Abstract
l-fucose is a dietary sugar that is used by cells in a process called fucosylation to post-translationally modify and regulate protein behavior and function. As fucosylation plays essential cellular functions in normal organ and immune developmental and homeostasis, it is perhaps not surprising that it has been found to be perturbed in a number of pathophysiological contexts, including cancer. Increasing studies over the years have highlighted key roles that altered fucosylation can play in cancer cell-intrinsic as well as paracrine signaling and interactions. In particular, studies have demonstrated that fucosylation impact tumor:immunological interactions and significantly enhance or attenuate antitumor immunity. Importantly, fucosylation appears to be a posttranslational modification that can be therapeutically targeted, as manipulating the molecular underpinnings of fucosylation has been shown to be sufficient to impair or block tumor progression and to modulate antitumor immunity. Moreover, the fucosylation of anticancer agents, such as therapeutic antibodies, has been shown to critically impact their efficacy. In this review, we summarize the underappreciated roles that fucosylation plays in cancer and immune cells, as well as the fucosylation of therapeutic antibodies or the manipulation of fucosylation and their implications as new therapeutic modalities for cancer.
Keywords: cancer, fucosylation, immunotherapy, l-fucose, tumor immunology
1 ∣. INTRODUCTION
The monosaccharide l-fucose (also known as 6-deoxy-l-galactose) is a dietary sugar that can play important roles in cellular biology. Although similar to hexose sugars such as glucose, l-fucose is structurally distinct in its l-configuration and lack of a hydroxyl group on the carbon at the 6-position (C-6). Also distinct is the way in which it is used by cells as a substrate that is conjugated as a posttranslational modifier onto proteoglycans (proteins subject to glycosylation, also known as glycoproteins). This process, called fucosylation, can regulate crucial aspects of protein behavior and function, and thus, cellular biology. Although l-fucose and fucosylation have been established to be essential for normal immune and organ development processes, precise underlying molecular mechanisms have largely not been delineated. Increasing numbers of studies have begun to elucidate and highlight fucosylation-regulated mechanisms that govern key aspects of pathogenic biology in cancer. In particular, numerous emerging studies highlight important pathophysiological roles and functions of l-fucose and fucosylation in tumor immunology. Importantly, unlike other posttranslational modifications (e.g., phosphorylation), the systemic augmentation or suppression of fucosylation using dietary l-fucose or small molecule inhibitors, respectively, has been shown to yield significant therapeutic potential. However, the precise extents of antitumor (or protumor) effects elicited by modulation of fucosylation appear to be tumor context-specific and remain to be explored and refined. Here, we review important findings regarding the roles of fucosylation in tumor biology and tumor immunology and highlight therapeutic insights and potential opportunities for much needed honing and development of fucosylation-centered modalities.
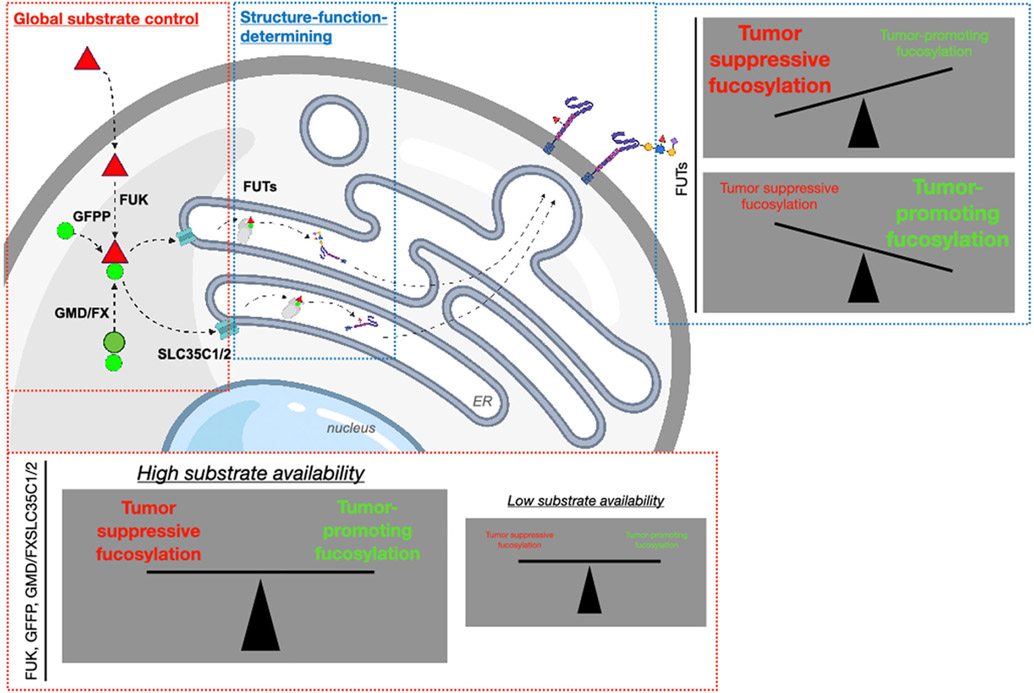
2 ∣. FUCOSYLATION: REGULATION OF SUBSTRATE AVAILABILITY AND STRUCTURE-FUNCTION
The process by which cells uptake, guanidine diphosphate (GDP)-couple, and conjugate l-fucose as a posttranslational modifier of proteins is called fucosylation. This process is regulated at the substrate availability and downstream structure–function levels (Figure 1).
FIGURE 1.
l-fucose (red triangle) uptaken into cells is phosphorylated by FUK and GDP-coupled (light green circle) by GFPP, yielding GDP-fucose, which is transported into the ER/Golgi via SLC35C1/2 transporters, where it is conjugated onto proteins by 13 ER/Golgi-resident fucosyltransferases (FUTs). GDP-fucose can also be supplied in the cell by conversion from GDP-mannose (dark green circle) by the GMD/FX enzymes. FUK, GFPP, and SLC35C1/2 regulate global GDP-fucose availability (i.e., functional amplitude of fucosylation), whereas the FUTs are structure–function determining, and in the context of tumors, delineate tumor-promoting versus tumor-suppressing functions. ER, endoplasmic reticulum; FUK, fucokinase; FS, GDP-4-keto-6-deoxymannose-3,5-epimerase-4-reductase; GDP, guanidine diphosphate; GFPP, GDP-fucose-pyrophosphorylase, GMD, GDP-mannose 4,6-dehydratase. The figure was created with BioRender.com
The first step of fucosylation comprises the sourcing of cellular l-fucose, from either endogenous or exogenous sources. Endogenous free l-fucose is derived from intracellular recycling via hydrolysis and liberation during the recycling of proteoglycans in lysosomes.1,2 In contrast, exogenous (dietary) l-fucose is found in a variety of plants. It has been detected in the cell walls of potato (Solanum tuberosum),3 cassava tuber (Manihot utilissima),4 kiwi (Actinidia arguta),5 and Thale cress (Arabidopsis thaliana)6; in seeds including soybeans (Glycine max),7 Winged beans (Psophocarpus tetragonolobus),8 and Rapeseed (Brassica napus)9 in mucilage of young leaves and stems of “Junsai” (Brasenia schreberi).10 Notably, l-fucose has been reported to be enriched at significantly higher amounts in seaweed in the form of sulfated l-fucose polymers known as fucoidan. Fucoidan, which has been subject to extensive commercialization as a nutraceutical supplement for an array of potential health-promoting purposes,11 is found in the intercellular mucilage of several species of seaweed. Seaweeds that have been used as sources of fucoidan have included Ecklonia kurome,12-14 Laminaria angustata var longissimi,15 Fucus vesiculosus,16 Kjellmaniella crassifolia,17 and Fucus serratus.18
Regardless of the source, l-fucose must be phosphorylated and then coupled to GDP, producing GDP-fucose, for subsequent conjugation onto proteins. Intracellular levels of GDP-fucose are maintained by two distinct pathways, the salvage and the de novo synthetic pathways. Via the salvage pathway, free l-fucose in the cytosol is phosphorylated by fucokinase (FUK), and the intermediate, fucose-1-phosphate, is further converted to GDP-fucose by GDP-fucose-pyrophosphorylase (GFPP).19,20 The salvage pathway is responsible for compensating and replenishing GDP-fucose to sustain physiological functions when the de novo pathway is defective.21
The de novo synthetic pathway is a highly conserved process by which intracellular GDP-mannose is converted into GDP-fucose (representing another endogenous source of l-fucose-derived substrate). In the cytoplasm, GDP-fucose is converted from GDP-mannose by GDP-mannose 4,6-dehydratase (GMD) and GDP-4-keto-6-deoxymannose-3,5-epimerase-4-reductase (FX) sequentially.22,23 This mechanism has been reported to produce up to 80%–90% of cellular GDP-fucose under “normal” (i.e., nondisease/pathological) circumstances,24,25 although the relative contribution of either de novo or salvage pathways to total intracellular GDP-fucose levels likely varies by pathophysiological and cell type-specific context. Together, by regulating fucosylation at the substrate level, the de novo synthetic and the salvage pathways regulate the functional amplitude of cellular fucosylation.
