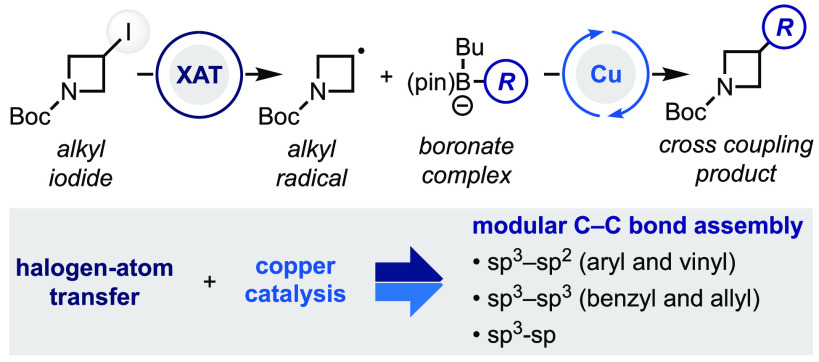
Abstract
We report here a mechanistically distinct approach to achieve Suzuki–Miyaura-type cross-couplings between alkyl iodides and aryl organoborons. This process requires a copper catalyst but, in contrast with previous approaches based on palladium and nickel systems, does not utilizes the metal for the activation of the alkyl electrophile. Instead, this strategy exploits the halogen-atom-transfer ability of α-aminoalkyl radicals to convert secondary alkyl iodides into the corresponding alkyl radicals that then are coupled with aryl, vinyl, alkynyl, benzyl, and allyl boronate species. These novel coupling reactions feature a simple setup and conditions (1 h at room temperature) and facilitate access to privileged motifs targeted by the pharmaceutical sector.
Introduction
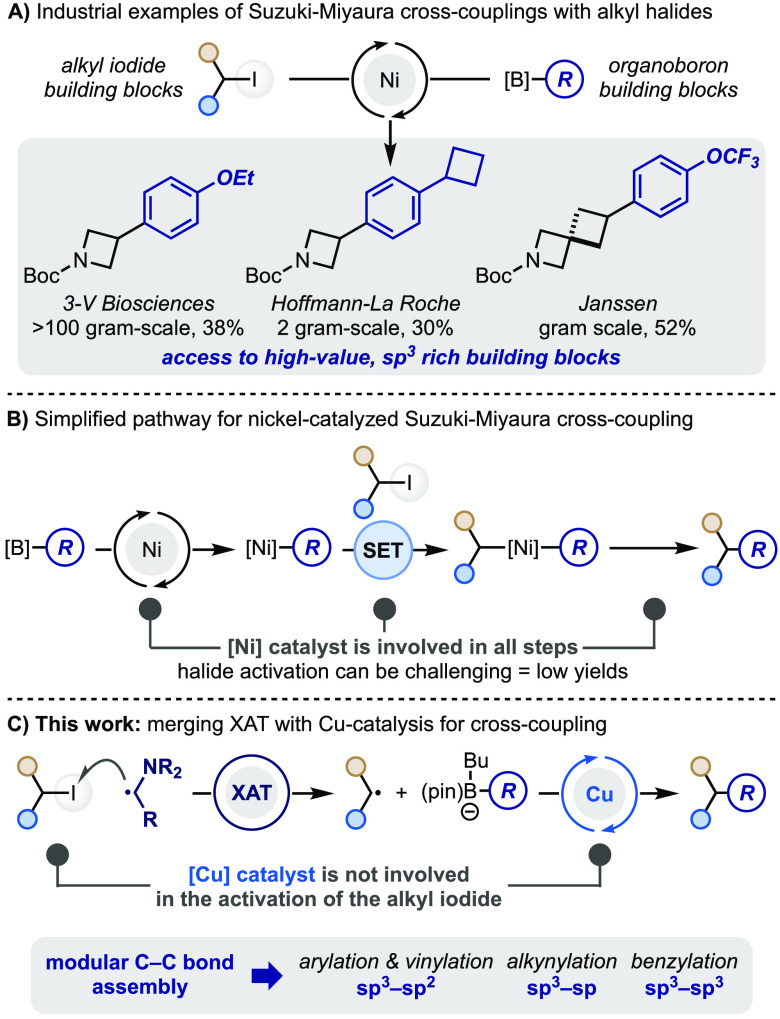
Among all cross-coupling approaches, the Nobel-Prize-winning Suzuki–Miyaura reaction has changed the way organic molecules are assembled.1 This process is widely used in both industrial and academic settings mostly due to its mild conditions and the commercial availability of both organic halides and organoboron building blocks.2 However, while aryl halides are a benchmarked class of coupling partners, the utilization of alkyl halides is less straightforward. Under palladium catalysis, the slower rate of oxidative addition and the increased chances of β-hydride elimination often render these reactions difficult to implement.3
Nickel catalysis has provided a workable solution to this challenge through the use of catalysts supported by either phenanthroline or aminoalcohol ligands.4 Other base metals (mostly Fe and Co) have been successfully applied in Suzuki-type cross-coupling, but their reactivity is less general.5 Overall, despite significant success in the area, coupling with secondary and unactivated alkyl electrophiles is still a relevant challenge, as these processes are usually low yielding, especially when polar functionalities are present.6 Notwithstanding these challenges and often suboptimal yields, the synthetic value provided by these coupling reactions makes them widely applied by the pharmaceutical and agrochemical sectors, where sp3-rich fragments have increased chances of biological activity (Scheme 1A).7 The development of novel strategies increasing our synthetic capacity for the assembly of these motifs has the potential to affect the discovery and manufacture of materials that ultimately can improve the quality of our lives.8
Scheme 1. (A) The Transition-Metal-Catalyzed (Mostly Nickel) Cross-Coupling between Alkyl Halide and Organoboron Building Blocks Is Often Used to Access sp3-Rich Materials, (B) Current Methods Require the Nickel Catalyst to Be Involved in the Halide Activation Step That Can Be Challenging, and (C) This Work Exploits α-Aminoalkyl Radical-Mediated XAT to Activate the Halide and Uses a Copper Catalyst.
