Abstract
Background
Common cold is among the main reasons patients visit a medical facility. However, few studies have investigated whether prescriptions for common cold in Japan comply with domestic and international evidence.
Objective
To determine whether prescriptions for common cold complied with domestic and international evidence.
Methods
This cross-sectional study was conducted between October 22, 2020, and January 16, 2021. Patients with cold symptoms who visited the two dispensing pharmacies and met the eligibility criteria were interviewed.
Main outcome measure
The pharmacists at each store and a physician classified the patients into two groups: the potentially inappropriate prescribing group and the appropriate prescribing group.
Results
Of the 150 selected patients, 14 were excluded and 136 were included in the analysis. Males accounted for 44.9% of the total study population, and the median patient age was 34 years (interquartile range [IQR], 27–42). The prevalence rates of potentially inappropriate prescriptions and appropriate prescriptions were 89.0% and 11.0%, respectively and the median drug costs were 602.0 yen (IQR, 479.7–839.2) [$5.2 (IQR, 4.2–7.3)] and 406.7 yen (IQR, 194.5–537.2) [$3.5 (IQR, 1.7–4.7)], respectively. The most common potentially inappropriate prescriptions were the prescription of oral cephem antibacterial agents to patients who did not have symptoms of bacterial infections (50.4%) and β2 stimulants to those who did not have respiratory symptoms due to underlying disease or history (33.9%).
Conclusions
Approximately 90% of prescriptions for common cold symptoms in the area were potentially inappropriate. Our findings could contribute to the monitoring of the use of medicines for the treatment of common cold symptoms.
Introduction
Common cold symptoms are one of the main reasons patients visit a medical facility both internationally and in Japan [1, 2]. In the past, physicians mainly prescribed antimicrobial agents for common cold symptoms. Numerous studies have reported the actual practice of prescribing antimicrobial agents [3, 4] and the harmful effects of consumption of these antimicrobial agents by patients with common cold symptoms [5, 6]. According to the reports published in the United States, antimicrobials should not be prescribed for common cold as much as possible owing to drug resistance (more than 2 million antibiotic-resistant diseases are reported each year), the financial burden on the patient and healthcare system (approximately 50% of antimicrobial prescriptions are unnecessary, totaling to medical expenses of more than $3 billion annually), and side effects (5%–25% of patients who use antimicrobials experience adverse events) [7]. In Japan, approximately 60% of outpatients with common cold symptoms were prescribed antimicrobials in 2005 [3], which led to the establishment of the government-led Action Plan to address antimicrobial resistance in 2016.
Several drugs intended to treat common cold symptoms in adults have been useful in relieving symptoms. However, their efficacy is limited [8], and their benefits and disadvantages should be considered. Previous studies have indicated that non-steroidal anti-inflammatory drugs (NSAIDs) and first-generation antihistamines may be inappropriate for older patients and should not be prescribed frequently for common cold symptoms in this population [9]. However, there is a lack of evidence on whether physicians’ prescriptions for common cold in Japan comply with the domestic and international evidence [10]. Therefore, we planned to conduct a study by examining prescriptions for common cold in Japan and conducting interviews with patients.
We aimed to investigate whether prescriptions for common cold at two dispensing pharmacies in Izumo City, Shimane Prefecture, Japan, comply with the domestic and international evidence and assess the appropriateness of the prescriptions from these pharmacies.
Materials and methods
Study design
This cross-sectional study was conducted for approximately 3 months, from October 22, 2020, to January 16, 2021. Two pharmacists from each of the two dispensing pharmacies in Izumo City conducted in-depth interviews with patients who had been prescribed medication to relieve cold symptoms.
Study protocol
The study researchers were four experienced pharmacists and one physician who was an expert in primary care. The pharmacists collected the data directly, and the physician set the definition of common cold and the classification criteria for the two groups, namely, potentially inappropriate and appropriate prescribing groups, using data from previous studies based on domestic and international evidence (S1 Appendix) [11, 12]. Next, a questionnaire was prepared for collecting data from the patients, which included the following variables: patient sex and age, doctor’s diagnosis and explanation of common cold, type of symptoms during the visit, duration of symptoms, presence of allergies, history, and current status of smoking, type of prescribed medication, and drug cost (S2 Appendix).