Once GDP-fucose is transported into the lumen of Golgi apparatus and endoplasmic reticulum (ER) via the SLC35C1 and SLC35C2 transporters,26 it serves as a substrate that is conjugated onto proteins on N-glycans by 11 fucosyltransferases (FUTs) in the Golgi27,28 and/or on O-glycans by two protein O-fucosyltransferases (POFUTs) in the ER.29,30 The 13 different FUTs catalyze a diverse range of fucose-conjugated structures. There are two well-established types of N-linked fucosylated structures, core fucosylation (α-1,6-linkage) catalyzed by FUT8 and terminal fucosylation (α-1,2/3/4-linkage) catalyzed by the other FUTs (FUT1–7 and FUT9–11), among which FUT1 and FUT2 are responsible for the synthesis of α-1,2 linkages, while FUT3–7/9–11 are involved in the generation of α-1,3/4 linkages.27,31,32 The diverse topologies of fucosylation are structure–function-regulating and therefore can result in diverse functional consequences. In cancer, for example, the profile of FUT expression delineates whether fucosylation elicits tumor-promoting or tumor-suppressing effects.
3 ∣. ABERRANT FUCOSYLATION IN CANCER
Protein fucosylation is crucial for maintaining the “normal” cell and tissue physiology and functions, including, for example, cellular adhesion, organ and immune development, and fertilization (Table 1). Suppression of global substrate-level fucosylation in mice (by FX knockout, which abrogates the de novo synthetic pathway) results in severe neutrophilia, myeloproliferation, and the absence of leukocyte selectin ligand expression.23 In melanoma, the downregulation of FUK also significantly enhances motility while altering tumor immunological profiles.33 Aberrant fucosylation resulting from abnormal or pathologically deregulated expression of specific FUTs has been reported to result in several pathological processes, such as inflammatory conditions, malignancy, and tumor metastasis (Table 1). In the context of cancer, our understanding of the specific mechanistic roles of FUTs has been generally limited, although increasing studies showcase the wide range of contributions that FUTs make to the pathogenesis of cancer.
TABLE 1.
Roles of fucosylation in normal cellular/organ development and biology versus cancer
| Organ/tissue | Normal cell/organ biology | FUTs | References (PMID) |
Cancer | FUTs | References (PMID) |
|---|---|---|---|---|---|---|
| Peripheral blood | FUT4 | 31097000 | ||||
| 24838610 | POFUT1 | 30344988 | ||||
| Leukocyte trafficking and homing | FUT1/2 | 8752218 | Myeloid leukemia | 20645416 | ||
| FUT4/7 | 8822932 | FUT8 | 27967290 | |||
| 11485743 | Lymphoma | - | 23493549 | |||
| MM | - | 30844485 | ||||
| Breast | - | - | - | Breast cancer | FUT1 | 27560716 |
| 27375041 | ||||||
| FUT4 | 23887626 | |||||
| 26701615 | ||||||
| 28692034 | ||||||
| FUT8 | 28982386 | |||||
| Lung | Pulmonary development | FUT8 | 16236725 | NSCLC | FUT4 | 32629386 |
| 31104223 | ||||||
| FUT7 | 28860805 | |||||
| FUT8 | 26443198 | |||||
| 23267084 | ||||||
| Liver | Hepatic development | FUT2 | 28056490 | Hepatocellular carcinoma | FUT4/6/8 | 24232099 |
| FUT7 | 11935309 | |||||
| 15666088 | ||||||
| FUT8 | 23352314 | |||||
| Brain | Synapse formation and neurite outgrowth | FUT1/2 | 15686343 | Glioblastoma | 29669750 | |
| FUT8 | 25727145 | |||||
| Gastrointestinal system | 33323540 | |||||
| Host–microbe interactions | FUT2 | 17673542 | Gastric cancer | FUT3/5 | 21978830 | |
| Gut microbiome maintaining | FUT3 | 25263220 | FUT8 | 24732908 | ||
| 25274297 | Colorectal cancer | FUT1 | 17459061 | |||
| 28988527 | FUT3/5/6 | 24253505 | ||||
| 28771224 | ||||||
| POFUT1 | 30250219 | |||||
| 9000578 | ||||||
| Skin | Keratinocyte migration (differentiation and epidermis renewal) | FUT1 | 23625752 | Melanoma | FUT1 | 29924834 |
| 17897065 | ||||||
| FUT4 | 25672851 | |||||
| 31961483 | ||||||
| FUT8 | 28609658 | |||||
| Prostate | Origination of Lewis/Core-type Fuco-PSAs | FUT3/8 | 27494861 | Prostate cancer | FUT3/6/7 | 24178760 |
| FUT8 | 24906821 | |||||
| Pancreatic | Lewis b, Lewis y, and H-type antigens | FUT1/2 | 10728712 | Pancreatic cancer | FUT1 | 10728712 |
| FUT3 | 11058871 | |||||
| 29963165 | ||||||
| Oral/head and neck | - | - | - | Oral squamous cell carcinoma | FUT1 | 23242548 |
| FUT3/6 | 25428916 | |||||
| Ovary | 29130097 | |||||
| FUT1 | 20003467 | |||||
| - | - | - | Ovarian cancer | 27240592 | ||
| FUT8 | 11093814 |
Abbreviations: FUT, fucosyltransferase; MM, multiple myeloma; NSCLC, non-small cell lung cancer; POFUT, protein O-fucosyltransferase; PSA, prostate-specific antigen.
The most extensively characterized aberration in FUT function in cancer is FUT8-mediated core fucosylation. FUT8 is frequently upregulated in human tumors and is associated with worse clinical outcomes in cancer patients.34,35 The crucial role of FUT8 in tumorigenesis has been clearly demonstrated in studies of breast, liver, lung cancer, and melanoma showing that depletion of FUT8 effectively blocks tumor growth and metastasis.36-39 Based on its protumorigenic function, extensive studies have highlighted corefucosylated proteins, suggesting their clinical utility as potential diagnostic and prognostic biomarkers in a wide range of cancer types (Table 2). In addition to core fucosylation, increasing studies report that α-1,3/4 fucosylation branching catalyzed by FUTs 3–7 and 9–11 is also upregulated in tumors, eliciting tumor-promoting functions when further assessed in cell-based assays and in vivo models (Table 1). Specifically, Lewis antigen structures comprising α-1,2/3/4-linkage fucosylation, which are known to decorate tumor cell membrane proteins, have been demonstrated to promote cellular adhesion and extravasation in the process of metastasis through binding to E/P-selectin expressed on endothelial cells.40 Consistent with this notion, FUTs that mediate this type of fucosylation topology, such as FUT4, are altered in cancers and promote tumorigenic events such as epithelial–mesenchymal transition.41 Conversely, α-1,2 terminal fucosylation mediated by FUT1–2 was reported to exhibit tumor-suppressive effects in melanoma, oral/head and neck, gastric, and hepatocellular carcinoma, whereas it promotes tumor progression in bladder, breast, epidermoid, ovarian, and prostate tumors. Our understanding of how FUTs regulate cancer development and progression is an actively growing area, it is becoming increasingly clear that their contributions are determined by specific expression profiles in different cancer types, which will dictate the functional balance of tumor-promoting versus tumor-suppressing forms of fucosylation.
TABLE 2.
