Abstract
Estrogen receptor (ER+) breast cancer is the most frequently diagnosed breast cancer subtype. Currently, adjuvant treatment for early stage disease consists of endocrine therapy, with or without chemotherapy and bone-targeted therapy, delivered in a risk-adapted manner. Despite this multimodal approach, a significant proportion of high risk patients will develop incurable distant recurrences. There is an ongoing need to develop new treatment strategies that address the biologic causes of treatment failure and to identify the individual patients who can benefit from such interventions. Here we review the clinical investigation of targeted and novel therapies, including inhibitors of the PI3K-AKT-mTOR pathway, oral selective estrogen receptor degraders (SERDs), and PARP-inhibitors for the treatment of early ER+ breast cancer. Furthermore, we highlight opportunities in biomarker development to help guide the delivery of escalated adjuvant strategies.
Keywords: Breast cancer, Adjuvant therapy, Endocrine therapy, Targeted agents, Estrogen receptor, mTOR, PI3K, SERD, AKT, Early breast cancer, PARP inhibitor
1. Introduction
Informed by an evolving understanding of disease biology, clinical investigation of systemic therapy for estrogen receptor positive (ER+)/HER2-negative breast cancer has yielded important treatment advances over the last two decades. Since the majority of cases of this most common breast cancer subtype are diagnosed as early stage disease, optimization of (neo)adjuvant systemic therapy has the potential for widespread impact. The development and clinical use of endocrine therapies, chemotherapy and bone targeted therapies, along with biomarkers to inform their application has enabled the risk-adapted delivery of adjuvant therapy to improve clinical outcomes while reducing overtreatment [[1], [2], [3]].
Unfortunately, however, metastatic recurrences of ER+ disease continue to inflict substantial morbidity and mortality, highlighting the need for more effective therapies for those at highest risk. The problem of late recurrence in ER+ breast cancer has received recent attention, with evidence of ongoing risk persisting for many years; in populations at high clinical-pathologic risk, late distant recurrence occurs in up to 41% [4,5]. While some of this disease burden may be preventable through more effective delivery of guideline-supported existing therapies and optimization of adherence, extended endocrine therapy has overall modest impacts on distant relapses and breast cancer-specific mortality. Tumor dormancy and the development of endocrine resistance are recognized contributors to the phenomenon of ER+ breast cancer recurrence, the biological basis of which continues to be investigated [6,7].
Existing adjuvant strategies have been developed by studying therapies shown to be safe and proven effective in the metastatic setting in early stage disease. Since the clinical introduction of tamoxifen in the 1980s, evidence supporting its use as chemoprevention, adjuvant, and palliative therapy in both pre- and post-menopausal women with ER+ breast cancer has been generated [[8], [9], [10], [11], [12], [13], [14]]. The uptake of tamoxifen into routine clinical practice has been made possible by its availability, low cost, and tolerable side-effect profile. Subsequently, third generation aromatase inhibitors (AI) were proven superior to tamoxifen in post-menopausal women with advanced disease and approved in the early 2000s, at which point their evaluation in early disease was well underway [[15], [16], [17], [18]]. The establishment of AIs as standard treatment for postmenopausal women with early ER+ breast cancer relied on large randomized trials showing improvement in treatment outcomes and were complemented by data from neoadjuvant trials demonstrating proof of biologic activity [[19], [20], [21]]. Follow-on randomized adjuvant studies have sought to address other clinical questions, such as treatment duration [17,22].
As new agents become available, there exists a strong desire to build upon and accelerate this paradigm, to deliver more effective curative treatment. Major classes of therapy recently developed, or under development for ER+ disease include agents targeting endocrine-related pathways, antibody drug conjugates (ADC), and PARP-inhibitors. The first group includes a wide range of targets up- and downstream of estrogen signalling (eg. PI3K-AKT-mTOR signalling, cyclin-dependent kinases, epigenetic regulators), as well as novel agents targeting the estrogen receptor directly (SERDs, SERCAs - selective estrogen receptor covalent antagonists). The successful development of new agents in the adjuvant setting depends on the identification of individuals with a persistent high-risk of relapse despite standard therapy, along with the availability of safe, tolerable and effective drugs that can achieve disease eradication (or permanent dormancy) leading to cure. Herein we review ongoing efforts to address these gaps and highlight future opportunities for adjuvant systemic therapy. CDK4/6 inhibitors have been the subject of ongoing intensive clinical investigation in early breast cancer, culminating in the recent approval of adjuvant abemaciclib for some high risk ER+ patients [23]. The role for CDK4/6 inhibitors is well described elsewhere in the issue. This review will therefore focus on other endocrine therapy partners in the PI3K-mTOR-AKT pathway, emerging ER-targeting agents (SERDs), and briefly address the role of PARP-inhibitors for early ER+ breast cancer in patients with germline BRCA1/2 mutations.
2. mTOR inhibitors
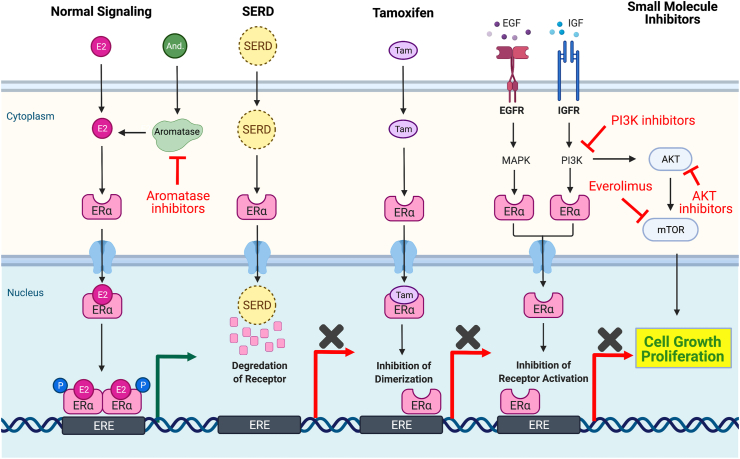
ER+ breast cancer cells have acquired dependence on aberrant metabolic and cellular signalling pathways [24,25]. Many pathways converge on mechanistic Target of Rapamycin (mTOR), a master regulator of cellular metabolism and growth [24]. De novo or acquired resistance to ER-dependent signalling has been attributed to activation of the PI3K/AKT/mTOR pathway and multiple approaches have been developed and tested to exploit mTOR signalling-dependence (Fig. 1) [26,27].
Fig. 1.
Estrogen-dependent signalling and targets of small-molecular inhibitors. Summary of normal estrogen-dependent signalling pathways and the method of pathway disruption with small molecule inhibitors. E2, estrogen. And., androgens. EGF(R), epithelial growth factor (receptor). IGF(R), insulin-like growth factor (receptor). ERα, estrogen receptor alpha. ERE, estrogen response elements. P., phosphorylation. MAPK, mitogen-activated protein kinase. AKT, protein kinase B. Tam., tamoxifen. PI3K, phosphoinositide 3-kinase. mTOR, mammalian target of rapamycin. PI3Kα, phosphoinositide 3-kinase alpha. SERD, selective estrogen receptor degraders. Created with BioRender.com.
