Abstract
Breast cancer is the most common malignancy among women worldwide, accordingly, numerous chemotherapeutic drugs have been discovered thus far. However, the development and application of these drugs is severely constrained because of their unclear mechanism. To address this issue, our previous work has defined 3-acyl isoquinolin-1(2H)-one derivatives as potent anti-tumor agents, among which the compound 4f possessed relatively higher activity in vitro. In this study, we aim to further explore the anti-cancer effect and the underlying molecular mechanism of 4f in breast cancer cells. Therefore, CCK8 assay was used to detect cell viability and flow cytometry was used to analyze cell cycle and apoptosis. Meanwhile, related proteins that regulate cell cycle and apoptosis were detected. The results showed that 4f induced cell apoptosis and inhibited cell proliferation in breast cancer cells in a dose-depended manner without significant toxicity to human normal mammary epithelial cell. The cell cycle was arrested at G2 phase with the suppressed expression of the CDK1 protein. Additionally, 4f was confirmed to induce the cell apoptosis with the up-regulation of bax, down-regulation of bcl-2, activation of cleaved-caspase3/7/9 and cleaved-PARP, together with the inhibition of MEK/ERK and p38 MAPK pathway. Moreover, the GSDME-mediated pyroptosis was also induced by 4f in breast cancer cells. Together, these results demonstrated that 4f could serve as a new and promising candidate for the treatment of breast cancer.
Introduction
Breast cancer represents the most frequently diagnosed and the leading cause of cancer death in women worldwide [1]. In the last two decades, incidence and mortality rates of breast cancer have climbed sharply in China [2]. Additionally, previous studies revealed that one in eight women in the United States would develop breast cancer in her lifetime [3]. Modern treatment of breast cancer is multimodal, which includes surgery, radiation and chemotherapy. Nevertheless, drug resistance often limits the application of chemotherapy. Hence, it is vital to develop novel effective drug therapies against breast cancer.
Cancer cells are characterized by uncontrollable cell proliferation due to dysregulation of cell cycle regulation influenced by the deregulation of the activity of cell cycle related proteins [4, 5]. The cyclin E/CDK2 complex plays a critical role in G1/S transition, the cyclin A/CDK2 complex is required in the progression of S phase and the cyclin B1 and cdc2 complex (CDK1) is the specific regulator in G2/M phase transition and processes during mitosis [6–8]. Evasion of apoptosis is another hallmark of most types of cancer and the deregulation of apoptosis will leads to the uncontrolled growth of cancers [4]. Compared to apoptosis, the pyroptosis is a newly identified programmed cell death initiated by inflammatory caspases and numerous chemotherapeutic agents have been discovered to exert anti-tumor activities by inducing pyroptosis [9–11]. The gasdermin D (GSDMD) acts as the substrate of caspase 1/4/5/11 and can be cleaved to generate GSDMD-N fragment, which induces pyroptosis via forming the membrane pores [12, 13]. Alternatively, the gasdermin E (GSDME) gene, belongs to gasdermin superfamily, originally identified as deafness autosomal dominant 5 (DNFA5) [14], can function as a tumor suppressor gene in colorectal, gastric, melanoma, and breast carcinomas [15–17]. Shao et al reported that chemotherapy drugs could induce pyroptosis via the specific cleavage of GSDME by caspase 3 in the linker, thus generating a GSDME-N fragment that perforated membranes for pyroptosis induction [10]. Therefore, drugs that disrupt cancer cell cycle, restore apoptosis or pyroptosis might be effective against many types of cancers.
Demonstrated as important building blocks and promising skeletons, 3-acyl isoquinolin-1(2H)-ones and analogues exert anti-tumor effect in many cancers including glioblastoma, colorectal cancer and breast cancer [18–21]. The literature precedent disclosed that isoquinolinone derivatives had anti-tumor effect in breast cancer via inhibiting MAPK/ERK pathway [21, 22], which is a significant cell signaling pathway and involved in multiple cellular processes including cell proliferation and apoptosis. By virtue of an efficient and scalable rhodium(III)-catalyzed redox-neutral C-H annulation of N-methoxybenzamides with propargyl cycloalkanols, a series of 3-acyl isoquinolin-1(2H)-ones were synthesized for anti-tumor agents screening. Preliminary evaluation revealed that these motifs possessed anti-tumor effects equivalent to or even better than 5-FU in three different cancers in vitro, and delightfully, the compound 4f exhibited the most potent anti-tumor activities against A549 and MCF-7 cell lines [23]. However, the anti-tumor mechanism of 4f in breast cancer cells is unclear, which limits the further modification and development of such skeleton as the anti-tumor drug.
To address this drawback and in continuation of our interest in the development of drug therapy against cancer disease, we herein disclosed the effect of 4f on cell proliferation, apoptosis and pyroptosis of breast cancer cells (MCF-7 and MDA-MB-213 cell lines) in vitro, as well as the underlying mechanisms. Our results demonstrated that 4f induces G2 phase arrest of cell cycle, cell apoptosis and GSDME-mediated pyroptosis in breast cancer cells without significant toxicity to human normal mammary epithelial cell. Therefore, 4f might serve as a lead compound for anti-tumor drug discovery.
Materials and methods
Chemicals and antibodies
The 3-acyl isoquinolin-1(2H)-one complex 4f was synthesized according to a known procedure developed in our previous work. Cell counting kits-8 (CCK8) was purchased from Beyotime Biotechnology, dimethyl sulfoxide (DMSO) and RIPA lysis buffer were purchased from Sigma (Beverly, MA, USA). Primary antibodies such as anti-caspase3 antibody (cat#14220T), anti-cleaved-caspase9 antibody (cat#52873), anti-cleaved-caspase7 antibody (cat#8438), anti-parp antibody (cat#9542), anti-bcl-2 antibody (cat#15071T), anti-bax antibody (cat#5023), anti-β-tubulin antibody (cat#2128s), anti-ERK1/2 antibody (cat#4695T), anti-p-ERK1/2 antibody (cat#4370T), anti-MEK1/2 antibody (cat#9126s), anti-p-MEK1/2 antibody (cat#9154T), (HRP)-labeled anti-rabbit secondary antibody (Cat#7074) and (HRP)-labeled anti-mouse secondary antibody (Cat#7076) were purchased from Cell Signaling Technology. Anti-GSDME antibody (ab215191), anti-GSDMD antibody (ab210070), anti-p-MLKL antibody (ab187091) and anti-MLKL antibody (ab184718) were purchased from Abcam. Anti-CDK1 antibody (AF1516) was purchased from Beyotime Biotechnology.
Cell culture
Human breast cancer cells MCF-7 and MDA-MB-231 were purchased from American Type Culture Collection (ATCC, Shanghai, China). Bother cells were cultured with Dulbecco’s Modified Eagle Medium cell culture medium (DMEM) (Gibco, USA) supplemented with 10% FBS (Gibco, USA), penicillin (100 μg/mL) and streptomycin (100 μg/mL, USA), all of them were cultured in 5% CO2 at 37°C in an incubator (Thermo Scientific, USA).
CCK8 assay
Cells were plated in 96-well microtiter plates at a density of 5×103/well and incubated in a humidified atmosphere with 5% CO2 at 37°C for 24 h. The tested compound was added into triplicate wells with different concentrations and 0.08% DMSO for the control. After they had been incubated for 24 h, 48 h or 72 h, respectively, 10 μL of CCK8 (Cell Counting Kit-8) solution was added into each well, and the plate was incubated for an additional 1 h. The absorbance (OD) was read on a microplate reader at 450 nm. The concentration causing 50% inhibition of cell growth (IC50) was determined by the Logit method using SPSS 13.0 software. All experiments were performed for three times.
