Abstract
Best practices in preclinical algesiometry (pain behaviour testing) have shifted over the past decade as a result of technological advancements, the continued dearth of translational progress and the emphasis that funding institutions and journals have placed on rigour and reproducibility. Here we describe the changing trends in research methods by analysing the methods reported in preclinical pain publications from the past 40 years, with a focus on the last 5 years. We also discuss how the status quo may be hampering translational success. This discussion is centred on four fundamental decisions that apply to every pain behaviour experiment: choice of subject (model organism), choice of assay (pain-inducing injury), laboratory environment and choice of outcome measures. Finally, we discuss how human tissues, which are increasingly accessible, can be used to validate the translatability of targets and mechanisms identified in animal pain models.
Pain remains a leading global health care problem1. Analgesic drugs are one of the primary tools used in clinical management of both acute pain and chronic pain. However, many of the current options in the pharmacological toolbox are riddled with unwanted side effects, high misuse potential and limited efficacy2. In recent years, many analgesic candidates directed against high-profile targets have failed in their translation to the clinic, with their failure routinely blamed on drug pharmacokinetics, clinical trial design or recruitment, on-target drug side effects or toxicity, and poor preclinical modelling2,3. With the last point in mind here, we review the status quo in preclinical pain modelling as revealed by our analysis of methods reported in articles recently published in the journal Pain, and discuss how current practices could be improved to increase translational success in our field.
Since the previous review of this topic by one of the current authors4, the only unambiguous pain drug development success story is that of calcitonin gene-related peptide (CGRP) antagonist use in migraine5. Although the anti-nerve growth factor drug tanezumab has shown efficacy in phase III clinical trials, it did not succeed into the clinic. As preclinical researchers, we might wish that improved animal models were a primary reason for the translational success of CGRP antagonists, but this is not the case. CGRP was originally identified as a key molecule in migraine pathology more than 30 years ago when elevated CGRP levels were measured in blood samples from patients with migraine6. It was later shown that injection of CGRP triggers headache in patients7. Parallel preclinical assessments of CGRP’s causative role in migraine were originally not as conclusive, as application of CGRP to rodent meningeal afferents, the peripheral nerve fibres presumed to underlie noxious sensory transmission in migraine, had very little effect on neuronal activity8, and the behavioural effects of CGRP administration varied widely across preclinical studies (reviewed in REF.9). Notably, these initial animal studies were performed exclusively in male rats; experimenters have only recently repeated these behavioural assessments in female mice and found that extremely low doses of CGRP induced robust migraine behaviours in them10. This case study is an example of how more appropriate animal modelling of clinical conditions (in this case, using female rodents to model a condition that predominantly affects women) could have potentially accelerated the identification, validation and implementation of a new pain target.
We believe that animal models can provide unparalleled mechanistic insight into complex biological phenomena, such as chronic pain, and should continue to be used in pain research in combination with human tissues. In this Review, we describe the decisions regarding model organism (subject), injury model (assay), testing environment and pain measurement that must be made before beginning a preclinical algesiometric experiment. The status quo of each of these factors is presented and compared with observations made in clinical patient populations with pain. Recognizing that human chronic pain is a complex, heterogeneous disease state, we end this Review by providing recommendations to assist in the design, execution and analysis of preclinical pain behavioural testing, so as to increase the translatability of every experiment.
Choice of subject (model organism)
When assessed in a 2009 review4, young adult male Sprague Dawley rats were the most commonly used model organism in preclinical pain research, although the typical patient with chronic pain was (and still is) a middle-aged woman11. The 2009 review also revealed that mice were being increasingly used in pain research4, a trend that began in the mid-1990s and was accelerated by the ever-expanding list of technologies that require the use of transgenic animals, such as optogenetics and chemogenetics. In our current analysis, we examined primary research articles published in the journal Pain between 2016 and 2020 (see Supplementary methods and Supplementary data 1 for details of the analysis) and found that in the past 5 years, the prevalence of mice and rats used in preclinical pain studies has been relatively similar; each species was used in approximately 50% of studies (FIG. 1a). Larger mammals, including dogs and pigs, are very rarely used in preclinical pain research, likely due to the size, cost and maintenance associated with these animals. Although increased use of large mammals was advocated for previously12,13, rodents are used far more frequently in preclinical pain research, and therefore will be the focus of this Review.
Fig. 1 |. Analysis of rodent use in pain studies from 1980 to 2020.
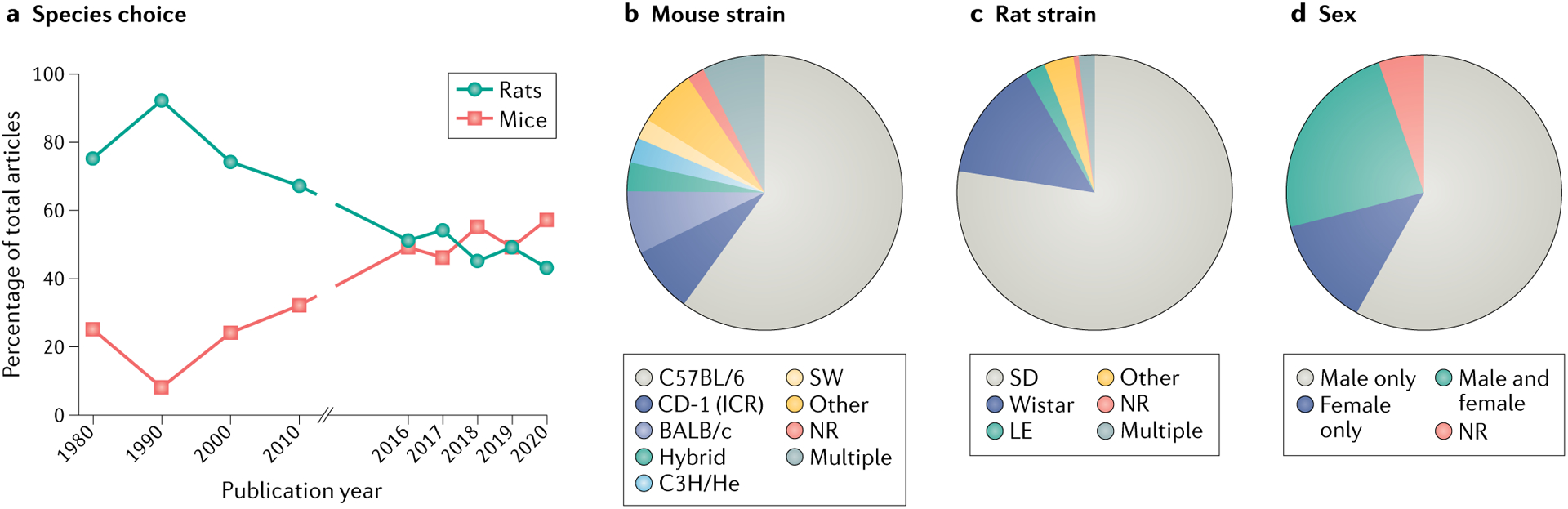
The methods and results sections of preclinical reports published in the journal Pain in the years 1980, 1990, 2000, 2010 and 2016–2020 (506 articles) were coded for a variety of terms, including ‘animal species’, ‘sex’ and ‘rodent strain’. Pain was selected as the representative journal for this analysis; however, it is recognized that the restriction of the analysis to a single journal may have implications for the generalizability of the findings to the wider pain literature. Inclusion was further restricted to studies that used inducible pain models and measured behaviours in awake non-human animals. Detailed coding methods and complete datasets are included in Supplementary methods and Supplementary data 1. a | Percentage of all studies published in Pain in the years listed that used rats or mice as the primary model organism. Between 2016 and 2020, only 4 of 320 studies analysed used non-rodent animals; dogs were used in 3 studies and pigs were used in 1 study. b | Mouse strain use in studies published in Pain between 2016 and 2020. The chart illustrates the percentage of mouse studies using each strain. c | Rat strain use in studies published in Pain between 2016 and 2020. The chart illustrates the percentage of rat studies using each strain. d | Use of male and female organisms in studies published in Pain studies between 2016 and 2020. The chart illustrates the percentage of all studies using male, female, or male and female organisms. LE, Long–Evans; NR, not reported; SD, Sprague Dawley; SW, Swiss Webster.
Until we have a more comprehensive understanding of the critical gene expression and electrophysiological differences between human tissues and mouse or rat tissues (see BOX 1), behavioural and anatomical differences will likely dictate whether mice or rats are used in a given experiment. Notable benefits exist for both species. Rats require shorter behaviour habituation periods and, although we are unaware of a research article directly comparing them with mice, rats are anecdotally easier to train in conditioning tasks. Similarly, although a direct species comparison has not been reported to our knowledge, rats are also believed to experience less experimenter-induced and procedure-induced stress than mice; for example, the Randall–Selitto deep pressure test requires extended periods of restraint, and therefore is almost exclusively performed in rats. The larger size of rats makes surgical injuries (see later) easier to perform. Alternatively, the smaller size of mice makes them more cost-efficient and space-efficient to house, greatly reduces the amount of drug required for pharmacological experiments and makes it easier to deliver light to or perform imaging in deep neural structures that are critical sites of pain modulation. The utility of mice is further increased by the number of transgenic lines that are commercially available and the fact that mouse tissues have been used more frequently than rat tissues in comparisons with human gene expression14.
Box 1 |. Which rodent (mouse or rat) is better for pain research?
Our analysis of articles published in the journal Pain between 1980 and 2020 shows that in the last decade the popularity of rats in pain research has markedly declined (FIG. 1a); rats were used in 67% of studies published in Pain in 2010, but in only 43% of studies published in 2020. Increased use of mice is completely responsible for this shift; mice were used in only 32% of studies published in Pain in 2010 but in almost twice as many studies (57% of publications) published in 2020. With the ultimate goal of clinical success in mind, this change begs the question of which rodent is better suited for modelling human pain. In the past decade, technical advances and increased affordability of gene expression technologies have allowed comprehensive comparisons of rodent and human tissues (reviewed in REF.14). Using these tools, multiple laboratories have demonstrated that classifiers traditionally used to delineate populations of sensory neurons in the mouse are not applicable in humans192–194. Expression differences in key therapeutic targets have also been observed between mice and humans194. These gene-level and population-level expression differences between species may be responsible for some of the unexpected side effects and limited analgesic efficacy of select targets in human clinical trials. Notably, rat tissue is absent from many of these genetic examinations but is routinely used in cross-species functional assessments. Direct comparisons of basic action potential properties193,195,196 and individual ion channel and G protein activities197–201 have been made between rodent and human dorsal root ganglion neurons and spinal cord202, but very rarely between rodent species. Exceptions to this include comparisons of peptidergic neuronal marker expression in dorsal root and trigeminal ganglia of both rodents203, comparisons of sciatic nerve anatomy in both rodents204, comparisons of cold responses in epidermal keratinocytes from both rodents and humans205, meta-analyses of chronic pain-related gene expression changes in both rodents206 and a small number of injury model and analgesic cross-species comparisons75,207–209. The magnitude of genetic and functional differences between human, rat and mouse tissues is currently unclear due to a lack of tri-species comparative analyses; before a definitive recommendation of which rodent tissue better models human tissue in vitro is provided, detailed gene expression analyses of rat tissue and tri-species electrophysiological experiments should be conducted.
Habituation periods.
Time spent in the testing room/apparatus before commencement of testing procedures.
Randall-Selitto deep pressure test.
A behaviour test that measures deep tissue mechanical sensitivity via the application of calipers or a weight to a part of the animal’s body (generally the hind paw of a rat).
In addition to species, rodent strain has significant effects on both baseline and injury-induced pain behaviours. In our analysis, less than 10% of mouse studies published in the journal Pain between 2016 and 2020 used outbred mice (such as the CD-1 strain; FIG. 1b). This is in stark contrast to rat studies published in Pain, of which more than 80% used outbred strains (mostly Sprague Dawley; FIG. 1c). C57BL/6 inbred mice were used in more than half of the studies published in Pain between 2016 and 2020, despite being a phenotypically outlying strain15,16; compared to other inbred strains, C57BL/6 animals exhibit heightened sensitivity to thermal stimuli at the baseline and resilience to injury-induced hypersensitivity15. One reason for continued use of C57BL/6 and other inbred mouse strains is the expectation of reduced response variability, and subsequently smaller required sample sizes. However, this argument was recently negated by a meta-analysis of biomedical studies in which inbred and outbred mice were directly compared; no differences in phenotypic variability were observed17. The argument that reduced variability in an animal model is desirable is also antithetical to the idea of modelling chronic pain; wide phenotypic variability is observed in patients with chronic pain receiving identical disease diagnoses or undergoing standardized pain tests. Therefore, pain in patient populations may be better modelled by outbred strains because of their genetic heterogeneity and phenotypic stability.
Outbred strains.
Strains in which direct brother–sister mating is avoided to minimize inbreeding.