The oral mTOR inhibitor everolimus was the first targeted therapy approved in combination with endocrine therapy for ER+ breast cancer in advanced disease. Its pivotal trial, BOLERO-2, comparing everolimus in combination with exemestane to exemestane alone, was conducted in the setting of endocrine resistance following progression on a first-line non-steroidal AI (NSAI) [28]. A significant improvement in median progression free survival was observed with the addition of everolimus (10.6 mo. vs. 4.1 mo; HR = 0.36, 95%CI: 0.27–0.47; P < 0.001), which led to its approval [28]. However, the addition of everolimus was associated with significant toxicity, with substantially higher rates of adverse events, treatment discontinuation (26.3%) and dose reductions (66.8%) [29]. As a result, the median duration of exposure to everolimus was 14.6 weeks [28]. The final analysis demonstrated no statistically significant improvement in overall survival and correlative analyses did not identify biomarkers predictive of everolimus benefit [29].
Nevertheless, given the biological rationale for mTOR inhibition in overcoming resistance to endocrine therapy and the positive primary result of BOLERO-2, evaluation of mTOR inhibitors in early breast cancer has been pursued (Table 1). Two large adjuvant trials were conducted, the first of which was very recently reported. The UNIRAD trial evaluated two years of everolimus in combination with adjuvant endocrine therapy vs. endocrine therapy (physician's choice of AI or tamoxifen) in 1278 patients with ER+/HER2-negative early breast cancer [30]. The trial was designed to enrich for high risk patients, requiring either involvement of ≥4 lymph nodes or 1–3 nodes with an EPclin score ≥3.3 (e.g. a high-risk gene expression score) [30]. The study eligibility allowed for up to 4 years of endocrine therapy prior to enrolment, to address the hypothesis that everolimus might overcome acquired resistance that develops over time [30]. However, 85% of patients enrolled were less than three years from surgery, limiting the ability of this trial to fully address this hypothesis [30]. The trial was stopped at the first pre-planned interim analysis for futility: median 3-years disease free survival (DFS) was 88% in both arms (HR = 0.95, 95%CI: 0.69–1.32) with no differences in metastasis-free survival (HR = 0.88, 95%CI: 0.62–1.25) or overall survival (HR = 1.09, 95%CI: 0.62–1.92) [30]. In this high-risk but early disease population, a very high rate of treatment discontinuation, primarily related to adverse events (mucositis, rash), was observed (53.4% vs. 22.3% of patients); 65% of patients started with a dose reduction (5 mg) with only 16% of patients achieving full dose everolimus (10 mg) at the time of discontinuation [30]. Median treatment duration was 9.2 mo in the everolimus arm versus 22.5 mo in the placebo arm [30]. Subgroup analyses did not identify any features associated with benefit of everolimus, with no difference in effect for participants enrolled strictly based on anatomical risk (≥4 nodes) vs. those with high-risk biology (1–3 nodes and high EPclin score) [30]. While follow-up and long term outcomes will be reported, the marked inability to deliver the experimental therapy represents an important cautionary finding: confirmation of tolerability and persistence on therapy in the target adjuvant population is essential for any therapeutic strategy to succeed in this setting. The large North American adjuvant everolimus study S1207, which differs slightly in risk criteria (using stage and Oncotype RS), duration of allowed prior endocrine therapy, and duration of everolimus (1 year), has completed accrual and results are pending [31].
Table 1.
| Drug Name | Sponsor | Study | Phase | Population | Intervention | Status | Sample Size (n) | Endpoints | Results |
|---|---|---|---|---|---|---|---|---|---|
| mTOR-inhibitors | |||||||||
| Everolimus [30] | UNICANCER; Ministry of Health, France | NCT01805271 | III | HR+/HER2- early BC (≥4 N or 1–3 N+ and EPclin score ≥3.3), ET up to3 years | Everolimus + ET vs. Placebo + ET | Complete | 1279 | DFS, OS, DMFS |
3-years DFS: 88% in both arms (HR = 0.95; 95%CI: 0.69–1.32) DMFS:(HR = 0.88; 95%CI: 0.62–1.25) OS:(HR = 1.09, 95CI: 0.62–1.92). |
| Everolimus [79] | Novartis Pharma AG | NCT00107016 | II | ER-positive, untreated, stage M0, cT > 2 cm | Everolimus + AI vs. AI | Complete | 270 | Clinical response by palpation, PIK3CA-mut., Ki67, phospho-S6, cyclin D1, PR |
Clinical response by palpation: Everolimus + AI = 68.1% vs. AI = 59.1% (p = 0.062) |
| Everolimus [80] | Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University | NCT02742051 | II | Neoadjuvant; post menopausal, cT2+, cN1 | Everolimus + Letrozole vs. FEC | Complete | 40 | Ultrasound response rate, pCR, change in Ki67 expression | Ultrasound response: Everolimus + Letrozole: 65% (95%CI: 40.8–84.6) vs. FEC: 40% (95%CI: 19.1–63.9) pCR: 0% in each arm |
| Everolimus [82] | Southwest Oncology Group, National Cancer Institute (NCI) | NCT01674140 | III | ER-positive, Oncotype DX® RS > 25, 1–3 N+ | Everolimus + ET vs. Placebo + ET | Accrual complete | 1900 | IDFS, OS, DRFS | Pending |
| PI3Kɑ-inhibitors | |||||||||
| Alpelisib [42] | Novartis Pharmaceuticals | NCT01923168 | II | Neoadjuvant; post menopausal, cT1c-T3 | Alpelisib + Letrozole vs. Placebo + Letrozole | Complete | 257 | ORR, pCR |
ORR: all; 43% (alpelisib) vs. 45% (placebo); PIK3CA -mutant: 63% (alpelisib) versus 61% (placebo) pCR: low, no difference |
| Taselisib [83] | Genentech, Inc., SOLTI Breast Cancer Research Group, Breast International Group, Austrian Breast and Colorectal Cancer Group | NCT02273973 | II | Neoadjuvant; post menopausal, cT1-3 | Taselisib + Letrozole vs. Placebo + Letrozole | Complete | 334 | mRECIST, ORR, pCR | ORR: all; 39% (placebo) vs. 50% (taselisib) OR = 1·55, (95%CI 1·00–2·38; p = 0·049); PIK3CA-mutant:38% (placebo) vs. 56% (taselisib) OR 2·03, (95%CI 1·06–3·88; p = 0·033) pCR: low, no difference |
| Oral SERDs | |||||||||
