Abstract
Background:
Household air pollution (HAP) from biomass fuel combustion remains a leading environmental risk factor for morbidity worldwide.
Objective:
Measure the effect of liquefied petroleum gas (LPG) interventions on HAP exposures in Puno, Peru.
Methods:
We conducted a 1-y randomized controlled trial followed by a 1-y pragmatic crossover trial in 180 women age 25–64 y. During the first year, intervention participants received a free LPG stove, continuous fuel delivery, and regular behavioral messaging, whereas controls continued their biomass cooking practices. During the second year, control participants received a free LPG stove, regular behavioral messaging, and vouchers to obtain LPG tanks from a nearby distributor, whereas fuel distribution stopped for intervention participants. We collected 48-h kitchen area concentrations and personal exposures to fine particulate matter (PM) with aerodynamic diameter (), black carbon (BC), and carbon monoxide (CO) at baseline and 3-, 6-, 12-, 18-, and 24-months post randomization.
Results:
Baseline (kitchen area concentrations vs. ; personal exposure vs. ), CO (kitchen vs. ; personal vs. ), and BC (kitchen vs. ; personal vs. ) were similar between control and intervention participants. Intervention participants had consistently lower concentrations at the 12-month visit for kitchen (, , and ) and personal exposures (, , and ) to , BC, and CO when compared to controls during the first year. In the second year, we observed comparable HAP reductions among controls after the voucher-based intervention for LPG fuel was implemented (24-month visit , BC, and CO kitchen mean concentrations of , , and and personal exposures of , , and , respectively), and average reductions were present among intervention participants even after free fuel distribution stopped (24-month visit , BC, and CO kitchen mean concentrations of , , and and personal exposures of , , and , respectively).
Discussion:
Both home delivery and voucher-based provision of free LPG over a 1-y period, in combination with provision of a free LPG stove and longitudinal behavioral messaging, reduced HAP to levels below 24-h World Health Organization air quality guidelines. Moreover, the effects of the intervention on HAP persisted for a year after fuel delivery stopped. Such strategies could be applied in LPG programs to reduce HAP and potentially improve health. https://doi.org/10.1289/EHP10054
Introduction
Household air pollution (HAP) from the combustion of solid fuels such as wood, crop waste, and animal dung is among the leading environmental risk factors for preventable disease (Bennitt et al. 2021; Bruce et al. 2000; Cohen et al. 2017; Smith et al. 2014; WHO 2016). Approximately people worldwide, mostly in low- and middle-income countries (LMICs), use biomass fuels as their primary energy source for cooking (Murray et al. 2020). Exposure to HAP from biomass cookstoves was estimated to be responsible for deaths and disability-adjusted life-years in 2019 (Murray et al. 2020).
The incomplete combustion of biomass fuels produces a complex mixture of gases and particulate pollutants (Fullerton et al. 2008). Particulate matter (PM) and carbon monoxide (CO) are the most commonly measured pollutants for cookstoves (Pope et al. 2017; Thomas et al. 2015). The World Health Organization (WHO) has developed indoor air pollution guidelines (WHO 2006, 2010, 2014) recognizing the health impacts of biomass cookstoves in low-income households. For example, the WHO recommends that 24-h indoor mean concentrations be (WHO 2014), although a growing body of evidence suggests that there is no safe level of exposure (Burnett et al. 2018; Liu et al. 2019; Raaschou-Nielsen et al. 2013; WHO 2006). Black carbon (BC), one of the main components of resulting from the incomplete combustion of carbonaceous materials, has been found to play an important role in a range of health effects, including cardiovascular and respiratory disease development (Kelly and Fussell 2012; WHO 2012). However, evidence on BC exposures from biomass cookstoves in LMICs is limited (Achilleos et al. 2017; Rohr and McDonald 2016; Soneja et al. 2016).
Early efforts to reduce HAP exposures have focused on improving the combustion efficiency of biomass cookstoves or directing smoke outdoors by building chimneys (Gordon et al. 2014; The World Bank 2010). Although improved biomass cookstove interventions have achieved important reductions in emissions and indoor concentrations, HAP concentrations remained several fold higher than the WHO recommended guidelines as shown in literature reviews (Balmes et al. 2014; Pope et al. 2017, 2021). Thus, intervention efforts are shifting toward stoves that use cleaner fuels such as liquefied petroleum gas (LPG) (Albalak et al. 2001; Anderman et al. 2015; Begum et al. 2009; Checkley et al. 2021; Clasen et al. 2020; Dutta et al. 2012, 2018; Naeher et al. 2000; Nie et al. 2016; Pollard et al. 2018; Sukhsohale et al. 2013). However, many previous HAP studies involving LPG stoves lack personal exposure measurements (Bruce et al. 2000; Pope et al. 2017), which could help reduce bias from measurement error when quantifying the relationship between HAP and health risks. Finally, few cookstove studies have collected direct-reading measurements (Bruce et al. 2000; Pope et al. 2017, 2021), which could enable assessment of temporal variability of short-term high-concentration exposures (Clark et al. 2013) and provide powerful information to detect relationships with health outcomes (Koehler and Peters 2015).
To address current gaps and better understand the effect of a biomass-to-LPG cleaner-cooking intervention on HAP exposures, we conducted a 1-y randomized controlled trial followed by a 1-y pragmatic crossover trial of two LPG intervention strategies (Fandiño-Del-Rio et al. 2017). In the first year, intervention participants were assigned to receive an LPG stove and education on its use and benefits, regular behavioral messaging, and free fuel delivered to their homes. Control participants were asked to continue their usual cooking practices in the first year. During the second year, control participants received the same LPG stove and education, regular behavioral messaging, and vouchers to obtain LPG tanks from a nearby distributor instead of home-based delivery of LPG; fuel delivery and behavioral messaging were discontinued in intervention participants, but they were permitted to keep their LPG stoves (Fandiño-Del-Rio et al. 2017). We have previously reported on reductions in mean kitchen area concentrations and personal exposures to HAP during the first year (Checkley et al. 2021). Specifically, averaged first-year postrandomization concentrations of 48-h time-weighted means were found to be lower in intervention participants when compared to controls for kitchen area concentrations of (58.0 vs. ), BC (5.0 vs. ), and CO (6.0 vs. ). We also reported lower personal exposures to (30.0 vs. ), BC (2.0 vs. ), and CO (2.4 vs. ) in intervention participants when compared to control participants.
Here, we present a more detailed summary of longitudinal HAP reductions resulting from our interventions, using the rich, direct-reading measurements and data on compliance with wearing the personal monitors. We also report on the effectiveness of the intervention in reducing HAP using data collected during the pragmatic trial in the second year, when we provided control participants with an LPG stove and behavioral messaging but asked them to pick up free fuel instead of delivering it to their homes, as we did with intervention participants during the first year. Specifically, this paper seeks to address the following questions: a) What was the efficacy of free home-based fuel delivery, as part of an LPG stove intervention, on mean and hourly maximum kitchen area concentrations and personal exposures to , CO, and BC among intervention participants in comparison with their prerandomization concentrations and in comparison with control participants’ longitudinal concentrations over 1 y? b) What is the effectiveness of a voucher-based system of free LPG fuel, within an LPG stove intervention, on mean and hourly maximum kitchen area concentrations and personal exposures to , CO, and BC in comparison with to prerandomization concentrations and in comparison with concentrations in homes that received LPG stoves but with discontinued free fuel provision? and c) What is the longer-term effect of a 1-y intervention on mean and hourly maximum kitchen area concentrations and personal exposures to , CO, and BC among intervention participants over the course of a year following discontinuation of free fuel provision and behavioral messaging?
Methods
Study Setting
The Cardiopulmonary outcomes and Household Air Pollution (CHAP) trial is an individually randomized, unblinded, controlled field trial of provision of an LPG stove, free fuel distribution, and behavioral messaging intervention (Checkley et al. 2021; Fandiño-Del-Rio et al. 2017). The CHAP trial was conducted in rural communities surrounding the city of Puno in southeastern Peru, near the shore of Lake Titicaca at above sea level. The area is characterized by a low population density (17.6 people) and low ambient air pollution due to few other sources of pollution beyond biomass-burning for cooking (Clasen et al. 2020). Median distance between houses in the study area was [interquartile range (IQR): 56–], and the median nearest distance to a main road was (IQR: 893–). Only 4% of houses were from a main road (Fandiño-Del-Rio et al. 2017). Individuals in these rural communities traditionally burn biomass fuels in open-fire stoves for cooking. However, the use of LPG has become more common since the introduction in 2012 of a government program [known as FISE (its acronym in Spanish)], which subsidizes the cost of LPG for poor families (Pollard et al. 2018). Consequently, some households owned LPG stoves prior to our study. Nonetheless, a recent study undertaken in our same study area found that of households with LPG stoves reported stove stacking with biomass-burning traditional stoves despite the government subsidy (Pollard et al. 2018).
Study Design
We enrolled 14–16 women each month between January 2017 and January 2018, ensuring that all seasons were represented at all monitoring points. Approximately half of these participants were randomly assigned to the intervention group. Inclusion criteria were: women age 25–64 y; primary household cook; full-time resident in Puno for months; ability to understand study procedures and provide informed consent; self-reported daily use of biomass fuels for cooking; and having separate kitchen and sleeping areas (Fandiño-Del-Rio et al. 2017). Prior ownership of an LPG stove was not a criterion for exclusion as long as women reported using biomass daily for cooking. Only one adult woman was enrolled per household. No changes were made to enrollment criteria after the start of the study. We drew a simple random sample of 569 households from a census of the study area to screen for eligible women, and we randomized a total of 181 women (Checkley et al. 2021).
Randomization to intervention or control groups was done with a 1:1 ratio using randomly permuted block sizes of two, four, and six based on the time and order of enrollment. Randomization envelopes were created by an investigator not involved in enrollment and blinded from field staff in sealed envelopes until baseline measurements were completed. Our study followed participants for 2 y. We conducted a 1-y randomized controlled trial followed by a 1-y pragmatic crossover trial of two LPG intervention strategies. During the first year, intervention participants received an LPG stove, a waist-high table (Figure 1A), free unlimited LPG fuel tanks for 1 y delivered to their homes, and a cooking demonstration and education to promote exclusive LPG stove use. The education component of the intervention covered how to use and maintain the LPG stove and emphasized the benefits of cooking with LPG compared to biomass. Cooking demonstrations used local ingredients to teach participants how to use the LPG stoves to prepare common dishes. The intervention also included continuous reinforcement visits to promote exclusive LPG stove use. Control participants were asked to continue with their usual cooking practices (Figure 1B) for the first year and did not receive education or behavioral messaging during this time.
Figure 1.
Kitchens of participants with an intervention LPG stove (A) and a traditional biomass cookstove in rural Peru. Note: LPG, liquefied petroleum gas. [Photo credit: (A) and (B): Kendra N. Williams; figures being reused with her permission as the copyright holder.]
During the first year, one participant, randomized to the control group, withdrew from the study before completing the 3-month evaluations, which left an intention-to-treat sample of 180 participants. An additional participant, whose data was included in the analysis (randomized to the intervention group), withdrew after completing the 9-month follow-up measurements (Figure S1). The first year of follow-up occurred between 1 March 2017 and 15 February 2019.
During the second year (at the end of the 12-month visit intervention period), we conducted a 1-y pragmatic effectiveness trial intended to follow a more practical, real-world scenario of an LPG stove intervention, where participants picked up the free LPG fuel from a nearby distributor (Schwartz and Lellouch 1967; Tunis et al. 2003; Ford and Norrie 2016). Intervention participants stopped receiving free fuel and behavioral messaging, but they were permitted to keep the stove and table. Control group participants received the same LPG stoves; tables, cooking demonstrations, and education on LPG stove use; continuous visits to reinforce exclusive LPG use, including the same behavioral messaging that intervention participants received during the first year; and vouchers to pick up free unlimited LPG fuel tanks from a nearby distributor. The main difference in the intervention delivered to control participants in the second year was the method of LPG fuel delivery: Instead of receiving the home-based delivery of free LPG that intervention participants received during the first year, control participants received vouchers that they could use to obtain LPG fuel tanks for free from a nearby distributor. Behavioral reinforcement was also provided less frequently to control participants during year two (approximately once per month on average) than to intervention participants during year one (approximately twice per month on average). Participants were followed for a second year to determine patterns of sustained use (intervention arm) or initial adoption under a different strategy of fuel provision (control arm). A total of seven participants withdrew from the study during the second year of follow-up (Figure S1): four were control participants (three withdrew after 18 months and one after 21 months), and three were intervention participants (two died after 15 and 24 months, and one participant withdrew after 18 months). The second year of follow-up occurred between 1 March 2018 and 15 February 2020.
We monitored potential harms and adverse events, including death and burns in both the intervention and control groups during the 2 y of follow-up time and any LPG stove or tank explosions in both groups once each group received the stove and intervention. Additional information has been described in detail elsewhere (Fandiño-Del-Rio et al. 2017). The trial is registered with ClinicalTrials.gov (ID: NCT02994680). We followed CONSORT guidelines for reporting nonpharmaceutical treatments (Table S1) (Boutron et al. 2017). The full study protocol has been published separately (Checkley 2021).
The trial was approved by the institutional review boards of A.B. PRISMA (CE2402.16) and Universidad Peruana Cayetano Heredia (66,780) in Lima, Peru, and by the Johns Hopkins Bloomberg School of Public Health (00007128) in Baltimore, Maryland, USA. All participants provided informed consent to participate at the time of enrollment.
Assessment of HAP Exposures
We measured kitchen area concentrations and personal exposures to and CO at 1-min intervals continuously over 48 h at baseline (preintervention) and at the 3-, 6-, 12-, 18-, and 24-month visits after randomization. At baseline, we conducted a questionnaire that included questions related to sociodemographics, kitchen characteristics (i.e., presence of chimney, roof material), and types of stoves and fuel used prior to the trial (including previous ownership of LPG stoves) (Fandiño-Del-Rio et al. 2020). Wealth status was estimated using the Demographic and Health Survey wealth quintiles, as described in more detail previously (Kephart et al. 2020).
Kitchen area concentrations were measured by placing and CO and monitors approximately one meter away from the combustion zone (of biomass cookstoves for control participants and of LPG stoves for intervention participants), above the floor (representing the breathing zone), and at least one meter from doors and windows when possible. Personal exposure was measured by placing a and CO monitor near each participant’s breathing zone using an adapted apron commonly worn by women in the study area and provided to the participants. Women were encouraged to wear the aprons throughout the duration of the sampling period and to keep the aprons close by while sleeping or bathing.