In our analysis of research articles published in Pain from 2016 to 2020, approximately 24% of preclinical pain researchers incorporated both male and female subjects in their studies (FIG. 1d). More detailed, temporal analyses regarding the use of both sexes in pain research were described in a recent review of this topic18. Inclusion of both sexes follows mandates from major research funding agencies (including the US National Institutes of Health, effective as of 2016 (REF.19)), and has resulted in reports of robust, and often qualitative, sex differences in neural and immune system pain mechanisms18. This is an extremely important development in the pain field, as female subjects were, for many years, rarely included in preclinical chronic pain studies despite the fact that women disproportionately receive a diagnosis of chronic pain, a phenomenon that is perhaps due in part to influences of sex and gender on clinical pain reporting20.
Experiences of acute and chronic pain vary greatly across the lifespan. In humans, the prevalence of chronic pain typically increases with age11. However, for certain disorders, such as migraine, pain prevalence declines later in life21. Although the average life expectancy of captive rodents is approximately 1/37 of the average human life expectancy, age-related effects on nociceptive processing have been grossly understudied by preclinical pain scientists22. Our analysis of articles published in Pain between 2016 and 2020 suggests that currently, most preclinical pain research is performed in rodents aged 2–3 months (Supplementary Fig. 1), an age range that roughly coincides to human years 15–20 (REF.23). The rare use of aged animals is likely due to the steep financial costs associated with housing animals for an extended period or purchasing aged animals; at the time of writing, 22-month-old C57BL/6 mice cost approximately 14 times as much as 3-month-old C57BL/6 mice on The Jackson Laboratory’s website. Notably, middle-aged and old-aged transgenic mice are often not commercially available. When aged animals are studied, one factor that should be considered is the homogeneous life history of captive-bred animals. The intentional standardization of laboratory rodent life history fails to appropriately model the complex life history of patients with chronic pain, and may contribute to the limited translational success in our field. In rodent pain models, for example, prior injury (even if seemingly resolved) often affects the susceptibility to and intensity of pain that results from subsequent injury (reviewed in REF.24). This priming effect is not exclusive to noxious tissue damage as early life stress can have similar effects on subsequent pain responses25–29. Therefore, our current approach of studying naive, young animals may skew therapeutic development towards acute pain targets but poorly model the complex, cumulative life history of patients with chronic pain.
In summary, our analysis suggests that young, naive, inbred C57BL/6 male mice and young, naive, outbred Sprague Dawley male rats are the current status quo for preclinical pain experimental subject selection. To increase the translatability of preclinical pain experiments, we provide the following recommendation regarding model organism selection: increase the heterogeneity of animal subjects to more accurately model the heterogeneity observed in patient populations with chronic pain. Although nociception is a critical biological process conserved in all animals, we are just beginning to understand the biology that allows variable perception and expression of nociceptive behaviours across species, sexes and an individual animal’s lifespan. At this time, mice and rats appear to offer similar levels of homology to humans with respect to modelling pain sensation and perception, and because of their predominance in the preclinical space for the last 50 years or more, rodent research infrastructure far exceeds that which is available for larger mammals. Therefore, we support continued use of (albeit not exclusive reliance on) both mice and rats in pain research, but we encourage mouse laboratories to consider using outbred strains and we argue for the inclusion of older animals and animals of both sexes, unless explicitly justified.
Choice of assay (injury model)
One of the leading concerns regarding animal pain models is whether the injury models (or assays) accurately mirror the temporal, anatomical and pathophysiological characteristics of patient phenotypes. According to our analysis of articles published in the journal Pain between 1980 and 2020, experimentally induced nerve injuries that result in neuropathic pain are currently the most popular method for inducing pain in rodents; one or more such injuries were used in 42% of all preclinical studies published in Pain in the last 5 years (FIG. 2a; Supplementary data 1). However, recent reviews estimate that only approximately 10% of human pain conditions have neuropathic qualities30,31. Although nervous system damage is difficult to assess in chronic musculoskeletal, cancer or nociplastic conditions, the current preclinical status quo appears to disproportionately favour neuropathic injury models relative to the prevalence of these conditions in patient populations. Our analysis shows that spinal nerve ligation32, spared nerve injury33 and chronic constriction injury34 were the three most commonly used mononeuropathic models (models in which damage is restricted to a single nerve) in articles published in Pain between 2016 and 2020 (FIG. 2b). These assays produce relatively localized pain in rodents, the intensity of which diminishes over time in the spinal nerve ligation and chronic constriction injury models, but curiously not in the spared nerve injury model32–34, at least in male mice35. Additionally, while these assays model causalgia (also known as chronic regional pain syndrome type II), it is unclear whether their mechanistic underpinnings recapitulate the most common types of human neuropathic pain, such as radiculopathy, postherpetic neuralgia, painful diabetic neuropathy and neuropathic low back pain. Thus, animal models likely oversimplify the spatial, temporal and biological complexity of human neuropathic pain conditions.
Fig. 2 |. Analysis of animal pain assays used from 1980 to 2020.
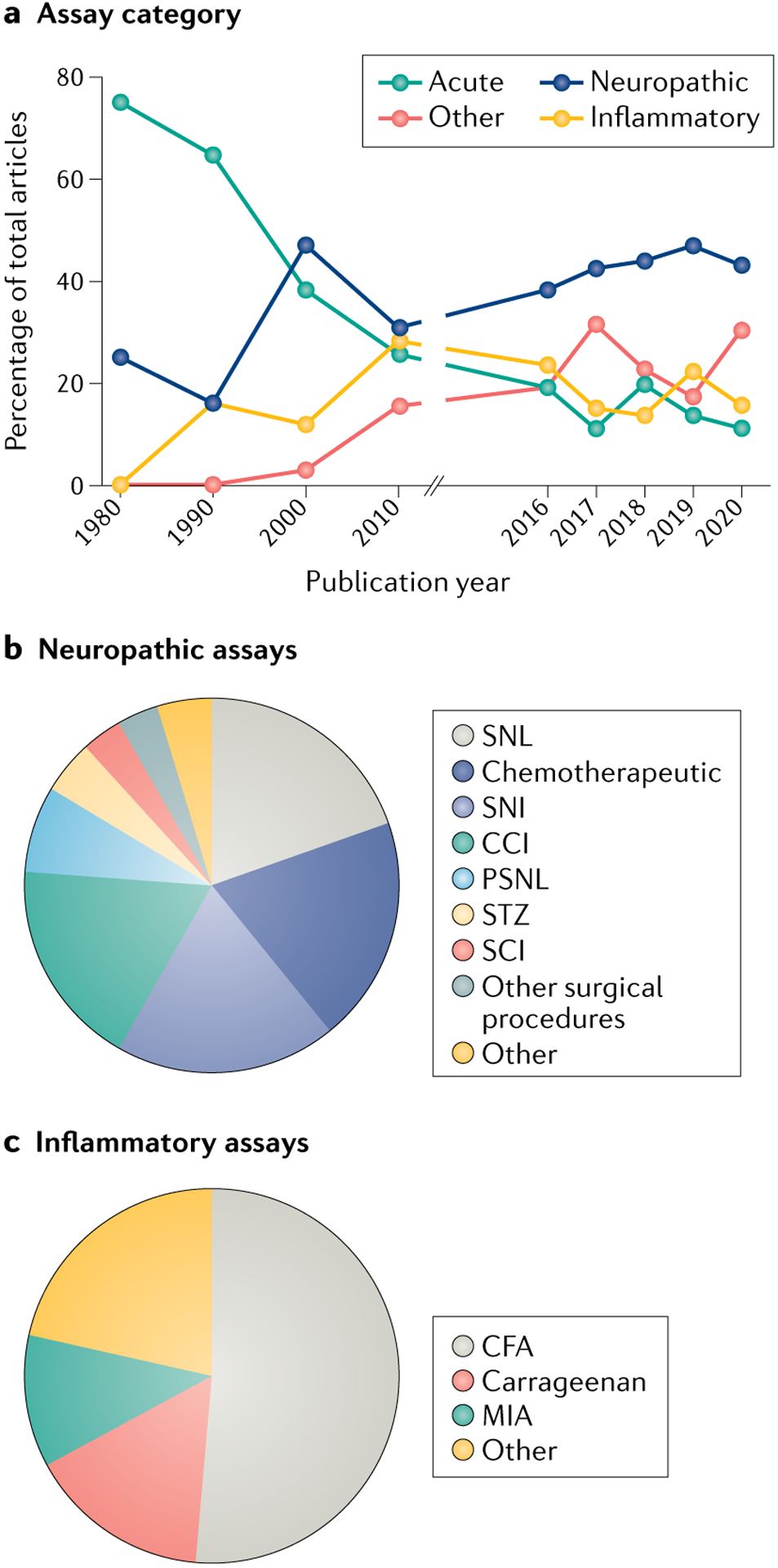
The methods and results sections of preclinical reports published in the journal Pain in the years 1980, 1990, 2000, 2010, and 2016–2020 (506 articles) were coded for a variety of terms, including ‘assay category’ and ‘injury model.’ Pain was selected as the representative journal for this analysis; however, it is recognized that the restriction of the analysis to a single journal may have implications for the generalizability of the findings to the wider pain literature. Assays were defined as belonging to one of 12 categories; detailed categorical definitions and complete datasets are included in Supplementary methods and Supplementary data 1. a | Percentage of all studies published in Pain in the years listed that included acute, neuropathic, inflammatory or one of the nine other remaining categories of assays. b | Comparison of different types of neuropathic assays used in studies published in Pain between 2016 and 2020. The chart illustrates the percentage of total studies using each assay. c | Comparison of different types of inflammatory assays used in studies published in Pain between 2016 and 2020. The chart illustrates the percentage of total studies using each assay. CCI, chronic constriction injury; CFA, complete Freund’s adjuvant; MIA, monosodium iodoacetate; PSNL, partial sciatic nerve ligation; SCI, spinal cord injury; SNI, spared nerve injury; SNL, spinal nerve ligation; STZ, streptozotocin.
Neuropathic pain.
Pain caused by a lesion or disease of the somatosensory nervous system.
Our analysis suggests that another popular means of inducing neuropathic injury is through use of chemotherapeutic drugs (reviewed in REF.36). Chemotherapy-induced peripheral neuropathy (CIPN) models have high face validity, because the compounds used in animals are the same as those that induce the dose-limiting side effect of (sometimes painful) neuropathy in patients with cancer. However, the vast majority of CIPN rodent studies are performed in cancer-free animals. The presence of a tumour, the induction of which is routinely modelled by those interested in cancer pain (reviewed in REF.37), should be considered when one is attempting to more accurately model CIPN pain.
Chemotherapy-induced peripheral neuropathy (CIPN) assays.
Neuropathic pain assays in which nerve damage is caused by the administration of chemotherapeutic drugs.
Face validity.
A subjective assessment of whether an assay replicates the patient population that it is attempting to model.
Inflammatory injuries are induced in rodents by introducing a chemical inflammogen or by mechanically or thermally damaging tissue. Our analysis of articles published in Pain between 2016 and 2020 suggests that the most widely used inflammatory assay in this period involves intraplantar injection of complete Freund’s adjuvant (CFA) (FIG. 2c). Two of the primary advantages of this method are the ease with which the injury can be induced and the concentration-dependent, extended period of hypersensitivity that follows injection22,38. As discussed later, the temporal duration of high-dose CFA pain may position CFA as one of the few inflammatory assays that actually allow investigation of the ‘chronic’ phase of pain. The primary concern with CFA use, however, is the clinical relevance of the associated injury. Accidental laboratory exposure39 notwithstanding, subcutaneous accumulation of dead bacteria is not a leading cause of chronic inflammatory pain in patients. Similar concerns exist with carrageenan40, a seaweed extract. The underlying immune and inflammatory processes in these models may or may not accurately reflect inflammatory mechanisms of pain in patients. To this end, the use of more-face-valid models of cutaneous inflammatory pain, such as the increasingly popular plantar incision model of post-operative pain41–43, in which both skin and muscle are incised, is recommended.
Many specific disease states are also modelled using direct infusion of inflammatory compounds into a target tissue or mechanical tissue damage that results in a robust immune response. Generic musculosketal pain is frequently induced by injecting CFA or carrageenan44 directly into a muscle or into a joint to model arthritis. More-face-valid osteoarthritis models, however, rely on either surgical manipulation of soft tissues in the joint45,46 or intra-articular injection of inflammatory compounds such as monosodium iodoacetate47. Similarly, gold standard rheumatoid arthritis models involve infusion of collagen into the joint to trigger an autoimmune response48; this response closely models that which is observed in patients with rheumatoid arthritis, making this a model with high face validity (reviewed in REF.49).
Musculosketal pain.
Pain affecting bones, joints, ligaments, tendons or muscles.
Osteoarthritis.
A form of arthritis involving the degeneration of joint cartilage and the underlying bone.
Rheumatoid arthritis.
A form of arthritis featuring inflammation in the joints and resulting in painful deformity and immobility.
