge, or opioid use for inadequate pain control. In a subgroup analysis of patients who experienced severe-range hypertension, the mean time to blood pressure control in the acetaminophen group was 68.4 hours and ibuprofen group was 56.7 hours (P = .26). At 6 weeks postpartum, there were no differences between groups in the rates of obstetric triage visits, hospital readmissions, continued opioid use, or continued antihypertensive use.
CONCLUSION:
The first-line use of ibuprofen rather than acetaminophen for postpartum pain did not lengthen the duration of severe-range hypertension in women with preeclampsia with severe features.
Keywords: blood pressure control, ibuprofen, nonsteroidal antiinflammatory drugs, postpartum pain control, preeclampsia
Introduction
Hypertensive disorders are important contributors to maternal morbidity and mortality, and women diagnosed with a hypertensive disorder of pregnancy are likely to meet criteria for postpartum antihypertensive therapy as well as require several days of postpartum observation.1–3 In addition, women with hypertensive disorders of pregnancy are at increased risk for cesarean delivery, with its attendant need for postoperative pain relief.4 Chronic use of nonsteroidal antiinflammatory drugs (NSAIDs), particularly cyclooxygenase inhibitors, is known to increase the risk of development of hypertension (HTN) in healthy, nonpregnant women as well as to antagonize the effects of some antihypertensive drugs in hypertensive patients receiving treatment after just a few days of NSAID use.5–13 The hypothesized mechanisms for this effect include the NSAID-mediated alteration of aldosterone metabolism,14 sodium retention,15 inhibition of prostaglandin-mediated vasodilation,16 and production of vaso-active metabolites of arachidonic acid via cytrochrome-P450 induction.17
Because of this concern, the American College of Obstetricians and Gynecologists (ACOG) suggests avoiding the postpartum use of NSAIDs in women diagnosed with preeclampsia who have postpartum HTN, though evidence in support of this recommendation is limited.18 Animal studies are limited to a single investigation in a rat model of preeclampsia, which showed that indomethacin had no effect on blood pressure (BP) while rats were still pregnant.19 In human beings, the limited data on the influence of NSAIDs on postpartum BP among women with preeclampsia are conflicting.3,20–23
NSAIDs are ideally suited for post-partum pain control and are still widely used for postpartum and postcesarean delivery pain management in non-hypertensive women because of their effectiveness.24–26 They are more effective than acetaminophen to alleviate pain from obstetric perineal injury, and have also been shown to decrease opioid use after cesarean delivery.21,24,25,27–29 Additionally, the analgesic alternatives to cyclooxygenase inhibitors have significant risks. The use of postpartum opioids may predispose to ongoing opioid dependence and is associated with neonatal central nervous system depression during breast-feeding. Although acetaminophen is an additional nonopioid alternative that may decrease the use of postcesarean opioids its use is contraindicated in the setting of severe, acute elevation of liver enzymes, a common occurrence among patients with severe variants of preeclampsia.30–32
It remains unclear whether the theoretical adverse effect of short-term NSAID use on postpartum BP is clinically significant and justifies forgoing their analgesic properties. We conducted this study to evaluate the effect of ibuprofen vs acetaminophen on post-partum BP control among women with preeclampsia with severe features. We hypothesized that ibuprofen would increase the duration of severe-range HTN in women with preeclampsia with severe features.
Materials and Methods
Details of ethics approval
This study was approved by Human Research Protections Office of the University of New Mexico (Albuquerque, NM) and was registered at clinicaltrials.gov prior to study onset (study identifier: NCT02911701). All enrolled participants provided written informed consent.
Eligibility
We performed a double-masked, randomized controlled trial of ibuprofen vs acetaminophen for postpartum pain control. We assessed for eligibility all women aged at least 18 years who were admitted to the University of New Mexico Hospital from Oct. 29, 2016, through Nov. 1, 2017, with any of the following diagnoses: preeclampsia with severe features, chronic HTN with superimposed preeclampsia with severe features, or HELLP syndrome (hemolysis, elevated liver function tests, and low platelets), as defined by ACOG.18 We included women with the above diagnoses even if their presentation did not include severe-range HTN as these women are still at risk of significant postpartum HTN, and make our results more generalizable. Women had to be able to give informed consent in either English of Spanish. Exclusion criteria were alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >200 mg/dL, serum creatinine >1.0 mg/dL, chronic kidney disease, chronic liver disease, infectious hepatitis, gastroesophageal reflux disease, peptic ulcer disease, bleeding disorder, known sensitivity or allergy to NSAIDs or acetaminophen, or current incarceration. Women were approached prior to delivery or within 6 hours after delivery.
