Abstract
We investigated the potential role of the three strains of Thiobacillus caldus (KU, BC13, and C-SH12) in arsenopyrite leaching in combination with a moderately thermophilic iron oxidizer, Sulfobacillus thermosulfidooxidans. Pure cultures of T. caldus and S. thermosulfidooxidans were used as well as defined mixed cultures. By measuring released iron, tetrathionate, and sulfur concentrations, we found that the presence of T. caldus KU and BC13 in the defined mixed culture lowered the concentration of sulfur, and levels of tetrathionate were comparable to or lower than those in the presence of S. thermosulfidooxidans. This suggests that T. caldus grows on the sulfur compounds that build up during leaching, increasing the arsenopyrite-leaching efficiency. This result was similar to leaching arsenopyrite with a pure culture of S. thermosulfidooxidans in the presence of yeast extract. Therefore, three possible roles of T. caldus in the leaching environment can be hypothesized: to remove the buildup of solid sulfur that can cause an inhibitory layer on the surface of the mineral, to aid heterotrophic and mixotrophic growth by the release of organic chemicals, and to solubilize solid sulfur by the production of surface-active agents. The results showed that T. caldus KU was the most efficient at leaching arsenopyrite under the conditions tested, followed by BC13, and finally C-SH12.
Bacterial leaching of sulfur-containing minerals in bioreactors has been commercialized in many parts of the world, including Australia, South Africa, and South America. Yet the microorganisms involved, and the role that they play in the bioleaching process, has only recently begun to be understood. For many years it was accepted that the most abundant microorganism involved in the mineral-leaching process at mesophilic temperatures was Thiobacillus ferrooxidans. More recently it has been established that under certain conditions Leptospirillium ferrooxidans is the more important of these two mineral sulfide-oxidizing species (20).
Bioleaching has also been shown to occur in the natural environment at temperatures between 40 and 50°C. Moderately thermophilic, acidophilic bacteria have been isolated from coal spoil heaps (16), which have elevated temperatures in interior zones and localized habitats of high temperature due to spontaneous and biologically mediated exergonic oxidative processes. These moderate thermophiles include iron- and sulfur-oxidizing acidophiles with growth temperature optima between 40 and 50°C and maximum growth temperatures of around 55°C.
We have previously reported that an undefined mixed culture of moderately thermophilic microorganisms was capable of oxidizing the arsenopyrite faster than T. ferrooxidans at 22°C, a temperature well below its optimum (24). Since that report, we have begun to investigate defined moderately thermophilic mixed cultures and, in particular, two of the dominant species which have been isolated as pure cultures.
The first of the isolated species was named Thiobacillus caldus, of which there are three known isolates: KU (9), BC13 (16), and C-SH12, which requires yeast extract for growth in pure culture. Despite its inability to oxidize iron sulfides in pure culture (8), T. caldus has been shown to be the most common sulfur (S0)-oxidizing bacterium isolated from continuous biooxidation reactors operating at temperatures between 40 and 50°C. The second isolated species was a moderately thermophilic iron oxidizer (designated MTFe-1) which was subsequently shown to be an isolate of Sulfobacillus thermosulfidooxidans (7a). S. thermosulfidooxidans has been observed to oxidize polythionates and S0, but consistently only under mixotrophic conditions (18).
In this study we have tested the hypothesis that the three strains of T. caldus may be growing on the S0 that builds up on the surface of the mineral or on tetrathionate formed by ferric iron oxidation of sulfidic minerals. By analyzing the concentrations of iron, tetrathionate, and S0, we have also attempted to address two main questions: what role does T. caldus play in mineral leaching, and especially, does the reduction of the level of S0 increase the efficiency of the leaching process; and are any of the three T. caldus strains more efficient in the leaching of iron from arsenopyrite when they are in mixed cultures with S. thermosulfidooxidans?
MATERIALS AND METHODS
Bacteria and growth conditions.
The three strains of T. caldus used were KU (DSM 8584; ATCC 51756), BC13 (ATCC 51577), and C-SH12 (DSM 9466). The moderately thermophilic iron oxidizer (MTFe-1) was isolated from a moderately thermophilic Kingsbury spoil enrichment culture (16) by streaking a single colony three times on iron-tetrathionate overlay plates (13).