| Giredestrant (GDC-9545) [84] | Roche | NCT04436744 | II | Neoadjuvant, window | Giredestrant (GDC-9545) + Palbociclib vs. Anastrozole + Palbociclib | Complete | 215 (83 results in interim analysis) | Change in Ki67 expression, CCCA, mRECIST | Ki67 reduction: giredestrant = 80%, 95%CI: 72%–85% vs. anastrozole = 67%, 95%CI: 56%–75%; p = 0.0222; Complete cell cycle arrest (CCCA): giredestrant = 25% vs. anastrozole = 5% (Δ 20%; 95% CI = −37%, −3%) |
| AZD9496 [85] | AstraZeneca | NCT03236974 | I | Neoadjuvant, window | AZD9496 vs. Fulvestrant | Complete | 49 (46 results) | Changes in ER, PR, Ki67 expression | AZD9496 was not superior to fulvestrant |
| Giredestrant (GDC-9545) [86] | Roche | NCT03916744 | I | Neoadjuvant, window | GDC-9545 (10 mg, 30 mg, 100 mg) | Complete | 75 (46 results) | Change in Ki67 expression |
Ki67 reduction: 79% (95% CI: 69–89; 10 mg: 80%; 30 mg: 76%; 100 mg: 80%) CCCA (Ki67 ≤ 2.7%): 51% |
| Amcenestrant (SAR439859) [87] | Sanofi | NCT04191382 | II | Neoadjuvant, window | SAR439859 vs. Letrozole | Recruiting | 126 | Change in Ki67, ER expression | Pending |
| Camizestrant (AZD9833) [88] | AstraZeneca | NCT04588298 | II | Neoadjuvant, window | AZD9833 vs. Fulvestrant | Recruiting | 92 | Changes in ER, PR, Ki67 expression | Pending |
| LY3484356 [89] | Eli Lilly | NCT04647487 | I | Neoadjuvant, window | LY3484356 | Recruiting | 60 | Change in ER, PK, Ki67, PR expression | Pending |
| Giredestrant (GDC-9545) [93] | Roche | NCT04961996 | III | Adjuvant therapy, medium- and high-risk Stage I-III | Giredestrant vs. Physician's choice endocrine therapy | Recruiting | 4100 (estimate) | IDFS, OS, DRFS | Pending |
| Amcenestrant (SAR439859) [96] | Sanofi | NCT05128773 | III | Adjuvant early ER+ breast cancer, discontinued adjuvant AI therapy due to treatment related toxicity | Amcenestrant vs. Tamoxifen | Not yet recruiting | 3738 (estimate) | IBCFS, IDFS, OS, DRFS | Pending |
| PARP-inhibitors | |||||||||
| Olaparib [64] | AstraZeneca | NCT02032823 | III | HER2-negative early breast cancer; BRCA1 or BRCA2 germline pathogenic or likely pathogenic variants; high risk clinical features | Olaparib vs. Placebo | Complete | 1836 | IDFS, DDFS, OS |
ITT: IDFS: HR = 0.58 (99.5%CI, 0.41–0.82) P < 0.001 DDFS: HR = 0.57 (99.5%CI, 0.39–0.83) P < 0.001 OS: HR = 0.68 (99%CI, 0.44–1.05) P = 0.02 HR+/HER2-negative: IDFS: HR = 0.70 (95%CI: 0.38–1.27) |
| Talazoparib [65] | Pfizer | NCT03499353 | II | HER2-negative, gBRCA-positive | Talazoparib | Complete | 20 (19 results; 5 HR+) | RCB |
RCB-0: 53% RCB-0/I: 63%. |
| Niraparib [90] | GlaxoSmithKline | NCT04915755 | III | TNBC, irrespective of BRCA status, HR+/HER2-negative with documented tBRCA mutation | Niraparib | Recruiting | 800 | DFS, OS | Pending |
Past and Future Studies Investigating mTOR-inhibitors, PI3Kα-inhibitors, oral SERDs, and PARP-inhibitors in early ER+ Breast Cancer. Summary of trials of mTOR, PI3Kα, SERDs, and PARP-inhibitors in early breast cancer in both neoadjuvant and adjuvant settings. DFS=disease-free survival, OS=overall survival, DMFS=distant metastasis-free survival, PR=progesterone receptor, pCR=pathologic complete response, PEPI=preoperative estrogen prognostic index, IDFS=invasive disease free survival, IBCFS=invasive breast cancer-free survival, DRFS=distant recurrence-free survival, ORR=overall response rate, mRECIST=Modified Response Evaluation Criteria in Solid Tumors, DDFS=distant disease-free survival, RCB=residual cancer burden, CCCA=complete cell cycle arrest, AI=aromatase inhibitor.
In the time since these studies were conducted, the management of everolimus-associated toxicities has improved [32]. It is conceivable that even with failed trials in relatively unselected patients, the identification of strong predictive biomarkers of everolimus benefit could provide an opportunity to revisit this strategy - particularly in patients at the highest risk of recurrence. Correlative studies of these trials, as well as ongoing basic investigation may yield future insights that could be applied towards this goal. While other agents targeting the mTOR complex have been developed, including ATP-competitive mTORC1/mTORC2 and dual PI3K/mTOR inhibitors, these have been met with limited success in advanced disease and thus there is presently no obvious path to application in early disease [[33], [34], [35]].
3. PI3K and AKT inhibitors
As with mTOR, there has been significant interest and major research investment over the last quarter century in targeting upstream signalling via inhibition of phosphoinositide 3-kinase (PI3K). Aberrantly activated PI3K initiates downstream signalling to activate AKT and mTOR, causing cancer cell growth and proliferation (Fig. 1) [36]. Activating oncogenic mutations in PIK3CA, encoding the PI3Kα subunit, are the most common somatic alteration in ER+ breast cancer. Occurring in up to 40% of primary and metastatic tumors, this target has potential for broad clinical and therapeutic impact [[37], [38], [39]].
Recently, alpelisib, an oral selective inhibitor of PI3Kα was approved for metastatic PIK3CA-mutated ER+ breast cancer in combination with fulvestrant on the basis of the SOLAR-1 trial [40]. This study enrolled patients with or without PIK3CA alterations, but identified clinical activity specifically in the mutated population where the addition of alpelisib was associated with an improvement in progression free survival (HR = 0.65, 95%CI: 0.50–0.85; P < 0.001), vs. the non-PIK3CA cohort (HR = 0.85, 95%CI: 0.58–1.25) [40]. Alpelisib, like other PI3K-inhibitors, is associated with on-target adverse effects, including hyperglycemia, rash and diarrhea. Treatment discontinuation in SOLAR-1 was higher in the alpelisib group (25.0% vs. 4.2%), where grade 3 toxicity (hyperglycemia, diarrhea) were common [40]. In the follow-up analysis of overall survival (OS) in the PIK3CA-mutated cohort, there was a numerical but not statistically-significant improvement in the alpelisib arm, 39.3 mo. vs. 31.4 mo (HR = 0.86, 95%CI: 0.64–1.15; P = 0.15) [41]. The results of this trial reinforce the value of a predictive biomarker strategy, but also the need to have well-established protocols for toxicity management.