Colony formation assay
MCF-7 and MDA-MB-231 cells in the logarithmic growth phase were plated in a 6-well plate at a density of 500 cells per well. After 48 h, cells were incubated with DMSO (0.1%) or compound 4f (1.25 μM, 2.5 μM, 5μM or 10 μM) for 72 h, and then were cultured in drug-free medium for 1 weeks. The cell colonies were fixed with 4% paraformaldehyde for 10 mins, stained with crystal violet for 20 mins, and then photographed. All experiments were performed for three times.
EDU incorporation assay
Two stable MCF-7 and MDA-MB-231 cells (5×104 cells/well) were plated in the 24-well plate. After treating with compound 4f for 48 h, cells were treated with 50 μM EDU (Ribobio, China), which was obtained through adding 1μL EDU primary liquid into 1 ml DMEM medium with 10% FBS and 1% penicillin-streptomycin according to manufacturer’s instructions, for 2 h, and then fixed with 4% paraformaldehyde for 30 min at room temperature, incubated with 2 mg/ml glycine and 0.5% TritonX-100 for 5 min and 10 min, respectively; Then, cells were incubated with Apollo dye reagent (red) for 30 min at room temperature to react with EDU; Hoechst was used to stain the cell nucleus (blue). 10 random views at 200× magnification were obtained using inverted fluorescence microscope and the number of EDU positive cells was measured per field of view by IOD.
Flow cytometry (FCM) analysis of cell cycle
After being cultured for 24 h, the MCF-7 and MDA-MB-231 cells were treated by different concentrations (0, 1.25, 2.5, 5, 10 μM) of compound 4f for 24 h. Afterwards, the cells were washed and resuspended in cold PBS and incubated in ice-cold 75% ethanol for 12 hours. The cells were then centrifuged at 1000 rpms for 10 mins and washed using cold PBS for 2 times, then resuspended in propidium iodide (PI) master mix (40 μg/ml PI and 100 μg/ml RNase A in PBS) at a density of 5×105 cells/ml and incubated at 37°C for 30 mins before analysis with flow cytometry (Beckman Coulter, USA) as described. The excitation and emission wavelengths of PI were 535/615 nm. The cell cycle phase analysis was performed by FlowJo 7.6 software.
Flow cytometry (FCM) analysis of cell apoptosis
Apoptotic cell death was determined by flow cytometry analysis using Annexin V-FITC and propidium iodide (PI) assay kit (BD, PharMingen, San Diego, CA). After being cultured for 24 h, the cells were treated by different concentrations (0, 1.25, 2.5, 5, 10 μM) of compound 4f for 48 h. MCF-7 and MDA-MB-231 cells were collected, washed with cold PBS, suspended in 5 μL of Annexin V binding buffer and stained with 5 μL of PI. The cells were mixed gently, incubated in the dark for 20 mins, and washed. The samples were analyzed with a FACS (Beckman Coulter, USA).
Quantitative real-time PCR
The total RNA of cells was extracted using TRIZOL reagent and purified by 75% ethyl alcohol. Complementary DNA was synthesized using iscript reverse transcription kit (Takara) according to manufacturer’s instructions. Quantitative real-time PCR was performed with SYBR Green Premix reagent (Takara) on the 7300 real-time PCR system (ABI). Two-step PCR amplification standard procedure was used according to manufacturer’s instructions. The first stage: PCR was initiated at 95°C for 30 seconds to hot-start DNA polymerase and denature the template. The second stage (PCR reaction): 40 cycles that consisted of 95°C for 5 seconds, 60°C for 31 seconds. Data were calculated using the 2-ΔΔCt method. The primers used in the quantitative real-time PCR were synthesized in Life Technologies (S2 Table).
LDH release assay
LDH release was measured using LDH Cytotoxicity Assay Kit (Beyotime Biotechnology) according to the manufacturer’s instructions. The absorbance value at 490 nm was then measured. Each experiment was repeated for three times.
Western blotting analysis
Equal amounts of cellular proteins were loaded onto SDS-polyacrylamide (Bio-Rad), electrophoresed, and transferred onto PVDF membranes (Bio-Rad) by wet electrophoretic transfer. The membranes were then blocked for 1 h at room temperature with 5% non-fat milk in TBST, and incubated with primary antibodies overnight at 4°C, followed by horseradish peroxidase-linked secondary antibody (Cell Signaling Technology) for 1 h at room temperature. The proteins were detected by using an enhanced chemiluminescence (ECL) substrate (Thermo), according to the manufacturer’s instructions. The β-tubulin was used as an internal control.
Measurement of mitochondrial membrance potential
The fluorescent dye JC-1 (Beyotime Institute of Biotechnology, China) was applied to evaluate the mitochondrial membrance potential (Δψm). Briefly, MCF-7 and MDA-MB-231 cells were seeded in the 24-well plates at a density of 1×104 cells/well for 24 h, followed by the treatment with different concentrations (0, 1.25, 2.5, 5, 10 μM) of compound 4f for 48 h. Then the cells were incubated with 0.5 mL JC-1 staining working solution for 20 min at 37°C. The cells were further washed twice with JC-1 staining buffer, then the fluorescence of monometric (green) or aggregate JC-1 (red) was examined at an emission wavelength of 525 or 597 nm, respectively, under a beckman flow cytometer. The assay was repeated in triplicate.
Statistical analyses
All data were analyzed with the unpaired study t-test by using GraphPad 5, and the data were presented as the mean ± SEM. All cell culture experiments were performed independently for at least three times and in triplicate each time. P value of <0.05 or <0.001 was considered statistically significant
Results
Effects of compound 4f on cancer cell viability
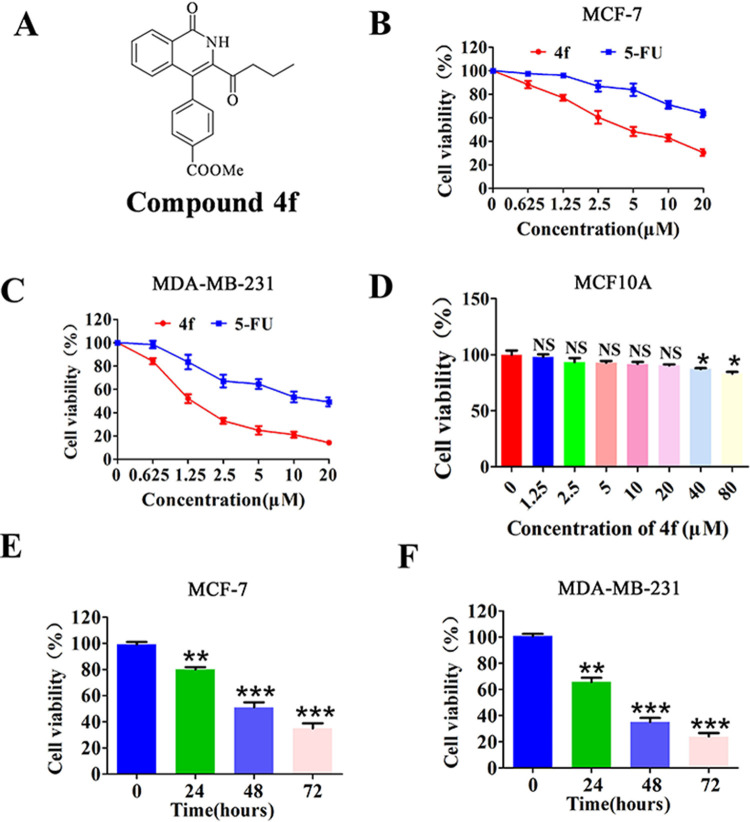
The efficient synthesis of diverse 3-acyl isoquinolin-1(2H)-ones and the preliminary evaluation of the anti-tumor activity against several human cancer cells (e.g. HepG2, A549, MCF-7 and SH-SY5Y) have been developed in our previous work, among which the compound 4f (Fig 1A) showed excellent anti-tumor effect in MCF-7 cells. Encouraged by this result, further examination of the anti-tumor activity of 4f against breast cancer cells including MCF-7 and MDA-MB-213 was next conducted. As shown in Fig 1B and 1C, 4f showed relatively higher anti-tumor activity than that of 5-FU in a dose-dependent manner determined by CCK8 assay in both breast cancer cells, with the indicated half-maximal inhibitory concentration (IC50) ranging from 2.39±0.63 μM to 5.65±0.57 μM (S2 Table). Further evaluation on the cytotoxicity of compound 4f against human normal mammary epithelial cell MCF10A demonstrated the selective killing effect of 4f on cancer cells (Fig 1D). Subsequently, the treatment of MCF-7 and MDA-MB-213 cells with 4f for 24, 48 and 72 h, respectively, indicated that this compound remarkably reduced cell viability in a time-dependent manner (Fig 1E and 1F).