Inflammatory approaches have also been used, albeit more controversially, to model low back pain and headache, the two leading causes of disability worldwide50. One common approach for inducing migraine in rodents is direct application of inflammatory agents onto the dura mater51,52. Other popular approaches include systemic administration of nitroglycerine53, a compound that also induces headache in patients, or triptans54, a class of migraine therapeutics that paradoxically induce headaches when overused. Similar to mononeuropathic assays, these models likely oversimplify the pathophysiology that leads to migraine in patients; experimental addition of stress paradigms or other common migraine triggers experienced by patients may increase the translational relevance of these models. In addition, assay-specific behavioural measures should be used for migraine studies to take into account the relatively unique set of symptoms experienced by patients: these could include photophobic, osmophobic and phonophobic behavioural tests.
Despite the obvious difference in bipedality versus quadrupedality between patients and rodents, rodent models are widely used to study low back pain. A number of inflammatory lumbar disc degeneration and soft tissue injuries have been developed for use in rodents (reviewed in REFS55,56). Clinically relevant outcomes such as decreased mobility or gait changes are not uniformly observed across these injuries. Therefore, a combination of different pathophysiological models or incorporation of additional behavioural measures should be considered. More successful modelling of low back pain in animals is one of the most pressing needs of the preclinical pain community given the high prevalence and associated disability in humans worldwide.
Most human chronic pain conditions do not adhere to the inflammatory versus neuropathic categorical dichotomy. A separate category of nociplastic pain conditions (previously called ‘functional pain disorders’, ‘dysfunctional pain’ or ‘idiopathic pain’) exists in humans and is defined as pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing activation of peripheral nociceptors or disease/lesion of the somatosensory nervous system57. While this term cannot be directly applied to any existing injury model in animals — by definition experimenters are inducing nociceptor activity or damage in the nervous system — many have attempted to model nociplastic conditions in rodents. Fibromyalgia is the most prevalent nociplastic clinical condition, affecting approximately 4% of women and 1% of men worldwide58. The most commonly used models of fibromyalgia59 employ various combinations of targeted muscle injections60,61 and fatigue62, reserpine-induced depletion of central monoaminergic signalling63 and a variety of stressors. A recently developed model that may have higher face validity is one in which antibodies are transferred from patients with fibromyalgia into naive rodents64.
Nociplastic pain.
Pain that arises form altered nociception despite no clear evidence of actual or threatened tissue damage.
Visceral nociplastic conditions, including irritable bowel syndrome, interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome, are another class of highly prevalent diseases65–67 that receive little preclinical attention; only 2% of studies published in Pain between 2016 and 2020 involved noxious stimuli directed to visceral organs (Supplementary data 1). Visceral hypersensitivity is most frequently induced through mechanical or chemical means. High fluid or air pressure can be used to distend any hollow organ to a level that is considered painful68,69; recordings of the reflexive abdominal contractions that accompany this distension (visceromotor responses) are commonly used visceral pain measurements in rodents70–72. Organ distension is frequently coupled with the instillation of a pro-inflammatory chemical or biological agent such as trinitrobenzenesulfonic acid73, dextran sulfate sodium74, cyclophosphamide75 or zymosan76, the combination of which often leads to prolonged visceral hypersensitivity.
Complex regional pain syndrome is another nociplastic condition that frequently develops following limb trauma77. Therefore, a rodent tibia fracture/cast model was developed78 that recapitulates many of the sex differences observed in patient populations with complex regional pain syndrome79. Additional assays of complex regional pain syndrome include chronic post-ischaemia pain80 and distal nerve injury81. Although all of these nociplastic models involve peripheral tissue hypersensitivity at one or more time points in the course of pain development, it is unclear how many are also associated with emotional distress or functional disability, two additional diagnostic criteria for chronic primary pain disorders listed in the most recent edition of the International Classification of Diseases (ICD-11)82. Since we do not understand the biological basis of many of these conditions, we deem our best nociplastic assays to be those that induce rodent behaviours that are similar to behaviours observed in patients. However, as described later, this anthropomorphization may be a leading reason for failed translational success.
In addition to the pathophysiological cause, injury models can be classified by their temporal duration. According to the International Association for the Study of Pain, chronic pain is defined as pain that persists or recurs for more than 3 months82. Only 3% of the preclinical pain studies considered in our analysis of articles published in Pain between 2016 and 2020 would meet this inclusion criterion; 86% of studies were terminated at 1 month or less following injury (Supplementary Fig. 2). Short data collection periods likely result from temporal, financial and external incentive (for example, publication or ‘scooping’) pressures, or the notion that biological processes happen on a more rapid timescale in rodents than in humans. Although it is true that rodent ageing happens more quickly than human ageing, it is unlikely that the same species-specific acceleration exists in pain-related processes. For instance, an 8-week-old mouse matures 45 times faster than a human at any point in their life23; with this time conversion, rodents would enter the chronic phase of pain only 2 days following injury. However, the basic biological processes associated with nociception do not happen this rapidly; injury-related immune cell recruitment, neuronal conduction velocity and synaptic transmission are not 45 times faster in rodents than in humans. And although wound healing and axon regeneration take less time in mice than in humans, this likely results from the smaller size or shorter distance over which these processes must occur, not from a fundamental difference in enzymatic time courses. Further complicating this issue is the fact that many acute pain processes occur on similar timescales in rodents and humans; capsaicin-induced pain occurs on similar timescales in humans83 and mice84, as does CFA-related mechanical allodynia22,39. Therefore, it is possible that the same temporal definition used for chronic pain in humans may also be appropriate for chronic pain in rodents. To the extent that this is true, very little extant preclinical research has actually studied chronic pain.
Mechanical allodynia.
Mechanical pain due to a stimulus that does not usually provoke pain.
Alternatively, the historical definition of chronic pain — that is, pain that persists past tissue healing85— could be used in rodent studies. However, concerns about this definition exist when considering pain in humans; residual tissue damage is difficult to assess in deep tissue or visceral injuries and many nociplastic conditions57, such as fibromyalgia, never present with obvious tissue damage. This criterion may be easier to assess in rodents; by definition, all injuries require some type of initial tissue insult and typical healing times could be assessed. However, as most of the injury models that we currently use have not been characterized in this manner, we are unsure how many models would meet this inclusion criterion.
In summary, our analysis suggests that intraplantar CFA injection and mononeuropathic surgical injury are the current status quo for inducing inflammatory and neuropathic pain, respectively, in rodents. These assays are highly reproducible between laboratories, but are relatively simplistic and not necessarily representative of the anatomical damage or underlying disease processes that occur in chronic inflammatory and neuropathic pain conditions in patients. To better model patient populations with chronic pain, we provide the following recommendation regarding pain assay selection: use multiple, face-valid, long-lasting pain assays in a given study. For example, if you are studying migraine, consider performing your initial experiments with the nitroglycerine assay and then confirm your results in a dural application priming model that has long-lasting behavioural effects. Regardless of outcome, these parallel assessments will provide insight into the generalizability of your findings; if a similar result is obtained in both assays, your manipulation may have broad applicability as a migraine therapy. Alternatively, if ‘positive’ results are obtained only in one assay, this may reveal important pathophysiology that distinguishes the injury models from one another. We also encourage further development of more valid nociplastic, low back pain (and other musculoskeletal) and headache models as these are the most prevalent, yet least studied, chronic pain conditions in patients. Lastly, researchers are encouraged to extend the temporal duration of their studies to better understand the mechanisms of chronic pain over a time course that better recapitulates that of chronic pain in patients.
Laboratory environmental factors
Whereas organism and assay selection are routinely described in pain research and widely recognized to affect behavioural results, variations in husbandry and testing environments are less frequently discussed as potential sources of behavioural outcome variability, despite their ability to impact results to a similar extent. In this section, we highlight important environmental variables (FIG. 3), most of which influence pain behaviours by modulating rodent stress systems. Stress can have opposing influences on nociceptive processing, sometimes manifesting itself as increases in pain (stress-induced hyperalgesia) and other times resulting in stress-induced analgesia (SIA) (reviewed in REFS86,87). The differential effects of stress are difficult to predict but may be related to the severity or predictability of a stressor or the length of stress exposure88,89. Unless the intention is to specifically add stress as an experimental variable, care should be taken to mitigate this factor to the greatest extent possible before and during behavioural testing.
Fig. 3 |. Environmental considerations for pain testing.
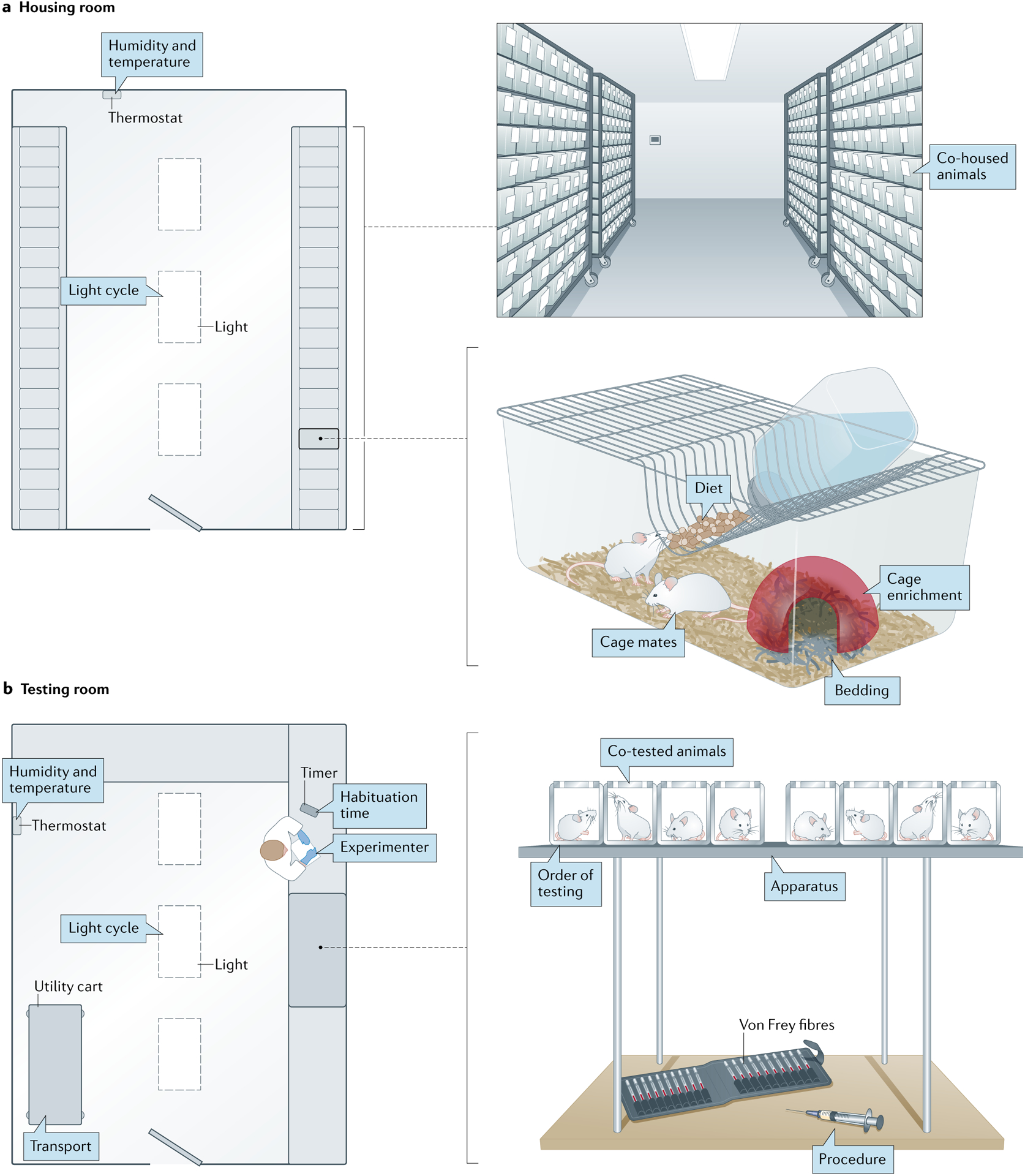
a | Factors in a standard vivarium rodent housing room that may affect pain behaviours. Animal behaviour can be influenced by room characteristics such as humidity, temperature and light cycle or factors such as the pain status of other animals housed nearby. Similarly, factors within the home cage can affect pain behaviours even when animals are tested in a different environment. b | Factors in a standard behaviour testing room that may affect pain measurements. As in the housing room, humidity, temperature, lighting and the presence of injured animals may affect test outcomes in a behaviour room. Stress induced by transport to the testing room, novelty of the testing apparatus, order of testing and accompanying procedures such as manual restraint or injection may also affect pain testing results. However, the presence, identity and experience level of an experimenter may be the largest factor that influences pain behaviours.
Stress-induced hyperalgesia.
Higher sensitivity to pain due to stress-induced activation of descending pain modulatory circuitry.
Stress-induced analgesia.
(SIA). Reduced sensitivity to pain due to stress-induced activation of descending pain modulatory circuitry.
