Abstract
Long-term follow-up data from multicenter phase III non-inferiority trials confirmed the safety of omission of axillary dissection in selected patients with clinically node-negative, sentinel node-positive breast cancer. Several ongoing trials investigate extended eligibility of the Z0011 protocol in the adjuvant setting. De-escalation of axillary surgery in patients with clinically node-positive breast cancer is currently limited to the neoadjuvant setting, where the sentinel procedure is used to determine nodal pathological complete response. Targeted axillary dissection lowers the false-negative rate of the sentinel procedure, which, however, is consistently associated with a very low risk of axillary recurrence in several recent single-center series. Axillary dissection remains standard care in patients with residual disease after neoadjuvant chemotherapy while the results of Alliance A011202 are pending. The TAXIS trial investigates the role of tailored axillary surgery in patients with clinically node-positive breast cancer, a novel concept designed to selectively remove positive nodes in the adjuvant and neoadjuvant setting.
Keywords: Breast cancer, Breast surgery, Axillary dissection, Sentinel lymph node procedure, Axillary staging
Highlights
-
•
Current indications for performing axillary lymph node dissection.
-
•
De-escalating axillary surgery strategies in node-positive breast cancer.
-
•
New concept for clinically node-positive breast cancer.
1. Summary
The evolution of axillary surgery is characterized by surgical de-escalation. Radical axillary lymph node dissection (ALND) was performed as standard in patients with breast cancer for almost a century. Since the identification of the sentinel nodes in the 90s, ALND was performed for clinically node-positive breast cancer and whenever cancer was found in the sentinel nodes. Today, we have learned from clinical trials that we can omit ALND in many patients with positive sentinel nodes [1,2]. In a situation with positive sentinel nodes and a high risk of recurrence, axillary radiation is increasingly preferred over ALND [3,4]. In the future, we will have to answer the question if we can omit the sentinel procedure in patients with negative ultrasound [[5], [6], [7], [8]]. We will also have to answer the question if we can omit ALND in all patients with positive sentinel nodes, even if there are additional risk factors [[9], [10], [11]]. Finally, we will have to find ways to omit ALND in clinically node-positive breast cancer with residual disease after neoadjuvant chemotherapy (NACT) (NCT01901094) and in the upfront surgery setting [12]. Current concepts use limited axillary surgery procedures, such as the sentinel procedure, to determine if the nodes are clear after NACT without removing them all [13,14].
2. Current indications for axillary dissection
Long-term follow-up data of several large phase III non-inferiority trials, randomizing clinically node-negative patients with positive sentinel nodes into one group with axillary dissection, compared to no axilla-specific treatment, have been published. Both the ACOSOG Z0011 and the IBCSG 23–01 trials found extremely low rates (<2%) of axillary recurrence, showing that many of these patients do not need axilla-specific treatment [1,2]. ALND is still considered standard practice in clinically node-positive breast cancer in the upfront surgery setting, in patients with residual nodal disease after NACT and in locally advanced breast cancer (cT3-4, inflammatory breast cancer, >2 positive sentinel nodes, gross extranodal disease). ALND is also indicated in sentinel node-positive patients with macrometastases undergoing mastectomy, but only if the positive sentinel node per se does not indicate postmastectomy radiotherapy (PMRT) or if irradiation does not include the lymph nodes (Table 1). If PMRT is performed and includes the axilla, sentinel node-positive patients do not require ALND, as radiation will suffice [3]. This has been demonstrated by the EORTC AMAROS trial, where the promising 10-year follow-up data were presented at the San Antonio Breast Cancer Symposium 2018, while full publication is pending [15]. In this trial, clinically node-negative, sentinel node-positive patients (n = 1425, of whom 17% underwent mastectomy) were randomized into a control group with ALND compared to axillary irradiation as experimental treatment. At a median follow-up of 10 years, axillary recurrence occurred in 0.9% in the ALND group vs. 1.8% in the irradiation group. Even though non-inferiority could not be proven statistically, from a clinical point of view looking at the very low rates of axillary recurrence, clinical non-inferiority was demonstrated. Therefore, in cases where treatment of the axilla is planned for patients within this population, irradiation can be an alternative to ALND. Patient selection for axillary radiation versus observation in this situation is a field of ongoing controversy.