Fig 1. Compound 4f inhibits breast cancer cell viability.
(A) The structure of compound 4f. (B-D) The effect of compound 4f on the viability of the breast cancer cells MCF-7 and MDA-MB-231 and human normal mammary epithelial cell MCF10A by CCK8 assay at the indicated concentration, n = 3. (E, F) Time-dependent of 4f on the viability of the MCF-7 and MDA-MB-231 cells assessed by CCK8 assay. NS, not significant different from 0 group, *significantly different from 0 group. *p<0.05, **p<0.01 and ***p<0.001, n = 3.
Compound 4f inhibits cell proliferation and induces cell cycle arrest in breast cancer
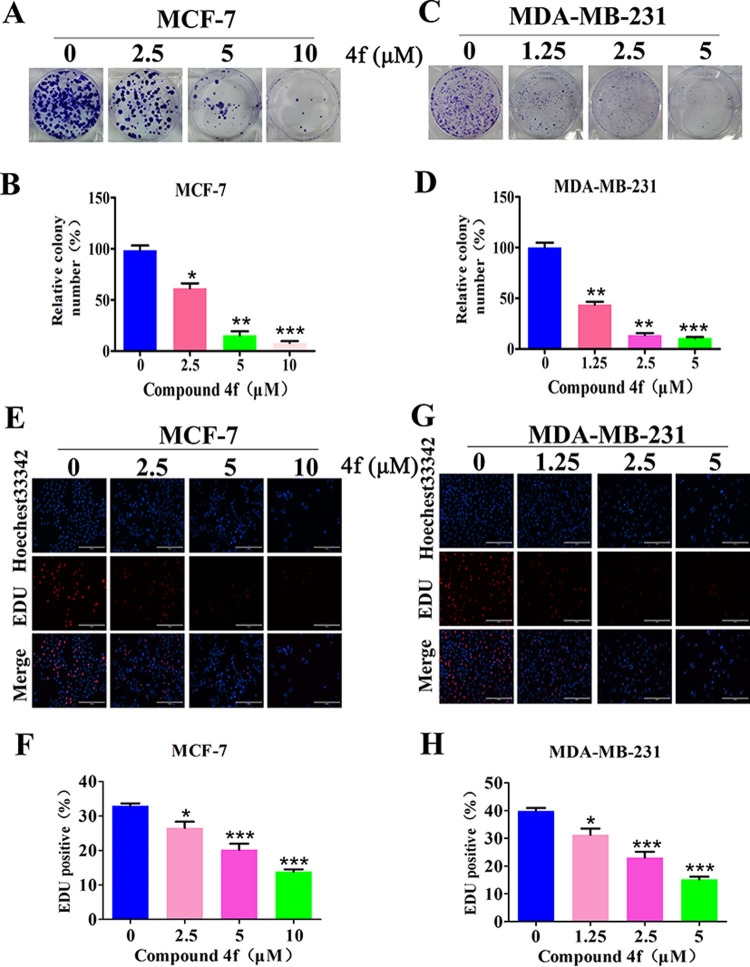
To gain more insight into the inhibitory effects of isoquinolinone 4f on cancer cell proliferation, we next conducted the colony formation assays. The results clearly showed that clone formation of the two tumor cell lines was reduced in a concentration-dependent manner after exposure to 4f (Fig 2A–2D). In addition, the EDU incorporation assay was performed to investigate the anti-proliferative effects of 4f against MCF-7 (Fig 2E and 2F) and MDA-MB-213 cells (Fig 2G and 2H). To the end, the percentages of EDU positive cells decreased with the increasing of the concentration of 4f, indicating that the proliferation of both breast cancer cells was strongly inhibited by compound 4f, which was consistent with the cell viability data.
Fig 2. Compound 4f inhibits cells proliferation in MCF-7 and MDA-MB-231 cell lines.
(A-D) The clonogenic assay and the number of colonies formation analyses. (E-H) The EDU incorporation assay on MCF-7 and MDA-MB-231 cells after treated with compound 4f for 48 h. The red fluorescent staining (EDU-positive) and blue fluorescent staining (Hoechst 33258 staining) cells represent the proliferating and total cells, respectively (E, G). Quantification of EDU-incorporating MCF-7 and MDA-MB-231 cells (F, H). *significantly different from 0 group.*p<0.05, **p<0.01 and ***p<0.001, n = 3.
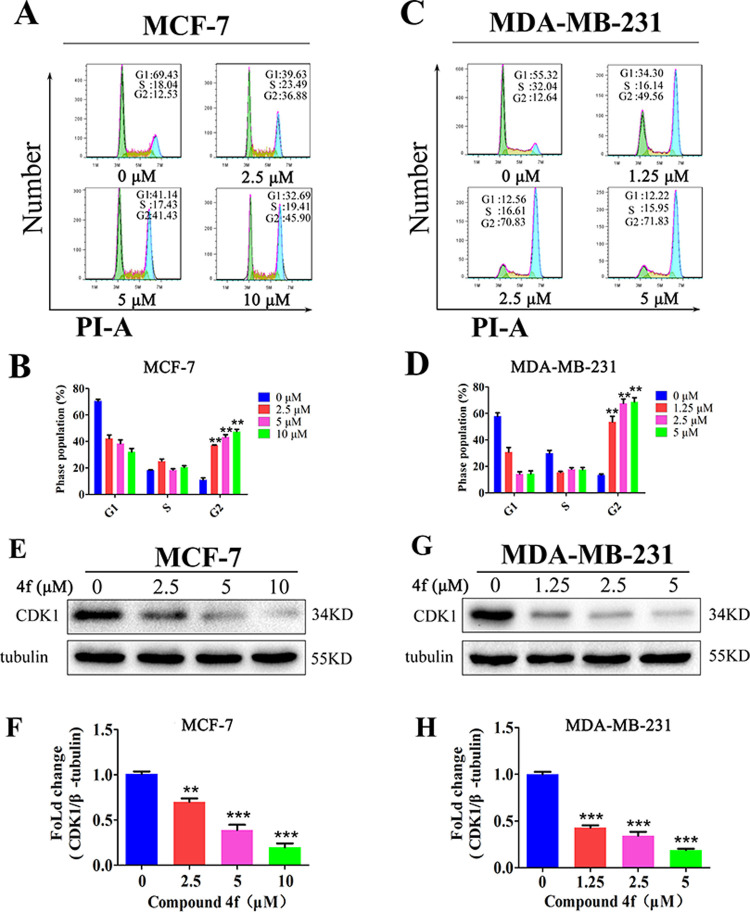
Subsequently, flow cytometry analysis of 4f-treated breast cancer cell lines was carried out to further determine whether 4f inhibited the cell growth by inducing cell cycle arrest. The results indicated that the proportions of MCF-7 and MDA-MB-231 cells were significantly increased in G2 phase in a dose-depended manner treated by 4f (Fig 3A–3D). Meanwhile, western blotting analysis further confirmed that the compound 4f induced cell cycle arrest in G2 phase in MCF-7 and MDA-MB-231 cells due to the reduction of the protein level expression of cyclin-dependent kinase 1 (CDK1), which represents an important regulator that control cells from G2 phase into M phase [8] (Fig 3E–3H).