Alpelisib has also been evaluated in the neoadjuvant setting in combination with standard endocrine therapy (Table 1). The NEO-ORB study evaluated alpelisib + letrozole vs. letrozole alone in patients with ER+/HER2-negative, cT1c-T3, operable breast breast cancer with or without PIK3CA mutations [42]. Objective response rate, the primary outcome, was not improved in either the PIK3CA mutant (ORR alpelisib + letrozole = 43% vs. placebo + letrozole = 45%), or wild-type (ORR alpelisib + letrozole = 63% vs. placebo + letrozole = 61%) cohorts [42]. The pathologic complete response rate in all patients was low and similar in both treatment arms [42]. Treatment interruptions and discontinuations were very common in this study, which may have impacted the primary results. Owing primarily to alpelisib treatment-related adverse events, dose reductions and interruptions occured in 53% and 65% of participants, and only 52% of those in the alpelisib group completed the 24-week treatment period [42]. Therefore, it is unclear whether sufficient treatment was delivered to provide a meaningful assessment of alpelisib effects in the PIK3CA-mutant population. These findings have significant implications for the potential of this strategy as an adjuvant therapy, where longer durations of treatment would typically be evaluated. While management of alpelisib-related toxicities has improved as more experience with its use has accumulated, it seems likely that adherence in the adjuvant setting would be challenging, based on the common decrement in treatment persistence that is observed with routine endocrine therapy and in the example of everolimus (UNIRAD).
One possible avenue to improve the risk benefit of adjuvant therapies is to direct treatment towards a selected group of patients with an increased potential to benefit. Here, PI3K-inhibitors have the advantage of an established (for advanced disease) predictive biomarker: PIK3CA mutations. While this would permit the selection of the ∼⅓ of ER+ breast cancer with activating mutations, it is possible that a predictive biomarker could be further enriched. It has recently been shown that a subset of these cancers, which carry two mutations in PIK3CA on the same allele (“double mutants in cis”), may have enhanced sensitivity to PI3K-inhibition and demonstrate increased clinical response in the metastatic setting [36]. In a retrospective analysis of the Phase 3 trial of taselisib (whose clinical development has been discontinued), patients with double mutations in cis had a higher response rate than those with single mutations (ORR 30.2% vs. 18.1%) [36]. This finding provides another example of the need to characterize and refine predictive biomarkers in order to optimize patient selection. Particularly in the setting of early disease, focussing the development of targeted therapies on the critical sensitive population may justify treatment and permit maximization of strategies to maintain adherence. While no adjuvant trials of alpelisib have been announced, these insights may be relevant as new PI3K-inhibitors, such as GDC-0077 (inavolisib) which has increased selectivity for mutant p110α that may confer a greater therapeutic index, are developed [43,44].
A further consideration relevant to the use of predictive-biomarker guided therapies in early disease is the potential prognostic role of those biomarkers with standard adjuvant therapy, eg. whether they are associated with greater or lesser risk of recurrence with standard endocrine therapy (+/− chemotherapy). Some evidence suggests that patients with PIK3CA-mutations have a lower risk of distant recurrence (HR = 0.57, 95%CI: 0.38–0.85; P = 0.006) with standard therapy and increased benefit with letrozole vs. tamoxifen [45]. Further characterization of the potential contribution of PIK3CA mutations to existing clinico-pathologic/gene-expression risk models and their interaction with endocrine therapy is important to better understand how they may be exploited for adjuvant therapy.
Inhibitors of AKT are also in development for advanced ER+ breast cancer in combination with endocrine therapy, with positive results (PFS, HR = 0.58, 95%CI: 0.39–0.84) reported from the randomized phase 2 FAKTION trial, which evaluated capivasertib in combination with fulvestrant as second line endocrine therapy in the metastatic setting [46]. While the biomarker analysis (alterations in PI3K/AKT/PTEN) in FAKTION suggested that activity of capivasertib did not depend on presence of those alterations, smaller biomarker-selected studies have specifically evaluated the less common but relevant AKT-mutant and PTEN-mutant populations, and further evaluation in the larger registrational trials now underway is necessary [[47], [48], [49], [50], [51]]. While there are differences in the toxicity of AKT inhibitors compared to PI3K inhibitors, diarrhea and rash are common, with 41% of participants requiring a capivasertib dose reduction in FAKTION [46]. Further insights from these ongoing trials will help to clarify the potential for use of AKT inhibitors in early breast cancer.
4. Selective estrogen receptor degraders (SERDs)
Exploiting a mechanism of action distinct from tamoxifen (selective estrogen receptor modulation) and aromatase inhibitors (estrogen suppression), SERDs have an established role in the treatment of advanced ER+ breast cancer, and a new oral class of SERDs is the subject of intense clinical development. SERDs bind the estrogen receptor, causing degradation and subsequent inhibition of intracellular signalling (Fig. 1). Fulvestrant, the first and only currently available SERD, gained clinical approval in the early 2000s after demonstrating similar outcomes to anastrozole in advanced disease when dosed 250 mg per month. Superiority to aromatase inhibitors was subsequently demonstrated with an increased dose (500 mg) and fulvestrant is now a well-established and well-tolerated monotherapy and combination partner for targeted agents. A principal disadvantage of fulvestrant is the need for large volume intramuscular injection [[52], [53], [54]]. Despite this mode of administration, fulvestrant has recently been evaluated in early ER+ disease. The ALTERNATE trial evaluated fulvestrant alone or in combination with anastrozole vs. anastrozole alone in the neoadjuvant and adjuvant settings [55]. The initial results of the neoadjuvant phase have been recently reported, where similar outcomes: endocrine-sensitive disease rate (ESDR; mPEPI 0 + pCR) and reduction in post-treatment Ki67, were observed in all treatment arms [55]. Consistent with previous studies, pCR was uncommon [20,55]. This study also included an adjuvant phase, where fulvestrant was continued for up to 1.5 years; results have yet to be reported [55]. As development of AI resistance through the acquisition of ESR1 mutations conferring ligand-independent ER activation is well-recognized and increases with duration of AI exposure, and fulvestrant has activity in the setting of some ESR1 mutations, an advantage of SERDs like fulvestrant may be more likely to emerge in this setting [56,57].
Given the complex pharmacokinetics and formulation of fulvestrant, which requires monthly intramuscular injections, there has been significant interest in the development of more potent and bioavailable oral SERDs, with broader activity against ESR1 mutants. More than a dozen oral SERDs have entered the clinic, with multiple Phase 2 and 3 trials underway as monotherapy or in combinations for metastatic ER+ disease. Early phase monotherapy studies have demonstrated clinical activity, including in ESR1 mutant, AI- and fulvestrant-resistant disease and a generally tolerable safety profile [58]. Compared to existing endocrine strategies, oral SERDs have been reported to have some distinct side-effects including gastrointestinal toxicities, diarrhea, bradycardia, and visual disturbances [[58], [59], [60]]. Bradycardia, which has been observed with some but not all agents, has attracted specific attention, and the frequency and severity of oral SERD toxicity, as well as long term tolerability, will be important outcomes of large ongoing studies and of particular importance to adjuvant strategies.