Table 1.
Current indications for axillary lymph node dissection.
| Clinical setting | Type of surgery | Nodal status | Primary axillary surgery procedure | Indication for ALND |
|---|---|---|---|---|
| After NACT |
cN0 | SLNB | Any residual diseasea | |
| cN1 → cN0 | SLNB (>2 neg. SLN) or TAD | Any residual diseasea, <3 negative SLNb | ||
| cN1 → cN1 |
ALND |
|||
| Upfront surgery | BCS +WBR | cN0 | SLNBc | ≥ 3 positive SLN, cT3-4 |
| cN1 | ALND | |||
| Mastectomy | cN0 | SLNB | SLN-macrometastasis if no PMRT is planned | |
| cN1 or inflammatory breast cancer | ALND |
NACT = neoadjuvant chemotherapy; SLNB = sentinel lymph node biopsy; SLN = sentinel lymph node; TAD = Targeted axillary dissection; ALND = axillary lymph node dissection; BCS = breast conserving surgery; WBR = whole breast radiotherapy; PMRT = postmastectomy radiotherapy.
Some centers omit ALND in case of isolated tumor cells.
Some centers omit ALND when <3 negative SLN are removed.
In case of 1 or 2 metastases with additional risk factors (e.g., microscopic extracapsular tumor extension, lymphovascular invasion), nodal irradiation can be considered.
3. Can Z0011 eligibility be broadened?
Because of the limitations of the ACOSOG Z0011 study, a series of randomized trials were initiated to validate the findings of Z0011 after the first results were published. In the meantime, however, the protocol has been validated by prospective observational studies and practice has been changed accordingly in many countries [16,17]. Therefore, the randomized trials adjusted their focus to patients that were excluded from Z0011. In the ERC/IPC 2012-001 SERC trial from France, they included 1855 patients at 53 sites according to Z0011 from 2012 to 2018 and started to select Z0011 non-eligible patients in August 2018 [9]. In the SENOMAC trial from Sweden, several countries started to focus on patients undergoing mastectomy [10]. All of these axillary surgery de-escalation studies encountered methodological challenges, primarily due to a lack of power based on lower than expected rates of events or accrual [[1], [2], [3],9,10,15].
4. Clinically node-positive breast cancer
Clinically node-positive patients are commonly defined by the occurrence of palpable disease at the time of diagnosis. Non-palpable disease detected solely on imaging can be considered clinically node-positive or imaging node-positive and refers to a frequent subpopulation in clinical practice where preoperative ultrasound or MRI is routinely used. Both groups are often jointly categorized as biopsy-proven node-positive breast cancer, as pathologic confirmation of malignancy is recommended [18]. Most of these patients still undergo ALND in the upfront surgery setting and in the event of residual nodal disease after NACT. The use of non-invasive imaging after NACT cannot replace axillary surgery. In a meta-analysis looking at how reliable imaging is in determining nodal pathological complete response (pCR), the outcome of 2380 patients in 13 studies with non-invasive imaging after NACT was compared with axillary surgery [19]. The study showed an axillary pCR of 39.5% (941/2380). Sensitivity for ultrasound, MRI, or PET-CT was far away from being reliable in terms of assessing accurate axillary response after NACT (65%, 60%, resp. 38%). At this point, microscopic analysis of at least a few nodes after NACT is needed to determine pCR in the lymph nodes, which de-escalated axillary surgery in current practice. A meta-analysis of 20 studies including 2217 patients investigated the false-negative rate (FNR) of the sentinel node procedure in biopsy-proven clinically node-positive patients with clinically node-negative sentinel lymph nodes after NACT undergoing back-up ALND [13]. The FNR was 22%; however, the FNR decreased to 8% when at least three negative nodes were removed and double tracing was used. The MARI procedure selectively marked and removed the sampled node with a radioactive seed, which showed a FNR of 7%. With the combination of the two techniques, selective localization and removal of the clipped node together with the sentinel procedure, the lowest FNR of 2–4% can be achieved [13]. This combination is called targeted axillary dissection (TAD). The prospective SenTa registry study included 473 patients with clipped nodes at 50 German centers. It showed that the clipped lymph node and sentinel node were identical in 64.8% and the detection rate of the clipped lymph node after NACT was 86.9% [14]. This means that the clip was left behind in 13% of patients, which can become problematic from a medical-legal aspect in case of regional recurrence. However, in terms of the FNR, performance was well with a FNR of 7.2% for the removal of just the localized node and a FNR of 4.3% when TAD was performed (Table 2).