Fig 3. Induction of cell cycle arrest at the G2 phase by compound 4f in breast cancer cells.
(A-D) Compound 4f increased the proportion of cells in G2 phase of the cell cycle. (E-H) Western blotting assay for the analysis of CDK1 expression in 4f-treated MCF-7 and MDA-MB-231 cells at indicated concentration for 24 h. *significantly different from 0 group. **p<0.01 and ***p<0.001, n = 3.
Compound 4f promotes breast cancer cells apoptosis
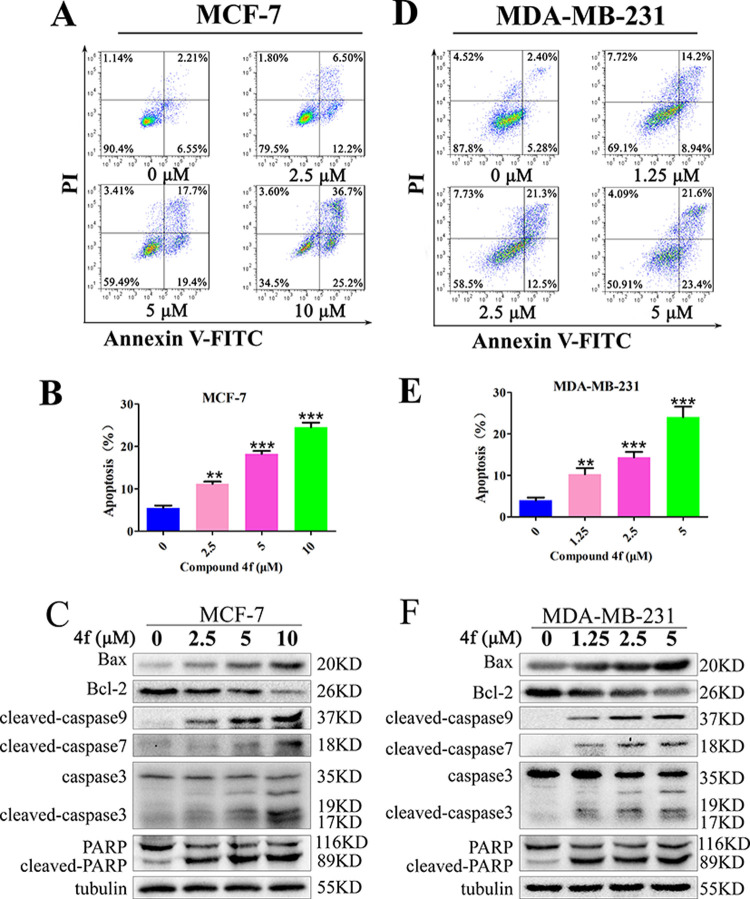
Except for the cell cycle arrest, cell apoptosis is also responsible for the anti-proliferation activity of chemotherapy drugs [24]. Consequently, the ability of isoquinolinone 4f on inducing apoptosis of MCF-7 and MDA-MB-231 cells was next explored to further probe the possible molecular mechanism for suppressing the breast cancer cells viability. Annexin V-FITC and Propidium Iodide (PI) assay kit were used to detect the cell apoptosis, which was analyzed by flow cytometry. The results displayed that 4f induced marked cell apoptosis in a concentration-depended manner, with the percentage of apoptotic cells ranged from 6.55% to 25.2% and 5.28% to 23.2% in MCF-7 (Fig 4A and 4B) and MDA-MB-231 cells (Fig 4D and 4E), respectively. Since mitochondria-mediated intrinsic and cell death receptor-mediated extrinsic paths represent the mainly two types of apoptosis pathway, a series of western blotting assay was next conducted to distinguish the apoptosis pathway induced by compound 4f in breast cancer cells. The results revealed that 4f had no effect on the cleavage of caspase 8, which is an important regulation factor for the extrinsic pathway. In sharp contrast, the compound 4f significantly up-regulated the pro-apoptotic protein Bax expression while reduced anti-apoptotic protein Bcl-2 expression, both of which as key factors to regulate the process of intrinsic apoptosis. Besides, the protein expression level of cleaved-PARP, cleaved-caspase 3, cleaved-caspase 7 and cleaved-caspase 9 in the breast cancer cells increased after treated with compound 4f (Fig 4C and 4F). Together, these results clearly demonstrated that the mitochondria-mediated intrinsic apoptosis pathway should be involved.
Fig 4. Compound 4f induced apoptosis in MCF-7 and MDA-MB-231 cells.
Annexin V-FITC and Propidium Iodide (PI) assay kit were used to detect cell apoptosis, which was analyzed by flow cytometry after the treatment with 4f for 48 h at different concentrations (0, 1.25, 2.5, 5, 10 μM) in MCF-7 (A, B) and MDA-MB-231 (D, E) cells. Western blotting analysis of the expression of apoptosis related proteins in MCF-7 (C) and MDA-MB-231 (F) cells after the treatment with 4f for 24 h. *significantly different from 0 group. **p<0.01 and ***p<0.001, n = 3.
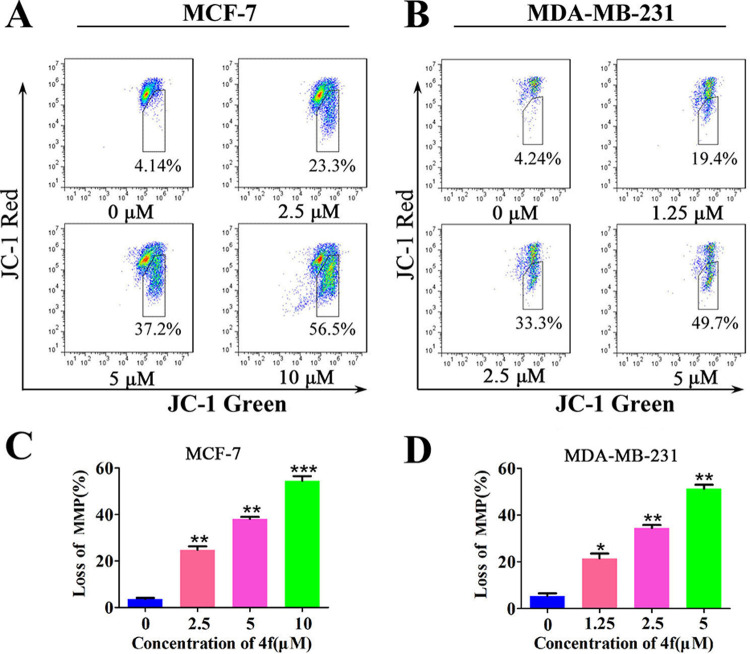
On the other hand, the decrease of mitochondrial membrane potential (MMP) is a key indicator of early apoptosis, which can cause the release of apoptotic factors and activate the downstream apoptotic pathways [25]. Consequently, the MMP was further analyzed in MCF-7 and MDA-MB-231 cells by flow cytometry using a JC-1 MMP Kit, which acts as a fluorescence probe and selectively enters into the mitochondria and reversibly change color from red (the aggregate form) to green (the monomeric form) when mitochondrial membrane potential declines [26]. As shown in Fig 5A–5D, the MMP reduced significantly after treated with compound 4f for 24 h in MCF-7 and MDA-MB-231 cells.