The three T. caldus strains were grown on tetrathionate plates made from 1.2% Oxoid no. 1 agar dissolved in mineral salts medium containing 5 mM tetrathionate (filter sterilized). The mineral salts medium consisted of the following basal salts (in grams per liter): (NH4)2SO4 (3.0), Na2SO4 · 10H2O (3.2), KCl (0.1), K2HPO4 (0.05), MgSO4 · 7H2O (0.5), and Ca(NO3)2 (0.01). The medium also contained the following trace elements (in milligrams per liter): FeCl3 · 6H2O (11.0), CuSO4 · 5H2O (0.5), HBO3 (2.0), MnSO4 · H2O (2.0), Na2MoO4 · 2H2O (0.8), CoCl2 · 6H2O (0.6), ZnSO4 · 7H2O (0.9), and Na2SeO4 (0.1) altered to pH 2.0. The basal salts were adjusted to pH 2.1 with H2SO4 and autoclaved before the filter-sterilized trace elements were added. The plates were incubated for 5 to 7 days at 45°C under a 2% (vol/vol) CO2-enriched atmosphere until colonies appeared.
From the plates, the three strains of T. caldus were inoculated into test tubes containing mineral salts solution (pH 2.1), 5 mM tetrathionate, and 10 mM Na2CO3. The cultures were grown in a 45°C shaking incubator until they reached an optical density of 0.2 at 440 nm and then inoculated into shake flasks containing 100 ml of mineral salts solution (pH 2.5). Yeast extract (0.02% [wt/vol]) was added to the C-SH12 culture, and 0.5% (wt/vol) S0 served as the energy source for all three strains. When mineral salts solution plus 0.5% (wt/vol) flowers of sulfur was prepared, the sulfur and medium were autoclaved at 105°C for 20 min on three consecutive days. The yeast extract was autoclaved separately prior to being added to the mineral salts solution. The growth temperature was 45 ± 1°C, and the growth medium was sparged with CO2-enriched (2% [vol/vol]) air.
MTFe-1 was inoculated from frozen stocks (−80°C) directly into shake flasks containing 100 ml of mineral salts medium (adjusted to pH 1.7 with H2SO4) containing 50 mM FeSO4, pH 2.0 (autoclaved separately), and 0.01% (wt/vol) yeast extract. The shake flask was incubated at 45 ± 1°C and sparged with CO2-enriched (2% [vol/vol]) air for approximately 48 h (until the culture became visibly turbid with bacteria and the culture turned from green to orange, indicating that ferrous iron was oxidized to ferric iron). After initial growth on ferrous iron, a 5-ml inoculum of MTFe-1 culture was transferred to shake flasks containing 100 ml of basal salts solution (pH 2.0) and 0.5% (wt/vol) autoclaved mineral. The basal salts solution and the mineral were autoclaved together, and the pH was readjusted to 2.0 with sterile 5 M H2SO4. The shake flasks were incubated for 3 to 5 days until a color change caused by jarosite formation from leached mineral was observed. The MTFe-1 was then further subcultured into 1.0% (wt/vol) mineral in basal salts solution and finally in 2.0% (wt/vol) mineral prior to inoculation into the stirred-tank reaction vessels.
Verification of cultures by SIMA.
Prior to the initiation of the leaching experiments the cultures were checked by slot immunobinding assay (SIMA) (3). The MTFe-1 culture was verified as containing no KU or BC13 prior to addition to the leaching vessel; unfortunately, it was not possible to verify the absence of C-SH12 by this method (10). The cultures were also tested at the end of the leaching run to ensure that no contamination had taken place during the course of the experiment.
Mineral and leaching experiments.
Boliden Mineral AB, Boliden, Sweden, provided the Rakkejaur concentrate employed in the experiments. Rakkejaur mineral is a complex fine-grained ore from northern Sweden. The Rakkejaur mineral concentrate is a combination of a number of batches that have been concentrated by flotation. It contained 1.9 g of Au/ton, 129 g of Ag/ton, 38.7% Fe, 5.85% Zn, 1.24% As, and 44.7% S and was passed through a sieve with a pore size of 28 μm. The leaching experiments were carried out in stirred-tank reaction vessels containing 1.5 liters of mineral salts medium (pH 2.0) and 5.0% (wt/vol) arsenopyrite. The reaction vessels were incubated at 45 ± 1°C and sparged with 2% (vol/vol) CO2-enriched air at 300 ml/min. To two of the experiments, 0.02% (wt/vol) yeast extract was added, and finally, in two different experiments, CO2-enriched air was replaced with pure air (300 ml/min) as specified.