Table 2.
| Procedure | n (patients) | Identification rate | FNR |
|---|---|---|---|
| SLNB | 2002 | 89% | Overall: 17% <3 SNL: 22% ≥3 SNL: 8% |
| MARI | 95 | 97% | 7% |
| TAD (seed) | 120 | 100% | 2–4% |
| TAD (no seed) | 473 | 86.% | 4.3% |
SLNB = sentinel lymph node biopsy; SNL = sentinel lymph node; FNR = false-negative rate; NACT = neoadjuvant chemotherapy; MARI = marking the axillary positive lymph node with an iodine seed, TAD = targeted axillary dissection.
The sentinel node procedure after NACT in clinically node-positive patients who turned clinically node-negative after NACT is the most commonly performed procedure today. For a long time, the importance of the FNR was unclear, since leaving chemo-resistant cancer in the nodes may increase axillary recurrence compared to the adjuvant setting. A retrospective single-center study from Canada investigated 102 patients in this setting [20]. Of these, 71% had regional irradiation and a medium of 4 negative sentinel nodes were removed. There was not a single case of axillary recurrence at a median follow-up of three years. However, since the authors insisted on having several negative nodes, the expected FNR was low and in combination with the broad use of axillary irradiation, these results were expected. On the other hand, a series from Milan also reported only two axillary recurrences at a very long median follow up of 9.2 years in 123 patients [21]. Importantly, they used only single tracer (99Tc), resulting in 74% of patients with less than 3 negative sentinel nodes, and the majority of patients did not get regional irradiation. Hence, the expected FNR was much higher, and yet, the vast majority of patients did not show recurrence. Similar results were confirmed in two retrospective studies from Brazil and the Mayo Clinic with removal of a median of 2 and 3 negative sentinel nodes, respectively, and very low rate of recurrence [22,23]. These results confirmed that the sentinel procedure is a valid treatment option in these patients (Table 3).
Table 3.
De-escalated axillary surgery procedure in clinically node positive-breast cancer. Axillary recurrence in node-negative patients after NACT [[20], [21], [22], [23]].
| First author | n (patients) | SLNB (median no.) | Double tracer | Irradiation | Axillary recurrence (absolute no.) | Median follow-up (y) |
|---|---|---|---|---|---|---|
| Wong | 102 | 4 | Yes | 71% | 0 | 3 |
| Kahler-Ribeiro-Fontana | 123 | 2 | No (only99Tc) | 42% | 2 | 9.2 |
| Damin | 38 | 2 | Yes | 87% | 1 | 4.7 |
| Piltin | 139 | 3 | NA | 78% | 1 | 2.8 |
5. Ongoing clinical trials in clinically node-positive breast cancer
ALND is the standard procedure when residual disease after NACT is detected in the sentinel nodes. The ongoing Alliance A011202 trial is randomizing this patient population into ALND compared to axillary radiation in the context of extended regional nodal irradiation (NCT01901094). Accrual is almost completed and the primary endpoint analysis is expected in a few years. Until then, the omission of ALND should be considered experimental in most of these patients. An analysis from the National Cancer Database looked at patients with up to 3 lymph nodes with residual disease and compared the sentinel procedure (defined as removal of ≤4 lymph nodes) with radiation (n = 304) versus ALND with radiation (n = 1313) [24]. Patients without ALND showed worse overall survival (71% vs. 77% at 5 years). Even though there is always selection bias in such studies-patients with more co-morbidities were spared ALND and the difference in outcome was due to the comorbidity and not the omission of the ALND-these results call for caution and confirmation by randomized trials. Interestingly, however, the authors found subgroups, primarily luminal tumors with only one lymph node metastasis, where the omission of ALND did not decrease survival.