Randomization and follow-up
Within 6 hours after delivery, we randomized study participants to receive scheduled, around-the-clock dosing with either 600 mg of ibuprofen or 650 mg of acetaminophen every 6 hours, starting immediately after delivery and continuing for the duration of their postpartum hospitalization. Randomization was performed by our investigational pharmacy using a block randomization scheme with block sizes of 10. Patients, nurses, and physicians were masked to study allocation. The investigational pharmacy executed study drug masking by either encapsulating the tablet form of the study drug or by measuring and encapsulating the appropriate quantity of ibuprofen or acetaminophen bulk powder and microcrystalline cellulose in unmarked, identical capsules. Ibuprofen tablets were manufactured by Ascend Laboratories LLC (Parsippany, NJ) and purchased from Cardinal Health Inc (Dublin, OH), and acetaminophen tablets were manufactured by Major Pharmaceuticals (Livonia, MI) and purchased from Cardinal Health Inc. Powdered forms of both study drugs along with the capsule shells and microcrystalline cellulose were purchased from Fagron Inc (St Paul, MN). Women undergoing cesarean delivery in both study arms did not receive ketorolac but were given 1000 mg of intravenous acetaminophen in the recovery room, and their study drug was initiated 6 hours after delivery. All study participants were given opioid analgesics upon request for breakthrough pain.
As per our institutional protocol and the ACOG guidelines, women diagnosed with preeclampsia with severe features received intravenous magnesium sulfate for 24 hours after delivery, were observed in-hospital for a minimum of 72 hours postpartum, and were not discharged until at least 24 hours after having either a systolic BP ≥160 or a diastolic BP ≥110 mm Hg. BP was measured hourly for the first 24 hours postpartum and every 4 hours thereafter. We initiated scheduled oral antihypertensive therapy with either labetalol or extended-release nifedipine for >1 BP ≥150/100 mm Hg in a 24-hour period, and acutely treated any BP ≥160/110 mm Hg with either oral labetalol or oral short-acting nifedipine, at the discretion of the care provider. Daily laboratory samples were drawn, and women with new-onset creatinine >1.1 mg/dL, AST/ALT >250 mg/dL, seizure activity, stroke, or focal neurologic findings requiring neuroimaging were automatically withdrawn and their physicians unmasked to their study allocation. A data safety monitoring board was created to convene in the event of any of the following sentinel events: stroke, seizure/eclampsia, posterior reversible encephalopathy syndrome, or death. Participants who received at least 8 doses of the study medication were considered to have completed the study intervention.
Study endpoints and sample size considerations
Our primary outcome was duration of severe-range HTN, defined as the time (in hours) from delivery to the last BP ≥160/110 mm Hg before discharge. For our sample size assumptions, we analyzed a pilot sample from our perinatal database of 31 gravidas meeting the same inclusion criteria as our study population who had both postpartum ibuprofen and acetaminophen made available on an as-needed basis. We found the duration of severe-range HTN was exponentially distributed, with an exponential mean of 35 hours (95% confidence intervals [CI], 25e51 hours). To have 80% power to detect the clinically significant difference of 24 hours between study arms with a type I error rate of 5%, 46 women in each group were required for our study. In addition to its previous use in comparing time-driven postpartum outcomes, we chose a difference of 24 hours between groups because this represents 1 additional day of hospitalization, which we considered to be generalizable in its clinical significance.2 To account for study group contamination, we obtained approval to recruit 100 women.
Secondary outcomes included time from delivery to last BP ≥150/100 mm Hg, postpartum mean arterial pressure (MAP), any BP ≥160/110 mm Hg, need for antihypertensive medication at discharge, prolongation of hospital stay for BP control, length of postpartum hospital stay, postpartum use of short-acting antihypertensives for acute BP control, need for opioid use stratified by postpartum day, and time to BP control in those who experienced severe-range HTN. At 6 weeks after delivery, participants were contacted and a questionnaire collected to determine the rates of obstetric triage visits, hospital readmissions, opioid medication use, and oral antihypertensive medication use.
Analysis plan
We differentiated between the primary endpoint of “duration of severe-range HTN,” which included all randomized patients (including those who never experienced severe-range HTN), and the secondary endpoint of “time to BP control,” which was only analyzed in the subset of patients who experienced postpartum severe-range HTN. The distribution of the primary endpoint was assessed with the Shapiro-Wilk test for exponentiality. Mean durations of severe-range HTN and their 95% CI from the exponential distribution were calculated for the 2 study arms and compared with a maximum likelihood test. An exponential regression model was fitted to duration of severe-range HTN that included arm, mode of delivery, and an interaction between them. Continuous secondary endpoints were assessed for normality. Means and SD were calculated for continuous data and compared between study arms with t tests with the exception of time variables, for which the mean and CIs for exponentially distributed data were calculated. Time to BP control among the subset with severe-range HTN was assessed by fitting an accelerated failure time model to compare arms, adjusted for mode of delivery and included an interaction between group and mode of delivery. Frequencies and percentages were calculated for categorical data and study arms were compared with χ2 or Fisher exact tests, as appropriate. The opioid use data were skewed, so the log-transformation of opioid use in morphine equivalents was used for analysis. Repeated measures logistic regression was used to compare any opioid use between arms over post-partum days 0–2, and repeated measures linear regression was used to compare mean morphine equivalents between arms over postpartum days 0–2. We analyzed all outcome data according to intention-to-treat principles but the per protocol analysis set was also analyzed to assess for any bias in the primary outcome. All analyses were performed in SAS 9.4 (SAS Institute Inc, Cary, NC) and R 3.4 (R Foundation for Statistical Computing, Vienna, Austria). The study protocol and outcomes were reported according to CONSORT guidelines.