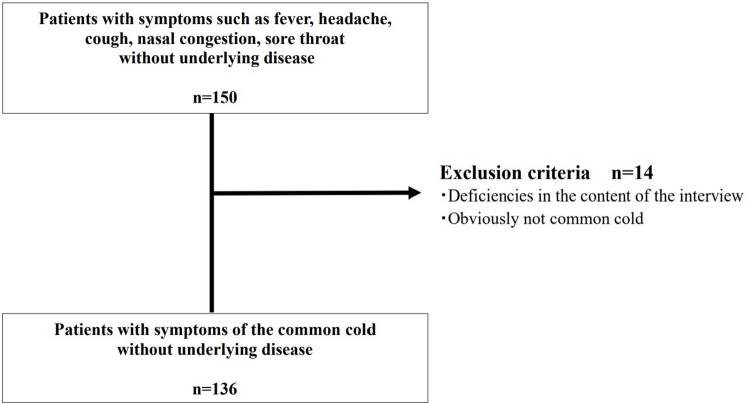
A flowchart of the patient selection process is shown in Fig 1. The patients visited a hospital or clinic, received a prescription, and then visited the pharmacy to pick up the medication. Next, the pharmacist assessed whether the patient met the inclusion criteria using the questionnaire (S2 Appendix) and confirmed the patients’ symptoms and details of the prescribed medication (the content of the questionnaire was based on the patients’ subjective opinions) (n = 150). The physician, who was not the prescribing physician, excluded patients who met the exclusion criteria, after which two pharmacists and a physician classified the patients with prescriptions into two groups: the potentially inappropriate prescribing group and the appropriate prescribing group (S1 Appendix) (N = 136).
Fig 1. Flowchart of the patient selection process.
Definitions
The term “potentially inappropriate prescribing” in this study refers to prescribing of medicines for which there is no clear evidence or indication or that are not suitable for the patient’s symptoms or are contraindicated or administered in inappropriate doses, all of which indicate that the disadvantages of prescribing are likely to outweigh the benefits [11, 12]. “Appropriate prescription” refers to prescribing drugs suitable for the patient’s symptoms where the benefits of prescribing for the patient’s condition are likely to outweigh the disadvantages for various reasons and the prescription is based on clear evidence and indications. The two pharmacists and the primary care expert physician made these judgments using the classification criteria developed in previous studies (S1 Appendix). The term “common cold symptoms” refers to one or more common cold symptoms, namely, acute low-grade fever, headache, cough symptoms, nasal congestion symptoms, and sore throat symptoms [1, 13]. ※ S1 Appendix includes reference [14–33].
Inclusion criteria
In this study, we included adult patients aged 20–80 years who reported that they had one or more typical symptoms of common cold, namely, low-grade fever, headache, cough, nasal congestion, and sore throat symptoms [1, 13] and that these symptoms were comparable to those they had previously. Prescriptions were included only for drugs intended to relieve cold symptoms (drugs included in S1 Appendix). We also ensured that the medications prescribed to the patients were their own medications.
Exclusion criteria
Patients with underlying diseases and those who were pregnant or breastfeeding were excluded. Since there is not enough scientific evidence for Chinese herbal medicines, also called “Kampo medicines,” prescriptions containing Kampo medicines were excluded from this study. Kampo medicine is a combination of herbal medicines made from a number of plants and minerals originally developed in China. It was introduced in Japan and developed into a unique treatment method in Japan. Through thousands of years of experience, the effects of various combinations of herbal medicines have been confirmed.
Patients who regularly took medicines other than those to relieve cold symptoms were also excluded to reduce the impact or interaction of other medicines on the results of our study. Patients with incomplete data, such as incomplete interviews, and patients who were judged by the prescribing physician or the physician involved in the study as not having evident symptoms of common cold were excluded from the study.
Outcome measures
The primary outcome was the frequency of "potentially inappropriate prescription " and "appropriate prescription." Other outcomes included the cost of each prescription drug (Japanese yen was converted to US dollars based on the exchange rate of 114.92 yen per 1 US dollar on December 29, 2021) in "potentially inappropriate prescriptions" and "appropriate prescriptions," types of symptomatic and antimicrobial drugs prescribed, and types of prescription drugs used for classifying prescriptions into the potentially inappropriate prescribing group based on patient interviews and prescription drug evidence.
Statistical analyses
We used standard descriptive statistics to calculate the number, percentage, median, and IQR (interquartile range). All statistical analyses were performed using JMP®, Version 15. SAS Institute Inc., Cary, NC, 1989–2021.
Ethics approval
The present study was conducted after obtaining approval from the Ethics Committee of the Shimane University School of Medicine (No. 20200622–2. Approval date: October 19, 2020). The included patients provided informed consent to participate in this study.
Results
Sample characteristics
The study included 150 patients with symptoms such as fever, headache, cough, nasal congestion, sore throat without underlying disease. Among them, 14 were excluded because the physician, who was not the prescribing physician, judged that the patients did not have cold symptoms, or the interview was incomplete (Fig 1). Patient characteristics are presented in Table 1. The median patient age was 34 years (interquartile range [IQR], 27–42), and the median duration of common cold symptoms was 2 days (IQR, 1.0–4.8). For 89.7% of the patients, the physician described the symptoms as “cold, upper respiratory tract infection, pharyngitis, and swollen tonsils.” Of all the patients, 18.4% had some kind of allergy, and 11.0% were smokers with median pack-years of 7.5% (IQR, 5–16).