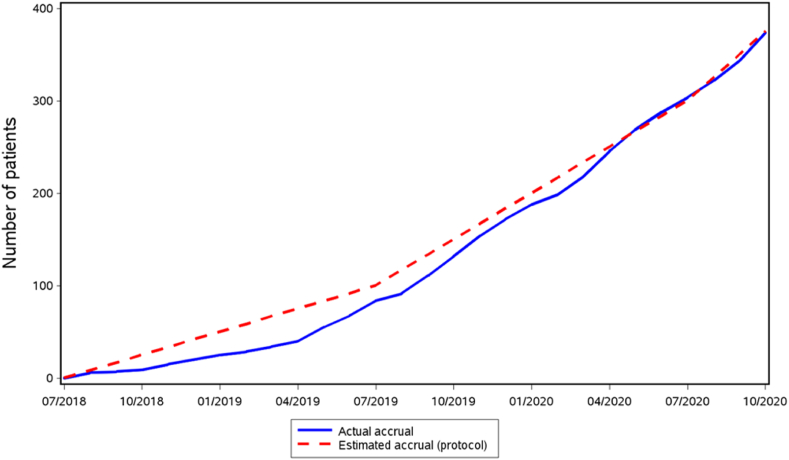
The European phase III randomized controlled TAXIS trial investigates the role of a novel concept called tailored axillary surgery (TAS) in patients with clinically node positive breast cancer in the neoadjuvant and the upfront surgery setting (NCT03513614). Accrual is running as estimated, with over 400 patients already randomized of the total planned sample size of 1500 (Fig. 1). TAS removes all palpably clearly suspicious lymph nodes together with the sentinel lymph nodes, whereas imaging-guided localization of the clipped node is optional. Main purpose of TAS is to reduce the tumor load in the axilla to the point where axillary irradiation can control it. Therefore, in a randomized manner, TAS with axillary irradiation is compared to standard ALND in the context of extended regional nodal irradiation. This non-inferiority trial investigates disease-free survival as primary endpoint and quality of life as most important secondary endpoint. Completion of accrual is expected in 2024 and analysis of the primary endpoint to be published in 2030.
Fig. 1.
Accrual of the TAXIS trial. The dotted line is the estimated accrual, the blue line is the actual accrual.
Declaration of competing interest
No commercial support was provided for this study. All authors except W.P. Weber have nothing to disclose. Outside the submitted work, W.P. Weber received research support from Takeda Pharmaceuticals International paid to the Swiss Group for Clinical Cancer Research (SAKK) and personal honoraria from Genomic Health, Inc., USA. Support for meetings was paid to his institution from Sandoz, Genomic Health, Medtronic, Novartis Oncology, Pfizer and Eli Lilly.
Footnotes
This article is published as part of a supplement supported by St. Gallen Oncology Conferences.
