Abstract
Background
Obesity has been shown to have a positive mortality benefit in patients undergoing percutaneous coronary intervention and dialysis and those with rheumatoid arthritis, chronic obstructive pulmonary disease, and various wasting diseases. Studies for this mortality benefit in ischemic stroke patients are conflicting and have not been well studied in hemicraniectomy patients. We sought to determine the impact of obesity on outcomes of hemicraniectomy patients.
Methods
We performed a retrospective case-control database analysis using a multi-institutional database (TriNetX) looking at obese versus non-obese patients with ischemic stroke undergoing hemicraniectomy. Our primary endpoint was mortality. Secondary endpoints included seizure, pulmonary embolism, myocardial infarction (MI), cerebral infarction, deep vein thrombosis, tracheostomy, and percutaneous endoscopic gastrostomy. Cohorts were propensity-score matched for confounders.
Results
After propensity score matching for basic demographics and common comorbidities, as well as indicators of stroke severity, 646 patients were identified that were obese and had an ischemic stroke with subsequent hemicraniectomy (cohort 1), and 646 patients were identified who were non-obese with ischemic stroke and hemicraniectomy (cohort 2). Thirty-day survival rate was 98.142% in the obese vs. 87.771% in the non-obese cohorts, 90-day survival was 85.15% vs. 79.35%, 180-day survival was 96.44% vs. 84.52%, 365-day survival was 94.272% vs. 81.734%, and five-year survival was 81.889% vs. 75.077%, respectively. At five years, risk difference was −7.276% (95% CI: −11.757, −2.794) and odds ratio was 0.666 (95% CI: 0.510, 0.871) (p = 0.0029). Despite a higher mortality rate, obese patients had a statistically significant increase in pulmonary embolism (11.61% vs. 5.108, p < 0.0001), deep venous thrombosis (16.873% vs. 9.133%, p < 0.0001), and MI (8.824% vs. 5.882%, p = 0.0428). There was no significant difference in intensive care unit length of stay, ventilator dependence, tracheostomy placement, percutaneous endoscopic gastrostomy placement, or intracerebral hemorrhage.
Conclusions
Despite the increased risk of ischemic stroke, obese patients who undergo hemicraniectomy have decreased mortality rates compared to their non-obese counterparts.
Keywords: craniectomy, obesity paradox, decompression, survival, outcomes, mortality rate, obesity, hemicraniectomy, ischemic stroke, neurosurgery
Introduction
The obesity pandemic has only gotten worse over the years without any end in sight. Numbers estimate that over two billion of the world’s population is considered obese [1]. This pandemic comes with numerous adverse outcomes in those affected including increased risks of various cancers, diabetes, cardiovascular disease, pulmonary disease, musculoskeletal problems, and premature death in addition to a range of stigma, discrimination, bias, and psychological factors that obese individuals experience. Every organ system is affected in obese individuals including the central nervous system (CNS). This effect on the CNS is believed to be from free fatty acid-induced lipotoxicity, oxidative stress, endoplasmic reticulum stress, sympathetic alterations, and metabolic dysfunction [2].
It has been well documented that obesity increases the risk of stroke [3,4]. It has been shown that for each increase in body mass index (BMI), the risk of stroke rises by roughly 6% [4]. However, paradoxically, it has been shown that obesity can provide a positive mortality benefit to these stroke patients although not all studies agree [4-16]. This paradox is known as the obesity paradox and it was first introduced in 2002 by Gruberg et al., who described an increased mortality rate in obese individuals with coronary artery disease [17]. Since then, numerous studies have been published backing the obesity paradox and showing an increased mortality benefit in conditions such as ischemic stroke, kidney disease, pulmonary disease exacerbation, and coronary artery disease [18-20]. The majority of literature outlining and detailing the obesity paradox has been shown through cardiologic, pulmonary, vascular, and renal diseases, but little is known surrounding the obesity paradox and neurosurgical operations, particularly brain operations. Physicians may not see the obesity paradox as a contributing factor in this domain as there is only a small amount of adipose tissue in and around the skull and brain compared to other areas of the body.