Stress is encountered with essentially every environmental change made in the course of a behavioural experiment, beginning with the delivery of animals to the housing facility90. Our analysis of articles published in Pain between 2016 and 2020 shows that only 10% of mouse studies and 11% of rat studies published in Pain used animals bred in-house (Supplementary Figs. 3, 4). Thus, the vast majority of animals are likely to experience stress associated with commercial vendor delivery, the physiological effects of which typically normalize within 48 hours90. Various factors within an animal’s home cage also influence pain behaviours (FIG. 3a); cage bedding can alter mechanical sensitivity91,92, the addition of items intended to enrich the animals’ environment such as igloos or tunnels can decrease periods of hypersensitivity following injury93–95 and different dietary compositions can affect behaviour96. In addition to these inanimate factors, an animal’s cage mates (or lack thereof) have perhaps the largest impact on nociception. SIA has been repeatedly demonstrated in socially isolated rodents97–99. Group-housed animals’ pain can also be bidirectly modulated by their conspecifics. Naive rodents will not only groom conspecifics exhibiting injury-evoked pain behaviours100 but will also develop hypersensitivity themselves in the absence of injury100–103. Likewise, injured mice exhibit more pain in the presence of a similarly injured cage mate104, less pain when observing analgesic-mediated reductions in cage mate pain behaviours102 and less pain in the presence of an injured stranger rodent105,106. This stranger-evoked analgesia is thought to be mediated by canonical stress hormone signalling, whereas the social transfer of pain between cage mates is mediated through olfactory and visual cues104,107. Notably, ‘social’ pain transfer has also been reported between mice housed in separate cages in the same room107. While these studies did not incorporate standard filter cage tops107 (the point of the studies was to increase olfactory cue dispersion), these data and other data108 suggest that — at a minimum — naive and injured rodents will influence observable injury phenotypes in one another if housed in the same cage. To mitigate stress in the home-cage environment, we therefore suggest housing animals with at least one additional phenotypically similar conspecific (that is, an injured animal with another injured animal or a naive animal with another naive animal), adding one or more inanimate sources of environmental enrichment to the cage and maintaining consistent food, water and bedding suppliers throughout an experiment. One caveat to this approach is the increased risk of cage or cohort effects, or changes in behaviour induced by environmental similarity in groups of mice that are bred, housed and/or transported together. To mitigate this problem, we recommend ensuring that cages are randomized for experimental group assignment.
The reduction of all stress-inducing stimuli is most critical shortly before and during behavioural testing. We acknowledge that stress also influences clinical pain and its reporting in patients, and one could therefore argue that minimization of stress in rodent pain testing is unnecessary. However, the magnitude of stress experienced by rodents in these situations likely far exceeds that experienced by patients — imagine how you might feel if a human predator, such as a grizzly bear, approached you with a von Frey fibre. Ideally, behaviour testing should occur in a dedicated humidity-controlled, light-controlled and temperature-controlled room109 (FIG. 3b). Performing euthanasia, tissue collection and/or blood draw procedures in the same room as behavioural tests is also strongly discouraged, considering the stress response that these procedures evoke in rodent bystanders110,111. Transport from the housing room to the testing room, whether by cart112 or by hand113, temporarily elevates circulating levels of the stress hormone corticosterone, as does removal from the home cage and placement into the testing apparatus114. Thus, it is critical that rodents be given proper time to acclimate to the testing apparatus before the start of testing. On average, rats require significantly less habituation time than mice; rats habituate to a wire mesh-floored surface within 10–15 minutes115, whereas mice do not reach a similar behavioural state for approximately 2–4 hours depending on the strain116. Our analysis of articles published in Pain between 2016 and 2020 showed that habituation times were not reported in more than 50% of articles; however, in those that did report this variable, 30–59 minutes was the most common habituation time frame (Supplementary Fig. 5). While appropriate for rats, these times are likely too short for mice. Not only are animals not reaching a steady behavioural state116 but — if they are being tested by a male experimenter — they are also likely exhibiting SIA at the beginning of the testing period. Olfactory cues extruded by male experimenters initiate stress responses in male and female mice, thus reducing pain sensitivity for the first 60 minutes of experimenter exposure117. Circadian fluctuations have also been reported in both heat and mechanical sensitivity in naive rodents109,118,119, so care should be taken to initiate habituation periods and complete behaviour tests (especially those within a sequential experiment) at the same time each day. In our analysis, only 4% of preclinical Pain studies reported housing animals on reverse light cycles (Supplementary Fig. 6), and we therefore assume that the vast majority of laboratories perform behaviour testing during the light (resting) phase of the cycle.
Variables inherent to the process of behavioural testing can also affect outcomes. As described in the next section, the vast majority of pain studies use stimulus-evoked reflexive behaviours as their primary outcome. While stimulus intensity and application site are obvious factors that should be controlled for in such tests119, the identity of the experimenter is the most influential factor in reflexive behavioural outcomes120. Interexperimenter variability impacts reflexive behavioural test outcomes to a greater extent than genetic variability or other environmental factors120 and, therefore, experimenters should not change from animal to animal or from day to day in sequential studies. Experimenter skill level can also affect the extent of stress induced by manipulations that are routinely used during behaviour testing. Handling, manual restraint, subcutaneous injections and oral gavage all induce measurable stress responses in rodents, the magnitude of which positively correlates with the length of time required by these activities (reviewed in REF.121). Specific details of testing apparatuses should also be reported in publications as these details can impact behavioural outcomes. For example, apparatus flooring can affect mechanical withdrawal thresholds. Higher, more variable thresholds were observed when rats were repeatedly tested on wire mesh as opposed to a plastic surface115. Furthermore, repeated testing in the same environment can also influence behavioural outcomes; male mice exhibit context-dependent hypersensitivity when behavioural measures are recorded in an environment associated with prior injury122.
The presence of other animals and the order in which they are tested120 can also influence pain behavioural test results. Similar to the phenomenon observed in home cages, rodents can transmit pain behaviours during the short periods associated with behaviour testing through visual and olfactory cues; male mice exhibit more or less stimulus-evoked pain when watching responses to the same stimulus in a familiar or stranger mouse, respectively104–106. Lingering stress-related odours prompt SIA in rats123, but the effects of other odours (such as pheromones from opposite-sex animals) are unknown. While it is difficult to minimize olfactory cue transfer during behaviour testing because many animals are typically acclimated on the same platform, it is advised to limit visual access between animals to at least prevent this means of pain transfer between familiar animals.
In summary, the current status quo regarding housing and testing environments in preclinical pain studies is unclear because these variables are often omitted or described in very little detail in article methods sections. We recommend the following guiding principal when one is considering environmental changes in a preclinical pain experiment: unless you are purposefully inducing stress as part of a pain model, great care should be taken to mitigate sources of animal stress, particularly during behavioural testing. Appropriate, and often lengthy, acclimation periods are critical for stress response normalization following any manipulation of the animal or its environment.
Choice of measures
Significant difficulties arise when one is attempting to measure pain (a sensation that is predominantly measured in humans with verbal ratings and/or descriptors) in non-verbal rodents. For this reason, measures of pain-associated symptoms, such as hypersensitivity, are the primary outcome measures in most animal behaviour tests (FIG. 4a). Currently, the most frequently used rodent pain measures are reflexive responses to experimenter-delivered stimuli (TABLE 1). Our analysis of all measurements reported in preclinical Pain articles between 2016 and 2020 showed that 53% were mechanical in nature (FIG. 4b). This current reliance on mechanical measures is a notable change from the historical use of heat-evoked responses, and may be attributed to the relatively large dynamic range of mechanically evoked behavioural outcomes or to the parallel rise in popularity of neuropathic injuries (FIG. 2a): in patient populations, these types of injury are more typically associated with mechanical allodynia or cold hypersensitivity than with heat hypersensitivity124. Our analysis showed that mechanical sensitivity is most frequently measured by stimulating the glabrous (bottom) surface of the rodent hind paw with von Frey monofilaments (Supplementary Fig. 7). Many variations of filament application exist, but more than 50% of Pain studies from between 2016 and 2020 used the manual ‘up/down’ psychophysical approach125,126 in which only the presence or absence of a withdrawal response is measured (Supplementary Fig. 8). Electronic von Frey tools were used in only 16% of Pain studies during this period.
Fig. 4 |. Behavioural measures of pain and an analysis of their use in studies from 1980 to 2020.
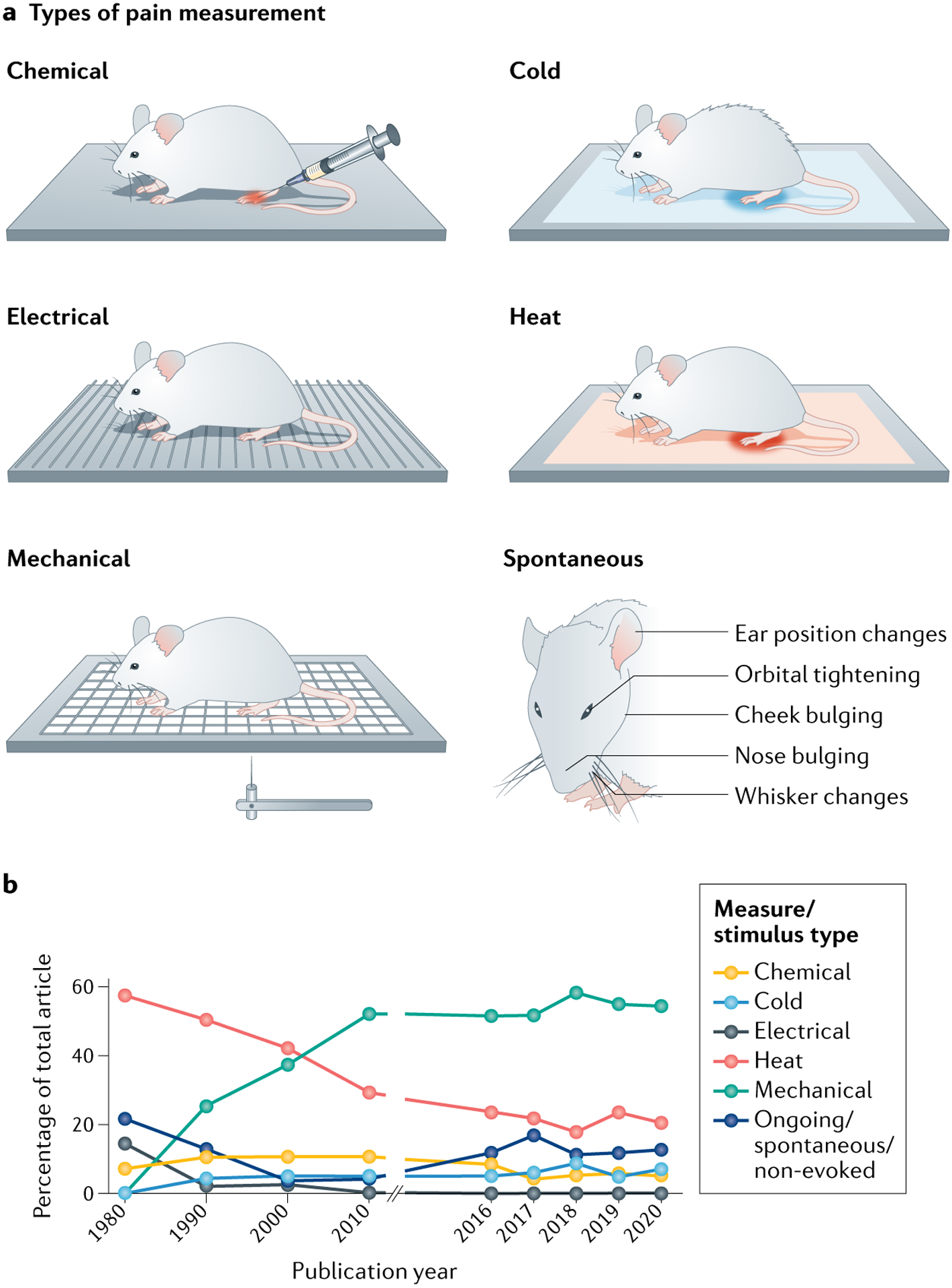
a | Depictions of frequently used pain measurements. Chemical measures typically consist of an animal tending to the part of its body into which an inflammatory agent was recently injected. In common cold measures, the amount of time required for an animal to withdrawal its paw from a cold stimulus is assessed. Electrical measures determine the timing and intensity of behavioural responses to electrical stimulation. Heat measures commonly assess the amount of time required for an animal to withdrawal its paw from a hot stimulus. Mechanical measures characterize the manner in which an animal responds to mechanical probing of a body part, or assess the amount of force required to elicit a behavioural response. Spontaneous measurements assess the quality and intensity of non-evoked behaviours exhibited at various bodily locations. The Mouse Grimace Scale is one such standardized tool that allows for measurement of facial expressions. b | The methods and results sections of preclinical reports published in the journal Pain in the years 1980, 1990, 2000, 2010 and 2016–2020 (506 articles) were coded for a variety of terms, including ‘stimulus modality’. Detailed modality definitions and complete datasets are included in Supplementary methods and Supplementary data 2. The graph shows the percentage of all studies published in Pain in the years listed that measured reflexive responses to chemical, cold, electrical, heat or mechanical stimuli or used spontaneous behavioural measures.