The first clinical evidence for the use of oral SERDs in early ER+/HER2-negative breast cancer was presented recently at the ESMO 2021 congress. In this study, giredestrant was compared to anastrozole in the neoadjuvant setting in a two week window of opportunity (WOO) study with primary outcomes evaluating reduction in Ki67 expression at day 14; all patients will subsequently continue with the addition of palbociclib at standard dosing to their assigned endocrine therapy after completion of the WOO phase [61]. At a planned interim analysis (n = 83) following completion of the WOO phase (receiving only endocrine therapy), giredestrant resulted in a further reduction of Ki67 from baseline when compared to anastrozole (Table 1; giredestrant = 80%, 95%CI: 72%–85% vs. anastrozole = 67%, 95%CI: 56%–75%; p = 0.0222) [61]. Furthermore, the frequency of complete cell cycle arrest (CCCA; pts with Ki67 score ≤2.7%) was significantly higher in the giredestrant vs. anastrozole group with fewer treatment related adverse events noted with giredestrant (Table 1) [61].
Preoperative evaluation of the oral SERD amcenestrant is also planned as part of the I-SPY2 Endocrine Optimization Protocol (EOP), where it will be evaluated as monotherapy or in combination with other targeted therapies. Additionally, several large adjuvant trials comparing oral SERDs to standard endocrine therapy have been announced [[62], [63], [93]]. The rapid transition of these agents to early disease investigation, prior to any approvals for advanced cancer, reflects enthusiasm for their clinical potential. However, large adjuvant trials will take many years to read out, and there remains uncertainty at this time about predictors of benefit and long term tolerability. Studies in specific settings (eg. late recurrence, with resistance to AI) or incorporating real-time risk or predictive biomarkers (discussed below) may have relevance to accelerating or refining the development of adjuvant oral SERD strategies.
5. PARP-inhibitors
The recently-reported OlympiA trial, which compared 1 year of olaparib vs. placebo as adjuvant therapy has established a new treatment option for patients with high risk germline BRCA1/2-related early breast cancer [64]. While the majority of participants in OlympiA had triple negative breast cancer (TNBC, 82%), 325 patients with ER+ disease were included: in this subgroup, the primary endpoint (invasive disease free survival, iDFS) was numerically improved (Table 1, HR = 0.70, 95%CI: 0.38–1.27). Given the temporal pattern of recurrences expected for ER+ disease, outcomes for this population are relatively immature, and longer followup will be important to better characterize the treatment impact for ER+ disease. Importantly, and in contrast to its use in the metastatic setting, olaparib was combined with standard adjuvant endocrine therapy for the majority of ER+ participants (olaparib: 86.9%, placebo: 90.4%) [64]. There has been additional interest in the use of PARP inhibitors in early BRCA-related breast cancer (Table 1) [65], including an ongoing study of niraparib in patients identified by the presence of molecular residual disease/circulating tumor DNA [NCT04915755]. Integration of these advances for patients with BRCA-related ER+ disease with other emerging treatment strategies will be an important area for continued research.
6. Measurable residual disease to guide new adjuvant strategies
Alongside the development of new breast cancer therapies and the characterization of markers of risk and response, there has been rapid evolution of novel tools that could inform or transform current approaches for the investigation and delivery of adjuvant therapy. Multiple tissue-based genomic classifiers have been developed that are prognostic for early stage ER+ breast cancer treated with endocrine therapy, enabling risk stratification and informing treatment decision making, for both chemotherapy and endocrine therapy [1,[66], [67], [68]]. However, such assays performed at the time of diagnosis or surgery and without prior exposure to standard therapy are limited by the single biologic snapshot that they can provide. Dynamic measurements that reflect response or lack thereof to standard therapy, or tools to detect minimal or measurable residual disease (MRD) following surgery may serve to better stratify individual risk, or predict benefit of a given therapeutic strategy. For example, as dynamic changes in Ki67 (or the resulting PEPI score) following exposure to neoadjuvant aromatase inhibitors are associated with risk of recurrence, measurement of gene expression or other cellular changes that reflect adaptive response in endocrine unresponsive disease may inform the likelihood of benefit from an alternative strategy.
Liquid biopsy, which permits non-invasive and serial assessment of a variety of blood-based analytes (eg. circulating tumour cells, CTCs; circulating tumour DNA, ctDNA; cell free microRNA, cfmiR; exosomes) has emerged as a promising tool for biomarker development [[69], [70], [71], [72]]. The development of circulating tumor DNA applications is an area of intense clinical investigation [69], including in ER+ breast cancer. Detection of circulating tumor DNA using a variety of techniques following definitive therapy has been strongly associated with risk of subsequent recurrence in early stage breast cancer [[73], [74], [75]]. While additional study of ctDNA MRD is required to fully characterize its performance, prevalence, lead time to clinical recurrence, relationship with standard imaging (i.e. clinically occult but radiologically detectable metastatic disease), and the relative performance of various assays, the opportunity to target escalated strategies to individuals at extreme risk of recurrence in an “interception” approach is attractive. Furthermore, longitudinal serial measurement of ctDNA kinetics after initiation of standard of care adjuvant therapy may provide the opportunity to further refine treatment selection, distinguishing patients who may be benefiting from current adjuvant treatment from those who are not.
Whether patients with ER+ breast cancer exhibiting MRD are those whose outcomes could be improved with escalated adjuvant therapy remains to be determined - it is of course possible that this state reflects escaped disease that cannot be eradicated. However, these rapidly evolving tools provide an important opportunity to overcome the need for enormously resource-intensive adjuvant trials seeking benefit in a tiny fraction of participants. Several trials for MRD + breast cancer have recently been launched, and it is likely that the adoption of such investigational strategies will be brisk [76,77]. Additionally, retrospective analyses of adjuvant trials that banked plasma for MRD analyses will provide important insights to guide future work and contribute to the biological characterization of disease recurrence, while hopefully identifying subsets of patients who benefited most from the study intervention [78].
7. Therapy selection and duration
The optimal duration of adjuvant therapy for patients with early ER+ breast cancer continues to be an area of active investigation even with standard of care endocrine therapy [17,22], and the duration of adjuvant therapy evaluated in escalation trials has been defined empirically. Where most interventions have chronic or cumulative toxicities as well as financial costs that ultimately influence their risk/benefit profiles and clinical adoption, defining optimal durations or surrogate markers that could inform individual management are important goals. Given the various novel therapies that have either emerged or remain under investigation for high risk ER+ disease, refined strategies that incorporate prognostic and predictive biomarkers seem necessary to maximize cures while minimizing overtreatment. Tumor and tissue based markers, together with repeated longitudinal measures and assessment of biomarker kinetics during or following curative intent therapy may inform both judicious use of escalated therapy and careful development of de-escalation approaches.