Although a recent study looked at mortality as an outcome for ischemic stroke patients undergoing mechanical thrombectomy and found that BMI is associated with decreased mortality, the effects of obesity on ischemic stroke patients undergoing hemicraniectomy are not well studied [21]. This study aims to determine the predictive role of obesity in stroke patients undergoing hemicraniectomy.
Materials and methods
This is a retrospective case-control study. A de-identified database network (TriNetX) was used to retrospectively query via the International Classification of Diseases, Tenth Revision (ICD-10) and Current Procedural Terminology codes to evaluate all patients with a diagnosis of ischemic stroke and obesity (ICD-10 E66) who subsequently underwent hemicraniectomy (cohort 1) and compared this to ischemic stroke patients without obesity who subsequently underwent hemicraniectomy (cohort 2). Data came from 58 healthcare organizations (HCOs) spanning six countries. This database includes demographic variables, diagnoses, medications, laboratory values, genomics, and procedures. The identity of the HCOs and patients is not disclosed to comply with ethical guidelines against patient re-identification. Because of the database's federated nature, an IRB waiver has been granted. Our use of this database and its validity was disclosed by previous literature, and exact details of the network have been previously described [22]. The index date (date from which time frames were based upon) was set at the date of hemicraniectomy.
The medical information included age at index date, as well as sex, race, and comorbidities such as hypertension, acute kidney injury, diabetes, ischemic heart disease, heart failure, atrial fibrillation, lipoprotein metabolism disorders, dyslipidemia, obesity, history of nicotine dependence, chronic respiratory disease, cirrhosis, alcohol abuse or dependence, and peripheral vascular disease, and was recorded up to the date of the index date. Antiplatelet and anticoagulation medications were likewise recorded. Our primary outcome of interest was mortality, with secondary outcomes of mechanical ventilation, tracheostomy, percutaneous endoscopic gastrostomy (PEG) tube placement, seizure, pulmonary embolism (PE), myocardial infarction (MI), ischemic stroke, and deep venous thrombosis (DVT). These outcomes were followed-up over a period of five years, with interval analysis at 90, 180, and 365 days. Analysis was performed using unmatched and propensity score-matched cohorts using the greedy-nearest neighbor algorithm with a caliper of 0.1 pooled standard deviations. Hazard ratios were calculated. Chi-square analysis was performed on categorical variables. The level of significance was set at a p-value of 0.05.
Results
A total of 1903 patients were identified with an ischemic stroke diagnosis with obesity and subsequent decompressive hemicraniectomy procedure (cohort 1), while 5530 were included in the non-obese group (cohort 2). A total of 646 patients remained in each group after propensity score matching. After matching, age at diagnosis was 53.79 ± 16.50 and 53.70 ± 19.10 years for cohorts 1 and 2, respectively. A total of 52.167% of patients in cohort 1 and 50.619% in cohort 2 were males. A total of 69.814% vs. 71.672% of patients were white, 21.517% vs. 20.124% were black or African American, and 7.121% vs. 6.037% were of unspecified race. Baseline demographics and characteristics such as diagnosis, acute thrombolytic medication, signs and symptoms, and invasive procedure status are shown in Table 1.
Table 1. Baseline demographics and characteristics after propensity score matching.
Cohort 1: Ischemic stroke diagnosis with obesity and subsequent decompressive hemicraniectomy procedure. Cohort 2: Ischemic stroke diagnosis without obesity and subsequent decompressive hemicraniectomy procedure.