The cross-coupling strategies discussed above revolve around the direct reaction of the nickel catalyst with an alkyl halide, which generally leads to the formation of transient radical species (Scheme 1B).9 While this might help in the case of difficult oxidative additions, it also means that the catalyst properties need to be carefully balanced to enhance halide activation without compromising the following elementary steps such as transmetalation and reductive elimination.6a,10
We recently speculated that a conceptually different strategy, where the metal catalyst is required to orchestrate the C–C bond formation but not to activate the alkyl halide, might provide synthetic advantages toward the assembly of challenging small-molecule building blocks. In this paper we report the realization of this goal and present a novel and general approach for the Suzuki–Miyaura-type cross-coupling between secondary alkyl iodides and a broad range of boronates. This method integrates α-aminoalkyl radical mediated halogen-atom transfer (XAT) with copper catalysis11 and provides a general entry into the modular assembly of challenging C(sp3)–C(sp2) as well as C(sp3)–C(sp3) and C(sp3)–C(sp) bonds.
Results and Discussion
We and the Doyle group have recently reported that alkyl and aryl iodides can be converted into the corresponding radicals via α-aminoalkyl radical mediated XAT.12 This reactivity benefits from a polarized transition state where the α-aminoalkyl unit stabilizes charge transfer, which kinetically accelerates the halide abstraction. This blueprint for radical generation can be exploited in different settings, which include the coupling of alkyl iodides with N-nucleophiles to assemble SN2-elusive C(sp3)–N bonds.13 This reactivity paradigm is possible by merging XAT with copper catalysis so that an alkyl radical is generated and then captured by a copper-bound N fragment.
In order to achieve C–C bond formation, we looked at the Chan–Lam cross-coupling that has pioneered the ability of aryl organoborons to undergo transmetalation with [Cu(II)] species.14 We therefore envisaged that the merger of XAT with [Cu] catalysis might enable a hybrid type of cross-coupling, which would be of the Suzuki type in terms of retrosynthetic disconnection but more Chan–Lam based in terms of mechanism (Scheme 1C).
Our proposed pathway for such XAT-mediated and Cu-catalyzed arylation is illustrated in Scheme 2A using 3-iodo-N-Boc-azetidine 1 and the generic Ph-organoboron derivative 2 as the coupling partners. Starting from a [Cu(I)] catalyst, ground-state SET with a stoichiometric oxidant such as cumO2TMS would deliver a Cu(II)] species that would transmetalate with 2 and give the Ph–[Cu(II)] complex A. The cumO• generated in the SET event would react with a stoichiometric amine reagent to give, upon polarity-matched H atom transfer (HAT),15 the key nucleophilic α-aminoalkyl radical B. Polarity-accelerated XAT between B and 1 would then be used to access the alkyl radical C, which could enter the [Cu(I/II/III)]-catalytic cycle and trap A.16 This step should deliver the alkyl,aryl–[Cu(III)] intermediate D, from which reductive elimination is facile17 and should therefore provide the cross-coupling product 3 while reinitiating the catalytic cycle.
Scheme 2. (A) Proposed Mechanism for the XAT-Mediated and Cu-Catalyzed Cross-Coupling of Alkyl Iodides and Aryl Organoborons, (B) Reaction Optimization and Key Control Experiments, and (C) Selected Mechanistic Experiments.