The inoculum was transferred from the 100-ml shake flasks where it was grown on mineral (MTFe-1) or flowers of sulfur (T. caldus KU, BC13, or C-SH12) to the 1.5-liter leaching vessels. The unoxidized mineral and flowers of sulfur were allowed to settle before the supernatant at the top of the shake flask was removed and the cells were counted and then used as inoculum to the stirred-tank reactor vessel. Cell counts were carried out to ensure that 2.5 · 107 cells of each species were added to the vessels (except the two uninoculated control experiments); i.e., MTFe-1 in pure culture would have 2.5 · 107 cells added to the reaction vessel, whereas for a mixed culture, 2.5 · 107 cells of MTFe-1 and 2.5 · 107 cells of the T. caldus strain were added. The culture was adjusted to pH 2.0 at the start of the experiment and was manually maintained at this pH by the addition of sterile H2SO4. Once the sulfur released from the arsenopyrite was oxidized, with the concurrent production of acid, the pH naturally dropped below 2.0 and was no longer adjusted. All experiments were carried out in duplicate and average values and standard deviations are presented.
Analysis of degree of leaching and levels of reduced inorganic sulfur compounds.
The progress of the mineral leaching was followed by monitoring the release of total iron (Fetot) and dissolved iron (Fesup), measured by atomic adsorption spectroscopy of acid-digested homogeneous samples removed from the bioreactors (0.2 ml of the sample plus 1.8 ml of 5 M HCl for 30 min at 65°C with dilution in 0.3 M HCl). Total iron is a measurement of both Fe2+ and Fe3+ in solution as well as the iron that had precipitated and subsequently been redissolved when mixed with 5 M HCl. Soluble iron (both Fe2+ and Fe3+) was measured by atomic adsorption spectroscopy of centrifuged samples, and soluble Fe2+ was monitored by titration with ceric sulfate (15). The titration was carried out by mixing 0.2 ml of supernatant with 1 ml of 1 M H2SO4 and 0.2 ml of indicator solution (15 mM 1,10-phenanthronine in 5 mM H2SO4). This was titrated with ceric sulfate [1 mM H4Ce(SO4)4 in 1 M H2SO4].
Soluble tetrathionate was analyzed by cyanolysis (22) as modified by Kelly et al. (14). Samples (1 ml) from the bioleaching vessel were centrifuged at 18,000 × g for 30 min to remove all mineral residue, precipitates, and S0, leaving the particle-free supernatant containing the soluble tetrathionate. The elemental sulfur concentration was determined by cyanolysis as described previously (11). A 100-μl culture sample was centrifuged at 18,000 × g for 30 min, and the supernatant was discarded. The pellet containing the S0 was dissolved in acetone. The remaining mineral was removed by centrifugation (3,000 × g for 30 s), and the acetone containing the S0 was analyzed. The pH and redox potential (a Pt electrode against a Ag0-AgCl reference) were also monitored during the progress of the leaching.
Chemicals.
All chemicals were of analytical grade and were purchased from commercial sources.
RESULTS
In order to test the effect of T. caldus on the kinetics of arsenopyrite leaching, pure and defined mixed cultures of S. thermosulfidooxidans and T. caldus were carried out in 1.5-liter stirred-tank reaction vessels with 5% (wt/vol) mineral at 45°C. The rate and total amount of iron leached was followed, along with the concentrations of tetrathionate and S0.
Testing of cultures by SIMA.
A SIMA with anti-KU antibodies confirmed that there was no contaminating T. caldus in any of the MTFe-1 inocula or at the end of the leaching experiment with MTFe-1 (data not shown). There was also no contamination of C-SH12 by KU or BC13 (data not shown).
Chemical leaching by uninoculated controls.