Table 1 |.
Classification of commonly used behavioural measures of pain
| Category | Evoked or non-evoked? | Example behaviours | Pros | Cons |
|---|---|---|---|---|
| Elective | Non-evoked | Cage hanging, nest building and social interaction | Similar to human pain
manifestations Can be tested in home cage |
May not be specific to pain |
| Nocifensive | Non-evoked | Grimace, writhing, gait changes and ultrasonic vocalizations | Automated scoring possible | Infrequently observed in chronic
models Stress associated with testing apparatus |
| Survival | Non evoked | Feeding and grooming | Easy to perform Can be tested in home cage |
May not be specific to pain |
| Conditioned | Non-evoked and evoked | CPP, CPA and operant measures | Measures affective/motivational aspect of pain | Requires specialized equipment Time intensive Stress associated with testing apparatus |
| Reflexive | Evoked | von Frey, radiant heat withdrawal and the acetone test | Easy to perform Can control stimulus intensity |
Not reflective of affective
state Experimenter presence, skill and bias affect results May reflect motor deficits, not analgesia Stress associated with testing apparatus |
CPA, conditioned place aversion; CPP, conditioned place preference.
Von Frey monofilaments.
A set of calibrated nylon filaments used for measuring mechanical sensitivity.
Our analysis suggests that there is an extremely wide range of observed naive von Frey withdrawal thresholds (Supplementary data 1), which can probably be attributed to the diversity of responses that are exhibited by naive and injured animals, and the lack of positive response standardization between experimenters and laboratories (for example, is toe flaring considered a withdrawal?). To this end, significant effort has recently been put into the development of automated, multidimensional withdrawal evaluation methods. High-speed video recordings have allowed individual components of stimulus-evoked paw withdrawals (such as paw height and velocity) to be analysed on a millisecond timescale127–129. Coupled with machine learning, these analyses provide unbiased assessments of paw withdrawal dynamics129,130 on a level of resolution that allows experimenters to simultaneously assess multiple behaviours such as withdrawal and shaking. This machine learning approach has also been used to assess behavioural responses to dynamic mechanical stimuli such as a brush or cotton swab128. The inclusion of both dynamic mechanical stimuli and innocuous and noxious static mechanical stimuli is encouraged in behaviour assessments, considering the divergent neuronal circuitry that mediates behavioural responses to these stimuli (reviewed in REF.131) and the high frequency of dynamic mechanical stimulation that occurs in routine day-to-day activities. Another less frequently used form of mechanical stimulation is deep tissue pressure: the Randall–Selitto test132 (performed on the hind paw) and the use of strain-gauged forceps on muscle133 are examples of this stimulus modality. However, one disadvantage of both of these tests is the stress associated with the required animal restraint.
Machine learning.
Computer systems that are able to learn without following explicit instructions, by using algorithms that analyse and draw inferences from patterns in data.
While not as prevalent as mechanical sensitivity assessments, heat and cold sensitivity assessments are still routinely used in pain research. Our analysis suggests that the use of the hot plate test134 has declined over time as the paw withdrawal to radiant heat (Hargreaves test)135, which allows separate determinations of heat sensitivity on each hind paw, has become the predominant method (Supplementary Fig. 7). Cold sensitivity is most commonly assessed via the acetone evaporation test136 or the cold plate test137. The cold plantar assay138 is a similar reflexive test that could be easily integrated into automated video analyses and, therefore, should be more widely adopted. Combined use of evoked mechanical and thermal behaviours accurately models the experience of clinical quantitative sensory testing. However, there is significant concern regarding reliance on these evoked response techniques as they do not reflect the most bothersome symptoms experienced by patients with chronic pain — which are, by far, spontaneous and movement-evoked pain139 — and evoked stimulus measures were recently shown to be unable to differentiate patients with painful and painless neuropathy140.
Hargreaves test.
An assay of thermal pain, also known as the radiant-heat paw withdrawal test.
For the purposes of this Review, all other pain behavioural measures are broadly defined as non-evoked; that is, elicited without an exogenous stimulus being applied by an experimenter at the time of measurement (TABLE 1). This class of measurements is extremely broad and contains reflexive, survival, elective and conditioned behaviours, all of which can be modified by ongoing or ‘spontaneous’ pain141. As noted earlier herein, ongoing pain (together with movement-evoked pain) is a significantly greater and far more common clinical problem than mechanical or cold hypersensitivity. Despite this, our analysis showed that only 13% of measurements reported in recent Pain articles investigated ongoing pain (FIG. 4b). Therefore, we recommend use of non-evoked behaviour measures in addition to evoked behaviours to better assess the entirety of an animal’s ongoing pain experience.
Algogen administration into body parts such as the hind paw or abdomen is associated with various time-limited spontaneous behavioural responses that have been termed ‘nocifensive’. More popular in the 1980s and 1990s (FIG. 2a), pain assays including acetic acid-induced writhing, the capsaicin test85 and the first phase of the formalin test142 measure reflexive behaviours that are maintained in decerebrate animals143,144. More relevant involuntary measures of ongoing pain include visual and audible assessments of animal well-being. Like humans, rodents are capable of exhibiting emotional states through facial expressions145–147. Similar to the automated paw withdrawal assessments described above, the mouse146 and rat147 grimace scales average individual facial features such as orbital tightening or ear position, to result in a cumulative pain score. Facial recognition software, automated scoring and machine learning platforms have recently been developed to standardize these measures between laboratories148 and allow the assessment of additional emotions145. Pain may149 (or may not150) also be visually relayed through anatomically specific protective behaviours, such as limb guarding and gait changes. Finally, audible and ultrasonic vocalizations have been used, albeit with mixed success, to measure ongoing pain151,152.
Writhing.
A measure of visceral pain, characterized by stereotypical abdominal constrictions.
Formalin test.
An assay of chemical/inflammatory pain in which dilute formaldehyde is injected into the hind paw; subsequent recuperative behaviours are directed towards the injected paw.
Grimace scales.
Scales to quantify animal pain levels on the basis of facial expressions.
A future goal of preclinical pain research should be the incorporation of more unbiased and long-lasting rodent behavioural assessments. Since the review of this topic by one of the current authors4, rapid technological progress has accelerated the parallel assessment of in vivo neural activity and naturalistic behaviours (neuroethology) in rodents. The ability to simultaneously measure nuanced facets of rodent behaviour while recording and/or manipulating nervous system activity will provide new insight into the biological basis of pain expression in rodents. Previously, complex survival and elective tasks were selected for study in pain models on the basis of anthropomorphized views of rodent behaviour. As predicted153, many of these behaviours are supressed following injury, including feeding153, burrowing154,155, wheel running156–159, grooming, nest building, cage lid hanging160 and reward seeking. However, suppression of these behaviours is not ubiquitously observed across injury modalities160–162, and sometimes a reduction in these behaviours arises independently of pain163. Far exceeding the analysis power of individual behaviour scoring, the development of home-cage monitoring systems has allowed unprecedented observation of naturalistic rodent behaviours before and following injury. Early versions of these systems could record only the activity of socially isolated animals and were trained to detect a limited number of behaviours, thus revealing little more than the previously described manual observations160,164–166. Very recent advances in high-speed videography, animal tracking software (such as DeepLabCut167 and two tools described in recent preprints — AlphaTracker168 and SLEAP169) and machine learning algorithms (such as MoSeq170 and two algorithms described in recent preprints — SimBA171 and DeepEthogram172), however, now allow the unbiased classification of subsecond behaviours from multiple rodents within the same cage. The power of this approach has already been capitalized to characterize unique rodent behaviours induced by psychoactive drug administration173; similar studies by pain researchers may result in the development of unique injury or analgesic behavioural profiles, the resolution of which far exceeds any manually scored behaviour. We warn, however, that just because it is increasingly possible to observe and quantify new, subtle behaviours, this does not mean they are specific to or selective for pain.
Operant and classical conditioning paradigms are also used in some pain research studies (Supplementary Fig. 9). Conditioned place aversion and analgesic conditioned place preference paradigms are two of the most commonly used tasks of this type. Relying on the principles of Pavlovian conditioning, conditioned place aversion and conditioned place preference paradigm design allows rodents to associate the affective state induced by a given manipulation with a distinct spatial environment; changes in manipulation-associated chamber residency time before and following conditioning trials depend on the appetitive174,175 or aversive176 nature of the manipulation. Conditioned place aversion is routinely performed in naive animals, using cell type-specific optogenetic and chemogenetic approaches to determine if activity (or lack thereof) of a certain brain region or cell population contributes to the negative affective state associated with pain177,178. Alternatively, conditioned place preference is primarily used in injured animals so that the analgesic or ‘relief of aversive state’ effects of pharmacological, optogenetic or chemogenetic manipulations can be ascertained174,179. Real-time versions of these approaches that do not rely on conditioning have also been used to directly assess the perception that arises as a result of a neuronal manipulation180–183 or the perception associated with evoked stimuli184–187. In addition to use of classical conditioning paradigms, the use of operant conditioning assays such as the mechanical conflict avoidance assay188, the operant plantar thermal assay189 and the operant facial thermal assay190 can be helpful for determining pain tolerance to complement the assessment of pain threshold which is routinely measured in evoked assays.
Conditioned place aversion.
An indirect measure of pain based on an animal learning an association between an environment with distinct cues and pain; the animal will avoid the environment paired with pain.
Conditioned place preference.
An indirect measure of pain based on an animal learning an association between an environment with distinct cues and pain relief via analgesic administration; the animal will spend more time in the environment paired with the analgesic.
Operant conditioning assays.
Associative learning processes through which the strength of a behaviour is modified by reinforcement or punishment (for example, pain).
Similarly to heterogeneous reports from patients with chronic pain, it is exceedingly unlikely that any one measure will ever be able to accurately and reproducibly assess pain across the diversity of injuries and assays described in the previous section. The current use of uniform measures across various injury modalities has likely biased model development to select for injuries that elicit maximum hypersensitivity, hence a robust dynamic range for treatment, in every animal (such as von Frey fibre-measured allodynia after spared nerve injury) as opposed to injuries that have variable symptoms from subject to subject, which more accurately reflect the variability in patient injuries and symptoms. The recent popularity of plotting individual subject data points has allowed a more thorough assessment of animal pain model heterogeneity. However, the question of what to do with so-called outliers still remains. Ultimately, injury-specific batteries of non-evoked and evoked assays, in addition to tests of pain co-morbidities such as anxiety and depression, will likely allow the most thorough assessment of pain-manipulated behaviours in rodents.
In addition to measurement selection, decisions regarding the number, frequency and timing of measurement collection should be made before beginning an experiment. According to our analysis, close to 90% of preclinical publications in Pain between 2016 and 2020 reported baseline measurements. Accurate reporting of these values is critical for the proper identification and interpretation of subsequent behavioural outcomes. Twenty-seven percent of reports in Pain between 2016 and 2020 reported data from only one time point, and 22% of reports included data from the baseline and a second time point (Supplementary Fig. 10). Longitudinal testing and area under the curve analyses are advantageous in that they measure the magnitude of a given effect over time; whereas an analgesic effect of a given manipulation may not appear to be significantly different from the effect of a control manipulation at any given time point, the cumulative effect of the drug may prove notably greater. This inherent power of longitudinal testing is essentially nullified, however, when different cohorts of animals are used for each time point. Consistency in both the experimenter and the subject are key to successful interpretation of time course experiments.
Conclusions and recommendations
As noted in BOX 2, the use of human tissue in pain research has great potential benefits. However, by definition, the experience of pain cannot be measured in a dish, but rather can be measured only in a living animal.
Box 2 |. Pain modelling in human tissues.
The use of primary or derived human tissues is steadily increasing in preclinical pain research. as described in BOX 1, primary peripheral tissues are now being more readily acquired from both living and deceased tissue donors, a trend that will hopefully continue in the future210. Despite problems inherent to studying tissue in vitro211, primary human tissues still allow analgesic target expression validation in tissues of interest, before proceeding to clinical trials. Because acquisition of freshly harvested human neural tissue for functional analysis remains geographically, financially and temporally challenging, laboratories have also developed protocols for differentiating human induced pluripotent stem cells into primary sensory neurons212–217 and central nervous system interneurons218,219 as an alternative. One benefit of this system is the increased capacity to compare functional responses between tissue derived from patients with pain and tissue derived from patients without pain, a strategy that is exceedingly difficult (albeit not impossible) to achieve with primary human tissues220. Similarly, tissues from painful and non-painful sites within the same patient can be generated. Needing only a skin biopsy sample from patients, this approach was recently leveraged to investigate the effects of a rare genetic mutation in the PIEZO2 gene on sensory neuron mechanical responsiveness221. The potential benefits of human tissue use in pain research are vast. In addition to the in vitro analysis of neural tissues, which will provide new insight into the mechanisms of human nociception, the use of plasma or other readily collectable materials may also aid in biomarker identification.