Table 1. Background characteristics of patients (n = 136).
| Male sex, No (%) | 61 (44.9) | |
| Age, median (IQR), y | 34 (27–42) | |
| Days of cold symptoms (IQR), d | 2.0 (IQR 1.0–4.8) | |
| Patients who received any explanation from a doctor, No (%) (Ex. common cold, upper respiratory tract inflammation, sore pharyngitis, swollen tonsils) | 122 (89.7) | |
| Any allergies, No (%) | 25 (18.4) | |
| Smoking | n (%) Pack-years, median (IQR) | 15 (11.0) 7.5 (5–16) |
IQR, interquartile range.
Patients’ symptoms
Table 2 shows the details of the patients’ symptoms. The most common symptom reported by the patients was sore throat (63.2%), followed by cough (52.2%), runny nose (46.3%), phlegm (41.9%), nasal congestion (36.8%), lethargy (35.3%), headache (30.1%), fever (28.7%), painful swallowing (26.5%), sneezing (18.4%), and abdominal pain (1.5%). The median body temperature of the patients who had a fever was 37.4°C (IQR, 37.1–38.0).
Table 2. Patients’ symptoms.
| n | % | |
|---|---|---|
| Sore throat | 86 | 63.2 |
| Cough | 71 | 52.2 |
| Runny nose | 63 | 46.3 |
| Phlegm | 57 | 41.9 |
| Nasal congestion | 50 | 36.8 |
| Lethargy | 48 | 35.3 |
| Headache | 41 | 30.1 |
| Fever | 39 | 28.7 |
| 37.4°C | (IQR 37.1–38.0) | |
| Painful swallowing | 36 | 26.5 |
| Sneezing | 25 | 18.4 |
| Abdominal pain | 2 | 1.5 |
| Others | 17 | 12.5 |
IQR, interquartile range.
Details of prescriptions
Among all the prescriptions (n = 136), the most commonly prescribed symptomatic medications were H1 receptor antagonists (chlorpheniramine maleate, bepotastine; 41.9%), followed by expectorants (carbocysteine, bromhexine, ambroxol; 41.2%), NSAIDs (diclofenac, thiaramide, loxoprofen; 40.0%), β2-stimulants (mainly tulobuterol patches, terbutaline; 36.0%) and decalinium chloride lozenges (indicated for sore throat in Japan; 36.0%), non-narcotic antitussives (dimethorphan, dextromethorphan, tipepidine; 22.1%), tranexamic acid (indicated for sore throat in Japan; 19.9%), acetaminophen (18.4%), multi-ingredient cold medication (non-pyrine cold remedy combination granules, pyrazolone antipyretic analgesic anti-inflammatory combination granules; 7.4%), and leukotriene receptor antagonists (pranlukast, montelukast; 3.7%). Regarding antimicrobial agents, 44.9% of all prescriptions contained oral cephem (cefcapene pivoxil, cefdinir), 25.0% contained new quinolones (galenoxacin, levofloxacin), 9.5% contained macrolides (azithromycin, clarithromycin), and 2.2% contained penicillin (amoxicillin) (Table 3).
Table 3. Details of prescription drugs (n = 136).
| Breakdown of all prescriptions | Breakdown of potentially inappropriate prescription drugs for each drug prescribed. | Percentage of potentially inappropriate prescriptions drugs for each drug in the total potentially inappropriate prescriptions (n = 121). | |||
|---|---|---|---|---|---|
| n | % | n | % | % | |
| Symptomatic medicine | |||||
| H1 receptor antagonist | 57 | 41.9 | 0 | 0 | 0 |
| Expectorant drug | 56 | 41.2 | 0 | 0 | 0 |
| NSAIDs | 53 | 40.0 | 6 | 11.3 | 5.0 |
| β2-stimulant | 49 | 36.0 | 41 | 83.7 | 33.9 |
| Decalinium chloride (cough drop) | 49 | 36.0 | 0 | 0 | 0 |
| Non-narcotic antitussive | 30 | 22.1 | 0 | 0 | 0 |
| Tranexamic acid | 27 | 19.9 | 0 | 0 | 0 |
| Acetaminophen | 25 | 18.4 | 0 | 0 | 0 |
| Multi-ingredient cold medication | 10 | 7.4 | 0 | 0 | 0 |
| #Non-pyrine cold remedy combination granules, Pyrazolone antipyretic analgesic anti-inflammatory combination granules | |||||
| Leukotriene receptor antagonist | 5 | 3.7 | 0 | 0 | 0 |
| Others | 13 | 9.4 | 3 | 23.1 | 2.5 |
| Antibacterial drugs | |||||
| Cephem (Oral) | 61 | 44.9 | 61 | 100 | 50.4 |
| New quinolone | 34 | 25.0 | 34 | 100 | 28.1 |
| Macrolide | 13 | 9.5 | 12 | 92.3 | 10.0 |
| Penicillin | 3 | 2.2 | 0 | 0 | 0 |
| Cephem (Nasal spray) | 1 | 0.7 | 0 | 0 | 0 |
NSAID, non-steroidal anti-inflammatory drug.