Fig 5. Compound 4f induces reduction of the MMP in breast cancer cells.
Cells were treated with 4f at a concentration of 0 μM, 2.5 μM, 5 μM, 10 μM in MCF-7 cell line (A) and 0 μM, 1.25 μM, 2.5 μM, 5 μM in MDA-MB-231 cell line (B) for 24 h, the MMP was measured by JC-1. (C, D) The ratio of decreased MMP. *significantly different from 0 group. *p<0.05, **p<0.01 and ***p<0.001, n = 3.
Compound 4f inhibits MEK/ERK and p38 MAPK pathways in breast cancer cells
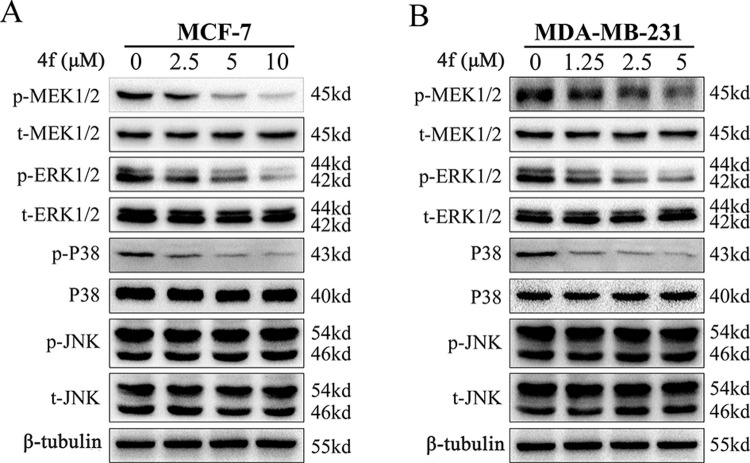
Numerous studies have reported that the induction of cell apoptosis is associate with MAPK pathway [24, 27, 28], among which the isoquinolinone derivatives were proved to present the anti-tumor effect in breast cancer via inhibiting MAPK/ERK pathway [21, 22]. Thus, the protein expression levels in MAPK pathway were detected in breast cancer cells to determine whether 4f affects the MAPK pathway by western blotting assays. As listed in Fig 6, the total protein expression of ERK1/2, p38 and JNK were not altered under the treatment of 4f, while the phosphorylation of ERK1/2 and p38 were reduced with the expression of phosphorylated JNK remained unchanged. ERK1/2 represents a serine/threonine kinase that positively regulated by phosphorylation and mediated by MEK1/2 [29]. The examination of the MEK1/2 protein revealed that 4f could reduce the phosphorylation of MEK1/2 without altering the total protein expression. These results confirmed that 4f inhibited MEK/ERK and p38 MAPK pathways in breast cancer cells.
Fig 6. 4f inhibits MEK/ERK and p38 MAPK pathways in breast cancer cells.
Cells were treated with 4f at a concentration of 0 μM, 2.5 μM, 5 μM, 10 μM in MCF-7 cell line (A) and a concentration of 0 μM, 1.25 μM, 2.5 μM, 5 μM in MDA-MB-231 cell line (B) for 24 h, the expression of phosphorlated and total ERK1/2, p38, JNK and MEK1/2 were detected by western blotting assays. All the data repeated for three times.
Compound 4f induces cell pyroptosis by caspase 3 mediated cleavaged of GSDME
Pyroptosis, a newly identified programmed cell death executed by gasdermins [12], is essentially different from apoptosis, a non-inflammatory form of programmed cell death. Precedented studies prove that pyroptosis plays a significant role in the treatment of tumors with chemotherapy drugs. Classical chemotherapy drugs including doxorubicin, peclitaxel, cisplatin, lobaplatin and 5-FU can induce pyroptosis effectively via caspase 3/GSDME in the treatment of tumors [9, 15, 16, 30–32]. Moreover, diverse bioactive molecules including chalcones, thiopyran derivatives and piperlongumine analogues have also been proven to induce pyroptosis [33–35], indicating that pyroptosis provides a novel therapeutic strategy for tumors. Considering the above observation that isoquinolinone 4f had an effect on the cleavage of caspase 3, we presumed that 4f might induce pyroptosis in breast cancer cells.
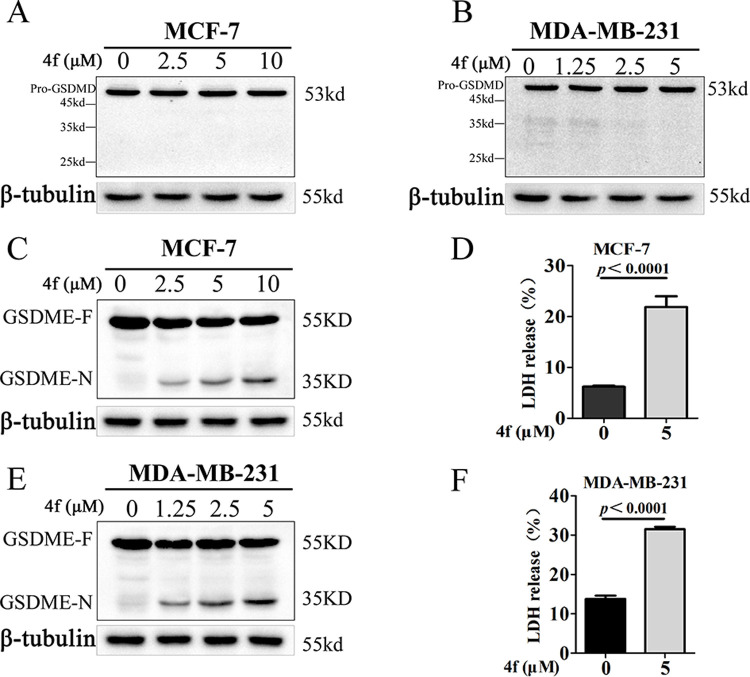
The GSDMD-N fragment generated by the cleavage of GSDMD via activating caspase 1/4/5/11 derives pyroptosis through its pore-forming activity [36–39]. Alternatively, GSDME-dependent cell death is another form of pyroptosis, which induced by TNF-α or chemotherapy drugs [11]. Recent studies reported that GSDME was specifically cleaved by caspase 3 in the linker, generating a GSDME-N fragment that perforated membranes for pyroptosis induction [10, 40]. To gain more insight into the effect of compound 4f on pyroptosis induction and clarify the pyroptosis pathway, a set of western blotting was conducted and the results showed that no cleavage of GSDMD occurred in MCF-7 and MDA-MB-231 cells treated by 4f (Fig 7A and 7B). Nevertheless, the GSDME cleavage was induced and increased by 4f in both breast cancer cell lines in a dose-depended manner (Fig 7C and 7E). In addition, the release of proinflammatory factor, lactate dehydrogenase (LDH), was significantly elevated compared with the control group, demonstrating the plasma membrane rupture and leakage under the treatment of 4f (Fig 7D and 7F). Moreover, MCF-7 and MDA-MB-231 cells were respectively treated with 4f at the concentration of 5 μM and 2.5 μM for 24 h, and then, the morphology of cells were photographed with microscope (S1 Fig). The cells that marked with red arrow exhibited large bubbles emerging from the plasma membrane and cell swelling, which are represent cells undergo pyroptosis. As the morphology and Annexin-V/PI character of necroptosis and pyroptosis are very similar, we also evaluated whether 4f induced necroptosis. p-MLKL is the key protein regulated necroptosis, for this concern, we detected the phosphorylation of MLKL signal that regulate necroptosis by western blotting analysis using the antibodies against MLKL and p-MLKL, necrosis inducer, a combination of TNF-a (T, 20 ng/ml), Smac mimetic (S, 100 nM), and a pan-caspase inhibitor z-VAD-FMK (Z, 20 μM) was used as positive control. The results indicated that no phos-MLKL signal was observed after treating with 4f (S2 Fig), suggesting that 4f did not induce necroptosis in MCF-7 and MDA-MB-231 cells after treating with 4f. Taken together, these results suggested that 4f could induce pyroptosis through the cleavage of GSDME rather than GSDMD.