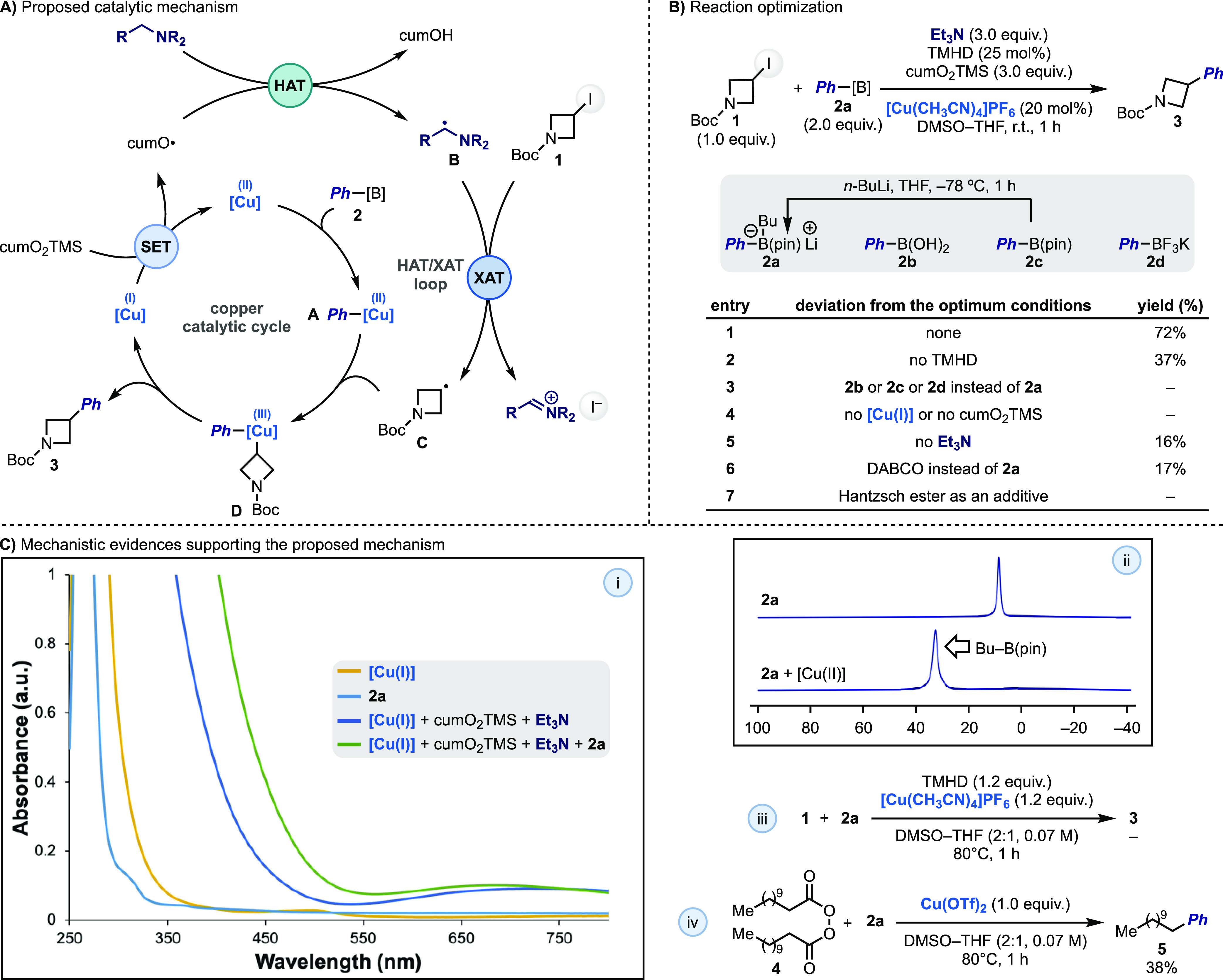
As was mentioned before, the key mechanistic difference between this approach and other metal-mediated cross-couplings is that the alkyl radical generation occurs in a HAT/XAT loop that is dissected by the copper-based catalytic cycle. This means that the alkyl halide does not need to engage in direct SET with the metal, which can be difficult due to its low reduction potential (for alkyl–I: Ered < −2 V vs SCE), or enter its coordination sphere for subsequent abstraction (or oxidative addition). This fundamental difference obviates the need of a highly reducing catalyst, which we hoped might translate into a broader substrate scope.
This proposal for the radical arylation of 1 was eventually implemented using boronate 2a as the coupling partner (prepared by addition of n-BuLi to 2c at −78 °C), [Cu(CH3CN)4]PF6 as the catalyst, Et3N as the XAT promoter, cumO2TMS as the oxidant, and 2,2,6,6-tetramethyl-3,5-heptanedione (TMHD) as the ligand in a DMSO–THF solvent mixture at room temperature (Scheme 2B, entry 1).18 Under these mild conditions, 3 was obtained in 72% yield in just 1 h. The TMHD ligand was important to improve the yield in this specific example (Scheme 2B, entry 2), but it was not necessary for all the cross-couplings presented below. Less activated organoborons (e.g., 2b–d) were evaluated, but they did not lead to product formation (Scheme 2B, entry 3), which we propose might result from their lower ability to transmetalate with [Cu(II)] species.18
In the absence of [Cu(I)] catalyst or the oxidant, no reactivity took place (Scheme 2B, entry 4) while the exclusion of Et3N provided 3 in 17% yield (Scheme 2B, entry 4). We were initially surprised by the success of this reaction, as the activation of 1 should not occur. An analysis of the crude reaction mixture revealed the trace formation of Ph–I and acetophenone. We propose that under these amine-free conditions other productive pathways based on SET oxidation of 2a to the corresponding Ph• and/or fragmentation of C to Me• might be operating. These reactivities would generate XAT-active species that can homolytically activate 1 and lead to product formation.18