Results
We assessed 154 women for eligibility. Of these, 100 women met all entry criteria and consented to participate. They were randomly assigned to receive ibuprofen or acetaminophen for postpartum pain control. Of the randomized participants, 93 subjects completed the study according to the protocol for their assigned group: 46 in the ibuprofen group and 47 in the acetaminophen arm. Of those who did not complete the protocol or who crossed over, details are as follows: in the ibuprofen arm, 1 woman did not receive any study drug because of inability to swallow capsules, and 3 women withdrew to ensure they were receiving ibuprofen and were also later given acetaminophen. In the acetaminophen arm, 1 woman was automatically withdrawn for postpartum AST and ALT that increased >250 mg/dL, and 2 others withdrew voluntarily to ensure they were being given ibuprofen, later receiving both ibuprofen and acetaminophen. In each group, 1 patient requested discharge on the day before the standard postpartum observation period of 72 hours had elapsed. Both patients had received ≥8 study drug doses and had already achieved adequate BP control, so were considered to have completed the study. Per our intention-to-treat plan, all women who withdrew or crossed over were included in the final analysis. This is summarized in the Figure. None of the sentinel safety events occurred, so the data safety monitoring board did not convene.
FIGURE. Enrollment, randomization, and follow-up.
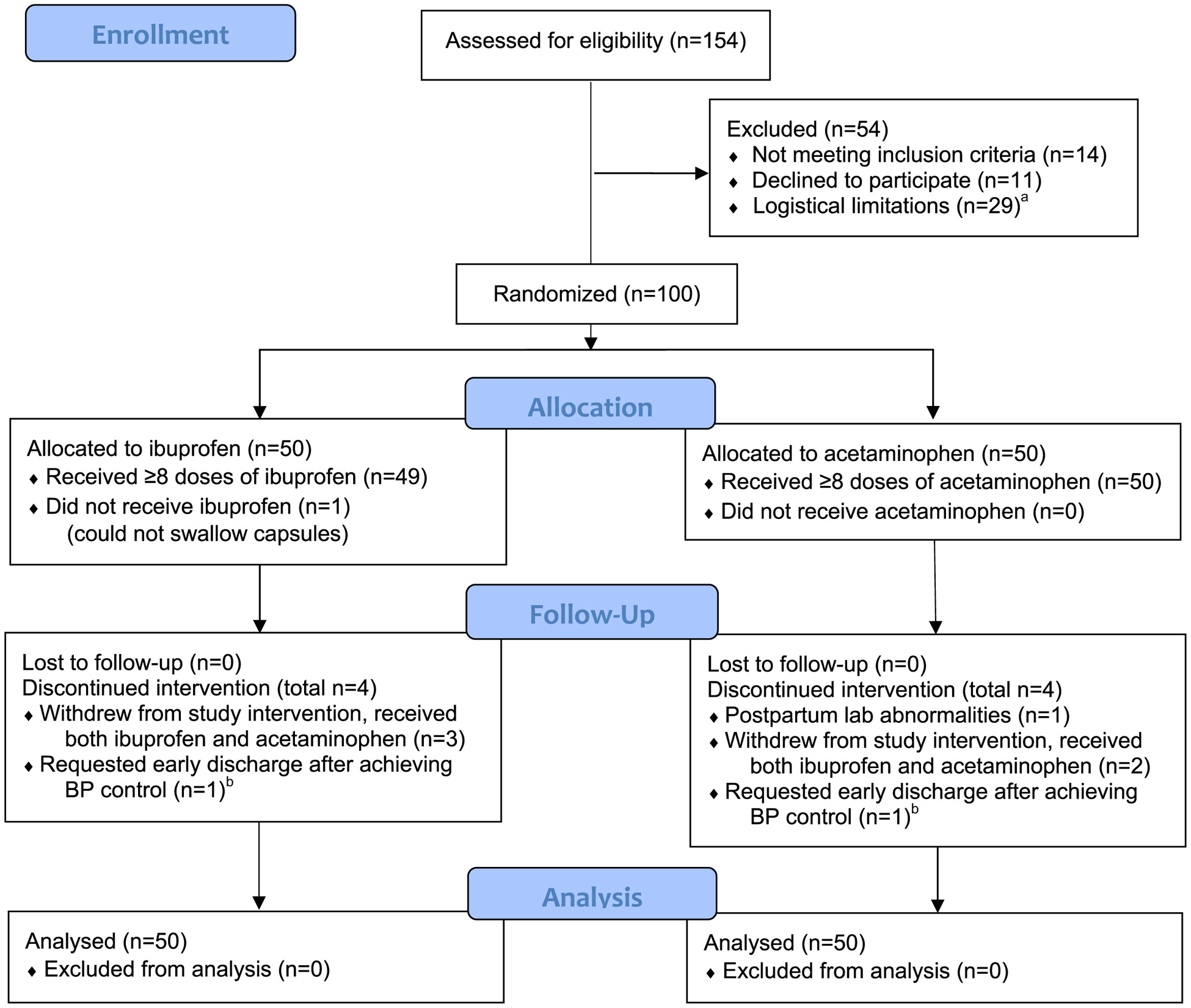
a Women not approached due to speaking language other than English or Spanish, limited availability of study personnel, or lack of prepared study drug. b Both patients requesting early discharge were considered to have completed study as they had both received ≥8 doses of study drug and had already achieved blood pressure (BP) control.