Therefore, we are confident that animal models will continue to play a vital role in preclinical pain research. Nevertheless, the suboptimal status quo revealed by our analysis and described in the previous sections suggests that changes are needed to increase the translational relevance of animal pain models. With this in mind, we outline several recommendations (FIG. 5).
Fig. 5 |. Recommendations for increased translational relevance of animal pain models.
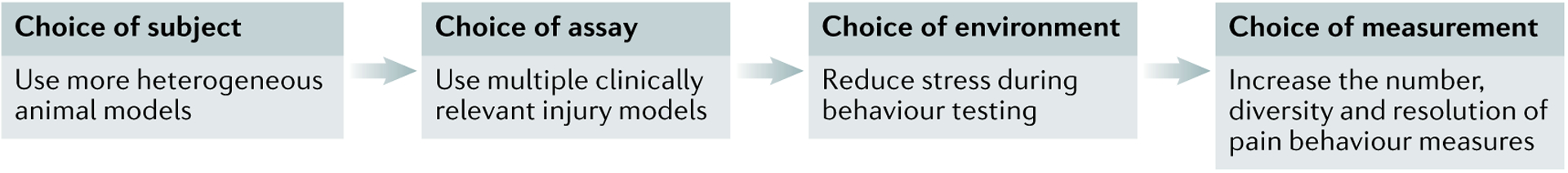
Before a preclinical pain experiment is begun, significant consideration should be given to the model organism, pain assay, holding and testing environments, and number and type of behavioural measures. Increased variability in model organism species, genetic background, age, sex and life history may increase the translatability of preclinical findings. To determine the generalizability of a new pain target or mechanism, experimenters should use multiple clinically relevant assays within a given study instead of a single, artificial injury. Specific care should be taken to minimize environmental stress before and during pain behaviour testing; appropriate habituation times are absolutely necessary to mitigate the effects of stress-induced analgesia or hyperalgesia on behavioural responses. Lastly, the number, type, and resolution of measures should be increased to allow more nuanced interpretations of pain behaviours during both the acute phase and the chronic phase of the injury.
Use more heterogeneous animal models.
Genetic, life-history and injury-specific factors undoubtedly influence pain in human patients. We should therefore consider letting these factors have a larger influence in our rodent models. By using an extremely reductionistic approach (for example, injecting 20 μl of CFA obtained from Sigma into the hind paw of an 8-week-old male C57BL/6 mouse purchased from The Jackson Laboratory), we are all studying the biology of a unique and artificial process that may have little if anything to do with human pain. After characterization of a potential analgesic in one assay, there is value in learning whether the analgesic is similarly efficacious in additional injury models, in both sexes and at various time points in the rodents’ life. Establishing the generalizability of a phenomenon (or the lack thereof) is just as important as the initial identification of that phenomenon in one defined context.
Increase use of non-neuropathic assays and related behavioural measures.
Back pain and migraine are the two leading causes of disability worldwide50, but animal models of these conditions are infrequently used in comparison with neuropathic and cutaneous inflammatory assays. To better match the needs of patients with chronic pain, musculoskeletal pain assays, migraine assays and associated pain measures such as deep tissue pressure tests for musculoskeletal pain or light aversion for migraine should be prioritized by basic pain researchers and not viewed as niche subfields studied by only a handful of laboratories.
Perform behavioural tests in more controlled environments.
Although increased variability in organisms and assays or injuries is encouraged, reduced variability in the act of pain behaviour testing is just as important. The impacts of stress on rodent pain behaviours cannot be overstated. If experimenters do not acknowledge and attempt to remedy the undue stress that they or the testing environment is causing animals, they may be unknowingly examining the effects of a given manipulation on stress biology as opposed to pain biology.
Collect more measurements over the time course of injury and healing.
The experience of pain changes over time. Therefore, any manipulation should be implemented and assessed at multiple time points during the injury trajectory. Furthermore, if one is assessing the effects of a pharmacological manipulation, multiple doses and multiple time points following dosing should be assessed; pharmacokinetic issues (plasma drug levels, target coverage, off-target effects) are vastly underappreciated by academic preclinical pain scientists, despite being a leading reason for translational failure.
Increase the diversity and resolution of pain behaviour measurements.
Touch hypersensitivity is rarely the primary concern of a patient with pain. Our over-reliance on dichotomous responses to von Frey stimulation is almost certainly due to the ease of assessment and the robust dynamic range it generates, but at what cost? Behavioural batteries involving unbiased and multidimensional pain measures should be tailored to the cause of pain. Ongoing pain measures may be more complicated and nuanced, but their increased use could lead to higher translation of pain targets.
Conclusions.
We recognize that these recommendations will require more time, money, effort and creativity on the part of experimenters. However, we and others191 believe that failed translational efforts are more costly in the long term. A specific recommendation that we believe will result in increased translational success is to increase collaborations between research teams with access to animal models and those with access to human tissues so as to bidirectionally validate new pain targets. Lastly, we encourage journals and funding agencies to incentivize and reward meticulously performed behavioural research in the same way that they reward and promote studies of novel molecular mechanism.
Supplementary Material
Acknowledgements
K.E.S. and C.L.S. are supported by grants from the US National Institutes of Health. J.S.M. is supported by funding from the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada and the Louise and Alan Edwards Foundation.
Footnotes
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41583-021-00536-7.
Competing interests
The authors declare no competing interests.
Data availability
The data supporting the findings of the analysis described in this Review are available in the files in Supplementary information.
References
- 1.Rice ASC, Smith BH & Blyth FM Pain and the global burden of disease. Pain 157, 791–796 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Woolf CJ Capturing novel non-opioid pain targets. Biol. Psychiatry 87, 74–81 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregory NS et al. An overview of animal models of pain: disease models and outcome measures. J. Pain 14, 1255–1269 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mogil JS Animal models of pain: progress and challenges. Nat. Rev. Neurosci 10, 283–294 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Yuan H, Spare NM & Silberstein SD Targeting CGRP for the prevention of migraine and cluster headache: a narrative review. Headache 59, 20–32 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Goadsby PJ, Edvinsson L & Ekman R Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol 28, 183–187 (1990). [DOI] [PubMed] [Google Scholar]
- 7.Lassen LH et al. CGRP may play a causative role in migraine. Cephalalgia 22, 54–61 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Levy D, Burstein R & Strassman AM Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann. Neurol 58, 698–705 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Wattiez AS, Wang M & Russo AF CGRP in animal models of migraine. Handb. Exp. Pharmacol 255, 85–107 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avona A et al. Dural calcitonin gene-related peptide produces female-specific responses in rodent migraine models. J. Neurosci 39, 4323–4331 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahlhamer J et al. Prevalence of chronic pain and high-impact chronic pain among adults — United States, 2016. MMWR 67, 1001–1006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klinck MP et al. Translational pain assessment: could natural animal models be the missing link? Pain 158, 1633–1646 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Lascelles BDX, Brown DC, Maixner W & Mogil JS Spontaneous painful disease in companion animals can facilitate the development of chronic pain therapies for humans. Osteoarthr. Cartil 26, 175–183 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Middleton SJ et al. Studying human nociceptors: from fundamentals to clinic. Brain 144, 1312–1335 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lariviere WR, Chesler EJ & Mogil JS Transgenic studies of pain and analgesia: mutation or background genotype? J. Pharmacol. Exp. Ther 297, 467–473 (2001). [PubMed] [Google Scholar]
- 16.Mogil JS et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain 80, 67–82 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Tuttle AH, Philip VM, Chesler EJ & Mogil JS Comparing phenotypic variation between inbred and outbred mice. Nat. Methods 15, 994–996 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mogil JS Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat. Rev. Neurosci 21, 353–365 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Clayton JA & Collins FS NIH to balance sex in cell and animal studies. Nature 509, 282–283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boerner KE et al. Conceptual complexity of gender and its relevance to pain. Pain 159, 2137–2141 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Bigal ME, Liberman JN & Lipton RB Age-dependent prevalence and clinical features of migraine. Neurology 67, 246–251 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Weyer AD et al. Nociceptor sensitization depends on age and pain chronicity. eNeuro 10.1523/ENEURO.0115-15.2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flurkey K, et al. ) in The Mouse in Biomedical Research 2nd edn Ch 20 (eds Fox JG et al. ) 637–672 (Academic Press, 2007). [Google Scholar]
- 24.Reichling DB & Levine JD Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 32, 611–618 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moloney RD et al. Early-life stress induces visceral hypersensitivity in mice. Neurosci. Lett 512, 99–102 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Coutinho SV et al. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am. J. Physiol. Gastrointest. Liver Physiol 282, G307–G316 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Pierce AN et al. Urinary bladder hypersensitivity and dysfunction in female mice following early life and adult stress. Brain Res. 1639, 58–73 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vilela FC, Vieira JS, Giusti-Paiva A & da Silva ML Experiencing early life maternal separation increases pain sensitivity in adult offspring. Int. J. Dev. Neurosci 62, 8–14 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Mizoguchi H et al. Maternal separation as a risk factor for aggravation of neuropathic pain in later life in mice. Behav. Brain Res 359, 942–949 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Smith BH, Hébert HL & Veluchamy A Neuropathic pain in the community: prevalence, impact, and risk factors. Pain 161, S127–S137 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Van Hecke O, Austin SK, Khan RA, Smith BH & Torrance N Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 155, 654–662 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Ho Kim S & Mo Chung J An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50, 355–363 (1992). [DOI] [PubMed] [Google Scholar]
- 33.Decosterd I & Woolf CJ Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Bennett GJ & Xie YK A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33, 87–107 (1988). [DOI] [PubMed] [Google Scholar]
- 35.Muralidharan A, Sotocinal SG, Austin J-S & Mogil JS The influence of aging and duration of nerve injury on the antiallodynic efficacy of analgesics in laboratory mice. Pain Rep. 5, e824 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gadgil S et al. A systematic summary and comparison of animal models for chemotherapy induced (peripheral) neuropathy (CIPN). PLoS ONE 14, e0221787 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pineda-Farias JB, Saloman JL & Scheff NN Animal models of cancer-related pain: current perspectives in translation. Front. Pharmacol 11, 1975 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donaldson LF, Seckl JR & McQueen DS A discrete adjuvant-induced monoarthritis in the rat: effects of adjuvant dose. J. Neurosci. Methods 49, 5–10 (1993). [DOI] [PubMed] [Google Scholar]
- 39.Gould HJ Complete Freund’s adjuvant-induced hyperalgesia: a human perception. Pain 85, 301–303 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Winter CA, Risley EA & Nuss GW Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med 111, 544–547 (1962). [DOI] [PubMed] [Google Scholar]
- 41.Brennan TJ, Vandermeulen EP & Gebhart GF Characterization of a rat model of incisional pain. Pain 64, 493–502 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Banik RK, Woo YC, Park SS & Brennan TJ Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology 105, 1246–1253 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Xu J & Brennan TJ Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology 112, 153–164 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radhakrishnan R, Moore SA & Sluka KA Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain 104, 567–577 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janusz MJ et al. Induction of osteoarthritis in the rat by surgical tear of the meniscus: inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthr. Cartil 10, 785–791 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Kamekura S et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr. Cartil 13, 632–641 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Van der Kraan PM, Vitters EL, Van de Putte LBA & Van den Berg WB Development of osteoarthritic lesions in mice by ‘metabolic’ and ‘mechanical’ alterations in the knee joints. Am. J. Pathol 135, 1001–1014 (1989). [PMC free article] [PubMed] [Google Scholar]
- 48.Courtenay JS, Dallman MJ, Dayan AD, Martin A & Mosedale B Immunisation against heterologous type II collagen induces arthritis in mice. Nature 283, 666–668 (1980). [DOI] [PubMed] [Google Scholar]
- 49.Caplazi P et al. Mouse models of rheumatoid arthritis. Vet. Pathol 52, 819–826 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Vos T et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burgos-Vega CC et al. Non-invasive dural stimulation in mice: a novel preclinical model of migraine. Cephalalgia 39, 123–134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oshinsky ML & Gomonchareonsiri S Episodic dural stimulation in awake rats: a model for recurrent headache. Headache 47, 1026–1036 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bates EA et al. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia 30, 170–178 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Felice M et al. Triptan-induced latent sensitization: a possible basis for medication overuse headache. Ann. Neurol 67, 325–337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi C et al. Animal models for studying the etiology and treatment of low back pain. J. Orthop. Res 36, 1305–1312 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daly C, Ghosh P, Jenkin G, Oehme D & Goldschlager T A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. BioMed. Res. Int 2016, 5952165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kosek E et al. Do we need a third mechanistic descriptor for chronic pain states? Pain 157, 1382–1386 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Queiroz LP Worldwide epidemiology of fibromyalgia. Curr. Pain Headache Rep 17, 356 (2013). [DOI] [PubMed] [Google Scholar]