To obtain more details on the process, we ran some mechanistic studies. UV/vis absorption spectroscopy studies demonstrated that [Cu(CH3CN)4]PF6 is oxidized upon treatment with cumO2TMS (new absorption band at λ ≈ 650 nm)19 and that transmetalation with 2a might be occurring (Scheme 2C-i). 11B NMR spectroscopy studies were used to further support the transmetalation of 2a with [Cu(II)], as evidenced by the formation of BuB(pin) (Scheme 2C-ii). The requirement of B to achieve XAT activation of 1 was revealed by the fact that replacing Et3N for DABCO, an amine that can be oxidized but cannot lead to the formation of an α-aminoalkyl radical,20 led to 3 in 17% yield, which is identical with the outcome observed under amine-free conditions (Scheme 2B, entry 6). Furthermore, the fact that a stoichiometric reaction of 1, 2a, and [Cu(I)] did not lead to any product formation demonstrates that a putative Ph–[Cu(I)] intermediate is not able to activate the alkyl halide by either SET or oxidative addition (Scheme 2C-iii). This clearly underscores the relevance of XAT as the alkyl iodide activation pathway. Finally, the thermal reaction of lauroyl peroxide 4 with 2a using a stoichiometric [Cu(II)] species gave 5 in 38% yield (Scheme 2C-iv). This experiment supports the generation of a primary alkyl radical (O–O homolysis then decarboxylation) that can intercept A and therefore enable the coupling process.18
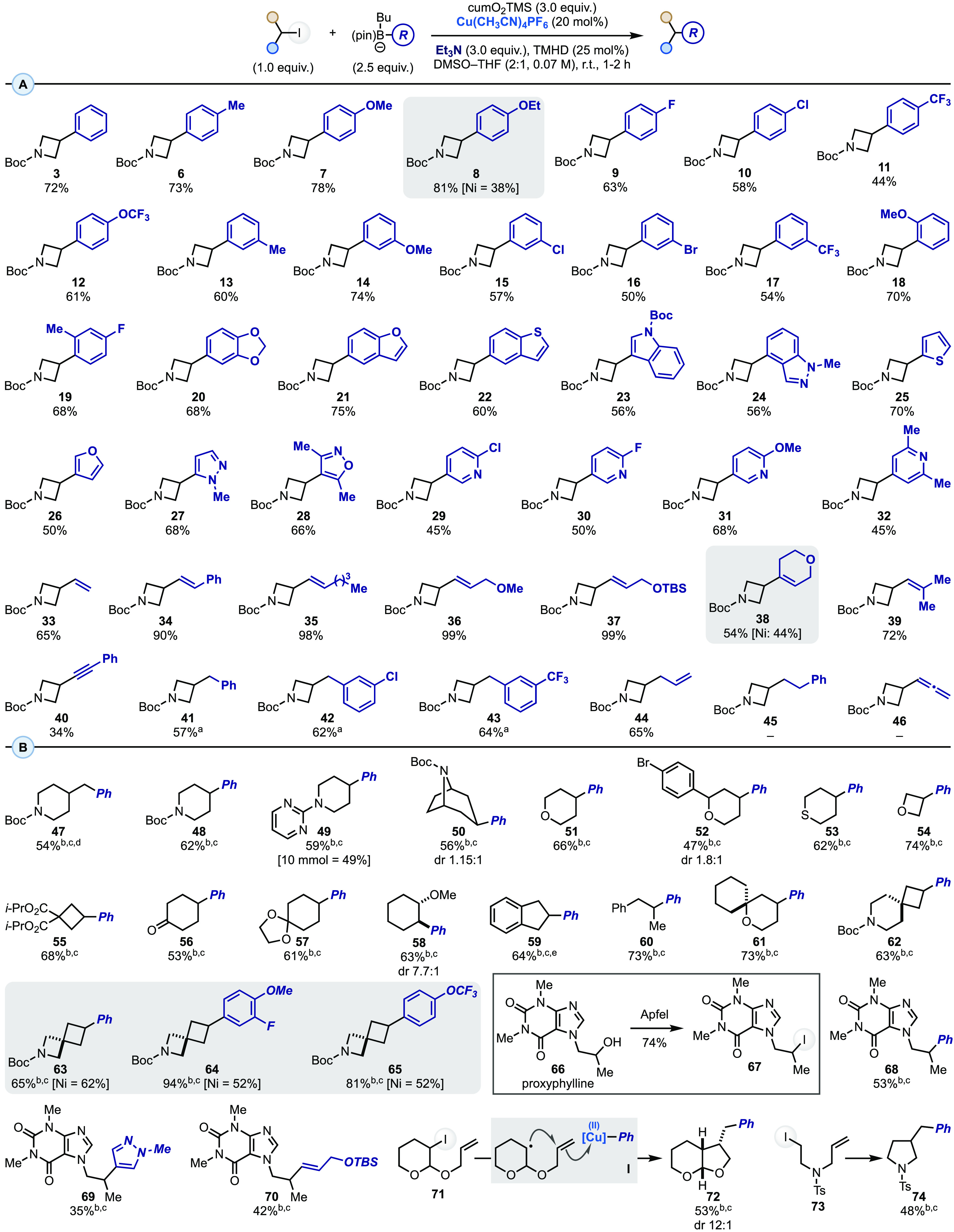
With the optimized reaction conditions in hand, we evaluated the scope of the transformation using 1 as the model alkyl iodide (Scheme 3A). We started by evaluating para-substituted aryl boronates and found that a variety of substituents were tolerated, delivering the desired products in good yields. These included substrates with electron-rich Me (6), OMe (7) and OEt (8) groups as well as electron-withdrawing Cl (9), F (10), CF3 (11), and OCF3 (12) functionalities. A similar trend was observed for the utilization of meta- and ortho-substituted derivatives (13–17 and 18, respectively), which also included aryl chloride and bromide functionalities that can be engaged in further modular diversifications.