ICD-10: International Classification of Diseases, Tenth Revision; CPT: Current Procedural Terminology.
| Before matching | After matching | ||||||
| Codes (ICD-10, medication, or CPT) | Diagnosis | Cohort 1, n (%) | Cohort 2, n (%) | Std. diff. | Cohort 1, n (%) | Cohort 2, n (%) | Std. diff. |
| AI | Age at index | 55.41 (100.000) | 56.26 (100.000) | - | 53.79 (100.000) | 53.69 (100.000) | - |
| M | Male | 920 (48.884) | 3230 (59.605) | 0.2165 | 337 (52.167) | 327 (50.619) | 0.030975 |
| F | Female | 962 (51.116) | 2189 (40.395) | 0.2165 | 309 (47.833) | 319 (49.381) | 0.030975 |
| 2106-3 | White | 1303 (69.235) | 3824 (70.567) | 0.0290 | 451 (69.814) | 463 (71.672) | 0.04084 |
| 2054-5 | Black or African American | 409 (21.732) | 910 (16.793) | 0.1255 | 139 (21.517) | 130 (20.124) | 0.034318 |
| 2131-1 | Unspecified race | 142 (7.545) | 564 (10.408) | 0.1003 | 46 (7.121) | 39 (6.037) | 0.043719 |
| 2028-9 | Asian | 20 (1.063) | 101 (1.864) | 0.0668 | 10 (1.548) | 13 (2.012) | 0.035126 |
| I10-I16 | Hypertensive diseases | 1476 (78.427) | 3268 (60.306) | 0.4009 | 418 (64.706) | 402 (62.229) | 0.051454 |
| E78 | Disorders of lipoprotein metabolism and other lipidemias | 982 (52.179) | 1828 (33.733) | 0.3793 | 244 (37.771) | 237 (36.687) | 0.022417 |
| E08-E13 | Diabetes mellitus, type 1 and type 2 | 786 (41.764) | 1097 (20.244) | 0.4784 | 181 (28.019) | 165 (25.542) | 0.055955 |
| R53 | Malaise and fatigue | 559 (29.702) | 1121 (20.686) | 0.2088 | 149 (23.065) | 119 (18.421) | 0.114722 |
| F17 | Nicotine dependence | 468 (24.867) | 1127 (20.797) | 0.0971 | 123 (19.040) | 104 (16.099) | 0.077343 |
| I20-I25 | Ischemic heart diseases | 525 (27.896) | 1090 (20.114) | 0.1829 | 120 (18.576) | 108 (16.718) | 0.048742 |
| R13 | Aphagia and dysphagia | 550 (29.224) | 1295 (23.897) | 0.1208 | 115 (17.802) | 108 (16.718) | 0.028677 |
| J40-J47 | Chronic lower respiratory diseases | 481 (25.558) | 825 (15.224) | 0.2586 | 107 (16.563) | 105 (16.254) | 0.00836 |
| N17-N19 | Acute kidney failure and chronic kidney disease | 501 (26.621) | 926 (17.088) | 0.2322 | 95 (14.706) | 86 (13.313) | 0.040148 |
| R40 | Somnolence, stupor, and coma | 459 (24.389) | 1214 (22.403) | 0.0469 | 82 (12.693) | 70 (10.836) | 0.057679 |
| I48 | Atrial fibrillation and flutter | 366 (19.447) | 785 (14.486) | 0.1325 | 76 (11.765) | 63 (9.752) | 0.06498 |
| I50 | Heart failure | 383 (20.351) | 610 (11.257) | 0.2513 | 71 (10.991) | 67 (10.372) | 0.020048 |