Note: Active ingredients in 1 g of Non-pyrine cold remedy combination granules (salicylamide, 270 mg; acetaminophen, 150 mg; caffeine anhydrous, 60 mg; promethazine methylene disalicylate, 13.5 mg); active ingredients in 1 g of Pyrazolone antipyretic analgesic anti-inflammatory combination granules (isopropylantipyrine, 150 mg; acetaminophen, 250 mg; allyl isopropylacetylurea, 60 mg; caffeine anhydrous, 50 mg).
Details of potentially inappropriate prescription
The breakdown of potentially inappropriate prescription drugs in terms of each prescribed drug is shown in Table 3. β2 stimulants (83.7%), followed by NSAIDs (11.3%), had the highest percentage among symptomatic medicines. Among the antimicrobial agents, oral cephems and new quinolones had the highest rates (100%), followed by macrolides (92.3%).
The percentage of each potentially inappropriate drug in the total number of potentially inappropriate prescriptions is shown in Table 3. β2 stimulants (33.9%), followed by NSAIDs (5.0%), had the highest percentage among symptomatic medicines. Among antimicrobial agents, oral cephem had the highest percentage (50.4%), followed by new quinolones (28.1%) and macrolides (10.0%).
There were two contraindicated doses (for patients with a history of β2-stimulant hypersensitivity), and the pharmacist posed questions regarding the prescription. There were no dosage errors.
Rates of potentially inappropriate prescription and medication costs
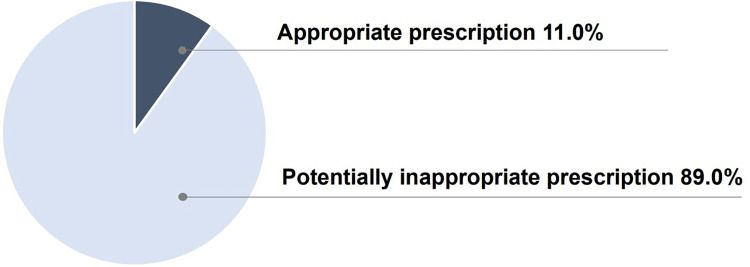
When the prescriptions were classified using the classification criteria (S1 Appendix), 11.0% (n = 15) fell under the appropriate prescription group and 89.0% (n = 121) under the potentially inappropriate prescription group (Fig 2). The median total cost of the prescribed drugs was 593.6 yen (IQR, 470–795.6) [$5.2 (IQR, 4.1–6.9)]. In particular, the median cost of drugs in the potentially inappropriate prescription group was 602.0 yen (IQR, 479.7–839.2) [$5.2 (IQR, 4.2–7.3)], and the median cost of drugs in the appropriate prescription group was 406.7 yen (IQR, 194.5–537.2) [$3.5 (IQR, 1.7–4.7)].
Fig 2. Classification of prescriptions based on criteria.
Discussion
This study suggests that approximately 90% of the prescriptions for common cold symptoms in this area are inappropriate and that antimicrobials and symptomatic drugs, such as β2-stimulants, which are not suitable for the respiratory symptoms in the absence of underlying disease or history, maybe inappropriately prescribed. This is the first cross-sectional study in Japan to investigate whether prescriptions for common cold comply with domestic and international evidence.
The rationale for classification as the potentially inappropriate prescribing group
Oral cephem antibiotic, β2-stimulants, and new quinolone antibacterials were the top three drugs used in classifying prescriptions into the potentially inappropriate prescribing group, based on patient interviews and prescribed drugs and prescription drug evidence. The rationale for classifying prescriptions of oral cephem antibiotics as potentially inappropriate is as follows: antimicrobial agents are not beneficial for common cold symptoms, and their side effects are notable [15]. In the case of suspected streptococcal infection, penicillin is recommended as the first choice because of its proven efficacy and safety, narrow spectrum, and low cost [31]. The rationale for classifying prescriptions containing β2-stimulants as potentially inappropriate is as follows: β2-stimulants are useful in patients with a history of cough, asthma, chronic obstructive pulmonary disease (COPD), and other diseases [27]. However, there is no clear benefit of these drugs for acute bronchitis and acute cough in adults, and side effects such as tremor and neurological symptoms may occur [28]. The tulobuterol patch, which was mainly used in this study, is approved for use only in Japan and Korea, and there is insufficient evidence in terms of its efficacy and safety [29]. Considering this information, we concluded that it is reasonable to consider a prescription for β2-stimulants for patients without underlying diseases such as asthma or COPD and with strongly suspected findings for acute cold symptoms as potentially inappropriate. The rationale for judging new quinolones as inappropriate is as follows. New quinolones are considered inappropriate as they are ineffective against viruses which cause common cold. The risk of aortic aneurysm and aortic dissection by inducing collagen degradation has been reported for some new quinolones [32]. Given the magnitude of the risk of adverse effects, it is unlikely that they need to be administered to patients with acute cold symptoms.