Among study participants, the mean maternal age was 31.2 years (SD 6.1). The ethnic/racial distribution was as follows: Hispanic, 40%; White, 24%; Native American, 20%; Black, 3%; Asian, 2%; other, 11%. In all, 41 were delivered by cesarean, 2 by operative vaginal delivery, and 57 vaginally. Between study arms, there were no differences in baseline demographic or obstetric characteristics (Table 1).
TABLE 1.
Demographic characteristics
| Baseline characteristic | Ibuprofen n = 50 | Acetaminophen n = 50 | P value |
|---|---|---|---|
| Maternal age, y, mean (SD) | 31.9 (5.9) | 30.5 (6.4) | .3a |
| BMI, mean (SD) | 36.5 (8.0) | 36.4 (7.5) | 1.0 |
| Parity, n (%) | |||
| Nulliparous | 18 (36) | 19 (38) | |
| Multiparous | 32 (64) | 31 (62) | |
| Ethnicity and race, n (%) | |||
| Hispanic | 20 (40) | 20 (40) | |
| White | 13 (26) | 11 (22) | |
| Black | 1 (2) | 2 (4) | |
| Asian | 0 (0) | 2 (4) | |
| Native American | 12 (24) | 9 (18) | |
| Otherd | 4 (8) | 6 (12) | |
| Mode of delivery, n (%) | |||
| Vaginal | 25 (50) | 32 (64) | |
| Operative vaginal | 1 (2) | 1 (2) | |
| Cesarean | 24 (48) | 17 (34) | |
| Chronic HTN requiring treatment, n (%) | 8 (16) | 7 (14) | .8a |
| Need for IV antihypertensives before delivery, n (%) | 37 (74) | 42 (84) | .2b |
| Maximum SBP before delivery, mean (SD) | 181 (16) | 183 (14) | .5a |
| Maximum DBP before delivery, mean (SD) | 107 (11) | 106 (11) | .7a |
BMI, body mass index; DBP, diastolic blood pressure; HTN, hypertension; IV, intravenous; SBP, systolic blood pressure.
Independent 2-sample t test;
χ2 Test;
Fisher exact test;
Includes women identifying as biracial, or another race or ethnicity not otherwise accounted for.
For the primary outcome of duration of severe-range HTN, we did not detect any difference between women assigned to receive ibuprofen and those assigned to acetaminophen (35.3 vs 38.0 hours, respectively; P =.30). There were also no differences between groups in secondary outcome measures of BP control: time from delivery to last BP ≥150/100 mm Hg, postpartum MAP, maximum post-partum systolic BP, maximum diastolic BP, any postpartum BP ≥160/110 mm Hg, need for short-acting antihypertensives for acute BP control, length of postpartum stay, need to extend post-partum stay for BP control, or requirement of antihypertensives at discharge (Table 2). To assess the impact of mode of delivery on duration of severe-range HTN, we fitted an exponential regression model that included study arm, mode of delivery, and the interaction between the two, which found no interaction (F = 0.43, P = .51). The primary outcome results were the same in the per protocol analysis, which excluded 7 patients who crossed over or never received study drug (see description of excluded patients in the first paragraph of “Results” section).
TABLE 2.