| Z87.891 | Personal history of nicotine dependence | 348 (18.491) | 587 (10.832) | 0.2178 | 65 (10.062) | 59 (9.133) | 0.031536 |
| R63 | Symptoms and signs concerning food and fluid intake | 177 (9.405) | 343 (6.330) | 0.1144 | 43 (6.656) | 42 (6.502) | 0.006244 |
| I73 | Other peripheral vascular diseases | 136 (7.226) | 232 (4.281) | 0.1267 | 35 (5.418) | 27 (4.180) | 0.057963 |
| F10.1 | Alcohol abuse | 88 (4.676) | 310 (5.721) | 0.0471 | 20 (3.096) | 16 (2.477) | 0.037629 |
| F10.2 | Alcohol dependence | 65 (3.454) | 220 (4.060) | 0.0319 | 14 (2.167) | 15 (2.322) | 0.01045 |
| K74 | Fibrosis and cirrhosis of the liver | 33 (1.753) | 60 (1.107) | 0.0544 | <10 (<1.548) | <10 (<1.548) | 0 |
| E65-E68 | Overweight, obesity, and other hyperalimentation | 1245 (66.153) | 10 (0.185) | 1.963531 | <10 (<1.548) | <10 (<1.548) | 0 |
| 1191 | Aspirin | 778 (41.339) | 1632 (30.116) | 0.2358213 | 215 (33.282) | 209 (32.353) | 0.019782 |
| 11289 | Warfarin | 172 (9.139) | 281 (5.185) | 0.1537802 | 36 (5.573) | 33 (5.108) | 0.020656 |
| 8410 | Alteplase | 140 (7.439) | 296 (5.462) | 0.08053 | 33 (5.108) | 27 (4.180) | 0.044147 |
| 1364430 | Apixaban | 65 (3.454) | 95 (1.753) | 0.1069543 | <10 (<1.548) | <10 (<1.548) | 0 |
| 1114195 | Rivaroxaban | 34 (1.807) | 67 (1.236) | 0.0465949 | <10 (<1.548) | <10 (<1.548) | 0 |
| 259280 | Tenecteplase | <10 (<0.531) | 16 (0.295) | 0.0368061 | <10 (<1.548) | <10 (<1.548) | 0 |
| 31500 | Intubation, endotracheal, emergency procedure | 173 (9.192) | 529 (9.762) | 0.0194479 | 33 (5.108) | 29 (4.489) | 0.028973 |
Thirty-day survival rates were 98.142% in the obese vs. 87.771% in the non-obese cohorts, 90-day survival rates were 85.15% vs. 79.35%, 180-day survival rates were 96.44% vs. 84.52%, 365-day survival rates were 94.272% vs. 81.734%, and five-year survival rates were 81.889% vs. 75.077%. At five years, risk difference was −7.276% (95% CI: −11.757, −2.794) and odds ratio was 0.666 (95% CI: 0.510, 0.871) (p = 0.0029).
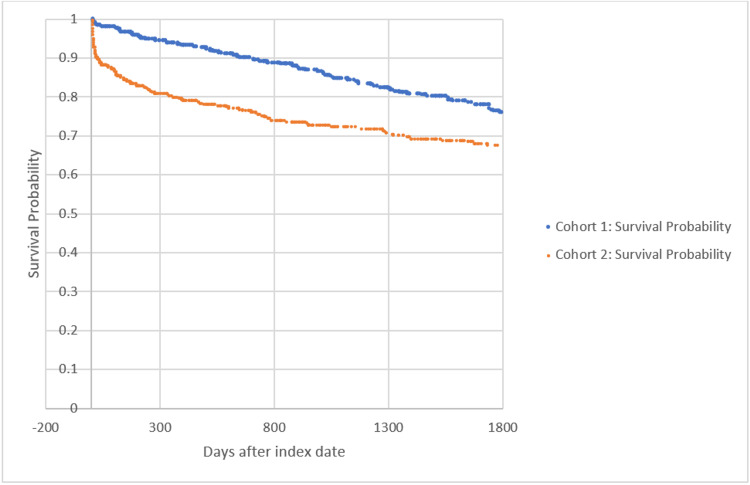
Figure 1 shows the Kaplan-Meier survival curve for mortality through five years comparing cohorts 1 and 2. The hazard ratio was 0.495, with 95% CI (0.390, 0.628) (p < 0.0001).
Figure 1. Kaplan-Meier survival analysis for primary outcome: deceased.
Cohort 1: Ischemic stroke diagnosis with obesity and subsequent decompressive hemicraniectomy procedure. Cohort 2: Ischemic stroke diagnosis without obesity and subsequent decompressive hemicraniectomy procedure.