Fig 7. GSDME rather than GSDMD is cleaved during cell pyroptosis of breast cancer cells induced by 4f.
(A, B) Full-length GSDMD (GSDMD-F) and GSDMD-C terminal (GSDMD-C) were detected by western blotting in MCF-7 and MDA-MB-231 cells treated with different doses of 4f. (C, E) Full-length GSDME (GSDME-F) and GSDME-N terminal (GSDME-N) were analyzed by western blotting in MCF-7 and MDA-MB-231 cells treated with different doses of 4f. (D, F) LDH release of MCF-7 and MDA-MB-231 cells treated with 4f at the concentration of 0 or 5 μM. All the data repeated for three times.
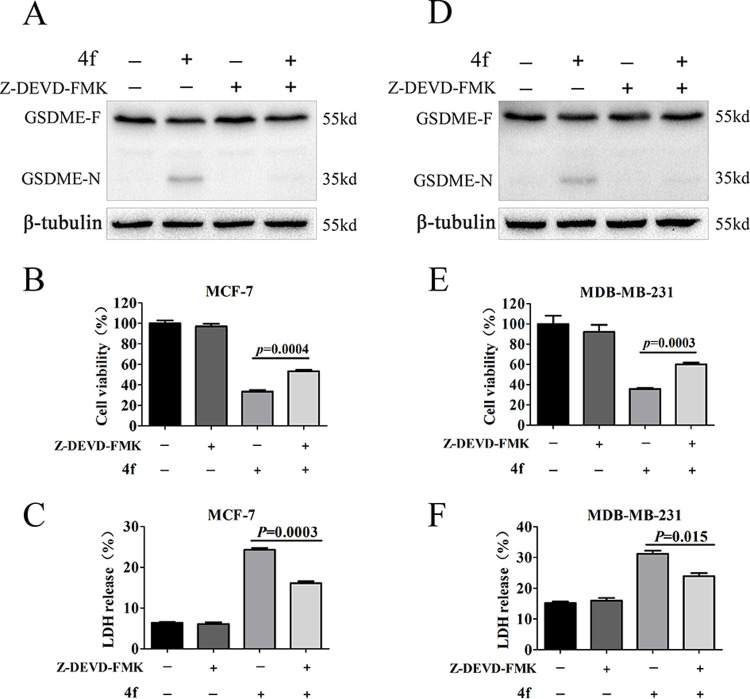
To verify the involvement of caspase 3 in 4f-triggered pyroptosis, the MCF-7 and MDA-MB-231 cells were pre-treated with caspase 3 specific inhibitor z-DEVD-FMK for 2 h and then treated with 4f for 24 h. The results revealed that z-DEVD-FMK could inhibit the cleavage of GSDME (Fig 8A and 8D) and reduce the release of LDH (Fig 8C and 8F) triggered by 4f. Moreover, CCK8 assay showed that z-DEVD-FMK reversed the cell viability induced by 4f (Fig 8B and 8E). These results demonstrated that the cleavage of GSDME by caspase 3 was responsible for 4f-induced pyroptosis in MCF-7 and MDA-MB-231 cells.
Fig 8. z-DEVD-FMK reduces the cleavage of GSDME.
(A, D) Western blotting analysis of full-length GSDME (GSDME-F) and GSDME-N terminal (GSDME-N) in MCF-7 and MDA-MB-231 cells treated by 4f (5 μM) in the presence or absence of z-DEVD-FMK (50 μM). (B, E) Cell viability analysis by CCK8 kit. (C, F) LDH release was measured using LDH cytotoxicity assay kit. All the data repeated for three times.
Discussion
Recent studies found that a number of isoquinolinone derivatives exhibited anti-tumor effect in many cancers by inducing cell cycle arrest and apoptosis [19, 20], and a series of 3-acyl isoquinolin-1(2H)-ones was obtained and identified as anti-tumor agents against four different cancer cell lines in our previous study [23], among which compound 4f showed potent anti-tumor effect in MCF-7 cell line. In this study, we demonstrate that 4f reduces cell viability in MCF-7 and MDA-MB-231 cell lines by CCK8 assay. Further investigation on cell proliferation indicates that 4f reduces the number of EDU positive cells and suppresses the colony formation in MCF-7 and MDA-MB-231 cell lines, and the results from flow cytometry demonstrate that 4f induces cell cycle arrest in G2 phase. Cyclin-dependent kinase 1 (CDK1), a complex of cyclin B1 and cdc2, is an important regulator that control cells from G2 phase into M phase [8]. Indeed, the reduction of CDK1 protein expression is observed in MCF-7 and MDA-MB-231 cells after treating with 4f, which is responsible for cell cycle arrest. These results demonstrate that 4f inhibits cell proliferation by inducing cell cycle arrest in G2 phase.
We further investigate whether 4f could induce apoptosis in breast cancer cells. The results showed that 4f induced marked cell apoptosis in a dose-depended manner in MCF-7 and MDA-MB-231 cells. In addition, 4f promoted the expression of cleaved-PARP, cleaved-caspase3, cleaved-caspase7, and cleaved-caspase9, as well as up-regulated the protein level of Bax while decreasing the protein level of Bcl-2, which are the key proteins that regulate intrinsic apoptosis pathway. Additionally, the results from flow cytometry showed that 4f could decrease the mitochondrial membrane potential, a feature of early apoptosis. Taken together, our data indicate that 4f promotes cell apoptosis via mitochondria pathway in breast cancer cells. Isoquinolinone derivatives have been reported to have anti-tumor effect in breast cancer via inhibiting MAPK/ERK pathway [21]. Thus, the changes in MAPK pathway in breast cancer cells after treating with 4f were analyzed by western blotting assays. In our study, we discovered that 4f inhibited the expression of phosphorylation of p38 and ERK1/2, as well as the phosphorylation of MEK1/2, which can positively regulate ERK1/2. These results confirmed that 4f inhibited MEK/ERK and p38MAPK pathways, which might be another reason for promoting cell apoptosis.