We used the Enhanced Child MicroPEM (ECM; RTI Inc.), an aerosol monitor that measures mass concentration via a nephelometer while simultaneously collecting a filter sample for gravimetric analysis. The ECM has a pump operating at to gravimetrically collect on a filter, a light-scattering laser for continuous-time assessment of , and an accelerometer to detect movement. We calibrated the ECM pump flow rate daily before sample collection and recorded the ECM flow rate before and after the collection of each sample using a TSI 4100 flowmeter (TSI Inc.). The ECM also logs temperature, relative humidity, and flow rate and reports humidity-corrected nephelometric concentration. Gravimetric samples were collected on Teflon filters with a pore size (Measurement Technology Laboratories LLC). Filters were pre- and post-weighed at the Exposure Sciences Laboratory of the Department of Environmental Health and Engineering at Johns Hopkins University (JHU), in a humidity () and temperature-controlled () room using a MT5 microbalance (Mettler Toledo). We continuously monitored the weight of filters selected exclusively as laboratory blanks for every batch of filters used, which we weighed before we performed measurements of samples. Laboratory blanks had to be within before performing any sample measurements. Every filter was weighed twice and recorded when the consecutive measurements were within . We then used the average of these two measurements.
Nephelometric concentrations of every sample were calibrated using the sample-specific gravimetric time-weighted average (TWA) filter samples. To avoid heavy loading of the filters given the high concentrations observed in our study, the ECMs were operated using intermittent duty cycles in households using biomass cookstoves: 30 contiguous seconds every minute for personal exposure samples and 20 contiguous seconds every 3 min for kitchen area samples. ECMs used to assess concentrations after randomization in intervention households were set to sample continuously to avoid being below the limit of detection (LOD); this change was made after 32% of intervention group samples during the first year had been collected. When ECMs presented heavily loaded filter issues that affected the logged flow rate, we used the average of the pre- and post-sampling flow rates measured in the field laboratory to estimate the total volume of air sampled ( of year-one samples; of these, approximately 60% were baseline samples that were evenly distributed between groups).
Compliance with personal monitor use during typical waking hours [0400 hours to 2030 hours (4:00 A.M. to 8:30 P.M.)] and during typical morning and evening cooking times [0430 hours to 0900 hours and 1700 hours to 1900 hours (4:30 A.M. to 9:00 A.M. and 5:00 P.M. to 7:00 P.M.)] was assessed using ECM logged accelerometer data. The awake times and customary cooking times for the current analysis were determined in consultation with field staff based on field observations; the same time window was assumed for all participants. Compliance was determined by estimating the arithmetic SD of the vector sum composed of the movement on the three axes (Rodes et al. 2012) for 15-min blocks (compliance threshold: ).
Direct-reading concentrations of CO were measured with the EL-USB-CO data logger (Lascar Electronics). Data from the CO monitors were calibrated using correction factors estimated every 3–4 months for each monitor. Calibration factors were derived by co-locating all CO monitors in a sealed chamber in the field laboratory. The monitors were exposed to clean air (nitrogen gas) and a CO concentration of . Individual slopes and intercepts were estimated for each device at each co-location time point to correct any drift in the devices. The LOD for the direct-reading CO monitors was estimated as , which was three times the SD of average concentrations logged during the regular clean air calibration checks in the field.
BC concentrations were determined by measuring infrared optical attenuation on the filter samples (a cumulative measure per sample) using a Magee OT21 SootScan transmissometer (Magee Scientific). Attenuation (ATN) units were converted to mass using the calibration algorithm (correction factor of 4.2 ATN units to micrograms per square centimeter) provided by Magee Scientific for Teflon filters using standard equations (Chow et al. 2010).
Quality Control Assessments of HAP Exposures
samples included 10% blanks and recorded concentrations were blank-corrected. The LOD for samples was estimated as three times the SD of the mass measured from field blanks, using cumulative gravimetric samples. In the first 6 months of the study, preweighed filters were loaded into cassettes in the field laboratory (these represented 15% of collected samples, were evenly split between groups, and were baseline samples). The LOD for each gravimetric sample was estimated to be (equivalent to approximately with a 48-h sample and flow rate). For the remainder of the study, filters were preloaded to individual ECM cassettes at the JHU laboratory, with an LOD of (equivalent to approximately with a 48-h sample and flow rate). The LOD for BC samples was estimated using the cumulative sample and estimated as three times the SD of the ATN readings recorded from field blanks. The LOD for BC was . Most of our BC sample readings () were within the manufacturer’s recommended range (0 to 125 ATN). Samples ATN (7%) were all below 275 ATN. A previous biomass cookstove study using the same type of optical transmissometer observed that the linear relationship between ATN and concentration was maintained up to 275 ATN (Garland et al. 2017).
Kitchen area samples of and CO included 10% duplicates. No duplicate samples were collected on personal samples to reduce burden on the participants and increase wearing compliance. We rotated monitors monthly to avoid systematic sampling of either biomass or LPG households by specific devices. High correlations were observed for duplicate samples (coefficients of determination using Spearman’s correlation coefficients of 0.93, 0.94, and 0.85 for , BC, and CO, respectively). The difference between the duplicate and the kitchen samples was below 10% for and BC and below 14% for CO.
Initially, a high proportion of samples among intervention households were below the LOD (approximately 70% of kitchen and 65% of personal samples). However, after duty cycles and the filter loading protocol were adjusted, the percent of samples below the LOD decreased to approximately 35%–47% for kitchen samples and 42%–58% for personal samples (Table S2). Furthermore, of kitchen area samples in control households were below the LOD for concentrations during the first year. Personal samples among participants in the control arm were below the LOD between 18% and 31% of the samples (during the first year of follow-up visits). Similar proportions were observed for BC (Table S2).
To increase the likelihood that each 24-h period was representative of a typical cooking day (i.e., capturing the typical number of cooking events), samples with fewer than 20 h were excluded (among samples with only the first day of valid sample, only three samples had durations between 20–24 h on the first year and eight samples on the second year, of samples). We truncated gravimetrically corrected real-time measurements for and CO samples at 48 h. Only 1% of samples were h. We estimated 48-h concentrations as the average of the two consecutive 24-h means. If the sample on the second day was h, we used the 24-h mean for the first day only ( of samples on the first year and on the second year). For BC, we estimated the integrated time-weighted average concentration from the time-integrated filter-based samples (entire sample duration).
Most short duration samples were due to battery issues and on isolated occasions because the participants tampered with the devices. When possible, we repeated sample collection for a maximum of two attempts. Missing samples represented 1.4% () of kitchen area and 0.8% () of personal exposure samples for the first year, and missing samples represented 2% () of kitchen area and 1% () of personal exposure samples for the second year. Missing CO samples represented 7% () of kitchen area and 9% () of personal exposure samples for the first year, and missing CO samples represented 8% () of kitchen area and 13% () of personal exposure samples for the second year. Half of the missing CO samples were due to short duration samples and the other half to technical issues, whereas most missing samples were due to technical issues. We did not identify any consistent patterns of missing samples due to device failures with time or by treatment group. All measurements below the LOD were replaced by the LOD divided by the square root of 2. These replacements were made to the direct-reading measurements for CO samples (Table S3) and to the integrated gravimetric samples for and BC (Table S2).
To provide context, concentrations were compared against 24-h WHO air quality guidelines (WHO 2006, 2010, 2014). The guideline used for CO in parts per million () is based on temperature and pressure conditions representative of Puno ( was adjusted to 10°C and 0.62 atm). We used the 24-h guideline for of and additionally estimated the number of participants below daily interim targets: 37.5 and . Although the WHO suggests 24-h guidelines, most of the HAP literature uses as a reference the annual interim target 1 of , which falls very close to the interim daily guideline of . We estimated the percentage of samples below the annual interim target 1 in addition to the 24-h guidelines. The WHO does not provide guidelines for BC. As part of quality control procedures, we calculated correlations between pollutants, exposures, and sampling days during the first year.
Assessment of Exposure Measurements
Exposures were represented with a daily mean metric estimated by averaging the two 24-h mean concentrations for each consecutive day, only including days with h of sampling. We used the first 24-h average when the full 48-h averages were not available (6% of samples). We calculated maximum hourly mean concentrations using centered 60-min rolling means (where means are placed at the center of the range rather than the end of it to position the moving average values at their central positions in time). We also calculated the proportion of samples of concentrations below the WHO daily guideline levels for and CO. For BC, we estimated the TWA concentration for the full sample duration due to the use of the filter samples. Using the direct-reading measurements of and CO, we estimated the percentage of samples that were within different concentration ranges for every minute of the sample duration.
Statistical Methods
According to our prespecified intention-to-treat analysis plan, we fitted linear regression models with generalized estimating equations (Zeger et al. 1988) to compare log-transformed pollutant concentrations (to help meet linear regression assumptions) between treatment groups as a function of follow-up visit and adjusted for baseline log-transformed pollutant concentration measured at the time of enrollment. We define prerandomization enrollment measurements as the baseline measurements. Linear regression equations were specified as:
In this equation, represents each study participant, represents the study visit, identifies the treatment group (0 for controls and 1 for intervention group), identify the study visits as indicator variables at times months, represents the log-transformed pollutant for the participant at the study visit, and represents the log-transformed pollutant for the participant at enrollment. To account for repeated measurements over time by participant, we used a compound symmetry correlation matrix and robust estimation of standard errors. The estimated exponentiated regression parameters represent the difference of geometric mean concentrations between intervention and control groups for the corresponding study visits (3-, 6-, 12-, 18- and 24-month visits, respectively). We used the above model to compare kitchen area concentrations and personal exposures of , BC, and CO between treatment groups.
In secondary analyses, we fitted separate linear models with generalized estimating equations by treatment group to compare postrandomization HAP concentrations against the HAP concentrations obtained at baseline. These generalized linear regression equations were specified as follows:
where represents each study participant, represents the study visit, the identify the study visits as indicator variables at times months, and represents the log-transformed pollutant for the participant at the study visit. To account for repeated measurements over time by participant, we used a compound symmetry correlation matrix and robust estimation of standard errors. The estimated exponentiated regression parameters ( represent the difference of geometric mean concentrations at follow-up study visits (3-, 6-, 12-, 18- and 24-month visits) when compared to baseline for either the intervention or control groups. In sensitivity analyses, we used Student’s -tests to compare log-transformed HAP concentrations between treatment groups at each study visit.
We used a Wilcoxon rank-sum test to compare ECM wearing compliance by treatment groups. To assess correlations between pollutants, type of exposures (personal or kitchen area), and duplicate samples, we used the Spearman’s rank correlation. Finally, we also compared concentrations of each pollutant at each time point separately using Student’s -test on the log transformed exposure concentrations comparing intervention and control groups to present along with summary statistics.
Data analyses and visualizations were conducted in MATLAB (The MathWorks, Inc.), STATA (version 14.2; StataCorp.) and R (R version 3.6.2, Development Core Team). Sample size calculations were based on clinical outcomes reported elsewhere before enrollment began (Fandiño-Del-Rio et al. 2017).
Results
Participant Characteristics at Enrollment
There were no differences in participant characteristics by treatment group at baseline (Table 1). Concentrations of , BC, and CO were also similar (Table 2 and Table S4). Mean concentrations of (kitchen 1,220 vs. , ; personal 126 vs. , ), BC (kitchen 180 vs. , ; personal 19 vs, , ), and CO (kitchen 53 vs. , ; personal 6.6 vs. , ), were similar between control and intervention participants, respectively (Table 2). We also did not identify differences in maximum hourly means (Table S4) between intervention and control participants or differences in wearing compliance by treatment group at any study visit [the median wearing compliance for all samples was 59% (IQR: 32%–80%); Table 3; Table S5]. Most baseline daily mean kitchen samples were above the WHO daily air quality guideline levels: 88% for CO in both groups, 99% for in controls, and 100% for in intervention participants (Table 2).
Table 1.
Baseline and household characteristics of study participants in rural Peru.
| Variable | Category | Intervention () | Control () | ||
|---|---|---|---|---|---|
| Household characteristics | — | — | — | — | — |
| Kitchen roof material [n (%)] | — | — | — | — | — |
| Corrugated metal roof | — | 36 | (40%) | 37 | (41%) |
| Natural/Light: straw, totora, reed, or similar | 54 | (60%) | 53 | (59%) | |
| Kitchen door permanently open [n (%)] | — | 13 | (14%) | 18 | (20%) |
| Kitchen door not permanently open [n (%)] | — | 77 | (86%) | 72 | (80%) |
| Windows permanently open [n (%)] | |||||
| 0 | 39 | (43%) | 33 | (37%) | |
| 1 | 14 | (16%) | 12 | (13%) | |
| 2 | 1 | (1%) | 6 | (7%) | |
| 5 | 0 | (0%) | 2 | (2%) | |
| Ventilation over biomass stove [n (%)] | |||||
| Stove with no ventilation | — | 30 | (33%) | 40 | (44%) |
| Stove with chimney | — | 7 | (8%) | 12 | (13%) |
| Stove in recessed area | — | 53 | (59%) | 38 | (42%) |
| Most common fuels used for cooking [n (%)] | |||||
| Alcohol/ethanol | — | 23 | (26%) | 13 | (14%) |
| Wood | — | 37 | (41%) | 38 | (42%) |
| Crop residue/grass/straw/bushes | 61 | (68%) | 66 | (73%) | |
| Cow dung | — | 90 | (100%) | 89 | (99%) |
| Previously owned secondary stove [n (%)] | |||||
| None | 26 | (29%) | 22 | (24%) | |
| LPG gas | 64 | (71%) | 68 | (76%) | |
| Electricity in household [n (%)] | 85 | (94%) | 90 | (100%) | |
| No electricity in household [n (%)] | 5 | (6%) | 0 | (0%) | |
| Participant characteristics | |||||
| Highest level of education achieved [n (%)] | |||||
| Without education or preschool only | — | 4 | (4%) | 3 | (3%) |
| Primary | — | 53 | (59%) | 53 | (59%) |
| Secondary | — | 33 | (37%) | 34 | (38%) |
| Non-university superior or university | 0 | (0%) | 0 | (0%) | |
| Wealth quintile [n (%)] | |||||
| 1 | 51 | (57%) | 50 | (56%) | |
| 2 | 32 | (36%) | 37 | (41%) | |
| 3 | 7 | (8%) | 3 | (3%) | |
| 4 and 5 | 0 | (0%) | 0 | (0%) | |
| Average years of education, mean (standard deviation) | 6 | 3 | 6 | 3 | |
Note: Data are complete for all observations. —, intentionally omitted.
Table 2.