Fucosylated glycoproteins as diagnostic/prognostic markers in cancer
| Fucosylated biomarker |
Type of cancer | FUTs | References (PMID) |
|---|---|---|---|
| EGFR | Lung cancer | FUT8 | 18754874 |
| 21709263 | |||
| PSA | Prostate cancer | FUT8 | 20861084 |
| 17432893 | |||
| AGP | Lung cancer | FUT6 | 31601857 |
| Esophagus, stomach, lung, breast, liver, pancreas, colon and rectum carcinomas | α(1,3) linkage | 23509786 | |
| 27295180 | |||
| 15536618 | |||
| AFP | Hepatocellular carcinoma | FUT8 | 2415789 |
| 31451706 | |||
| 24899827 | |||
| A1AT | Lung cancer | FUT8 | 25347993 |
| Hepatocellular carcinoma | α(1,2/3/4) linkage | 20811639 | |
| Ovarian cancer | 3265332 | ||
| HP | Pancreatic cancer | FUT8 | 16385567 |
| α(1,3/4) linkage | 18646007 | ||
| Colorectal Cancer | 22006099 | ||
| Gastric cancer | 28052004 | ||
| NSCLC | 24018448 | ||
| Prostate cancer | 24802742 | ||
| Ovarian cancer | 1511415 | ||
| 1451094 | |||
| Breast cancer | 18218651 | ||
| Hepatocellular carcinoma | 24807840 | ||
| 26503433 | |||
| PON1 | Hepatocellular carcinoma | FUT8 | 25702281 |
| 22751611 | |||
| SCLC | 24085812 | ||
| 30619732 | |||
| GM1 | SCLC | FUT1/2 | 16880505 |
| 9334808 | |||
| E-cadherin | Lung cancer | FUT8 | 22634079 |
| Colorectal Cancer | 19302290 | ||
| CD147 | OSCC | FUT8 | 23078850 |
| ESCC | 32474852 | ||
| CA19-9 | Pancreatic cancer | FUT3 | 31221853 |
| CP | Hepatocellular carcinoma | FUT8 | 24799124 |
| Pancreatic cancer | Sialyl-Lewis X | 25595436 | |
| Fetuin-A | Cholangiocarcinoma | α(1,3/6) linkage | 28561948 |
| L1CAM | Melanoma | FUT8 | 28609658 |
Abbreviations: A1AT, α-1-antitrypsin; AFP, α-fetoprotein; AGP, α1-acid glycoprotein; CP, ceruloplasmin; EGFR, epidermal growth factor receptor; ESCC, esophageal squamous cell carcinoma; FUTs, fucosyltransferases; GM1, monosialotetrahexosylganglioside; HP, haptoglobin; L1CAM, L1 cell adhesion molecule; NSCLC, non-small cell lung cancer; OSCC, oral squamous cell carcinoma; PON, paraoxonase 1; PSA, prostate-specific antigen; SCLC, small cell lung cancer.
4 ∣. ROLES OF FUCOSYLATION AT THE TUMOR:IMMUNE INTERFACE
Importantly, whereas studies on the roles of FUTs and fucosylation in cancer have largely focused on tumor cell autonomous signaling, fucosylation plays key functional roles in other cells within the tumor microenvironment—particularly immune cells—that are significant determinants of tumor progression and therapeutic responses. Immunotherapies are among the most effective emerging treatment modalities for many tumor types. However, across all cancers, their efficacy is hampered by a number of underlying reasons ranging from the paucity of tumor-infiltrating immune cells to the lack of target protein expression to immunosuppressed tumor microenvironment. These therapeutic hurdles are areas of active study aimed at increasing immunotherapy efficacy. Fucosylation can mechanistically regulate aspects of immune cell biology that are highly relevant to the mechanisms underlying multiple types of immunotherapies. However, this remains an emerging area and our increased understanding of how fucosylation regulates tumor:immune interactions and immunology is expected to help delineate how fucosylation might itself be leveraged as a therapeutic approach to help improve immunotherapeutic efficacy and clinical outcomes.
Interactions between tumor and immune cells are fundamentally governed by the maturation and differentiation of individual immune cell types—processes that are stringently orchestrated to ensure specific, timely, and durable innate and adaptive immune responses. To precisely control such aspects of immune cell biology, the cells implement diverse posttranslational modifications to rapidly and precisely establish spatiotemporal control of key proteins that can regulate and alter these processes. As stated above, one such modifier is the addition of l-fucose to proteins through the process of fucosylation. Unlike other posttranslational modifications including phosphorylation, ubiquitination, degradation, or proteolytic processing, which have been subject to extensive study in the context of tumor microenvironments and their potential impact on immunotherapy efficacy, fucosylation is another important form of posttranslational modification, the contributions of which have generally been underappreciated. Previous studies have highlighted a number of important roles that fucosylation plays in normal immune cell biology. These functions range from myeloid and lymphocyte development (e.g., thymic B- and T-cell maturation) to immune cell signaling and behaviors in several key immune cell lineages, demonstrating the broad regulatory impact of this modification in the immune system (Table 3).42
TABLE 3.
Roles of fucosylation in immune cell biology
| Immune cell lineage | Role of fucosylation | FUTs | References (PMID) |
|---|---|---|---|
| Immature B cell | Mediates BCR development via u heavy chain fucosylation | FUT8 | 22084235 |
| 20622883 | |||
| Hematopoietic stem cell | Pushes development toward leukocyte lineages via fucosylation of Notch | POFUT1 | 15653671 |
| 15692013 | |||
| 11524432 | |||
| 21464368 | |||
| Mature B cell | Promotes signaling of BCR | POFUT1 | 22269039 |
| 17714731 | |||
| 1688813 | |||
| 16888140 | |||
| Monocyte | Adhesion and migration of monocytes to areas of infection | FUT1/2 | 24665114 |
| 10075584 | |||
| Macrophages | Shifts polarization towards M1 phenotype | FUT1/2 | 24838610 |
| Developing lymphocyte | Signals proper developmental patterns of T and B cells in the thymus. Suppresses B cells in the thymus | POFUT1 | 21464368 |
| Neutrophils | E- and P-selectin-mediated adhesion and extravasation | FUT1/2 | 31530991 |
| 11133780 |
Abbreviations: BCR, B-cell receptor; FUT, fucosyltransferase; POFUT, protein O-fucosyltransferase.
In addition to its contributions to normal immune cell development, the pathological deregulation of fucosylation is implemented in a number of immunological diseases. One well-established example is leukocyte adhesion deficiency ll (LAD-ll).43-46 Patients suffering from LAD-ll possess mutations in the GDP-fucose transporter gene SLC35C1, which results in the attenuation of cellular protein fucosylation rates.45,47 Although LAD-ll may manifest through several phenotypes, the leukocyte compartment is significantly impaired as these cells lose their ability to bind to vessel walls, leading to uncontrolled rolling of leukocytes and failure of leukocyte extravasation.48 Patients with LAD-ll present with chronic bacterial infections due to the lack of immune infiltration that would normally deal with such infections.49 Notably, the administration of l-fucose to children suffering from the disorder is sufficient to at least partially mitigate symptoms of the disease.50 Another example of a fucosylation-related immune disease is rheumatoid arthritis (RA), a pathology that is characterized by joint pain resulting from immune-mediated destruction of cartilage.50 Intriguingly, several studies have reported that RA patients generally exhibit reduced serum levels of l-fucose and fucosylated proteoglycans compared with healthy individuals, despite overexpression of all FUTs except FUT8 and FUT13 (also POFUT2), suggesting potentially altered substrate-level perturbations in fucosylation that play key roles in the generation and/or maintenance of immune tolerance in lymphocytes.28,42,51-55
Whereas fucosylation has been demonstrated to play pivotal pathogenic roles in the progression of immunological diseases, less is known about the immunological roles of l-fucose and fucosylation in cancer pathogenesis. Moreover, in the context of tumor immunity, studies on the roles of fucosylation have predominantly focused on T cells.
To date, a number of studies—focusing largely on FUT8, which mediates tumor-promoting core fucosylation in tumor cells—have reported both tumor-promoting and tumor-suppressing effects of fucosylation in the context of tumor immunity. Several studies have highlighted the significant roles of fucosylation in the regulation of T-cell activity. FUT8 knockout mice exhibit impaired development and increased apoptosis of thymic T cells, indicating the requirement of core fucosylation for the development and viability.56 Further, fucosylation of the CD4+ T-cell receptor (TCR), is required for full activation of CD4+ T cells.57,58 A study by Fujii et al.58 identified FUT8 as a FUT that fucosylates TCR, critically mediating CD4+ T activation; knockout of FUT8 significantly attenuates CD4+ T-cell activation. Consistent with the notion of pathological T-cell activation by aberrant fucosylation, core fucosylation of CD4+ TCR was reported to be increased in tissues of patients with irritable bowel syndrome and systemic lupus erythematosus.57,58 Collectively, these findings indicate that FUT8-mediated core fucosylation is crucial for general T-cell development, viability, and CD4+ T-cell activation—and importantly, that aberrant TCR fucosylation is associated with the pathogenesis of specific immunological diseases.
However, core fucosylation has also been reported by Okada et al.59 to conversely induce CD8+ T-cell exhaustion by stabilizing cell surface PD1. Moreover, Huang et al.60 found that FUT8-mediated core fucosylation stabilizes cell surface B7H3 on tumor cells, enforcing multipronged immune checkpoint signaling to suppress T-cell activity in breast tumors. These findings illustrate the complex and divergent roles of fucosylation within the tumor immune microenvironment, including potentially differential effects on CD4+ versus CD8+ T cells, as well as on tumor and other stromal cells within the tumor microenvironment. Consistent with these possibilities, FUT8 has previously been reported to significantly impact both immune tolerance and T-cell autoreactivity.