Table 2 shows outcomes after propensity score matching. Despite a higher mortality rate, obese patients had a statistically significant increase in PE (11.61% vs. 5.108, p < 0.0001), DVT (16.873% vs. 9.133%, p < 0.0001), and MI (8.824% vs. 5.882%, p = 0.0428). Intensive care unit length of stay was also longer in obese patients at 8.441 ± 10.674 days vs. 7.522 ± 8.110 days, but this was not statistically significant (p = 0.1796). There was no significant difference in ventilator dependence, tracheostomy, PEG, or intracerebral hemorrhage.
Table 2. Outcomes after propensity score matching.
Cohort 1: Ischemic stroke diagnosis with obesity and subsequent decompressive hemicraniectomy procedure. Cohort 2: Ischemic stroke diagnosis without obesity and subsequent decompressive hemicraniectomy procedure.
| Outcome | Cohort 1, n (%) | Cohort 2, n (%) | Odds ratio (95% CI) | P-value |
| Mortality | 117 (18.111) | 161 (24.923) | 0.666 (0.510, 0.871) | 0.0029 |
| Ventilator dependence | 84 (13.003) | 75 (11.610) | 1.138 (0.816, 1.587) | 0.4459 |
| Tracheostomy | 73 (11.300) | 69 (10.681) | 1.065 (0.752, 1.510) | 0.722 |
| Percutaneous endoscopic gastrostomy | 87 (13.467) | 72 (11.146) | 1.241 (0.889, 1.732) | 0.204 |
| Pulmonary embolism | 75 (11.610) | 33 (5.108) | 2.44 (1.595, 3.732) | <0.0001 |
| Deep venous thrombosis | 109 (16.873) | 59 (9.133) | 2.019 (1.441, 2.831) | <0.0001 |
| Intracerebral hemorrhage | 213 (32.972) | 187 (28.947) | 1.207 (0.953, 1.529) | 0.1177 |
| Myocardial infarction | 57 (8.824) | 38 (5.882) | 1.548 (1.011, 2.370) | 0.0428 |
| Intensive care unit length of stay | 8.441 ± 10.674 days | 7.522 ± 8.110 days | - | 0.1796 |
Discussion
Unfortunately, the problem of obesity is likely only going to increase [4]. Ever since the concept of the obesity paradox was first introduced, studies have continued to be published detailing the increased mortality benefit in patients with higher BMI in diseases such as heart failure, percutaneous coronary intervention, and dialysis, and those with rheumatoid arthritis, chronic obstructive pulmonary disease, and various wasting diseases [4,23-27]. However, the obesity paradox is still controversial and often literature against the obesity paradox will cite confounding factors associated with the findings. Examples include how BMI is calculated and individuals with a higher muscle mass will have a higher BMI and will be considered obese despite less adipose tissue. Another confounding variable is that most conditions studied have been chronic in nature, which tends to favor older individuals and may not accurately depict the condition [28].
When assessing the obesity paradox in neurological conditions, it has been shown that this paradox exists for conditions such as intracerebral hemorrhage and subarachnoid hemorrhage [29,30]. Researchers found 12 relevant studies that assess stroke and the obesity paradox. Of these 12 studies, 10 have shown higher BMI to be associated with lower mortality [4-14]. Two of them did not show such an association, when adjusted for stroke severity and when looking at mortality only within a 30-day period [15,16]. To our knowledge, this is the first study looking at and approaching a possible outcome correlation between ischemic stroke and obesity in patients' mortality after hemicraniectomy.
Our results demonstrate a significant decrease in mortality in patients with obesity who have undergone decompressive hemicraniectomy after ischemic stroke, as seen in the Kaplan-Meier survival curve in Figure 1, despite an increased rate of PE, DVT, and MI in the obese cohort. This study could be expanded on using prospective longitudinal cohort analysis to assess the validity of the data presented.