References
- 1.Galimberti V., Cole B.F., Viale G., Veronesi P., Vicini E., Intra M., et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018 doi: 10.1016/S1470-2045(18)30380-2. http://www.thelancet.com/article/S1470204518303802/fulltext [Internet] Oct 1 [cited 2021 Mar 21];19(10):1385–93. Available from: [DOI] [PubMed] [Google Scholar]
- 2.Giuliano A.E., Ballman K.V., Mccall L., Beitsch P.D., Brennan M.B., Kelemen P.R., et al. Effect of axillary dissection vs No axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial HHS public access. J Am Med Assoc. 2017;318(10):918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donker M., van Tienhoven G., Straver M.E., Meijnen P., van de Velde C.J.H., Mansel R.E., et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014 doi: 10.1016/S1470-2045(14)70460-7. [Internet] Nov 1 [cited 2021 Mar 21];15(12):1303–10. Available from:/pmc/articles/PMC4291166/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sávolt Péley G., Polgár C., Udvarhelyi N., Rubovszky G., Kovács E., et al. Eight-year follow up result of the OTOASOR trial: the Optimal Treatment of the Axilla – surgery or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: a randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol. 2017 doi: 10.1016/j.ejso.2016.12.011. [Internet] Apr 1 [cited 2021 Apr 11];43(4):672–9. Available from: [DOI] [PubMed] [Google Scholar]
- 5.Hughes K.S., Schnaper L.A., Bellon J.R., Cirrincione C.T., Berry D.A., McCormick B., et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 Years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.45.2615. www.jco.org [Internet] [cited 2021 Apr 11];31:2382–7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentilini O., Botteri E., Dadda P., Sangalli C., Boccardo C., Peradze N., et al. Physical function of the upper limb after breast cancer surgery. Results from the SOUND (Sentinel node vs. Observation after axillary Ultra-souND) trial. Eur J Surg Oncol. 2016 doi: 10.1016/j.ejso.2016.01.020. [Internet] May 1 [cited 2021 Apr 11];42(5):685–9. Available from: [DOI] [PubMed] [Google Scholar]
- 7.Reimer A.T., Stachs A., Nekljudova V., Loibl S., Hartmann S., Wolter K., et al. Restricted axillary staging in clinically and sonographically node-negative early invasive breast cancer (c/iT1-2) in the context of breast conserving therapy: first results following commencement of the intergroup-sentinel-mamma (INSEMA) trial eingeschrä. Axillary Staging … Geburtsh Frauenheilk [Internet] 2017;77:149–157. doi: 10.1055/s-0042-122853. http://dx Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Roozendaal L.M., Vane M.L.G., Van Dalen T., Van Der Hage J.A., Strobbe L.J.A., Boersma L.J., et al. Clinically node negative breast cancer patients undergoing breast conserving therapy, sentinel lymph node procedure versus follow-up: a Dutch randomized controlled multicentre trial. https://clinicaltrials.gov/ct2/show/NCT02271828 BOOG 2013-08). Available from: [DOI] [PMC free article] [PubMed]
- 9.Houvenaeghel G., Cohen M., Raro P., De Troyer J., De Lara C.T., Gimbergues P., et al. Overview of the pathological results and treatment characteristics in the first 1000 patients randomized in the SERC trial: axillary dissection versus no axillary dissection in patients with involved sentinel node. BMC Canc. 2018;18(1) doi: 10.1186/s12885-018-5053-7. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Boniface J., Ahlgren J., Andersson Y., Bergkvist L., Frisell J., Lundstedt D., et al. The generalisability of randomised clinical trials: an interim external validity analysis of the ongoing SENOMAC trial in sentinel lymph node-positive breast cancer behalf of the SENOMAC Trialists' Group. Breast Canc Res Treat. 2020;180:167–176. doi: 10.1007/s10549-020-05537-1. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal A., Dodwell D. Vol. 27. Clinical Oncology. Elsevier Ltd; 2015. POSNOC: a randomised trial looking at axillary treatment in women with one or two sentinel nodes with macrometastases. [Internet] [cited 2021 Apr 11]. pp. 692–5. Available from: [DOI] [PubMed] [Google Scholar]