Postpartum outcomes
| Outcome | Ibuprofen n = 50 | Acetaminophen n = 50 | P value |
|---|---|---|---|
| Duration of severe-range HTN, h, mean (95% CI)a | 35.3 (27.2−47.5) | 38.0 (29.4−51.3) | .3b |
| Time from delivery to last BP ≥150/100 mm Hg, h, mean (95% CI) | 58.3 (48.0−68.6) | 57.1 (45.8−68.5) | .9b |
| Time to BP control, h, mean (95% CI)c | 56.7 (45.1−71.2) | 68.8 (54.0−86.7) | .3d |
| Postpartum MAP, mean (SD) | 97.6 (6.2) | 97.3 (9.1) | .9e |
| Maximum postpartum SBP, mean (SD) | 168 (16) | 165 (15) | .3e |
| Maximum postpartum DBP, mean (SD) | 99 (10) | 96 (10) | .2e |
| Any postpartum BP ≥160/110 mm Hg, n (%) | 34 (68) | 31 (62) | .5f |
| Any postpartum meds for acute BP control, n (%) | 30 (60) | 26 (52) | .4f |
| Postpartum stay, d, mean (SD) | 3.8 (1.4) | 4.0 (1.3) | .5e |
| Postpartum stay extended for BP control, n (%) | 18 (36) | 23 (46) | .3f |
| On antihypertensives at discharge, n (%) | 33 (66) | 31 (62) | .7f |
BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; HTN, hypertension; MAP, mean arterial pressure; SBP, systolic blood pressure.
Time from delivery to last BP ≥160/110 mm Hg;
Maximum likelihood test for exponential means;
Subgroup analysis in only patients who experienced any severe-range HTN, n = 31 in acetaminophen group and n = 34 in ibuprofen group;
Wald χ2 test;
Independent 2-sample t test;
χ2 Test.
For the subgroup analysis of time to BP control, there were 30 (60%) patients in the acetaminophen group who experienced severe-range HTN and 33 (66%) in the ibuprofen arm. We excluded from this analysis the 2 study participants (1 from each group) who were delivered vaginally with forceps or vacuum because their small number would disproportionately influence the test for interaction between mode of delivery and study arm. There were no differential effects in time to BP control between the arms when accounting for mode of delivery (accelerated failure time model: interaction between study group and mode of delivery Wald χ2 0.50, P = .48). The adjusted mean time (95% CI) to BP control in the acetaminophen group was 68.4 hours (54.0e86.7), and 56.7 hours (45.1e71.2) in the ibuprofen arm.
Though it was not a planned outcome measure, we also analyzed the frequency of at least 1 postpartum BP ≥150/100 mm Hg to more directly compare our results with findings published after our trial was initiated.23 We also found no difference between groups for this BP cutoff (ibuprofen 92% vs acetaminophen 84%, P = .22). Comparisons of postpartum opioid use also revealed no difference between groups overall or over time (Table 3). At 6 weeks postpartum, there was no difference in the rates of obstetric triage visits, hospital readmissions, or continued use of opioids or antihypertensive medications (Table 4).
TABLE 3.
Pain control and opioid use
| Any opioid use | Ibuprofen n = 50 | Acetaminophen n = 50 | P value | ||
|---|---|---|---|---|---|
| Postpartum d 0, n (%) | 22 (44) | 20 (40) | |||
| Postpartum d 1, n (%) | 27 (54) | 25 (50) | |||
| Postpartum d 2, n (%) | 26 (52) | 18 (36) | |||
| Opioid requirement (in MEQ), among those requiring opioids | Ibuprofen | Acetaminophen | |||
|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | ||
| Postpartum d 0, mg | 22.1 (25.1) | 15.0 | 22.6 (13.9) | 18.8 | |
| Postpartum d 1, mg | 27.5 (18.7) | 30.0 | 32.3 (17.5) | 30.0 | |
| Postpartum d 2, mg | 28.9 (18.9) | 22.5 | 45.4 (32.3) | 41.3 | |
| Total MEQ dose | 77.4 (64.8) | 60.0 | 88.4 (108.1) | 30.0 | .6c |
MEQ, morphine equivalent.
χ2 Test for interaction effect of group and postpartum day on any opioid use from repeated measures logistic regression;
F test for interaction effect of group and postpartum day on log-transformed opioid MEQs from repeated measures linear regression;
t Test for 2 independent samples on log-transformed total MEQ dose.
TABLE 4.
Outcomes at 6 weeks postpartum
| Outcome, n (%) | Ibuprofen n = 43 | Acetaminophen n = 34 | P value |
|---|---|---|---|
| Continued antihypertensive use | 15 (34.9) | 7 (20.6) | .2a |
| Continued opioid use | 2 (4.7) | 0 (0) | .3b |
| OB triage visits after discharge | 7 (16.3) | 5 (15.7) | .6b |
| Hospital readmission | 3 (7.0) | 0 (0) | .2b |
| Readmission related to preeclampsia | 1 (2.3) | 0 (0) | .6b |
OB, obstetric.
χ2 Test;
Fisher exact test.