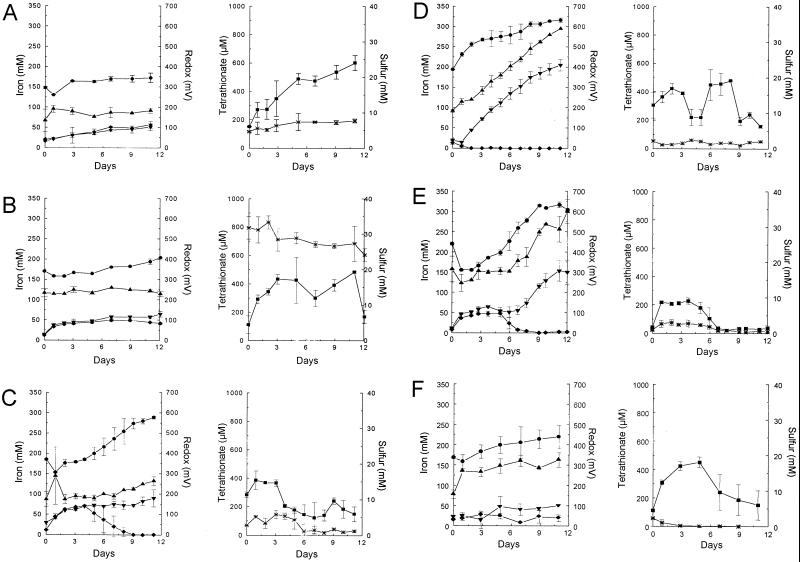
The increase in Fetot as a result of spontaneous chemical leaching of a sterile uninoculated mineral suspension was very small (14% of available Fe [Fig. 1A]). The small increase over the first 10 days coincided with an increase in the tetrathionate and S0 concentrations. In contrast to the Fetot, the Fe2+ continued to rise at a constant rate over the duration of the experiment, reaching 66 mM after 26 days. The redox potential of the solution never rose above 400 mV, which is indicative of a lack of Fe2+-to-Fe3+ oxidation. The S0 concentration rose from 6.1 ± 0.1 mM to a peak of 24.1 ± 2.1 mM after 11 days before remaining stable at approximately 18 mM (Fig. 1A and Table 1). The lack of oxidation of reduced inorganic sulfur compound was reflected in the pH, which increased over the entire experiment (data not shown).
FIG. 1.
Arsenopyrite leaching by the uninoculated control (A), BC13 (B), a pure culture of MTFe-1 (C), MTFe-1 with yeast extract (D), MTFe-1 plus BC13 (E), and BC13 without additional CO2 (F). Symbols: •, redox potential; ▴, Fetot; ▾, Fesup; ⧫, Fe2+; ∗, tetrathionate, and ■, S0. The error bars indicate standard deviations.
TABLE 1.
Total Fe released and maximum rates of release
| Culture | Rate of total Fe released (mM/day)a
|
Total Fe yield (mM)b
|
||
|---|---|---|---|---|
| Average | SDc | Average | SD | |
| MTFe-1 | 7.5 | 1.77 | 133 | 11 |
| MTFe-1 + 0.02% yeast extract | 18.2 | 0.2 | 295 | 1 |
| MTFe-1 + KU | 40.5 | 4.1 | 360 | 8 |
| MTFe-1 + BC13 | 30.0 | 4.0 | 306 | 4 |
| MTFe-1 + C-SH12 | 7.8 | 1.8 | 147 | 38 |
| KU | 2.6 | 0.2 | 108 | 4 |
| BC13 | 2.2 | 0.1 | 115 | 7 |
| C-SH12 | 2.2 | 0.2 | 120 | 7 |
| MTFe-1 + KU (no additional CO2) | 11.6 | 1.5 | 178 | 18 |
| MTFe-1 + BC13 (no additional CO2) | 5.1 | 1.1 | 163 | 18 |
| Uninoculated | 2.3 | 0.1 | 87 | 8 |
| Uninoculated + 0.02% yeast extract | 4.6 | 1.8 | 125 | 7 |
Release of iron was plotted for each of the replicates, and the slope of the line at the maximum rate was calculated.
An average value of the maximum total Fe released at the end point of the experiments or after 11 days (the approximate time for the fastest experiments to reach a plateau).
SD, standard deviation (n = 2).
The uninoculated control plus 0.02% (wt/vol) yeast extract showed an increase in both the mineral-leaching rate and total mineral dissolved compared to the uninoculated control without yeast extract (Table 1). The rates of increase in the S0 concentrations were very similar in the two uninoculated controls until day 7, when both sets of flasks had approximately 18 mM S0. After day 7, the concentration of sulfur in the control flask containing yeast extract remained constant whereas the control flasks without yeast extract continued to accumulate sulfur until day 11, when a peak sulfur concentration of 24 mM was reached (data not shown).