8. Conclusion
Existing and emerging targeted therapies for ER+ breast cancer present opportunities to address the need for improved adjuvant strategies to reduce metastatic recurrence. It is clear from available clinical data that successful strategies for adjuvant treatment escalation must be safe, tolerable, and delivered to patients who are both at high risk of recurrence and with disease responsive to the intervention. Building on the success of predictors for chemo- and endocrine therapy, novel agents demand careful attention to biomarker development. Assays that incorporate response to standard endocrine therapy, and those that detect measurable residual disease in real time may enable a transformation of existing approaches to adjuvant therapy. Successful development of these strategies will require integrated basic, translational and clinical investigation to identify not only the patients who will benefit the most, but also biomarkers to inform emerging therapies. Future research must further delineate the biology of resistance to existing adjuvant therapies, and interrogate the relationships between clinical, pathologic, genomic and patient-related factors in high risk individuals to understand the role of current and future biomarkers and their potential utility. Thorough clinical characterization of new technologies is necessary to understand their operating characteristics and ability to guide adjuvant therapy delivery. The major interest and investment in these areas will undoubtedly facilitate a further transition to more personalized adjuvant therapy for early breast cancer, reshaping clinical trials and clinical care.
Declaration of competing interest
David Cescon was supported by the Canadian Cancer Society Research Institute (CCSRI), the Breast Cancer Research Foundation (BCRF), The Princess Margaret Cancer Foundation and the DH Gales Family Foundation. DWC reports consultancy and advisory fees from AstraZeneca, Exact Sciences, Eisai, Gilead, GlaxoSmithKline, Merck, Novartis, Pfizer and Roche; research funding (to institution) from GlaxoSmithKline, Inivata, Merck, Pfizer and Roche; is a member of a trial steering committee for Merck and GlaxoSmithKline; and holds a holds a patent (US62/675,228) for methods of treating cancers characterized by a high expression level of spindle and kinetochore associated complex subunit 3 (ska3) gene.
Footnotes
This article is published as part of a supplement supported by St. Gallen Oncology Conferences.
References
- 1.Sparano J.A., et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalinsky K., et al. Abstract GS3-00: first results from a phase III randomized clinical trial of standard adjuvant endocrine therapy (ET)/- chemotherapy (CT) in patients (pts) with 1-3 positive nodes, hormone receptor-positive (HR ) and HER2-negative (HER2-) breast cancer (BC) with recurrence score (RS) < 25: SWOG S1007 (RxPonder) General Session Abstracts. 2021 doi: 10.1158/1538-7445.sabcs20-gs3-00. [DOI] [Google Scholar]
- 3.Denduluri N., et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO guideline update. J Clin Oncol. 2021;39:685–693. doi: 10.1200/JCO.20.02510. [DOI] [PubMed] [Google Scholar]
- 4.Pan H., et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richard Gray and Early Breast Cancer Trialists’ Collaborative Group . 2018 san antonio breast cancer symposium GS3–03. 2018. Effects of prolonging adjuvant aromatase inhibitor therapy beyond five years on recurrence and cause-specific mortality: an EBCTCG meta-analysis of individual patient data from 12 randomised trials including 24,912 women. [Google Scholar]
- 6.Dowling R.J.O., et al. vol. 3. JNCI Cancer Spectrum; 2019. (Toronto workshop on late recurrence in estrogen receptor-positive breast cancer: Part 2: approaches to predict and identify late recurrence, research directions). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowling R.J.O., et al. vol. 3. JNCI Cancer Spectrum; 2019. (Toronto workshop on late recurrence in estrogen receptor–positive breast cancer: Part 1: late recurrence: current understanding, clinical considerations). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher B., et al. Tamoxifen for the prevention of breast cancer: current status of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 9.Powles T.J., Ashley S., Tidy A., Smith I.E., Dowsett M. vol. 99. JNCI Journal of the National Cancer Institute; 2007. pp. 283–290. (Twenty-year follow-up of the royal marsden randomized, double-blinded tamoxifen breast cancer prevention trial). [DOI] [PubMed] [Google Scholar]
- 10.Cuzick J., et al. First results from the international breast cancer intervention study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 11.Powles T., et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. 1998;352:98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]
- 12.Cuzick J., et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827–1834. doi: 10.1016/S0140-6736(13)60140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuzick J., Baum M. Tamoxifen and contralateral breast cancer. Lancet. 1985;2:282. doi: 10.1016/s0140-6736(85)90338-1. [DOI] [PubMed] [Google Scholar]
- 14.Orr J.D., Macdonald J.A., Thomson J.W. Tamoxifen in the palliative treatment of advanced breast cancer. A clinical review. J R Coll Surg Edinb. 1979;24:141–147. [PubMed] [Google Scholar]
- 15.Mouridsen H., et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the international letrozole breast cancer group. J Clin Oncol. 2001;19:2596–2606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 16.Bonneterre J., et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the tamoxifen or arimidex randomized group efficacy and tolerability study. J Clin Oncol. 2000;18:3748–3757. doi: 10.1200/JCO.2000.18.22.3748. [DOI] [PubMed] [Google Scholar]
- 17.Francis P.A., et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379:122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 19.Smith I.E., et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23:5108–5116. doi: 10.1200/JCO.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Spring L.M., et al. Neoadjuvant endocrine therapy for estrogen receptor–positive breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2:1477–1486. doi: 10.1001/jamaoncol.2016.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alba E., et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. 2012;23:3069–3074. doi: 10.1093/annonc/mds132. [DOI] [PubMed] [Google Scholar]
- 22.Gnant M., et al. Duration of adjuvant aromatase-inhibitor therapy in postmenopausal breast cancer. N Engl J Med. 2021;385:395–405. doi: 10.1056/NEJMoa2104162. [DOI] [PubMed] [Google Scholar]
- 23.Harbeck N., et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and ki-67 analysis from the monarchE study. Ann Oncol. 2021 doi: 10.1016/j.annonc.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Mossmann D., Park S., Hall M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018;18:744–757. doi: 10.1038/s41568-018-0074-8. [DOI] [PubMed] [Google Scholar]
- 25.Burstein H.J. Systemic therapy for estrogen receptor-positive, HER2-negative breast cancer. N Engl J Med. 2020;383:2557–2570. doi: 10.1056/NEJMra1307118. [DOI] [PubMed] [Google Scholar]
- 26.Ma C.X., Reinert T., Chmielewska I., Ellis M.J. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer. 2015;15:261–275. doi: 10.1038/nrc3920. [DOI] [PubMed] [Google Scholar]
- 27.Martin L.-A., et al. Enhanced estrogen receptor (ER) α, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem. 2003;278:30458–30468. doi: 10.1074/jbc.M305226200. [DOI] [PubMed] [Google Scholar]
- 28.Baselga J., et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yardley D.A., et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870–884. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachelot T., et al. Efficacy of everolimus in patients with HR+/HER2- high risk early stage breast cancer. Ann Oncol. 2021;32:574–575. [Google Scholar]