Comment
Principal findings
The main finding of our study is that compared to acetaminophen, ibuprofen did not extend the duration of severe-range HTN in women with preeclampsia with severe features. Our findings do not lend support to the recommendation put forth ACOG’s 2013 Hypertension in Pregnancy Task Force monograph to avoid NSAIDS in women with preeclampsia with severe features.18 When comparing women randomized to receive ibuprofen rather than acetaminophen for first-line pain control, we did not identify a difference in any metric of postpartum BP control, including the time to BP control among those who experienced postpartum severe-range HTN. Additionally, we did not find any difference in the need for antihypertensive use either at hospital discharge or at 6 weeks postpartum.
We chose the primary outcome measure of duration of severe-range HTN because it conveys the effect of elevated BP on a woman’s postpartum care more accurately than other outcome measures. Alternative outcomes, such as the presence of any BP ≥160/110 mm Hg, MAP, or rate of persistent postpartum BP ≥150/100 mm Hg, treat a woman with the specified degree of HTN for a few hours postpartum as categorically equal to a woman with the same degree of HTN for 48 or 72 hours after delivery. In the management of women with postpartum HTN, the role of timing is important. The clinical significance and implications of a BP ≥160/110 mm Hg occurring 6 hours after delivery is different from when it occurs 72 hours after delivery, as the woman with severe-range HTN 72 hours after delivery is considered to have more persistent, severe disease, and is more likely to require prolonged observation and treatment with scheduled antihypertensive medication.
Strengths and weaknesses
Our study had several strengths. It was a double-masked, randomized controlled design with an intention-to-treat analysis. Additionally, we chose outcome measures that were clinically relevant and included additional measures that were reported in other studies to facilitate comparison. The use of around-the-clock study drug dosing helped ensure that the inherent potential for type II error would be related to limited power rather than underestimation of NSAID effect because of the less frequent dosing due to PRN dosing. Lastly, the high proportion of our study population with clinically significant postpartum HTN makes our findings generalizable to women with persistent postpartum HTN, the population among which ACOG initially advised caution.
Our study also had several limitations. The superiority design does not allow us to infer equivalence between treatment arms, and the relatively small sample size limited our ability to assess rare adverse outcomes. We chose a superiority design because our objective was to identify a difference between groups rather than prove equivalence. Equivalence or noninferiority designs are powered to rule out a narrower margin of difference between groups with more confidence, and so require much larger sample sizes. Lastly, the unique racial and ethnic makeup of our study population, with high proportions of Native American and Hispanic participants and a low proportion of Black participants, may limit the generalizability of our findings.
Comparison with existing literature
Our findings are consistent with 2 of 3 previous publications addressing this research question. The 2 studies consistent with our findings were retrospective in design and did not identify any association between postpartum NSAID use and persistent BP ≥150/100 mm Hg, MAP, antihypertensive use, or length of postpartum hospital stay.3,22
Our findings are not consistent with the 1 previously published open-label, randomized controlled trial, which reported that women with preeclampsia with severe features who were given ibuprofen after vaginal delivery had at least 1 BP ≥150/100 mm Hg at more than twice the rate of those given acetaminophen (63.1 vs 28.6%, P = .01).23 While such a difference is impressive, this outcome measure may not have signified clinically relevant postpartum hypertensive morbidity. The investigators did not report whether the HTN was persistent and whether it led to antihypertensive use or extended post-partum hospital stay.
Significant differences in study methodology include their use of open-label randomization, limitation of the study population to those having a vaginal delivery, use of a primary outcome that would not affect clinical management according to ACOG clinical guidelines, and less frequent BP monitoring (every 4 hours during the first 24 hours post-partum and every 12 hours thereafter). In contrast, our study employed a double-masked design, included all mode of delivery types, used a clinically meaningful primary outcome that accurately assessed the difficulty of HTN control, and more frequent BP monitoring (hourly for first 24 hours post-partum and every 4 hours thereafter).
A principle difference between the studies is that in their randomized controlled trial, the rate of HTN in the acetaminophen group was only 28.6% compared to 84% in our cohort, which may be related to differences in study design or attributable to their cohort having less severe disease. This may suggest that patients with less severe disease are more susceptible to NSAID-induced changes in BP. By comparison, our study’s primary and secondary outcomes were specifically chosen to address the question of whether any NSAID-associated increase in BP would alter care. The above randomized controlled trial is the only study we identified that supports the recommendation to avoid NSAID use in women with postpartum HTN.
Conclusion
In conclusion, we were unable to identify any detrimental effect of ibuprofen on postpartum BP. Our study does not support the hypothesis that postpartum use of NSAIDs adversely affects BP control in women with preeclampsia with severe features. A larger trial is still required to establish noninferiority and exclude differences in rare, adverse outcomes. Additionally, these findings are limited to women diagnosed with preeclampsia with severe features, therefore a trial to assess the effect of NSAIDs on postpartum BP in the setting of preeclampsia without severe features is warranted.