Factors responsible for prescriptions of potentially inappropriate drugs
Based on previous studies, four major factors may be involved in the prescriptions becoming potentially inappropriate: The first factor is that patients may be seeking medications from doctors [34]. The Japanese healthcare system provides easy access to higher medical institutions and clinics. Due to the low cost of medical care in Japan, patients may be inclined to receive some kind of medication and feel relieved [35]. The second factor may be the physician’s attitude toward consultation. For example, doctors intend to satisfy patients by prescribing them medications [36] and quickly end the consultation [37]; their prescribing patterns are fixed to some extent according to the age and sex of the patient [38]. The third factor is the lack of evidence on physicians’ standard of care for the treatment of common cold. In Japan, despite the publication of national guidelines in 2016 regarding the appropriate use of antimicrobials, there was no significant change in the trend of antimicrobial use among outpatients with acute upper respiratory tract infection before and after the publication [39]. In this study, the percentage of antimicrobials in prescriptions (83%) was higher than that reported in previous studies (60%) [3]. Furthermore, third-generation oral cephem antibacterial agents were used in approximately 50% of the prescriptions for common cold symptoms, followed by new quinolone and macrolide antibacterials, and the proportion of prescriptions was approximately the same as that in previous studies [3]. This information suggests that there is insufficient awareness regarding the need to refrain from prescribing unnecessary antimicrobials for common cold symptoms. Fourth, there is a lack of primary care education for physicians. It is common for Japanese primary care physicians to specialize in organ-specific treatment and start private practices, taking on the role of primary care providers in their respective regions [40]. In Japan, many physicians start their own clinical practice without undergoing any special training for minor illnesses [41]. Therefore, it is possible that many physicians in this region also routinely prescribe the same drugs that they prescribe for organ-specific treatment. However, it is unlikely that any single solution will alter the prescribing habits of physicians [42]. Cultural factors may also be involved in addressing this problem, and steps other than continuing medical and patient education are necessary.
Pharmacists’ views on potentially inappropriate prescription
In Japan, the importance of the pharmacists’ intervention in prescribing has been highlighted in recent years [43]. Pharmacists in Japan are proactive in making inquiries regarding the "safety and dosage volume" of drugs [43]. In contrast, in cases where there is a possibility of inappropriate prescribing but the pharmacist is unsure about making an inquiry, the pharmacist often does not make an inquiry, taking the prescribing physician’s intentions into consideration. One of the reasons underlying this is that a certain number of physicians consider the pharmacist’s frequent inquiries to be bothersome, and pharmacists, who place importance on a good relationship with the local physician, are hesitant to make such inquiries. Such psychological barriers between pharmacists and doctors may be detrimental to patients. This is an issue that needs to be gradually addressed in the future.
Considerations for drug costs
The median cost of drugs for potentially inappropriate prescriptions and appropriate prescriptions were 602.0 yen (IQR, 479.7–839.2) [$5.2 (IQR, 4.2–7.3)] and 406.7 yen (IQR, 194.5–537.2) [$3.5 (IQR, 1.7–4.7)], respectively. As shown in the previous study, "Japan’s universal health insurance system covers all prescription drugs generally; therefore, patient co-payments are minimal, and this is a factor that contributes towards doctors prescribing drugs easily” [44, 45]. This makes it easier for doctors to prescribe unnecessary drugs leading to a higher cost of drugs. As part of the Choosing Wisely Campaign, it is generally recommended that patients ask their doctors the following five questions [46]. (1) Do I really need this test or procedure? (2) What are the risks? (3) Are there simpler, safer options? (4) What happens if I do not do anything? (5) What are the costs? Patients must be aware of their own "treatment" by referring to the above questions to promote the appropriate use of medicines in Japan. It is necessary to incorporate education and measures that consider the interactive opinions of the medical professionals and patients.