- 31.Chavez-MacGregor M., et al. Abstract OT1-03-11: phase III randomized, placebo-controlled clinical trial evaluating the use of adjuvant endocrine therapy/- one year of everolimus in patients with high-risk, hormone receptor (HR) positive and HER2-negative breast cancer (BC): SWOG/NRG/Alliance S1207 ( NCT01674140) Ongoing Clinical Trials. 2016 doi: 10.1158/1538-7445.sabcs15-ot1-03-11. [DOI] [Google Scholar]
- 32.Rugo H.S., et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol. 2017;18:654–662. doi: 10.1016/S1470-2045(17)30109-2. [DOI] [PubMed] [Google Scholar]
- 33.Koca E., et al. Open label, phase II trial of neoadjuvant TAK-228 plus tamoxifen in patients with estrogen receptor (ER)-positive, human epidermal growth factor receptor type 2 (HER2)-negative breast cancer-ANETT. J Clin Oncol. 2019;37 584–584. [Google Scholar]
- 34.Dowsett M., et al. 104P phase II randomized study of pre-operative pf-04691502 plus letrozole compared with letrozole (L) in patients with estrogen receptor-positive, her2-negative early breast cancer (bc) Ann Oncol. 2012;23:ii44. [Google Scholar]
- 35.Schmid P., et al. Fulvestrant plus vistusertib vs fulvestrant plus everolimus vs fulvestrant alone for women with hormone receptor-positive metastatic breast cancer: the MANTA phase 2 randomized clinical trial. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2019.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasan N., et al. Double PIK3CA mutations in cis increase oncogenicity and sensitivity to PI3Kα inhibitors. Science. 2019;366:714–723. doi: 10.1126/science.aaw9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isakoff S.J., et al. Breast cancer–associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 38.Zhao J.J., et al. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell. 2003;3:483–495. doi: 10.1016/s1535-6108(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 39.Razavi P., et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell. 2018;34:427–438. doi: 10.1016/j.ccell.2018.08.008. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.André F., et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 41.André F., et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol. 2021;32:208–217. doi: 10.1016/j.annonc.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Mayer I.A., et al. vol. 25. Clinical Cancer Research; 2019. pp. 2975–2987. (A phase II randomized study of neoadjuvant letrozole plus alpelisib for hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer (NEO-ORB)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juric D., et al. Abstract OT1-08-04: a first-in-human phase Ia dose escalation study of GDC-0077, a p110a-selective and mutant-degrading PI3K inhibitor, in patients with PIK3CA-mutant solid tumors. Cancer Res. 2020;80 OT1–08–04–OT1–08–04. [Google Scholar]
- 44.Turner N., et al. 355TiP Phase III study of GDC-0077 or placebo (pbo) with palbociclib (P) fulvestrant (F) in patients (pts) with PIK3CA-mutant/hormone receptor-positive/HER2-negative locally advanced or metastatic breast cancer (HR/HER2– LA/MBC) Ann Oncol. 2020;31:S391–S392. [Google Scholar]
- 45.Luen S.J., et al. Association of somatic driver alterations with prognosis in postmenopausal, hormone receptor--positive, HER2-negative early breast cancer: a secondary analysis of the BIG 1-98 randomized clinical trial. JAMA oncology. 2018;4:1335–1343. doi: 10.1001/jamaoncol.2018.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones R.H., et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020;21:345–357. doi: 10.1016/S1470-2045(19)30817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nct A. Double-Blind placebo controlled randomized phase III trial of fulvestrant and ipatasertib as treatment for advanced HER-2 negative and estrogen receptor positive (ER+) breast cancer following progression on first line CDK 4/6 inhibitor and aromatase inhibitor (FINER) ( NCT04650581) Canadian Clinical Trials Group (CCTG) 2016 https://www.ctg.queensu.ca/public/all-disease-sites [Google Scholar]
- 48.Turner N., et al. A phase III trial of capivasertib and fulvestrant versus placebo and fulvestrant in patients with HR+/HER2- breast cancer (CAPItello-291) Ann Oncol. 2020;31:S388–S389. [Google Scholar]
- 49.Smyth L.M., et al. Selective AKT kinase inhibitor capivasertib in combination with fulvestrant in PTEN-mutant ER-positive metastatic breast cancer. NPJ Breast Cancer. 2021;7:44. doi: 10.1038/s41523-021-00251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalinsky K., et al. Effect of capivasertib in patients with an AKT1 E17K-mutated tumor: NCI-match subprotocol EAY131-Y nonrandomized trial. JAMA Oncol. 2021;7:271–278. doi: 10.1001/jamaoncol.2020.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner N.C., et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020;21:1296–1308. doi: 10.1016/S1470-2045(20)30444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howell A., et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 53.Osborne C.K., et al. Double-Blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20:3386–3395. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 54.Robertson J.F.R., et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388:2997–3005. doi: 10.1016/S0140-6736(16)32389-3. [DOI] [PubMed] [Google Scholar]
- 55.Ma C.X., et al. ALTERNATE: neoadjuvant endocrine treatment (NET) approaches for clinical stage II or III estrogen receptor-positive HER2-negative breast cancer (ER HER2- BC) in postmenopausal (PM) women: alliance A011106. J Clin Oncol. 2020;38 504–504. [Google Scholar]
- 56.Fribbens C., et al. Plasma ESR1 mutations and the treatment of estrogen receptor–positive advanced breast cancer. J Clin Oncol. 2016;34:2961–2968. doi: 10.1200/JCO.2016.67.3061. [DOI] [PubMed] [Google Scholar]
- 57.Turner N.C., et al. ESR1 mutations and overall survival on fulvestrant versus exemestane in advanced hormone receptor–positive breast cancer: a combined analysis of the phase III SoFEA and efect trials. Clin Cancer Res. 2020;26:5172–5177. doi: 10.1158/1078-0432.CCR-20-0224. [DOI] [PubMed] [Google Scholar]
- 58.Bardia A., et al. Phase I study of elacestrant (RAD1901), a novel selective estrogen receptor degrader, in ER-positive, HER2-negative advanced breast cancer. J Clin Oncol. 2021;JCO2002272 doi: 10.1200/JCO.20.02272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim E., et al. A phase Ib study to evaluate the oral selective estrogen receptor degrader GDC-9545 alone or combined with palbociclib in metastatic ER-positive HER2-negative breast cancer. J Clin Oncol. 2020;38 1023–1023. [Google Scholar]
- 60.Hamilton E.P., et al. A phase I dose escalation and expansion study of the next generation oral SERD AZD9833 in women with ER-positive, HER2-negative advanced breast cancer. J Clin Onc. 2020;38 doi: 10.1016/j.annonc.2024.04.012. 1024–1024. [DOI] [PubMed] [Google Scholar]
- 61.Hurvitz S.A., et al. LBA14 Neoadjuvant giredestrant (GDC-9545)+ palbociclib (palbo) vs anastrozole (A)+ palbo in post-menopausal women with oestrogen receptor-positive, HER2-negative, untreated early breast cancer (ER+/HER2--eBC): interim analysis of the randomised, open-label, phase II coopERA BC study. Ann Oncol. 2021;32:S1285–S1286. [Google Scholar]
- 62.Campone M., et al. Phase II preoperative window study of SAR439859 versus letrozole in post-menopausal women with newly diagnosed estrogen receptor-positive (ER )/human epidermal growth factor receptor 2-negative (HER2-) breast cancer. J Clin Oncol. 2020;38 TPS1108–TPS1108. [Google Scholar]
- 63.Myshko, D. Trial explores preoperative window for amcenestrant therapy in early breast cancer.