Household air pollution exposure of daily means summary statistics on control and intervention groups in rural Peru.
| Control | Intervention | Relative difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up visit (months)a | b | Mean (SD) | Median (25th, 75th) | GM (GSD) | b | Mean (SD) | Median (25th, 75th) | GM (GSD) | -Valuec | ||
| Kitchen | |||||||||||
| Baseline | 89 | 1,220 (1,010) | 970 (390, 1,790) | 770 (3) | 1% | 89 | 1,190 (880) | 990 (500, 1,880) | 800 (3) | 0% | 0.865 |
| 3 | 89 | 1,140 (980) | 840 (320, 1,730) | 670 (3) | 1% | 90 | 82 (52) | 71 (40, 140) | 61 (2) | 18% | |
| 6 | 88 | 1,180 (1,130) | 770 (330, 1,830) | 670 (3) | 0% | 90 | 50 (30) | 62 (21, 72) | 39 (2) | 28% | |
| 12 | 89 | 1,420 (1,220) | 1,080 (430, 2,230) | 830 (3) | 0% | 87 | 41 (59) | 21 (8, 41) | 22 (3) | 59% | |
| 18 | 88 | 63 (183) | 17 (8, 41) | 22 (3) | 67% | 87 | 447 (649) | 74 (70, 635) | 150 (5) | 14% | |
| 24 | 84 | 34 (74) | 14 (8, 23) | 17 (3) | 76% | 84 | 561 (1,251) | 74 (70, 708) | 140 (6) | 17% | |
| Personal | |||||||||||
| Baseline | 90 | 126 (214) | 71 (44, 127) | 76 (2) | 8% | 90 | 104 (100) | 77 (37, 137) | 73 (2) | 11% | 0.782 |
| 3 | 87 | 92 (186) | 57 (29, 85) | 53 (3) | 21% | 90 | 26 (18) | 25 (16, 32) | 22 (2) | 50% | |
| 6 | 90 | 96 (111) | 61 (28, 113) | 60 (3) | 23% | 89 | 35 (55) | 16 (15, 33) | 22 (2) | 65% | |
| 12 | 90 | 103 (108) | 71 (33, 117) | 65 (3) | 18% | 87 | 26 (34) | 15 (8, 23) | 16 (2) | 76% | |
| 18 | 90 | 16.7 (15.1) | 8.8 (8, 18.9) | 13.2 (2) | 84% | 88 | 46 (102) | 16 (16, 38) | 25 (2) | 59% | |
| 24 | 85 | 17 (15) | 11 (8, 16) | 13 (2) | 84% | 84 | 35 (38) | 17 (16, 43) | 24 (3) | 58% | |
| Kitchen CO | |||||||||||
| Baseline | 84 | 53.4 (48.5) | 38.8 (21.3, 71.8) | 33.5 (3.1) | 12% | 85 | 50.2 (40.8) | 42.1 (17.8, 69.1) | 32.5 (3) | 12% | 0.874 |
| 3 | 84 | 46.4 (37.4) | 38.4 (18.7, 67.1) | 28.5 (3.4) | 17% | 80 | 4.6 (6) | 2.5 (1, 5.4) | 2.6 (2.8) | 89% | |
| 6 | 86 | 49.9 (43) | 40.9 (18.3, 67.8) | 30.5 (3.3) | 12% | 82 | 5.1 (5.5) | 2.9 (1.4, 5.7) | 3.2 (2.6) | 82% | |
| 12 | 86 | 48.7 (38.6) | 41.2 (15.2, 70.5) | 29.9 (3.4) | 17% | 77 | 8.1 (13.2) | 4.1 (1.8, 8.3) | 4.1 (3.1) | 78% | |
| 18 | 83 | 7.1 (10.6) | 3.1 (1.1, 7.9) | 3.3 (3.4) | 77% | 79 | 21.3 (25.8) | 12.8 (1.9, 30.3) | 8.6 (4.6) | 46% | |
| 24 | 80 | 5.7 (6.2) | 4.1 (1.2, 6.7) | 3.3 (2.9) | 84% | 79 | 23 (28.2) | 12.4 (3.3, 33.3) | 10.2 (4.1) | 47% | |
| Personal CO | |||||||||||
| Baseline | 81 | 6.6 (8.2) | 3.2 (1.6, 8) | 3.9 (2.7) | 79% | 79 | 7.1 (8.4) | 4.4 (1.9, 8.7) | 4.3 (2.7) | 77% | 0.526 |
| 3 | 79 | 6.3 (8.7) | 3.5 (1.7, 7.5) | 3.7 (2.7) | 81% | 76 | 2.5 (3.3) | 1.2 (0.9, 2.6) | 1.7 (2.2) | 96% | |
| 6 | 81 | 7 (8.1) | 4.3 (1.8, 8.8) | 4.2 (2.7) | 75% | 79 | 1.7 (2.6) | 1 (0.8, 1.3) | 1.2 (1.9) | 97% | |
| 12 | 81 | 6.7 (8.9) | 3.8 (1.8, 7.8) | 4.1 (2.7) | 80% | 79 | 2.7 (3.9) | 1.2 (0.8, 2.1) | 1.6 (2.4) | 91% | |
| 18 | 75 | 2.6 (4.2) | 1.2 (0.8, 2.2) | 1.6 (2.4) | 95% | 80 | 4.5 (7.3) | 1.6 (0.8, 4.9) | 2.2 (3) | 88% | 0.033 |
| 24 | 73 | 2.7 (4.4) | 1.1 (0.8, 2.5) | 1.6 (2.4) | 93% | 76 | 3.9 (5.4) | 1.6 (0.9, 5) | 2.1 (2.8) | 91% | 0.042 |
| Kitchen BC | |||||||||||
| Baseline | 89 | 180 (120) | 170 (90, 270) | 130 (3) | — | 89 | 210 (150) | 170 (80, 330) | 140 (3) | — | 0.672 |
| 3 | 89 | 200 (130) | 210 (110, 290) | 130 (3) | — | 90 | 7.6 (4.2) | 10.4 (2.3, 10.7) | 5.6 (2) | — | |
| 6 | 88 | 200 (140) | 160 (100, 290) | 130 (3) | — | 90 | 5.8 (4.5) | 2.8 (1.3, 10.8) | 3.8 (3) | — | |
| 12 | 89 | 220 (150) | 210 (100, 300) | 150 (3) | — | 87 | 3 (5.7) | 1.2 (1.2, 2.2) | 1.8 (2) | — | |
| 18 | 88 | 4.4 (9.5) | 1.6 (1.6, 1.7) | 2.3 (2) | — | 87 | 99 (133) | 15 (14, 147) | 31 (6) | — | |
| 24 | 84 | 2.7 (4.8) | 1.6 (1.6, 1.7) | 1.9 (2) | — | 84 | 82 (124) | 14 (14, 127) | 23 (6) | — | |
| Personal BC | |||||||||||
| Baseline | 90 | 19.1 (16.7) | 15.2 (6.1, 26.8) | 12.5 (2.7) | — | 90 | 21.3 (22.3) | 15.6 (5.9, 31.3) | 13.4 (2.8) | — | 0.646 |
| 3 | 87 | 14.4 (15.3) | 9.1 (2.4, 21.5) | 8.8 (2.8) | — | 90 | 2.2 (1.2) | 2.3 (1.2, 2.4) | 2 (1.5) | — | |
| 6 | 90 | 14.7 (18.3) | 8.8 (2.6, 14.8) | 8.5 (2.8) | — | 89 | 2.3 (4.5) | 2 (1.2, 2.4) | 1.8 (1.6) | — | |
| 12 | 90 | 17.6 (18.9) | 12 (4.5, 20.9) | 10.8 (2.8) | — | 87 | 1.9 (2.6) | 1.2 (1.2, 1.3) | 1.4 (1.7) | — | |
| 18 | 90 | 2.2 (3.6) | 1.6 (1.6, 1.7) | 1.8 (1.5) | — | 88 | 7.9 (11.2) | 3.2 (3.1, 7.4) | 4.7 (2.4) | — | |
| 24 | 85 | 2.1 (1.7) | 1.6 (1.6, 1.7) | 1.9 (1.5) | — | 84 | 5.9 (6.1) | 3.2 (3.1, 5.9) | 3.9 (2.6) | — | |
Note: —, no data; BC, black carbon; CO, carbon monoxide; GM, geometric mean; GSD, geometric SD; , percent of samples Health Organization daily guidelines ( , ) (There is no current guideline for BC.). LOD, limit of detection; , fine particulate matter with aerodynamic diameter of ; SD, standard deviation.
During the second year (follow-up visits:18- and 24 months) the control participants received the LPG stove intervention and vouchers for 1-y supply of free fuel and intervention participants stopped receiving free fuel but kept the LPG stove.
n: Represents nonmissing samples in each time point; each sample represents the daily mean metric defined as the mean of the two consecutive 24-h average concentrations, where available.
-Values obtained using Student’s -test on the log-transformed exposure concentrations comparing intervention and control groups. Samples were replaced by : 7 and for and BC gravimetric integrated samples, respectively; and for direct reading CO measurements.
Table 3.
Percent of time personal monitors were worn by 180 study participants during awake time in rural Peru.
| Visit | Group | a | Full 48-h sample | Day 1 | Day 2 | **-Value day 1 vs.day 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 25th pct | 75th pct | *-Value | Mean | Median | 25th pct | 75th pct | *-Value | Mean | Median | 25th pct | 75th pct | -Value | ||||
| Baseline | Intervention | 89 | 62.0 | 65.8 | 45.1 | 84.1 | — | 64.7 | 76.2 | 39.4 | 87.1 | — | 58.6 | 69.9 | 26.0 | 85.6 | — | |
| Baseline | Control | 90 | 66.6 | 75.1 | 42.0 | 88.9 | 0.09 | 66.2 | 77.2 | 49.1 | 88.1 | 0.66 | 67.1 | 80.4 | 41.4 | 91.2 | 0.02 | 0.82 |
| Month 3 | Intervention | 90 | 56.5 | 59.8 | 35.6 | 79.5 | — | 57.3 | 71.1 | 24.5 | 83.4 | — | 55.2 | 64.4 | 24.4 | 83.8 | — | |
| Month 3 | Control | 88 | 57.1 | 62.1 | 33.3 | 83.9 | 0.74 | 58.2 | 68.6 | 26.6 | 87.2 | 0.66 | 55.7 | 60.3 | 23.7 | 87.0 | 0.78 | 0.51 |
| Month 6 | Intervention | 89 | 56.4 | 64.3 | 29.6 | 79.8 | — | 56.0 | 67.5 | 24.9 | 83.8 | — | 56.6 | 65.1 | 23.6 | 85.7 | — | — |
| Month 6 | Control | 90 | 51.5 | 54.3 | 20.5 | 79.3 | 0.23 | 51.1 | 54.4 | 24.0 | 78.8 | 0.20 | 50.8 | 50.6 | 15.9 | 82.9 | 0.30 | 0.72 |
| Month 12 | Intervention | 88 | 54.0 | 54.9 | 35.5 | 73.6 | — | 54.2 | 57.1 | 30.6 | 79.6 | — | 51.3 | 59.0 | 23.6 | 79.2 | — | — |
| Month 12 | Control | 90 | 57.7 | 61.7 | 35.8 | 83.8 | 0.29 | 58.5 | 66.2 | 30.2 | 84.3 | 0.28 | 55.0 | 55.9 | 27.1 | 87.6 | 0.26 | 0.42 |
| Month 18 | Intervention | 87 | 48.1 | 43.3 | 24.0 | 73.7 | — | 52.7 | 54.0 | 23.1 | 81.3 | — | 42.0 | 29.6 | 15.7 | 75.3 | — | — |
| Month 18 | Control | 90 | 52.6 | 52.1 | 31.1 | 75.2 | 0.28 | 55.1 | 61.4 | 32.5 | 78.4 | 0.73 | 51.5 | 48.6 | 25.9 | 81.1 | 0.04 | 0.05 |
| Month 24 | Intervention | 85 | 47.9 | 47.2 | 18.7 | 72.9 | — | 48.9 | 50.5 | 18.8 | 78.3 | — | 47.5 | 47.1 | 13.7 | 78.1 | — | — |
| Month 24 | Control | 85 | 52.2 | 53.0 | 28.4 | 78.8 | 0.32 | 53.7 | 48.0 | 28.1 | 84.8 | 0.19 | 46.9 | 44.2 | 18.7 | 75.3 | 0.93 | 0.21 |
| All samples | 1,061 | 55 | 59 | 32 | 80 | — | 56 | 63 | 28 | 84 | — | 53 | 59 | 22 | 84 | — | 0.50 | |
Note: —, intentionally omitted; pct, percentile; , fine particulate matter with aerodynamic diameter of .
represents nonmissing samples in each timepoint; *-Values obtained using the Wilcoxon rank-sum test to compare the intervention group and control group in each follow-up visit. **-Value using the Wilcoxon rank-sum test to compare percent for time wearing the device on day 1 vs. day 2. Awake time: 0400 hours to 2030 hours (4:00 A.M. to 8:30 P.M.).
Effect of Free, Home-Based LPG Delivery on HAP Exposures during the First Year of the Trial
During the first year, when intervention participants received home-based delivery of free LPG and control participants continued cooking primarily with biomass, we found consistently lower postrandomization mean kitchen area concentrations and personal exposures to , CO, and BC in intervention participants in comparison with controls in intention-to-treat analysis [at the 12-month visit for intervention participants, average postrandomization kitchen area concentrations: (SD ), (SD ), and (SD ) and personal exposures: (SD ), (SD ), and (SD ), respectively; Table 2] (Checkley et al. 2021). Specifically, we observed relative differences in month-12 follow-up visit kitchen concentrations of for [95% confidence interval (CI): , ], of for BC (95% CI: , ), and of for CO (95% CI: , ), in intervention participants when compared to controls (Table 4). See Table 2 and Table S4 for kitchen area concentrations and personal exposures to , BC, and CO by treatment group and study visit.
Table 4.