Pyroptosis, a newly identified programmed cell death [12], which is essentially different from apoptosis. Recent advances have shown all Gasdermin family members except for a shortened one, DFNB59, can mediate pyroptosis. And GSDME-N is specifically cleaved by caspase3 in the linker, generating GSDME-N fragment, which can induce pyroptosis [10, 40]. Recent studies have reported that pyroptosis plays a significant role in the treatment of tumors with chemotherapy drugs. Besides, the newly synthesized compounds including chalcone analogues, a thiopyran derivative, a piperlongumine analogue can also induce pyroptosis [38–40], indicating that pyroptosis provides a novel therapeutic strategy for tumors. The results in our study indicated that 4f promoted the release of LDH and induces the cleavage of GSDME while fail to promote the cleavage of GSDMD. What’s more, the cleavage of GSDME and the release of LDH could be inhibited by Z-DEVD-FMK, a specific inhibitor of caspase3, meanwhile, cell viability is restored in 4f treated breast cancer cells after treating with Z-DEVD-FMK. These results indicated that 4f induced pyroptosis through caspase3/GSDME pathway. As caspase3 is also a core protein in regulating apoptosis, the correlation between apoptosis and pyroptosis in breast cancer cells treated with 4f need to be further explored.
In summary, having developed the efficient synthetic approach of diverse 3-acyl isoquinolin-1(2H)-ones and preliminarily explored their bioactivity as anti-tumor agents, we herein further evaluated the pharmacodynamics of the most active compound 4f in vitro against the breast cancer cell lines including MCF-7 and MDA-MB-231 cells. The results demonstrated that 4f inhibited cell proliferation by inducing cell cycle arrest in G2 phase and promoted cell apoptosis via mitochondria-mediated intrinsic apoptosis pathway. Meanwhile, the MEK/ERK and p38 MAPK pathways were also inhibited by 4f. Additionally, the pyroptosis induction triggered by 4f was also confirmed and the caspase 3/GSDME pathway was verified. Taken together, these results suggested that the isoquinolinone complex 4f may serve as a lead compound for anti-tumor drug discovery against breast cancer, and more importantly, it also provide a mechanistic basis for employing such isoquinolinone skeleton as the anti-cancer discovery and development. Having these solid data in hand, further investigation on targeted structural modifications together with the comprehensive in vivo studies of 4f is in progress.
Supporting information
Representative microscopic images of MCF-7 and MDA-MB-231 cells after treated with 4f for 24 h with cell swelling. Red arrowheads indicate the characteristic balloon in the cell membrane, which are represent cells undergo pyroptosis.
(TIF)
MCF-7 and MDA-MB-231 cells were treated with 4f or TSZ for 24 h, the total protein was extracted respectively, and then, the protein expression of p-MLKL and MLKL were analysized by western blotting analysis. TSZ is a combination of TNF-a (T, 20 ng/ml), Smac mimetic (S, 100 nM), and a pan-caspase inhibitor z-VAD-FMK (Z, 20 μM), which is used as necrosis inducer.
(TIF)
(DOCX)
Cells were treated with different concentrations of compound 4f for 48 h. IC50 values are the mean±SD (n = 3).
(DOC)
HPLC conditions: Thermo Fisher C18 column HYPERSIL GOLD ODS, 250 x 4.6 mm I.D, S-5 μm (30:70 MeCN: H2O, 1.0 mL/min, 30°C, 254 nm); tr = 20.2 min, 99.3%. Instrument model: C18 chromatographic column HYPERSIL GOLD ODS, 250×4.6 mm I.D, S-5 μm (Thermo Fisher Scientific).
(PDF)
(XLSX)
(PDF)
Data Availability
All relevant data are within the article and its Supporting Information files.
Funding Statement
This research was supported by NSFC (21877020, 22007020), Guangdong Natural Science Funds for Distinguished Young Scholar (2017A030306031) and Natural Science Foundation of Guangdong Province (2019A1515010935). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017; 1(1): CD010802. doi: 10.1002/14651858.CD010802.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu YX, Ji R, Li JY, Gu Q, Zhao XH, Sun T, et al. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J Exp Clin Cancer Res. 2010; 29(1): 16. doi: 10.1186/1756-9966-29-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSantis C, Ma JM, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014; 64(1): 52–62. doi: 10.3322/caac.21203 [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144(5): 646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 5.Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017; 17(2): 93–115. doi: 10.1038/nrc.2016.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu YY, Wang W, Fang B, Ma FY, Zheng Q, Deng PY, et al. Anti-tumor effect of germacrone on human hepatoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Eur J Pharmacol. 2013; 698(1–3): 95–102. doi: 10.1016/j.ejphar.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-Martínez C, Gelbert LM, José Lallena M, Dios AD. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs. Bioorg Med Chem Lett. 2015; 25(17): 3420–3435. doi: 10.1016/j.bmcl.2015.05.100 [DOI] [PubMed] [Google Scholar]
- 8.Zhang ZY, Wang CZ, Du GJ, Qi LW, Calway T, He TC, et al. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int J Oncol. 2013; 43(1): 289–296. doi: 10.3892/ijo.2013.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang CC, Li CG, Wang YF, Xu LH, He XH, Zeng QZ, et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019; 24(3–4): 312–325. doi: 10.1007/s10495-019-01515-1 [DOI] [PubMed] [Google Scholar]
- 10.Wang YP, Gao WQ, Shi XY, Ding JJ, Liu W, He HB, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017; 547(7661): 99–103. doi: 10.1038/nature22393 [DOI] [PubMed] [Google Scholar]
- 11.Ding QY, Zhang WD, Cheng C, Mo FB, Chen L, Peng GF, et al. Dioscin inhibits the growth of human osteosarcoma by inducing G2/M-phase arrest, apoptosis, and GSDME-dependent cell death in vitro and in vivo. J Cell Physiol. 2020; 235(3): 2911–2924. doi: 10.1002/jcp.29197 [DOI] [PubMed] [Google Scholar]
- 12.Shi JJ, Zhao Y, Wang K, Shi XY, Wang Y, Huang HW, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015; 526(7575): 660–665. doi: 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- 13.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015; 526(7575): 666–671. doi: 10.1038/nature15541 [DOI] [PubMed] [Google Scholar]
- 14.Kim MS, Chang X, Yamashita K, Nagpal JK, Baek JH, Wu G, et al. Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma. Oncogene. 2008; 27(25): 3624–3634. doi: 10.1038/sj.onc.1211021 [DOI] [PubMed] [Google Scholar]
- 15.Yu JH, Li S, Qi J, Chen ZL, Wu YH, Guo J, et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 2019; 10(3): 193. doi: 10.1038/s41419-019-1441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang YB, Yin B, Li DN, Wang GJ, Han XD, Sun XJ. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem Biophys Res Commun. 2018; 495(1): 1418–1425. doi: 10.1016/j.bbrc.2017.11.156 [DOI] [PubMed] [Google Scholar]