Limitations
This study had several limitations. First, the sample size was small, and the survey was limited to hospitals and clinics in Izumo City; therefore, there may have been strong influences of the characteristics of doctors in the area, such as biases in prescribing. Second, because the survey was conducted in a dispensing pharmacy, the actual conversations between the doctors and patients in the clinic, patients’ physical findings, and information in their medical records were not captured. Third, the study excluded prescriptions of Kampo medicines. Since Kampo medicines are often prescribed for common cold symptoms in Japan, the results of this study do not fully reflect the actual situation of prescriptions for common cold symptoms in Japan. Fourth, the criteria used to classify prescriptions were not complete. Several previous overseas studies have evaluated the efficacy of cold medicines; however, the efficacy of many of these medicines is unclear, which may have led to the misclassification of the prescriptions. Fifth, there may have been influences of changes in the epidemiology of common cold caused by the novel coronaviruses. Fortunately, however, during the study period (October 2020 to January 2021), we had the lowest incidence of coronavirus disease in Japan, with the lowest positivity rate in polymerase chain reaction (S3 Appendix). Therefore, although we believe that the impact of the epidemiological changes caused by the new coronavirus infection in this study was quite limited, we cannot deny the possibility of its impact on the study. Sixth, the details regarding the prescribing physician were not investigated in this study as the Ethics Committee stipulates that “the content that identifies the prescribing physician (specialty, affiliation, and the number of individuals) must not be added to the content of the interview because it may cause disadvantages to the prescribing physician.” We believe that the prescribing physician’s details are important factors and further research is needed. Lastly, this study was limited to adult patients with no underlying medical conditions. The content of prescriptions for common cold patients is likely to be affected by the presence or absence of underlying diseases and the age of the patients. Therefore, the results of this study may not fully reflect the current status of prescribing for common cold symptoms in Japan.
Conclusion
Approximately 90% of the prescriptions for common cold symptoms in this local area were potentially inappropriate, and antimicrobials and symptomatic drugs such as β2-stimulants, which are not suitable for the patients’ respiratory symptoms in the absence of underlying diseases or history, may be inappropriately prescribed. This finding is expected to help promote the appropriate use of medicines for common cold symptoms and improve the quality of medical care. However, this was a pilot study at the regional level, and it is necessary to conduct a nationwide survey in the future.
Supporting information
(XLSX)
(DOCX)
(PPTX)
Acknowledgments
We are very grateful to Midori Pharmacy Co. Ltd. for their cooperation in interviewing the target patients.
Data Availability
The data that support the findings of this study are available for anyone from the General Medicine Center, Shimane University (contact information; tel +81-853-20-2217, e-mail: shimanegp@gmail.com) upon reasonable request.
Funding Statement
YES T.W. is supported by grants from the National Academic Research Grant Funds (JSPS KAKENHI: 20H03913). The sponsor of the study had no role in the study design, data collection, analysis, or preparation of the manuscript.
References
- 1.Fashner J, Ericson K, Werner S. Treatment of the common cold in children and adults. Am Fam Phys. 2012;86: 153–159. [PubMed] [Google Scholar]
- 2.Tsutsumi M, Shaku F, Ozone S, Sakamoto N, Maeno T. Reasons for the preference of clinic visits to self‐medication by common cold patients in Japan. J Gen Fam Med. 2017;18: 336–340. doi: 10.1002/jgf2.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higashi T, Fukuhara S. Antibiotic prescriptions for upper respiratory tract infection in Japan. Intern Med. 2009;48: 1369‒1375. doi: 10.2169/internalmedicine.48.1893 [DOI] [PubMed] [Google Scholar]
- 4.Dekker ARJ, Verheij TJM, van der Velden AW. Inappropriate antibiotic prescription for respiratory tract indications: most prominent in adult patients. Fam Pract. 2015;32: 401–407. doi: 10.1093/fampra/cmv019 [DOI] [PubMed] [Google Scholar]
- 5.Huntington MK, VanKeulen S, Hoffman WW. What ever happened to the common cold? Improving antibiotic utilization. S D. Med. 2013;66: 136–139, 141–143, 145–146. [PubMed] [Google Scholar]