Relative differences in pollutant concentration of the intervention group in comparison with control group (first year) and control group compared to intervention group (second year) at the same time point using a daily mean metric in rural Peru ( participants).
| Visit | Percent relative differencea (95% CI) | -Value |
|---|---|---|
| Kitchen | ||
| Month 3 | (, ) | |
| Month 6 | (, ) | |
| Month 12 | (, ) | |
| Month 18 | 85 (77, 90) | |
| Month 24 | 88 (82, 92) | |
| Personal | ||
| Month 3 | (, ) | |
| Month 6 | (, ) | |
| Month 12 | (, ) | |
| Month 18 | 48 (34, 58) | |
| Month 24 | 45 (30, 57) | |
| Kitchen BC | ||
| Month 3 | (, ) | |
| Month 6 | (, ) | |
| Month 12 | (, ) | |
| Month 18 | 92 (89, 95) | |
| Month 24 | 92 (87, 94) | |
| Personal BC | ||
| Month 3 | (, ) | |
| Month 6 | (, ) | |
| Month 12 | (, ) | |
| Month 18 | 61 (52, 68) | |
| Month 24 | 52 (40, 61) | |
| Kitchen CO | ||
| Month 3 | (, ) | |
| Month 6 | (, ) | |
| Month 12 | (, ) | |
| Month 18 | 62 (41, 75) | |
| Month 24 | 70 (56, 80) | |
| Personal CO | ||
| Month 3 | (, ) | |
| Month 6 | (, ) | |
| Month 12 | (, ) | |
| Month 18 | 25 (, 45) | 0.076 |
| Month 24 | 33 (7, 51) | 0.015 |
Note: BC, black carbon; CI, confidence interval; CO, carbon monoxide; LOD, limit of detection; , fine particulate matter with aerodynamic diameter of .
Generalized estimating equation models were used using the log transformed pollutant concentrations as the response adjusted for baseline concentrations, visit, randomization group, and interaction terms between categorical variables for visit and randomization group. Samples were replaced by : 7 and for and BC gravimetric integrated samples, respectively; and for direct reading CO measurements. Daily mean metric used is defined as the mean of the two consecutive 24-h average concentrations, where available. During the second year (follow-up visits:18- and 24 months) the control participants received the LPG stove intervention and vouchers for 1-y supply of free fuel and intervention participants stopped receiving free fuel but kept the LPG stove. The reference group for the first-year relative differences is the control group (C), and for second year it is the intervention group (I): (I–C)/C and (I–C)/I, respectively.
In secondary analyses, we observed that intervention participants had lower postrandomization kitchen area concentrations [month-12 visit relative differences in daily means : (95% CI: , ); BC: (95% CI: , ); CO: (95% CI: , )] and personal exposures [month-12 visit kitchen relative difference in daily means : (95% CI: , ); BC: 89% (95% CI: , ); CO: (95% CI: , )] to all pollutants during the first year when compared to their baseline measurements (Table 5). We also observed consistent reductions in maximum hourly averages during the first year of follow-up in comparison with baseline (Table 5). These reductions were greater over time for both kitchen area concentrations of and personal exposures to and BC, and we found lower reductions for kitchen area concentrations of exposures to CO (Table S6), which were, however, still below the CO daily WHO air quality guidelines.
Table 5.
Pollutant concentration differences across follow-up visits for the intervention group in comparison with baseline using a daily mean metric and maximum hourly means in rural Peru ( participants).
| Visitb | Differences in daily meansa | Differences in maximum hourly means | ||
|---|---|---|---|---|
| Kitchen area percent differences (95% CI) | Personal exposure percent differences (95% CI) | Kitchen area percent differences (95% CI) | Personal exposure percent differences (95% CI) | |
| 3 | (, ) | (, ) | (, ) | (, ) |
| 6 | (, ) | (, ) | (, ) | (, ) |
| 12 | (, ) | (, ) | (, ) | (, ) |
| 18 | (, ) | (, ) | (, ) | (, ) |
| 24 | (, ) | (, ) | (, ) | (, ) |
| CO | ||||
| 3 | (, ) | (, ) | (, ) | (, ) |
| 6 | (, ) | (, ) | (, ) | (, ) |
| 12 | (, ) | (, ) | (, | (, ) |
| 18 | (, ) | (, ) | (, ) | (, ) |
| 24 | (, ) | (, ) | (, ) | (, ) |
| BC | ||||
| 3 | (, ) | (, ) | — | — |
| 6 | (, ) | (, ) | — | — |
| 12 | (, ) | (, ) | — | — |
| 18 | (, ) | (, ) | — | — |
| 24 | (, ) | (, ) | — | — |
Note: BC, black carbon; CI, confidence interval; CO, carbon monoxide; LOD, limit of detection; LPG, liquefied petroleum gas; , fine particulate matter with aerodynamic diameter of .
Daily mean metric used is defined as the mean of the two consecutive 24-h average concentrations, where available. All coefficients: .
Follow-up visits: 3-, 6-, 12-, 18-, and 24-months. BC consisted of time weighted integrated samples therefore hourly maximums were not evaluated for this pollutant. Samples were replaced by : 7 and for and BC gravimetric integrated samples, respectively; and for direct reading CO measurements. Generalized estimating equation models using the log transformed pollutant concentrations were used. During the second year (follow-up visits:18- and 24 months) the intervention participants stopped receiving free fuel but kept the LPG stove.
Control participants also had lower postrandomization HAP concentrations in comparison with baseline. Although kitchen area concentrations from daily mean samples for control participants were 14% lower for [ (95% CI: , 9%) and (95% CI: , 7%)] and 15% and 9% lower for CO [ (95% CI: , 6%) and (95% CI: , 12%)] during the 3- and 6-month visits when compared with baseline, these differences were not significant (Table 6); and, although personal exposures at the 3- and 6-month visits were 20%–30% lower in comparison with baseline, these differences did not remain statistically significant at the 12-month visit (Table 6).
Table 6.
Pollutant concentration changes across follow-up visits for the control group compared to baseline using a daily mean metric and maximum hourly means in rural Peru ().a
| Differences in daily means a | Differences in maximum hourly means | |||||||
|---|---|---|---|---|---|---|---|---|
| Visitb | Kitchen area percent differences (95% CI) | -Value | Personal exposure % differences (95% CI) | -Value | Kitchen area percent differences (95% CI) | -Value | Personal exposure percent differences (95% CI) | -Value |
| 3 | ( ,9) | 0.22 | (, ) | 0.001 | (, ) | 0.004 | (, ) | 0.004 |
| 6 | (, 7) | 0.17 | (, ) | 0.01 | (, ) | 0.039 | (, ) | 0.02 |
| 12 | 7 (, 31) | 0.54 | (, 4) | 0.12 | (, 12) | 0.470 | (, 7) | 0.16 |
| 18 | (, ) | (, ) | (, ) | (, ) | ||||
| 24 | (, ) | (, ) | (, ) | (, ) | ||||
| CO | ||||||||
| 3 | (, 6) | 0.14 | (, 25) | 0.76 | (, 7) | 0.206 | 1 (, 36) | 0.97 |
| 6 | (, 12) | 0.38 | 9 (, 45) | 0.55 | (, 16) | 0.688 | 17 (, 59) | 0.32 |
| 12 | (, 9) | 0.26 | 6 (, 35) | 0.63 | (, 12) | l0.615 | 37 (1, 86) | 0.04 |
| 18 | (, ) | (, ) | (, ) | (, ) | ||||
| 24 | (, ) | (, ) | (, ) | (, ) | ||||
| BC | ||||||||
| 3 | (, 31) | 0.96 | (, ) | 0.01 | — | — | — | — |
| 6 | (, 24) | 0.84 | (, ) | 0.001 | — | — | — | — |
| 12 | 15 (, 41) | 0.18 | (, 6) | 0.16 | — | — | — | — |
| 18 | (, ) | (, ) | — | — | — | — | ||
| 24 | (, ) | (, ) | — | — | — | — | ||
Note: —, no data; BC, black carbon; CI, confidence interval; CO, carbon monoxide; , fine particulate matter with aerodynamic diameter of .
Daily mean metric used is defined as the mean of the two consecutive 24-h average concentrations, where available.
Follow-up visits: 3-, 6-, 12-, 18-, and 24-months. BC consisted of time weighted integrated samples therefore hourly maximums were not evaluated for this pollutant. Samples were replaced by : 7 and for and BC gravimetric integrated samples, respectively; and for direct reading CO measurements. Generalized estimating equation models using the log transformed pollutant concentrations were used. During the second year (follow-up visits:18- and 24 months) the control participants received the LPG stove intervention and vouchers for 1-y supply of free fuel.
During the first year of follow-up, the LPG stove intervention with home-based delivery of free LPG reduced most daily mean samples of personal and kitchen and CO concentrations below the respective WHO daily air quality guideline levels (Figure 2 and Figure 3) (Checkley et al. 2021). At the 12-month follow-up visit, more than 76% and 91% of the personal exposure samples (59% and 78% of kitchen samples) were below WHO air quality guideline levels for 24-h () and CO (), respectively (Table 2). When compared with the interim target-3 recommended by the WHO (WHO 2006, 2010) (, only one target above the 24-h guideline), 82% of personal exposure samples (72% of kitchen samples) were below this threshold (Table S7).
Figure 2.
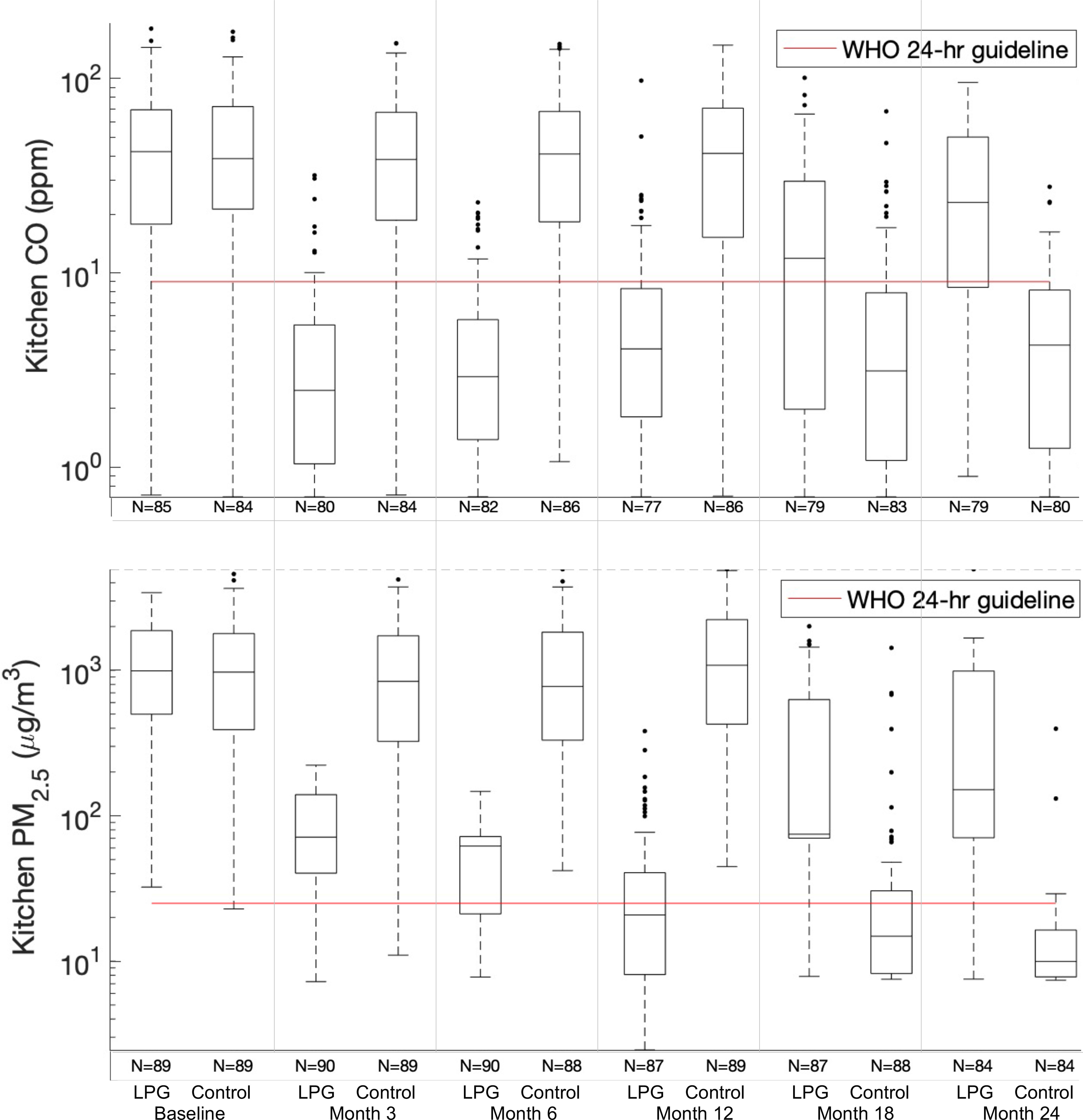
and CO box plots of daily mean kitchen area exposure concentrations at each follow-up visit (baseline, 3-, 6-, 12-, 18-, and 24-months) for LPG stove intervention participants (LPG) and control participants (Control). Daily mean metric used is defined as the mean of the two consecutive 24-h average concentrations, where available. During the second year (follow-up visits:18- and 24 months) the control participants received the LPG stove intervention and vouchers for 1-y supply of free fuel and intervention participants stopped receiving free fuel but kept the LPG stove. Interquartile ranges of the box plots represent the 25th and the 75th percentiles of the daily means for each group; the middle line of the box represents the 50th percentile. Numeric data is provided in Table 2. Note: CO, carbon monoxide; LPG, liquefied petroleum gas; , fine particulate matter with aerodynamic diameter of ; WHO 24-h guideline, World Health Organization daily guideline (: , CO: ).
Figure 3.