- 17.Beeck KOD, Laer LV, Camp GV. DFNA5, a gene involved in hearing loss and cancer: a review. Ann Otol Rhinol Laryngol. 2012; 121(3): 197–207. doi: 10.1177/000348941212100310 [DOI] [PubMed] [Google Scholar]
- 18.Beeck KOD, Laer LV, Camp GV. Synthesis and biological evaluation of 2,3-diaryl isoquinolinone derivatives as anti-breast cancer agents targeting ERα and VEGFR-2. Bioorg Med Chem Lett. 2014; 24(9): 2129–2133. doi: 10.1016/j.bmcl.2014.03.042 [DOI] [PubMed] [Google Scholar]
- 19.He LJ, Yang DL, Li SQ, Zhang YJ, Tang Y, Lei J, et al. Facile construction of fused benzimidazole-isoquinolinones that induce cell-cycle arrest and apoptosis in colorectal cancer cells. Bioorg Med Chem. 2018; 26(14): 3899–3908. doi: 10.1016/j.bmc.2018.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen HY, He LJ, Li SQ, Zhang YJ, Huang JH, Qin HY, et al. A derivate of benzimidazole-isoquinolinone induces SKP2 transcriptional inhibition to exert anti-tumor activity in glioblastoma cells. Molecules. 2019; 24(15): 2722. doi: 10.3390/molecules24152722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang ZC, Wu CZ, Wang TL, Lao KJ, Wang YJ, Liu LY, et al. Design, synthesis and evaluation of 6-aryl-indenoisoquinolone derivatives dual targeting ERα and VEGFR-2 as anti-breast cancer agents. Eur J Med Chem. 2016; 118: 328–339. doi: 10.1016/j.ejmech.2016.04.029 [DOI] [PubMed] [Google Scholar]
- 22.Asano Y, Kitamura S, Ohra T, Itoh F, Kajino M, Tamura T, et al. Discovery, synthesis and biological evaluation of isoquinolones as novel and highly selective JNK inhibitors (2). Bioorg Med Chem. 2008; 16(8): 4699–4714. doi: 10.1016/j.bmc.2008.02.028 [DOI] [PubMed] [Google Scholar]
- 23.Bian MY, Ma L, Wu M, Wu LX, Gao H, Yi W, et al. Rh(III)-catalyzed redox-neutral [4+2] annulation for direct assembly of 3-acyl isoquinolin-1(2H)-ones as potent antitumor agents. ChemPlusChem. 2020; 85(3): 405–410. doi: 10.1002/cplu.201900616 [DOI] [PubMed] [Google Scholar]
- 24.Xiao WQ, Xu ZJ, Chang SK, Li B, Yu DD, Wu HQ, et al. Rafoxanide, an organohalogen drug, triggers apoptosis and cell cycle arrest in multiple myeloma by enhancing DNA damage responses and suppressing the p38 MAPK pathway. Cancer Lett. 2019; 444: 45–59. doi: 10.1016/j.canlet.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 25.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000; 6(5): 513–519. doi: 10.1038/74994 [DOI] [PubMed] [Google Scholar]
- 26.Song J, Lin CC, Yang X, Xie YQ, Hu P, Li HT, et al. Mitochondrial targeting nanodrugs self-assembled from 9-O-octadecyl substituted berberine derivative for cancer treatment by inducing mitochondrial apoptosis pathways. J Control Release. 2019; 294: 27–42. doi: 10.1016/j.jconrel.2018.11.014 [DOI] [PubMed] [Google Scholar]
- 27.Dai Y, Chen S, Pei XY, Almenara JA, Kramer LB, Venditti CA, et al. Interruption of the Ras/MEK/ERK signaling cascade enhances Chk1 inhibitor-induced DNA damage in vitro and in vivo in human multiple myeloma cells. Blood. 2008; 112(6): 2439–2449. doi: 10.1182/blood-2008-05-159392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burmistrova O, Quintana J, Díaz JG, Estévez F. Astragalin heptaacetate-induced cell death in human leukemia cells is dependent on caspases and activates the MAPK pathway. Cancer Lett. 2011; 309(1): 71–77. doi: 10.1016/j.canlet.2011.05.018 [DOI] [PubMed] [Google Scholar]
- 29.Chang FJ, Lee T, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003; 17(3): 590–603. doi: 10.1038/sj.leu.2402824 [DOI] [PubMed] [Google Scholar]
- 30.Lu HJ, Zhang SZ, Wu J, Chen MJ. Molecular targeted therapies elicit concurrent apoptotic and GSDME-dependent pyroptotic tumor cell death. Clin Cancer Res. 2018; 24(23): 6066–6077. doi: 10.1158/1078-0432.CCR-18-1478 [DOI] [PubMed] [Google Scholar]
- 31.Zheng XB, Zhong T, Ma YH, Wan XY, Qin A, Yao BF, et al. Bnip3 mediates doxorubicin-induced cardiomyocyte pyroptosis via caspase-3/GSDME. Life Sci. 2020; 242: 117186. doi: 10.1016/j.lfs.2019.117186 [DOI] [PubMed] [Google Scholar]
- 32.Mai FY, He PY, Ye JZ, Xu LH. Caspase-3-mediated GSDME activation contributes to cisplatin- and doxorubicin-induced secondary necrosis in mouse macrophages. Cell Prolif. 2019; 52(5): e12663. doi: 10.1111/cpr.12663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu M, Wang JB, Xie JW, Chen LP, Wei XY, Jiang X, et al. Design, synthesis, and evaluation of chalcone analogues incorporate α,β-Unsaturated ketone functionality as anti-lung cancer agents via evoking ROS to induce pyroptosis. Eur J Med Chem. 2018; 157: 1395–1405. doi: 10.1016/j.ejmech.2018.08.072 [DOI] [PubMed] [Google Scholar]
- 34.Chen LP, Weng BX, Li HM, Wang HN, Li Q, Wei XY, et al. A thiopyran derivative with low murine toxicity with therapeutic potential on lung cancer acting through a NF-κB mediated apoptosis-to-pyroptosis switch. Apoptosis. 2019; 24(1–2): 74–82. doi: 10.1007/s10495-018-1499-y [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Chen LP, Dong ZJ, Zhao Y, Deng H, Wu JZ, et al. Piperlongumine analogue L50377 induces pyroptosis via ROS mediated NF-κB suppression in non-small-cell lung cancer. Chem Biol Interact. 2019; 313: 108820. doi: 10.1016/j.cbi.2019.108820 [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Zhang ZB, Ruan JB, Pan YD, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016; 535(7610): 153–158. doi: 10.1038/nature18629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi JJ, Zhao Y, Wang YP, Gao WQ, Ding JJ, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014; 514(7521): 187–192. doi: 10.1038/nature13683 10.1038/nature13683 [DOI] [PubMed] [Google Scholar]
- 38.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013; 341(6151): 1250–1253. doi: 10.1126/science.1240988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014; 157(5): 1013–1022. doi: 10.1016/j.cell.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 40.Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017; 8:14128. doi: 10.1038/ncomms14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative microscopic images of MCF-7 and MDA-MB-231 cells after treated with 4f for 24 h with cell swelling. Red arrowheads indicate the characteristic balloon in the cell membrane, which are represent cells undergo pyroptosis.
(TIF)
MCF-7 and MDA-MB-231 cells were treated with 4f or TSZ for 24 h, the total protein was extracted respectively, and then, the protein expression of p-MLKL and MLKL were analysized by western blotting analysis. TSZ is a combination of TNF-a (T, 20 ng/ml), Smac mimetic (S, 100 nM), and a pan-caspase inhibitor z-VAD-FMK (Z, 20 μM), which is used as necrosis inducer.
(TIF)
(DOCX)
Cells were treated with different concentrations of compound 4f for 48 h. IC50 values are the mean±SD (n = 3).
(DOC)
HPLC conditions: Thermo Fisher C18 column HYPERSIL GOLD ODS, 250 x 4.6 mm I.D, S-5 μm (30:70 MeCN: H2O, 1.0 mL/min, 30°C, 254 nm); tr = 20.2 min, 99.3%. Instrument model: C18 chromatographic column HYPERSIL GOLD ODS, 250×4.6 mm I.D, S-5 μm (Thermo Fisher Scientific).
(PDF)
(XLSX)
(PDF)
Data Availability Statement
All relevant data are within the article and its Supporting Information files.