Although the aforementioned studies focused exclusively on core fucosylation mediated by FUT8, it is important to recognize that the 12 other FUTs mediate a range of diverse fucosylated glycan structures. As the individual FUTs can elicit divergent effects in cancer cell-autonomous signaling and biology (Table 1), the precise expression profiles of the individual FUTs specific to individual immune cell subtypes is expected to similarly elicit different immunological effects mediated by the different fucosylated glycan structures. Consistent with these possibilities, in contrast to the CD8+ T-cell exhaustion mediated by FUT8, FUT7-mediated noncore fucosylation enhances CD8+ T-cell activity in models of leukemia, breast cancer, and melanoma.61
Thus, rather than a binary on/off signal, the impact of fucosylation on T cells (and other immune cells in the tumor microenvironment) likely exists as a multifaceted balance of regulation. Future studies are needed to address how subtle changes in the fucosylation landscape in immune cells can significantly change immunological outcomes.
5 ∣. AFUCOSYLATED THERAPEUTIC ANTIBODIES FOR THE TREATMENT OF CANCER
In addition to impacting the biology of tumor and immune cells, fucosylation of therapeutic molecules such as antibodies has been reported to impact efficacy, and fucosylation itself has been modulated as an experimental anticancer approach.
Therapeutic antibodies are a highly effective class of anticancer therapy that has been demonstrated to suppress tumor growth and spread via a number of mechanisms including direct inhibition of key signaling pathways by blocking ligand:receptor binding or receptor dimerization/activation, and immune-mediated antitumor activity, including (i) induction of complement-mediated toxicity, (ii) phagocytosis of tumor cells, and (iii) antibody-dependent cytotoxicity (ADCC) 62,63
Importantly, the direct glycosylation of antibodies can vary depending on the cells in which they are produced and can significantly influence a given antibody's ability to recruit immune effector cells and to trigger target neutralization and cytotoxicity.64 For example, the differential glycosylation of CAMPATH-1H, a humanized antibody used for the treatment of leukemia, lymphoma, and RA, was found to associate with differential extents of ADCC, depending on the cell line that the antibody was produced in [65]. Initial studies of therapeutic antibodies against human immunodeficiency virus (HIV) revealed that differential glycosylation of the Fc regions is a significant determinant of their therapeutic efficacy. Specifically, profiling of natural Fc region glycosylation variants (a.k.a., glycoforms) of anti-HIV antibodies revealed that galactosylation or fucosylation of the Fc region is associated with significantly enhanced or reduced anti-viral activity and HIV control, respectively.66,67 The removal of core-linked fucose from Fc has been recognized as a method to improve the immune-mediated activity of therapeutic antibodies. Initial structural modeling studies suggested that differential glycosylation at a conserved Asn297 residue on the IgG Fc region impacts the structural conformation of the FC receptor:FC interaction that mediates ADCC responses.68 Further studies revealed that FC fucosylation significantly enhances FC:FC receptor interaction in an FC receptor-dependent manner, as among FCγ receptor isoforms, FCγ receptor IIIa binds with 50-fold increased affinity to afucosylated IgG1 FC compared with other FCγ receptor isoforms including IIA and IIB. Importantly, the increased binding affinity also synergistically increased ADCC.69 Indeed, depletion of l-fucose from IgG1 enhances the binding of IgG1 to FCγ receptor IIIa.70 Notably, N-glycosylation of FCγ receptor IIIa at Asn162 was found to be required for enhanced binding affinity to afucosylated IgG FC, indicating that differential glycosylation and fucosylation of Fc and cognate receptors can significantly impact binding affinity and consequent immunological effects.71
Numerous efforts have been undertaken to produce afucosylated antibodies to improve immune-mediated therapeutic efficacy.72 To reduced fucosylation on antibodies, glycoengineering efforts have targeted fucosylation mechanisms in the cells used to produce the antibodies. Efforts targeting GDP-fucose substrate-level production/availability have included the knockout of GMD/FX enzymes (which mediate de novo synthesis of GDP-fucose from GDP-mannose) or SLC35C1 (Golgi transporter of l-fucose).73-76 Downstream structure–function-targeted efforts have included knockdown or knockout of FUT8.77,78 In each case, the targeted perturbation in cellular fucosylation resulted in increased production of antibodies exhibiting reduced fucosylation. However, these approaches can be limited by generally incomplete abrogation of fucosylation, resulting in increased ratios of afucosylated:fucosylated antibodies produced.
A number of afucosylated antibodies have been tested in clinical trials for the treatment of various cancers, and a small number are commercially produced (Table 4).
TABLE 4.
Afucosylated anticancer antibodies
| Commercial name | Target | Pathology | Commercial/clinical trial phase | References (PMID) |
|---|---|---|---|---|
| ARGX-111 | c-MET | Multiple cancers | Phase 1 (completed): NCT02055066 | 29733746 |
| Bemarituzumab | FGFR2b | Gastric cancer, gastroesophageal junction adenocarcinoma | Phases 1, 2, and 3: NCT05111626; NCT05052801; NCT02213289; NCT03694522; NCT03343301 | 29733746 |
| Transitional cell carcinoma of the bladder | Phase 1: NCT02318329 | 29733746 | ||
| BMS-986218 | CTLA4 | Prostate cancer | Early Phase 1: NCT04301414 | 29733746 |
| ALC; advanced malignant solid neoplasm; primary and metastatic liver cancer; metastatic lung carcinoma; metastatic malignant solid neoplasm | Phases 1 and 2: NCT04785287 | 29733746 | ||
| Cusatuzumab | CD70 | AML | Phase 2: NCT04023526 | 29733746 |
| Gatipotuzumab/PankoMab-GEX | Glycoepitope of Muc1 (TA-Muc1 | Ovarian epithelial cancer, primary peritoneal cancer recurrent fallopian tube cancer | Phase 2 (completed): NCT01899599 | 29733746 |
| Solid tumors | Phase 1 (completed) | 29733746 | ||
| GSK2849330 | HER3 | Multiple cancers, neoplasms | Phase 1: NCT01222624; NCT01966445; NCT02345174 | 29733746 |
| Inebilizumab | CD19 | Advanced B-cell malignancies; blood cancer; relapsed/refractory aggressive BCL; CLL; DLBCL |
Phases 1 and 2 (completed): NCT01453205 | 29733746 |
| Lumretuzumab | HER3 | Breast cancer; neoplasms | Phase 1 (completed): NCT01918254; NCT01482377 | 29733746 |
| MEDI-570 | ICOS (CD278) | Angioimmunoblastic T-cell lymphoma; T-cell lymphoma follicular variant | Phase 1: NCT02520791 | 29733746 |
| Mogamulizumab | CC chemokine receptor 4 (CCR4) | CTL; relapsed or refractory CCR4-positive adult T-cell leukemia-lymphoma | Commercially available (POTELIGEO): NCT04745234 | 29733746 |
| DLBCL; HCC; recurrent and/refractory Hodgkin lymphoma; recurrent/refractory NHL | Phases 1 and 2: NCT03309878 | 29733746 | ||
| Stage IB–IIB CTL | Phase 2: NCT04128072 | 29733746 | ||
| Obinutuzumab (a.k.a., GA101 or Gazyva) | CDC20 | CLL, NHL | Commercially available (GA101): NCT04908228; NCT03059251 | 29733746 |
| BCL | Trial Phases 1, 2, and 3 | 25143487; 23835718; 22431570; 24401022 | ||
| SEA-BCMA | B-cell maturation antigen | Relapsed or refractory multiple myeloma | Phase 1: NCT03582033 | 29733746 |
| SEA-CD40 | CD40 | Hodgkin's disease; melanoma; NSCLC | Phase 2: NCT04993677 | 29733746 |
| Tomuzotuximab/CetuGEX | EGFR | Head and neck squamous cell carcinoma | Phase 2: NCT02052960 | 29733746 |
| Solid tumor | Phase 1 | 29733746 | ||
| TrasGex | HER2 | Solid tumor | Phase 1 | 29733746 |
| TRX518 | Glucocorticoid-induced TNF receptor | Unresectable Stage III or Stage IV MM or other solid tumor malignancies | Phase 1 (completed): NCT01239134 | 29733746 |
| Ublituximab | CD20 | BCL; CLL; marginal zone lymphoma; NHL; primary central nervous system lymphoma; SLL; Waldenstrom's macroglobulinemia | Phases 1 and 2: NCT01647971; NCT01744912l | 29733746 |
| CLL | Phase 2: NCT02656303 | 29733746 | ||
| CLL, Mantle cell lymphoma | Phase 1: NCT02013128 | 29733746 | ||
| CLL; NHL | Phase 2: NCT03207256 | 29733746 | ||
| DLBCL; follicular lymphoma; marginal zone lymphoma; mediastinal large BCL; SLL | Phase 1: NCT04806035 | 29733746 | ||
| Follicular lymphoma, SLL | Phase 2: NCT03828448 | 29733746 |
Abbreviations: ALC, advanced lung carcinoma; AML, acute myeloid leukemia; BCL, B-cell lymphoma; CLL, chronic lymphocytic leukemia; CTL, cutaneous T-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; HCC, hepatocellular carcinoma; MM, malignant melanoma; NHL, non-Hodgkin's lymphoma; NSCLC, non-small cell lung cancer; SLL, small lymphocytic lymphoma.