AJOG at a Glance.
Why was this study conducted?
We sought to generate evidence for or against American College of Obstetricians and Gynecologists (ACOG) recommendation to avoid nonsteroidal antiinflammatory drugs in women with postpartum hypertension (HTN).
Key findings
Postpartum ibuprofen use did not adversely affect any metric of postpartum blood pressure control, including the duration of severe-range HTN, need for antihypertensive medication at discharge, or need to extend hospital stay for blood pressure control.
What does this add to what is known?
The ACOG recommendation to avoid nonsteroidal antiinflammatory use in women with postpartum HTN is not based on substantive clinical evidence.
Acknowledgment
The authors would like to thank to Dr Eve Espey and Dr Rebecca Rogers for their guidance and support; Dr Luis Izquierdo for his assistance with the institutional review board; as well as Dr Heather Riese for her contribution to patient recruitment.
This study received support from the following sources: internal grant award from the Department of Obstetrics and Gynecology, University of New Mexico; University of New Mexico Clinical and Translational Science Center Biostatistics Core (National Institutes of Health [NIH] award UL1TR001449); and REDCap data management funded by Department of Health and Human Services/NIH/National Center for Research Resources grant 8UL1TR000041.
Footnotes
The authors report no conflict of interest.
Presented in the late-breaking oral session at the 38th Annual Pregnancy Meeting of the Society for Maternal-Fetal Medicine, Dallas, TX, Jan. 29-Feb. 3, 2018.
References
- 1.Matthews Z World health report 2005: make every mother and child count. World Health 2005;33:409–11. [DOI] [PubMed] [Google Scholar]
- 2.Sharma KJ, Greene N, Kilpatrick SJ. Oral labetalol compared to oral nifedipine for post-partum hypertension: a randomized controlled trial. Hypertens Pregnancy 2017;36:44–7. [DOI] [PubMed] [Google Scholar]
- 3.Viteri OA, England JA, Alrais MA, et al. Association of nonsteroidal antiinflammatory drugs and postpartum hypertension in women with preeclampsia with severe features. Obstet Gynecol 2017;130:1. [DOI] [PubMed] [Google Scholar]
- 4.Kim LH, Cheng YW, Delaney S, Jelin AC, Caughey AB. Is preeclampsia associated with an increased risk of cesarean delivery if labor is induced? J Matern Fetal Neonatal Med 2010;23: 383–8. [DOI] [PubMed] [Google Scholar]
- 5.Dedier J, Stampfer MJ, Hankinson SE, Willett WC, Speizer FE, Curhan GC. Nonnarcotic analgesic use and the risk of hypertension in US women. Hypertension 2002;40:604–8. [DOI] [PubMed] [Google Scholar]
- 6.Curhan GC, Willett WC, Rosner B, Stampfer MJ. Frequency of analgesic use and risk of hypertension in younger women. Arch Intern Med 2002;162:2204–8. [DOI] [PubMed] [Google Scholar]
- 7.White WB, Kent J, Taylor A, Verburg KM, Lefkowith JB, Whelton A. Effects of celecoxib on ambulatory blood pressure in hypertensive patients on ACE inhibitors. Hypertension 2002;39: 929–34. [DOI] [PubMed] [Google Scholar]
- 8.Morgan TO, Anderson A, Bertram D. Effect of indomethacin on blood pressure in elderly people with essential hypertension well controlled on amlodipine or enalapril. Am J Hypertens 2000;13:1161–7. [DOI] [PubMed] [Google Scholar]
- 9.Klassen DK, Jane LH, Young DY, Peterson CA. Assessment of blood pressure during naproxen therapy in hypertensive patients treated with nicardipine. Am J Hypertens 1995;8:146–53. [DOI] [PubMed] [Google Scholar]
- 10.Whelton A, White WB, Bello AE, Puma JA, Fort JG. Effects of celecoxib and rofecoxib on blood pressure and edema in patients > or =65 years of age with systemic hypertension and osteoarthritis. Am J Cardiol 2002;90:959–63. [DOI] [PubMed] [Google Scholar]
- 11.Izhar M, Alausa T, Folker A, Hung E, Bakris GL. Effects of COX inhibition on blood pressure and kidney function in ACE inhibitor-treated blacks and Hispanics. Hypertension 2004;43:573–7. [DOI] [PubMed] [Google Scholar]
- 12.Floor-Schreudering A, De Smet PAGM, Buurma H, et al. NSAID-antihypertensive drug interactions: which outpatients are at risk for a rise in systolic blood pressure? Eur J Prev Cardiol 2015;22:91–9. [DOI] [PubMed] [Google Scholar]