Scheme 3. XAT-Mediated and Cu-Catalyzed Cross-Coupling of Alkyl Iodides and Aryl Organoborons: (A) Organoboron Scope and Limitations and (B) Alkyl Iodide Scope and Limitations.
cumO2Sit-BuPh was used in place of cumO2TMS.
cumO2TES was used in place of cumO2TMS.
Reaction run with no TMHD.
10 mol % of Cu(CH3CN)4PF6.
30 mol % of Cu(CH3CN)4PF6.
Polysubstituted aryl boronates were screened next, and they provided the desired products in good yields (19 and 20). Electron-rich heteroaryl boronates were competent coupling partners, and they enabled the introduction of medicinally relevant benzofuran (21), bezothiophene (22), indole (23), and indazole (24) units, as well as thiophene (25), furan (26), pyrazole (27), and isoxazole (28). Pyridines are some of the most prevalent motifs in bioactive leads,21 and pleasingly, our copper-catalyzed approach successfully engaged both C-3 (29–31) and C-4 (32) borylated derivatives.
Having benchmarked this reactivity on a diverse set of aromatic coupling partners, we evaluated its feasibility with respect to vinyl derivatives. Pleasingly, the utilization of several commercial boronic esters enabled, upon boronate formation, the introduction of vinyl (33) and styrenenyl (34) as well as other mono- and disubstituted olefin (35–37 and 38, 39 respectively) units in high yields. The initial results demonstrated that alkynyl boronates are viable partners (40) to achieve C(sp3)–C(sp) bond formation, albeit in lower yields.
The formation of C(sp3)–C(sp3) linkages via cross-coupling strategies is still a challenging task. We were pleased to find that activated benzylic22 and allylic boronates performed well under the reaction conditions, delivering 41–43 and 44 in good yields. In terms of limitations, allenyl and unactivated alkyl boronates failed to to provide the desired products (45 and 46).
Of the substrates presented in Scheme 3A, 8 and 38 have been recently prepared by the pharmaceutical sector (8, 3 V-Biosciences;23 and 38, GSK24) by Ni-catalyzed Suzuki–Miyaura cross-coupling on 1. The approach reported here utilized the same iodide and provided the desired products in higher yields. We hope this might highlight the complementarity that this strategy can provide to mainstream approaches in the case of challenging arylations.
Evaluation of the alkyl iodide scope was performed using the Ph–B(pin)-based boronate 2a as the coupling partner, which revealed that a wide range of unactivated alkyl iodides can be engaged (Scheme 3B). This was showcased by the arylation of several piperidine derivatives, either N-Boc protected (47 and 48) or part of a 2-aminopyrimidine unit (49, also on a 10 mmol scale). The chemistry was then applied to the preparation of 50, which is an analogue of the alkaloid nortropine. Other commercial small-molecule building blocks were successfully engaged, as demonstrated by the arylation of 4-iodo(thio)pyrans (51–53), 3-iodooxetane (54), and a cyclobutene derivative (55) recently disclosed by Merck for the preparation of trifluoromethylated cyclobutanes.25
The coupling reactivity was compatible with ketone, acetal, and ether functionalities (56–59), while the formation of 59 and 60 demonstrated that HAT-labile benzylic positions are tolerated. This chemoselectivity is noteworthy, considering the ability of cumO• to promote HAT reactions on activated benzylic C(sp3)–H positions.26
Spirocycles are interesting chemotypes in drug development campaigns due to their high C(sp3) content.27 Pleasingly, radical cross-couplings with several commercially available iodides were high-yielding (61–65). Of these substrates, 63–65 have been recently prepared by Janssen using Ni catalysis on the corresponding iodides, albeit in lower yields and, in the case of 65, as a mixture with the demethoxylated product,28 which was not observed under our conditions.
Complex alkyl iodides can be easily generated by Appel reactions on secondary alcohols, which are a large class of commercial materials. We therefore converted the cardiac stimulant proxyphylline (66) into linear alkyl iodide 67 and demonstrated that this derivative can undergo divergent arylation and alkenylation reactivity (68–70).
Finally, all the examples presented so far have dealt with the direct arylation of a secondary alkyl iodide. An interesting avenue for molecular construction offered by the utilization of carbon radicals is that these species can undergo other types of reactivities before engaging in the final cross-coupling event. This might provide a synthetic opportunity with respect to standard Suzuki–Miyaura reactivity, as simplified building blocks can be used to access more complex products. A preliminary illustration of this was demonstrated by the use of 71 and 73 that upon XAT activation underwent 5-exo-trig cyclization onto the tethered olefin followed by Cu-catalyzed arylation (i.e., I) to give 72 and 74 in useful yields.
Conclusions
In conclusion, the results reported here demonstrate that α-aminoalkyl radical mediated halogen-atom transfer can be integrated with copper catalysis to enable the modular assembly of C–C bonds. This reactivity provides a mechanistically distinct tactic to engage alkyl iodides in general cross-coupling reactions with aryl, vinyl, alkynyl, benzyl, and allyl organborons. Future developments will be aimed at engaging unactivated alkyl organometallic partners as well as translating the chemistry into asymmetric settings.
Acknowledgments
D.L. thanks the EPSRC for a Fellowship (EP/P004997/1), the European Research Council for a research grant (758427), and the Leverhulme Trust for an Award (Philip Leverhulme). Z.Z. thanks the Marie Curie Actions for a Fellowship (840318).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c12649.