- 6.Kenealy T, Arroll B. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2013;2013: CD000247. doi: 10.1002/14651858.CD000247.pub3 [DOI] [PubMed] [Google Scholar]
- 7.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163: 487–494. doi: 10.1001/archinte.163.4.487 [DOI] [PubMed] [Google Scholar]
- 8.Deckx L, De Sutter AI, Guo L, Mir NA, van Driel ML. Nasal decongestants in monotherapy for the common cold. Cochrane Database Syst Rev. 2016;10: CD009612. doi: 10.1002/14651858.CD009612.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fixen DR. AGS Beers Criteria for older adults. Pharm Today. 2019;25: 42–54. 10.1016/j.ptdy.2019.10.022 [DOI] [Google Scholar]
- 10.Komagamine J. Characteristics of the use of cold combination products among older ambulatory patients at the National Hospital Organization Tochigi Medical Center in Japan: a retrospective single-center observational study. BMC Res Notes. 2017;10: 728. doi: 10.1186/s13104-017-3070-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Mahony D, Gallagher PF. Inappropriate prescribing in the older population: need for new criteria. Age Ageing. 2008;37: 138–141. doi: 10.1093/ageing/afm189 [DOI] [PubMed] [Google Scholar]
- 12.Barry PJ, Gallagher P, Ryan C, O’mahony D. START (screening tool to alert doctors to the right treatment)—an evidence-based screening tool to detect prescribing omissions in elderly patients. Age Ageing. 2007;36: 632–638. doi: 10.1093/ageing/afm118 [DOI] [PubMed] [Google Scholar]
- 13.Kirkpatrick GL. The Common Cold. Community-acquired respiratory infections in children. Prim Care. 1996;23: 657–675. doi: 10.1016/s0095-4543(05)70355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scaglione F, Petrini O. Mucoactive agents in the therapy of upper respiratory airways infections: fair to describe them just as mucoactive? Clin Med Insights Ear Nose Throat. 2019;12: 1179550618821930. doi: 10.1177/1179550618821930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186: 190–199. doi: 10.1503/cmaj.121442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterrantino C, Duarte G, Costa J, Vaz-Carneiro A. Analysis of the Cochrane review: antihistamines for the common cold. Análise da Revisão Cochrane: Antihistamínicos para a Constipação. Cochrane Database Syst Rev. 2015;11: CD009345 [Analysis of the Cochrane Review: Antihistamines for the Common Cold. Cochrane Database Syst Rev. 2015;11:CD009345]. Acta Med Port. 2016;29: 164–167. doi: 10.20344/amp.7526 [DOI] [PubMed] [Google Scholar]
- 17.Arroll B. Non-antibiotic treatments for upper-respiratory tract infections (common cold). Respir Med. 2005;99: 1477–1484. doi: 10.1016/j.rmed.2005.09.039 [DOI] [PubMed] [Google Scholar]
- 18.Ida H. The nonnarcotic antitussive drug dimemorfan: a review. Clin Ther. 1997;19: 215–31. doi: 10.1016/s0149-2918(97)80111-7 [DOI] [PubMed] [Google Scholar]
- 19.Jungersen M, Wind A, Johansen E, Christensen JE, Stuer-Lauridsen B, Eskesen D. The science behind the probiotic strain Bifidobacterium animalis subsp lactis BB-12(®). Microorganisms. 2014;2:92–110. doi: 10.3390/microorganisms2020092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Issa I, Moucari R. Probiotics for antibiotic-associated diarrhea: do we have a verdict? World J Gastroenterol. 2014;20: 17788–17795. doi: 10.3748/wjg.v20.i47.17788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douma JAJ, Smulders YM. [Loperamide for acute infectious diarrhoea]. Ned Tijdschr Geneeskd. 2015;159: A9132. [PubMed] [Google Scholar]
- 22.Ziegler TR, Szeszycki EE, Estívariz CF, Puckett AB, Leader LM. Affiliations expand. Glutamine: from basic science to clinical applications. Nutrition. 1996;12: S68–S70. doi: 10.1016/s0899-9007(96)00019-6 [DOI] [PubMed] [Google Scholar]
- 23.Katona G, Sultész M, Farkas Z, Gyimesi A, Hirschberg A, Huszka J, et al. Treatment of acute rhinitis with a nasal spray containing tramazoline and essential oils: a multicenter, uncontrolled, observational trial. Clin Transl Allergy. 2015;5: 38. doi: 10.1186/s13601-015-0084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niño-Serna LF, Acosta-Reyes J, Veroniki A-A, Florez ID. Antiemetics in children with acute gastroenteritis: A meta-analysis. Pediatrics. 2020;145: e20193260. doi: 10.1542/peds.2019-3260 [DOI] [PubMed] [Google Scholar]
- 25.Satomura K, Kitamura T, Kawamura T, Shimbo T, Watanabe M, Kamei M, et al. Great Cold Investigators-I. Prevention of upper respiratory tract infections by gargling: a randomized trial. Am J Prev Med. 2005;29: 302–307. doi: 10.1016/j.amepre.2005.06.013 [DOI] [PubMed] [Google Scholar]
- 26.Horiguchi T, Ohira D, Kobayashi Kashin, Hirose M, Miyazaki J, Kondo R, et al. Clinical evaluation of leukotriene receptor antagonists in preventing common cold-like symptoms in bronchial asthma patients. Allergol Int. 2007;56: 263–267. doi: 10.2332/allergolint.O-06-473 [DOI] [PubMed] [Google Scholar]