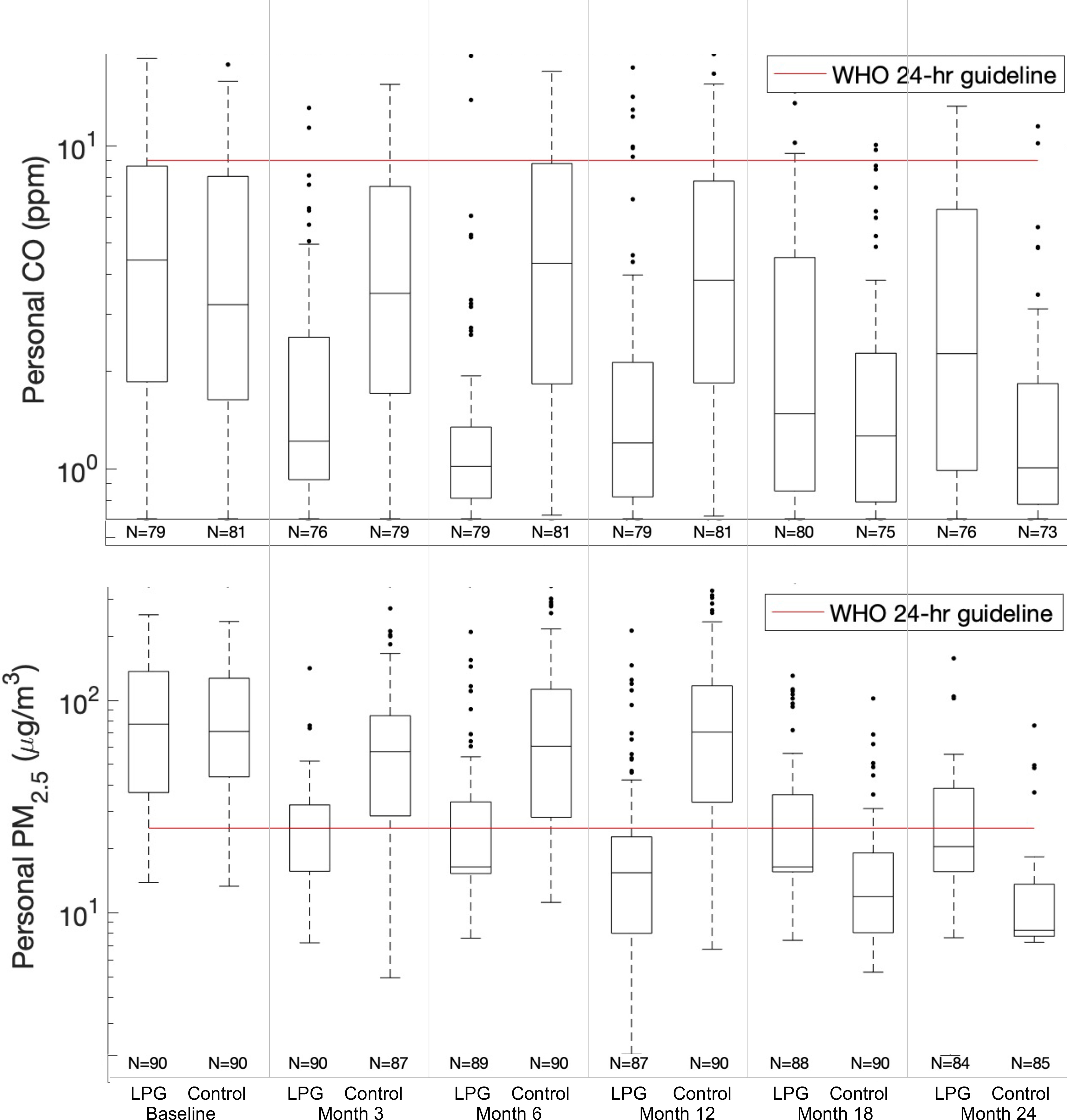
and CO box plots of daily mean personal exposure concentrations at each follow-up visit (baseline, 3-, 6-, 12-, 18-, and 24-months) for LPG stove intervention participants (LPG) and control participants (Control). Daily mean metric used is defined as the mean of the two consecutive 24-h average concentrations, where available. During the second year (follow-up visits:18- and 24 months) the control participants received the LPG stove intervention and vouchers for 1-y supply of free fuel and intervention participants stopped receiving free fuel but kept the LPG stove. Interquartile ranges of the box plots represent the 25th and the 75th percentiles of the daily means for each group; the middle line of the box represents the 50th percentile. Numeric data is provided in Table 2. Note: CO, carbon monoxide; LPG, liquefied petroleum gas; PM, particulate matter; WHO 24-h guideline, World Health Organization daily guideline (: , CO: ).
Reductions were also evident when evaluating the daily variability in concentrations. The percentage of samples that fell within five concentration ranges for every minute of the day are shown in Figures 4–7. Direct reading samples show two cooking events each day. Baseline concentrations in kitchen area concentrations show that at approximately 0600 hours (6:00 A.M.), only 20% of the samples experienced concentrations below for and below for CO. At that time of the day, more than 60% of households had concentrations above for and above for CO. Large reductions during the first year of follow-up were observed in the daily patterns of concentrations for the LPG intervention group for both kitchen concentrations and personal exposures in comparison with baseline (Figures 4D, 5D, 6D, and 7D). Specifically, of the first year follow-up kitchen area samples and of personal exposure samples in the LPG stove intervention group were in the lowest concentration categories ( and CO ) during cooking periods.
Figure 4.
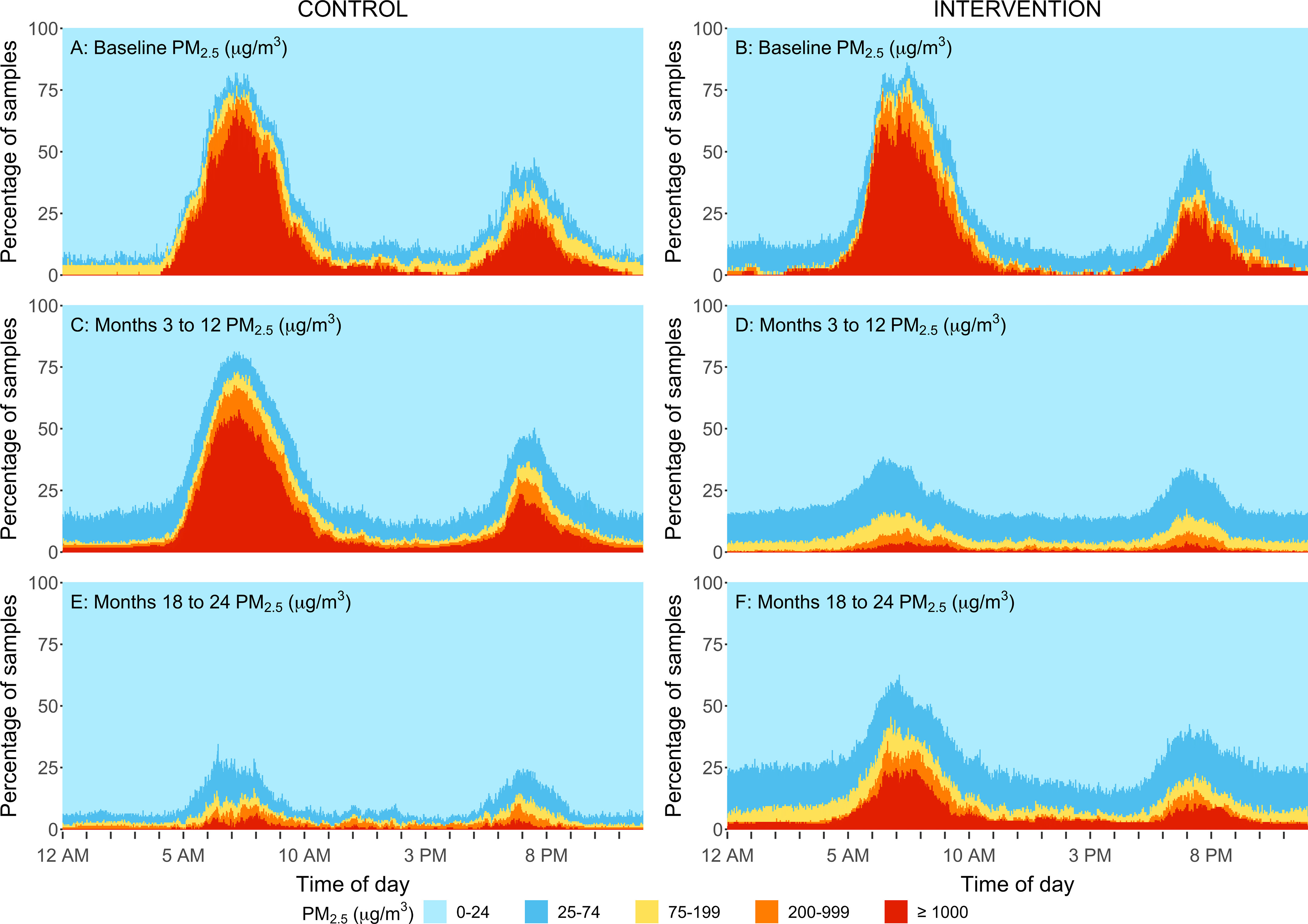
Percent of kitchen area samples within different concentration ranges by minute of a day for the intervention group (B,D,F) and control group (A,C,E) at baseline (A,B), at follow-up samples taken at the 3-, 6-, 12-month time points (C,D), and 18-, and 24-month time points (E,F) in rural Peru. Samples were replaced by : for gravimetric integrated samples, respectively. The sample size for baseline, 3-, 6-, 12, 18-, and 24-month visits for control and intervention households are: and 89, and 90, and 90, and 87, and 87, and 84. During the second year (follow-up visits:18- and 24 months), the control participants received the LPG stove intervention and vouchers for 1-y supply of free fuel and intervention participants stopped receiving free fuel but kept the LPG stove. Note: LOD, limit of detection; LPG, liquefied petroleum gas; PM, particulate matter.
Figure 7.
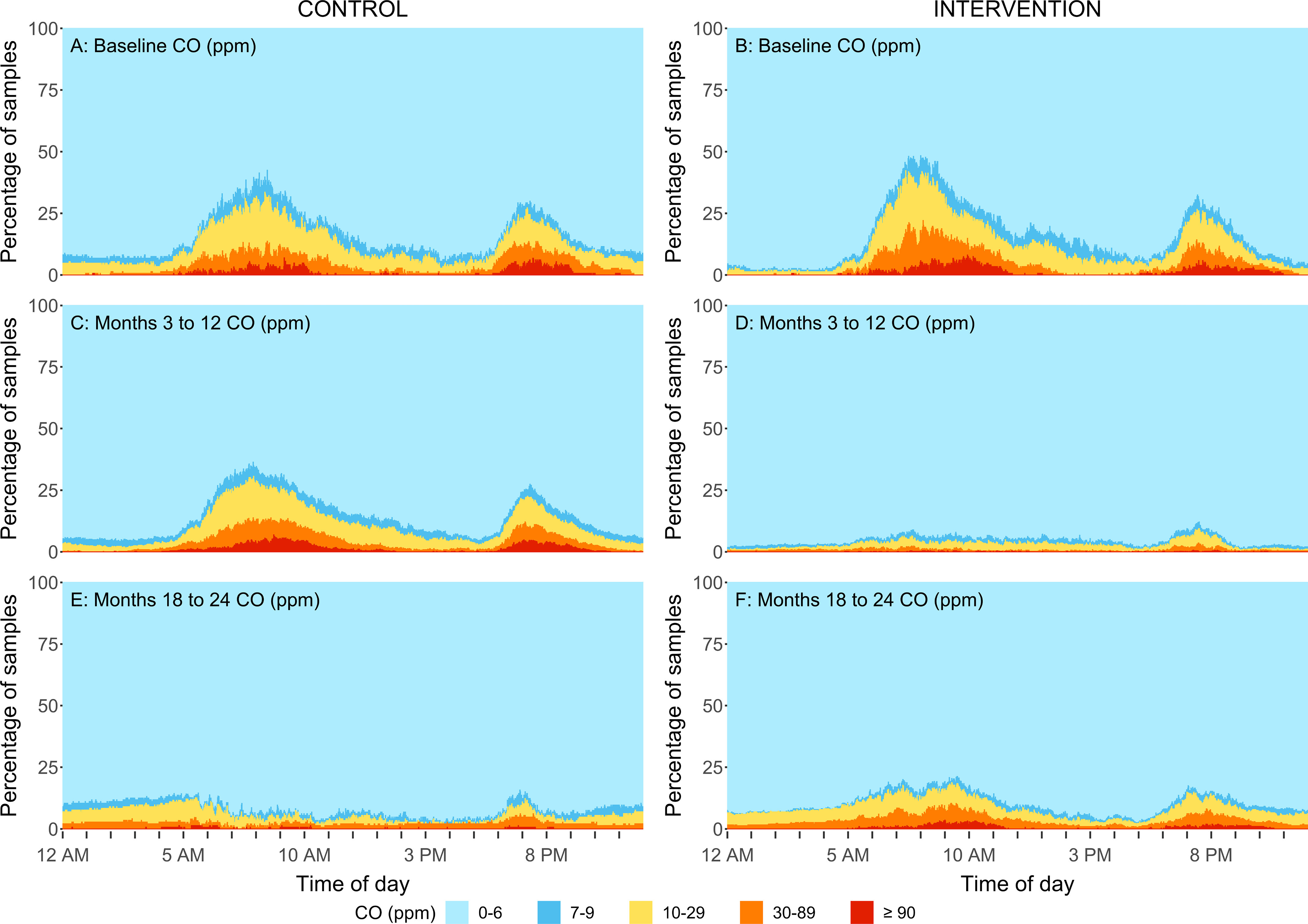
Percent of personal exposure samples within different CO concentration ranges by minute of a day for the intervention group (B,D,F) and control group (A,C,E) at baseline (A,B), at follow-up samples taken at the 3-, 6-, 12-month time points (C,D) and at 18-, and 24-month time points (E,F) in rural Peru. Samples were replaced by ): for direct reading CO measurements. The sample size for baseline, 3-, 6-, 12, 18-, and 24-month visits for control and intervention households are: and 79, and 76, and 79, and 79, and 80, and 76. During the second year (follow-up visits:18- and 24 months) the control participants received the LPG stove intervention and vouchers for 1-y supply of free fuel and intervention participants stopped receiving free fuel but kept the LPG stove. Note: CO, carbon monoxide; LPG, liquefied petroleum gas; LOD, limit of detection.
Figure 5.