6 ∣. TARGETING FUCOSYLATION IN CANCER
In parallel to the development of afucosylated antibodies with enhanced therapeutic potential for anticancer activities, systemic inhibition of protein fucosylation has also been an area of active study as a potential anticancer therapeutic modality. A few small molecule inhibitors of fucosylation have been studied and developed in recent years that inhibit fucosylation at the substrate level (FUK/GMD/FX) and at the downstream FUT level.79,80
Substrate-level fucosylation inhibitors have been developed based on fluorinated analogs of l-fucose, which can principally be uptaken into cells and converted into inhibitor analogs of GDP-fucose that bind tightly to and inhibit FUTs.79,81 The most studied inhibitor analog is 2-fluoro-fucose, 2FF.82 This inhibitor has been preclinically tested for therapeutic effects in the context of a number of different cancer types including liver and breast cancers.83,84 Recent studies have developed higher potency inhibitors of fucosylation based on 2FF. These include, for example, unnatural α-anomers and endogenous α-anomers of 2-fluoro-fucose-1-phosphate (A2FF1P and B2FF1P, respectively), which exhibit 4–7-fold increased potency for inhibiting fucosylation compared with 2FF.85 In parallel, fluorinated versions of GDP-mannose-1-phosphate, Fucotrim I/II, have recently been developed to suppress fucosylation via de novo synthetic pathway conversion to fluorinated GDP-fucose-1-phosphate.86 Fluorinated rhamnosides were also recently developed and reported to robustly inhibit fucosylation.85
In preclinical breast cancer models, 2FF inhibited tumor growth and increased the antitumor capability of immune cells.87 These data promoted a phase I clinical trial for solid tumors, which resulted in one complete response in head and neck squamous cell carcinoma and stable disease in 36% of patients. However, the clinical trial was discontinued due to significant rates of thromboembolic events.88 Given the pleiotropic roles of the 13 FUTs across the different cell and tissue types, it is not necessarily surprising that systemic and chronic inhibition of fucosylation without FUT-specific targeting would result in deleterious adverse events and co-morbidities. Thus, although these 2FF and 2FF analogs represent potent substrate-level inhibitors of fucosylation and important tools for studying fucosylation, their implementation as therapeutic agents need to be carefully and contextually considered given the potential side effects.
Based on the potential and unintended deleterious effects elicited by systemic inhibition of fucosylation, FUT-targeted efforts have been anticipated to provide more tumor-specific suppression. Given previously reported protumorigenic roles of FUT8 in driving tumor cell-autonomous signaling (e.g., increase metastatic capacity), FUT8, in particular, has been the subject of study as an antitumor target.89 Other inhibitors with narrower FUT-specific affinities have recently been developed, as derivatives of GDP-fucose, and have been reported to exhibit enhanced inhibitory specificity for FUTs including FUT6 and FUT8.79,89,90 It is important to acknowledge, however, the divergent roles that individual FUTs have been reported to play—divergence for which it might be detrimental to inhibit their functions. For example, FUT8 has been reported to be required for T-cell development, viability, and activation.56-58 However, it has also been reported to induce CD8+ T-cell exhaustion56 and to stabilize tumor cell immune checkpoint proteins, enforcing immune checkpoint inhibition of T-cell activity.60 Thus, specific targeting of FUT8 (and other individual FUTs) might inadvertently abrogate the balance of antitumor immunity and tumor autonomous signaling, promoting tumor progression. Given the pleiotropic roles of FUT8 (and other individual FUTs), further studies are required to delineate the key determinants of response (i.e., FUT expression, sex, age, type, and stage of cancer) and are expected to guide the clinical utility of FUT inhibitors.
In contrast to the efforts aimed at inhibiting fucosylation for the treatment of cancers, increasing fucosylation has also been reported to elicit therapeutically potent antitumor effects with the potential to augment immunotherapies. As mentioned above, clinical-grade l-fucose has been implemented as an experimental treatment for leukocyte adhesion deficiency-II (LAD-II), a rare genetic disorder in children driven by mutations in the de novo GDP-fucose synthetic pathway that abrogates fucosylation in leukocyte precursors, impairing their ability to extravasate from the vasculature.91 Oral administration of l-fucose, which increases fucosylation via the fucose salvage pathway, is sufficient to compensate for cellular fucosylation that is suppressed by de novo pathway mutations in LAD-II patients, at least partially reverting symptoms of this pathology, demonstrating the potential clinical utility and the safety of l-fucose supplementation.50
In the context of cancer, preclinical in vivo mouse models of breast cancer, Ehrlich carcinoma, and melanoma have demonstrated antitumorigenic effects of l-fucose.33,92,93 In the melanoma models performed by our laboratory, oral l-fucose results in net decreases in tumor growth, suggesting that despite divergent and potentially tumor-promoting functions of individual FUTs, the systemic dosing of l-fucose tips the functional balance of fucosylation toward tumor suppression. Notably, the fucosylation-triggered tumor suppression might be immune-mediated, as overall immune infiltration of melanomas was increased by l-fucose supplementation.33 Consistent with this notion, the ex vivo treatment of CD8+ T cells with exogenous recombinant FUT7 before adoptive cell transfer into melanomabearing mice resulted in increased T-cell fucosylation associated with enhanced cytotoxic activities in vitro and melanoma tumor suppression in vivo.61 Studies elucidating how l-fucose triggers antitumor immunity are forthcoming. Increasing our knowledge of (1) what is being fucosylated and (2) how l-fucose affects specific immune cell populations in the tumor microenvironment is expected to further delineate the rationale and contexts for the clinical utility of l-fucose.
In addition to supplementation with l-fucose in preclinical cancer models, a seaweed-derived l-fucose-rich extract called fucoidan has exhibited antitumorigenic properties in vivo and in vitro.94-101 In the context of immunotherapy, this is particularly exciting as fucoidan has been shown to enhance the efficacy of immune checkpoint blockade.102
7 ∣. CONCLUSION
In conclusion, growing bodies of study have highlighted l-fucose and fucosylation as key components in cancer biology and in tumor immunology. Although it is clear that fucosylation plays crucial roles in the regulation of immune cell biology, particularly in the context of the tumor immune microenvironment, how fucosylation can be most effectively modulated to suppress tumors remains unclear. Specifically, there is a need to increase our understanding of how to leverage fucosylation to (i) suppress tumor autonomous signaling, (ii) enhance antitumor immune biology, and (iii) enhance immunotherapeutic molecules (e.g., afucosylated therapeutic anti-bodies) and modalities (e.g., coadministration of l-fucose vs. fucosylation inhibitors with other antitumor therapeutics). Further studies are expected to help deconvolute the complexities of tumor:immune contexts that will therapeutically benefit from modulating fucosylation and provide contextual frameworks in which increasing or inhibiting fucosylation will enhance the efficacies of specific immunotherapeutic modalities.
ACKNOWLEDGMENTS
We thank the members of the Lau laboratory for critical reading of this manuscript, as well as members of the Moffitt Tumor Biology department for mentorship and critical reading. We would also like to thank Ms. Susan Sharpe (Moffitt Biomedical Library) for her assistance with citations and references. We would like to acknowledge support from NIH (R01CA241559) to Eric Lau.
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
DATA AVAILABILITY STATEMENT
This manuscript did not generate any new data; it is a review of previously published scientific studies for which the data are available on a study-by-study basis.