Experimental procedures, optimization and mechanistic studies, and characterization data (PDF)
Author Contributions
† Z.Z. and B.G. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Suzuki A. Cross-Coupling Reactions Of Organoboranes: An Easy Way To Construct C–C Bonds (Nobel Lecture). Angew. Chem., Int. Ed. 2011, 50 (30), 6722–6737. 10.1002/anie.201101379. [DOI] [PubMed] [Google Scholar]; b Blakemore D., Chapter 1 Suzuki–Miyaura Coupling. In Synthetic Methods in Drug Discovery; The Royal Society of Chemistry: 2016; Vol. 1, pp 1–69. [Google Scholar]
- Brown D. G.; Boström J. Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone?. J. Med. Chem. 2016, 59 (10), 4443–4458. 10.1021/acs.jmedchem.5b01409. [DOI] [PubMed] [Google Scholar]
- a Campeau L.-C.; Hazari N. Cross-Coupling and Related Reactions: Connecting Past Success to the Development of New Reactions for the Future. Organometallics 2019, 38 (1), 3–35. 10.1021/acs.organomet.8b00720. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kambe N.; Iwasaki T.; Terao J. Pd-catalyzed cross-coupling reactions of alkyl halides. Chem. Soc. Rev. 2011, 40 (10), 4937–4947. 10.1039/c1cs15129k. [DOI] [PubMed] [Google Scholar]
- a Perez Garcia P. M.; Di Franco T.; Epenoy A.; Scopelliti R.; Hu X. From Dimethylamine to Pyrrolidine: The Development of an Improved Nickel Pincer Complex for Cross-Coupling of Nonactivated Secondary Alkyl Halides. ACS Catal. 2016, 6 (1), 258–261. 10.1021/acscatal.5b02324. [DOI] [Google Scholar]; b González-Bobes F.; Fu G. C. Amino Alcohols as Ligands for Nickel-Catalyzed Suzuki Reactions of Unactivated Alkyl Halides, Including Secondary Alkyl Chlorides, with Arylboronic Acids. J. Am. Chem. Soc. 2006, 128 (16), 5360–5361. 10.1021/ja0613761. [DOI] [PubMed] [Google Scholar]; c Zhou J.; Fu G. C. Suzuki Cross-Couplings of Unactivated Secondary Alkyl Bromides and Iodides. J. Am. Chem. Soc. 2004, 126 (5), 1340–1341. 10.1021/ja039889k. [DOI] [PubMed] [Google Scholar]
- a Ludwig J. R.; Simmons E. M.; Wisniewski S. R.; Chirik P. J. Cobalt-Catalyzed C(sp2)–C(sp3) Suzuki–Miyaura Cross Coupling. Org. Lett. 2021, 23 (3), 625–630. 10.1021/acs.orglett.0c02934. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Crockett M. P.; Wong A. S.; Li B.; Byers J. A. Rational Design of an Iron-Based Catalyst for Suzuki–Miyaura Cross-Couplings Involving Heteroaromatic Boronic Esters and Tertiary Alkyl Electrophiles. Angew. Chem., Int. Ed. 2020, 59 (13), 5392–5397. 10.1002/anie.201914315. [DOI] [PubMed] [Google Scholar]; c Goetzke F. W.; Mortimore M.; Fletcher S. P. Enantio- and Diastereoselective Suzuki–Miyaura Coupling with Racemic Bicycles. Angew. Chem., Int. Ed. 2019, 58 (35), 12128–12132. 10.1002/anie.201906478. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Daifuku S. L.; Kneebone J. L.; Snyder B. E. R.; Neidig M. L. Iron(II) Active Species in Iron–Bisphosphine Catalyzed Kumada and Suzuki–Miyaura Cross-Couplings of Phenyl Nucleophiles and Secondary Alkyl Halides. J. Am. Chem. Soc. 2015, 137 (35), 11432–11444. 10.1021/jacs.5b06648. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Bedford R. B.; Brenner P. B.; Carter E.; Clifton J.; Cogswell P. M.; Gower N. J.; Haddow M. F.; Harvey J. N.; Kehl J. A.; Murphy D. M.; Neeve E. C.; Neidig M. L.; Nunn J.; Snyder B. E. R.; Taylor J. Iron Phosphine Catalyzed Cross-Coupling of Tetraorganoborates and Related Group 13 Nucleophiles with Alkyl Halides. Organometallics 2014, 33 (20), 5767–5780. 10.1021/om500518r. [DOI] [Google Scholar]; f Hatakeyama T.; Hashimoto T.; Kondo Y.; Fujiwara Y.; Seike H.; Takaya H.; Tamada Y.; Ono T.; Nakamura M. Iron-Catalyzed Suzuki–Miyaura Coupling of Alkyl Halides. J. Am. Chem. Soc. 2010, 132 (31), 10674–10676. 10.1021/ja103973a. [DOI] [PubMed] [Google Scholar]