Our analysis was not without limitations. The major limitation of this study was that it was retrospective. Furthermore, due to the nature of the database, we were unable to collect patient-level data on specific outcomes. We were unable to report on radiology information. We do not have information on the type of diagnostic test used for confirmation of disease. The BMI is not disclosed, and we do not know the adipose to muscle ratio. In addition, some misidentification is inevitable in database studies.
Conclusions
Obesity is a health condition that is becoming more prominent. And with increased obesity comes an increased risk of stroke. In this study, we examined the impact of obesity on stroke patients who undergo hemicraniectomy as a life-saving procedure. Our results denote that, paradoxically, obesity is a protective factor against the mortality of this procedure, as compared to an obese patient's non-obese counterpart. Further studies are needed to understand the physiological explanation of these findings, for recommendations to be made concerning patient weight in this patient population.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Humans against obesity: who will win? Caballero B. Adv Nutr. 2019;10:0–9. doi: 10.1093/advances/nmy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neurological consequences of obesity. O'Brien PD, Hinder LM, Callaghan BC, Feldman EL. Lancet Neurol. 2017;16:465–477. doi: 10.1016/S1474-4422(17)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Effect of body mass index on outcomes of mechanical thrombectomy in acute ischemic stroke. Chen SH, McCarthy D, Saini V, Brunet MC, Peterson EC, Yavagal D, Starke RM. World Neurosurg. 2020;143:0–15. doi: 10.1016/j.wneu.2020.07.220. [DOI] [PubMed] [Google Scholar]
- 4.Obesity paradox in stroke - myth or reality? A systematic review. Oesch L, Tatlisumak T, Arnold M, Sarikaya H. PLoS One. 2017;12:0. doi: 10.1371/journal.pone.0171334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Body mass index and poststroke mortality. Olsen TS, Dehlendorff C, Petersen HG, Andersen KK. Neuroepidemiology. 2008;30:93–100. doi: 10.1159/000118945. [DOI] [PubMed] [Google Scholar]
- 6.Overweight and obesity are associated with improved survival, functional outcome, and stroke recurrence after acute stroke or transient ischaemic attack: observations from the TEMPiS trial. Doehner W, Schenkel J, Anker SD, Springer J, Audebert HJ. Eur Heart J. 2013;34:268–277. doi: 10.1093/eurheartj/ehs340. [DOI] [PubMed] [Google Scholar]
- 7.The obesity paradox in stroke: lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Andersen KK, Olsen TS. Int J Stroke. 2015;10:99–104. doi: 10.1111/ijs.12016. [DOI] [PubMed] [Google Scholar]
- 8.The impact of body mass index on mortality after stroke. Towfighi A, Ovbiagele B. Stroke. 2009;40:2704–2708. doi: 10.1161/STROKEAHA.109.550228. [DOI] [PubMed] [Google Scholar]
- 9.Association between obesity and mortality after acute first-ever stroke: the obesity-stroke paradox. Vemmos K, Ntaios G, Spengos K, et al. Stroke. 2011;42:30–36. doi: 10.1161/STROKEAHA.110.593434. [DOI] [PubMed] [Google Scholar]
- 10.Paradoxical longevity in obese patients with intracerebral hemorrhage. Kim BJ, Lee SH, Ryu WS, Kim CK, Lee J, Yoon BW. Neurology. 2011;76:567–573. doi: 10.1212/WNL.0b013e31820b7667. [DOI] [PubMed] [Google Scholar]
- 11.Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Kim BJ, Lee SH, Jung KH, Yu KH, Lee BC, Roh JK. Neurology. 2012;79:856–863. doi: 10.1212/WNL.0b013e318266fad1. [DOI] [PubMed] [Google Scholar]