REFERENCES
- 1.Winchester B Lysosomal metabolism of glycoproteins. Glycobiology. 2005;15(6):1R–15R. doi: 10.1093/glycob/cwi041 [DOI] [PubMed] [Google Scholar]
- 2.O'Brien JS, Willems PJ, Fukushima H, et al. Molecular biology of the alpha-l-fucosidase gene and fucosidosis. Enzyme. 1987;38(1-4):45–53. doi: 10.1159/000469189 [DOI] [PubMed] [Google Scholar]
- 3.Hoff J, Castro MD. Chemical composition of potato cell wall. J Agricult Food Chem. 1969;17(6):1328–31. [Google Scholar]
- 4.Salvador LD, Suganuma T, Kitahara K, Tanoue H, Ichiki M. Monosaccharide composition of sweetpotato fiber and cell wall polysaccharides from sweetpotato, cassava, and potato analyzed by the high-performance anion exchange chromatography with pulsed amperometric detection method. J Agric Food Chem. 2000;48(8):3448–3454. doi: 10.1021/jf991089z [DOI] [PubMed] [Google Scholar]
- 5.Redgwell RJ, Melton LD, Brasch DJ, Coddington JM. Structures of the pectic polysaccharides from the cell walls of kiwifruit. Carbohydr Res. 1992;226(2):287–302. [Google Scholar]
- 6.Reiter WD, Chapple CC, Somerville CR. Altered growth and cell walls in a fucose-deficient mutant of Arabidopsis. Science. 1993;261(5124):1032–1035. doi: 10.1126/science.261.5124.1032 [DOI] [PubMed] [Google Scholar]
- 7.Perkins EG. Composition of soybeans and soybean products Elsevier. In: Practical Handbook of Soybean Processing and Utilization. Elsevier; 1995:9–28 [Google Scholar]
- 8.Ravindran G, Palmer JK, Gajameragedara SM. Seed polysaccharides of some winged bean varieties. J Agricult Food Chem. 1989;37(2):327–329. [Google Scholar]
- 9.Slominski BA, Campbell LD. Non-starch polysaccharides of canola meal: quantification, digestibility in poultry and potential benefit of dietary enzyme supplementation. J Sci Food Agric. 1990;53(2):175–184. [Google Scholar]
- 10.Kim H, Wang Q, Shoemaker CF, Zhong F, Bartley GE, Yokoyama WH. Polysaccharide gel coating of the leaves of Brasenia schreberi lowers plasma cholesterol in hamsters. J Tradit Complement Med. 2015;5(1):56–61. doi: 10.1016/j.jtcme.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitton HJ, Stringer DS, Park AY, Karpiniec SN. Therapies from Fucoidan: new developments. Mar Drugs. 2019;17(10), doi: 10.3390/md17100571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishino T, Yokoyama G, Dobashi K, Fujihara M, Nagumo T. Isolation, purification, and characterization of fucose-containing sulfated polysaccharides from the brown seaweed Ecklonia kurome and their blood-anticoagulant activities. Carbohydr Res. 1989;186(1):119–129. [DOI] [PubMed] [Google Scholar]
- 13.Nishino T, Kiyohara H, Yamada H, Nagumo T. An anticoagulant fucoidan from the brown seaweed Ecklonia kurome. Phytochemistry. 1991;30(2):535–539. doi: 10.1016/0031-9422(91)83722-w [DOI] [PubMed] [Google Scholar]
- 14.Nishino T, Nagumo T, Kiyohara H, Yamada H. Structural characterization of a new anticoagulant fucan sulfate from the brown seaweed Ecklonia kurome. Carbohydr Res. 1991;211(1):77–90. doi: 10.1016/0008-6215(91)84147-7 [DOI] [PubMed] [Google Scholar]
- 15.Teruya T, Takeda S, Tamaki Y, Tako M. Fucoidan isolated from Laminaria angustata var. longissima induced macrophage activation. Biosci Biotechnol Biochem. 2010;74(9):1960–1962. doi: 10.1271/bbb.100294 [DOI] [PubMed] [Google Scholar]
- 16.Nishino T, Nishioka C, Ura H, Nagumo T. Isolation and partial characterization of a novel amino sugar-containing fucan sulfate from commercial Fucus vesiculosus fucoidan. Carbohydr Res. 1994;255:213–224. doi: 10.1016/s0008-6215(00)90980-7 [DOI] [PubMed] [Google Scholar]
- 17.Nishide E, Tsukayama K. Elimination of water-soluble alginate from the fucose-containing polysaccharides of brown alga, Kjellmaniella crassifolia. Bull Japan Soc Sci Fish. 1982;48:1771–1773. [Google Scholar]
- 18.Green JR, Stafford CJ, Jones JL, Wright PJ, Callow JA. Binding of monoclonal antibodies to vegetative tissue and fucose-containing polysaccharides of Fucus serratus L. New Phytol. 1993;124(3):397–408. [Google Scholar]
- 19.Park SH, Pastuszak I, Drake R, Elbein AD. Purification to apparent homogeneity and properties of pig kidney L-fucose kinase. J Biol Chem. 1998;273(10):5685–5691. doi: 10.1074/jbc.273.10.5685 [DOI] [PubMed] [Google Scholar]
- 20.Pastuszak I, Ketchum C, Hermanson G, Sjoberg EJ, Drake R, Elbein AD. GDP-l-fucose pyrophosphorylase. Purification, cDNA cloning, and properties of the enzyme. J Biol Chem. 1998;273(46):30165–30174. doi: 10.1074/jbc.273.46.30165 [DOI] [PubMed] [Google Scholar]
- 21.Thiel C, Korner C. Therapies and therapeutic approaches in gongenital disorders of glycosylation. Glycoconj J. 2013;30(1):77–84. doi: 10.1007/s10719-012-9447-5 [DOI] [PubMed] [Google Scholar]
- 22.Sullivan FX, Kumar R, Kriz R, et al. Molecular cloning of human GDP-mannose 4,6-dehydratase and reconstitution of GDP-fucose biosynthesis in vitro. J Biol Chem. 1998;273(14):8193–8202. doi: 10.1074/jbc.273.14.8193 [DOI] [PubMed] [Google Scholar]
- 23.Smith PL, Myers JT, Rogers CE, et al. Conditional control of selectin ligand expression and global fucosylation events in mice with a targeted mutation at the FX locus. Cell Biol. 2002;158(4):801–815. doi: 10.1083/jcb.200203125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yurchenco PD, Atkinson PH. Fucosyl-glycoprotein and precursor polls in HeLa cells. Biochemistry. 1975;14(14):3107–3114. doi: 10.1021/bi00685a011 [DOI] [PubMed] [Google Scholar]
- 25.Kuettel S, Wadum MC, Guther ML, Marino K, Riemer C, Ferguson MA. The de novo and salvage pathways of GDP-mannose biosynthesis are both sufficient for the growth of bloodstream-form Trypanosoma brucei. Mol Microbiol. 2012;84(2):340–351. doi: 10.1111/j.1365-2958.2012.08026.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu L, Hou X, Shi S, Korner C, Stanley P. Slc35c2 promotes Notch1 fucosylation and is required for optimal Notch signaling in mammalian cells. J Biol Chem. 2010;285(46):36245–36254. doi: 10.1074/jbc.M110.126003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13(7):41R–53R. doi: 10.1093/glycob/cwg054 [DOI] [PubMed] [Google Scholar]
- 28.Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16(12):158R–184RR. doi: 10.1093/glycob/cwl040 [DOI] [PubMed] [Google Scholar]
- 29.McMillan BJ, Zimmerman B, Egan ED, et al. Structure of human POFUT1, its requirement in ligand-independent oncogenic Notch signaling, and functional effects of Dowling–Degos mutations. Glycobiology. 2017;27(8):777–786. doi: 10.1093/glycob/cwx020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holdener BC, Haltiwanger RS. Protein O-fucosylation: structure and function. Curr Opin Struct Biol. 2019;56:78–86. doi: 10.1016/j.sbi.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider M, Al-Shareffi E, Haltiwanger RS. Biological functions of fucose in mammals. Glycobiology. 2017;27(7):601–618. doi: 10.1093/glycob/cwx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem. 2008;143(6):725–729. doi: 10.1093/jb/mvn011 [DOI] [PubMed] [Google Scholar]
- 33.Lau E, Feng Y, Claps G, et al. The transcription factor ATF2 promotes melanoma metastasis by suppressing protein fucosylation. Sci Signal. 2015;8(406):ra124. doi: 10.1126/scisignal.aac6479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastian K, Scott E, Elliott DJ, Munkley J. FUT8 alpha-(1,6)-fucosyltransferase in cancer. Int J Mol Sci. 2021;22(1), doi: 10.3390/ijms22010455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taniguchi N, Kizuka Y. Glycans and cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv Cancer Res. 2015;126:11–51. doi: 10.1016/bs.acr.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 36.Ji J, Gu X, Fang M, et al. Expression of alpha 1,6-fucosyltransferase 8 in hepatitis B virus-related hepatocellular carcinoma influences tumour progression. Dig Liver Dis. 2013;45(5):414–421. doi: 10.1016/j.dld.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 37.Geng F, Shi BZ, Yuan YF, Wu XZ. The expression of core fucosylated E-cadherin in cancer cells and lung cancer patients: prognostic implications. Cell Res. 2004;14(5):423–433. doi: 10.1038/sj.cr.7290243 [DOI] [PubMed] [Google Scholar]