Leaching experiments with pure cultures of T. caldus strains.
Arsenopyrite leaching by the three strains of T. caldus in pure culture showed only limited variations. The rates of total iron release were similar for all three cultures, as well as being virtually identical to that of the uninoculated control (with no yeast extract) (Table 1). The maximum rate of Fe2+ production occurred over the first 3 to 5 days, reaching a concentration of over 40 mM before leveling off for all three cultures, with the rate of Fe2+ production greater than that observed for MTFe-1 in pure culture. The S0 concentration in all three cultures was kept below 20 mM, with a maximum of 17.1 ± 0.1 mM, dropping to 5.43 ± 1.1 mM at the end of the experiment (day 15) for KU. The peak S0 value for BC13 was 17.37 ± 1.34 mM, dropping to 6.58 ± 4.9 mM after day 15 (Fig. 1B). C-SH12 had a peak S0 value after 1 day of 11.1 ± 1.0 mM, which decreased to 5.8 ± 1.7 mM after 15 days. The redox potential did not rise much over 400 mV, indicating that none of the T. caldus strains can use Fe2+ or arsenopyrite as an energy source.
Leaching experiments with S. thermosulfidooxidans in pure culture.
MTFe-1 in pure culture did not leach arsenopyrite effectively (Table 1 and Fig. 1C) in the absence of yeast extract, even though the redox potential rose to 578 ± 7 mV (Fig. 1C). The redox potential increased due to the high Fe3+/Fe2+ ratio that is the main contributor to the redox potential in leaching systems, indicating that MTFe-1 was growing (Fig. 1C). The total mineral dissolution rate and the total mineral dissolved were greater than those observed in the uninoculated control (Fig. 1A and C and Table 1). The reduced inorganic sulfur compound concentrations initially increased and then decreased as a result of oxidation to sulfate, with the concurrent production of H+ producing a net decrease in the pH (from 1.73 ± 0.02 at time zero to 1.24 ± 0.06 after 47 h; data not shown).
MTFe-1 leached much more efficiently in pure culture with the addition of 0.02% (wt/vol) yeast extract (Fig. 1D) than in culture without yeast extract (85 and 38% of the available Fe, respectively, was leached). The lack of Fe2+ accumulation was reflected in the redox potential, where the value increased immediately from time zero, as opposed to the small drop that was evident in all other experiments (Fig. 1D). The level of S0 was slightly higher than without yeast extract, but as a far greater amount of the mineral was leached, the MTFe-1 culture with yeast extract must have been oxidizing S0 at a higher rate. The pH in the culture was indicative of reduced sulfur compound oxidation, as it fell from 1.73 ± 0.02 to 1.34 ± 0.01 after 48 h and was then stable for the remainder of the experiment (data not shown).
Leaching experiments with S. thermosulfidooxidans in mixed cultures with the T. caldus strains.
If KU is added to MTFe-1, then both the maximum leaching rate and the amount of iron released were increased in comparison to those with MTFe-1 in pure culture (Table 1). The rate of increase in the redox potential was also greater, and this was reflected in a smaller transient accumulation of Fe2+ (data not shown). The Fetot concentration at the end of the experiment in the mixed culture containing KU was 99.9% of the available iron, in comparison to 36% in the MTFe-1 pure culture. In the presence of KU there was no characteristic increase in S0 observed in any of the other experiments. Even though no S0 accumulated, there was still an increase in the level of tetrathionate from day 0 (from 40 ± 15 to 192 ± 7 μM after 1 day) before the concentration was reduced to approximately 42 ± 20 μM at the end of the experiment. The addition of KU also caused a large reduction in the concentration of S0 present at the end of leaching (5.9 ± 2.1 mM in the pure culture compared to 0.5 ± 0.01 mM in the MTFe-1 and KU mixed culture).
Although the maximum leaching rate and total iron dissolved were not as high in the mixed culture of MTFe-1 and BC13 as in the MTFe-1 and KU culture (Table 1), 88% of the available iron was still leached. The Fe2+ concentration rose to 48.4 ± 9.3 mM (Fig. 1E), and MTFe-1 and BC13 were a little slower in oxidizing the Fe2+ than MTFe-1 and KU (data not shown). The levels of S0 and tetrathionate in the MTFe-1 and BC13 mixed culture (Fig. 1E) were comparable to those found for MTFe-1 and KU (data not shown).