- 12.Prestroke factors associated with poststroke mortality and recovery in older women in the Women's Health Initiative. Bell CL, LaCroix A, Masaki K, et al. J Am Geriatr Soc. 2013;61:1324–1330. doi: 10.1111/jgs.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Association of body mass index and mortality after acute ischemic stroke. Skolarus LE, Sanchez BN, Levine DA, et al. Circ Cardiovasc Qual Outcomes. 2014;7:64–69. doi: 10.1161/CIRCOUTCOMES.113.000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favorable functional recovery in overweight ischemic stroke survivors: findings from the China National Stroke Registry. Zhao L, Du W, Zhao X, et al. J Stroke Cerebrovasc Dis. 2014;23:0–6. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Body mass index and death by stroke: no obesity paradox. Dehlendorff C, Andersen KK, Olsen TS. JAMA Neurol. 2014;71:978–984. doi: 10.1001/jamaneurol.2014.1017. [DOI] [PubMed] [Google Scholar]
- 16.Body mass index, initial neurological severity and long-term mortality in ischemic stroke. Ryu WS, Lee SH, Kim CK, Kim BJ, Yoon BW. Cerebrovasc Dis. 2011;32:170–176. doi: 10.1159/000328250. [DOI] [PubMed] [Google Scholar]
- 17.The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? Gruberg L, Weissman NJ, Waksman R, et al. J Am Coll Cardiol. 2002;39:578–584. doi: 10.1016/s0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- 18.Obesity and mortality after the first ischemic stroke: is obesity paradox real? Chaudhary D, Khan A, Gupta M, Hu Y, Li J, Abedi V, Zand R. PLoS One. 2021;16:0. doi: 10.1371/journal.pone.0246877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patients with obesity have better long-term outcomes after hospitalization for COPD exacerbation. DeLapp DA, Glick C, Furmanek S, Ramirez JA, Cavallazzi R. COPD. 2020;17:373–377. doi: 10.1080/15412555.2020.1781805. [DOI] [PubMed] [Google Scholar]
- 20.Obesity paradox in advanced kidney disease: from bedside to the bench. Naderi N, Kleine CE, Park C, et al. Prog Cardiovasc Dis. 2018;61:168–181. doi: 10.1016/j.pcad.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obesity and mechanical thrombectomy. Hallan DR. Cureus. 2021;13:0. doi: 10.7759/cureus.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Using a federated network of real-world data to optimize clinical trials operations. Topaloglu U, Palchuk MB. JCO Clin Cancer Inform. 2018;2:1–10. doi: 10.1200/CCI.17.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Body mass index and mortality in heart failure: a meta-analysis. Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Obesity paradox in a cohort of 4880 consecutive patients undergoing percutaneous coronary intervention. Hastie CE, Padmanabhan S, Slack R, et al. Eur Heart J. 2010;31:222–226. doi: 10.1093/eurheartj/ehp317. [DOI] [PubMed] [Google Scholar]
- 25.Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 26.Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Escalante A, Haas RW, del Rincón I. Arch Intern Med. 2005;165:1624–1629. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 27.Risk factor paradox in wasting diseases. Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, Morley JE. Curr Opin Clin Nutr Metab Care. 2007;10:433–442. doi: 10.1097/MCO.0b013e3281a30594. [DOI] [PubMed] [Google Scholar]
- 28.The obesity paradox in cancer: clinical insights and perspectives. Trestini I, Carbognin L, Bonaiuto C, Tortora G, Bria E. Eat Weight Disord. 2018;23:185–193. doi: 10.1007/s40519-018-0489-y. [DOI] [PubMed] [Google Scholar]
- 29.Obesity paradox in intracerebral hemorrhage. Persaud SR, Lieber AC, Donath E, et al. Stroke. 2019;50:999–1002. doi: 10.1161/STROKEAHA.119.024638. [DOI] [PubMed] [Google Scholar]
- 30.Body mass index and aneurysmal subarachnoid hemorrhage: decreasing mortality with increasing body mass index. Hughes JD, Samarage M, Burrows AM, Lanzino G, Rabinstein AA. World Neurosurg. 2015;84:1598–1604. doi: 10.1016/j.wneu.2015.07.019. [DOI] [PubMed] [Google Scholar]