- 12.Henke G, Knauer M, Ribi K, Hayoz S, Gérard M-A, Ruhstaller T, et al. Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (TAXIS): study protocol for a multicenter, randomized phase-III trial. Available from: 10.1186/s13063-018-3021-9. [DOI] [PMC free article] [PubMed]
- 13.Simons J.M., van Nijnatten T.J.A., van der Pol C.C., Ejt Luiten, Koppert L.B., Smidt M.L. Diagnostic accuracy of different surgical procedures for axillary staging after neoadjuvant systemic therapy in node-positive breast cancer. Ann Surg. 2019 doi: 10.1097/SLA.0000000000003075. https://journals.lww.com/00000658-201903000-00010 [Internet] Mar 1 [cited 2021 Mar 21];269(3):432–42. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuemmel S., Heil J., Rueland A., Seiberling C., Harrach H., Schindowski D., et al. A prospective, multicenter registry study to evaluate the clinical feasibility of targeted axillary dissection (TAD) in node-positive breast cancer patients. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004572. [Publish Ah] [DOI] [PubMed] [Google Scholar]
- 15.Exner R, Exner FREBS. Post San Antonio Breast Cancer Symposium 2018 Local and operative therapy, radiotherapy. memo - Mag Eur Med Oncol [Internet]. Available from: 10.1007/s12254-019-0513-6. [DOI]
- 16.Hennigs A., Köpke M., Feißt M., Riedel F., Rezai M., Nitz U., et al. Which patients with sentinel node-positive breast cancer after breast conservation still receive completion axillary lymph node dissection in routine clinical practice? Breast Canc Res Treat. 2019 doi: 10.1007/s10549-018-5009-2. [Internet] Jan 30 [cited 2021 Apr 11];173(2):429–38. Available from: [DOI] [PubMed] [Google Scholar]
- 17.Morrow M., Van Zee K.J., Patil S., Petruolo O., Mamtani A., Barrio A.V., et al. Axillary dissection and nodal irradiation can Be avoided for most node-positive z0011-eligible breast cancers: a prospective validation study of 793 patients. Ann Surg. 2017 doi: 10.1097/SLA.0000000000002354. [Internet] Sep 1 [cited 2021 Apr 11];266(3):457–62. Available from:/pmc/articles/PMC5649371/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macdonald S., Oncology R., General M. Breast cancer breast cancer. J R Soc Med. 2016;70(8):515–517. https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf [Internet] Available from: [Google Scholar]
- 19.Samiei S., de Mooij C.M., Lobbes M.B.I., Keymeulen K.B.M.I., van Nijnatten T.J.A., Smidt M.L. Diagnostic performance of noninvasive imaging for assessment of axillary response after neoadjuvant systemic therapy in clinically node-positive breast cancer: a systematic review and meta-analysis. Ann Surg. 2021 doi: 10.1097/SLA.0000000000004356. http://www.ncbi.nlm.nih.gov/pubmed/33201095 [Internet] Apr 1 [cited 2021 Mar 21];273(4):694–700. Available from: [DOI] [PubMed] [Google Scholar]
- 20.Wong S.M., Basik M., Florianova L., Margolese R., Dumitra S., Muanza T., et al. Oncologic safety of sentinel lymph node biopsy alone after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2020 doi: 10.1245/s10434-020-09211-0. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 21.Kahler-Ribeiro-Fontana S., Pagan E., Magnoni F., Vicini E., Morigi C., Corso G., et al. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol. 2020 doi: 10.1016/j.ejso.2020.10.014. [Internet] Apr 1 [cited 2021 Apr 7]; Available from: [DOI] [PubMed] [Google Scholar]
- 22.Damin A.P., Zancan M., Melo M.P., Biazus J.V. vol. 186. 2021. pp. 527–534. (Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with node-positive breast cancer: guiding a more selective axillary approach). Available from: [DOI] [PubMed] [Google Scholar]
- 23.Piltin MA, Hoskin TL, Day CN, Davis J, Boughey JC. Oncologic Outcomes of Sentinel Lymph Node Surgery After Neoadjuvant Chemotherapy for Node-Positive Breast Cancer. Available from: 10.1245/s10434-020-08900-0. [DOI] [PubMed]
- 24.Almahariq M.F., Levitin R., Quinn T.J., Chen P.Y., Dekhne N., Kiran S., et al. Omission of axillary lymph node dissection is associated with inferior survival in breast cancer patients with residual N1 nodal disease following neoadjuvant chemotherapy. Ann Surg Oncol. 2021;28:930–940. doi: 10.1245/s10434-020-08928-2. [Internet] Available from: [DOI] [PubMed] [Google Scholar]