- 13.Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? Ann Intern Med 1994;121: 289–300. [DOI] [PubMed] [Google Scholar]
- 14.Knights KM, Mangoni AA, Miners JO. Non-selective nonsteroidal anti-inflammatory drugs and cardiovascular events: is aldosterone the silent partner in crime? Br J Clin Pharmacol 2006;61:738–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whelton A, Schulman G, Wallemark C, et al. Effects of celecoxib and naproxen on renal function in the elderly. Arch Intern Med 2000;160:1465–70. [DOI] [PubMed] [Google Scholar]
- 16.Narumiya S, Sugimoto Y, Ushikubi F. Pros-tanoid receptors: structures, properties, and functions. Physiol Rev 1999;79:1193–226. [DOI] [PubMed] [Google Scholar]
- 17.Rahman M, Wright JT, Douglas JG. The role of the cytochrome P450-dependent metabolites of arachidonic acid in blood pressure regulation and renal function. Am J Hypertension 1997;7061:356–65. [DOI] [PubMed] [Google Scholar]
- 18.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Executive summary: hypertension in pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- 19.Zlatnik MG, Buhimschi I, Chwalisz K, Liao QP, Saade GR, Garfield RE. The effect of indomethacin and prostacyclin agonists on blood pressure in a rat model of preeclampsia. Am J Obstet Gynecol 1999;180:1191–5. [DOI] [PubMed] [Google Scholar]
- 20.Makris A, Thornton C, Hennessy A. Postpartum hypertension and nonsteroidal analgesia. Am J Obstet Gynecol 2004;190:577–8. [DOI] [PubMed] [Google Scholar]
- 21.Schachtel BP, Thoden WR, Baybutt RI. Ibuprofen and acetaminophen in the relief of postpartum episiotomy pain. J Clin Pharmacol 1989;29:550–3. [DOI] [PubMed] [Google Scholar]
- 22.Wasden SW, Ragsdale ES, Chasen ST, Skupski DW. Impact of non-steroidal anti-inflammatory drugs on hypertensive disorders of pregnancy. Pregnancy Hypertens 2014;4:259–63. [DOI] [PubMed] [Google Scholar]
- 23.Vigil-De Gracia P, Solis V, Ortega N. Ibuprofen versus acetaminophen as a post-partum analgesic for women with severe preeclampsia: randomized clinical study. J Matern Neonatal Med 2017;30:1279–82. [DOI] [PubMed] [Google Scholar]
- 24.Lowder JL, Shackelford DP, Holbert D, Beste TM. A randomized, controlled trial to compare ketorolac tromethamine versus placebo after cesareansection toreducepainand narcotic usage. Am J Obstet Gynecol 2003;189:1559–62. [DOI] [PubMed] [Google Scholar]
- 25.Sammour RN, Ohel G, Cohen M, Gonen R. Oral naproxen versus oral tramadol for analgesia after cesarean delivery. Int J Gynecol Obstet 2011;113:144–7. [DOI] [PubMed] [Google Scholar]
- 26.Deussen AR, Ashwood P, Martis R. Analgesia for relief of pain due to uterine cramping/involution after birth. Cochrane Database Syst Rev 2011;5:CD004908. [DOI] [PubMed] [Google Scholar]
- 27.Bhounsule SA, Nevreker PR, Agshikar NV, Pal MN, Dhume VG. A comparison of four analgesics in post-episiotomy pain. Indian J Physiol Pharmacol 1990;34:34–8. [PubMed] [Google Scholar]
- 28.Wilasrusmee S, Chittachareon A, Jirasiritum S, Srisangchai P. Naproxen suppository for perineal pain after vaginal delivery. Int J Gynaecol Obstet 2008;102:19–22. [DOI] [PubMed] [Google Scholar]
- 29.El-Tahan MR, Warda OM, Yasseen AM, Attallah MM, Matter MK. A randomized study of the effects of preoperative ketorolac on general anesthesia for cesarean section. Int J Obstet Anesth 2007;16:214–20. [DOI] [PubMed] [Google Scholar]
- 30.Towers CV, Shelton S, van Nes J, et al. Preoperative cesarean delivery intravenous acetaminophen treatment for postoperative pain control: a randomized double-blinded placebo control trial. Am J Obstet Gynecol 2018;218: 353.e1–4. [DOI] [PubMed] [Google Scholar]
- 31.Lam J, Kelly L, Ciszkowski C, et al. Central nervous system depression of neonates breastfed by mothers receiving oxycodone for postpartum analgesia. J Pediatr 2012;160: 33–7.e2. [DOI] [PubMed] [Google Scholar]
- 32.Altenau B, Crisp CC, Devaiah CG, Lambers DS. Randomized controlled trial of intravenous acetaminophen for postcesarean delivery pain control. Am J Obstet Gynecol 2017;217:362.e1–6. [DOI] [PubMed] [Google Scholar]