In the presence of C-SH12, 42% dissolution of the mineral was achieved (data not shown). The accumulation of Fe2+ (peak concentration, 55 ± 4 mM after 3 days) was lower than that obtained with MTFe-1 in pure culture (Fig. 1C) but greater than that with the MTFe-1 and KU mixed culture (peak concentration, 37 ± 3 mM after 1 day). The increase in S0 was much higher (from 9.6 ± 4.3 to 34 ± 15.7 mM after 4 days) than those in all of the other cultures. The oxidation of tetrathionate and S0 to sulfate was accompanied by a drop in pH from 2.00 ± 0.02 after 4 days to 1.18 ± 0.08 after 7 days (data not shown); during this time, no H2SO4 was added to the vessels.
Leaching experiments with mixed cultures without additional CO2.
In an industrial process, addition of growth supplements to the leaching vessel raises the cost and renders the process more complex. Therefore, the abilities of different mixed cultures to leach arsenopyrite in the absence of additional CO2 were compared. The results suggested that both MTFe-1 and KU in mixed culture kept growing as the levels of Fe2+ and reduced inorganic sulfur compounds were reduced to nearly zero, but the mineral was not leached as efficiently as with the addition of CO2 (Table 1). The lag time observed before leaching started suggested that the microorganisms had to adapt to the new environment with a reduced level of CO2 (data not shown).
The mineral-leaching efficiency of MTFe-1 and BC13 in mixed culture without additional CO2 was slightly lower than that of the MTFe-1 and KU mixed culture without additional CO2 (Table 1 and Fig. 1F). The leaching rate was not as great as for MTFe-1 plus KU without additional CO2, but at the end of the experiment a higher percentage of the total available iron had been released (51% after 13 days and 58% after 22 days for MTFe-1 plus KU and MTFe-1 plus BC13, respectively). The difference in peak S0 concentrations in the two sets of mixed cultures without additional CO2 was insignificant when the standard errors were compared (26 ± 6 and 18 ± 2 mM S0 for MTFe-1 plus KU and MTFe-1 plus BC13, respectively).
DISCUSSION
This study is the first attempt to elucidate the role that T. caldus plays in the moderately thermophilic mineral-leaching environment and whether its presence increases the efficiency of the process. Sulfur is formed as an intermediate in chemical leaching, according to the “classical” model (equation 1) or via the model proposed by Schippers et al. (21):
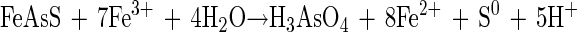 |
(1)
If the S0 is not metabolized, then its accumulation will cause a layer to form on the surface of the mineral, as well as S0 globules in the liquid medium and in the periplasms of the bacteria (19, 23). It is apparent from the transient nature of the S0 accumulation in the S. thermosulfidooxidans pure cultures that a portion of the S0 was biologically oxidized. The concentration of S0 never reaches zero, and therefore some is available to support the presence of T. caldus. This is made evident by the addition of KU or BC13 to the MTFe-1 culture, as the sulfur accumulation is lower in the mixed cultures.
It can be hypothesized that the addition of T. caldus affects arsenopyrite-leaching efficiency via three mechanisms. In the first mechanism, oxidation of S0 increases the leaching rate by removing the S0 that builds up on the mineral surface, allowing bacterial and chemical (Fe3+) access to the mineral. A close contact between the bacteria and the mineral is required, as it has been shown that the bacteria need to be attached to the surface of the mineral to be able to leach (4). This has also been shown for ferric iron precipitation, which inhibits leaching by preventing contact between the leaching agent and the mineral (1). The results suggest that a possible value for the inhibitory concentration of S0 is approximately 20 mM for 5% (wt/vol) arsenopyrite. It is at this point that leaching is inhibited in the uninoculated controls, as well as in the MTFe-1 and C-SH12 mixed culture, where metal solubilization does not proceed until the concentration of S0 is reduced below 20 mM (data not shown).