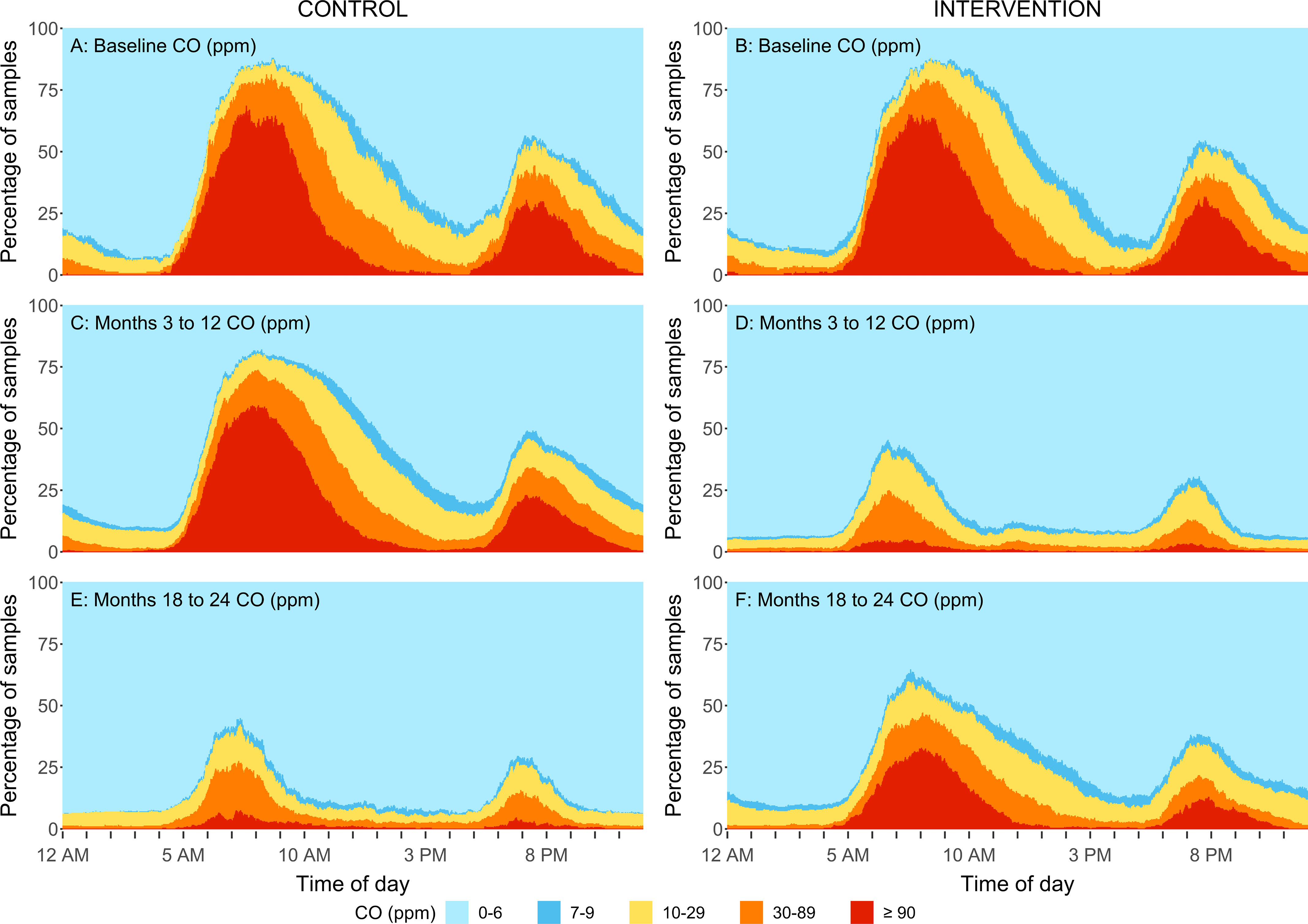
Percent of kitchen area samples within different CO concentration ranges by minute of a day for the intervention group (B,D,F) and control group (A,C,E) at baseline (A,B), at follow-up samples taken at the 3-, 6-, 12-month time points (C,D) and at 18-, and 24-month time points (E,F) in rural Peru. Samples were replaced by : for direct reading CO measurements. The sample size for baseline, 3-, 6-, 12, 18-, and 24-month visits for control and intervention households are: and 85, and 80, and 82, and 77, and 79, and 79. During the second year (follow-up visits:18- and 24 months) the control participants received the LPG stove intervention and vouchers for 1-y supply of free fuel and intervention participants stopped receiving free fuel but kept the LPG stove. Note: CO, carbon monoxide; LOD, limit of detection; LPG, liquefied petroleum gas.
Figure 6.
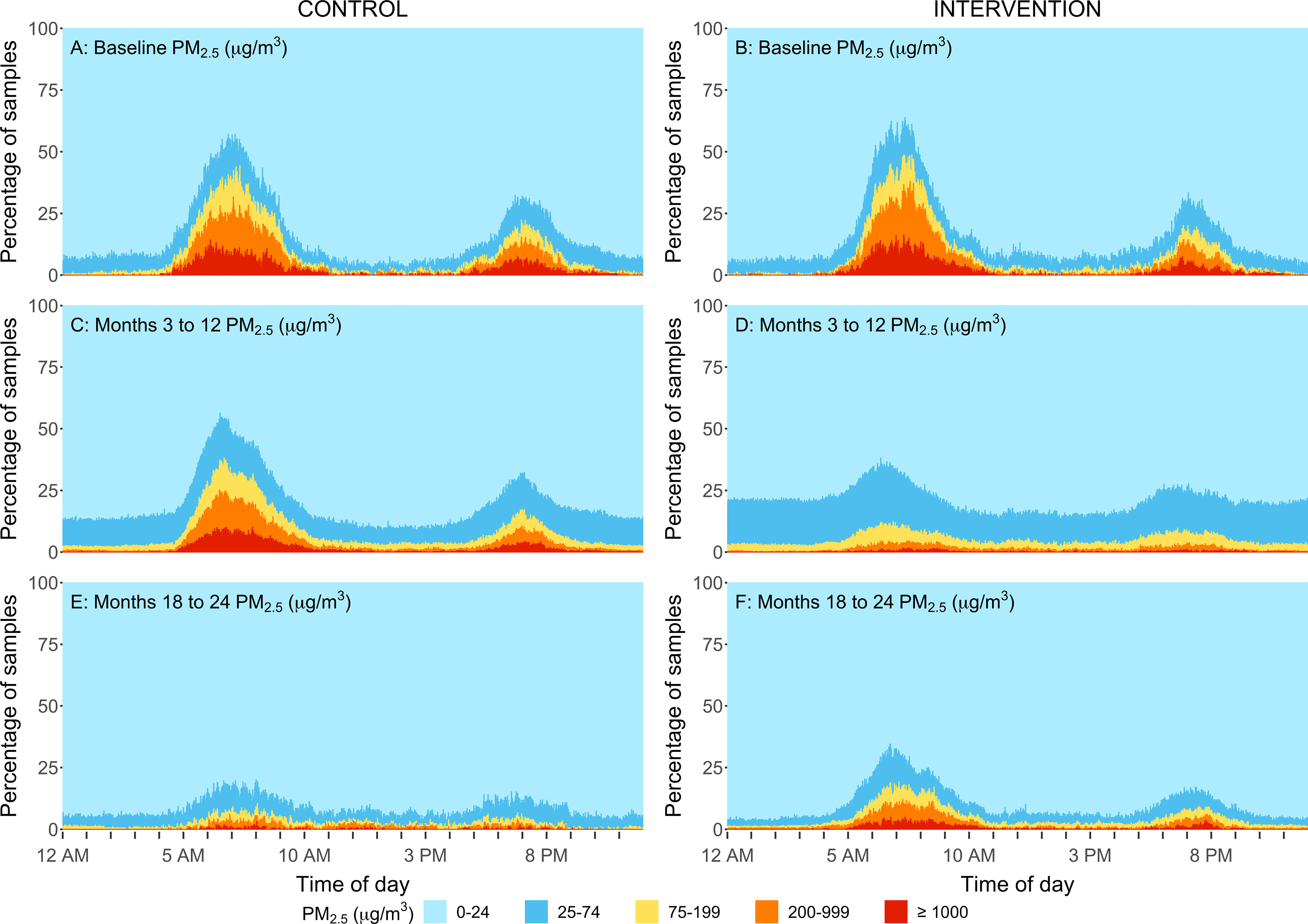
Percent of personal exposure samples within different concentration ranges by minute of a day for the intervention group (B,D,F) and control group (A,C,E) at baseline (A,B), at follow-up samples taken at the 3-, 6-, 12-month time points (C,D) and at 18-, and 24-month time points (E,F) in rural Peru. Samples were replaced by for gravimetric integrated samples, respectively. The sample size for baseline, 3-, 6-, 12, 18-, and 24-month visits for control and intervention households are: and 90, and 90, and 89, and 87, and 88, and 84. During the second year (follow-up visits:18- and 24 months) the control participants received the LPG stove intervention and vouchers for 1-y supply of free fuel and intervention participants stopped receiving free fuel but kept the LPG stove. Note: LOD, limit of detection; LPG, liquefied petroleum gas.
Effect of Voucher-Based Provision of LPG on HAP Exposures during the Second Year of the Trial
During the second year, when intervention participants stopped receiving free LPG and control participants received vouchers to obtain free LPG from local distributors, we observed lower , BC, and CO concentrations in comparison with baseline (at the 24-month visit, average kitchen area concentrations of , , and and personal exposures of , , and , respectively; Table 2), and we observed reductions in HAP exposure for the control group in the second year of the trial that were similar to those found in the intervention group during the first year (who received fuel delivery) (Figures 2 and 3; Figure S2). and CO daily mean concentrations in controls during the second year were below the WHO daily air quality guideline levels (Table 2).
In the intention-to-treat analysis of the second year of the trial, we found lower mean kitchen area concentrations and personal exposures in controls (receiving fuel vouchers) in comparison with intervention participants (no longer receiving a fuel subsidy) for the three pollutants measured (Table 4). For example, the relative differences in kitchen area concentrations show an 88% reduction in the control group in comparison with those of the intervention group for and 70% for CO at the 24-month follow-up visit [: 88% (95% CI: 82%, 92%); CO: 70% (95% CI: 56%, 80%), ]. In secondary analysis, we observed significant reductions between the second-year concentrations in the control group in comparison with their baseline levels (all ) in kitchen area daily mean concentrations [month-24 visit daily mean differences : (95% CI: , ); CO: (95% CI: , ); BC: (95% CI: , )] and personal exposures [between 83% to 85% for and BC and 60% for CO; month-24 visit daily mean differences : (95% CI: , ); CO: (95% CI: , ); BC: 85% (95% CI: , )] to all pollutants (Table 6). Consistent proportions were observed for maximum hourly means (Table 6). Reductions between baseline and measurements taken at 18-mo and 24-mo follow-up visits among control participants were comparable; no consistent time trends were identified for any of the pollutants throughout the second year (Table S6).
At the end of the second year of follow-up, more than 80% and 90% of the personal exposure samples (67% and 84% of kitchen samples) in the control group were below 24-h WHO air quality guideline levels for () and CO (), respectively (Table 2). When compared with the interim target-3 recommended by the WHO (, only one target above the 24-h guideline), 90% of personal exposure samples (82% of kitchen samples) were below this threshold (Table S7).
Important and sustained reductions in kitchen area concentrations of and personal exposures to all pollutants were also evident when evaluating the daily variability in concentrations among controls during the second year in comparison with their concentration during the first year and in comparison with baseline measurements (Figures 4E, 5E, 6E, and 7E). Similar to what was observed for intervention participants in the first year of the intervention (receiving fuel delivery), of the kitchen area samples and of personal exposure samples in the control group during the second year (receiving fuel vouchers) were in the lowest concentration category ( ppm and ) during cooking periods.
HAP Exposures among Intervention Participants during the Second Year of the Trial
During the second year of the trial, after intervention participants had completed 1 y of receiving free LPG and had to begin purchasing their own LPG if desired, kitchen area and personal exposures to all pollutants were higher in comparison with measurements for , BC, and CO concentrations during the first year with the LPG stove intervention (at the 24-month visit average kitchen area concentrations of , , and and personal exposures of , , and , respectively; Table 2). However, they were still significantly lower than baseline concentrations among intervention participants (Figures 2 and 3; Figure S2; Figures 4F, 5F, 6F, and 7F). Daily mean kitchen area concentrations in the intervention group were between 78% to 83% lower for and BC during the 18- and 24-month visits and between 69% to 74% lower for CO in comparison with baseline (Table 5). Daily mean personal exposures at 18- and 24-month visits in the intervention group were also lower (reductions between 65% to 71% for and BC and 48% to 50% for CO) in comparison with baseline (Table 5). The percentages of samples below the WHO daily air quality guideline levels among intervention households at the 24-month visit were 90% and 58% for personal samples (47% and 17% for kitchen samples) for CO and , respectively (Table 2).
Correlations
Correlation between kitchen area CO and was low among intervention participants and high among controls (Table S8). We also observed low correlations between kitchen area concentrations and personal exposures for CO and . When comparing the consecutive days of sampling within pollutants, personal exposure to had the lowest intraclass correlation coefficient (ICC) between the first and second day of measurements at each visit (0.36 for controls and 0.5 for intervention), whereas the ICC for personal exposure to CO was the highest (0.77 for controls and 0.84 for intervention).
Discussion
Our study demonstrated that both home-based delivery and voucher-based supply of free LPG resulted in significant and sustained reductions in kitchen concentrations and personal exposures to , BC, and CO to levels below the 24-h WHO air quality guideline levels for most homes in comparison with daily biomass use. Mean kitchen area concentrations of and personal exposures to CO were below the 24-h WHO air quality guideline levels when free fuel was delivered (intervention, year 1) and when free fuel vouchers were provided without delivery (controls, year 2). Reductions among the control group during the second year (under provision of free LPG through vouchers) were comparable to the reductions obtained for the intervention group during the first year (under home-based delivery of free LPG). This finding suggests that, combined with a behavioral and education intervention, a voucher system for provision of free LPG would be sufficient to achieve HAP exposures within daily WHO air quality guidelines.
Over the course of the first year of the trial, we observed that exposure reductions among intervention participants were not only substantial during the first follow-up visit but were consistent over the entire 12 months of free LPG delivery. Our observation of greater reductions for and BC at the end of the first year in comparison with measurements taken during the first few months after the start of the intervention suggests a trend of enhanced reductions over time. Our study also demonstrates that the HAP reductions were sustained beyond the intervention year for intervention participants (year 2) after their fuel subsidy had ended. Specifically, 1 year after intervention participants stopped receiving LPG deliveries, 58% of personal exposures (17% of kitchen samples) were still below 24-h WHO air quality guideline levels for the intervention group (in comparison with a baseline of 11% of personal samples and 0% of kitchen samples below the guideline level). This finding suggests that provision of a free LPG stove accompanied by a short-term program of free LPG delivery with behavioral messaging and education may result in longer-term exposure reductions in resource-limited settings such as Peru. However, it is important to note that achievement of the WHO air quality guidelines was much higher among participants receiving free LPG, suggesting that financial subsidies are essential to sustain the near-exclusive LPG use required to maintain exposures within guideline levels more consistently.
Preliminary analyses of stove and fuel use during the second year demonstrate that intervention participants continued using the LPG stove after they stopped receiving free fuel, but less exclusively in comparison with the first year (details will be published separately). Possible factors influencing the levels of sustained LPG use observed include time savings and other benefits perceived by participants (Williams et al. 2020b), the relative proximity () of LPG retailers to participant homes (Fandiño-Del-Rio et al. 2017), the governmental LPG subsidization program in Peru (Pollard et al. 2018), and the experience participants gained with LPG during the first year of the trial. A qualitative investigation into the specific factors motivating sustained LPG use among the participants is currently under preparation.