- 27.Tamura G, Ohta K. Adherence to treatment by patients with asthma or COPD: comparison between inhaled drugs and transdermal patch. Respir Med. 2007;101: 1895–1902. doi: 10.1016/j.rmed.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 28.Smucny J, Becker LA, Glazier R. Beta2‐agonists for acute bronchitis. Cochrane Database Syst Rev. 2004: CD001726. doi: 10.1002/14651858.CD001726.pub2 [DOI] [PubMed] [Google Scholar]
- 29.Ebihara S, Ebihara T, Arai H. Cough and transdermal long-acting β2 agonist in Japan. Respir Med. 2008;102: 1497; author reply 1498. doi: 10.1016/j.rmed.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 30.Cazzola M, Matera MG. The effect of doxofylline in asthma and COPD. Respir Med. 2020;164: 105904. doi: 10.1016/j.rmed.2020.105904 [DOI] [PubMed] [Google Scholar]
- 31.Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of Group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55: e86–e102. doi: 10.1093/cid/cis629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee C-C, Lee MG, Hsieh R, Porta L, Lee WC, Lee SH, et al. Oral fluoroquinolone and the risk of aortic dissection. J Am Coll Cardiol. 2018;72: 1369–1378. doi: 10.1016/j.jacc.2018.06.067 [DOI] [PubMed] [Google Scholar]
- 33.Lemiengre MB, van Driel ML, Merenstein D, Young J, De Sutter AI. Antibiotics for clinically diagnosed acute rhinosinusitis in adults. Cochrane Database Syst Rev. 2012;10: CD006089. doi: 10.1002/14651858.CD006089.pub4 [DOI] [PubMed] [Google Scholar]
- 34.Bradley CP. Uncomfortable prescribing decisions: a critical incident study. BMJ. 1992;304: 294‒296. doi: 10.1136/bmj.304.6822.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blaiss MS, Dicpinigaitis PV, Eccles R, Wingertzahn MA. Consumer attitudes on cough and cold: US (ACHOO) survey results. Curr Med Res Opin. 2015;31: 1527‒1538. doi: 10.1185/03007995.2014.1002558 [DOI] [PubMed] [Google Scholar]
- 36.Fenton JJ, Jerant AF, Bertakis KD, Franks P. The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172: 405–411. doi: 10.1001/archinternmed.2011.1662 [DOI] [PubMed] [Google Scholar]
- 37.Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA. 2012;307:1 377–1378. 10.1001/jama.2012.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández-Liz E, Modamio P, Catalán A, Lastra CF, Rodríguez T, Mariño EL. Identifying how age and gender influence prescription drug use in a primary health care environment in Catalonia, Spain. Br J Clin Pharmacol. 2008;65: 407–417. doi: 10.1111/j.1365-2125.2007.03029.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato D, Goto T, Uda K, Kumazawa R, Matsui H, Yasunaga H. Impact of national guidelines for antimicrobial stewardship to reduce antibiotic use in upper respiratory tract infection and gastroenteritis. Infect Control Hosp Epidemiol. 2021;42: 280–286. doi: 10.1017/ice.2020.427 [DOI] [PubMed] [Google Scholar]
- 40.Yukishige Ishibashi MD. Why is family medicine needed in Japan? J Fam Pract. 1987;25: 83‒86. [PubMed] [Google Scholar]
- 41.Murai M, Kitamura K, Fetters MD. Lessons learned in developing family medicine residency training programs in Japan. BMC Med Educ. 2005;5: 33. doi: 10.1186/1472-6920-5-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butler CC, Rollnick S, Pill R, Maggs-Rapport F, Stott N. Understanding the culture of prescribing: qualitative study of general practitioners’ and patients’ perceptions of antibiotics for sore throats. BMJ. 1998;317: 637‒642. doi: 10.1136/bmj.317.7159.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shikamura Y, Mano Y, Komoda M, Negishi K, Sato T, Miyazaki S. [Reduction of Medical Cost through Pharmaceutical Inquiries by Community Pharmacists and Relation with Iyaku Bungyo Rates: A Nationwide Survey on Prescription Inquiries.] Yakugaku Zasshi 2016;136: 1263‒1273. doi: 10.1248/yakushi.16-00024 [DOI] [PubMed] [Google Scholar]
- 44.Onda M, Imai H, Takada Y, Fujii S, Shono T, Nanaumi Y. Identification and prevalence of adverse drug events caused by potentially inappropriate medication in homebound elderly patients: a retrospective study using a nationwide survey in Japan. BMJ Open. 2015;5: e007581. doi: 10.1136/bmjopen-2015-007581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannon KT, Choi MM, Zuniga MA. Potentially inappropriate medication use in elderly patients receiving home health care: a retrospective data analysis. Am J Geriatr Pharmacother. 2006;4: 134‒143. doi: 10.1016/j.amjopharm.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 46.Muscat DM, et al. Evaluation of the Choosing Wisely Australia 5 Questions resource and a shared decision-making preparation video: protocol for an online experiment. BMJ Open 2019;9: e033126. doi: 10.1136/bmjopen-2019-033126 [DOI] [PMC free article] [PubMed] [Google Scholar]