The second possible mechanism by which T. caldus may affect the arsenopyrite-leaching rate is the production of organic growth factors that may stimulate heterotrophic and mixotrophic growth of bacteria. The level of S0 in the pure culture of MTFe-1 (without yeast extract) was reduced after the initial increase, and therefore MTFe-1 must be scavenging for growth factors in the medium. Possible sources for the organic material could be air- and waterborne contaminants (7) and microorganisms present on the mineral prior to autoclaving. It has been observed that some autotrophic acidophiles release organic material into the medium (5). Therefore, it can be hypothesized that another role of T. caldus might be to provide organic material in a form of cross-feeding that aids mixotrophic growth. This cross-feeding could also be in the form of a symbiotic relationship, as MTFe-1 may reduce the concentration of organic chemicals that can inhibit the growth of some chemolithoautotrophic acidophiles by accumulation in the cytoplasm (2, 12, 17). The fact that the three strains of T. caldus do not efficiently oxidize S0 and tetrathionate in pure culture lends weight to this hypothesis. Cross-feeding of S. thermosulfidooxidans by other acidophilic species has been observed during mixed culture ferrous iron oxidation with Acidimicrobium ferrooxidans (6).
The third hypothetical mechanism by which T. caldus affects arsenopyrite leaching is the production of surface-active agents to solubilize the S0. The yeast extract may act as a wetting agent to allow S0 to be dispersed in the medium, thereby allowing further oxidation of the mineral. T. caldus has also been observed to release surface-active agents to aid in oxidation of S0 (unpublished observation).
The leaching efficiency was also increased in the defined mixed culture by the addition of any of the three strains of T. caldus, with KU being the best, followed by BC13, and finally C-SH12, which had little effect under the experimental conditions tested. This is in agreement with many studies that suggest that a mixed culture of specialized bacteria is more efficient at leaching than a pure culture (24, 25).
S. thermosulfidooxidans and T. caldus both require supplemental CO2 for optimum growth; therefore, the leaching of arsenopyrite by a mixed culture of MTFe-1 and BC13 without additional CO2 was in conditions that were below optimal, resulting in a lower leaching rate. The growth of moderate thermophiles without additional CO2 was particularly affected, as the solubility of CO2 is reduced at higher temperatures.
ACKNOWLEDGMENTS
The technical assistance of Siv Sääf is gratefully appreciated, and we also thank Kevin Hallberg for critical discussions.
This research was funded by Boliden Mineral AB and the Alf Åkerman fund. Mark Dopson also thanks the J. C. Kempe Foundation for support.
REFERENCES
- 1.Ahonen L, Tuovinen O H. Bacterial leaching of complex sulfide ore samples in bench-scale column reactors. Hydrometallurgy. 1995;37:1–21. [Google Scholar]
- 2.Alexander B, Leach S, Ingledew W J. The relationship between chemiosmotic parameters and sensitivity to anions and organic acids in the acidophile Thiobacillus ferrooxidans. J Gen Microbiol. 1987;133:1171–1179. [Google Scholar]
- 3.Amaro A M, Hallberg K B, Lindström E B, Jerez C A. An immunological assay for detection and enumeration of thermophilic biomining microorganisms. Appl Environ Microbiol. 1994;60:3470–3473. doi: 10.1128/aem.60.9.3470-3473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arredondo R, Garcia A, Jerez C A. Partial removal of lipopolysaccharide for Thiobacillus ferrooxidans affects its adhesion to solids. Appl Environ Microbiol. 1994;60:2846–2851. doi: 10.1128/aem.60.8.2846-2851.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borichewski R M. Keto acids as growth-limiting factors in autotrophic growth of Thiobacillus thiooxidans. J Bacteriol. 1967;93:597–599. doi: 10.1128/jb.93.2.597-599.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark D A, Norris P R. Acidimicrobium ferrooxidans gen. nov., sp. nov.: mixed-culture ferrous iron oxidation with Sulfobacillus species. Microbiology. 1996;142:785–790. doi: 10.1099/00221287-142-4-785. [DOI] [PubMed] [Google Scholar]
- 7.Geller A. Growth of bacteria in inorganic medium at different levels of airborne organic substances. Appl Environ Microbiol. 1983;46:1258–1262. doi: 10.1128/aem.46.6.1258-1262.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Hallberg, K. B. Personal communication.