- a Uehling M. R.; King R. P.; Krska S. W.; Cernak T.; Buchwald S. L. Pharmaceutical diversification via palladium oxidative addition complexes. Science 2019, 363, 405–408. 10.1126/science.aac6153. [DOI] [PubMed] [Google Scholar]; b Buitrago Santanilla A.; Regalado E. L.; Pereira T.; Shevlin M.; Bateman K.; Campeau L.-C.; Schneeweis J.; Berritt S.; Shi Z.-C.; Nantermet P.; Liu Y.; Helmy R.; Welch C. J.; Vachal P.; Davies I. W.; Cernak T.; Dreher S. D. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules. Science 2015, 347, 49–53. 10.1126/science.1259203. [DOI] [PubMed] [Google Scholar]
- a Lovering F.; Bikker J.; Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52 (21), 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]; b Beutner G. L.; Simmons E. M.; Ayers S.; Bemis C. Y.; Goldfogel M. J.; Joe C. L.; Marshall J.; Wisniewski S. R. A Process Chemistry Benchmark for sp2–sp3 Cross Couplings. J. Org. Chem. 2021, 86 (15), 10380–10396. 10.1021/acs.joc.1c01073. [DOI] [PubMed] [Google Scholar]; c Campbell P. S.; Jamieson C.; Simpson I.; Watson A. J. B. Practical synthesis of pharmaceutically relevant molecules enriched in sp3 character. Chem. Commun. 2018, 54 (1), 46–49. 10.1039/C7CC08670A. [DOI] [PubMed] [Google Scholar]; d Duncton M. A. J.; Estiarte M. A.; Tan D.; Kaub C.; O’Mahony D. J. R.; Johnson R. J.; Cox M.; Edwards W. T.; Wan M.; Kincaid J.; Kelly M. G. Preparation of Aryloxetanes and Arylazetidines by Use of an Alkyl–Aryl Suzuki Coupling. Org. Lett. 2008, 10 (15), 3259–3262. 10.1021/ol8011327. [DOI] [PubMed] [Google Scholar]
- a Campos K. R.; Coleman P. J.; Alvarez J. C.; Dreher S. D.; Garbaccio R. M.; Terrett N. K.; Tillyer R. D.; Truppo M. D.; Parmee E. R. The importance of synthetic chemistry in the pharmaceutical industry. Science 2019, 363, 244. 10.1126/science.aat0805. [DOI] [PubMed] [Google Scholar]; b Blakemore D. C.; Castro L.; Churcher I.; Rees D. C.; Thomas A. W.; Wilson D. M.; Wood A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018, 10, 383–394. 10.1038/s41557-018-0021-z. [DOI] [PubMed] [Google Scholar]; c Wild H.; Heimbach D.; Huwe C. Editorial: The Importance of Chemistry for the Future of the Pharma Industry. Angew. Chem., Int. Ed. 2011, 50 (33), 7452–7453. 10.1002/anie.201103888. [DOI] [PubMed] [Google Scholar]
- Hu X. Nickel-catalyzed cross coupling of non-activated alkyl halides: a mechanistic perspective. Chem. Sci. 2011, 2 (10), 1867–1886. 10.1039/c1sc00368b. [DOI] [Google Scholar]
- Torres G. M.; Liu Y.; Arndtsen B. A. A dual light-driven palladium catalyst: Breaking the barriers in carbonylation reactions. Science 2020, 368 (6488), 318–323. 10.1126/science.aba5901. [DOI] [PubMed] [Google Scholar]
- a Zhu X.; Chiba S. Copper-catalyzed oxidative carbon–heteroatom bond formation: a recent update. Chem. Soc. Rev. 2016, 45 (16), 4504–4523. 10.1039/C5CS00882D. [DOI] [PubMed] [Google Scholar]; b Jiang S.-P.; Dong X.-Y.; Gu Q.-S.; Ye L.; Li Z.-L.; Liu X.-Y. Copper-Catalyzed Enantioconvergent Radical Suzuki–Miyaura C(sp3)–C(sp2) Cross-Coupling. J. Am. Chem. Soc. 2020, 142 (46), 19652–19659. 10.1021/jacs.0c09125. [DOI] [PubMed] [Google Scholar]; c Nakamura K.; Hara R.; Sunada Y.; Nishikata T. Radical-Organometallic Hybrid Reaction System Enabling Couplings between Tertiary-Alkyl Groups and 1-Alkenyl Groups. ACS Catal. 2018, 8 (8), 6791–6795. 10.1021/acscatal.8b01572. [DOI] [Google Scholar]
- a Constantin T.; Zanini M.; Regni A.; Sheikh N. S.; Julia F.; Leonori D. Aminoalkyl radicals as halogen-atom transfer agents for activation of alkyl and aryl halides. Science 2020, 367 (6481), 1021–1026. 10.1126/science.aba2419. [DOI] [PubMed] [Google Scholar]; b Neff R. K.; Su Y. L.; Liu S.; Rosado M.; Zhang X.; Doyle M. P. Generation of Halomethyl Radicals by Halogen Atom Abstraction and Their Addition Reactions with Alkenes. J. Am. Chem. Soc. 2019, 141 (42), 16643–16650. 10.1021/jacs.9b05921. [DOI] [PubMed] [Google Scholar]
- a Górski B.; Barthelemy A.-L.; Douglas J. J.; Juliá F.; Leonori D. Copper-catalysed amination of alkyl iodides enabled by halogen-atom transfer. Nat. Catal. 2021, 4 (7), 623–630. 10.1038/s41929-021-00652-8. [DOI] [Google Scholar]; b Su Y.-L.; Tram L.; Wherritt D.; Arman H.; Griffith W. P.; Doyle M. P. α-Amino Radical-Mediated Diverse Difunctionalization of Alkenes: Construction of C–C, C–N, and C–S Bonds. ACS Catal. 2020, 10 (22), 13682–13687. 10.1021/acscatal.0c04243. [DOI] [Google Scholar]