To our knowledge, the concentration reductions observed in this field intervention trial are among the largest achieved in any published clean fuel intervention trial targeting users of biomass cookstoves. Only a couple of recent nonrandomized pilot studies, with a similar LPG stove and behavioral interventions in India, have been able to achieve comparable reductions (Pillarisetti et al. 2019; Sambandam et al. 2020). Studies in Guatemala, Bangladesh, and India demonstrated PM indoor area reductions with LPG stove use in comparison with biomass use, which ranged from 45% to 75% (achieving concentrations between 50 to ) (Albalak et al. 2001; Banerjee et al. 2012; Begum et al. 2009; Dutta et al. 2012; Naeher et al. 2000). Most of these previous studies were cross-sectional, some measured exposures on a small number of participants (Albalak et al. 2001; Begum et al. 2009; Naeher et al. 2000), and many participants continued to use both LPG and biomass stoves (Albalak et al. 2001; Gould et al. 2020; Naeher et al. 2000).
During the first year of the trial, our free, home-based delivery of LPG fuel and behavioral reinforcement intervention resulted in kitchen area and CO reductions (97% and 86%, respectively) greater than those previously estimated for advanced combustion stoves (41% and 39%, respectively) and ethanol stoves (83% and 82%, respectively) (Kumar et al. 2021; Pope et al. 2017). Furthermore, our LPG intervention resulted in concentrations below WHO air quality guideline levels, in contrast to previous studies (Kumar et al. 2021; Pope et al. 2017, 2021; Saleh et al. 2020). A combination of low ambient air pollution concentrations (Clasen et al. 2020; Pollard et al. 2014), low population density, large distance from main roads, and near-exclusive use of LPG stoves among intervention participants who used LPG for over 98% of cooking minutes (Williams et al. 2020b) may explain our success in achieving lower HAP levels than the levels achieved in previous studies. We also achieved HAP levels lower than what has been estimated through modeled scenarios with LPG stoves (personal exposures to of ) (Steenland et al. 2018).
Our results indicate levels of , BC, and CO that could be attained with unlimited, free supply of LPG in areas with low levels of ambient air pollution. However, achieving near-exclusive use of LPG and reducing indoor air concentrations requires careful consideration of local characteristics (including population density); the economic, social, and behavioral conditions that influence cooking practices; and fuel choices, which will vary both within and between countries (Fandiño-Del-Rio et al. 2020; Williams et al. 2020a).
In addition to the significant exposure reductions from free, delivered LPG in the first year of the trial (70%–90%), we observed some reductions (20%–30%) in and BC personal exposure within the control arm during the same period. These reductions in personal exposures were much smaller than the reductions observed for the intervention participants and did not persist throughout the year. Although kitchen area concentrations in the control group were also lower during the first year in comparison with the baseline measurements, the differences were not statistically significant. Other intervention trials have found that the control group often experiences some of the intervention benefits when not blinded (Gal et al. 2019; Magill et al. 2019). For example, an energy package intervention that included a semi-gasifier cookstove, water heater, chimney, and supply of processed biomass fuel in rural China resulted in reductions in air pollution exposures in both the treatment and control groups (Clark et al. 2019). One possible explanation is the increased use of previously owned LPG stoves among control participants that may have occurred as a result of learning about LPG benefits over the course of the trial (Williams et al. 2020b).
Our analysis of monitor-wearing compliance showed that participants wore personal monitors for 55% of awake time, similar to what some studies have observed in other rural settings (66% in the eastern Tibetan Plateau; 45% in rural Malawi) (Cho et al. 2016; Ye et al. 2020). Although higher compliance has been achieved in a cookstove study in Sri Lanka (83%–87%) (Chartier et al. 2017), lower compliance is not uncommon. A study in Ecuador observed only 12% compliance in daytime hours (Gould et al. 2020). Previous studies have considered a wearing compliance as representative of personal exposures (Lawless et al. 2012; Rodes et al. 2010). Wearing compliance did not differ by study arm, which increases our confidence that the differences observed in personal exposures between study arms were due to the intervention and not to differences in wearing compliance.
Low ICC between the consecutive first and second days of personal samples could be due to differences in movement patterns across days. If this is the case, then one 24-h sample might not always be sufficient to capture the typical concentrations in these types of settings. Also, because kitchen CO samples were similar on consecutive sampling days, a second day of sampling might not be necessary for CO kitchen area concentrations but might be valuable for .
Strengths and Limitations
We faced several challenges during sampling due to the high concentrations observed with biomass stoves that caused some of our filters to overload, draining the battery and resulting in shorter than intended sample durations. Additionally, concentrations below our detection limits in the intervention group were common. However, because the impact of HAP with the LPG stove in comparison with biomass cookstoves was so dramatic, it is unlikely that the sampling limitations affected the interpretation of our results. In contrast to and CO, BC samples were only measured on the integrated samples. Thus, it was not possible to identify peaks or daily patterns for this pollutant. Another limitation of our study was the inability to fully quantify LPG stove use by control participants during the first year of the trial. Although all participants reported using biomass daily at baseline, 70 control participants had observed, reported, or monitored LPG use during the first year of the trial, but only 24 participants had monitors installed on their LPG stoves during this period (Williams et al. 2020b). Exposure contrasts may have been even greater if control participants had used biomass stoves exclusively during the first year of the trial. Additionally, because we used intermittent duty cycles on the devices for participants who used biomass stoves, we might have missed short duration peaks in concentration within the sampling cycle. However, it is unlikely that this limitation affected our conclusions because it would result in biomass group concentrations estimations that are conservative. Therefore, this would bias our effect estimates toward the null, meaning that the effect estimates might be even more extreme that what we found.
Successes of this study include high retention of participants, near-exclusive use of the LPG fuel intervention by intervention participants during the first year of the trial, and significant reduction of HAP under conditions of provision of free LPG through home-based delivery and a voucher system. Our use of small, lightweight, portable sampling devices allowed us to measure personal exposures, which are expected to be more relevant for understanding the relationship between exposures and health outcomes than kitchen area concentrations. The collection of pollutant concentrations at high temporal resolution at multiple time points over the course of 2 y and the gravimetric correction for nephelometric samples provided high-quality data. We observed important reductions of , BC, and CO concentrations; however, future directions should incorporate quantification of other hazardous air pollutants associated with LPG stoves such as . Although we have previously shown significant concentration reductions for a subset of participants in this trial, we have also demonstrated that concentrations with LPG stove use can still remain above those of the WHO air quality guidelines (Kephart et al. 2021).
Conclusion
We observed significant and sustained HAP reductions from free provision of LPG through both home-delivery and voucher-based delivery systems, when combined with a free LPG stove and continuous behavioral reinforcement. Home-based delivery of free LPG resulted in personal exposures to HAP at levels approaching those recommended by the WHO for and CO. We also observed that, combined with a behavioral and education intervention, provision of free LPG through vouchers was sufficient to encourage participants to use LPG stoves and achieve similar low exposures. Moreover, we found that home-based delivery of free LPG for 1 y motivated many households to sustain lower levels of exposure in comparison with baseline and in comparison with levels experienced by primary biomass users who had not received such an intervention (control participants during the first year of the trial). These results support efforts to promote exclusive LPG stove use over improved biomass stoves. Further research on the feasibility and cost-effectiveness of the intervention, in addition to specific contributions of the behavioral and education intervention components, is needed to understand its potential for implementation and scale-up in larger-scale national programs. Results of this trial will inform the feasibility of LPG stove intervention programs to reduce HAP and improve health in resource-limited settings such as Peru. Our results highlight the need for more efforts to improve the feasibility and accessibility of exclusive use of LPG stoves to achieve levels specified by the WHO and CO guidelines globally.
Supplementary Material
Acknowledgments
CHAP Trial Investigators. Steering Committee: W.C. MD PhD (Johns Hopkins University, Baltimore, MD, USA), G.F.G. MD (Universidad Peruana Cayetano Heredia, Lima, Peru), L.P.N. PhD (University of Georgia, Athens, GA, USA), J. Rosenthal PhD (National Institutes of Health, Bethesda, MD, USA), and N.K.S. PhD (Emory University, Atlanta, GA, USA).
JHU Investigators: T. Aguilar, V. Burrowes PhD, M.F. PhD, E.C. Fung MSPH, D. Goodman MSPH, S.A. Harvey PhD, P. Herrera MD, J.L. Kephart PhD, K. Koehler PhD, A. Lee, K.A. Lee MPH, C.H. Miele MD MPH, M. Moazzami MSPH, L. Moulton PhD, S. Nangia, L. Nicolaou PhD, C.J. O’Brien MSPH, S. Simkovich MD MS, T. Shade, L. Stashko MSPH, A. Villegas-Gomez MSPH, K.N. Williams PhD, A. Winiker MSPH.
Asociación Benéfica PRISMA Investigators: M.C. MD MPH, G. Malpartida, and C. Tarazona-Meza MPH.
Washington University Investigators: V. Davila-Roman MD, L. de las Fuentes MD. Emory University Investigators: D. Barr Boyd PhD, M. Jolly MSPH, and A. Rozo MS.
RTI International: R.T.C. PhD.
The trial was approved by the institutional review boards of A.B. PRISMA (CE2402.16) and Universidad Peruana Cayetano Heredia (66780) in Lima, Peru, and by the Johns Hopkins Bloomberg School of Public Health (00007128) in Baltimore, Maryland, USA. The trial is registered in ClinicalTrials.gov (identifier NCT02994680).
There were no adverse events related to the intervention during the year-long intervention. One intervention participant’s pressure cooker exploded while cooking with the LPG stove. No people were harmed, but the LPG stove and table were destroyed and had to be replaced. Another intervention participant had reported a fire in her kitchen after using her biomass stove. No people were harmed, but the LPG stove and table were burned and had to be replaced. Both episodes were reported as adverse events.
Funding was provided by the U.S. NIH (ClinicalTrials.gov ID NCT02994680).
Research reported in this publication was supported by the U.S. National Institutes of Health (NIH) through the following Institutes and Centers: Fogarty International Center, National Institute of Environmental Health Sciences (NIEHS), National Cancer Institute, and Centers for Disease Control under award numbers U01TW010107 and U2RTW010114 [Multiple principal investigators (MPIs): W.C., G.F.G., L.P.N., K.S.]. This trial was additionally supported in part by the Clean Cooking Alliance of the United Nations Foundation UNF-16-810 [Principal investigator (PI): W.C.]. M.F. was further supported by the Global Environmental and Occupational Health (GEOHealth), Fogarty International Center, and by the David Leslie Swift Fund of the Bloomberg School of Public Health, JHU. K.N.W. and J.L.K. were supported by the NIH Research Training Grant D43TW009340 (MPIs: Buekens, W.C., Chi, Kondwani) funded by U.S. NIH through the following Institutes and Centers: Fogarty International Center; National Institute of Neurological Disorders and Stroke; National Institute of Mental Health; National Heart, Lung, and Blood Institute; and the NIEHS. J.L.K., K.N.W., and M.F. were supported by a Global Established Multidisciplinary Sites award from the Center for Global Health at JHU (PI: W.C.). J.L.K. was further supported by the NIEHS of the NIH under award number T32ES007141 (PI: Wills-Karp). K.N.W. was supported by the National Heart, Lung, and Blood Institute of the NIH under award number T32HL007534 (PI: Wise). Our Global Non-Communicable Disease Research and Training field center in Puno, Peru, also received generous support from William and Bonnie Clarke III and the COPD Discovery Award from JHU.
References
- Achilleos S, Kioumourtzoglou M-A, Wu C-D, Schwartz JD, Koutrakis P, Papatheodorou SI. 2017. Acute effects of fine particulate matter constituents on mortality: a systematic review and meta-regression analysis. Environ Int 109:89–100, PMID: , 10.1016/j.envint.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albalak R, Bruce N, McCracken JP, Smith KR, De Gallardo T. 2001. Indoor respirable particulate matter concentrations from an open fire, improved cookstove, and LPG/open fire combination in a rural Guatemalan community. Environ Sci Technol 35(13):2650–2655, PMID: , 10.1021/es001940m. [DOI] [PubMed] [Google Scholar]
- Anderman TL, DeFries RS, Wood SA, Remans R, Ahuja R, Ulla SE. 2015. Biogas cook stoves for healthy and sustainable diets? A case study in southern India. Front Nutr 2:28, PMID: , 10.3389/fnut.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmes J, Pope D, Dherani M, Zhang J, Duan X, Bates M, et al. . 2014. WHO Indoor Air Quality Guidelines: Household Fuel Combustion. Review 4: Health effects of household air pollution (HAP) exposure [WWW Document], 2014. https://www.who.int/publications/m/item/who-indoor-air-quality-guidelines-household-fuel-combustion [accessed 2 May 2022]. [Google Scholar]
- Banerjee A, Mondal NK, Das D, Ray MR. 2012. Neutrophilic inflammatory response and oxidative stress in premenopausal women chronically exposed to indoor air pollution from biomass burning. Inflammation 35(2):671–683, PMID: , 10.1007/s10753-011-9360-2. [DOI] [PubMed] [Google Scholar]
- Begum BA, Paul SK, Dildar Hossain M, Biswas SK, Hopke PK. 2009. Indoor air pollution from particulate matter emissions in different households in rural areas of Bangladesh. Build Environ 44(5):898–903, 10.1016/j.buildenv.2008.06.005. [DOI] [Google Scholar]
- Bennitt FB, Wozniak SS, Causey K, Burkart K, Brauer M. 2021. Estimating disease burden attributable to household air pollution: new methods within the Global Burden of Disease Study. Lancet Glob Health 9:S18, 10.1016/S2214-109X(21)00126-1. [DOI] [Google Scholar]
- Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P, CONSORT NPT Group. 2017. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med 167(1):40–47, PMID: , 10.7326/M17-0046. [DOI] [PubMed] [Google Scholar]
- Bruce N, Perez-Padilla R, Albalak R. 2000. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull World Health Organ 78:1078–1092, PMID: . [PMC free article] [PubMed] [Google Scholar]
- Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA, et al. . 2018. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci USA 115(38):9592–9597, PMID: , 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier R, Phillips M, Mosquin P, Elledge M, Bronstein K, Nandasena S, et al. . 2017. A comparative study of human exposures to household air pollution from commonly used cookstoves in Sri Lanka. Indoor Air 27(1):147–159, PMID: , 10.1111/ina.12281. [DOI] [PubMed] [Google Scholar]
- Checkley W, Williams KN, Kephart JL, Fandiño-Del-Rio M, Steenland NK, Gonzales GF, et al. . 2021. Effects of a household air pollution intervention with liquefied petroleum gas on cardiopulmonary outcomes in Peru. A randomized controlled trial. Am J Respir Crit Care Med 203(11):1386–1397, PMID: , 10.1164/rccm.202006-2319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Chartier RT, Mortimer K, Dherani M, Tafatatha T. 2016. A personal particulate matter exposure monitor to support household air pollution exposure and health studies. In: 2016 IEEE Global Humanitarian Technology Conference (GHTC). 13–16 October 2016. Seattle, Washington: IEEE Global Humanitarian Technology Conference, 817–818.