- 8.Hallberg K B, Dopson M, Lindström E B. Reduced sulfur compound oxidation by Thiobacillus caldus. J Bacteriol. 1996;178:6–11. doi: 10.1128/jb.178.1.6-11.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallberg K B, Lindström E B. Characterization of Thiobacillus caldus, sp. nov., a moderately thermophilic acidophile. Microbiology. 1994;140:3451–3456. doi: 10.1099/13500872-140-12-3451. [DOI] [PubMed] [Google Scholar]
- 10.Hallberg K B, Lindström E B. Multiple serotypes of the moderate thermophile Thiobacillus caldus, a limitation of immunological assays for biomining microorganisms. Appl Environ Microbiol. 1996;62:4243–4246. doi: 10.1128/aem.62.11.4243-4246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazeu W, Batenburg-van der Vegte W H, Bos P, van der Pas R K, Kuenen J G. The production and utilization of intermediary elemental sulfur during the oxidation of reduced sulfur compounds by Thiobacillus ferrooxidans. Arch Microbiol. 1988;150:574–579. [Google Scholar]
- 12.Ingledew W J. Thiobacillus ferrooxidans. The bioenergetics of an acidophilic chemolithotroph. Biochim Biophys Acta. 1982;683:89–117. doi: 10.1016/0304-4173(82)90007-6. [DOI] [PubMed] [Google Scholar]
- 13.Johnson D B. Selective solid media for isolating and enumerating acidophilic bacteria. J Microbiol Methods. 1995;23:205–218. [Google Scholar]
- 14.Kelly D P, Chambers L A, Trudinger P A. Cyanolysis and spectrophotometric estimation of trithionate in mixture with thiosulfate and tetrathionate. Anal Chem. 1969;41:898–902. [Google Scholar]
- 15.Kolthoff J M, Sandell E B. Textbook of quantitative inorganic chemistry. New York, N.Y: MacMillan Publishing Co.; 1963. [Google Scholar]
- 16.Marsh R M, Norris P R. The isolation of some thermophilic, autotrophic, iron- and sulfur-oxidizing bacteria. FEMS Microbiol Lett. 1983;17:311–315. [Google Scholar]
- 17.Matin A. Organic nutrition of chemolithotrophic bacteria. Annu Rev Microbiol. 1978;32:433–468. doi: 10.1146/annurev.mi.32.100178.002245. [DOI] [PubMed] [Google Scholar]
- 18.Norris P R, Clark D A, Owens J P, Waterhouse S. Characteristics of Sulfobacillus acidophilus sp. nov. and other moderately thermophilic mineral-sulphide-oxidizing bacteria. Microbiology. 1996;142:775–783. doi: 10.1099/00221287-142-4-775. [DOI] [PubMed] [Google Scholar]
- 19.Rojas J, Giersig M, Tributsch H. Sulfur colloids as temporary energy reservoirs for Thiobacillus ferrooxidans during pyrite oxidation. Arch Microbiol. 1995;163:352–356. [Google Scholar]
- 20.Sand W, Rohde K, Sobotke B, Zenneck C. Evaluation of Leptospirillum ferrooxidans for leaching. Appl Environ Microbiol. 1992;58:85–92. doi: 10.1128/aem.58.1.85-92.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schippers A, Jozsa P G, Sand W. Sulfur chemistry in bacterial leaching of pyrite. Appl Environ Microbiol. 1996;62:3424–3431. doi: 10.1128/aem.62.9.3424-3431.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorbö B. A colorimetric method for the determination of thiosulfate. Biochim Biophys Acta. 1957;23:412–416. doi: 10.1016/0006-3002(57)90346-3. [DOI] [PubMed] [Google Scholar]
- 23.Steudel R, Holdt G, Göbel T, Hazeu W. Chromatographic separation of higher polythionates SnO62−(n = 3−22) and their detection in cultures of Thiobacillus ferrooxidans; molecular composition of bacterial sulfur excretions. Angew Chem Int Ed Engl. 1987;26:151–153. [Google Scholar]
- 24.Tuovinen O H, Bhatti T M, Bigham J M, Hallberg K B, Garcia O, Jr, Lindström E B. Oxidative dissolution of arsenopyrite by mesophilic and moderately thermophilic acidophiles. Appl Environ Microbiol. 1994;60:3268–3274. doi: 10.1128/aem.60.9.3268-3274.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuovinen O H, Kelley B C, Groudev S N. Mixed cultures in biological leaching processes and mineral biotechnology. In: Zeikus J G, Johnson E A, editors. Mixed cultures in biotechnology. New York, N.Y: McGraw-Hill; 1991. pp. 373–425. [Google Scholar]