- a West M. J.; Fyfe J. W. B.; Vantourout J. C.; Watson A. J. B. Mechanistic Development and Recent Applications of the Chan–Lam Amination. Chem. Rev. 2019, 119, 12491–12523. 10.1021/acs.chemrev.9b00491. [DOI] [PubMed] [Google Scholar]; b Fyfe J. W. B.; Watson A. J. B. Recent Developments in Organoboron Chemistry: Old Dogs, New Tricks. Chem. 2017, 3 (1), 31–55. 10.1016/j.chempr.2017.05.008. [DOI] [Google Scholar]; c Thapa S.; Shrestha B.; Gurung S. K.; Giri R. Copper-catalysed cross-coupling: an untapped potential. Org. Biomol. Chem. 2015, 13 (17), 4816–4827. 10.1039/C5OB00200A. [DOI] [PubMed] [Google Scholar]
- a Fontana F.; Kolt R. J.; Huang Y.; Wayner D. D. M. Organic Reducing Agents: Some Radical Chain Reactions of Ketyl and 1,3-Dioxolanyl Radicals with Activated Bromides. J. Org. Chem. 1994, 59 (16), 4671–4676. 10.1021/jo00095a050. [DOI] [Google Scholar]; b Lalevée J.; Allonas X.; Fouassier J.-P. N–H and α(C–H) Bond Dissociation Enthalpies of Aliphatic Amines. J. Am. Chem. Soc. 2002, 124 (32), 9613–9621. 10.1021/ja0204168. [DOI] [PubMed] [Google Scholar]
- Kochi J. K.; Subramanian R. V. Kinetics of Electron-Transfer Oxidation of Alkyl Radicals by Copper(II) Complexes. J. Am. Chem. Soc. 1965, 87 (21), 4855–4866. 10.1021/ja00949a033. [DOI] [Google Scholar]
- Casitas A.; Ribas X. The role of organometallic copper(iii) complexes in homogeneous catalysis. Chem. Sci. 2013, 4 (6), 2301–2318. 10.1039/c3sc21818j. [DOI] [Google Scholar]
- See the Supporting Information for more information.
- Wiese S.; Badiei Y. M.; Gephart R. T.; Mossin S.; Varonka M. S.; Melzer M. M.; Meyer K.; Cundari T. R.; Warren T. H. Catalytic C–H Amination with Unactivated Amines through Copper(II) Amides. Angew. Chem., Int. Ed. 2010, 49 (47), 8850–8855. 10.1002/anie.201003676. [DOI] [PubMed] [Google Scholar]
- Griller D.; Howard J. A.; Marriott P. R.; Scaiano J. C. Absolute rate constants for the reactions of tert-butoxyl, tert-butylperoxyl, and benzophenone triplet with amines: the importance of a stereoelectronic effect. J. Am. Chem. Soc. 1981, 103 (3), 619–623. 10.1021/ja00393a020. [DOI] [Google Scholar]
- Taylor R. D.; MacCoss M.; Lawson A. D. G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- Russell R. W.; Barker T. J. Transition Metal-Free sp3–sp3 Carbon-Carbon Coupling between Benzylboronic Esters and Alkyl Bromides. Eur. J. Org. Chem. 2021, 2021 (19), 2782–2784. 10.1002/ejoc.202100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagman A. S.; Johnson R. J.; Zaharia C. A.; Cai H.; Lily W.; Duke G.; Ohoi Y.; Heuer T.; O’Farrell M.. Heterocyclic modulators of lipid synthesis for use against cancer and viral infections. WO 2015/105860 A1, 2015.
- Porras De Francisco E.; Remuinan-Blanco M. J.; Bourotte M.; Deprez B.; Dequirez G.; Willand N.. Novel compounds. WO 2019/034702 A9, 2019.
- Song Z. J.; Qi J.; Emmert M. H.; Wang J.; Yang X.; Xiao D. Two Scalable Syntheses of 3-(Trifluoromethyl)cyclobutane-1-carboxylic Acid. Org. Process Res. Dev. 2021, 25 (1), 82–88. 10.1021/acs.oprd.0c00422. [DOI] [Google Scholar]
- Salamone M.; Galeotti M.; Romero-Montalvo E.; van Santen J. A.; Groff B. D.; Mayer J. M.; DiLabio G. A.; Bietti M. Bimodal Evans–Polanyi Relationships in Hydrogen Atom Transfer from C(sp3)–H Bonds to the Cumyloxyl Radical. A Combined Time-Resolved Kinetic and Computational Study. J. Am. Chem. Soc. 2021, 143 (30), 11759–11776. 10.1021/jacs.1c05566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira E. M.; Fessard T. C. Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114, 8257. 10.1021/cr500127b. [DOI] [PubMed] [Google Scholar]
- Guillemont J. E. G.; Motte M. M. S.; Raboisson P. J.-M. B.; Tahri A., Antibacterial compounds. WO 2017/001660 A1, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.