- 64.Tutt A.N.J., et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Litton J.K., et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. J Clin Oncol. 2020;38:388–394. doi: 10.1200/JCO.19.01304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paik S., et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 67.Cardoso F., et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 68.Gluz O., et al. West German study group phase III PlanB trial: first prospective outcome data for the 21-gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol. 2016;34:2341–2349. doi: 10.1200/JCO.2015.63.5383. [DOI] [PubMed] [Google Scholar]
- 69.Cescon D.W., Bratman S.V., Chan S.M., Siu L.L. Circulating tumor DNA and liquid biopsy in oncology. Nat Can. 2020;1:276–290. doi: 10.1038/s43018-020-0043-5. [DOI] [PubMed] [Google Scholar]
- 70.Lim S.B., Di Lee W., Vasudevan J., Lim W.-T., Lim C.T. Liquid biopsy: one cell at a time. NPJ Precis Oncol. 2019;3:23. doi: 10.1038/s41698-019-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ignatiadis M., Sledge G.W., Jeffrey S.S. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18:297–312. doi: 10.1038/s41571-020-00457-x. [DOI] [PubMed] [Google Scholar]
- 72.Zhou B., et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduction and Targeted Therapy. 2020;5 doi: 10.1038/s41392-020-00258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magbanua M.J.M., et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol. 2021;32:229–239. doi: 10.1016/j.annonc.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cavallone L., et al. Prognostic and predictive value of circulating tumor DNA during neoadjuvant chemotherapy for triple negative breast cancer. Sci Rep. 2020;10:14704. doi: 10.1038/s41598-020-71236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cullinane C., et al. Association of circulating tumor DNA with disease-free survival in breast cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.26921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.ClinicalTrials. gov Criterium, inc. DNA-guided second line adjuvant therapy for high residual risk, stage II-III, hormone receptor positive, HER2 negative breast cancer (DARE) ClinicalTrials.gov. 2021 https://clinicaltrials.gov/ct2/show/NCT04567420 [Google Scholar]
- 77.ClinicalTrials. gov National institute for health research biomedical research centre at the royal marsden/institute of cancer research UK merck sharp & dohme corp. A trial using ctDNA blood tests to detect cancer cells after standard treatment to trigger additional treatment in early stage triple negative breast cancer patients (c-TRAK-TN) ClinicalTrials.gov. 2020 https://clinicaltrials.gov/ct2/show/NCT03145961 [Google Scholar]
- 78.Powles T., et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595:432–437. doi: 10.1038/s41586-021-03642-9. [DOI] [PubMed] [Google Scholar]
- 79.Baselga J., et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor--positive breast cancer. J Clin Oncol. 2009;27:2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 80.Wu W., et al. Neoadjuvant everolimus plus letrozole versus fluorouracil, epirubicin and cyclophosphamide for ER-positive, HER2-negative breast cancer: a randomized pilot trial. BMC Cancer. 2021;21:862. doi: 10.1186/s12885-021-08612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chavez-Mac Gregor M., et al. Phase III randomized, placebo-controlled clinical trial evaluating the use of adjuvant endocrine therapy +/- one year of everolimus in patients with high-risk, hormone receptor (HR) positive and HER2-negative breast cancer (BC): SWOG/NRG/Alliance S1207 ( NCT01674140) J Clin Onc. 2015;33 TPS637–TPS637. [Google Scholar]
- 83.Saura C., et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen receptor-positive, HER2-negative, early-stage breast cancer (LORELEI): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2019;20:1226–1238. doi: 10.1016/S1470-2045(19)30334-1. [DOI] [PubMed] [Google Scholar]
- 84.A Study Evaluating the Efficacy, Safety, and Pharmacokinetics of Giredestrant Plus Palbociclib Compared With Anastrozole Plus Palbociclib for Postmenopausal Women With Estrogen Receptor-Positive and HER2-Negative Untreated Early Breast Cancer (coopERA Breast Cancer). https://clinicaltrials.gov/ct2/show/NCT04436744.
- 85.Robertson J.F.R., et al. A Randomized, Open-label, Presurgical, Window-of-Opportunity Study Comparing the Pharmacodynamic Effects of the Novel Oral SERD AZD9496 with Fulvestrant in Patients with Newly Diagnosed ER+ HER2- Primary Breast Cancer. Clin. Cancer Res. 2020;26:4242–4249. doi: 10.1158/1078-0432.CCR-19-3387. [DOI] [PubMed] [Google Scholar]
- 86.Moore H.M., et al. Evaluation of pharmacodynamic (PD) and biologic activity in a preoperative window-of-opportunity (WOO) study of giredestrant (GDC-9545) in postmenopausal patients (pts) with estrogen receptor-positive, HER2-negative (ER/HER2–) operable breast cancer (BC) J Clin Oncol. 2021;39 577–577. [Google Scholar]
- 87.Campone M., et al. Phase II preoperative window study of SAR439859 versus letrozole in post-menopausal women with newly diagnosed estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2-) breast cancer. J Clin Onc. 2020;38 TPS1108–TPS1108. [Google Scholar]
- 88.A Study to Investigate the Biological Effects of AZD9833 in Women With ER-positive, HER2 Negative Primary Breast Cancer - Full Text View - ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT04588298.
- 89.A Study of LY3484356 in Women With Breast Cancer Before Having Surgery - Full Text View - ClinicalTrials.Gov. https://clinicaltrials.gov/ct2/show/NCT04647487.
- 90.Efficacy and Safety Comparison of Niraparib to Placebo in Participants With Either Human Epidermal Growth Factor 2 Negative (HER2-) Breast Cancer Susceptibility Gene Mutation (BRCAmut) or Triple-Negative Breast Cancer (TNBC) With Molecular Disease - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04915755.
- 93.A Study Evaluating the Efficacy and Safety of Adjuvant Giredestrant Compared With Physician’s Choice of Adjuvant Endocrine Monotherapy in Participants With Estrogen Receptor-Positive, HER2-Negative Early Breast Cancer (lidERA Breast Cancer) ClinicalTrials.gov. 2021 https://clinicaltrials.gov/ct2/show/NCT04961996 [Google Scholar]
- 96.2021. Study of Amcenestrant (SAR439859) Versus Tamoxifen for Patients With Hormone Receptor-positive (HR+) Early Breast Cancer, Who Have Discontinued Adjuvant Aromatase Inhibitor Therapy Due to Treatment-related Toxicity (AMEERA-6)