- 38.Tu CF, Wu MY, Lin YC, Kannagi R, Yang RB. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-beta receptor core fucosylation. Breast Cancer Res. 2017;19(1):111. doi: 10.1186/s13058-017-0904-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agrawal P, Fontanals-Cirera B, Sokolova E, et al. A systems biology approach identifies FUT8 as a driver of melanoma metastasis. Cancer Cell. 2017;31(6):804–19. e7. doi: 10.1016/j.ccell.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin X, Rana K, Ponmudi V, King MR. Knockdown of fucosyltransferase III disrupts the adhesion of circulating cancer cells to E-selectin without affecting hematopoietic cell adhesion. Carbohydr Res. 2010;345(16):2334–2342. doi: 10.1016/j.carres.2010.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Liu S, Yan Q. Role of fucosyltransferase IV in epithelial–mesenchymal transition in breast cancer cells. Cell Death Dis. 2013;4:e735. doi: 10.1038/cddis.2013.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Hsu HC, Mountz JD, Allen JG. Unmasking fucosylation: from cell adhesion to immune system regulation and diseases. Cell Chem Biol. 2018;25(5):499–512. doi: 10.1016/j.chembiol.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 43.Hanna S, Etzioni A. Leukocyte adhesion deficiencies. Ann N Y Acad Sci. 2012;1250:50–55. doi: 10.1111/j.1749-6632.2011.06389.x [DOI] [PubMed] [Google Scholar]
- 44.Etzioni A Adhesion molecule deficiencies and their clinical significance. Cell Adhes Commun. 1994;2(3):257–260. doi: 10.3109/15419069409004445 [DOI] [PubMed] [Google Scholar]
- 45.Etzioni A, Phillips LM, Paulson JC, Harlan JM. Leukocyte adhesion deficiency (LAD) II. Ciba Found Symp. 1995;189:51–58. discussion 8-62, 77-8. doi: 10.1002/9780470514719.ch5 [DOI] [PubMed] [Google Scholar]
- 46.Helmus Y, Denecke J, Yakubenia S, et al. Leukocyte adhesion deficiency II patients with a dual defect of the GDP-fucose transporter. Blood. 2006;107(10):3959–3966. doi: 10.1182/blood-2005-08-3334 [DOI] [PubMed] [Google Scholar]
- 47.Yakubenia S, Frommhold D, Schölch D, et al. Leukocyte trafficking in a mouse model for leukocyte adhesion deficiency II/congenital disorder of glycosylation IIc. Blood. 2008;112(4):1472–1481. doi: 10.1182/blood-2008-01-132035 [DOI] [PubMed] [Google Scholar]
- 48.Etzioni A, Sturla L, Antonellis A, et al. Leukocyte adhesion deficiency (LAD) type II/carbohydrate deficient glycoprotein (CDG) IIc founder effect and genotype/phenotype correlation. Am J Med Genet. 2002;110(2):131–135. doi: 10.1002/ajmg.10423 [DOI] [PubMed] [Google Scholar]
- 49.Wolach B, Gavrieli R, Wolach O, et al. Leucocyte adhesion deficiency—a multicentre national experience. Eur J Clin Invest. 2019;49(2):e13047. doi: 10.1111/eci.13047 [DOI] [PubMed] [Google Scholar]
- 50.Marquardt T, Luhn K, Srikrishna G, Freeze HH, Harms E, Vestweber D. Correction of leukocyte adhesion deficiency type II with oral fucose. Blood. 1999;94(12):3976–3985. [PubMed] [Google Scholar]
- 51.Brinkman-van der Linden EC, de Haan PF, Havenaar EC, van Dijk W. Inflammation-induced expression of sialyl LewisX is not restricted to alpha1-acid glycoprotein but also occurs to a lesser extent on alpha1-antichymotrypsin and haptoglobin. Glycoconj J. 1998;15(2):177–182. doi: 10.1023/a:1006972307166 [DOI] [PubMed] [Google Scholar]
- 52.Goodarzi MT, Axford JS, Varanasi SS, et al. Sialyl Lewis(x) expression on IgG in rheumatoid arthritis and other arthritic conditions: a preliminary study. Glycoconj J. 1998;15(12):1149–1154. doi: 10.1023/a:1006920007227 [DOI] [PubMed] [Google Scholar]
- 53.Li J, Hsu HC, Ding Y, et al. Inhibition of fucosylation reshapes inflammatory macrophages and suppresses type II collagen-induced arthritis. Arthritis Rheumatol. 2014;66(9):2368–2379. doi: 10.1002/art.38711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryden I, Pahlsson P, Lundblad A, Skogh T. Fucosylation of alpha1-acid glycoprotein (orosomucoid) compared with traditional bio-chemical markers of inflammation in recent onset rheumatoid arthritis. Clin Chim Acta. 2002;317(1-2):221–229. doi: 10.1016/s0009-8981(01)00803-8 [DOI] [PubMed] [Google Scholar]
- 55.Thompson S, Kelly CA, Griffiths ID, Turner GA. Abnormally-fucosylated serum haptoglobins in patients with inflammatory joint disease. Clin Chim Acta. 1989;184(3):251–258. doi: 10.1016/0009-8981(89)90058-2 [DOI] [PubMed] [Google Scholar]
- 56.Liang W, Mao S, Li M, et al. Ablation of core fucosylation attenuates the signal transduction viaT cell receptor to suppress theT cell development. Mol Immunol. 2019;112:312–321. doi: 10.1016/j.molimm.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 57.Fujii H, Shinzaki S, Iijima H, et al. Core fucosylation on T cells, required for activation of T-cell receptor signaling and induction of colitis in mice, is increased in patients with inflammatory bowel disease. Gastroenterology. 2016;150(7):1620–1632. doi: 10.1053/j.gastro.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 58.Liang W, Mao S, Sun S, et al. Core fucosylation of the T cell receptor is required for T cell activation. Front Immunol. 2018;9:78. doi: 10.3389/fimmu.2018.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okada M, Chikuma S, Kondo T, et al. Blockage of core fucosylation reduces cell-surface expression of PD-1 and promotes anti-tumor immune responses of T cells. Cell Rep. 2017;20(5):1017–1028. doi: 10.1016/j.celrep.2017.07.027 [DOI] [PubMed] [Google Scholar]
- 60.Huang Y, Zhang HL, Li ZL, et al. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat Commun. 2021;12(1):2672. doi: 10.1038/s41467-021-22618-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alatrash G, Qiao N, Zhang M, et al. Fucosylation enhances the efficacy of adoptively transferred antigen-specific cytotoxic T lymphocytes. Clin Cancer Res. 2019;25(8):2610–2620. doi: 10.1158/1078-0432.CCR-18-1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zahavi D, Weiner L. Monoclonal antibodies in cancer therapy. Antibodies. 9, 2020;3. doi: 10.3390/antib9030034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carter P Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1(2):118–129. doi: 10.1038/35101072 [DOI] [PubMed] [Google Scholar]
- 64.Wright A, Morrison SL. Effect of glycosylation on antibody function: implications for genetic engineering. Trends Biotechnol. 1997;15(1):26–32. doi: 10.1016/S0167-7799(96)10062-7 [DOI] [PubMed] [Google Scholar]
- 65.Lifely MR, Hale C, Boyce S, Keen MJ, Phillips J. Glycosylation and biological activity of CAMPATH-1H expressed in different cell lines and grown under different culture conditions. Glycobiology. 1995;5(8):813–822. doi: 10.1093/glycob/5.8.813 [DOI] [PubMed] [Google Scholar]
- 66.Ackerman ME, Crispin M, Yu X, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123(5):2183–2192. doi: 10.1172/JCI65708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masuda K, Kubota T, Kaneko E, et al. Enhanced binding affinity for FcgammaRIIIa of fucose-negative antibody is sufficient to induce maximal antibody-dependent cellular cytotoxicity. Mol Immunol. 2007;44(12):3122–3131. doi: 10.1016/j.molimm.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 68.Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcgamma receptor in complex with Fc. J Biol Chem. 2001;276(19):16469–16477. doi: 10.1074/jbc.M100350200 [DOI] [PubMed] [Google Scholar]
- 69.Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–26740. doi: 10.1074/jbc.M202069200 [DOI] [PubMed] [Google Scholar]
- 70.Okazaki A, Shoji-Hosaka E, Nakamura K, et al. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J Mol Biol. 2004;336(5):1239–1249. doi: 10.1016/j.jmb.2004.01.007 [DOI] [PubMed] [Google Scholar]
- 71.Ferrara C, Stuart F, Sondermann P, Brunker P, Umana P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem. 2006;281(8):5032–5036. doi: 10.1074/jbc.M510171200 [DOI] [PubMed] [Google Scholar]