- 59.DeSantana JM, da Cruz KML & Sluka KA Animal models of fibromyalgia. Arthritis Res. Ther 15, 222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sluka KA, Kalra A & Moore SA Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 24, 37–46 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Dina OA, Levine JD & Green PG Muscle inflammation induces a protein kinase Cε-dependent chronic-latent muscle pain. J. Pain 9, 457–462 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gregory NS, Gibson-Corley K, Frey-Law L & Sluka KA Fatigue-enhanced hyperalgesia in response to muscle insult: induction and development occur in a sex-dependent manner. Pain 154, 2668–2676 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagakura Y, Oe T, Aoki T & Matsuoka N Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: a putative animal model of fibromyalgia. Pain 146, 26–33 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Goebel A et al. Passive transfer of fibromyalgia symptoms from patients to mice. J. Clin. Invest 131, e144201 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oka P et al. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol 5, 908–917 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Berry SH et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the united states. J. Urol 186, 540–544 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Liang CZ, Shang X & Li H Chronic prostatitis/chronic pelvic pain syndrome: a disease or symptom? Current perspectives on diagnosis, treatment, and prognosis. Am. J. Men’s Health 14, 155798832090320 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ness TJ, Richter HE, Varner RE & Fillingim RB A psychophysical study of discomfort produced by repeated filling of the urinary bladder. Pain 76, 61–69 (1998). [DOI] [PubMed] [Google Scholar]
- 69.Ness TJ, Metcalf AM & Gebhart GF A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain 43, 377–386 (1990). [DOI] [PubMed] [Google Scholar]
- 70.Ness TJ & Elhefni H Reliable visceromotor responses are evoked by noxious bladder distention in mice. J. Urol 171, 1704–1708 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Ness TJ, Lewis-Sides A & Castroman P Characterization of pressor and visceromotor reflex responses to bladder distention in rats: sources of variability and effect of analgesics. J. Urol 165, 968–974 (2001). [PubMed] [Google Scholar]
- 72.Ness TJ & Gebhart GF Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 450, 153–169 (1988). [DOI] [PubMed] [Google Scholar]
- 73.Morris GP et al. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96, 795–803 (1989). [PubMed] [Google Scholar]
- 74.Okayasu I et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98, 694–702 (1990). [DOI] [PubMed] [Google Scholar]
- 75.Bon K, Lichtensteiger CA, Wilson SG & Mogil JS Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J. Urol 170, 1008–1012 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Coutinho SV, Meller ST & Gebhart GF Intracolonic zymosan produces visceral hyperalgesia in the rat that is mediated by spinal NMDA and non-NMDA receptors. Brain Res. 736, 7–15 (1996). [DOI] [PubMed] [Google Scholar]
- 77.Schwartzman RJ, Erwin KL & Alexander GM The natural history of complex regional pain syndrome. Clin. J. Pain 25, 273–280 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Guo TZ, Offley SC, Boyd EA, Jacobs CR & Kingery WS Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain 108, 95–107 (2004). [DOI] [PubMed] [Google Scholar]
- 79.Tajerian M et al. Sex differences in a murine model of complex regional pain syndrome. Neurobiol. Learn. Mem 123, 100–109 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coderre TJ, Xanthos DN, Francis L & Bennett GJ Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain 112, 94–105 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Siegel SM, Lee JW & Oaklander AL Needlestick distal nerve injury in rats models symptoms of Complex Regional Pain Syndrome. Anesth. Analg 105, 1820–1829 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Treede R-D et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain 160, 19–27 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Simone DA, Baumann TK & LaMotte RH Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain 38, 99–107 (1989). [DOI] [PubMed] [Google Scholar]
- 84.Sakurada T, Katsumata K, Tan-No K, Sakurada S & Kisara K The capsaicin test in mice for evaluating tachykinin antagonists in the spinal cord. Neuropharmacology 31, 1279–1285 (1992). [DOI] [PubMed] [Google Scholar]
- 85.Bonica J The Management of Pain (Lea & Febiger, 1953). [Google Scholar]
- 86.Butler RK & Finn DP Stress-induced analgesia. Prog. Neurobiol 88, 184–202 (2009). [DOI] [PubMed] [Google Scholar]
- 87.Imbe H, Iwai-Liao Y & Senba E Stress-induced hyperalgesia: animal models and putative mechanisms. Front. Biosci 11, 2179–2192 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Gamaro GD et al. The effects of acute and repeated restraint stress on the nociceptive response in rats. Physiol. Behav 63, 693–697 (1998). [DOI] [PubMed] [Google Scholar]
- 89.Gameiro GH et al. Nociception- and anxiety-like behavior in rats submitted to different periods of restraint stress. Physiol. Behav 87, 643–649 (2006). [DOI] [PubMed] [Google Scholar]
- 90.Obernier JA & Baldwin RL Establishing an appropriate period of acclimatization following transportation of laboratory animals. ILAR J. 47, 364–369 (2006). [DOI] [PubMed] [Google Scholar]
- 91.Moehring F, O’Hara CL & Stucky CL Bedding material affects mechanical thresholds, heat thresholds, and texture preference. J. Pain 17, 50–64 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robinson I, Dowdall T & Meert TF Development of neuropathic pain is affected by bedding texture in two models of peripheral nerve injury in rats. Neurosci. Lett 368, 107–111 (2004). [DOI] [PubMed] [Google Scholar]
- 93.Gabriel AF et al. Enriched environment and the recovery from inflammatory pain: social versus physical aspects and their interaction. Behav. Brain Res 208, 90–95 (2010). [DOI] [PubMed] [Google Scholar]
- 94.Vachon P et al. Alleviation of chronic neuropathic pain by environmental enrichment in mice well after the establishment of chronic pain. Behav. Brain Funct 9, 1–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tall JM Housing supplementation decreases the magnitude of inflammation-induced nociception in rats. Behav. Brain Res 197, 230–233 (2009). [DOI] [PubMed] [Google Scholar]
- 96.Shir Y, Ratner A & Seltzer Z Diet can modify autotomy behavior in rats following peripheral neurectomy. Neurosci. Lett 236, 71–74 (1997). [DOI] [PubMed] [Google Scholar]
- 97.Puglisi-Allegra S & Oliverio A Social isolation: effects on pain threshold and stress-induced analgesia. Pharmacol. Biochem. Behav 19, 679–681 (1983). [DOI] [PubMed] [Google Scholar]
- 98.Tuboly G, Benedek G & Horvath G Selective disturbance of pain sensitivity after social isolation. Physiol. Behav 96, 18–22 (2009). [DOI] [PubMed] [Google Scholar]
- 99.Meng Q, Li N, Han X, Shao F & Wang W Peri-adolescence isolation rearing alters social behavior and nociception in rats. Neurosci. Lett 480, 25–29 (2010). [DOI] [PubMed] [Google Scholar]
- 100.Lu YF et al. Social interaction with a cagemate in pain increases allogrooming and induces pain hypersensitivity in the observer rats. Neurosci. Lett 662, 385–388 (2018). [DOI] [PubMed] [Google Scholar]
- 101.Baptista-De-Souza D et al. Mice undergoing neuropathic pain induce anxiogenic-like effects and hypernociception in cagemates. Behav. Pharmacol 26, 664–672 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Smith ML, Asada N & Malenka RC Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science 371, 153–159 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Z et al. Social interaction with a cagemate in pain facilitates subsequent spinal nociception via activation of the medial prefrontal cortex in rats. Pain 155, 1253–1261 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Langford DJ et al. Social modulation of pain as evidence for empathy in mice. Science 312, 1967–1970 (2006). [DOI] [PubMed] [Google Scholar]
- 105.Martin LJ et al. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr. Biol 25, 326–332 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Langford DJ et al. Varying perceived social threat modulates pain behavior in male mice. J. Pain 12, 125–132 (2011). [DOI] [PubMed] [Google Scholar]
- 107.Smith ML, Hostetler CM, Heinricher MM & Ryabinin AE Social transfer of pain in mice. Sci. Adv 2, e1600855 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raber P & Devor M Social variables affect phenotype in the neuroma model of neuropathic pain. Pain 97, 139–150 (2002). [DOI] [PubMed] [Google Scholar]
- 109.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL & Mogil JS Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci. Biobehav. Rev 26, 907–923 (2002). [DOI] [PubMed] [Google Scholar]
- 110.Sharp J, Zammit T, Azar T & Lawson D Are ‘by-stander’ female Sprague-Dawley rats affected by experimental procedures? Contemp. Top. Lab. Anim. Sci 42, 19–27 (2003). [PubMed] [Google Scholar]
- 111.Kugler J, Lange KW & Kalveram KT Influence of bleeding order on plasma corticosterone concentration in the mouse. Exp. Clin. Endocrinol. Diabetes 91, 241–243 (1988). [DOI] [PubMed] [Google Scholar]
- 112.Drozdowicz CK, Bowman TA, Webb ML & Lang CM Effect of in-house transport on murine plasma corticosterone concentration and blood lymphocyte populations. Am. J. Vet. Res 51, 1841–1846 (1990). [PubMed] [Google Scholar]
- 113.Tuli JS, Smith JA & Morton DB Stress measurements in mice after transportation. Lab. Anim 29, 132–138 (1995). [DOI] [PubMed] [Google Scholar]
- 114.Brown GM & Martin JB Corticosterone, prolactin, and growth hormone responses to handling and new environment in the rat. Psychosom. Med 36, 241–247 (1974). [DOI] [PubMed] [Google Scholar]
- 115.Pitcher GM, Ritchie J & Henry JL Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. J. Neurosci. Methods 87, 185–193 (1999). [DOI] [PubMed] [Google Scholar]
- 116.Callahan BL, Gil ASC, Levesque A & Mogil JS Modulation of mechanical and thermal nociceptive sensitivity in the laboratory mouse by behavioral state. J. Pain 9, 174–184 (2008). [DOI] [PubMed] [Google Scholar]
- 117.Sorge RE et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods 11, 629–632 (2014). [DOI] [PubMed] [Google Scholar]
- 118.Konecka AM & Sroczynska I Circadian rhythm of pain in male mice. Gen. Pharmacol 31, 809–810 (1998). [DOI] [PubMed] [Google Scholar]
- 119.Minett MS, Eijkelkamp N & Wood JN Significant determinants of mouse pain behaviour. PLoS ONE 9, e104458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL & Mogil JS Influences of laboratory environment on behavior. Nat. Neurosci 5, 1101–1102 (2002). [DOI] [PubMed] [Google Scholar]
- 121.Balcombe JP, Barnard ND & Sandusky C Laboratory routines cause animal stress. Contemp. Top. Lab. Anim. Sci 43, 42–51 (2004). [PubMed] [Google Scholar]
- 122.Martin LJ, Acland EL, Carlson EN, Schweinhardt P & Mogil Correspondence JS Male-specific conditioned pain hypersensitivity in mice and humans. Curr. Biol 29, 192–201 (2019). [DOI] [PubMed] [Google Scholar]
- 123.Fanselow MS Odors released by stressed rats produce opioid analgesia in unstressed rats. Behav. Neurosci 99, 589–600 (1985). [DOI] [PubMed] [Google Scholar]
- 124.Backonja MM & Stacey B Neuropathic pain symptoms relative to overall pain rating. J. Pain 5, 491–497 (2004). [DOI] [PubMed] [Google Scholar]
- 125.Chaplan SR, Bach FW, Pogrel JW, Chung JM & Yaksh TL Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994). [DOI] [PubMed] [Google Scholar]
- 126.Dixon WJ Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol 20, 441–462 (1980). [DOI] [PubMed] [Google Scholar]
- 127.Fried NT, Chamessian A, Zylka MJ & Abdus-Saboor I Improving pain assessment in mice and rats with advanced videography and computational approaches. Pain 161, 1420–1424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abdus-Saboor I et al. Development of a mouse pain scale using sub-second behavioral mapping and statistical modeling. CellReports 28, 1623–1634 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jones JM et al. A machine-vision approach for automated pain measurement at millisecond timescales. Elife 9, e57258 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burdge J, Fried NT & Abdus-Saboor I Using high-speed videography for objective and reproducible pain measurement on a mouse pain scale. Star. Protoc 2, 100322 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.La JH & Chung JM Peripheral afferents and spinal inhibitory system in dynamic and static mechanical allodynia. Pain 158, 2285–2289 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Randall LO & Selitto JJ A method for measurement of analgesic activity on inflamed tissue. Arch. Int. Pharmacodyn. Ther 111, 409–419 (1957). [PubMed] [Google Scholar]
- 133.Skyba DA, Radhakrishnan R & Sluka KA Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. J. Pain 6, 41–47 (2005). [DOI] [PubMed] [Google Scholar]
- 134.Woolfe G & Macdonald AD The evaluation of the analgesic action of pethidine hydrochloride (Demerol). J. Pharmacol. Exp. Ther 80, 300–307 (1944). [Google Scholar]
- 135.Hargreaves K, Dubner R, Brown F, Flores C & Joris J A new and sensitive method for measuring thermal nociception. Pain 32, 77–88 (1988). [DOI] [PubMed] [Google Scholar]
- 136.Yoon C, Young Wook Y, Heung Sik N, Sun Ho K & Jin Mo C Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 59, 369–376 (1994). [DOI] [PubMed] [Google Scholar]
- 137.Allchorne AJ, Broom DC & Woolf CJ Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol. Pain 1, 36 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brenner DS, Golden JP & Gereau IV RW A novel behavioral assay for measuring cold sensation in mice. PLoS ONE 7, e39765 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schmelz M What can we learn from the failure of quantitative sensory testing? Pain 162, 663–664 (2021). [DOI] [PubMed] [Google Scholar]