- Chow JC, Watson JG, Green MC, Frank NH. 2010. Filter light attenuation as a surrogate for elemental carbon. J Air Waste Manag Assoc 60(11):1365–1375, 10.3155/1047-3289.60.11.1365. [DOI] [PubMed] [Google Scholar]
- Clark ML, Peel JL, Balakrishnan K, Breysse PN, Chillrud SN, Naeher LP, et al. . 2013. Health and household air pollution from solid fuel use: the need for improved exposure assessment. Environ Health Perspect 121(10):1120–1128, PMID: , 10.1289/ehp.1206429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SN, Schmidt AM, Carter EM, Schauer JJ, Yang X, Ezzati M, et al. . 2019. Longitudinal evaluation of a household energy package on blood pressure, central hemodynamics, and arterial stiffness in China. Environ Res 177:108592, PMID: , 10.1016/j.envres.2019.108592. [DOI] [PubMed] [Google Scholar]
- Clasen T, Checkley W, Peel JL, Balakrishnan K, McCracken JP, Rosa G, et al. . 2020. Design and rationale of the HAPIN study: a multicountry randomized controlled trial to assess the effect of liquefied petroleum gas stove and continuous fuel distribution. Environ Health Perspect 128(4):047008, PMID: , 10.1289/EHP6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. . 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389(10082):1907–1918, PMID: , 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A, Khramtsova G, Brito K, Alexander D, Mueller A, Chinthala S, et al. . 2018. Household air pollution and chronic hypoxia in the placenta of pregnant Nigerian women: a randomized controlled ethanol cookstove intervention. Sci Total Environ 619-620:212–220, 10.1016/j.scitotenv.2017.11.091. [DOI] [PubMed] [Google Scholar]
- Dutta A, Ray MR, Banerjee A. 2012. Systemic inflammatory changes and increased oxidative stress in rural Indian women cooking with biomass fuels. Toxicol Appl Pharmacol 261(3):255–262, PMID: , 10.1016/j.taap.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Fandiño-Del-Rio M, Goodman D, Kephart JL, Miele CH, Williams KN, Moazzami M, et al. . 2017. Effects of a liquefied petroleum gas stove intervention on pollutant exposure and adult cardiopulmonary outcomes (CHAP): study protocol for a randomized controlled trial. Trials 18(1), 10.1186/s13063-017-2179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandiño-Del-Rio M, Kephart JL, Williams KN, Moulton LH, Steenland K, Checkley W, et al. . 2020. Household air pollution exposure and associations with household characteristics among biomass cookstove users in Puno, Peru. Environ Res 191:110028, PMID: , 10.1016/j.envres.2020.110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford I, Norrie J. 2016. Pragmatic trials. N Engl J Med 375(5):454–463, PMID: , 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- Fullerton DG, Bruce N, Gordon SB. 2008. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg 102(9):843–851, PMID: , 10.1016/j.trstmh.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal R, Monninkhof EM, van Gils CH, Groenwold RHH, van den Bongard DHJG, Peeters PHM, et al. . 2019. The Trials within Cohorts design faced methodological advantages and disadvantages in the exercise oncology setting. J Clin Epidemiol 113:137–146, PMID: , 10.1016/j.jclinepi.2019.05.017. [DOI] [PubMed] [Google Scholar]
- Garland C, Delapena S, Prasad R, L’Orange C, Alexander D, Johnson M. 2017. Black carbon cookstove emissions: a field assessment of 19 stove/fuel combinations. Atmos Environ 169:140–149, 10.1016/j.atmosenv.2017.08.040. [DOI] [Google Scholar]
- Gordon SB, Bruce NG, Grigg J, Hibberd PL, Kurmi OP, Lam K-B.H, et al. . 2014. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med 2(10):823–860, PMID: , 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould CF, Schlesinger SB, Molina E, Lorena Bejarano M, Valarezo A, Jack DW. 2020. Long-standing LPG subsidies, cooking fuel stacking, and personal exposure to air pollution in rural and peri-urban Ecuador. J Expo Sci Environ Epidemiol 30(4):707–720, PMID: , 10.1038/s41370-020-0231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly FJ, Fussell JC. 2012. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos Environ 60:504–526, 10.1016/j.atmosenv.2012.06.039. [DOI] [Google Scholar]
- Kephart JL, Fandiño-Del-Rio M, Williams KN, Malpartida G, Lee A, Steenland K, et al. . 2021. Nitrogen dioxide exposures from LPG stoves in a cleaner-cooking intervention trial. Environ Int 146:106196, PMID: , 10.1016/j.envint.2020.106196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kephart JL, Fandiño‐Del‐Rio M, Williams KN, Malpartida G, Steenland K, Naeher LP, et al. . 2020. Nitrogen dioxide exposures from biomass cookstoves in the Peruvian Andes. Indoor Air 30(4):735–744, PMID: , 10.1111/ina.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler KA, Peters TM. 2015. New methods for personal exposure monitoring for airborne particles. Curr Environ Health Rep 2(4):399–411, PMID: , 10.1007/s40572-015-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Phillip E, Cooper H, Davis M, Langevin J, Clifford M, et al. . 2021. Do improved biomass cookstove interventions improve indoor air quality and blood pressure? A systematic review and meta-analysis. Environ Pollut 290, 117997. 10.1016/j.envpol.2021.117997. [DOI] [PubMed] [Google Scholar]
- Lawless P, Thornburg J, Rodes C, Williams R. 2012. Personal exposure monitoring wearing protocol compliance: an initial assessment of quantitative measurement. J Expo Sci Environ Epidemiol 22(3):274–280, PMID: , 10.1038/jes.2012.8. [DOI] [PubMed] [Google Scholar]
- Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, et al. . 2019. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med 381(8):705–715, PMID: , 10.1056/NEJMoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill N, Knight R, McCrone P, Ismail K, Landau S. 2019. A scoping review of the problems and solutions associated with contamination in trials of complex interventions in mental health. BMC Med Res Methodol 19(1), 10.1186/s12874-018-0646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. . 2020. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396(10258):1223–1249, 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeher LP, Leaderer BP, Smith KR. 2000. Particulate matter and carbon monoxide in highland Guatemala: indoor and outdoor levels from traditional and improved wood stoves and gas stoves. Indoor Air 10(3):200–205, PMID: , 10.1034/j.1600-0668.2000.010003200.x. [DOI] [PubMed] [Google Scholar]
- Nie P, Sousa-Poza A, Xue J. 2016. Fuel for life: domestic cooking fuels and women’s health in rural China. Int J Environ Res Public Health 13(8):810, 10.3390/ijerph13080810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillarisetti A, Ghorpade M, Madhav S, Dhongade A, Roy S, Balakrishnan K, et al. . 2019. Promoting LPG usage during pregnancy: a pilot study in rural Maharashtra, India. Environ Int 127:540–549, PMID: , 10.1016/j.envint.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard SL, Williams DL, Breysse PN, Baron PA, Grajeda LM, Gilman RH, et al. . 2014. A cross-sectional study of determinants of indoor environmental exposures in households with and without chronic exposure to biomass fuel smoke. Environ Health 13(1):21, PMID: , 10.1186/1476-069X-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard SL, Williams KN, O’Brien CJ, Winiker A, Puzzolo E, Kephart JL, et al. . 2018. An evaluation of the Fondo de Inclusión Social Energético program to promote access to liquefied petroleum gas in Peru. Energy Sustain Dev 46:82–93, 10.1016/j.esd.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope D, Bruce N, Dherani M, Jagoe K, Rehfuess E. 2017. Real-life effectiveness of ‘improved’ stoves and clean fuels in reducing PM2.5 and CO: systematic review and meta-analysis. Environ Int 101:7–18, PMID: , 10.1016/j.envint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Pope D, Johnson M, Fleeman N, Jagoe K, Duarte R, Maden M, et al. . 2021. Are cleaner cooking solutions clean enough? A systematic review and meta-analysis of particulate and carbon monoxide concentrations and exposures. Environ Res Lett 16(8):083002, 10.1088/1748-9326/ac13ec. [DOI] [Google Scholar]
- Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, et al. . 2013. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol 14(9):813–822, PMID: , 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- Rodes CE, Chillrud SN, Haskell WL, Intille SS, Albinali F, Rosenberger M. 2012. Predicting adult pulmonary ventilation volume and wearing compliance by on-board accelerometry during personal level exposure assessments. Atmos Environ (1994) 57:126–137, 10.1016/j.atmosenv.2012.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodes CE, Lawless PA, Thornburg JW, Williams RW, Croghan CW. 2010. DEARS particulate matter relationships for personal, indoor, outdoor, and central site settings for a general population. Atmos Environ 44(11):1386–1399, 10.1016/j.atmosenv.2010.02.002. [DOI] [Google Scholar]
- Rohr A, McDonald J. 2016. Health effects of carbon-containing particulate matter: focus on sources and recent research program results. Crit Rev Toxicol 46(2):97–137, PMID: , 10.3109/10408444.2015.1107024. [DOI] [PubMed] [Google Scholar]
- Saleh S, Shepherd W, Jewell C, Lam NL, Balmes J, Bates MN, et al. . 2020. Air pollution interventions and respiratory health: a systematic review. Int J Tuberc Lung Dis 24(2):150–164, PMID: , 10.5588/ijtld.19.0417. [DOI] [PubMed] [Google Scholar]
- Sambandam S, Mukhopadhyay K, Sendhil S, Ye W, Pillarisetti A, Thangavel G, et al. . 2020. Exposure contrasts associated with a liquefied petroleum gas (LPG) intervention at potential field sites for the multi-country Household Air Pollution Intervention Network (HAPIN) trial in India: results from pilot phase activities in rural Tamil Nadu. BMC Public Health 20(1):1799, PMID: , 10.1186/s12889-020-09865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D, Lellouch J. 1967. Explanatory and pragmatic attitudes in therapeutical trials. J Chronic Dis 20(8):637–648, PMID: , 10.1016/0021-9681(67)90041-0. [DOI] [PubMed] [Google Scholar]
- Smith KR, Bruce N, Balakrishnan K, Adair-Rohani H, Balmes J, Chafe Z, et al. . 2014. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health 35:185–206, PMID: , 10.1146/annurev-publhealth-032013-182356. [DOI] [PubMed] [Google Scholar]
- Soneja SI, Tielsch JM, Khatry SK, Curriero FC, Breysse PN. 2016. Highlighting uncertainty and recommendations for improvement of black carbon biomass fuel-based emission inventories in the Indo-Gangetic plain region. Curr Environ Health Rep 3(1):73–80, PMID: , 10.1007/s40572-016-0075-2. [DOI] [PubMed] [Google Scholar]
- Steenland K, Pillarisetti A, Kirby M, Peel J, Clark M, Checkley W, et al. . 2018. Modeling the potential health benefits of lower household air pollution after a hypothetical liquified petroleum gas (LPG) cookstove intervention. Environ Int 111:71–79, PMID: , 10.1016/j.envint.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhsohale ND, Narlawar UW, Phatak MS, Agrawal SB, Ughade SN. 2013. Effect of indoor air pollution during cooking on peak expiratory flow rate and its association with exposure index in rural women. Indian J Physiol Pharmacol 57(2):184–188, PMID: . [PubMed] [Google Scholar]
- The World Bank. 2010. Household Cookstoves, Environment, Health, and Climate change: A New Look at an Old Problem. http://documents.worldbank.org/curated/en/732691468177236006/Household-cookstoves-environment-health-and-climate-change-a-new-look-at-an-old-problem [accessed 7 January 2022].
- Thomas E, Wickramasinghe K, Mendis S, Roberts N, Foster C. 2015. Improved stove interventions to reduce household air pollution in low and middle income countries: a descriptive systematic review. BMC Public Health 15:650, PMID: , 10.1186/s12889-015-2024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunis SR, Stryer DB, Clancy CM. 2003. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA 290(12):1624–1632, PMID: , 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- Williams KN, Kephart JL, Fandiño-Del-Rio M, Condori L, Koehler K, Moulton LH, et al. . 2020a. Beyond cost: exploring fuel choices and the socio-cultural dynamics of liquefied petroleum gas stove adoption in Peru. Energy Research & Social Science 66:101591, 10.1016/j.erss.2020.101591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KN, Kephart JL, Fandiño-Del-Rio M, Simkovich SM, Koehler K, Harvey SA, et al. . 2020b. Exploring the impact of a liquefied petroleum gas intervention on time use in rural Peru: a mixed methods study on perceptions, use, and implications of time savings. Environ Int 145:105932, PMID: , 10.1016/j.envint.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2006. Air Quality Guidelines Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide. https://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/pre2009/air-quality-guidelines.-global-update-2005.-particulate-matter,-ozone,-nitrogen-dioxide-and-sulfur-dioxide [accessed 7 January 2022]. [PubMed]
- WHO. 2010. WHO Guidelines for Indoor Air Quality: Selected Pollutants. http://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/2010/who-guidelines-for-indoor-air-quality-selected-pollutants [accessed 7 January 2022]. [PubMed]
- WHO. 2012. Health Effects of Black Carbon (2012). http://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/2012/health-effects-of-black-carbon-2012 [accessed 7 January 2022].
- WHO. 2014. WHO Guidelines for Indoor Air Quality: Household Fuel Combustion. https://apps.who.int/iris/handle/10665/141496 [accessed 7 January 2022].
- WHO. 2016. Preventing Disease Through Healthy Environments: A Global Assessment of the Burden of Disease from Environmental Risks. https://www.who.int/publications/i/item/9789241565196 [accessed 7 January 2022].
- Ye W, Saikawa E, Avramov A, Cho S-H, Chartier R. 2020. Household air pollution and personal exposure from burning firewood and yak dung in summer in the eastern Tibetan Plateau. Environ Pollut 263(pt B):114531, PMID: , 10.1016/j.envpol.2020.114531. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang K-Y, Albert PS. 1988. Models for longitudinal data: a generalized estimating equation approach. Biometrics 44(4):1049–1060, PMID: , 10.2307/2531734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