- 72.Pereira NA, Chan KF, Lin PC, Song Z. The “less-is-more” in therapeutic antibodies: afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs. 2018;10(5):693–711. doi: 10.1080/19420862.2018.1466767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohyama C, Smith PL, Angata K, Fukuda MN, Lowe JB, Fukuda M. Molecular cloning and expression of GDP-D-mannose-4,6-dehydratase, a key enzyme for fucose metabolism defective in Lec13 cells. J Biol Chem. 1998;273(23):14582–14587. doi: 10.1074/jbc.273.23.14582 [DOI] [PubMed] [Google Scholar]
- 74.Louie S, Haley B, Marshall B, et al. FX knockout CHO hosts can express desired ratios of fucosylated or afucosylated antibodies with high titers and comparable product quality. Biotechnol Bioeng. 2017;114(3):632–644. doi: 10.1002/bit.26188 [DOI] [PubMed] [Google Scholar]
- 75.Chan KF, Shahreel W, Wan C, et al. Inactivation of GDP-fucose transporter gene (Slc35c1) in CHO cells by ZFNs, TALENs and CRISPR-Cas9 for production of fucose-free antibodies. Biotechnol J. 2016;11(3):399–414. doi: 10.1002/biot.201500331 [DOI] [PubMed] [Google Scholar]
- 76.Bardhi A, Wu Y, Chen W, et al. Potent in vivo NK cell-mediated elimination of HIV-1-infected cells mobilized by a gp120-bispecific and hexavalent broadly neutralizing fusion protein. J Virol. 2017;91(20), doi: 10.1128/JVI.00937-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malphettes L, Freyvert Y, Chang J, et al. Highly efficient deletion of FUT8 in CHO cell lines using zinc-finger nucleases yields cells that produce completely nonfucosylated antibodies. Biotechnol Bioeng. 2010;106(5):774–783. doi: 10.1002/bit.22751 [DOI] [PubMed] [Google Scholar]
- 78.Shinkawa T, Nakamura K, Yamane N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278(5):3466–3473. doi: 10.1074/jbc.M210665200 [DOI] [PubMed] [Google Scholar]
- 79.Rillahan CD, Antonopoulos A, Lefort CT, et al. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat Chem Biol. 2012;8(7):661–668. doi: 10.1038/nchembio.999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Okeley NM, Alley SC, Anderson ME, et al. Development of orally active inhibitors of protein and cellular fucosylation. Proc Natl Acad Sci USA. 2013;110(14):5404–5409. doi: 10.1073/pnas.1222263110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burkart MD, Vincent SP, Duffels A, Murray BW, Ley SV, Wong CH. Chemo-enzymatic synthesis of fluorinated sugar nucleotide: useful mechanistic probes for glycosyltransferases. Bioorg Med Chem. 2000;8(8):1937–1946. doi: 10.1016/s0968-0896(00)00139-5 [DOI] [PubMed] [Google Scholar]
- 82.Villalobos JA, Yi BR, Wallace IS. 2-Fluoro-l-fucose Is a Metabolically incorporated inhibitor of plant cell wall polysaccharide fucosylation. PLoS ONE. 2015;10(9):e0139091. doi: 10.1371/journal.pone.0139091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Y, Fukuda T, Hang Q, et al. Inhibition of fucosylation by 2-fluorofucose suppresses human liver cancer HepG2 cell proliferation and migration as well as tumor formation. Sci Rep. 2017;7(1):11563. doi: 10.1038/s41598-017-11911-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carrascal MA, Silva M, Ramalho JS, et al. Inhibition of fucosylation in human invasive ductal carcinoma reduces E-selectin ligand expression, cell proliferation, and ERK1/2 and p38 MAPK activation. Mol Oncol. 2018;12(5):579–593. doi: 10.1002/1878-0261.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pijnenborg JFA, Rossing E, Merx J, et al. Fluorinated rhamnosides inhibit cellular fucosylation. Nat Commun. 2021;12(1):7024. doi: 10.1038/s41467-021-27355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pijnenborg J, Rossing E, Noga M, et al. Fluorinated mannosides inhibit cellular fucosylation. Nat Commun. 2020;12(1):7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Disis ML, Corulli LR, Gad EA, et al. Therapeutic and prophylactic antitumor activity of an oral inhibitor of fucosylation in spontaneous mammary cancers. Mol Cancer Ther. 2020;19(5):1102–1109. doi: 10.1158/1535-7163.MCT-19-0500 [DOI] [PubMed] [Google Scholar]
- 88.Do KT, Chow LQM, Reckamp K, et al. First-in-human, first-in-class, phase I trial of the fucosylation inhibitor SGN-2FF in patients with advanced solid tumors. Oncologist. 2021;26(11):925–e1918. doi: 10.1002/onco.13911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manabe Y, Kasahara S, Takakura Y, et al. Development of alpha1,6-fucosyltransferase inhibitors through the diversity-oriented syntheses of GDP-fucose mimics using the coupling between alkyne and sulfonyl azide. Bioorg Med Chem. 2017;25(11):2844–2850. doi: 10.1016/j.bmc.2017.02.036 [DOI] [PubMed] [Google Scholar]
- 90.Maeda T, Nishimura S. FRET-based direct and continuous monitoring of human fucosyltransferases activity: an efficient synthesis of versatile GDP-l-fucose derivatives from abundant d-galactose. Chemistry. 2008;14(2):478–487. doi: 10.1002/chem.2007007602 [DOI] [PubMed] [Google Scholar]
- 91.Vestweber D, Luhn K, Marquardt T, Wild M. The role of fucosylation in leukocyte adhesion deficiency II. Ernst Schering Res Found Workshop. 2004;(44):53–74. doi: 10.1007/978-3-662-05397-3_4 [DOI] [PubMed] [Google Scholar]
- 92.Weiland G, Taylor P. Ligand specificity of state transitions in the cholinergic receptor: behavior of agonists and antagonists. Mol Pharmacol. 1979;15(2):197–212. [PubMed] [Google Scholar]
- 93.Tomsik P, Soukup T, Cermakova E, et al. l-rhamnose and l-fucose suppress cancer growth in mice. Cent Eur J Biol. 2011;6(1):1–9. [Google Scholar]
- 94.Chen H, Cong Q, Du Z, et al. Sulfated fucoidan FP08S2 inhibits lung cancer cell growth in vivo by disrupting angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Cancer Lett. 2016;382(1):44–52. doi: 10.1016/j.canlet.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 95.Lin J, Wang K, Wang H, et al. Fucoidan reduced the invasion of oral squamous cell carcinoma cells and modified their effects to macrophages. Med Oncol. 2017;34(1):9. doi: 10.1007/s12032-016-0858-1 [DOI] [PubMed] [Google Scholar]
- 96.Hsu HY, Lin TY, Lu MK, Leng PJ, Tsao SM, Wu YC. Fucoidan induces Toll-like receptor 4-regulated reactive oxygen species and promotes endoplasmic reticulum stress-mediated apoptosis in lung cancer. Sci Rep. 2017;7:44990. doi: 10.1038/srep44990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang ZJ, Xu W, Liang JW, Wang CS, Kang Y. Effect of Fucoidan on B16 murine melanoma cell melanin formation and apoptosis. Afr J Tradit Complement Altern Med. 2017;14(4):149–155. doi: 10.21010/ajtcam.v14i4.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rui X, Pan HF, Shao SL, Xu XM. Anti-tumor and anti-angiogenic effects of Fucoidan on prostate cancer: possible JAK-STAT3 pathway. BMC Complement Altern Med. 2017;17(1):378. doi: 10.1186/s12906-017-1885-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ishikawa C, Mori N. In vitro and in vivo anti-primary effusion lymphoma activities of fucoidan extracted from Cladosiphon okamuranus Tokida. Oncol Rep. 2017;38(5):3197–3204. doi: 10.3892/or.2017.5978 [DOI] [PubMed] [Google Scholar]
- 100.Thakur V, Lu J, Roscilli G, et al. The natural compound fucoidan from New Zealand Undaria pinnatifida synergizes with the ERBB inhibitor lapatinib enhancing melanoma growth inhibition. Oncotarget. 2017;8(11):17887–17896. doi: 10.18632/oncotarget.14437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shen HY, Li LZ, Xue KC, Hu DD, Gao YJ. Antitumor activity of fucoidan in anaplastic thyroid cancer via apoptosis and anti-angiogenesis. Mol Med Rep. 2017;15(5):2620–2624. doi: 10.3892/mmr.2017.6338 [DOI] [PubMed] [Google Scholar]
- 102.Yang J, Yang X, Pan W, et al. Fucoidan-supplemented diet potentiates immune checkpoint blockage by enhancing antitumor immunity. Front Cell Dev Biol. 2021;9:733246. doi: 10.3389/fcell.2021.733246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This manuscript did not generate any new data; it is a review of previously published scientific studies for which the data are available on a study-by-study basis.