- 140.Forstenpointner J et al. No pain, still gain (of function): the relation between sensory profiles and the presence or absence of self-reported pain in a large multicenter cohort of patients with neuropathy. Pain 162, 718–727 (2021). [DOI] [PubMed] [Google Scholar]
- 141.Bennett GJ What is spontaneous pain and who has it? J. Pain 13, 921–929 (2012). [DOI] [PubMed] [Google Scholar]
- 142.Hunskaar S & Hole K The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30, 103–114 (1987). [DOI] [PubMed] [Google Scholar]
- 143.Matthies BK & Franklin KBJ Formalin pain is expressed in decerebrate rats but not attenuated by morphine. Pain 51, 199–206 (1992). [DOI] [PubMed] [Google Scholar]
- 144.Woolf CJ Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain 18, 325–343 (1984). [DOI] [PubMed] [Google Scholar]
- 145.Dolensek N, Gehrlach DA, Klein AS & Gogolla N Facial expressions of emotion states and their neuronal correlates in mice. Science 368, 89–94 (2020). [DOI] [PubMed] [Google Scholar]
- 146.Langford DJ et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 7, 447–449 (2010). [DOI] [PubMed] [Google Scholar]
- 147.Sotocinal SG et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 7, 1744–8069–7–55 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tuttle AH et al. A deep neural network to assess spontaneous pain from mouse facial expressions. Mol. Pain 14, 1744806918763658 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Coulthard P, Pleuvry BJ, Brewster M, Wilson KL & Macfarlane TV Gait analysis as an objective measure in a chronic pain model. J. Neurosci. Methods 116, 197–213 (2002). [DOI] [PubMed] [Google Scholar]
- 150.Mogil JS et al. Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Mol. Pain 6, 1744–8069–6–34 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wallace VCJ, Norbury TA & Rice ASC Ultrasound vocalisation by rodents does not correlate with behavioural measures of persistent pain. Eur. J. Pain 9, 445 (2005). [DOI] [PubMed] [Google Scholar]
- 152.Han JS, Bird GC, Li W, Jones J & Neugebauer V Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J. Neurosci. Methods 141, 261–269 (2005). [DOI] [PubMed] [Google Scholar]
- 153.Negus SS, Bilsky EJ, Do Carmo GP & Stevenson GW Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. Methods Mol. Biol 617, 79–91 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jirkof P et al. Burrowing behavior as an indicator of post-laparotomy pain in mice. Front. Behav. Neurosci 4, 165 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Andrews N et al. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur. J. Pain 16, 485–495 (2012). [DOI] [PubMed] [Google Scholar]
- 156.Kandasamy R, Calsbeek JJ & Morgan MM Home cage wheel running is an objective and clinically relevant method to assess inflammatory pain in male and female rats. J. Neurosci. Methods 263, 115–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cobos EJ et al. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain 153, 876–884 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grace PM, Strand KA, Maier SF & Watkins LR Suppression of voluntary wheel running in rats is dependent on the site of inflammation: evidence for voluntary running as a measure of hind paw-evoked pain. J. Pain 15, 121–128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stevenson GW et al. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol. Biochem. Behav 98, 35–42 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang H et al. Cage-lid hanging behavior as a translationally relevant measure of pain in mice. Pain 162, 1416–1425 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sheahan TD et al. Inflammation and nerve injury minimally affect mouse voluntary behaviors proposed as indicators of pain. Neurobiol. Pain 2, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shepherd AJ, Cloud ME, Cao YQ & Mohapatra DP Deficits in burrowing behaviors are associated with mouse models of neuropathic but not inflammatory pain or migraine. Front. Behav. Neurosci 12, 124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jirkof P et al. Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Lab. Anim 47, 153–161 (2013). [DOI] [PubMed] [Google Scholar]
- 164.Urban R, Scherrer G, Goulding EH, Tecott LH & Basbaum AI Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 152, 990–1000 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brodkin J et al. Validation and implementation of a novel high-throughput behavioral phenotyping instrument for mice. J. Neurosci. Methods 224, 48–57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roughan JV, Wright-Williams SL & Flecknell PA Automated analysis of postoperative behaviour: assessment of homecagescan as a novel method to rapidly identify pain and analgesic effects in mice. Lab. Anim 43, 17–26 (2009). [DOI] [PubMed] [Google Scholar]
- 167.Mathis A et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci 21, 1281–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 168.Chen Z et al. AlphaTracker: a multi-animal tracking and behavioral analysis tool. bioRxiv 10.1101/2020.12.04.405159 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pereira TD et al. SLEAP: multi-animal pose tracking. bioRxiv. 10.1101/2020.08.31.276246 (2020). [DOI] [Google Scholar]
- 170.Wiltschko AB et al. Mapping sub-second structure in mouse behavior. Neuron 88, 1121–1135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nilsson SRO et al. Simple Behavioral Analysis (SimBA) – an open source toolkit for computer classification of complex social behaviors in experimental animals. bioRxiv 10.1101/2020.04.19.049452 (2020). [DOI] [Google Scholar]
- 172.Bohnslav JP et al. DeepEthogram: a machine learning pipeline for supervised behavior classification from raw pixels. bioRxiv 10.1101/2020.09.24.312504 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wiltschko AB et al. Revealing the structure of pharmacobehavioral space through motion sequencing. Nat. Neurosci 23, 1433–1443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.King T et al. Unmasking the tonic-aversive state in neuropathic pain. Nat. Neurosci 12, 1364–1366 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sufka KJ Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain 58, 355–366 (1994). [DOI] [PubMed] [Google Scholar]
- 176.Johansen JP, Fields HL & Manning BH The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc. Natl Acad. Sci. USA 98, 8077–8082 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Daou I et al. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J. Neurosci 33, 18631–18640 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Samineni VK, Grajales-Reyes JG, Sundaram SS, Yoo JJ & Gereau RW Cell type-specific modulation of sensory and affective components of itch in the periaqueductal gray. Nat. Commun 10, 1–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lax NC, George DC, Ignatz C & Kolber BJ The mGluR5 antagonist fenobam induces analgesic conditioned place preference in mice with spared nerve injury. PLoS ONE 9, e103524 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Massaly N et al. Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron 102, 564–573 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cowie AM, Moehring F, O’Hara C & Stucky CL Optogenetic inhibition of CGRPα sensory neurons reveals their distinct roles in neuropathic and incisional pain. J. Neurosci 38, 5807–5825 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang Y et al. Battery-free, fully implantable optofluidic cuff system for wireless optogenetic and pharmacological neuromodulation of peripheral nerves. Sci. Adv 5, eaaw5296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Samineni VK et al. Optogenetic silencing of nociceptive primary afferents reduces evoked and ongoing bladder pain. Sci. Rep 7, 15865 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Labuda CJ & Fuchs PN A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp. Neurol 163, 490–494 (2000). [DOI] [PubMed] [Google Scholar]
- 185.Wu HE, Gemes G, Zoga V, Kawano T & Hogan QH Learned avoidance from noxious mechanical simulation but not threshold Semmes Weinstein filament stimulation after nerve injury in rats. J. Pain 11, 280–286 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Moqrich A et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307, 1468–1472 (2005). [DOI] [PubMed] [Google Scholar]
- 187.Lee H, Iida T, Mizuno A, Suzuki M & Caterina MJ Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J. Neurosci 25, 1304–1310 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Harte SE, Meyers JB, Donahue RR, Taylor BK & Morrow TJ Mechanical Conflict System: a novel operant method for the assessment of nociceptive behavior. PLoS ONE 11, e0150164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Reker AN et al. The operant plantar thermal assay: a novel device for assessing thermal pain tolerance in mice. eNeuro 10.1523/ENEURO.0210-19.2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Neubert JK et al. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain 116, 386–395 (2005). [DOI] [PubMed] [Google Scholar]
- 191.Shansky RM & Murphy AZ Considering sex as a biological variable will require a global shift in science culture. Nat. Neurosci 24, 457–464 (2021). [DOI] [PubMed] [Google Scholar]
- 192.Rostock C, Schrenk-Siemens K, Pohle J & Siemens J Human vs. mouse nociceptors – similarities and differences. Neuroscience 387, 13–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Davidson S et al. Human sensory neurons: membrane properties and sensitization by inflammatory mediators. Pain 155, 1861–1870 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shiers S, Klein RM & Price TJ Quantitative differences in neuronal subpopulations between mouse and human dorsal root ganglia demonstrated with RNAscope in situ hybridization. Pain 161, 2410–2424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Han C et al. Human Nav1.8: enhanced persistent and ramp currents contribute to distinct firing properties of human DRG neurons. J. Neurophysiol 113, 3172–3185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang X, Priest BT, Belfer I & Gold MS Voltage-gated Na+ currents in human dorsal root ganglion neurons. eLife 6, e23235 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sheahan TD et al. Metabotropic glutamate receptor 2/3 (mGluR2/3) activation suppresses TRPV1 sensitization in mouse, but not human, sensory neurons. eNeuro 10.1523/ENEURO.0412-17.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang XL, Lee KY, Priest BT, Belfer I & Gold MS Inflammatory mediator-induced modulation of GABAA currents in human sensory neurons. Neuroscience 310, 401–409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang X et al. Nicotine evoked currents in human primary sensory neurons. J. Pain 20, 810–818 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Davidson S et al. Group II mGluRs suppress hyperexcitability in mouse and human nociceptors. Pain 157, 2081–2088 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Moy JK et al. Distribution of functional opioid receptors in human dorsal root ganglion neurons. Pain 161, 1636–1649 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dedek A et al. Loss of STEP61 couples disinhibition to N-methyl-d-aspartate receptor potentiation in rodent and human spinal pain processing. Brain 142, 1535–1546 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Price TJ & Flores CM Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J. Pain 8, 263–272 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rigaud M et al. Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain 136, 188–201 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sadler KE, Moehring F & Stucky CL Keratinocytes contribute to normal cold and heat sensation. eLife 9, e58625 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lacroix-Fralish ML, Austin JS, Zheng FY, Levitin DJ & Mogil JS Patterns of pain: meta-analysis of microarray studies of pain. Pain 152, 1888–1898 (2011). [DOI] [PubMed] [Google Scholar]
- 207.Kostowski W, Członkowski A, Rewerski W & Piechocki T Morphine action in grouped and isolated rats and mice. Psychopharmacology 53, 191–193 (1977). [DOI] [PubMed] [Google Scholar]
- 208.Macolino CM, Daiutolo BV, Albertson BK & Elliott MB Mechanical allodynia induced by traumatic brain injury is independent of restraint stress. J. Neurosci. Methods 226, 139–146 (2014). [DOI] [PubMed] [Google Scholar]
- 209.Kerstein PC, del Camino D, Moran MM & Stucky CL Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol. Pain 5, 19 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.W R et al. Human cells and networks of pain: transforming pain target identification and therapeutic development. Neuron 109, 1426–1429 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wangzhou A et al. Pharmacological target-focused transcriptomic analysis of native versus cultured human and mouse dorsal root ganglia. Pain 161, 1497–1517 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schrenk-Siemens K et al. PIEZO2 is required for mechanotransduction in human stem cell-derived touch receptors. Nat. Neurosci 18, 10–16 (2015). [DOI] [PubMed] [Google Scholar]
- 213.Wainger BJ et al. Modeling pain in vitro using nociceptor neurons reprogrammed from fibroblasts. Nat. Neurosci 18, 17–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schoepf CL et al. Selected ionotropic receptors and voltage-gated ion channels: more functional competence for human induced pluripotent stem cell (IPSC)-derived nociceptors. Brain Sci. 10, 344 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chambers SM et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat. Biotechnol 30, 715–720 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.McDermott LA et al. Defining the functional role of NaV1.7 in human nociception. Neuron 101, 905–919 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xiong C et al. Human induced pluripotent stem cell derived sensory neurons are sensitive to the neurotoxic effects of paclitaxel. Clin. Transl. Sci 14, 568–581 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gupta S et al. Deriving dorsal spinal sensory interneurons from human pluripotent stem cells. Stem Cell Rep. 10, 390–405 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kim TG et al. Efficient specification of interneurons from human pluripotent stem cells by dorsoventral and rostrocaudal modulation. Stem Cell 32, 1789–1804 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.North RY et al. Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain 142, 1215–1226 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nickolls A et al. Transcriptional programming of human mechanosensory neuron subtypes. SSRN Electron. J 30, 932–946 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of the analysis described in this Review are available in the files in Supplementary information.


